Abstract
Aim
We aimed to investigate the effect of resveratrol (Rsv) on expression of genes regulating triglyceride (TG) accumulation and consumption in differentiated 3T3-L1 preadipocytes.
Methods
3T3-L1 preadipocytes were cultured in DMEM supplemented with 10% fetal calf serum. Upon reaching confluence, cells were induced to differentiate for 4 days, cultured for 10 days for TG accumulation, and then incubated with Rsv (0, 25 or 50 μM) for 3 days. TG accumulation was analyzed by Oil Red-O staining. To understand how Rsv regulates TG accumulation and consumption, changes in gene and protein expressions of several factors associated with free fatty acid (FFA) uptake and β-oxidation were investigated by real-time RT-PCR and Western blot. For further elucidation of underlying mechanisms, we also investigated gene expressions using Sirtuin1 (Sirt1) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) siRNA.
Results
Rsv dose dependently enhanced Sirt1 expression and reduced TG accumulation. Rsv-induced reduction of TG accumulation was abolished by inhibition of Sirt1 and PGC1α. Rsv also enhanced expressions of genes involved in FFA uptake [peroxisome proliferator-activated receptor-gamma (PPARγ) and lipoprotein lipase] and in β-oxidation regulation [PGC1-α and carnitine palmitoyl-transferase 1a (CPT1a)]. All these effects were abolished by Sirt1 inhibition.
Conclusion
The present results suggest that Rsv may augment synthesis and oxidation of fatty acid, and possibly increases energy utilization efficiency in adipocytes through activation of Sirt1. The present study may provide meaningful evidence supporting the efficacy of Rsv in the treatment of obesity.
Keywords: Resveratrol, Lipoprotein lipase, β-oxidation, TG accumulation
1. Introduction
Sirtuin1 (Sirt1) functions as a protein deacetylase to remove the acetyl groups of diverse proteins in a NAD-dependent manner [1]. Sirt1 participates in multiple cellular processes such as modulation of the cell cycle, metabolism and aging, through interaction with diverse substrates [2], [3], [4]. In adipose tissue, Sirt1 enhances metabolic efficiency by regulating adipokines [5]. Conversely, adipocyte Sirt1 expression is suppressed by high-fat feeding in rodents, and is also markedly reduced in the adipose tissue of obese humans and genetically obese rodents [6], [7].
Resveratrol (Rsv) is a widely studied phytochemical present in grapes, peanuts and red wine, and has potent pharmacological effects. Recently, Rsv has become available in tablet form and is recommended as a dietary supplement. The compound has been reported to have potent anti-obesity effects and to improve insulin sensitivity [8]. Recent data derived from animal studies have opened a new, promising perspective for the potential use of Rsv to prevent serious metabolic disorders such as obesity and diabetes [9]. Rsv supplementation in mice fed a high fat diet increased mitochondrial content and activity in skeletal muscle brown adipose tissue and the liver, thereby preventing the development of diet-induced obesity and improving metabolic disturbances [10]. Moreover, Rsv has attracted much attention for its ability to enhance the deacetylase activity of Sirt1 [11]. However, it remains controversial how Rsv, which mediates both synthesis and oxidation of fatty acid, exerts anti-obesity effect.
Obesity is linked to impaired glucose tolerance, dyslipidemia and hypertension, and contributes to the occurrence of atherosclerosis [12]. Environment, lifestyle, and genetic susceptibility are involved in the increased risk of obesity [13]. The condition is characterized by an increased fat mass mainly due to enlarged adipocytes. Adipocytes play an important role in maintaining metabolic health by functioning not only as an energy storage, but also as an active endocrine organ, regulating whole body metabolic homeostasis, appetite and energy consumption through secreting beneficial adipokines such as adiponectin and leptin under normal conditions.
Previous studies have demonstrated that enlarged adipocytes are associated with substantial changes in adipokines [14]. Tumor necrosis factor-alpha (TNF-α) is chronically elevated in adipose tissues of obese rodents and humans. Increased levels of TNF-α are implicated in the induction of chronic inflammation and oxidative stress, atherogenic adipokines such as plasminogen activator inhibitor-1 (PAI-1) and IL-6, and inhibition of the anti-atherogenic adipokine, adiponectin. Several studies reported that Rsv may inhibit chronic inflammation and oxidative stress by attenuating the TNF-α-induced changes of adipokines [15], [16].
The present study was conducted to evaluate the effect of Rsv on regulators involved in lipid metabolism using mouse 3T3-L1 adipocytes.
2. Materials and methods
2.1. Culture of 3T3-L1 preadipocytes
Mouse 3T3-L1 preadipocytes were cultured in Dulbecco modified Eagle medium (DMEM; Gibco BRL Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum (FCS) at 37 °C under 5% CO2. The cells (20,000 cells/well) were seeded in 6-well plate and incubated for 48 h. Cell differentiation was induced by supplementing the medium with dexamethasone (0.25 μmol/L), 3-isobutyl-1-methyx-anthine (0.5 mmol/L), and insulin (10 μg/mL) for 48 h. The differentiating media was replaced with 10% FBS/DMEM medium containing 167 nM insulin for another 48 h. Then, the cells were cultured in DMEM containing 10% FCS for 10 days, with medium change every 48 h. Thereafter, the cells were treated with or without Rsv (endowed by Sigma Chemical, St Louis, MO) at a final concentration of 25 or 50 mmol/L for 6 days.
2.2. Concentrations of Rsv tested
Rsv was purchased from Sigma Chemical, St Louis, MO. Commercial Rsv tablets are available at doses between 50 μg and 300 mg. A 25 mg oral dose results in peak plasma Rsv levels of approximately 2 μM and plasma half-life of 9.2 ± 0.6 h in human subjects [15]. These data show that the bioavailability of Rsv is very low, and only a small proportion reaches the blood and tissues. High concentration of Rsv treatment may induce cytotoxicity in 3T3-L1 adipocytes [17].
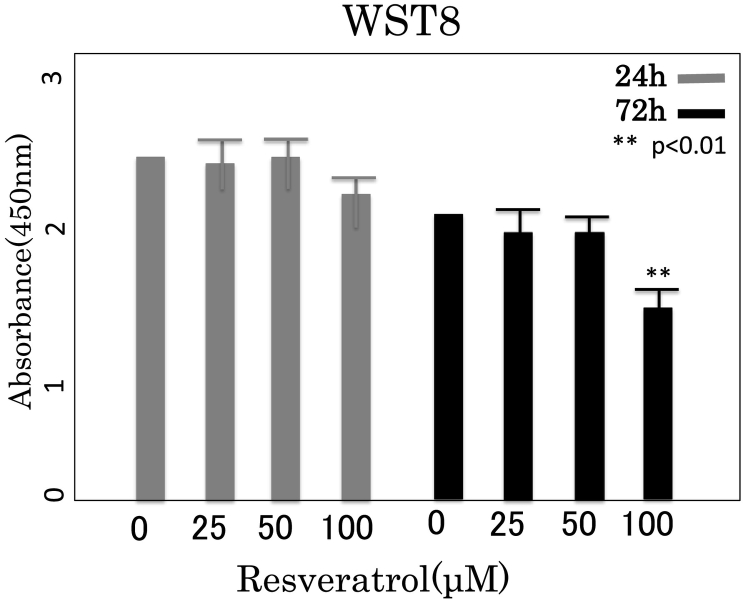
Viability of 3T3-L1 cells with resveratrol treatment was examined by the WST-8 (2-(2-methoxy-4-ni-trophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H tetrazolium, monosodium salt) assay which is reduced by dehydrogenases in cells to give a water-soluble orange colored product (formazan). 2 × 104 cells were incubated in 96-well microtiter cell culture plates, in a final volume of 100 μL. After 24 h and 72 h incubation, 10 μM of WST-8 solution was added to each well for an additional 1 h. A microplate reader was used to test the absorbance of each well at 450 nm. Cell viability was expressed as a percentage of control. Data are shown as the mean ± standard deviation of triplicate cultures. Therefore, we set the upper limit of Rsv concentration for testing to be 50 μM (Fig. 1).
Fig. 1.
Dosage effect of Rsv on cytotoxicity, proliferation, and adipogenesis in 3T3-L1 cells. Rsv decreased survival rate was examined by WST8 in pre-adipocyte.
The data are expressed as mean ± SD of three experiments. *P < 0.01 vs 0 μM.
2.3. Oil Red-O staining
To determine the triglycerides accumulated in 3T3-L1 cells, Oil Red-O staining was performed using 2-well chamber slide. Cells seeded in the wells (10,000 cells/well) were cultured until reaching confluency, and were stimulated to differentiate for 4 days. After removing the medium and washing twice with PBS, the cells were fixed with 4% paraformaldehyde and 8% saccharose in phosphate buffer saline (PBS) (1 mL/well) for 30 min. After washing with distilled water for 30 s, 60% isopropanol (1 mL/well) was added for 1 min. Oil Red-O (800 μL/well) was added and incubated at 37 °C for 15 min. After adding 60% isopropanol (1 mL/well) for 2 min, the wells were washed twice with distilled water. The cells were counterstained with Meyer hematoxylin solution (500 μL/well) for 20 s, and washed twice with distilled water. Phase contrast photomicrographs (20 × magnification) were recorded on digital camera. The area ratio (%) of Oil-red O staining as determined by ImageJ.
2.4. Reverse transcriptase polymerase chain reactions
Expressions of Sirt1, lipoprotein lipase (LPL), peroxisome proliferator-activated receptor-gamma (PPARγ), carnitine palmitoyl-transferase 1a (CPT1a), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) genes in 3T3-L1 cells were measured using reverse transcriptase polymerase chain reaction (PCR). RNeasy Mini Kit (QIAGEN, Hilden, Germany) was used to isolate total RNA from 3T3-L1 cells grown in 6-well plates. The amount of RNA was measured as absorbance at 260 nm. Synthesis of complementary DNA (cDNA) and PCR were performed using the PrimeScript™ RT reagent Kit (TaKaRa Bio Inc., Japan). Total RNA from treated 3T3-L1 cells was isolated using the RNeasy Kit (QIAGEN). Quantification of mRNA expression was performed using SYBR®Premix Ex Taq™ II (TaKaRa) with an Mx3005P Real-Time QPCR System (Agilent Technologies). The primers used for Sirt1, LPL, PPARγ, PGC1α, CPT1a, and 18s are shown in Table 1.
Table 1.
Primers used in PCR amplification of each gene studied.
| Gene symbol | Sense primer | Antisense primer |
|---|---|---|
| Sirt1 | CAGACCCTCAAGCCATGTTTGATA | TTGGATTCCTGCAACCTGCTC |
| 18-S | TTCTGGCCAACGGTCTAGACAAC | CCAGTGGTCTTGGTGTGCTGA |
| PPARγ | CCGAGTCTGTGGGGATAAAG | GGATCCGGCAGTTAAGATCA |
| LPL | AGGACCCCTGAAGACAC | GGCACCCAACTCTCATA |
| PGC1α | CCCTCAGGAGGCAGAAGAG | AGTGCTAAGACCGCTGCATT |
| CPT1α | TCAATCGGACCCTAGACACC | TGGTAGGAGAGCAGCACCTT |
2.5. Western blots analysis
Protein expressions of Sirt1, LPL, and PGC-1α were detected by Western blot analysis. Cells were suspended in a lysis buffer containing 50 mM HEPES (pH 7.0), 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 0.1% sodium vanadate and pepstatin A (Sigma) at 4 °C, and sonicated. After centrifugation at 10,000 rpm, protein concentration in the supernatant was measured using the Pierce BCA Protein Assay Kit (Thermo Scientific). Equal amounts of protein (20 to 60 μg) were resolved by SDS-PAGE on a 10% acrylamide gel and transferred to PVDF membranes. The membranes were incubated with the following primary antibodies: goat antiserum against mouse Sirt1 (1:1000; Santa Cruz Biotechnology), rabbit antiserum against mouse LPL (1:1000; Santa Cruz Biotechnology), rabbit antiserum against mouse PGC1α (1:1000; Santa Cruz Biotechnology), and mouse antiserum against β-actin (1:2000; Santa Cruz Biotechnology) as negative control; and the following secondary antibodies: donkey antiserum against goat IgG (1:2000; R&D system), donkey antiserum against rabbit IgG (1:2000; GE Healthcare), and sheep antiserum against mouse IgG (1:2000; GE Healthcare). Bound antibodies were visualized using ECL Prime Western Blotting Detection Reagent (GE Healthcare) and imaged by ChemiDoc XRS + imaging system (Bio Rad).
2.6. Knockdown of Sirt1 and PGC1α by RNA interference
Confluent 3T3-L1 cells (plated and cultured as described in the cell culture section) were transfected with Sirt1 and PGC1α siRNA oligonucleotide duplexes. One day after reaching confluence, the cells were transfected with the respective siRNA and lipofectamine RNAiMAX reagent (Thermo Fisher Scientific). For a 6-well plate, 10 nM of siRNA and 6 μL/well of lipofectamine were generally used. Lipofectamine RNAiMAX and siRNA were individually diluted in 100 μl Opti-MEM medium, mixed, incubated for 5 min at room temperature, and then added to each well. The cells were incubated at 37 °C for 3 days. At the same time of transfection, 25 or 50 μM of Rsv was added to the cell culture. The effect of siRNA knockdown was determined after 3 days of cell differentiation by measuring the expression of Sirt1 and PGC1α genes using real-time PCR.
2.7. Statistical analysis
SPSS 15.0 software (SPSS Inc., Chicago, Ill, USA) was used for all statistical analyses. Paired t-test was performed to determine if the differences between groups were statistically significant. P < 0.05 was considered to be significant.
3. Results
3.1. Effect of resveratrol on TG accumulation
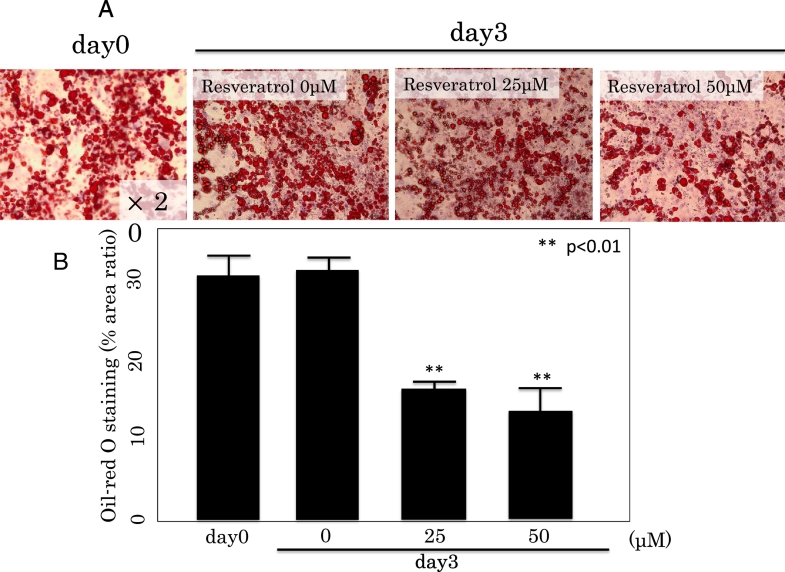
The effect of Rsv in preventing lipid accumulation was examined by Oil Red-O staining of 3T3-L1 adipocytes. After 10 days of TG accumulation, the differentiated 3T3-L1 cells were treated with or without 25 or 50 μM Rsv for 3 days (Fig. 2). As shown in Fig. 3, Rsv reduced lipid accumulation apparently in a dose-dependent manner, as indicated by a decrease in Oil Red-O staining with increase in Rev concentration.
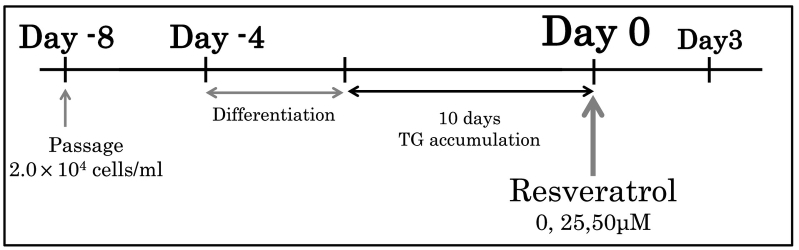
Fig. 2.
Time course of cell passage, culture induction, cell recovery and Oil-Red O staining.
Fig. 3.
A. Oil Red-O staining for triglycerides in 3T3L1 cells. The 3T3L1 cells were treated with or without 25 or 50 μM of Rsv for 3 days after differentiation.
B. Lower panels show graphs depicting the area ratio (%) of Oil-red O staining as determined by ImageJ.
3.2. Effect of resveratrol on Sirt1 gene expression in adipocytes
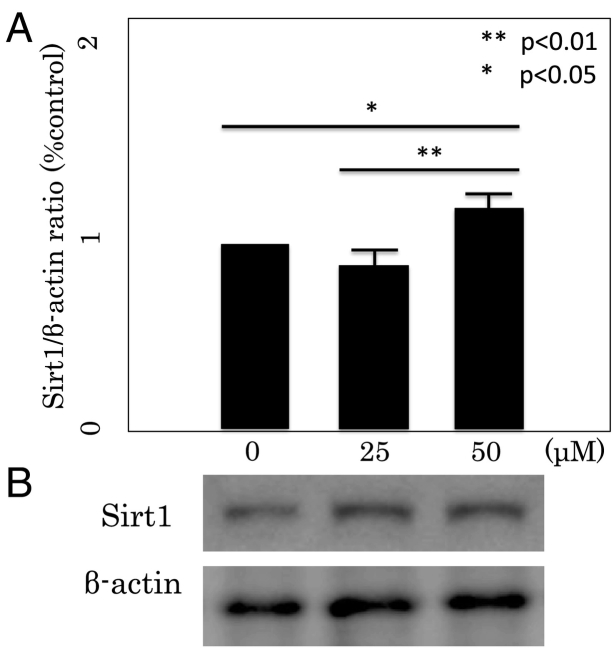
Quantitative real-time PCR analysis showed that Sirt1 mRNA expression was enhanced by 50 μM Rsv in differentiated 3T3-L1 cells (Fig. 4), and the increase was significant compared with 25 μM Rsv and with control cells. Sirt1 protein expression in 3T3-L1 cells cultured in the presence of Rsv was studied by Western blotting. Resveratrol at both 25 and 50 μM increased Sirt1 protein expression (Fig. 4).
Fig. 4.
Sirt1 mRNA (A) and protein (B) expressions in differentiated 3T3L1 cells. (A) Sirt1 mRNA expression was measured by real-time PCR. The data are expressed as mean ± SD of three experiments. *P < 0.05, **P < 0.01 vs 0 μM. (B) Sirt1 protein expression was measured by Western blot. The result shown is representative of three experiments.
3.3. Effect of resveratrol on expression of adipocyte-specific genes
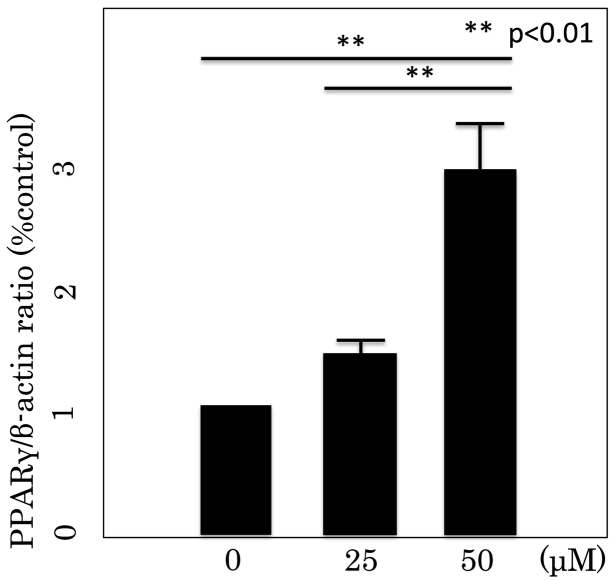
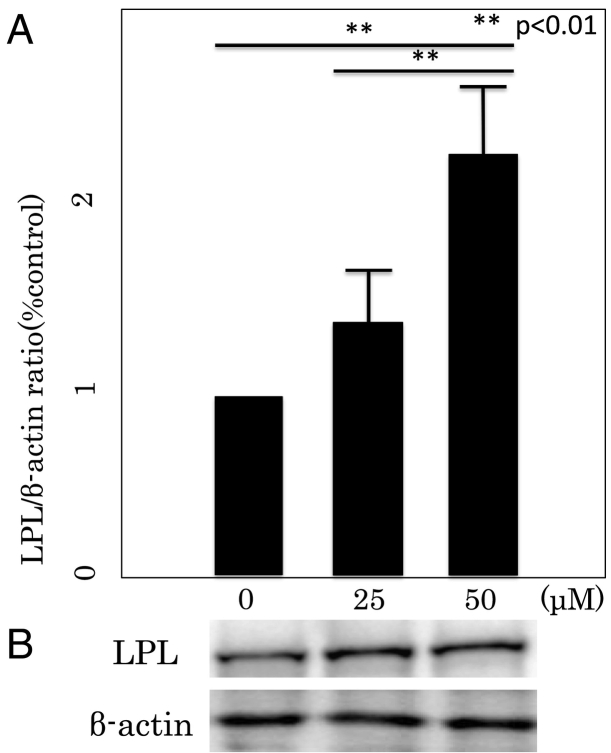
RT-PCR and Western blotting analysis were performed to examine the effect of Rsv on the expression of two transcriptional factors; PPARγ and LPL, in differentiated 3T3-L1 cells (Fig. 5, Fig. 6). Rsv increased PPARγ and LPL mRNA expressions. The expression levels of PPARγ and LPL genes following treatment with 50 μM Rsv were significantly higher compared with those of untreated cells and cells treated with 25 μM Rsv (P < 0.01, both).
Fig. 5.
PPARγ mRNA expression in differentiated 3T3L1 cells. PPARγ mRNA expression was measured by real-time PCR. The data are expressed as mean ± SD of three experiments. **P < 0.01 vs 0 μM.
Fig. 6.
Lipoprotein lipase (LPL) mRNA (A) and protein (B) expressions in differentiated 3T3L1 cells. (A) LPL mRNA expression was measured by real-time PCR. The data are expressed as mean ± SD of three experiments. **P < 0.01 vs 0 μM. (B) LPL protein expression was measured by Western blot. The result shown is representative of three experiments.
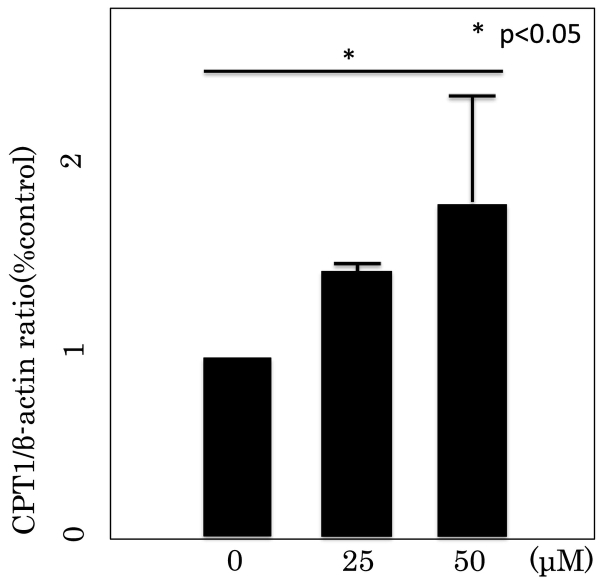
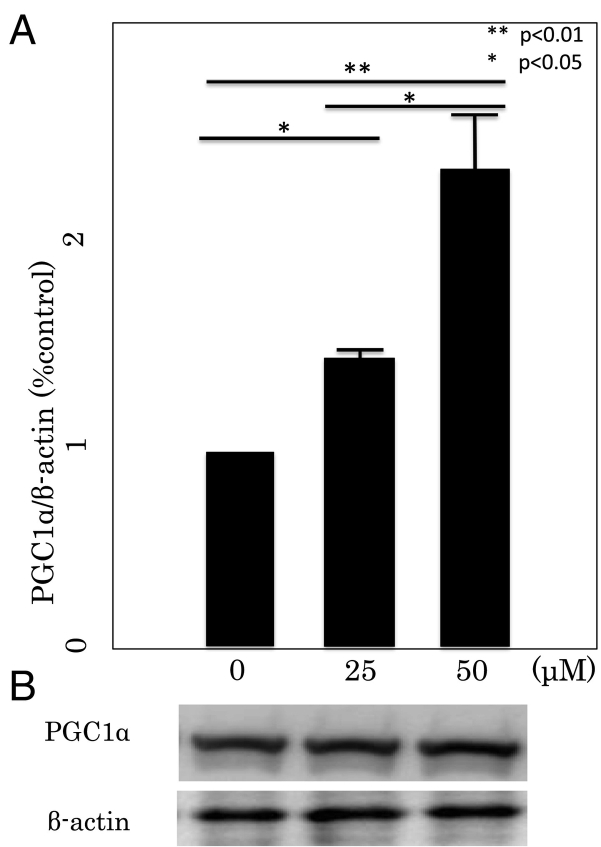
RT-PCR was performed to determine the effect of Rsv on the expression of two β-oxidation target genes; PGC1α and CPT1a (Fig. 7, Fig. 8). Rsv at 25 and 50 μM dose-dependently and significantly upregulated PGC1α gene expressions, while Rsv at 50 μM upregulated CPT1a gene expression.
Fig. 7.
CPT1 mRNA expression in differentiated 3T3L1 cells. CPT1mRNA expression was measured by real-time PCR. The data are expressed as mean ± SD of three experiments. *P < 0.05 vs 0 μM.
Fig. 8.
PGC1α mRNA (A) and protein (B) expressions in differentiated 3T3L1 cells. (A) Sirt1 mRNA expression was measured by real-time PCR. The data are expressed as mean ± SD of three experiments. *P < 0.05, **P < 0.01 vs 0 μM. (B) PGC1α protein expression was measured by Western blot. The result shown is representative of three experiments.
3.4. Effects of Sirt1 and PGC1α siRNA on TG accumulation
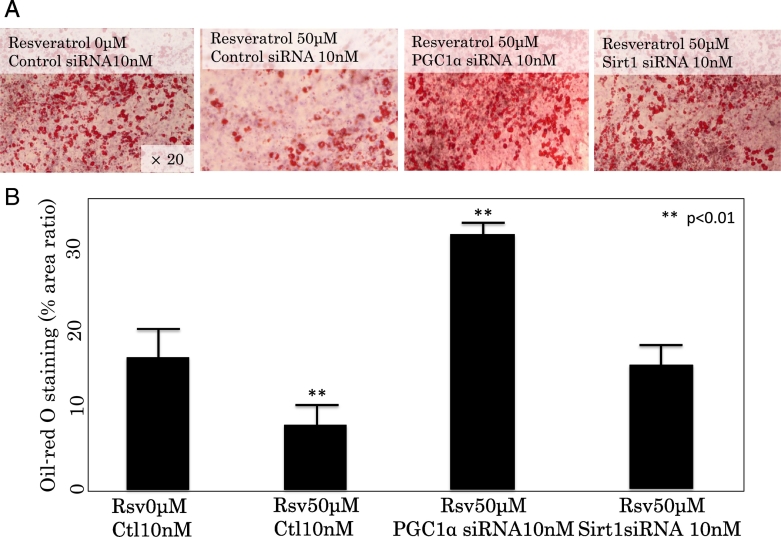
The effects of interfering Sirt1 and PGC1-α signaling by siRNA on Rsv attenuated triglyceride accumulation in 3T3-L1 cells was examined. The siRNA-transfected 3T3-L1 cells were induced to differentiate, cultured for 10 days for TG accumulation, and then treated with or without 50 μM of Rsv. As shown in Fig. 9, when treated with Rev 50 μM, TG accumulation increased in cells transfected with Sirt1siRNA and also in cells transfected with PGC1α siRNA, as indicated by increased Oil Red-O staining, compared with control siRNA.
Fig. 9.
A. Effects of interfering Sirt1 and PGC1-α signaling by the respective siRNAs on resveratrol-attenuated triglyceride accumulation in 3T3L1 cells. The siRNA-transfected 3T3L1 cells were treated with or without 50 μM of resveratrol for 3 days after differentiation.
B. Lower panels show graphs depicting the area ratio (%) of Oil-Red O staining as determined by ImageJ.
3.5. Effects of Sirt1 siRNA on the expression of adipocyte-specific genes
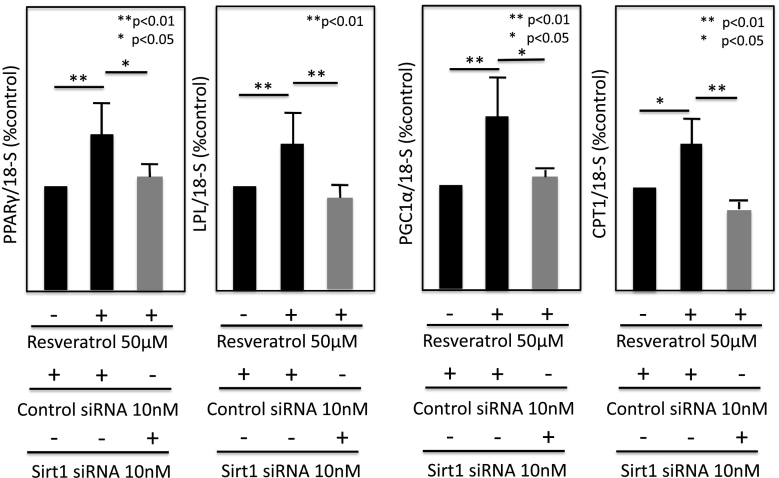
To clarify the relationship between Sirt1 and PPARγ, LPL, PGC1α, or CPT1a in 3T3-L1 adipocytes, we interfered with Sirt1 signaling using Sirt1 siRNA (Fig. 10). The Sirt1 siRNA-transfected 3T3-L1 cells were treated with or without 50 μM of Rsv.
Fig. 10.
Effects of interfering signaling by Sirt1 siRNA on resveratrol-regulated PPARγ, LPL, PGC1-α and CPT1 mRNA expressions in 3T3L1 cells. The Sirt1 siRNA-transfected 3T3L1 cells were treated with or without 50 μM of resveratrol for 3 days after differentiation. Messenger RNA expressions were measured by real-time PCR.
PPARγ, LPL, PGC1α, and CPT1a mRNA expressions were significantly enhanced in the presence of 50 μmol/L Rsv, and all these increases were abolished by transfection of Sirt1 siRNA.
4. Discussion
To examine the effects of Rsv on TG accumulation and expression of several genes regulating lipid metabolism, this study was conducted using 3T3-L1 adipocytes [18]. In the present study, decreased TG accumulation and increased Sirt1 gene and protein expressions were observed after treating 3T3-L1 adipocytes with 50 μM Rsv. Colitti and Stefanon reported that resveratrol was significantly strong inhibitor of adipogenesis in human omental fat cells [19]. The expression of PGC1α and CPT1a was also increased by Rsv through activation of Sirt1. Several studies focused on the potential anti-adipogenic effect of Rsv under in vitro conditions. Lasa et al. [20] reported that Rsv increased fatty acid consumption via enhancement of CPT1a and PGC1α expressions in differentiated 3T3-L1 adipocytes. Furthermore, Wang et al. [21] reported that Rsv induced brown-like adipocyte formation in white fat tissue through the increased expression of uncoupling protein. These findings were consistent with enhanced fatty acid oxidation observed in 3T3-L1 adipocytes in the present study.
Several studies reported the effects of Rsv on adipogenesis and adipokine expression [22], [23]. It is important to know whether Rsv contributes to the beneficial effect of energy homeostasis in adipokines. Several positive effects of Rsv are mediated by changes in adipokine production. Adipokines can act in an autocrine or in a paracrine manner to modify metabolic pathways involved in lipid metabolism and glucose homeostasis [24]. For instance, leptin increases energy expenditure and reduces food intake [25]. Furthermore, physiological increase in plasma leptin level has been shown to increase insulin secretion [26]. Adiponectin increases glucose uptake in muscles and insulin sensitivity, suppresses gluconeogenesis in hepatocytes and increases β-oxidation [27]. With regard to these adipokines, Rsv has been reported to reduce leptin expression and secretion and increase adiponectin expression in both in vitro and in vivo studies [28]. Because of these positive effects, Rsv is marketed as a supplement with anti-obesity effect.
Adipocytes are known to release various chemokines and enzyme including LPL, which regulate lipid metabolism accompanied by change in adipocyte size. LPL is mainly produced by adipocytes and catalyzes TG hydrolysis in blood [29], [30]. Fatty acid is taken up and driven to TG accumulation in adipocytes. Thus, LPL expression may function to metabolize excess energy in adipocytes [31].
Adipogenesis is the developmental process by which a multipotent mesenchymal stem cell differentiates into a mature adipocyte. This process involves a highly regulated and coordinated cascade of transcription factors including members of the PPAR families, which together lead to development of the differentiated state [32]. PPARγ, a lipid-activated transcription factor, is present in adipocytes and regulates genes involved in lipid metabolism [33]. Modulation of PPARγ activity may be an effective way to regulate obesity. In our study, Rsv significantly and dose-dependently increased PPARγ mRNA expression. Therefore, Rsv may contribute to increase synthesis of fatty acid through upregulation of Sirt1 expression in 3T3-L1 adipocytes.
On the other hand, previous studies reported that Rsv decreased LPL expression in adipocytes [23], while we observed that Rsv induced up-regulation of LPL expression in 3T3-L1 adipocytes. Our study differed from previous reports in that Rsv was added to 3T3-L1 adipocytes after a relatively long maturation period for the purpose of adequate TG accumulation. In the present study, after adipogenic differentiation, the differentiation medium was replaced with DMEM containing 10% FCS. The differentiated 3T3-L1 cells were cultured for 10 days, and thereafter treated with Rsv for 3 days(Fig. 2). This protocol may contribute to the discrepancy between our result and previous reports. Several reports have demonstrated the effects of Rsv on adipogenic differentiation. Chang et al. [34] reported that low doses (1 and 10 μM) of Rsv suppressed adipogenic differentiation, although Hu et al. [35] showed that the same concentrations enhanced 3T3-L1 adipogenic differentiation. Chang et al. [34] also found that high doses of Rsv exerted cytotoxicity and induced apoptosis. Therefore, the impact of Rev on adipogenic differentiation was probably not relevant in our experimental setting.
In this study, we found that Rsv increased factors associated with fatty acid synthesis and may contribute to increased β-oxidation in differentiated 3T3-L1 adipocytes. In the present study, however, it was not enough to conclude that Rsv-up-regulated PGC1α actually resulted in increased β-oxidation. Further examination is needed to elucidate the effect of Rsv on β-oxidation in adipocyte. Yang et al. [36] demonstrated that a combination of Rsv and quercetin down-regulated PPARγ and C/EBPα and induced apoptosis, resulting in attenuated fat accumulation. Floyd et al. [37] showed that Rsv treatment modulated insulin sensitivity by suppressing PPARγ gene and protein expression in 3T3-L1 adipocytes. Chen et al. [38] reported that inhibition of PPARγ expression by Rsv treatment led to improved insulin sensitivity, which might be associated with decreased lipid accumulation and increased lipid oxidation.
Qiang et al. reported that Sirt1 gain-of-function induces BAT-like remodeling of white adipocytes by deacetylating PPARγ [39]. Vernochet et al. reported that activation of PPARγ by thiazolidinediones can also induce a brown-like phenotype in white adipocytes by promoting expression of brown adipocyte- specific genes and suppressing visceral WAT genes. These reports suggested that browning of white adipocytes via PPARγ by Rsv also contributes to TG reduction [40]. In this study, UCP1, a factor involved in browning, has not been measured, but it is suggested that browning may be mediated through PPARγ expression. These findings indicate that up-regulation of PPARγ, LPL, PGC-1α and CPT1a by Rsv treatment may enhance fatty acid synthesis and β-oxidation, resulting in the inhibition of lipid accumulation. Furthermore, Rsv may modulate energy metabolism of adipose tissue, and it is a potential therapeutic allocation in the treatment or prevention of obesity related metabolic disorders.
This manuscript has several limitations. Mouse preadipocytes were used in this study. The results cannot necessarily be translated to human cells. Furthermore, some reports suggest that Rsv improves metabolism through enhancing brown-like or beige adipogenesis [41], [42]. The present study does not provide information on the separate effects of Rsv on different types of adipose tissues. Further investigations are necessary. We adopted only one housekeeping gene, β-actin, to normalize quantitative PCR, whereas several genes should be selected. Furthermore, we found that Rsv increased factors associated with fatty acid synthesis and may contribute to increased β-oxidation in differentiated 3T3-L1 adipocytes. However, it was not enough to conclude that Rsv-up-regulated PGC1α actually resulted in increased β-oxidation. Further examination is needed to elucidate the effect of Rsv on β-oxidation in adipocyte.
In conclusion, the present results suggest that Rsv may increase synthesis and oxidation of fatty acids and possibly increases the energy utilization efficiency in adipocytes through activation of Sirt1. The present study provides valuable evidence supporting the use of Rsv in obese individuals.
References
- 1.Michan S., Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem. J. 2007;404(1):1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunet A., Sweeney L.B., Sturgill J.F., Chua K.F., Greer P.L., Lin Y., Tran H., Ross S.E., Mostoslavsky R., Cohen H.Y., Hu L.S., Cheng H.L., Jedrychowski M.P., Gygi S.P., Sinclair D.A., Alt F.W., Greenberg M.E. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 3.Langley E., Pearson M., Faretta M., Bauer U.M., Frye R.A., Minucci S., Pelicci P.G., Kouzarides T. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21(10):2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaziri H., Dessain S.K., Ng Eaton E., Imai S.I., Frye R.A., Pandita T.K., Guarente L., Weinberg R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107(2):149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 5.Banks A.S., Kon N., Knight C., Matsumoto M., Gutiérrez-Juárez R., Rossetti L., Gu W., Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiao L., Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J. Biol. Chem. 2006;281:39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- 7.Costa C.D., Hammes TO, Rohden F., Margis R., Bortolotto J.W., Padoin A.V., Mottin C.C., Guaragna R.M. SIRT1 transcription is decreased in visceral adipose tissue of morbidly obese patients with severe hepatic steatosis. Obes. Surg. 2009;20:633–639. doi: 10.1007/s11695-009-0052-z. [DOI] [PubMed] [Google Scholar]
- 8.Szkudelska K., Szkudelski T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010;635:1–8. doi: 10.1016/j.ejphar.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 9.Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K., Pistell P.J., Poosala S., Becker K.G., Boss O., Gwinn D., Wang M., Ramaswamy S., Fishbein K.W., Spencer R.G., Lakatta E.G., Le Couteur D., Shaw R.J., Navas P., Puigserver P., Ingram D.K., de Cabo R., Sinclair D.A. Resveratrol improves health and survival of mice on a high- calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Borra M.T., Smith B.C., Denu J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005;280(17):17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 12.Kannel W.B., Cupples L.A., Ramaswami R., Stokes J., Kreger B.E., Higgins M. Regional obesity and risk of cardiovascular disease; the Framingham Study. J. Clin. Epidemiol. 1991;44:183–190. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- 13.Ogden C.L., Carroll M.D., Curtin L.R., McDowell M.A., Tabak C.J., Flegal K.M. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 14.Eseberri I., Lasa A., Churruca I., Portillo M.P. Resveratrol metabolites modify adipokine expression and secretion in 3T3-L1 pre-adipocytes and mature adipocytes. PLoS One. 2013;8(5):e63918. doi: 10.1371/journal.pone.0063918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen G.C., Chen Y.C., Chang W.T., Hsu C.L. Effects of polyphenolic compounds on tumor necrosis factor-α (TNF-α)-induced changes of adipokines and oxidative stress in 3T3-L1 adipocytes. J. Agric. Food Chem. 2011;59(2):546–551. doi: 10.1021/jf1036992. [DOI] [PubMed] [Google Scholar]
- 16.Ahn J., Lee H., Kim S., Ha T. Resveratrol inhibits TNF-alpha-induced changes of adipokines in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2007;364(4):972–977. doi: 10.1016/j.bbrc.2007.10.109. [DOI] [PubMed] [Google Scholar]
- 17.Walle T., Hsieh F., DeLegge M.H., Oatis J.E., Jr., Walle U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 18.Rayalam S., Della-Fera M.A., Yang J.Y., Park H.J., Ambati S., Baile C.A. Resveratrol potentiates genistein's anti- adipogenic and proapoptotic effects in 3T3-L1 adipocytes. J. Nutr. 2007;137:2668–2673. doi: 10.1093/jn/137.12.2668. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H.Y., Du Z.X., Meng X. Resveratrol prevents TNFα-induced suppression of adiponectin expression via PPARγ activation in 3T3-L1 adipocytes. Clin. Exp. Med. 2013;13(3):193–199. doi: 10.1007/s10238-012-0189-2. [DOI] [PubMed] [Google Scholar]
- 20.Lasa A., Churruca I., Eseberri I., Andrés-Lacueva C., Portillo M.P. Delipidating effect of resveratrol metabolites in 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2012;56:1559–1568. doi: 10.1002/mnfr.201100772. [DOI] [PubMed] [Google Scholar]
- 21.Wang S., Liang X., Yang Q., Fu X., Rogers C.J., Zhu M., Rodgers B.D., Jiang Q., Dodson M.V., Du M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int. J. Obes. 2015;39:967–976. doi: 10.1038/ijo.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos J.C., Gotardo E.M., Brianti M.T., Piraee M., Gambero A., Ribeiro M.L. Effects of yerba maté, a plant extract formulation (“YGD”) and resveratrol in 3T3-L1 adipogenesis. Molecules. 2014;19(10):16909–16924. doi: 10.3390/molecules191016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X.H., Huang B., Choi S.K., Seo J.S. Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation. Nutr. Res. Pract. 2012;6(4):286–293. doi: 10.4162/nrp.2012.6.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liaw J.J., Peplow P.V. Differential effect of electroacupuncture on inflammatory adipokines in two rat models of obesity. J. Acupunct. Meridian Stud. 2016;9(4):183–190. doi: 10.1016/j.jams.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Kuryszko J., Sławuta P., Sapikowski G. Secretory function of adipose tissue. Pol. J. Vet. Sci. 2016;19(2):441–446. doi: 10.1515/pjvs-2016-0056. [DOI] [PubMed] [Google Scholar]
- 26.Vatier C., Fetita S., Boudou P., Tchankou C., Deville L., Riveline J., Young J., Mathivon L., Travert F., Morin D., Cahen J., Lascols O., Andreelli F., Reznik Y., Mongeois E., Madelaine I., Vantyghem M., Gautier J., Vigouroux C. One-year metreleptin improves insulin secretion in patients with diabetes linked to genetic lipodystrophic syndromes. Diabetes Obes. Metab. 2016;18(7):693–697. doi: 10.1111/dom.12606. [DOI] [PubMed] [Google Scholar]
- 27.Chang C.S., Lu Y.J., Chang H.H., Hsu S.H., Kuo P.H., Shieh C.C. Role of adiponectin gene variants, adipokines and hydrometry-based percent body fat in metabolically healthy and abnormal obesity. Obes Res Clin Pract. May 25, 2016 doi: 10.1016/j.orcp.2016.05.003. (pii: S1871-403X(16)30031-X [Epub ahead of print]) [DOI] [PubMed] [Google Scholar]
- 28.Tsuda T., Ueno Y., Aoki H., Koda T., Horio F., Takahashi N., Kawada T., Osawa T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004;316(1):149–157. doi: 10.1016/j.bbrc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 29.Shirai K., Itoh I., Sasaki H., Totsuka M., Murano T., Watanabe H. The effect of insulin sensitizer, troglitazone, on lipoprotein lipase mass in preheparin serum. Diabetes Res. Clin. Pract. 1999;46:35–41. doi: 10.1016/s0168-8227(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 30.Totsuka M., Miyashita Y., Ito Y., Watanabe H., Murano T., Shirai K. Enhancement of preheparin serum lipoprotein lipase mass by bezafibrate administration. Atherosclerosis. 2000;153:175–179. doi: 10.1016/s0021-9150(00)00394-4. [DOI] [PubMed] [Google Scholar]
- 31.Ohira M., Miyashita Y., Ebisuno M., Saiki A., Endo K., Koide N., Oyama T., Murano T., Watanabe H., Shirai K. Effect of metformin on serum lipoprotein lipase mass levels and LDL particle size in type 2 diabetes mellitus patients. Diabetes Res. Clin. Pract. 2007;78:34–41. doi: 10.1016/j.diabres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 32.He Y., Li Y., Zhao T., Wang Y., Sun C. Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PLoS One. 2013;8:e70135. doi: 10.1371/journal.pone.0070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., Zhu X.X., Liu L., Xue Y., Yang X., Zou H.J. SIRT1 prevents hyperuricemia via the PGC-1α/PPARγ-ABCG2 pathway. Endocrine. 2016;53(2):443–452. doi: 10.1007/s12020-016-0896-7. [DOI] [PubMed] [Google Scholar]
- 34.Chang C.C., Lin K.Y., Peng K.Y., Day Y.J., Hung L.M. Resveratrol exerts anti-obesity effects in high-fat diet obese mice and displays differential dosage effects on cytotoxicity, differentiation, and lipolysis in 3T3-L1 cells. Endocr. J. 2016;63(2):169–178. doi: 10.1507/endocrj.EJ15-0545. [DOI] [PubMed] [Google Scholar]
- 35.Hu P., Zhao L., Chen J. Physiologically achievable doses of resveratrol enhance 3T3-L1 adipocyte differentiation. Eur. J. Nutr. 2015;54(4):569–579. doi: 10.1007/s00394-014-0738-4. [DOI] [PubMed] [Google Scholar]
- 36.Yang J.Y., Della-Fera M.A., Rayalam S., Ambati S., Hartzell D.L., Park H.J., Baile C.A. Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci. 2008;82:1032–1039. doi: 10.1016/j.lfs.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Floyd Z.E., Wang Z.Q., Kilroy G., Cefalu W.T. Modulation of peroxisome proliferator-activated receptor γ stability and transcriptional activity in adipocytes by resveratrol. Metabolism. 2008;57:S32–S38. doi: 10.1016/j.metabol.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L.L., Zhang H.H., Zheng J., Hu X., Kong W., Hu D., Wang S.X., Zhang P. Resveratrol attenuates high-fat diet-induced insulin resistance by influencing skeletal muscle lipid transport and subsarcolemmal mitochondrial β-oxidation. Metabolism. 2011;60:1598–1609. doi: 10.1016/j.metabol.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Qiang L., Wang L., Kon N., Zhao W., Lee S., Zhang Y., Rosenbaum M., Zhao Y., Gu W., Farmer Stephen R., Accili Domenico. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell. 2012;150(3):620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farmer S.R. Obesity: be cool, lose weight. Nature. 2009;458:839–840. doi: 10.1038/458839a. [DOI] [PubMed] [Google Scholar]
- 41.Wang S., Liang X., Yang Q., Fu X., Rogers C.J., Zhu M., Rodgers B.D., Jiang Q., Dodson M.V., Du M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int. J. Obes. 2015 Jun;39(6):967–976. doi: 10.1038/ijo.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ku C.R., Cho Y.H., Hong Z.Y., Lee H., Lee S.J., Hong S.S., Lee E.J. The effects of high fat diet and resveratrol on mitochondrial activity of Brown adipocytes. Endocrinol. Metab. (Seoul) 2016 Jun;31(2):328–335. doi: 10.3803/EnM.2016.31.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]