Abstract
Rationale: South Africa has a high prevalence of tuberculosis (TB) and HIV-coinfected adults in whom TB is often diagnosed late in the course of disease.
Objectives: Improved case-finding approaches for TB and HIV are needed to reduce mortality and prevent transmission.
Methods: We identified newly diagnosed index TB cases in a rural district and enrolled their households in a TB-HIV contact-tracing study. A group of randomly selected control households were enrolled to determine community prevalence of undetected TB and HIV. Field teams screened participants for TB symptoms, collected sputum specimens for smear microscopy and culture, provided HIV counseling and testing, and collected blood for CD4 testing. Participants were referred to public clinics for TB treatment and antiretroviral therapy.
Measurements and Main Results: We evaluated 2,843 household contacts of 727 index patients with TB and 983 randomly selected control household members. The prevalence of TB in household contacts was 6,075 per 100,000 (95% confidence interval, 5,789–6,360 per 100,000), whereas the prevalence detected in randomly selected households was 407 per 100,000 (95% confidence interval, 0–912 per 100,000; prevalence difference, 5,668 per 100,000; P < 0.001). TB detected among contacts was less likely to be smear-positive than in the index patients (6% vs. 22%; P < 0.001). Most contacts with culture-confirmed TB were asymptomatic. At least one case of undiagnosed TB was found in 141 (19%) of 727 contact versus 4 (1%) of 312 control households. HIV testing was positive in 166 (11%) of 1,568 contacts tested versus 76 (14%) of 521 control participants tested (odds ratio, 1.48; P = 0.02).
Conclusions: Active case finding in TB contact households should be considered to improve TB and HIV case detection in high-prevalence settings, but sensitive diagnostic tools are necessary.
Keywords: tuberculosis, HIV, AIDS, case detection, contact tracing
At a Glance Commentary
Scientific Knowledge on the Subject
In areas with high tuberculosis (TB) and HIV prevalence, passive case finding is insufficient in detecting TB and HIV early enough to prevent substantial transmission, morbidity, and mortality.
What This Study Adds to the Field
Screening household contacts of patients with TB in high-TB-prevalence areas detects TB and HIV in a high proportion of contacts, and when TB culture is used TB is detected before it is symptomatic. Targeting households is more efficient than unselected community-based screening.
The World Health Organization (WHO) global tuberculosis (TB) control policy advocates the DOTS strategy, which relies on passive case detection in symptomatic individuals presenting to health services (1). Despite the success of DOTS in reducing TB mortality in settings of low HIV prevalence, evidence suggests that additional strategies are necessary for TB control in high-HIV-prevalence areas (2, 3). Intensified case finding in individuals infected with HIV has recently been incorporated into HIV-TB control strategies (4), but this approach still relies on patient presentation at a health facility. Obstacles to self-presentation, such as distance to the nearest health facility, sex, and age, have all been associated with delayed time to diagnosis of TB from onset of symptoms (5–9). Additionally, HIV-associated TB is more likely to be smear-negative than TB in individuals who are HIV-negative (10).
The case detection strategy in South Africa has been heavily dependent on sputum smear microscopy and passive case detection. Although mycobacterial culture is available, its use is incomplete, particularly for patients at higher risk of smear-negative TB, such as patients infected with HIV (11).
Contacts of patients with active TB have an increased risk of prevalent TB, and in settings with a high burden of HIV, these contacts may also have higher rates of HIV infection (12). This study was undertaken to determine the prevalence of undiagnosed TB and HIV in household contacts of recently diagnosed patients with TB, and to compare the case detection yield of TB and HIV in contact households with randomly selected noncontact households, to determine the efficiency of targeting households of patients with TB for active case-finding interventions. Some of the results of these studies have been previously reported in the form of an abstract (13).
Methods
Study Setting
The study took place in the Matlosana municipality, North West Province, South Africa. The area, consisting of a town and five residential townships, is served by a public tertiary care hospital and 16 primary care clinics. During the study period, a single public outpatient facility provided initiation of antiretroviral therapy (ART). TB smear microscopy and mycobacterial culture samples are collected at the hospital and clinics and processed at a central reference laboratory. TB treatment is administered at each primary care clinic. Clinic-based treatment supporters assist with directly observed therapy and trace patients who are nonadherent with treatment. Patients with multidrug-resistant TB (MDR-TB) are admitted to the MDR-TB unit at Klerksdorp Hospital for treatment.
TB Index Cases and Contacts
Adults with newly diagnosed TB were recruited by study staff between February and September 2009 in the hospital medical wards and 16 clinics. Eligibility criteria for index participants were age greater than or equal to 18 years; recorded diagnosis of TB based on clinical evaluation and radiographic studies (with or without sputum smear and culture); TB treatment initiation within the past 30 days; residence in Matlosana for the past 6 months; living with at least one person; and consent to a home visit. Index patients completed a questionnaire assessing sociodemographic factors, TB risk-factors, and self-reported prior TB and symptom review at the time of enrollment. Clinical and laboratory data were abstracted from patient medical records. A household was defined as all persons sharing the same structure as the index case, or living in a separate structure on the same residential plot and sharing meals with the index case.
Control Household Selection
A survey of 312 area control households was conducted after recruitment of the contact survey was completed. Control households were selected to evaluate the competing district-level screening approaches of randomly selected households for screening versus targeted contact tracing in households of known TB cases. Geotagged aerial photographs of the district were used to establish coordinate boundaries of the five residential townships after excluding lots without dwellings. The target total enrollment was divided proportionate to each township’s population. Random geographic coordinates within each boundary were sequentially generated using E-pop software Version 1.2.4.17 (Epicentre, Paris, France), then located using hand-held GPS units. The house closest to each randomly generated coordinate was approached by a study team for enrollment. Houses were sequentially enrolled until the predefined total in each township was reached. Households were excluded from this randomized survey if no adult could be found after three visits; if it was a participant household in the contact-tracing study; or if there was a registered patient with TB living in the home (i.e., the house was eligible but not enrolled in the contact-tracing study). Household definitions and study procedures after enrollment were the same for the contact-tracing survey and control household survey.
Study Procedures
Study teams consisting of a nurse and lay counselors visited patient households, typically within 2 weeks of index enrollment. At least three visit attempts were made to find all household residents. Household members were eligible if they were willing to attempt to provide a sputum specimen (unless currently on TB treatment), regardless of symptoms. HIV testing was not a condition of participation and consent for HIV testing was obtained separately. Study staff administered verbal questionnaires including demographic and TB symptom information according to standardized study protocol. The study nurse collected a sputum specimen from each participant; sputum induction using hypertonic saline was performed if a participant was unable to produce sputum. Specimens were collected outdoors to minimize aerosol exposure to household members and study staff. All participants were offered HIV counseling and testing and results were confidentially returned to the participant. For participants with a positive HIV rapid test, venous blood was collected for ELISA testing and CD4 cell count determination. Noncontact household members were also offered anonymous oral HIV testing if they declined voluntary counseling and testing (VCT), for research purposes only and without receipt of results. Household TB contacts less than or equal to 5 years old were referred to the local TB clinic for TB evaluation, including tuberculin skin testing, regardless of enrollment in accordance with national guidelines. Participants who reported any symptoms (cough, fever, weight loss, or night sweats) and were sputum smear-negative were referred for chest radiography at Tshepong hospital while awaiting sputum culture results. Treatment and clinical follow-up for TB and HIV was provided free of charge by all primary care clinics in Matlosana and the district ART clinic according to the district and national guidelines.
Laboratory Testing
Sputum specimens were decontaminated and underwent fluorescence microscopy with auramine staining. Culture was performed using the BACTEC MGIT 960 Detection System (Becton Dickinson, Sparks, MD) at the public sector National Health Laboratory Service (NHLS) laboratory site at Tshepong Hospital or Johannesburg NHLS facility. Drug susceptibility testing was performed by MGIT for all cultures testing positive for Mycobacterium tuberculosis. Whenever possible, additional sputum specimens were collected from participants whose samples were contaminated. HIV testing was performed at household visits using two rapid tests (FirstResponse [PMC Medical, India] and Sensa [Pantech, Durban, South Africa]) in parallel. All reactive tests and any inconclusive tests were confirmed using whole-blood ELISA at the Tshepong NHLS. CD4 T-cell counts were enumerated by flow cytometry. Specimens collected during the noncontact study were tested using the same procedures at Contract Laboratory Services in Johannesburg, affiliated with the NHLS. Oral fluid specimens for HIV testing (OraSure, Bethlehem, PA) from noncontact households were also processed at Contract Laboratory Services.
Clinical Follow-up
TB cases were defined as follows: confirmed, culture-positive specimen in which M. tuberculosis was identified; or probable, smear-positive sample without culture confirmation, or a culture-positive specimen without definitive speciation. Participants with confirmed or probable TB were visited by the study team and referred to the nearest local clinic to initiate treatment. The clinic and district TB program were notified of all positive results and referrals. Referrals for HIV follow-up care were made based on CD4 count and staging. Study staff obtained appointments and referrals to the ART clinic for participants infected with HIV with CD4 less than 250. Participants infected with HIV not yet eligible for ART were referred to their local clinics for repeat CD4 testing in 6 months and ongoing monitoring according to the national guidelines (14).
Statistical Methods
In univariate analysis, variables were compared using logistic regression (binary covariates) or linear regression (continuous covariates), accounting for household clustering using a random-effects term for the household as the unit of clustering. A threshold of P equal to 0.05 was used for statistical significance. Confidence intervals (CI) for prevalence were adjusted using the design effect for clustering because of nonindependence of members of the same household. Similarly, risk factors for TB disease in individual household contacts were determined using logistic regression with a random-effects term to control for the effect of shared household status.
Multivariate logistic regression models were built using variables that reached a significance of P less than 0.1 in univariate analyses or a priori epidemiologic significance.
Ethical Considerations
The study was approved by the ethics committees of the University of the Witwatersrand, the Research Committee of the Klerksdorp/Tshepong Hospital Complex, and the Johns Hopkins School of Medicine institutional review board. All study participants gave individual written informed consent (parental consent was obtained for all participants <18, with assent if 7–17 years old) for study participation, and separate consent for HIV testing or oral specimen collection.
Results
Contact Households
From February through September 2009, 878 index TB cases were evaluated for inclusion in the study. Some contacts could not be enrolled because of household refusal to participate (n = 49) or inability to locate an address or find people at home (n = 54). An additional 26 index patients were found to have relocated from their stated address and were not traceable. At the time of home visit, 22 index patients were found to be living alone. The final study population included 727 index cases enrolled from the hospital (n = 374) and 16 clinics (n = 353), representing 18% of all TB cases diagnosed in the Matlosana subdistrict during this time period. Index TB cases were predominantly diagnosed by sputum smear (161; 22%) or clinical criteria of TB signs and symptoms with or without chest radiograph (531; 73%) (Table 1). Among index patients, 629 (87%) were HIV-infected and 224 (31%) reported a prior TB infection. Three index patients had MDR-TB; however, because not all index patients had a positive TB culture, not all were tested for MDR-TB. Most index patients were unemployed (567; 78%) and 197 (27%) lived in shack housing, indicating a substantial poverty rate among these patients.
TABLE 1.
INDEX TB CASE CHARACTERISTICS (N = 727)
| Index Case Characteristics | N (%) |
| Sex | |
| Male | 314 (43) |
| Female | 413 (57) |
| Age | |
| 18–29 | 169 (23) |
| 30–43 | 359 (49) |
| 44+ | 199 (27) |
| HIV infection status | |
| Positive | 629 (87) |
| Negative | 94 (13) |
| Unknown | 4 (1) |
| Median (IQR) CD4 count (cells/mm3)* | 101 (30–183) |
| Prior TB | 224 (31) |
| MDR-TB | 3 (0.4) |
| Diagnosis | |
| Sputum smear positive | 161 (22) |
| Culture positive | 16 (2) |
| Other laboratory-based diagnosis (e.g., cerebrospinal fluid, biopsy) | 19 (3) |
| Clinical diagnosis | 531 (73) |
| Shack house | 195 (27) |
| Unemployed | 567 (78) |
| Median (IQR) number of contacts enrolled | 3 (2–5) |
| Number of additional TB cases found in household | |
| 0 | 586 (81) |
| 1 | 115 (16) |
| 2 | 24 (3) |
| 3 | 2 (0.3) |
| Number of new HIV cases found in household | |
| 0 | 582 (80) |
| 1 | 127 (17) |
| 2 | 16 (2) |
| 3 | 1 (0.1) |
| 4 | 1 (0.1) |
Definition of abbreviations: IQR = interquartile range; MDR = multidrug resistant; TB = tuberculosis.
HIV-infected only.
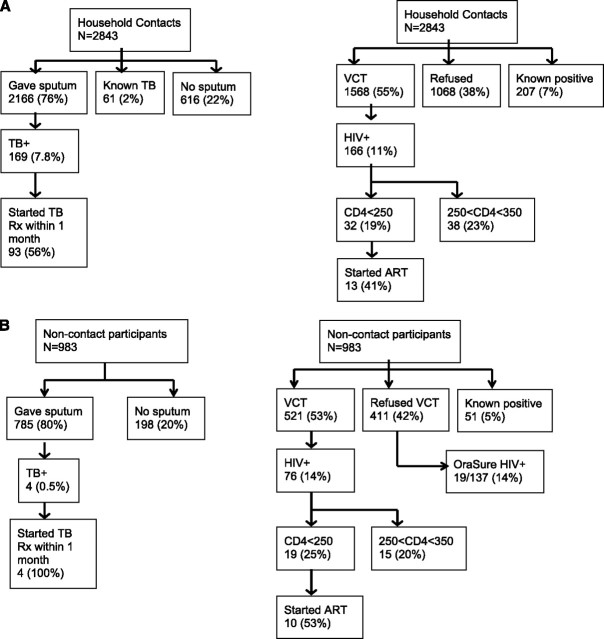
In the 727 index case households, 2,843 contacts were enrolled (Figure 1A, Table 2), with a median of three (interquartile range [IQR], 2–5) contacts enrolled per index. Sputum specimens were collected from 2,166 (76%) contacts, including 2,147 (94%) of all contacts more than 5 years of age. Sixty-one household contacts (2%) were receiving TB treatment at the time of the household visit and did not give a specimen, and 616 (22%) were unable to produce a specimen. Most participants not able to give a specimen were young children; 468 (76%) were younger than 5 and 114 (19%) were between 5 and 17 years old. HIV counseling and testing was provided to 1,568 (55%) contacts, with 1,068 (38%) refusing, and 207 (7%) already known to be HIV-infected at the time of visit.
Figure 1.
Operational flow: diagnoses, referrals, and treatment initiations. (A) Household contacts. (B) Randomly selected households. ART = antiretroviral therapy; Rx = treatment; TB = tuberculosis; VCT = voluntary counseling and testing.
TABLE 2.
TB CONTACT AND NON-TB CONTACT HOUSEHOLD MEMBER CHARACTERISTICS
| Participant Household Characteristics | Contact N = 727 | Noncontact N = 312 | P Value |
| Median household size (IQR) (including nonenrolled) | 5 (3–7) | 4 (3–5) | <0.001 |
| Shack house (%) | 195 (27) | 76 (24) | 0.407 |
| Participant Individual Characteristics | N = 2,843 (%) | N = 983 (%) | |
| Sex | |||
| Male | 1,199 (42) | 414 (42) | |
| Female | 1,644 (58) | 569 (58) | 0.975 |
| Age | |||
| <5 | 511 (18) | 123 (13) | |
| 5–17 | 864 (30) | 253 (26) | |
| 18–29 | 589 (21) | 209 (21) | |
| 30–43 | 363 (13) | 175 (18) | |
| 44+ | 516 (18) | 223 (23) | <0.001 |
| Unemployed (18+) | 805 (58) | 100 (17) | <0.001 |
| Prior TB | 207 (7) | 31 (3) | <0.001 |
| Known HIV infection | 207 (7) | 51 (5) | 0.03 |
| Known current TB | 61 (2) | — | — |
Definition of abbreviations: IQR = interquartile range; TB = tuberculosis.
Active case finding identified 169 undiagnosed TB cases in households of known TB cases, for a prevalence of 6,075 per 100,000 (95% CI, 5,789–6,360 per 100,000); 141 contact households (19%) had at least one undiagnosed TB case (Table 1). Among all contacts who submitted a sputum specimen, the prevalence of undiagnosed TB was 7,802 per 100,000 (95% CI, 6,643–8,962 per 100,000). Most TB found by active case finding was sputum smear-negative, culture-positive TB (Table 3). Only 18 (11%) contacts with undiagnosed TB reported any symptom (cough, fever, weight loss, or night sweats) at the time of specimen collection (Table 3). Among newly diagnosed TB cases in contacts, 26 (15%) were HIV-infected, including 16 newly diagnosed at the household visit. Index cases were nearly six times as likely to have HIV compared with contacts (P < 0.001). Five contacts were diagnosed with MDR-TB, but none were contacts of index patients with known MDR-TB.
TABLE 3.
ACTIVELY VERSUS PASSIVELY DETECTED TB, HIV, AND TB-HIV
| Contact N (%) | Index N (%) | P Value | |
| Total TB cases | 169 | 727 | |
| Basis for TB diagnosis | |||
| Smear | 10 (6) | 161 (22) | |
| Culture | 159 (94) | 16 (2) | |
| Other | 0 (0) | 550 (76) | <0.001 |
| MDR | 5 (3) | 3 (0.4) | 0.007 |
| Any symptoms | 18 (11) | 687 (95) | <0.001 |
| HIV-infected | 26 (15) | 634 (87) | <0.001 |
Definition of abbreviations: MDR = multidrug resistant; TB = tuberculosis.
Among contacts tested for HIV, 166 (11%) were positive (Table 4). The median CD4 count of new HIV diagnoses was 383/mm3 (IQR, 285–561) and 32 (19%) were referred to initiate ART because of a CD4 count less than 250/mm3. In contrast, the median CD4 count among index TB cases diagnosed with HIV at the time of their TB diagnosis was 80 cells/mm3 (IQR, 36–180). In contacts with newly diagnosed HIV, 16 (10%) also had undiagnosed TB, with a median CD4 count of 323 (IQR, 278–542) in these coinfected participants. Two contacts with newly diagnosed HIV were already receiving TB treatment at the study visit. In contacts with known HIV at the time of the study visit, 24 had CD4 cell counts less than 250, but only 12 (50%) were receiving ART.
TABLE 4.
HOUSEHOLD VCT RESULTS, TB CONTACT VERSUS NONCONTACT
| Contact N (%) | Noncontact N (%) | P Value | |
| HIV | |||
| Positive | 166 (6) | 76 (8) | |
| Negative | 1,402 (53) | 447 (48) | |
| Refused VCT | 1,068 (41) | 412 (44) | <0.001 |
| For HIV-infected only | |||
| CD4 count/mm3 median (IQR) | 383 (285–561) | 381 (236–567) | |
| <250 | 32 (19) | 19 (25) | |
| 250–350 | 38 (23) | 15 (20) | |
| >350 | 88 (53) | 37 (49) | |
| Not available | 8 (5) | 5 (7) | 0.67 |
| TB coinfected | 18 (11) | 0 (0) | 0.003 |
| Started ART within 2 mo | 3 (9) | 8 (42) | 0.006 |
Definition of abbreviations: ART = antiretroviral therapy; IQR = interquartile range; TB = tuberculosis; VCT = voluntary counseling and testing.
Noncontact Control Households
We approached 425 randomly selected control houses between February and April 2009; 92 refused enrollment, 7 had no adults present to consent after three attempts, and 14 had a known patient with TB in the house, leaving 312 noncontact households that enrolled at least one adult. A total of 983 household members were enrolled (Figure 1B) and 785 (80%) provided sputum specimens. Fifty-one (5%) noncontact household members reported they were HIV-infected; 411 (42%) refused VCT; and 521 (53%) agreed to HIV testing, of whom 76 (14%) tested positive. Among participants who refused to receive counseling or HIV test results, 137 participants subsequently agreed to provide an oral fluid specimen for off-site HIV testing; 19 (14%) of these individuals tested positive.
Four TB cases were found in four noncontact households, a prevalence of 407 of 100,000 (95% CI, 0–912 of 100,000). Three tested negative for HIV and one refused testing. The prevalence of TB within control households was 1.3% (4 of 312), versus 19% of contact households (P < 0.001).
The proportion of household members with HIV infection detected by home-based testing was similar in the contact and noncontact households (Table 4). Among people who consented to testing, noncontacts were more likely to be HIV-positive than TB contacts (odds ratio [OR], 1.48; P = 0.02). Mean CD4 counts at diagnosis were similar in contacts and noncontacts (461 vs. 456; P = 0.90). TB coinfection was more common in contacts than noncontacts. Significantly more noncontacts who were referred for ART at the time of diagnosis had documented ART initiation within 2 months (42% vs. 9%; P = 0.006).
Short-Term Follow-up
Ninety-seven persons (57%) diagnosed with TB at a household visit were confirmed to have initiated TB treatment within 1 month of notification of results. Eleven (22%) persons diagnosed with HIV at household VCT and with CD4 less than 250 initiated ART within 2 months of the initial visit.
Risk Factors
We examined characteristics of index cases and contacts to determine the risk of secondary cases being detected in a household (Table 5). Among contact households, having an HIV-positive index TB case was associated with a lower likelihood of having an additional case of TB (OR, 0.65; 95% CI, 0.39–1.079) (Table 5), although this was not statistically significant in univariate analyses or after adjusting for potential confounders. Smear-positive index households were slightly more likely to have at least one other case of TB than households with a smear-negative index (OR, 1.30; 95% CI, 0.874–1.94), but this association was not statistically significant.
TABLE 5.
RISK FACTORS FOR ≥1 UNDETECTED TUBERCULOSIS CASE IN CONTACT HOUSEHOLDS
| Index/Household-Level Factors | Odds Ratio | P Value | Adjusted Odds Ratio | P Value |
| Male | 1.0 | 1.0 | ||
| Female | 0.90 | 0.55 | 0.86 | 0.46 |
| Index age 18–29 | 1.0 | 1.0 | ||
| Index age 30–43 | 0.77 | 0.24 | 0.83 | 0.41 |
| Index age 44+ | 0.62 | 0.07 | 0.63 | 0.08 |
| Smear-positive index | 1.30 | 0.19 | 1.18 | 0.43 |
| Smear-negative index | 1.0 | 1.0 | ||
| HIV-positive index | 0.65 | 0.10 | 0.67 | 0.16 |
| HIV-negative index | 1.0 | 1.0 | ||
| Diagnosed in hospital | 0.75 | 0.13 | ||
| Diagnosed in clinic | 1.0 | |||
| Index cough | 0.89 | 0.66 | 0.88 | 0.64 |
| No cough | 1.0 | 1.0 | ||
| Shack housing | 1.31 | 0.19 | 1.28 | 0.24 |
| Nonshack housing | 1.0 | 1.0 |
We also examined factors in the index TB case that might be associated with individual contacts’ risk of TB (Table 6). Age, sex, HIV status, smoking status, and symptomatic cough were not associated with risk of being a contact TB case among persons who provided a sputum specimen; similarly, being the contact of a smear-positive index was not associated with statistically significant increased odds of having undiagnosed TB.
TABLE 6.
RISK FACTORS FOR TUBERCULOSIS FOR INDIVIDUAL CONTACTS
| Individual-Level Factors | Odds Ratio | P Value | Adjusted Odds Ratio | P Value |
| Age | ||||
| <5 | 1.0 | 1.0 | ||
| 5–17 | 1.45 | 0.35 | 1.45 | 0.73 |
| 18–29 | 1.54 | 0.41 | 1.54 | 0.69 |
| 30–43 | 1.29 | 0.24 | 1.26 | 0.82 |
| 44+ | 1.75 | 0.53 | 1.78 | 0.59 |
| Male | 1.0 | 1.0 | ||
| Female | 0.93 | 0.65 | 0.91 | 0.59 |
| HIV status | ||||
| Positive | 1.03 | 0.91 | 1.10 | 0.71 |
| Unknown | 1.03 | 0.89 | 1.05 | 0.80 |
| Negative | 1.0 | 1.0 | ||
| Smoker | 0.91 | 0.72 | ||
| Nonsmoker | 1.0 | |||
| Cough | 1.36 | 0.30 | ||
| No cough | 1.0 | |||
| Index/household-level factors | ||||
| Smear-positive | 1.15 | 0.45 | 0.97 | 0.88 |
| Smear-negative | 1.0 | 1.0 | ||
| HIV-positive | 0.86 | 0.51 | 0.92 | 0.73 |
| HIV-negative | 1.0 | 1.0 | ||
| Diagnosis in hospital | 0.71 | 0.05 | 0.71 | 0.07 |
| Diagnosis in clinic | 1.0 | 1.0 | ||
| Cough | 0.98 | 0.92 | ||
| No cough | 1.0 | |||
Discussion
This community-based study in South Africa found a prevalence of undetected TB of 6,075 per 100,000 (95% CI, 5,789–6,360 per 100,000) cases among household contacts of recently diagnosed patients with TB. In households without a known patient with TB, the prevalence of undetected TB was less than 500 per 100,000, suggesting that TB is clustering in households and that targeting contacts is a far more efficient approach to case finding than population-wide screening. The prevalence of TB found in contacts is likely an underestimate of the true prevalence, because the prevalence among all contacts who gave a sputum specimen was 7,802 per 100,000. This TB prevalence is approximately nine times the national prevalence of TB in South Africa and is nearly double the upper estimate of household TB prevalence found in a metaanalysis of household contact TB studies (12). Additionally, TB detected in household contacts was more likely to be in persons uninfected with HIV than in persons infected with HIV, and HIV disease in household contacts was less advanced than in index patients, suggesting that morbidity and mortality may be reduced by the early intervention provided by active case finding. Although HIV prevalence was slightly higher in noncontact households compared with contacts of patients with TB, HIV prevalence was higher in contact households if the index patient with TB was included in the household.
We did not identify typical risk factors for TB in household contacts. Previous studies have found that contacts of smear-positive index cases are more likely to be infected with TB and develop TB (15, 16). Our study found no difference in the odds of TB disease between contacts of smear-negative and smear-positive index patients. A possible explanation is that this study tested sputum specimens from all contacts (regardless of symptoms) using culture, whereas previous studies focused primarily on infection alone, relied on less-sensitive sputum microscopy, or only tested symptomatic contacts for active TB.
Of the TB cases in household contacts found by contact tracing, 89% of cases did not report symptoms at the time of the household visit, and 93% of cases were diagnosed by sputum culture alone. This is a higher proportion of asymptomatic, culture-positive TB than has been previously reported. It is possible that some of the TB detected may represent a transient infection that does not progress to clinical illness, as has been proposed to occur particularly in immunocompetent adults (17), the suspicion for true TB is high in this at-risk population with known exposure. However, it was not possible to determine which culture-positive patients might ultimately resolve infection without illness, so all were considered to have clinically active TB. Importantly, Lawn and colleagues (18) observed a rate of 75% progression to symptomatic, active TB in persons infected with HIV found to have asymptomatic, culture-positive TB. These results highlight the need for expanded use of culture or similarly sensitive diagnostic tool (19) in screening and diagnostic workups for TB. The DETECTB study, which compared active case-finding strategies that relied on sputum smear only, found that community-based case finding reduced the prevalence of TB in Zimbabwe, particularly among individuals uninfected with HIV (20). Given the low rate of smear-positive TB detected in the present study, it is unlikely that case finding using smear alone would have as large an effect in this setting. Smear-negative, culture-positive TB can nonetheless account for approximately 12% of new infections (21) and in individuals positive for HIV, TB may progress to death without an individual ever becoming smear-positive; therefore, use of culture in TB case-finding programs should further decrease infection, transmission, and incidence.
An important aspect of active case finding for TB is the negative predictive value (NPV) of the screening algorithm. In particular, individuals infected with HIV without active TB and child contacts less than 5 years of age should receive isoniazid prophylaxis therapy (IPT) according to local and international guidelines (22). A screening algorithm to rule out TB in individuals infected with HIV that uses the absence of any of four symptoms to rule out active TB in individuals positive for HIV has recently been endorsed by the WHO (22–24). Studies in Southeast Asia and Africa have indicated that this approach would be highly specific and would detect TB-free individuals who may safely be started on IPT (23, 24). The algorithm performed well in control participants in our study with an NPV of 99.6%, but the NPV dropped to 91.6% in HIV-infected contacts of known patients with TB and to 92.4% in contacts infected and uninfected with HIV. This result suggests that the WHO screening algorithm is reliable to rule out active TB in people with no known TB contact, but among household contacts of patients with TB in high-prevalence settings, the symptom screen is inadequate to rule out active TB, and all contacts should be investigated for TB with sputum culture to identify TB suspects and safely provide IPT for individuals infected with HIV.
The study has several limitations. Among index patients initially recruited, 17% were not able to have household members evaluated for TB. There was no indication that these index cases or their household members were systematically different from those who were ultimately evaluated. The diagnostic approach used relied on sputum culture, and because it is difficult to obtain sputum specimens from children, this approach may have missed cases in children who are at high risk of contracting TB from household contacts (25). Even when obtained, sputum culture is not optimally sensitive for diagnosing TB in children. Any inaccuracy in prevalence estimates as a result is likely to be an underestimate of the true prevalence of TB. We also experienced a relatively high rate of contamination of TB cultures, resulting in some positive cultures unable to be definitively speciated as M. tuberculosis. This may have resulted in some overdiagnosis of TB, although in several instances contaminated specimens recollected before the individual initiated treatment grew M. tuberculosis, and we believe that the effect of overdiagnosis is not a major contributor to the high prevalence observed.
Appropriate case detection is a necessary but not sufficient first step to reducing the community burden of TB; ultimately, case finding must be followed by treatment and cure to impact the epidemic. Preliminary follow-up results suggest that not all patients are accessing treatment appropriately after diagnosis and referral. In particular, only 9% of contacts and 42% of noncontacts referred for ART initiation based on CD4 count were able to start ART within the first 2 months after referral. Initiating ART is a multistage process, requiring multiple clinic visits before receiving a first prescription, and there is substantial drop-out between referral and initiation. Further studies will evaluate the effectiveness of household case finding for facilitating entry into care and long-term survival. Use of new sensitive diagnostic tools, such as the GeneXpert rapid nucleic amplification platform, instead of TB culture may also be of use in reducing the time to detection of TB and drug resistance, and should be investigated for feasibility in a contact-screening approach (19). Of note, a recent South African investigation of household contacts of known MDR and extensively drug-resistant TB found similarly elevated incidences of MDR and extensively drug-resistant TB (30–40 times higher than the population incidence) among household contacts (26), highlighting the urgent need for combined innovation in diagnostic tools and screening strategies.
In addition to improved case detection within a high-risk group, home-based active case finding may have other benefits. Household-based screening may serve as an infection control measure by not requiring HIV-positive and other susceptible contacts to come to a clinic for TB screening, where risk of exposure to TB is high (27). Conversely, with home-based screening, household contacts who are at higher risk for having active TB are not required to go to clinics for screening where they may potentially expose others in a vulnerable congregate setting.
Study procedures used existing laboratory facilities and were conducted by individuals with training comparable with that received by nurses and counselors at local clinics, suggesting that currently available resources could be adapted to expand the scope of these efforts. Indeed, in the study area, most clinics are partnered with local volunteer organizations that provide home-based care and patient tracing for TB and HIV; supplemental training of these personnel could replicate the screening provided in this study. Further cost-effectiveness analysis, currently underway, is also necessary to evaluate the relative costs and impact of household case finding and better inform health districts as to how to allocate resources.
This comprehensive approach to the HIV and TB epidemics that are devastating such districts as Matlosana may provide an intervention to mitigate the high morbidity and mortality caused by these epidemics. This strategy may also lead to the interruption of the transmission cycle by diagnosing and treating individuals who would continue to be at risk for transmitting HIV and TB while their disease remained undetected. Active case finding in households should be considered as a component of integrated HIV-TB care in high-prevalence settings.
Acknowledgments
The authors are grateful to the study participants and the nurses, counselors, and other staff of the Matlosana Department of Health and Klerksdorp/Tshepong Hospital Complex, especially the management, Ms. K. Randeree and Ms. Z. Motala. They thank Ms. B. Shezi for data management and Ms. R. Msandiwa for research oversight.
Footnotes
Supported by USAID through URC and ANOVA Health Institute. A.E.S. is supported in part by an NIH MSTP grant T32GM007309. R.E.C., J.E.G., and N.A.M. are supported by NIH grant R01 HL090312. E.V., B.L., and M.H.R. received TB/HIV research training through Fogarty International Center grants 2RTW007370 and 2RTW007373. The opinions herein do not necessarily reflect those of USAID or the U.S. government.
Author Contributions: N.A.M., E.V., A.E.S., R.E.C., and J.E.G. designed the study; N.A.M., A.E.S., M.H.R., E.V., N.M., B.L., and S.S. were responsible for data collection, study coordination, and data management; A.E.S. did the data analysis with contributions from N.A.M., R.E.C., J.E.G., and E.V; A.E.S., R.E.C., N.A.M., J.E.G., and E.V. drafted the manuscript; and all authors contributed to the writing of the report.
Originally Published in Press as DOI: 10.1164/rccm.201111-1941OC on March 15, 2012
References
- 1.Obermeyer Z, Abbott-Klafter J, Murray CJ. Has the DOTS strategy improved case finding or treatment success? An empirical assessment. PLoS ONE 2008;3:e1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havlir DV, Getahun H, Sanne I, Nunn P. Opportunities and challenges for HIV care in overlapping HIV and TB epidemics. JAMA 2008;300:423. [DOI] [PubMed] [Google Scholar]
- 3.Cock KMD, Chaisson RE. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis 1999;3:457–465. [PubMed] [Google Scholar]
- 4.World Health Organization. WHO Three I’s Meeting: report of a joint WHO HIV/AIDS and TB department meeting, 2–4 April 2008 [Internet]. c2008 [accessed 2011 Oct 1]. Available from: http://www.who.int/hiv/pub/tb/3is_mreport/en/index.html.
- 5.Meintjes G, Schoeman H, Morroni C, Wilson D, Maartens G. Patient and provider delay in tuberculosis suspects from communities with a high HIV prevalence in South Africa: a cross-sectional study. BMC Infect Dis 2008;8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health 2008;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JL, Vjecha MJ, Okwera A, Hatanga E, Byekwaso F, Wolski K, Aisu T, Whalen CC, Huebner R, Mugerwa RD, et al. Impact of human immunodeficiency virus type-1 infection on the initial bacteriologic and radiographic manifestations of pulmonary tuberculosis in Uganda. Makerere University-Case Western Reserve University Research Collaboration. Int J Tuberc Lung Dis 1998;2:397–404. [PubMed] [Google Scholar]
- 8.Koblin BA, Husnik MJ, Colfax G, Huang Y, Madison M, Mayer K, Barresi PJ, Coates TJ, Chesney MA, Buchbinder S. Risk factors for HIV infection among men who have sex with men. AIDS 2006;20:731–739. [DOI] [PubMed] [Google Scholar]
- 9.Bakari M, Arbeit RD, Mtei L, Lyimo J, Waddell R, Matee M, Cole BF, Tvaroha S, Horsburgh CR, Soini H, et al. Basis for treatment of tuberculosis among HIV-infected patients in Tanzania: the role of chest x-ray and sputum culture. BMC Infect Dis 2008;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet 2007;369:2042. [DOI] [PubMed] [Google Scholar]
- 11.Dowdy DW, Chaisson RE, Maartens G, Corbett EL, Dorman SE. Impact of enhanced tuberculosis diagnosis in South Africa: a mathematical model of expanded culture and drug susceptibility testing. Proc Natl Acad Sci USA 2008;105:11293–11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 2008;8:359–368. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro AE, Rakgokong MH, Chaisson RE, Modiba P, Msandiwa R, Golub JE, Variava E, Martinson NA. Targeting at-risk households: intensified case-finding for TB and HIV in contacts of TB patients in South Africa. Presented at the 18th International AIDS Conference. July 18–23, 2010, Vienna, Austria. Abstract FRLBC106.
- 14.South Africa National Department of Health. National antiretroviral treatment guidelines [Internet]. c2004 [accessed 2011 Oct 1]. Available from: http://www.doh.gov.za/list.php?type=HIV%20and%20AIDS&year=2004
- 15.Verver S, van Loenhout-Rooyackers JH, Bwire R, Annee-van Bavel JA, de Lange HJ, van Gerven PJ, Borgdorff MW. Tuberculosis infection in children who are contacts of immigrant tuberculosis patients. Eur Respir J 2005;26:126–132. [DOI] [PubMed] [Google Scholar]
- 16.Radhakrishna S, Frieden TR, Subramani R, Santha T, Narayanan PR. Additional risk of developing TB for household members with a TB case at home at intake: a 15-year study. Int J Tuberc Lung Dis 2007;11:282–288. [PubMed] [Google Scholar]
- 17.Marais BJ. Does finding M. tuberculosis in sputum always equal tuberculosis disease? Am J Respir Crit Care Med 2010;181:195–196. [DOI] [PubMed] [Google Scholar]
- 18.Lawn SD, Kerkhoff AD, Wood R. Progression of subclinical culture-positive tuberculosis to symptomatic disease in HIV-infected individuals. AIDS 2011;25:2190–2191. [DOI] [PubMed] [Google Scholar]
- 19.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbett EL, Bandason T, Duong T, Dauya E, Makamure B, Churchyard GJ, Williams BG, Munyati SS, Butterworth AE, Mason PR, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet 2010;376:1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tostmann A, Kik SV, Kalisvaart NA, Sebek MM, Verver S, Boeree MJ, van Soolingen D. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis 2008;47:1135–1142. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Guidelines for intensive case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings [Internet]. WHO Department of HIV/AIDS, Stop TB Department, editors. c2011 [accessed 2011 Oct 1]. Available from: http://www.who.int/hiv/pub/tb/9789241500708/en/index.html
- 23.Getahun H, Kittikraisak W, Heilig CM, Corbett EL, Ayles H, Cain KP, Grant AD, Churchyard GJ, Kimerling M, Shah S, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med 2011;8:e1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cain KP, McCarthy KD, Heilig CM, Monkongdee P, Tasaneeyapan T, Kanara N, Kimerling ME, Chheng P, Thai S, Sar B, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med 2010;362:707–716. [DOI] [PubMed] [Google Scholar]
- 25.Marais BJ, Graham SM, Cotton MF, Beyers N. Diagnostic and management challenges for childhood tuberculosis in the era of HIV. J Infect Dis 2007;196:S76–S85. [DOI] [PubMed] [Google Scholar]
- 26.Vella V, Racalbuto V, Guerra R, Marra C, Moll A, Mhlanga Z, Maluleke M, Mhlope H, Margot B, Friedland G, et al. Household contact investigation of multidrug-resistant and extensively drug-resistant tuberculosis in a high HIV prevalence setting. Int J Tuberc Lung Dis 2011;15:1170–1175. [DOI] [PubMed] [Google Scholar]
- 27.Harries AD, Zachariah R, Tayler-Smith K, Schouten EJ, Chimbwandira F, Van Damme W, El-Sadr WM. Keeping health facilities safe: one way of strengthening the interaction between disease-specific programmes and health systems. Trop Med Int Health 2010;15:1407–1412. [DOI] [PubMed] [Google Scholar]