Abstract
Rationale: The course of lung function decline for smokers with early airflow obstruction remains undefined. It is also unclear which early spirometric characteristics identify individuals at risk for rapid decline and increased mortality.
Objectives: To determine the association between spirometric measures and 5-year decline in FEV1 and 12-year mortality.
Methods: We analyzed longitudinal data from the Lung Health Study, a clinical trial of intensive smoking cessation intervention with or without bronchodilator therapy in 5,887 smokers with mild to moderate airflow obstruction. Participants were stratified into bins of baseline FEV1 to FVC ratio, using bins of 5%, and separately into bins of Z-score (difference between actual and predicted FEV1/FVC, normalized to SD of predicted FEV1/FVC). Associations between spirometric measures and FEV1 decline and mortality were determined after adjusting for baseline characteristics and time-varying smoking status.
Measurements and Main Results: The cohort was approximately two-thirds male, predominantly of white race (96%), and with mean age of 49 ± 7 years. In general, individuals with lower lung function by any metric had more rapid adjusted FEV1 decline. A threshold for differential decline was present at FEV1/FVC less than 0.65 (P < 0.001) and Z-score less than −2 (2.3 percentile) (P < 0.001). At year 12, 575 (7.2%) of the cohort had died. Lower thresholds of each spirometric metric were associated with increasing adjusted hazard of death.
Conclusions: Smokers at risk or with mild to moderate chronic obstructive pulmonary disease have accelerated lung function decline. Individuals with lower baseline FEV1/FVC have more rapid decline and worse mortality.
Keywords: chronic obstructive pulmonary disease, spirometry, disease progression, prognosis, mortality
At a Glance Commentary
Scientific Knowledge on the Subject
The traditional model of accelerated decline in lung function in chronic obstructive pulmonary disease (COPD) has been recently challenged by several studies showing heterogeneity in lung function decline. It is important to identify early spirometric characteristics that can determine who is at risk for the most rapid rate of lung function decline and worse mortality.
What This Study Adds to the Field
In smokers at risk or with mild to moderate COPD, worse baseline lung function is associated with more rapid long-term FEV1 decline and higher risk of death. The threshold of FEV1/FVC that placed people at risk for excessive lung function decline and mortality was lower than current thresholds used to define COPD.
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality, recently becoming the third leading cause of death in the United States (1). Expiratory airflow limitation, the hallmark physiologic abnormality in COPD, is determined by a reduction in the ratio of FEV1 to the FVC. The traditional model of COPD as defined by Fletcher and Peto is one of accelerated decline in lung function, resulting in reduced FEV1 over many years (2). Recently, this notion has been challenged by several clinical trials describing substantial heterogeneity in lung function decline, with normal or near-normal rates of decline in some individuals with established COPD (3–6). It is unclear if these observations reflect a plateau of lung function decline in those with moderate to severe COPD, the natural course of treated COPD, or that not all COPD patients experience the historically accepted trajectory of FEV1 decline.
As most clinical trials included patients with established and symptomatic COPD, the course of lung function decline for individuals with early airflow obstruction remains unclear. It is important to identify early spirometric characteristics that can identify those at risk for the most rapid rate of lung function decline as well as those with inconsequential disease. Moreover, current spirometric guidelines used to diagnose COPD have not been validated in terms of predicting long-term outcomes (7–11). The Lung Health Study (LHS) was a longitudinal multicenter randomized clinical trial of an intensive smoking cessation intervention with or without inhaled bronchodilator therapy compared with usual care in smokers recruited from the community with mild to moderate airflow obstruction (12). The longitudinal data from LHS offer a unique opportunity to explore how a range of baseline spirometric measures are associated with longitudinal FEV1 decline and mortality. In this analysis, we stratify the 5,885 LHS participants into bins of baseline FEV1/FVC and FEV1% predicted, determining the association between spirometric measures and the annual decline in FEV1 over 5 years and mortality at 12 years.
Methods
Participant Selection
The design of LHS has been previously described (12–14). LHS I was a multicenter, randomized three-arm trial of smoking cessation intervention combined with inhaled ipratropium or placebo versus usual care (Clinialtrials.gov NCT00000568). The study enrolled 5,887 active smokers from the community aged 35 to 60 years with prebronchodilator FEV1/FVC less than 0.70 and prebronchodilator FEV1 between 55 and 90% predicted who were not regularly using physician-prescribed bronchodilators. Lung function was measured annually over 5 years. LHS III extended the follow-up of 98.3% of LHS I participants to December 31, 2001 or 14.5 years (whichever was earlier), to determine long-term impact of the smoking cessation intervention (15, 16). For this analysis, all LHS I participants with post-bronchodilator FEV1/FVC measurements (n = 5885) were included. Because of the observed increase in FEV1 during the first year of LHS (13), and potential bias related to deaths and loss to follow-up with LHS III, analysis of rate of decline of FEV1 was limited to annual visit one through annual visit five. For mortality analyses, LHS III death status was used. Written informed consent was obtained from all participants originally enrolled in LHS. Institutional Review Board approval was waived as all data were previously collected and deidentified.
Defining Spirometric Thresholds
The procedure for spirometry measurements has been described elsewhere (17). Spirometric values collected at the second screening visit were used in this analysis. Participants were stratified into bins of baseline post-bronchodilator FEV1/FVC as a percent, using bins of 5%. Separately, participants were stratified by baseline post-bronchodilator FEV1/FVC into bins of Z-score, using reference formulas of Hankinson (18). The Z-score represents the difference between the actual FEV1/FVC and the predicted FEV1/FVC, normalized to the SD of the predicted FEV1/FVC. For example, a Z-score of −1.0 means that the measured FEV1/FVC is 1 SD below the mean of the reference population (the 16th percentile of the population). A Z-score of −1.645 represents the 5th percentile of the population (the lower limit of normal [LLN] threshold) used to define COPD by some guidelines (8). Table E1 in the online supplement provides a summary of the correlation between Z-scores and normally distributed population percentiles. Participants were also stratified into bins of baseline FEV1% predicted.
Modeling of Longitudinal FEV1 Decline
To test for the association between bins of baseline spirometric measurements and mean annual FEV1 decline, generalized estimating equations (19) with a robust exchangeable variance-covariance matrix were used. Lung function decline was assessed via the interaction of the spirometric measure and time. Predictors associated with FEV1 measurements and thus included in modeling were randomization group, race, sex, age, height, body mass index, and time-varying smoking patterns. Time-varying smoking pattern was modeled with three covariates: (1) average number of cigarettes smoked per day over the previous year at each annual visit, (2) subject’s smoking status for the current year, (3) and subject’s smoking status for the previous year (defined as smoker or nonsmoker). Full details on predictors and model construction are available in the online supplement. Graphs of adjusted mean FEV1 change (expressed in milliliters per year) across bins of different spirometric measures were generated. Methacholine reactivity was defined as previously described (20). To determine the association between different spirometric thresholds and the hazard of death at LHS III follow-up, adjusted Cox proportional hazard regression models were generated using duration of follow-up as the time metric. A P value of less than 0.05 was used to infer statistical significance. Stata version 10.0 (Stata Corp, College Station, TX), was used for statistical analyses.
Results
Participant Characteristics at Baseline
A total of 5,885 LHS participants were included in this analysis, representing 99.9% of the original LHS cohort. (Table 1) Two individuals were excluded due to the lack of post-bronchodilator spirometry. The cohort was predominantly of white race (96%), with mean age of 49 ± 6.8 years. Approximately two-thirds were men. All participants were active smokers at the time of enrollment, with an average smoking history of 41 ± 19 pack-years. The mean prebronchodilator FEV1/FVC was 0.63 ± 0.06, with post-bronchodilator FEV1/FVC increasing to 0.65 ± 0.06. Although all participants had a prebronchodilator FEV1/FVC less than 0.70, 1,245 (21%) had a post-bronchodilator FEV1/FVC greater than or equal to 0.70. The mean post-bronchodilator FEV1% predicted of the cohort was 78 ± 9% predicted.
TABLE 1.
CHARACTERISTICS OF LUNG HEALTH STUDY COHORT (N = 5,885)
| Baseline | |
| Age, yr | 48.5 (6.8) |
| Male, n (%) | 3,701 (63) |
| Race/ethnicity, n (%) | |
| White | 5,636 (95.8) |
| Black | 225 (3.8) |
| Other | 24 (0.4) |
| BMI, kg/m2 | 25.6 (3.9) |
| Pack-years smoking | 40.5 (19) |
| Cigarettes per day | 21 (12) |
| FEV1/FVC ratio, post-BD | 0.65 (0.06) |
| FEV1, post-BD | |
| Absolute, L | 2.75 (0.63) |
| % Predicted | 78.3 (9.1) |
| FVC, post-BD, L | 4.24 (0.95) |
| Longitudinal Follow-up | |
| Smoking status at last visit | |
| Continuous smoker | 2,695 (45.8) |
| Intermittent smoker | 2,275 (38.7) |
| Sustained quitter | 915 (15.5) |
| Alive | |
| 5-yr | 5,738 (97.5) |
| LHS3 | 5,310 (90.2) |
| Average annual change in post-BD FEV1, ml | |
| 5-yr | −53.1 (0.78) |
| LHS3 | −53.9 (0.51) |
Definition of abbreviations: BD = bronchodilator; BMI = body mass index; LHS3 = Lung Health Study III.
Values are presented as mean (SD) unless otherwise indicated.
Five-Year and LHS III Outcomes
At 5-year follow-up, 147 (2.5%) of the cohort had died (Table 1). Of those still living, 3,890 (68%) were active smokers. At LHS III, 575 participants had died, representing 9.8% of the original LHS cohort. Data regarding cause of death have been previously reported (13, 15, 21). At the last visit before study completion, loss to follow-up, or death, 2,695 (46%) of the cohort were active smokers, whereas 915 (15.5%) were sustained quitters. The unadjusted annual decline in absolute FEV1 for the entire cohort was 53.1 ± 0.6 ml at year 5 and 53.9 ± 0.5 ml at year 12.
Adjusted Mean FEV1 Decline Stratified by Bins of Baseline Spirometric Measures
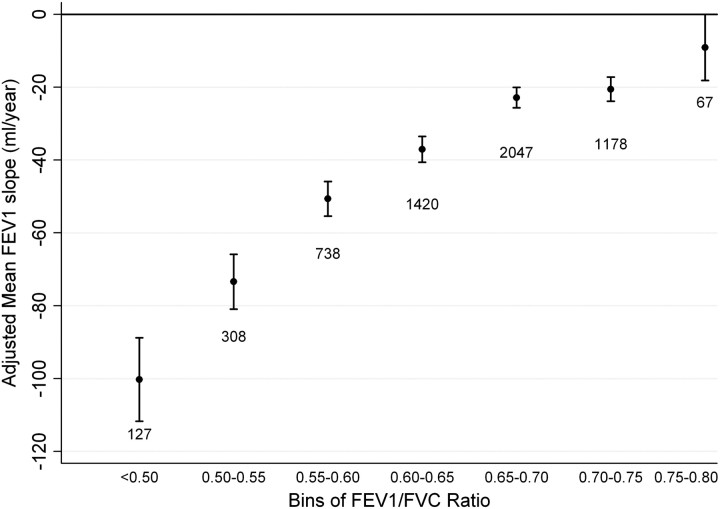
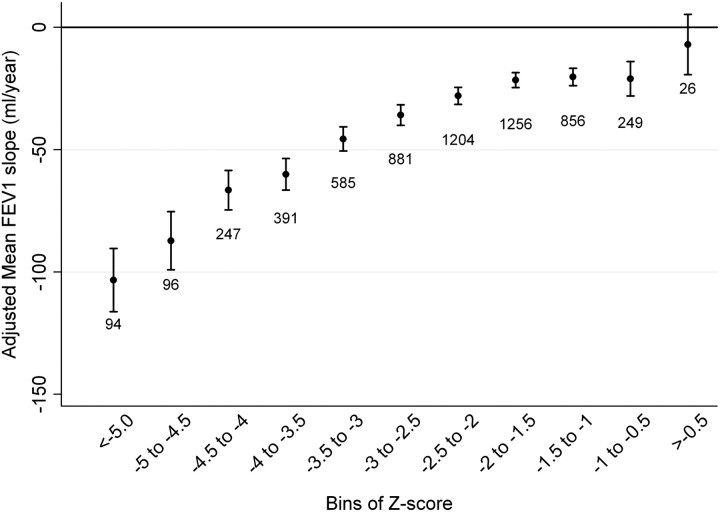
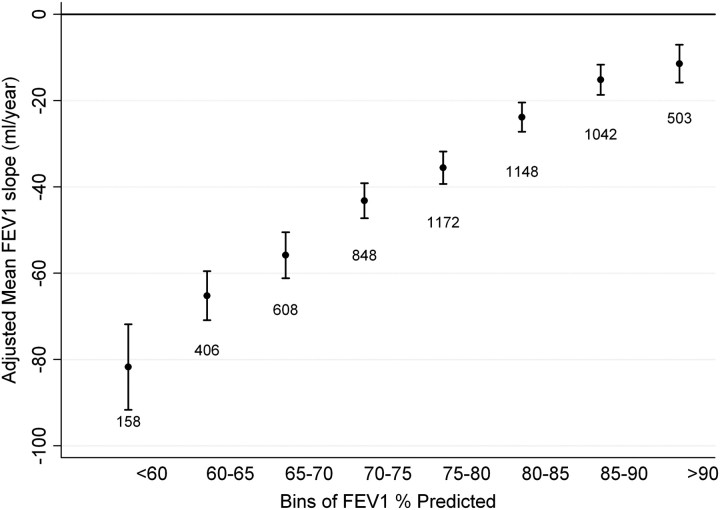
In exploratory analyses, several participant baseline characteristics were identified to contribute to the longitudinal decline in absolute FEV1 and were incorporated in a multivariate model. The relative adjusted effect of these covariates on absolute FEV1 is summarized in Table E2. In general, individuals with lower lung function by any metric had more rapid adjusted FEV1 decline. When stratifying participants into bins of baseline absolute FEV1/FVC, all but the highest bin was associated with an adjusted mean FEV1 slope less than zero (Figure 1). There appeared to be a threshold of differential decline at the 0.60 to 0.65 bin, with a statistically greater FEV1 decline compared with participants in the next highest bin (P < 0.001). For the bins less than 0.65 to 0.70, there was a significantly greater FEV1 decline for lower FEV1/FVC bins (see Figure 1 and Table E3). When stratifying participants into bins of Z-score, a similar pattern was observed with a threshold of differential decline occurring between the −2.5 to −2 Z-score bin and the −2 to −1.5 bin (P < 0.001) (Figure 2). There was no statistical difference in annual FEV1 decline between participants whose baseline FEV1/FVC Z-score includes the LLN 5th percentile threshold (−2 to −1.5 bin) compared with the next highest bin (P = 0.52). Subsequently lower Z-score bins were associated with greater adjusted FEV1 slope of decline (Table E3). Individuals with baseline FEV1% predicted that ranged from 80 to 85% had a greater annual decline than participants in the 85 to 90% FEV1% predicted bin (P < 0.001) (Figure 3). Each subsequently lower bin of baseline FEV1% predicted was associated with a statistically greater decline in annual FEV1 (Table E3). The observed trends in FEV1 slope of decline across bins of different spirometric measures were not substantially altered when including methacholine reactivity or baseline FEV1 in the primary model (Figures E1–E5). When evaluating the usual care group separately (to completely remove any potential impact of smoking cessation intervention and ipratropium on the FEV1 slope) or including the 11-year follow-up data, similar trends were present (Figures E6–E11). When stratifying the study cohort by smoking pattern (sustained quitter, intermittent quitter, or continuous smoker), the association between lower baseline lung function and accelerated decline was present in all three groups, but attenuated in the sustained quitters. Intermittent smokers and continuous smokers had similar trends when baseline lung function was normal or mildly impaired. At levels of more severe baseline lung impairments, intermittent smokers demonstrated less rapid lung function decline than continuous smokers (Figures E12–E14).
Figure 1.
Adjusted mean FEV1 slope stratified by bins of baseline FEV1/FVC ratio. Model adjusted for randomization group, race, sex, sex–time interaction, age, height, body mass index, average number of cigarettes smoked per day over the previous year at each annual visit, subject’s smoking status in each of the 2 prior years (defined as smoker or nonsmoker), and smoking status–time interaction. Error bars represent 95% confidence intervals.
Figure 2.
Adjusted mean FEV1 slope stratified by bins of baseline FEV1/FVC Z-score. The Z-score represents the difference between the actual FEV1/FVC and the predicted FEV1/FVC, normalized to the SD of the predicted FEV1/FVC. Model adjusted for randomization group, race, sex, sex–time interaction, age, height, body mass index, average number of cigarettes smoked per day over the previous year at each annual visit, subject’s smoking status in each of the 2 prior years (defined as smoker or nonsmoker), and smoking status–time interaction. Error bars represent 95% confidence intervals.
Figure 3.
Adjusted mean FEV1 slope stratified by bins of baseline post-bronchodilator FEV1% predicted. Model adjusted for randomization group, race, sex, sex–time interaction, age, height, body mass index, average number of cigarettes smoked per day over the previous year at each annual visit, subject’s smoking status in each of the 2 prior years (defined as smoker or nonsmoker), and smoking status–time interaction. Error bars represent 95% confidence intervals.
Hazard of Death with Different Spirometric Thresholds
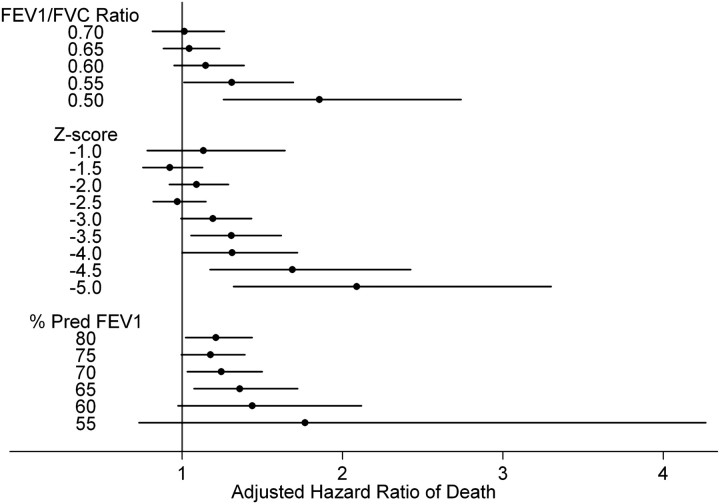
As seen in Figure 4 and Table E4, a consistent trend was seen with lower thresholds of spirometric measures being associated with increasing adjusted hazard of death. Specifically, lower FEV1/FVC ratios increased the hazard of death, with statistical significance being met when comparing an FEV1/FVC less than 0.55 to greater than 0.55 (hazard ratio [HR], 1.31; 95% confidence interval [CI], 1.01–1.70; P = 0.04). When evaluating different Z-score thresholds, the hazard of death began increasing at the −3.0 threshold (HR, 1.19; 95% CI, 0.99–1.43; P = 0.06) and reached statistical significance at the −3.5 threshold (HR, 1.31; 95% CI, 1.06–1.62; P = 0.014). Progressively lower Z-score thresholds were associated with an increasing hazard of death. An increased hazard of death was seen across the range of baseline FEV1% predicted thresholds, with the greatest risk observed when comparing those with a baseline FEV1% predicted less than 65% to those greater than 65% predicted (HR, 1.36; 95% CI, 1.08–1.72; P = 0.01). When stratifying by cause of death, no clear spirometric thresholds existed to identify an increased risk of death. (Table E5).
Figure 4.
Adjusted hazard ratio of death comparing different thresholds of baseline spirometric predictors. Point estimate and 95% error bars represent the hazard ratio comparing spirometric indices above the threshold to below the threshold, adjusted for baseline age, race, sex, and smoking status at last visit.
Discussion
We have demonstrated that spirometry testing in a population at risk or with mild to moderate lung disease provides important prognostic information. Some smokers with mild to moderate airflow limitation do have an increased rate of lung function decline. In smokers at risk or with mild to moderate COPD, worse lung function at baseline is associated with more rapid long-term decline in FEV1 and higher risk of death. In our analysis, excessive lung function decline occurred below FEV1/FVC of 0.65 or Z-score of −2.0 (2.3 percentile). The risk of death did not increase until even lower thresholds (FEV1/FVC of 0.55 or Z-score of −3.5 [0.023 percentile]). The thresholds we observed that placed people at risk for excessive lung function decline and mortality were lower than current thresholds used to define COPD (FEV1/FVC < 0.70 or fifth percentile LLN). These findings highlight the usefulness of screening spirometry in at-risk groups, allowing for risk stratification of at-risk individuals to avoid overtreatment of those not at risk for accelerated lung function decline or death. Moreover, these data can inform screening criteria for guideline development.
Understanding the predictors and significance of the rate of decline in lung function in individuals with COPD has been the focus of several recent studies (3–6, 22–24). When examining individuals with established COPD, it was observed that lung function decline is heterogeneous (5). A recent report observed that only 18% of patients with COPD experienced a statistically significant FEV1 decline assessed by linear regression (6). It is unclear if the observation of less than anticipated FEV1 decline in those studies was attributable to alterations in disease pathobiology or response to medical therapy. By analyzing a group of individuals with mild to moderate disease relatively free of treatment, we have expanded the understanding of lung function decline in smokers. Our results demonstrate that some smokers with minimal to moderate lung function impairment do have an accelerated decline of lung function. Both the baseline FEV1/FVC ratio and the FEV1% predicted were informative in this regard. These findings were present even after accounting for longitudinal smoking habits of the LHS cohort. Although the most accelerated lung function decline was seen in continuous smokers, the association between lower baseline lung function and more rapid FEV1 decline was also present in intermittent quitters and sustained quitters. Our findings confirm the previously described “horse racing effect” in established COPD, whereby those with the most severe FEV1 impairment, on average, have the most accelerated future FEV1 decline (25). Our findings also complement the reported observation that lower lung function at young age is highly predictive of low lung function in middle age (23). We have now expanded this observation by including at-risk individuals as well. We observed that at thresholds of FEV1% predicted above 60%, those below that level had a higher risk of death than those above. These observations can help practitioners identify those individuals who are at greatest risk for future lung impairment and death.
Consistent with recent reports describing the heterogeneity of FEV1 decline in COPD, in addition to identifying a subset of individuals with accelerated lung function decline we also observed that many smokers did not have a rapid decline in lung function over time. We observed that differential FEV1 decline did not occur unless the baseline FEV1/FVC was below 0.65 or Z-score less than −2.0, and the mean rate of decline of FEV1 increased with further reduction in the baseline ratio. As identified by the writing committees of the major pulmonary organizations, the currently selected spirometric thresholds lack clinical validation (7, 8). These data suggest that the current thresholds to define COPD may be identifying individuals who are not at risk of accelerated FEV1 decline and thus may not warrant pharmacotherapy. The use of a diagnostic threshold that identifies individuals who may not be at risk of accelerated decline could lead to potential overdiagnosis of nonsignificant disease.
Current guidelines recommend against spirometric screening of asymptomatic individuals, regardless of COPD risk factors (26). Such recommendations are based on the lack of evidence for beneficial pharmacotherapy to treat asymptomatic persons with or without airflow obstruction. Our analysis shows that spirometry can inform the prognosis of smokers. Albeit limited by post hoc subgroup analysis, reports demonstrate that pharmacotherapies can improve symptoms, exacerbation frequency, rate of FEV1 decline, and mortality in individuals with mild and moderate COPD (27–29). It remains unclear if therapies for mild COPD will definitely improve outcomes, and thus the findings presented here do not contradict current screening guidelines. If pharmacotherapy that reduces rate of decline in more advanced disease is found to be useful in milder disease, there may be therapeutic benefit of screening. This is not the case currently. Smoking cessation should be recommended to all individuals regardless of lung function status. However, if resources for high intensity smoking cessation are limited, those with the greatest risk for lung function decline would be highest priority.
In our analysis, we did not observe a statistically significant increase in the risk of death for participants with a baseline FEV1/FVC less than 0.70 or LLN encompassing the fifth percentile compared with those above that threshold. The majority of studies evaluating the relationship between spirometry and mortality focus on comparing the fixed ratio and LLN criteria (30–33). Few have explored different thresholds above and below the current criteria (34, 35). Moreover, all LHS participants were active smokers at baseline with careful follow-up of biochemically validated smoking status, allowing us to account for the confounding effect of smoking on mortality. Vaz Fragoso and colleagues examined the association between different thresholds of LLN criteria (ranging from 5th to 25th percentile) and 12-year mortality in 3,502 participants of the Third National Health and Nutrition Examination Survey (NHANES III) (34). They observed an increase in the adjusted hazard of death only for those below the 5th percentile compared with those above the 25th percentile. The authors did not evaluate thresholds below the fifth percentile. Mannino and colleagues evaluated the association between FEV1/FVC less than 0.70 and LLN fifth percentile criteria with 11-year mortality in an elderly community-based cohort (35). These authors observed a 40% increase in the hazard of death (HR, 1.40; 95% CI, 1.1–1.7) comparing individuals with FEV1/FVC less than 0.70 and below LLN to individuals with normal spirometry. In a recent analysis of the Lung Health Study cohort, increased 15-year mortality was seen only in those with modified GOLD stage three or four lung disease (36). Our findings support and refine these observations by demonstrating that a better threshold to determine mortality risk may exist, specifically an FEV1/FVC < 0.55 or Z-score less than −3.5.
Our analysis has limitations. The data for this analysis are collected in the setting of a clinical trial, and thus the characteristics of participants in this cohort may not reflect the general population. Even though evaluation of the usual care group alone was similar to the overall findings, the data presented here may reflect changes related to trial interventions rather than the natural course of COPD. LHS did not collect radiographic or pulmonary function measures of emphysema, which has been shown to be important in determining prognosis in patients with COPD (24). Because the LHS did not enroll subjects with severe airflow limitation, these analyses do not exclude the possibility that individuals with severe airflow obstruction experience a slower rate of decline in lung function than those with moderate obstruction, resulting in a sigmoid curve with mild and severe obstruction showing the slowest rates of decline. The participants of LHS were predominantly white, limiting generalizability to other demographic groups. LHS had relatively few deaths and relatively mild airflow obstruction, potentially leading to an underpowering for detection of a consistent association between lower spirometric values and mortality, particularly when stratifying by cause of death. This analysis did not evaluate other endpoints important in the conceptual definition of disease, specifically the association between baseline FEV1 and respiratory symptoms and quality of life.
In summary, we have demonstrated that smokers at risk or with mild to moderate COPD have accelerated lung function decline. Individuals with lower FEV1/FVC have more rapid decline and worse mortality. The current spirometric thresholds used to define COPD, either an FEV1/FVC less than 0.70 or below the fifth percentile, are above a level that predicts more rapid lung function decline and increased risk of death. In a group of active smokers, it may be necessary to lower the threshold to an FEV1/FVC less than 0.65 or Z-score less than −2.0 to identify those at increased risk for more rapid fall in FEV1, with even lower thresholds potentially necessary to identify those at increased risk of mortality. In addition to demonstrating the value of screening spirometry in smokers to predict long-term outcomes, we have provided information allowing for risk stratification of at-risk individuals.
Footnotes
Author Contributions: M.B.D. was responsible for manuscript concept, data analysis, drafting of the manuscript, and revisions. N.N.H. was responsible for study design, data analysis, and revisions for intellectual content. J.E.C., P.D.S., D.P.T., and R.A.W. were responsible for data collection, study design, data analysis, and revisions for intellectual content. All authors approved the final manuscript.
Supported by National Institutes of Health–National Heart, Lung, and Blood Institute grant K23HL103192 (M.B.D.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201202-0223OC on May 3, 2012
References
- 1.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H. Deaths: preliminary data for 2009. Natl Vital Stat Rep 2011;59:1–51. [PubMed] [Google Scholar]
- 2.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ 1977;1:1645–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543–1554. [DOI] [PubMed] [Google Scholar]
- 4.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775–789. [DOI] [PubMed] [Google Scholar]
- 5.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011;365:1184–1192. [DOI] [PubMed] [Google Scholar]
- 6.Casanova C, de Torres JP, Aguirre-Jaime A, Pinto-Plata V, Marin JM, Cordoba E, Baz R, Cote C, Celli BR. The progression of chronic obstructive pulmonary disease is heterogeneous: the experience of the BODE cohort. Am J Respir Crit Care Med 2011;184:1015–1021. [DOI] [PubMed] [Google Scholar]
- 7.From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011. [Internet; accessed 2012 Jan 15]. Available from: http://www.goldcopd.org/. [Google Scholar]
- 8.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–968. [DOI] [PubMed] [Google Scholar]
- 9.Marks GB. Are reference equations for spirometry an appropriate criterion for diagnosing disease and predicting prognosis? Thorax 2011;67:85–87. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed Hoesein FA, Zanen P, Lammers JW. Lower limit of normal or FEV1/FVC < 0.70 in diagnosing COPD: an evidence-based review. Respir Med 2011;105:907–915. [DOI] [PubMed] [Google Scholar]
- 11.Mannino DM. Defining chronic obstructive pulmonary disease… and the elephant in the room. Eur Respir J 2007;30:189–190. [DOI] [PubMed] [Google Scholar]
- 12.Anthonisen NR. Lung Health Study. Am Rev Respir Dis 1989;140:871–872. [DOI] [PubMed] [Google Scholar]
- 13.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA, Enright PL, Kanner RE, O’Hara P, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994;272:1497–1505. [PubMed] [Google Scholar]
- 14.Connett JE, Kusek JW, Bailey WC, O’Hara P, Wu M. Design of the Lung Health Study: a randomized clinical trial of early intervention for chronic obstructive pulmonary disease. Control Clin Trials 1993;14:3S–19S. [DOI] [PubMed] [Google Scholar]
- 15.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005;142:233–239. [DOI] [PubMed] [Google Scholar]
- 16.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 2002;166:675–679. [DOI] [PubMed] [Google Scholar]
- 17.Enright PL, Johnson LR, Connett JE, Voelker H, Buist AS. Spirometry in the Lung Health Study. 1. Methods and quality control. Am Rev Respir Dis 1991;143:1215–1223. [DOI] [PubMed] [Google Scholar]
- 18.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 19.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–130. [PubMed] [Google Scholar]
- 20.Tashkin DP, Altose MD, Connett JE, Kanner RE, Lee WW, Wise RA. Methacholine reactivity predicts changes in lung function over time in smokers with early chronic obstructive pulmonary disease. The Lung Health Study Research Group. Am J Respir Crit Care Med 1996;153:1802–1811. [DOI] [PubMed] [Google Scholar]
- 21.Anthonisen NR, Connett JE, Enright PL, Manfreda J. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med 2002;166:333–339. [DOI] [PubMed] [Google Scholar]
- 22.Kesten S, Celli B, Decramer M, Liu D, Tashkin D. Adverse health consequences in COPD patients with rapid decline in FEV1 - evidence from the UPLIFT trial. Respir Res 2011;12:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalhan R, Arynchyn A, Colangelo LA, Dransfield MT, Gerald LB, Smith LJ. Lung function in young adults predicts airflow obstruction 20 years later. Am J Med 2010;123:468.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimura M, Makita H, Nagai K, Konno S, Nasuhara Y, Hasegawa M, Shimizu K, Betsuyaku T, Ito YM, Fuke S, et al. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;185:44–52. [DOI] [PubMed] [Google Scholar]
- 25.Burrows B, Knudson RJ, Camilli AE, Lyle SK, Lebowitz MD. The “horse-racing effect” and predicting decline in forced expiratory volume in one second from screening spirometry. Am Rev Respir Dis 1987;135:788–793. [DOI] [PubMed] [Google Scholar]
- 26.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schunemann H, Wedzicha W, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155:179–191. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, Yates JC, Willits LR, Vestbo J. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res 2009;10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet 2009;374:1171–1178. [DOI] [PubMed] [Google Scholar]
- 29.Dusser D, Bravo ML, Iacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur Respir J 2006;27:547–555. [DOI] [PubMed] [Google Scholar]
- 30.Vaz Fragoso CA, Concato J, McAvay G, Van Ness PH, Rochester CL, Yaggi HK, Gill TM. Chronic obstructive pulmonary disease in older persons: a comparison of two spirometric definitions. Respir Med 2010;104:1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vollmer WM, Gislason T, Burney P, Enright PL, Gulsvik A, Kocabas A, Buist AS. Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J 2009;34:588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirtcliffe P, Weatherall M, Marsh S, Travers J, Hansell A, McNaughton A, Aldington S, Muellerova H, Beasley R. COPD prevalence in a random population survey: a matter of definition. Eur Respir J 2007;30:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen JE, Sun XG, Wasserman K. Spirometric criteria for airway obstruction: use percentage of FEV1/FVC ratio below the fifth percentile, not < 70%. Chest 2007;131:349–355. [DOI] [PubMed] [Google Scholar]
- 34.Vaz Fragoso CA, Concato J, McAvay G, Van Ness PH, Rochester CL, Yaggi HK, Gill TM. The ratio of FEV1 to FVC as a basis for establishing chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;181:446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mannino DM, Sonia Buist A, Vollmer WM. Chronic obstructive pulmonary disease in the older adult: what defines abnormal lung function? Thorax 2007;62:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mannino DM, Diaz-Guzman E, Buist S. Pre- and post-bronchodilator lung function as predictors of mortality in the Lung Health Study. Respir Res 2011;12:136. [DOI] [PMC free article] [PubMed] [Google Scholar]