Abstract
Rationale: The survival benefit of lung transplantation (LT) in adult patients with cystic fibrosis (CF) is debated.
Objectives: We sought to assess the survival benefit of LT in adult patients with CF.
Methods: We used data from the United Network for Organ Sharing Registry to identify adult patients with CF on a wait list for LT in the United States between 2005 and 2009. Survival times while on the wait list and after LT were modeled by use of a Cox model that incorporated transplantation status as a time-dependent covariate. Evolution in lung allocation score (LAS) while on the wait list was used as a surrogate for disease severity. We fitted a model for the joint distribution of survival and longitudinal disease process (LAS over time).
Measurements and Main Results: A total of 704 adult patients with CF were registered on a wait list during the study period. The cumulative incidence of LT was 39.3% (95% confidence interval, 35.6–42.9%) at 3 months and 64.7% (61.0–68.4%) at 12 months, whereas the incidence of death while on the wait list at the same times was 8.5% (6.4–10.6%) and 12.9% (10.3–15.5%), respectively. Survival after LT was 96.5% (94.7–98.2%) at 3 months; 88.4% (85.1–91.8%) at 12 months; and 67.8% (59.9–76.8%) at 3 years. LT conferred a 69% reduction in the instantaneous risk of death (51–80%). The interaction between LAS and LT was significant: the higher the LAS, the greater the survival benefit of LT (P < 0.001).
Conclusions: LT confers a survival benefit for adult patients with CF.
Keywords: lung transplantation, cystic fibrosis, epidemiology
At a Glance Commentary
Scientific Knowledge on the Subject
Cystic fibrosis (CF) is the most common lethal genetic disease among whites. Most patients with CF are considered for lung transplantation (LT) at some point in their life. Previous studies assessing the survival benefit of LT in these patients have produced conflicting results, possibly because of the use of inappropriate statistical approaches that were unable to account for disease evolution after patients were placed on a wait list for LT. Moreover, the results of these studies may no longer be applicable to LT performed in the modern era.
What This Study Adds to the Field
We used data from the United Network of Organ Sharing Registry to identify adult patients with CF on a wait list for LT in the United States after the implementation of the lung allocation score (May 2005). We used newly developed statistical methods that allowed us to model the survival benefit of LT taking into account disease evolution after registration on the wait list. We showed that LT significantly improved the survival of patients with CF, with a 69% reduction in instantaneous risk of death.
Cystic fibrosis (CF) is the most common lethal genetic disease in white populations (1). Although CF affects the function of multiple organs, respiratory failure remains the most frequent cause of death and impairment. Lung transplantation (LT) can extend and substantially improve the quality of life in properly selected patients, and most patients with CF are considered for LT at some point in their life. According to the 2011 report of the International Society for Heart and Lung Transplantation, patients with CF comprise the third largest group undergoing LT (2, 3).
However, despite improvements in survival over time, survival after LT lags far behind that after other solid-organ transplantation, with median survival of 7.4 years for patients with CF according to the International Society for Heart and Lung Transplantation Registry (2). At the same time, the outlook for patients with the disease has improved substantially. For instance, the US Cystic Fibrosis Foundation’s projected life expectancy for patients has increased from 31 to 37 years over the past decade (1), and new therapeutics are under development (4). Consequently, the survival benefit of LT has been the subject of vigorous debate. Several studies have questioned the use of LT for CF and suggested that only a small fraction of patients with CF may actually benefit from LT in terms of survival (5–10). With lack of convincing evidence, healthcare organizations, such as the Arizona Health Care Cost Containment System (http://www.azahcccs.gov/), have stopped funding LT for adults, including those with CF.
Therefore, studies assessing the survival benefit of LT are of critical importance, because they are likely to drive healthcare organization benefits and thus hospital activity. In the absence of randomized controlled trials, deemed unfeasible and possibly unethical in this context, assessment of the survival benefit of LT relies on statistical modeling. The aim is to estimate whether transplantation is associated with improved survival in a cohort of patients on a wait list for LT (11). In general, this modeling involves a Cox model, with a time-dependent variable indicating whether or not a patient has received a transplant included in the model. In this model, other covariates are measured at the time of registration and are not allowed to vary over time. One of the main caveats of these techniques is that they do not take into account disease variables that change between registration and transplantation, which may occur during months or years after listing.
We aimed to assess the survival benefit of LT in adult patients with CF by using newly developed techniques to model the evolution in disease severity from registration on a wait list to transplantation (12).
Methods
This study was classified as exempt from review by the Mayo Clinic institutional review board.
Patients
Because spontaneous and post-transplant survivals evolve over time, we investigated only patients who were listed for a LT after the lung allocation score (LAS) was implemented in the United States (May 2005) (13). All data were supplied by the United Network for Organ Sharing (UNOS) as a Standard Analysis and Research file based on Organ Procurement and Transplantation Network data as of February 2009 that included a coded transplant center identifier and the full LAS history for each patient. The registry contains data for all patients who underwent LT in the United States since the registry’s inception, in 1987. We included patients if (1) they had a diagnosis of CF (UNOS code 1602); (2) they were 18 or older at the time of registration; (3) the first registration date was between May 4, 2005 and February 2009; (4) the date of LT was known; (5) the date of the last follow-up was known; and (6) the vital status at the last follow-up was known.
We used data from the UNOS registry on LT recipients from registration to transplantation, including the whole LAS record for all patients. Data related to donor and transplantation were also recorded. We excluded variables for which data were sparse or that described rare characteristics.
Outcomes
The primary outcome was survival time. Mortality data are validated with the social security death index in the UNOS STAR file.
Study Design
We assessed the survival benefit of LT by using a Cox model that incorporated the time of transplantation as a time-dependent covariate as previously described (11). Because patients who did and did not receive a graft may differ by disease severity, all models were adjusted on potential confounders. We adjusted our models on the LAS, a major marker of disease severity. The Cox model assumed that excluded patients (because of LT) shared the same risk of death as patients with the same covariate values who were not excluded (still waiting). The model assumed that the selection of LT recipients with the same LAS score was random. Thus, after the LAS score was included in the model, the concern about potential biases (e.g., informative censoring) in the analysis because of exclusion at the time of LT was minimized.
In a first set of analyses, we used the traditional approach, in which the only time-dependent covariate is transplantation; the other covariates included in the model are measured at baseline and are supposed to be time independent. This approach assumes that differences in patient severity measured at baseline account for the differences in severity between patients who will and will not eventually undergo LT.
In a second set of analyses, we included in our model the evolution of LAS over time. Because of the fact that LAS measurements over time contain biologic variation (i.e., LAS does not remain constant between two successive measurements of the patient), we used a joint model for longitudinal and survival data. To estimate the association between a single LAS measurement and survival time, standard statistical tools, such as Cox regression, are applicable. However, when it comes to the analysis of serial LAS measurements for estimation of survival time, the time-dependent Cox model is not appropriate (14–16). Problems arise from the fact that the LAS measurements contain temporal variation; that is, LAS does not remain constant in between two successive measurements of the patient, which is what the Cox model assumes (see Figure E1, bottom panel, dashed line, in the online supplement). The problem with ignoring this biologic variation and using the time-dependent Cox model is that derived results may be substantially biased (17, 18).
A relevant modeling framework capable of resolving these issues is the joint model for longitudinal and survival data described in Tsiatis and Davidian (16). This is a relatively new and powerful method that takes into account special features. The basic idea behind these models is to construct a suitable mixed-effects model to describe the evolution in time for the marker, and then to use these estimated evolutions as a time-dependent covariate in a Cox model instead of the observed marker. This idea is depicted graphically in Figure E1 where at each time point we associate the level of the marker as estimated from the mixed-effects models (bottom, solid line) with the risk for an event (top) (14, 16). The longitudinal process (LAS over time) was fitted by use of a linear mixed-effects model that included age, body mass index, sex, and functional status as fixed effects. In the random effects design matrix, we included an intercept and a time term, with the time effect modeled flexibly using splines to allow possibly nonlinear subject-specific evolutions. All variables in Table 1 were tested for inclusion in the longitudinal model. Decisions regarding the final model were based on the Akaike Information Criterion. For the survival submodel, the LAS as estimated from the longitudinal model, the LT covariate, and an interaction of these two variables were included, together with the following variables: age, body mass index, sex, and functional status.
TABLE 1.
MAIN CHARACTERISTICS OF PATIENTS AT REGISTRATION AND AT TRANSPLANTATION
| At Registration
(n =
704) |
At Transplantation
(n = 454)* |
|||
|---|---|---|---|---|
| Characteristics | Nonmissing Data (%) | Values | Nonmissing Data (%) | Values |
| Recipient | ||||
| Age, yr | 100 | 30.0 (9.4) | 100 | 30.3 (9.4) |
| Age distribution | 100 | 100 | ||
| 18–22 | 201 (28.6) | 114 (25.1) | ||
| 23–27 | 180 (25.6) | 131 (28.9) | ||
| 28–34 | 149 (21.2) | 100 (22.0) | ||
| >34 | 174 (24.7) | 109 (24.0) | ||
| Sex, male | 100 | 355 (50.4) | 100 | 251 (55.3) |
| Functional status† | 99.0 | 98.0 | ||
| Class I | 141 (20.3) | 86 (19.2) | ||
| Class II | 415 (59.8) | 246 (55.0) | ||
| Class III | 138 (19.9) | 115 (25.7) | ||
| 6-min-walk distance, ft, median (IQR) | 87.0 | 1,120 (800–1,360) | 98.0 | 950.0 (565–1,238) |
| Diabetes | 98.0 | 335 (48.6) | 98.0 | 225 (50.6) |
| FVC, % predicted | 94.0 | 40.0 (11.8) | 100 | 38.8 (13.4) |
| Pulmonary artery pressure, mm Hg | 75.0 | 38.1 (11.4) | 79.0 | 39.0 (11.5) |
| Body mass index, kg/m2 | 100 | 19.4 (3.6) | 100 | 19.3 (3.0) |
| Receiving mechanical ventilation | 100 | 40 (5.7) | 100 | 34 (7.5) |
| ECMO | 100 | 0 | 100 | 3 (0.7) |
| Surgery | ||||
| Ischemic time, h | 87.0 | 5.8 (1.7) | ||
| Distance for graft retrieval, miles, median (IQR) | 100 | 161.9 (23.6–316.6) | ||
| Donor | ||||
| Age, yr | 100 | 31.0 (13.5) | ||
| Sex, male | 100 | 275 (60.6) | ||
| Body mass index, kg/m2 | 99.9 | 24.4 (4.5) | ||
| Diabetes | 100 | 22 (4.9) | ||
| Cause of death | 100 | |||
| Anoxia | 39 (8.6) | |||
| Stroke | 140 (30.9) | |||
| Head trauma | 259 (57.2) | |||
| CNS tumor | 6 (1.3) | |||
| Others | 9 (2.0) | |||
Definition of abbreviations: CNS = central nervous system; ECMO = extracorporeal membrane oxygenation; IQR = interquartile range.
Data are from the United Network for Organ Sharing based on Organ Procurement and Transplantation Network data as of February 2009. Data are mean (SD) or number (%) unless otherwise indicated.
A total of 473 patients underwent lung transplantation, but data were not available for 19 (4.0%).
Ranges from class I to III, whether the patient performs activity of daily living with no, some, or total assistance, respectively.
We tested the proportional hazard assumption using a test based on Schoenefeld residuals. We also fitted additional Cox models with LT coded as a piece-wise, constant, time-dependent covariate.
All models were stratified on LT center, allowing for different baseline hazard function in each center.
Statistical Software
Data management involved use of Stata MP v12.0 (StataCorp, College Station, TX) and data analyses R 2.9.2 (R Foundation for Statistical Computing, Vienna, Austria). Stratification was by use of the “coxph” function (package “survival”) with the “strata” option. Joint models were fitted by use of the JM package (version 0.9–3) developed by the last author (D.R.) (19).
Results
Patients
There were 859 patients with CF on the waiting list during the study period; data for 155 patients (18.0%) younger than 18 years were eliminated. We used data for 704 adults registered on a wait list in 60 centers after LAS implementation. The main characteristics of these patients are in Table 1.
Among these patients, 473 underwent LT (67.2%); 100 (14.2%) died while on the wait list; and 131 (18.6%) were still waiting for LT at the end of the study period. At wait-list registration, 75 patients had a LAS set to 0, which indicated inactive status. The median LAS for the 629 remaining patients was 36.5 (range, 26.3–92.1; interquartile range [IQR], 34.4–40.0). The median LAS at transplantation (n = 473) was 39.3 (29.2–93.4 [36.6–45.2]). The LAS measured at the time of registration and at LT varied significantly among centers (P < 0.001).
Outcome while on the LT Wait List
The cumulative incidence of LT was 39.3% (35.6–42.9%) at 3 months; 53.9% (50.1–57.7%) at 6 months; and 64.7% (61.0–68.4%) at 12 months after registration. Conversely, the cumulative incidence of death while on the wait list at the same times was 8.5% (6.4–10.6%); 11.5% (9.1–13.9%); and 12.9% (10.3–15.5%), respectively. Figure E2 shows the cumulative incidence of transplantation and death while on the wait list by quartiles of the median LAS at registration; data for patients with a LAS of 0 at registration (n = 75) were removed because they were not eligible for LT (inactive status). As expected, cumulative incidence of LT and death were largely associated with LAS. For instance, 6 months after list registration, the proportion of patients who underwent LT ranged from 33.3% (25.7–40.1%) to 70.8% (63.3–78.3%) (P < 0.001) for the first and fourth LAS quartiles, respectively, whereas mortality while on the list at the same time ranged from 6.0% (2.2–9.8%) to 21.0% (14.3–27.6%) (P < 0.001).
Post-Transplant Survival
Among the 473 patients who underwent LT, post-transplant survival was not available for 19 (4.0%), who were lost to follow-up, and 70 patients died. Survival after LT was 96.5% (94.7–98.2%) at 3 months; 93.3% (90.9–95.8%) at 6 months; 88.4% (85.1–91.8%) at 12 months; and 67.8% (59.9–76.8%) at 3 years (Figure 1). In a Cox model stratified on center, the LAS at the time of LT was associated with decreased survival: hazard ratio (HR) 1.14 (95% confidence interval, 1.02–1.27) for each five-point increment in LAS score (P = 0.02). Residual plot results were consistent, with a linear relationship between LAS at LT and the log hazard for death (see Figure E3).
Figure 1.
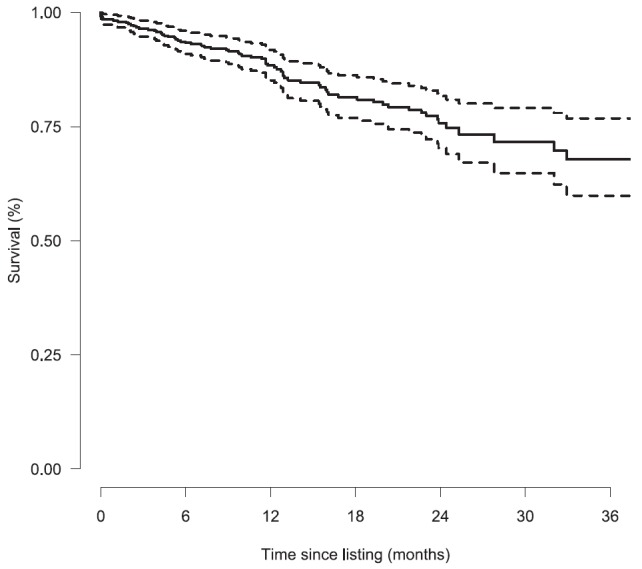
Kaplan-Meier estimator (with 95% confidence interval) for survival after lung transplantation for the 454 patients.
Survival Benefit of LT Using the Traditional Approach
In unadjusted models, LT was associated with a statistically significant reduction in the instantaneous risk of death (HR, 0.56 [0.36–0.87]; P < 0.01) (Table 2). On multivariable analysis, with adjustment for LAS at registration, age, 6-minute-walk distance, diabetes, and mechanical ventilation, and stratified on center, the HR for LT was 0.51 (0.32–0.81) (Table 3). The test of the proportional hazard assumption based on Schoenfeld residuals did not detect any departure from proportionality (P = 0.36) (see Figure E4). A piece-wise constant model gave similar results (data not shown).
TABLE 2.
UNIVARIATE ANALYSIS OF FACTORS ASSOCIATED WITH OUTCOME WHILE ON THE WAIT LIST FOR LUNG TRANSPLANTATION (UNADJUSTED MODEL)
| Hazard Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|
| Age, yr | 0.98 | 0.96–1.03 | 0.15 |
| Gender, male | 0.90 | 0.63–1.27 | 0.54 |
| Functional status | 1 | 0.25* | |
| Class II | 1.19 | 0.70–2.01 | |
| Class III | 1.71 | 0.87–3.34 | |
| 6-min-walk distance | 0.99 | 0.99–1.00 | 0.02 |
| Diabetes | 1.25 | 0.86–1.82 | 0.24 |
| Forced vital capacity | 1.00 | 0.99–1.02 | 0.36 |
| Body mass index | 1.02 | 0.97–1.08 | 0.36 |
| Pulmonary arterial pressure | 1.00 | 0.98–1.02 | 0.76 |
| Mechanical ventilation | 1.77 | 0.70–4.43 | 0.23 |
| Lung transplantation† | 0.56 | 0.36–0.87 | 0.01 |
All models included the lung allocation score measured at registration and were stratified on center.
Likelihood ratio test.
Indicator of lung transplantation status, modeled as a time-dependent covariate.
TABLE 3.
SURVIVAL BENEFIT OF LUNG TRANSPLANTATION ACCORDING TO TRADITIONAL COX MODEL OR JOINT MODEL
| Traditional Cox
Model |
Joint Model |
|||
|---|---|---|---|---|
| Characteristics | Hazard Ratio | P Value | Hazard Ratio | P Value |
| Model 1 | ||||
| LT | 0.51 | 0.32–0.81 | 0.31 | 0.20–0.49 |
| Model 2 | ||||
| LT | 0.54 | 0.34–0.86 | 1.20 | 0.72–2.0 |
| LT x LAS | 0.94 | 0.90–0.98 | 0.92 | 0.89–0.94 |
Definition of abbreviations: LAS = lung allocation score; LT = lung transplantation.
In these models, LAS is centered. Models are adjusted on LAS at registration, age, 6-minute-walk distance, diabetes, and mechanical ventilation, and stratified on center.
We tested whether the survival benefit of LT was associated with LAS measured at listing and found that the interaction of LAS at transplantation and LT was significant (P = 0.004). The impact of LT on survival depicted in Figure 2 suggests that LT confers a survival benefit for patients with LAS greater than 30. All but one patient who underwent LT had a LAS at transplant more than 30.
Figure 2.
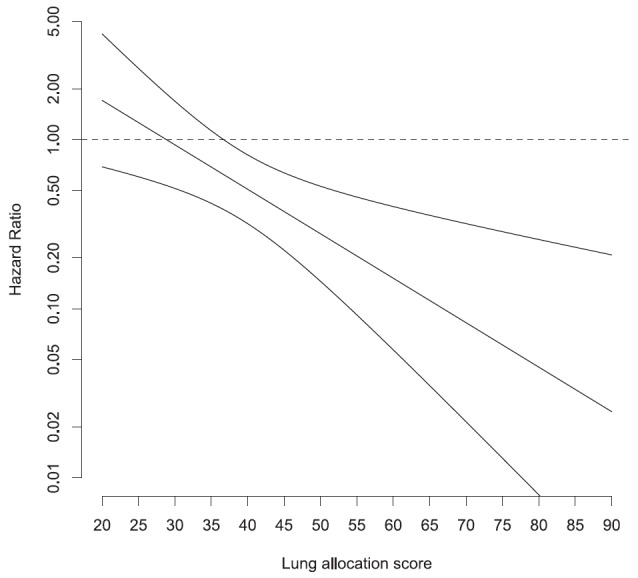
Hazard ratio for lung transplantation survival effect (with 95% confidence interval) by lung allocation score measured at registration for the wait list. Hazard ratio below 1 indicates a survival benefit.
Survival Benefit of LT Using the Joint Model
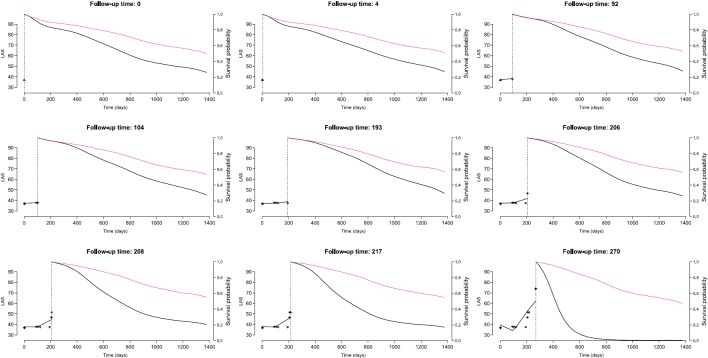
Length of stay on the wait list ranged from 1 to 1,377 days. The median number of LAS measurements per patient was 4 (range, 1–174; IQR, 2–9). The course of LAS varied substantially among patients as illustrated in Figure E5. The median increase in LAS over time was 0.24 points per month (IQR, 0.01–2.14). As assessed by the joint model, with adjustment for age, 6-minute-walk distance, diabetes, and mechanical ventilation, and stratified on center, the HR for LT was 0.31 (0.20–0.49), for a 69% reduction in the instantaneous risk of death with LT (Table 3). The interaction between transplantation LAS and LT was significant: the higher the LAS, the higher the survival benefit of LT (P < 0.001). Figure 3 gives the conditional probability of surviving after the last observed time for which a longitudinal measurement was available, either with or without a lung transplant for one of the patients on the waiting list. This figure shows that the expected benefit of LT for a given patient varies over time on the waiting list.
Figure 3.
Conditional probability of surviving later times than the last observed time for which a longitudinal measurement was available with (red) or without (black) a lung transplant. These probabilities are computed based on the true lung allocation score (LAS) history of one of the patients enrolled in this study. Expected survival is computed at each time a LAS measurement is available (asterisks). The black curve on the left is the fit of the mixed-effect model.
Discussion
LT is the best treatment available for selected patients with end-stage lung diseases. CF is a major indication for LT, accounting for 16.8% of all LTs performed worldwide in the last 15 years (2). Because the survival benefit of LT has been questioned in patients with CF, the LT community is committed to demonstrating that LT is effective in improving the outcome of these patients. So far, 13 studies have assessed the survival benefit of LT; nine included patients with CF (3). Results of these studies were mixed, some suggesting that patients with CF may not benefit from LT in terms of survival. These studies cast some doubt on the value of LT for patients with CF, which has in turn led some healthcare programs to deny patients with CF access to LT. Using appropriate statistical models in the post-LAS era, we present evidence of a survival benefit for adult patients with CF with LT.
Assessing the survival benefit of a complex procedure like LT is not straightforward (20). Randomized controlled trials are deemed the most appropriate method for comparing different intervention strategies and has been advocated to assess the survival benefit of LT in pediatric patients with CF (8). Such a trial has been conducted to assess the usefulness of liver transplantation in patients with Child-Pugh Stage B alcoholic cirrhosis (21). However, the external validity of such trials is debatable because we cannot assume that patients who agree to participate in such a trial form a random subsample of the target population. Because patients on an LT wait list are a highly selected subsample of all patients with end-stage respiratory disease, the most popular approach is to assess how LT affects the instantaneous risk of death of cohorts of patients on a wait list (8, 11). In this analysis, the transplantation status is incorporated in the model as a time-dependent variable. For instance, for a patient who spends 200 days on the list before LT, the transplantation status is 0 from Day 1 to 200 on the list and 1 from the date of transplantation onward. The exponential of the coefficient (i.e., the HR) for this time-dependent transplantation status measures the impact of LT on the instantaneous risk of death. This traditional model must account for the many other prognostic factors of survival while on the list, because patients who undergo LT are unlikely to form a random subsample of all patients on the wait list. In a sense, they have been selected by their ability to survive long enough to undergo LT. This approach has gained wide popularity in the field, probably because it is straightforward to implement and results are easy to interpret (8, 22, 23).
However, understanding the implications of this modeling is complex, and in general, the implications are largely overlooked. The first implication is the proportional hazard assumption. The model assumes that patients receiving transplantation face an instantaneous risk of death that is proportional to the instantaneous risk of death of patients on a wait list (multiplied by a constant quantity), whatever the time elapsed since the surgery. The beneficial effect of LT, if any, is supposed to take effect immediately after transplantation. This assumption does not hold true in many transplant settings. This has led several authors to use nonproportional hazards models (24). However, in the present study, we were unable to demonstrate a violation of this assumption with whatever technique we used.
The second assumption involves computing the impact of LT on the instantaneous risk of death by directly comparing the death rates after LT and while on the wait list. The model assumes that for given values of the covariates, transplanted patients would have faced the same risk of death while on the list as those who are not on the wait list. The model must then incorporate covariates that allow a sensible assessment of the risk of death while on the list to obtain a reasonable estimate of the effect of LT. Most prior studies using this methodology have adjusted only for baseline covariates. For instance, if LAS is used as a proxy for patient spontaneous prognosis, we assume that two patients with the same LAS at registration will share the same risk of death throughout. In general, this assumption is not true. Figure E5 shows that the LAS may not change for some patients indicating no increase in risk of death while on the list, but it may greatly increase for other patients, for a steep increase in risk of death.
In this study we used the entire LAS history for each patient up until the time of transplant to account for the evolution of patients’ prognosis over time. Because the LAS is updated frequently, we can adjust on the most recent LAS value. However, because LAS over time is directly correlated with the survival process, the inclusion of the LAS as a traditional, continuous, time-dependent covariate would provide biased estimates. Thus, a model for the joint distribution of longitudinal and survival outcomes is required to produce valid inferences (14, 16). Using such an approach, we could demonstrate that LT can confer a survival benefit for most patients with CF.
This study has limitations. First, we used the LAS as a measure of patients’ severity on the waiting list, whereas the LAS has been developed as a scoring system aiming to prioritize patients according to their expected survival benefit from LT. However, this study found a strong association between the LAS and survival on the list, making the LAS a reasonable proxy for patients’ severity. Second, we restricted our analysis to patients registered for a lung transplant. Because patients listed for a lung transplant form a selected subset of all patients with CF, our approach is likely to provide a more accurate estimate of the survival impact of LT; however, this approach limits the generalizability of our results in the sense that they apply only to patients who are eligible to a transplantation (25). Third, there is a large variability in the kinetics of LAS between individual patients (see Figure E5), with some patients having rather stable LAS values over a long period of time, whereas in others the LAS values rise quickly. Because our models did not take into account LAS slopes, we were not able to determine whether these two groups of patients have different survival benefits from lung transplant. Fourth, our assessment of the survival benefit is based on the HR for death between patients with and those without a lung transplant. It is possible that, although beneficial in terms of survival, LT performed in patients with extreme LAS values may result in unacceptable survival from a societal perspective. Fifth, we assessed only the benefit of LT on patients’ survival, whereas LT may be beneficial on other endpoints, such as quality of life. Other studies are required to assess these endpoints.
In conclusion, this study suggests that LT is beneficial in patients with CF in the modern era.
Footnotes
Author Contributions: Conception and design, G.T., J.D.C., H.M., M.F., O.B., Y.C., G.L., and D.R. Acquisition of data, G.T., J.D.C., and D.R. Analysis and interpretation of data, G.T., J.D.C., H.M., M.F., O.B., Y.C., G.L., and D.R. Drafting or revising the manuscript for important intellectual content, G.T., J.D.C., H.M., M.F., O.B., Y.C., G.L., and D.R. Final approval of the version to be published, G.T., J.D.C., H.M., M.F., O.B., Y.C., G.L., and D.R.
Supported by a grant from Vaincre la Mucoviscidose and by NIH grant HL115354. The funding sources had no role in design, conduct, or analysis of the study or the decision to submit the manuscript for publication. This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Originally Published in Press as DOI: 10.1164/rccm.201303-0429OC on April 13, 2013
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The registry of the International Society for Heart and Lung Transplantation: twenty-eighth adult lung and heart-lung transplant report–2011. J Heart Lung Transplant. 2011;30:1104–1122. doi: 10.1016/j.healun.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med. 2011;184:159–171. doi: 10.1164/rccm.201101-0134CI. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liou TG, Adler FR, Cahill BC, Cox DR. Correction: lung transplantation and survival in children with cystic fibrosis. N Engl J Med. 2008;359:536. doi: 10.1056/NEJMc086289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liou TG, Adler FR, Cahill BC, FitzSimmons SC, Huang D, Hibbs JR, Marshall BC. Survival effect of lung transplantation among patients with cystic fibrosis. JAMA. 2001;286:2683–2689. doi: 10.1001/jama.286.21.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liou TG, Adler FR, Cahill BC, FitzSimmons SC, Huang D, Hibbs JR, Marshall BC.Priorities for lung transplantation among patients with cystic fibrosis JAMA 20022871523–1524.author reply 1524–1525 [DOI] [PubMed] [Google Scholar]
- 8.Liou TG, Adler FR, Cox DR, Cahill BC. Lung transplantation and survival in children with cystic fibrosis. N Engl J Med. 2007;357:2143–2152. doi: 10.1056/NEJMoa066359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liou TG, Adler FR, Huang D. Use of lung transplantation survival models to refine patient selection in cystic fibrosis. Am J Respir Crit Care Med. 2005;171:1053–1059. doi: 10.1164/rccm.200407-900OC. [DOI] [PubMed] [Google Scholar]
- 10.Liou TG, Woo MS, Cahill BC. Lung transplantation for cystic fibrosis. Curr Opin Pulm Med. 2006;12:459–463. doi: 10.1097/01.mcp.0000245716.74385.3f. [DOI] [PubMed] [Google Scholar]
- 11.Crowley J, Hu M. Covariance analysis of heart transplant survival data. JASA. 1977;72:27–36. [Google Scholar]
- 12.Rizopoulos D. Dynamic predictions and prospective accuracy in joint models for longitudinal and time-to-event data. Biometrics. 2011;67:819–829. doi: 10.1111/j.1541-0420.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 13.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, Coke MA, Garrity ER, Sweet SC, Heiney DA, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol. 2010;28:2796–2801. doi: 10.1200/JCO.2009.25.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalbfleisch JD, Prentice RL. New York: John Wiley; 2002. The statistical analysis of failure time data, 2nd ed. [Google Scholar]
- 16.Tsiatis A, Davidian M. Joint modeling of longitudinal and time to event data: an overview. Stat Sin. 2004;14:809–834. [Google Scholar]
- 17.Prentice RL. Covariate measurement errors and parameter estimates in a failure time regression model. Biometrika. 1982;69:331–342. [Google Scholar]
- 18.Sweeting MJ, Thompson SG. Joint modelling of longitudinal and time-to-event data with application to predicting abdominal aortic aneurysm growth and rupture. Biom J. 2011;53:750–763. doi: 10.1002/bimj.201100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizopoulos D. JM: an R package for the joint modelling of longitudinal and time-to-event data. J Stat Softw. 2010;35:1–33. [Google Scholar]
- 20.Thabut G, Fournier M. Assessing survival benefits from lung transplantation. Rev Mal Respir. 2011;28:e1–e6. doi: 10.1016/j.rmr.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Vanlemmens C, Di Martino V, Milan C, Messner M, Minello A, Duvoux C, Poynard T, Perarnau JM, Piquet MA, Pageaux GP, et al. TRANSCIAL Study Group. Immediate listing for liver transplantation versus standard care for Child-Pugh stage B alcoholic cirrhosis: a randomized trial. Ann Intern Med. 2009;150:153–161. doi: 10.7326/0003-4819-150-3-200902030-00004. [DOI] [PubMed] [Google Scholar]
- 22.Thabut G, Mal H, Castier Y, Groussard O, Brugière O, Marrash-Chahla R, Lesèche G, Fournier M. Survival benefit of lung transplantation for patients with idiopathic pulmonary fibrosis. J Thorac Cardiovasc Surg. 2003;126:469–475. doi: 10.1016/s0022-5223(03)00600-7. [DOI] [PubMed] [Google Scholar]
- 23.Aurora P, Spencer H, Moreno-Galdó A. Lung transplantation in children with cystic fibrosis: a view from Europe. Am J Respir Crit Care Med. 2008;177:935–936. doi: 10.1164/rccm.200801-019ED. [DOI] [PubMed] [Google Scholar]
- 24.Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet. 1998;351:24–27. doi: 10.1016/S0140-6736(97)06405-2. [DOI] [PubMed] [Google Scholar]
- 25.Kotloff RM. Does lung transplantation confer a survival benefit? Curr Opin Organ Transplant. 2009;14:499–503. doi: 10.1097/MOT.0b013e32832fb9f8. [DOI] [PubMed] [Google Scholar]