Abstract
Angiogenesis is a multi‐step process that culminates in vascular maturation whereby nascent vessels stabilize to become functional, and mural cells play an essential role in this process. Recent studies have shown that mural cells in tumors also promote and maintain vascular integrity, with wide‐reaching clinical implications including the regulation of tumor growth, metastases, and drug delivery. Various regulatory signaling pathways have been hitherto implicated, but whether regulation of Fas‐dependent apoptotic mechanisms is involved has not yet been fully investigated. We first compared endothelial FAS staining in human pancreatic ductal adenocarcinomas and colon carcinomas and show that the latter, characterized by lower mural cell coverage of tumor vasculature, demonstrated higher expression of FAS than the former. Next, in an in vitro coculture system of MS‐1 and 10T1/2 cells as endothelial and mural cells respectively, we show that mural cells decreased endothelial Fas expression. Then, in an in vivo model in which C26 colon carcinoma cells were inoculated together with MS‐1 cells alone or with the further addition of 10T1/2 cells, we demonstrate that mural cells prevented hemorrhage. Finally, knockdown of endothelial Fas sufficiently recapitulated the protection against hemorrhage seen with the addition of mural cells. These results together suggest that regulation of endothelial Fas signaling is involved in the promotion of vascular integrity by mural cells in tumors.
Keywords: Apoptosis, endothelial cells, Fas, mural cells, vascular integrity
Aberrant angiogenesis is a hallmark of cancer.1, 2 Angiogenesis is a multi‐step process culminating in vessel maturation in general, an indispensable step for nascent vessels to become functional.3 Mural cells, which include pericytes and vascular smooth muscle cells, play a key role in vessel maturation via promotion of vascular integrity.4, 5, 6, 7, 8 Knockout studies have highlighted the quintessentially important role of mural cells, as mural cell defective mice are often embryonic lethal due to extensive hemorrhage.9 Recent studies have established that mural cells in tumors also participate in the promotion and maintenance of vascular integrity, with wide‐ranging clinical implications including regulation of tumor growth,10 metastases,11, 12 and drug delivery.13
Numerous reports to date have investigated and elucidated various pro‐survival signaling pathways crucial to the regulation of vascular integrity.7 However, whether anti‐apoptotic mechanisms via regulation of endothelial Fas signaling, suggested to be important in angiogenic processes,14 are involved has not yet been fully addressed. Fas, also known as CD95 or APO‐1, is a death receptor which induces apoptosis via the extrinsic pathway.15 Previous reports have described Fas‐mediated endothelial apoptosis in several physiological16, 17, 18, 19, 20 and pathological21, 22, 23 conditions, but whether mural cells also engage these mechanisms is unknown.
In the present study we show that human colon carcinomas, which are characterized by low mural cell coverage of vasculature, demonstrate higher FAS expression in endothelium compared to pancreatic ductal adenocarcinomas which are characterized by high mural cell coverage. Then, we show, both in vitro and in vivo, that mural cells decrease endothelial Fas expression, suggesting that endothelial cells are more resistant to apoptosis in the presence of mural cells. By establishing a novel tumor vessel model in which mural cell coverage of blood vessels can be experimentally manipulated, we demonstrate that blood vessels without mural cell coverage are more prone to hemorrhage, which can be rescued either by the presence of mural cells or knockdown of endothelial Fas expression.
Materials and Methods
Immunohistochemistry: human histopathology
The histological analysis of human pathological specimens in this study was performed with the approval of the Internal Review Board on Ethical Issues of Hokkaido University Graduate School of Medicine, Sapporo, Japan. The samples and the patients’ information were obtained under a blanket written informed consent. The samples analyzed in this study were obtained from patients who underwent surgery in related hospitals in Hokkaido, whose final pathological diagnosis was made in Hokkaido University Graduate School of Medicine, Department of Cancer Pathology. Samples were embedded in paraffin, subjected to hematoxylin and eosin (HE) staining or immunohistochemistry (performed by Morphotechnology, Co. Ltd., Hokkaido, Japan) using primary antibodies against CD34 (Nichirei Bioscience Inc., Tokyo, Japan), smooth muscle actin (Dako Japan Co., Kyoto, Japan), and/or FAS (Cell Signaling Technology, Danvers, MA, USA), and analyzed with a Keyence BIOREVO BZ‐9000 fluorescence microscope (Osaka, Japan). To quantify mural cell coverage and FAS positive vessels, vessels were manually traced and the length of vessels with mural cell coverage or FAS staining was divided by the total length of vessels using ImageJ software (NIH, Bethesda, MD, USA).
Cell culture
The Mile Sven 1 (MS‐1) cell line, 10T1/2 cell line, and C26 cell line were obtained from American Type Culture Collection (Manassas, VA, USA), the Cancer Institute of the Japanese Foundation for Cancer Research (Tokyo, Japan), and National Cancer Center Research Institute (Tokyo, Japan), respectively. All cell lines were cultured in Dulbecco's modified Eagle medium (DMEM), high‐glucose (Gibco/Life Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS). Lentiviral vector systems, a kind gift from Dr. Hiroyuki Miyoshi (RIKEN, Ibaraki, Japan), were used to express the enhanced green fluorescent protein (eGFP) gene and the DsRed gene in MS‐1 cells and 10T1/2 cells, respectively. The sequence for DsRed was obtained from pDsRed‐Express Vector (Takara Bio Inc., Shiga, Japan).
RNA isolation and quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR)
Total RNA was extracted from cells using the RNeasy Mini Kit (Qiagen, Venlo, Netherlands) or Sepasol‐RNA I Super G (Nacalai Tesque, Kyoto, Japan). First‐strand cDNAs were synthesized by PrimeScript 1st strand cDNA Synthesis Kit (Takara Bio Inc.) or ReverTra Ace ‐α‐ (TOYOBO, Osaka, Japan) with the respective random primers supplied by the manufacturer. qRT‐PCR analyses were carried out using FastStart Universal SYBR Green Master (ROX) (Roche Applied Science, Upper Bavaria, Germany) or THUNDERBIRD SYBR qPCR Mix (TOYOBO), and the StepOne Plus Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA). The expression of each gene was normalized by hypoxanthine phosphoribosyl transferase 1 (Hprt1) gene expression. The primer sequences used in this study were as follows: GTTCTTTGCTGACCTGCTGGAT and CTTTTATGTCCCCCGTTGACTG for murine Hprt1, GTCCGCCCTGAGCAAAGA and TCCAGCAGGACCATGTGATC for eGFP, AAGTCCATCTACATGGCCAAGAA and TCCAGCTTGGAGTCCACGTAGT for DsRed, and TTCTCCTGGCTGTGAACACTGT and CACGGCTCAAGGGTTCCAT for murine Fas.
In vitro coculture system
MS‐1 cells stably expressing eGFP and 10T1/2 cells stably expressing DsRed were seeded separately or together in a 1.3:1 ratio on cell culture dishes. After incubation for 2 days, cells at confluence were collected in cold PBS with 0.2% bovine serum albumin (BSA) (Sigma‐Aldrich, St Louis, MO, USA) and separated by fluorescence‐activated cell sorting (FACS) based on eGFP or DsRed fluorescence by a MoFlo Astrios cell sorter (Beckman Coulter, Brea, CA, USA). For quantification of Fas protein, MS‐1 cells and 10T1/2 cells without eGFP or DsRed expression were seeded, cultured, and collected as above. Cells were then incubated on ice with Alexa Fluor 647‐labelled anti‐PECAM1 antibody (clone MEC13.3; Sony Biotechnology, Tokyo, Japan) and PE‐labelled anti‐Fas antibody (clone 15A7; eBioscience/affymetrix, San Diego, CA, USA) for 30 min, washed thrice with cold PBS, and analyzed by a BD C6 Accuri flow cytometer (BD Biosciences, Bedford, MA, USA). The obtained data were exported for analysis using FlowJo version 10 (FlowJo, LLC, Ashland, OR, USA).
Induction and detection of apoptosis
MS‐1 cells and 10T1/2 cells were seeded in μ‐Slide VI0.4 (ibiTreat) from Ibidi (Martinsried, Germany). After 2 days of culture in DMEM with 10% FBS, the medium was replaced with fresh DMEM containing 1% FBS, and cells at 75% confluence were incubated for 24 h to induce apoptosis using 20 μg/mL of anti‐mouse Fas monoclonal antibody (clone Jo2) or isotype control (BD Biosciences Pharmingen, San Diego, CA, USA). For quantification of cleaved Caspase‐3, the incubated cells were fixed for 3 min with Mildform 10N (Wako, Osaka, Japan) after 24 h of apoptosis induction. Cells were immunostained by the same procedure as for immunohistochemistry described below, using rat anti‐platelet endothelial cell adhesion molecule‐1 (PECAM‐1) monoclonal antibody (clone MEC 13.3; 1:150; BD Biosciences Pharmingen) and rabbit anti‐cleaved Caspase‐3 (Asp175) polyclonal antibody (1:400; Cell Signaling Technology) and 4′,6‐diamidino‐2‐phenylindole (DAPI) (1 μg/mL; Invitrogen/Molecular Probes, Eugene, OR, USA), and were observed with a LSM510 Meta confocal microscope (Carl Zeiss, Oberkochen, Germany) and/or BIOREVO BZ‐9000. The apoptosis index was calculated by counting the number of cleaved Caspase‐3 positive endothelial cells for each condition and then normalizing by cell number.
siRNA oligonucleotides transfection
The 23 nucleotide siRNA duplexes were designed using Enhanced siDirect (RNAi, Inc., Tokyo, Japan). The specific target sequence for mouse Fas siRNA, 5′‐CAGAAATCGCCTATGGTTGTTGA‐3′, and the negative control siRNA were purchased from RNAi, Inc. MS‐1 cells were transfected with siRNA oligonucleotides using Lipofectamine RNAiMAX (Life Technologies) reagent according to manufacturer's instructions. The medium was replaced 24 h after transfection.
Animals
BALB/c‐nu/nu male mice at 5 weeks of age were bought from Charles River Laboratories Japan (Kanagawa, Japan) or Japan SLC (Shizuoka, Japan). All animal experiments were approved and carried out in accordance with the policies of the Animal Ethics Committee at the University of Tokyo and with the Policy on the Care and Use of the Laboratory Animals, Okayama University.
In vivo model for angiogenesis
BALB/c‐nu/nu mice at 5 weeks of age were inoculated subcutaneously in the left flank with C26 murine colon carcinoma cells simultaneously with MS‐1 murine endothelial cells with or without the further addition of 10T1/2 murine mural precursor cells.24, 25 10T1/2 cells with DsRed expression (M for mural cells; 1.5–2 × 106 cells), MS‐1 cells with eGFP expression (E for endothelial cells; 1.5–2 × 106 cells) or eGFP expressing MS‐1 with Fas knocked down (E‐FasKD; 1.5–2 × 106 cells), and C26 cancer cells (5 × 105 cells) were suspended in PBS and mixed with an equal volume of Matrigel (BD Biosciences) for a total of 200 μL for subcutaneous inoculation. For the sake of brevity, we named the condition in which MS‐1 cells were inoculated together with C26 cells as the E (for “endothelial cells only”) condition and the condition with the further addition of 10T1/2 cells as E+M (for “endothelial cells + mural cells”) condition. Macroscopic photographs of xenograft tumors were obtained on day 4, and the presence and degree of hemorrhage was scored. The degree of hemorrhage was scored as follows: “moderate” if tumors appeared purplish and “severe” if overt subcutaneous hemorrhage emanating from the xenograft was observed. Samples were harvested on day 3 for analysis of endothelial Fas expression and apoptosis, and day 7–10 for analyzing hemorrhage. Samples were cut in half upon harvesting. One half was fixed overnight in Mildform 10N, embedded in paraffin, subjected to HE staining or immunohistochemistry (performed by Morphotechnology, Co. Ltd.), and analyzed with BIOREVO BZ‐9000. The other half was embedded in O.C.T. Compound (Sakura Finetek/Tissue‐Tek, Torrance, CA, USA) and frozen in dry‐iced hexane for fluorescent immunostaining, either immediately when MS‐1 cells and 10T1/2 cells were not labeled, or after the following procedure when eGFP‐labeled MS‐1 cells or DsRed‐labeled 10T1/2 cells were used: samples were fixed for 30 min at room temperature with Mildform 10N and washed overnight at 4°C thrice, in PBS with 10%, 15%, and 20% sucrose sequentially.
Immunohistochemistry: animal model
Frozen samples were sectioned at a thickness of either 5 or 10 μm with a cryostat, fixed with Mildform 10N for 3 min, blocked with Blocking One (Nacalai Tesque), and were incubated for 2 h with one or more primary antibodies listed below: rat anti‐PECAM‐1 monoclonal antibody (1:150), mouse anti‐actin, alpha‐smooth muscle (α‐SMA)‐Cy3 monoclonal antibody (clone 1A4; 1:100; Sigma‐Aldrich), rabbit anti‐NG2 chondroitin sulfate proteoglycan polyclonal antibody (1:200; Chemicon/Merck Millipore, Darmstadt, Germany), rabbit anti‐cleaved Caspase‐3 (Asp175) polyclonal antibody (1:600), and rabbit anti‐CD95 (Fas) polyclonal antibody (1:50; Abcam, Cambridge, UK). Subsequently, specimens were incubated for 1.5 h with the appropriate secondary antibodies labeled with Alexa Fluor 488, Alexa Fluor 594, or Alexa Fluor 647 (1:200; Invitrogen/Molecular Probes). Cell nuclei were then counterstained with DAPI (1 μg/mL) for 5 min and mounted with Fluorescent Mounting Medium (Dako, Glostrup, Denmark). Terminal deoxynucleotidyl Transferase (TdT)‐mediated dUTP nick end labeling (TUNEL) staining, together with staining for eGFP using anti‐GFP polyclonal antibody (Medical & Biological Laboratories, Nagoya, Japan) was performed by Morphotechnology, Inc. Stained samples were observed with LSM510 Meta and/or BIOREVO BZ‐9000.
Image quantification and statistical analysis
Collected images were processed or quantified by ImageJ software, BZ‐II analyzer (Keyence), LSM Image Browser (Carl Zeiss), and/or Photoshop (Adobe systems, Inc., San Jose, CA, USA). To quantify the proportion of 10T1/2 cells which become NG2 positive and also the proportion of NG2 positive cells derived from 10T1/2 cells, area doubly positive for NG2 and DsRed were divided by area positive for DsRed or NG2, respectively. For quantification of cleaved Capsase‐3 positive vessels, the number of cleaved Caspase‐3 positive cells were counted and normalized by PECAM‐1 positive area in 3D projected images. To quantify TUNEL positive vessels, TUNEL positive area was divided by PECAM1 positive area. For quantification of the degree of hemorrhage, we divided the sum of the lengths of hemorrhagic vessels by total vessel length using the HE stained samples. Hemorrhagic vessel was defined as vessel having extravasated red blood cells (RBCs) within 25 μm outside the vessel wall. Vessel lengths were measured by manual tracing using Photoshop followed by quantification using ImageJ software. Statistical analysis was performed with GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Data were analyzed by Student's t‐test or analysis of variance (ANOVA) followed by Tukey's multiple comparison test.
Results
We have previously investigated mural cell coverage of tumor vasculature in various human tumors26 and noted significantly different levels of coverage depending on tumor type. Pancreatic ductal adenocarcinomas, for example, show significantly greater mural cell coverage of tumor vessels than colon carcinomas. Building on this observation, we first performed immunohistochemical analysis of human pancreatic ductal adenocarcinoma and colon carcinoma tissue samples to assess whether degree of mural cell coverage may correlate with endothelial Fas expression. We first confirmed, consistent with our previous analyses, that colon carcinomas were characterized with vasculature (visualized by CD34) with a lower level of coverage by α‐smooth muscle actin (α‐SMA) positive mural cells than pancreatic ductal adenocarcinomas (Fig. 1a–d). Comparison of Fas expression between these tumors revealed that many Fas‐positive blood vessels were present in colon carcinomas but not in pancreatic ductal adenocarcinoma (Fig. 1e–h). Quantification of α‐SMA positive mural cell coverage (Fig. 1i) and Fas positivity (Fig. 1j) of CD34 positive tumor neovessels confirmed the above observations.
Figure 1.
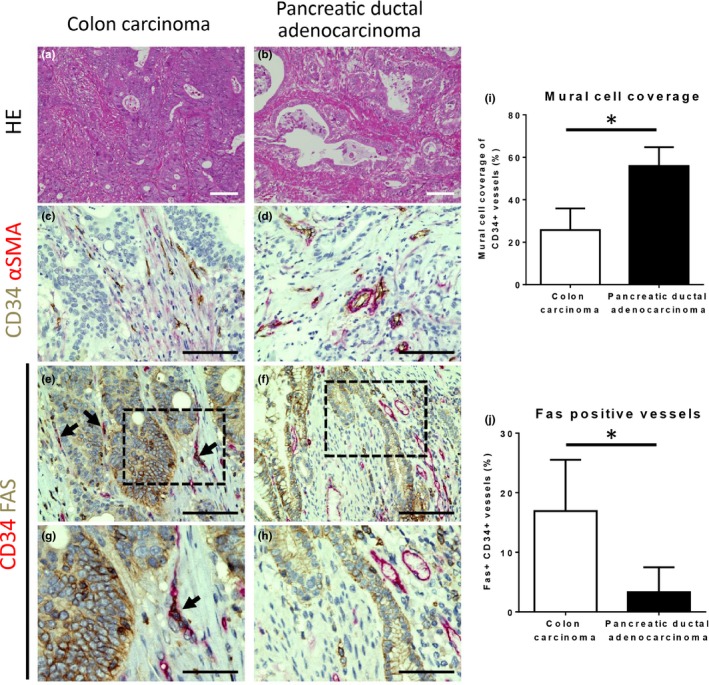
Correlation between mural cell coverage of tumor vasculature and endothelial Fas expression in human colon carcinoma and pancreatic ductal adenocarcinoma. (a,b) Representative images of hematoxylin and eosin (HE) staining of human colon carcinoma (a) and pancreatic ductal adenocarcinoma (b) tissue samples. (c–h) Representative images of immunohistochemical analyses of tumor vasculature in human colon carcinoma (c,e, and g) and pancreatic ductal adenocarcinoma (d,f, and h) tissue samples. Double staining for the endothelial marker CD34 (brown) and the mural cell marker α‐smooth muscle actin (α‐SMA; red) revealed little mural cell coverage in colon carcinoma (c), whereas coverage was prominent in pancreatic ductal adenocarcinoma (d). Double staining for CD34 (red) and FAS (brown) demonstrated many blood vessels positive for FAS in colon carcinoma (e; dark red due to double positive for red and brown, black arrows) but not in pancreatic ductal adenocarcinoma (f). (g) and (h) are higher magnification images of the area indicated by the dotted black lines in images (e) and (f), respectively. Scale bars = 100 μm (a–f), 50 μm (g, h). (i,j) Quantification of α‐SMA positive mural cell coverage as shown in (c,d and i) and FAS positive vessels as shown in (e–h, j). Data represented as mean ± SD in both graphs. *P < 0.05, Student's t‐test.
We therefore asked whether a causative relationship exists between mural cell coverage and Fas expression of tumor vessels. To this end, we first cocultured MS‐1 endothelial cells fluorescently labeled by eGFP and 10T1/2 mesenchymal stem cells labeled by DsRed as mural cell progenitors. After 2 days of coculture in vitro (Fig. 2a), MS‐1 cells and 10T1/2 cells were separated by FACS and subjected to qRT‐PCR analysis. By quantifying eGFP and DsRed mRNA expression, we confirmed successful segregation of the two cell types (Fig. 2b). We then quantified Fas mRNA expression in MS‐1 cells and found that it was significantly decreased in the presence of 10T1/2 cells (Fig. 2c). We then wondered whether the decrease in Fas mRNA expression is due to a general down‐regulation of Fas in MS‐1 cells cocultured with 10T1/2 cells or alternatively due to down‐regulation within a select subpopulation of MS‐1 cells. We thus performed flow cytometry and discovered that Fas expression was mildly decreased in the whole cocultured MS‐1 population compared to monocultured MS‐1 cells (Fig. S1). We next sought to address the functional significance of the down‐regulation of Fas expression in MS‐1 cells by treating both culture conditions with Jo2, a Fas agonist monoclonal antibody. Jo2 significantly induced the apoptosis of monocultured MS‐1 cells, but the effect was largely abrogated in MS‐1 cells cocultured with 10T1/2 cells (Fig. 2d) suggesting that the down‐regulation of MS‐1 Fas expression conferred enhanced resistance to Fas‐mediated apoptosis and was thus indeed functionally important. The above data suggest that the presence of mural cells result in decreased endothelial Fas expression and lessened endothelial susceptibility to Fas‐mediated apoptosis.
Figure 2.
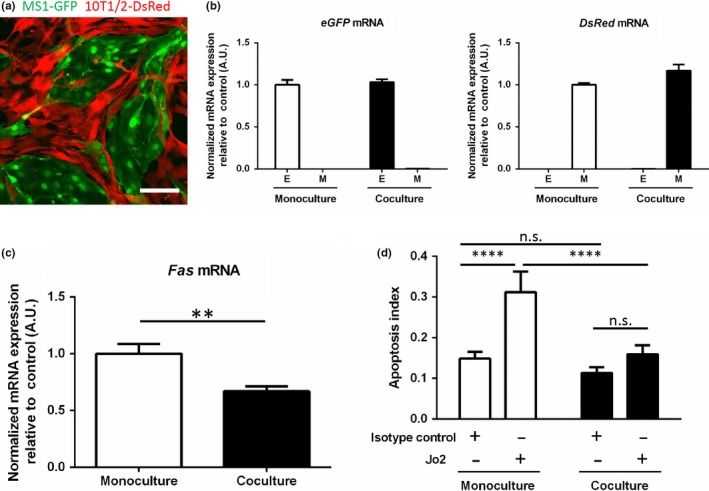
Fas gene expression and susceptibility to Fas‐dependent apoptosis of MS‐1 cells were decreased in the presence of 10T1/2 cells in vitro. (a) Representative image of the in vitro coculture of Mile Sven 1 (MS‐1) endothelial cells (green) and 10T1/2 mural progenitor cells (red) stably expressing enhanced green fluorescent protein (eGFP) and DsRed, respectively. Scale bar = 100 μm. (b) Successful segregation of MS‐1 (E, for endothelial cells) and 10T1/2 cells (M, for mural cells) after coculture by fluorescence‐activated cell sorting (FACS) was confirmed by quantifying eGFP and DsRed mRNA expression which, respectively, is exclusively expressed in MS‐1 and 10T1/2 cells by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). (c) Fas mRNA expression was down‐regulated in MS‐1 cells separated by FACS after coculture with 10T1/2 cells compared to monocultured MS‐1 cells. (d) Endothelial susceptibility to apoptosis was determined by comparing the change in the apoptosis index upon the addition of Jo2, a Fas agonist antibody, compared to isotype control. Though the apoptotic index at baseline was not significantly different with the presence of 10T1/2 cells, a clear suppression of apoptosis was seen in the cocultured MS‐1 cells upon Jo2 stimulation. Data represented as mean ± SD in all graphs. **P < 0.01, ****P < 0.0001, n.s.: not significant; Student's t‐test (c), Tukey's multiple comparison test (d).
We next asked whether a similar mechanism may be at play in tumors in vivo. To this end, we established a novel xenograft model in which the presence of mural cell coverage can be easily manipulated by inoculating C26 murine colon carcinoma cells with MS‐1 cells (E, for endothelial cell) only or MS‐1 cells together with 10T1/2 cells (M, for mural cell), to test whether endothelial‐mural cell interactions result in the decrease of endothelial Fas expression. Implantation of C26 tumor cells with MS‐1 cells only (E condition) results in a tumor with neovasculature hardly covered by mural cells, but the 10T1/2 cells added in the E+M condition successfully associated with the induced neovasculature and resulted in a conspicuous increase in NG2‐positive mural cell coverage of neovessels (Fig. 3a,b). By quantifying DsRed and NG2 double positive area as a percentage of DsRed positive or NG2 positive area, we found that approximately 45% of 10T1/2 cells became NG2 positive and that 25% of NG2 cells originated from the inoculated 10T1/2 cells (Fig. 3c,d). Taken together, these results suggest that the addition of 10T1/2 cells in the E+M condition indeed results in increased mural cell coverage both directly by differentiation of 10T1/2 cells into NG2 positive mural cells and indirectly via a 10T1/2 cell non‐autonomous mechanism. Notably, fluorescent immunostaining of Fas revealed a significant decrease of Fas expression in the endothelium of E+M condition xenografts compared to that of E condition (Fig. 3e,f). Furthermore, the degree of endothelial apoptosis, defined as cleaved Caspase‐3/PECAM‐1 double‐positive cells,27, 28 was significantly decreased in the E+M condition than in the E condition (Fig. 3g,h). We additionally performed TUNEL staining to compare the number of apoptotic endothelial cells, and found that there were significantly more TUNEL positive MS‐1 cells in the E condition xenografts than in the E+M condition xenografts (Fig. S2a,b). Taken together, these findings, which were very much in line with the results obtained from the in vitro coculture system, together suggest that the presence of mural cells also decreased endothelial Fas expression and conferred strengthened resistance to Fas‐mediated apoptosis in vivo even within tumors.
Figure 3.
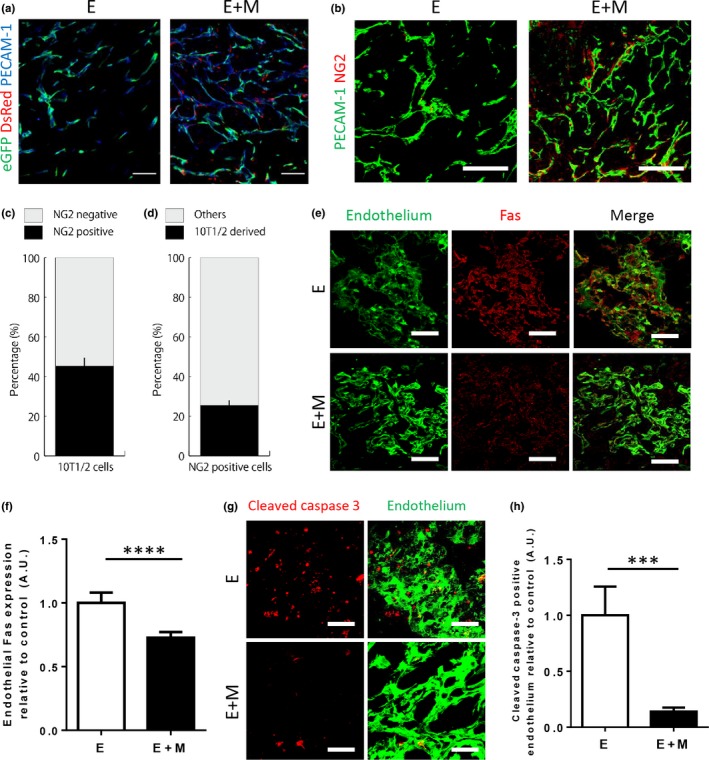
Fas expression and endothelial apoptosis were decreased in the presence of 10T1/2 cells in vivo. (a) Representative images of the E and E+M xenografts harvested on day 10. The inoculated MS‐1 cells (eGFP, green) successfully formed a vascular network (PECAM‐1, blue) in both conditions. The addition of 10T1/2 cells (DsRed, red) in the E+M condition further resulted in the coverage of the network by these cells. Scale bar = 100 μm. (b) Staining for the pericyte marker NG2 (red) together with PECAM‐1 (green) revealed that pericyte coverage in the E condition was scarce, whereas the addition of 10T1/2 cells increased pericyte coverage in the E+M condition. Scale bars = 100 μm. (c,d) To assess the contribution of 10T1/2 cells to NG2 positivity in the E+M condition, the proportion of DsRed cells expressing NG2 (c) and NG2 positive cells expressing DsRed (d) were quantified. We found that approximately 45% of the inoculated 10T1/2 cells became NG2 positive, and about 25% of NG2 positive cells originated from the inoculated 10T1/2 cells. (e) E and E+M condition xenografts harvested on day 3 were stained for Fas (light blue). To quantify Fas expression in endothelium, endothelial cells (green) were visualized by both eGFP and staining for PECAM‐1. Scale bars = 100 μm. (f) Quantification of Fas expression of endothelial cells as stained in B. Fas expression was significantly diminished in the E+M condition. (g) Representative 3D projection images of E and E+M condition xenografts stained for cleaved Caspase‐3 (blue) and endothelium (green) on day 3. Scale bars = 50 μm. (h) Quantification of cleaved Caspase‐3 positive endothelial cells as stained in D revealed a marked reduction in the E+M condition xenografts. Data represented as mean ± SD in all graphs. ***P < 0.001, ****P < 0.0001; Student's t‐test.
Finally, we assessed the effect of the presence of mural cells and the resultant down‐regulation of endothelial Fas expression on vascular integrity by quantifying hemorrhage, an indicator of immature vasculature, in the xenografts (Fig. 4a). E+M condition xenografts showed a significant reduction of hemorrhage compared to E condition xenografts (Fig. 4b), in line with a previous report demonstrating the importance of mural cells in vascular integrity.4 We further exploited this model to definitively establish the functional significance of mural cell‐induced endothelial Fas down‐regulation by siRNA‐mediated knockdown of Fas expression in MS‐1 cells (E‐FasKD, Fig. 4c). Indeed, we discovered that E‐FasKD condition xenografts showed a significant reduction in hemorrhage compared to E condition xenografts, to a level much comparable to E+M condition xenografts (Fig. 4a,b). These findings were in line with macroscopic observations which revealed the occurrence of severe hemorrhage in E condition xenografts, while E+M and E‐FasKD condition xenografts demonstrated hemorrhage of diminished severity (Fig. S3). Altogether, these results suggest that the presence of mural cells decrease the expression of endothelial Fas and that this alone sufficiently recapitulates the promotion of vascular integrity by mural cells at least as determined by extent of hemorrhage.
Figure 4.
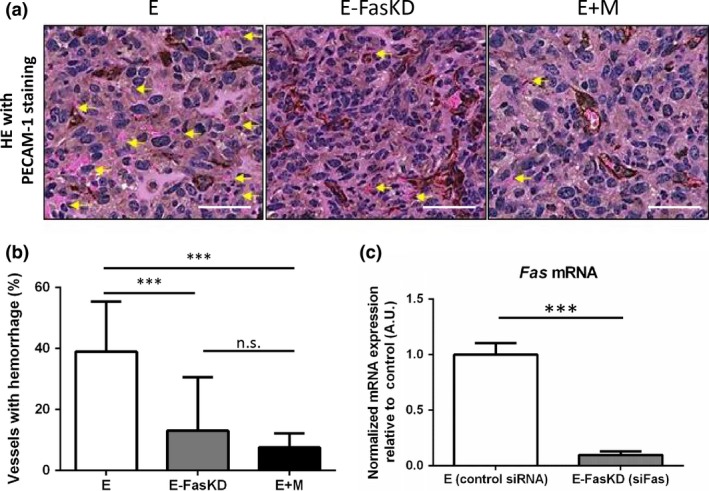
siRNA‐mediated knockdown of endothelial Fas recapitulated the reduction in hemorrhage seen in the presence of 10T1/2 cells in vivo. (a) Representative HE stain images of the E, E‐FasKD, and E+M condition xenografts. The slides were also stained for PECAM‐1 (brown). Yellow arrows indicate hemorrhagic vessels identified by extravasated red blood cells (RBCs). Scale bars = 50 μm. (b) Quantification of hemorrhagic vessels as seen in (a). The addition of 10T1/2 cells markedly reduced hemorrhage compared to MS‐1 cells alone. The knockdown of Fas in MS‐1 cells recapitulated the reduction of hemorrhage to levels comparable to that seen in the E+M xenografts. (c) Fas expression in MS‐1 cells was quantified by qRT‐PCR to confirm successful knockdown by siRNA. Data represented as mean ± SD in all graphs. ***P < 0.001, n.s.: not significant; Tukey's multiple comparison test (b), Student's t‐test (c).
Discussion
Fas‐mediated apoptosis in endothelium has been observed in several physiological and pathological conditions,16, 17, 18, 19, 20, 21, 22, 23 but its role and regulation in the promotion and maintenance of vascular integrity was unclear. Mural cells have been reported to be an essential player in the regulation of vessel integrity,4 but little was hitherto known about how death signals or death receptors in endothelium are affected by the presence of mural cells in tumors.
The present study first shows correlation between mural cell coverage of tumor vasculature and endothelial Fas expression in human pancreatic and colon carcinoma tissue samples. However, we have interestingly noticed that not all vessels without mural cell coverage were positive for Fas. Alternative mechanisms governing endothelial Fas expression29 independent of mural cell coverage may be one reason for this discrepancy. We further surmised that histological analysis of human surgical specimens, which allows observation of only a singular given point in time for a given patient, might not be optimally suited for the visualization and dissection of the highly dynamic and complex process of vascular maturation.
We therefore demonstrate both in vitro and in vivo using a novel coculture system, which allows the manipulation of mural cell coverage against a uniform background that the presence of mural cells results in endothelial resistance to apoptosis at least partly via a decrease in endothelial Fas expression. We further show that forced down‐regulation of endothelial Fas functioned to attenuate the extent of hemorrhage in vivo in a C26 tumor xenograft model even in the absence of mural cells, thus providing preliminary evidence that mural cell‐induced decrease of endothelial Fas is indeed involved in the regulation of vascular integrity. We have adopted hemorrhage as a marker of vascular integrity since previous reports establishing the essential role of mural cells have demonstrated that mural cell‐loss leads to embryonic lethality and is characterized by extensive hemorrhage.9, 30
Currently, how down‐regulation of endothelial Fas leads to decreased hemorrhage remains unclear. Taking into account the well‐known role of Fas in the extrinsic pathway of apoptosis, we surmise that enhanced resistance of endothelial cells to apoptosis results in increased endothelial survival, thus enabling the formation of functional endothelial‐endothelial junctions from an earlier stage. Recent reports, however, have also described apoptosis‐independent roles of Fas signaling such as regulation of calcium influx in lymphocytes and dendritic branching in neurons.31, 32 Further research is thus warranted to elucidate how mural cells downregulate endothelial Fas expression and how, in turn, decreased Fas expression in endothelium results in enhanced barrier integrity in tumors. A related question of importance is the nature of the Fas ligand expressing cells, which would presumably be necessary for Fas‐mediated regulation of endothelial cell death. Preliminary immunohistochemical analyses revealed that Fas ligand expression was fairly ubiquitously seen throughout the xenograft, including in endothelial cells themselves (data not shown). Though we have not yet clearly delineated the exact nature of the Fas ligand expressing cells and further the specific contribution of each cell population in death signaling mediated through endothelial Fas, it at least seems reasonable to conclude that regulation of Fas expression in endothelial cells is of great importance in such an environment.
The findings presented in this work were obtained via the establishment of a new in vivo model. Previous works have analyzed the function of mural cells by, for example, respectively implanting different cancer cells with varying capacities to induce vasculature with mural cell coverage,33 genetic ablation of specific mural cell markers,4 or pharmacologic disruption of endothelial‐mural cell association.34 Though these works have shed light on important aspects of endothelial‐mural cell interaction, the difference between vessels with or without mural cells could not simply be attributed to the function of mural cells. We have addressed this difficulty by generating a new additive xenograft model, where C26 tumor cells, MS‐1 endothelial cells, and 10T1/2 mural cell progenitors were xenografted together into nude mice. Two main limitations of our model, however, should especially be noted. First, the induced vasculature of the xenografts does not solely consist of the co‐inoculated MS‐1 cells, and there is a significant contribution of host endothelial cells. Thus for example in the E‐FasKD condition, Fas knockdown is not uniformly accomplished in endothelial cells within the xenografts. Even then, it seems that the extraneously injected MS‐1 cells with siRNA‐mediated down‐regulation of Fas expression were sufficient to prevent hemorrhage. Secondly, we have only tested a particular tumor cell/endothelial cell/mural cell combination in this study. Further studies comparing various combinations may be useful in elucidating both conserved and context‐specific mechanisms governing the regulation of vascular integrity.
Disclosure
The authors have no conflicts of interests.
Supporting information
Fig. S1. Flow cytometric quantification of Fas expression in MS‐1 cells in monoculture or in coculture with 10T1/2 cells. (a) MS‐1 cells were selected by gating for PECAM1 positivity. The threshold for the gating was determined by comparing PECAM1 staining in MS‐1 cells (red) and 10T1/2 cells (light blue). (b) Using the threshold set in (a), MS‐1 cells in monoculture (red) or in coculture with 10T1/2 cells (blue) were gated. Two peaks in PECAM1 fluorescence were observed in the coculture condition: the major peak which coincided with the peak seen in monocultured MS‐1 cells, and a minor peak of lower fluorescence intensity resulting from the presence of 10T1/2 cells. (c) Comparison of Fas expression of the PECAM1 positive cells selected from (b) demonstrated a modest decrease in Fas fluorescence intensity in cocultured MS‐1 cells as opposed to MS‐1 cell in monoculture.
Fig. S2. TUNEL staining of xenografts revealed decreased apoptosis of MS‐1 cells in E+M condition xenografts. (a) Representative images of Terminal deoxynucleotidyl Transferase (TdT)‐mediated dUTP nick end labeling (TUNEL) staining (red) performed on E and E+M condition xenografts on day 7 together with staining for eGFP expressed in the injected MS‐1 cells (green). Inset: higher magnification of the area indicated by white dashed lines. White arrowheads indicate TUNEL positive MS‐1 cells. (b) Quantification of TUNEL positive vessels as shown in (a). TUNEL positive vessels were markedly reduced in the E+M condition xenografts. Scale bars = 100 μm. Data represented as mean ± SD in all graphs. *P < 0.05; Student's t‐test.
Fig. S3. Macroscopic evaluation of xenografts revealed that the presence of 10T1/2 cells or siRNA‐mediated knockdown of Fas in MS‐1 cells attenuated the severity of hemorrhage in vivo. (a) Representative macroscopic images of xenografts obtained on day 4. Black dotted lines and yellow arrows indicate tumor borders and severe hemorrhage, respectively. (b) The degree of hemorrhage was scored and summarized as a table. While all E condition xenografts presented with severe hemorrhage, the presence of 10T1/2 cells in the E+M condition prevented severe hemorrhage. The siRNA‐mediated knockdown of Fas in MS‐1 cells in the E‐FasKD condition showed an intermediate phenotype: not all xenografts presented with severe hemorrhage.
Acknowledgments
We would like to thank Professor K. Miyazono at the University of Tokyo for his kind comments and suggestions and for the generous provision of experimental facilities. We further thank Dr. H. Matsubara and Dr. A. Ogawa at the National Hospital Organization Okayama Medical Center for kindly allowing the use of experimental facilities. This work was financially supported by KAKENHI (23790433 and 26293119), Okayama University, Health Labor Sciences Research Grant, and the Japan Society for the Promotion of Science (JSPS) through the “Funding Program for World‐Leading Innovative R&D on Science and Technology (FIRST Program),” initiated by the Council for Science and Technology Policy (CSTP).
Cancer Sci 108 (2017) 1080–1088
Funding Information
This work was financially supported by KAKENHI (23790433 and 26293119), Okayama University, Health Labor Sciences Research Grant, and the Japan Society for the Promotion of Science (JSPS) through the “Funding Program for World‐Leading Innovative R&D on Science and Technology (FIRST Program),” initiated by the Council for Science and Technology Policy (CSTP).
References
- 1. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 3. Carmeliet P. Angiogenesis in health and disease. Nat Med 2003; 9: 653–60. [DOI] [PubMed] [Google Scholar]
- 4. Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial‐mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 2009; 29: 630–8. [DOI] [PubMed] [Google Scholar]
- 5. Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol 2011; 55: 261–8. [DOI] [PubMed] [Google Scholar]
- 6. Fukuhara S, Sako K, Noda K, Zhang J, Minami M, Mochizuki N. Angiopoietin‐1/Tie2 receptor signaling in vascular quiescence and angiogenesis. Histol Histopathol 2010; 25: 387–96. [DOI] [PubMed] [Google Scholar]
- 7. Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis 2009; 12: 125–37. [DOI] [PubMed] [Google Scholar]
- 8. Cascone T, Heymach JV. Targeting the angiopoietin/Tie2 pathway: cutting tumor vessels with a double‐edged sword? J Clin Oncol 2012; 30: 441–4. [DOI] [PubMed] [Google Scholar]
- 9. Levéen P, Pekny M, Gebre‐Medhin S et al Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 1994; 8: 1875–87. [DOI] [PubMed] [Google Scholar]
- 10. Furuhashi M, Sjöblom T, Abramsson A et al Platelet‐derived growth factor production by B16 melanoma cells leads to increased pericyte abundance in tumors and an associated increase in tumor growth rate. Cancer Res 2004; 64: 2725–33. [DOI] [PubMed] [Google Scholar]
- 11. Xian X, Håkansson J, Ståhlberg A et al Pericytes limit tumor cell metastasis. J Clin Invest 2006; 116: 642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yonenaga Y, Mori A, Onodera H et al Absence of smooth muscle actin‐positive pericyte coverage of tumor vessels correlates with hematogenous metastasis and prognosis of colorectal cancer patients. Oncology 2005; 69: 159–66. [DOI] [PubMed] [Google Scholar]
- 13. Kano MR, Bae Y, Iwata C et al Improvement of cancer‐targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF‐beta signaling. Proc Natl Acad Sci USA 2007; 104: 3460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chavakis E, Dimmeler S. Regulation of endothelial cell survival and apoptosis during angiogenesis. Arterioscler Thromb Vasc Biol 2002; 22: 887–93. [DOI] [PubMed] [Google Scholar]
- 15. Grivennikov SI, Kuprash DV, Liu Z‐G, Nedospasov SA. Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: from simple paradigms to complex mechanisms. Int Rev Cytol 2006; 252: 129–61. [DOI] [PubMed] [Google Scholar]
- 16. Volpert OV, Zaichuk T, Zhou W et al Inducer‐stimulated Fas targets activated endothelium for destruction by anti‐angiogenic thrombospondin‐1 and pigment epithelium‐derived factor. Nat Med 2002; 8: 349–57. [DOI] [PubMed] [Google Scholar]
- 17. Ashton SV, Whitley GSJ, Dash PR et al Uterine spiral artery remodeling involves endothelial apoptosis induced by extravillous trophoblasts through Fas/FasL interactions. Arterioscler Thromb Vasc Biol 2005; 25: 102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aoudjit F, Vuori K. Matrix attachment regulates Fas‐induced apoptosis in endothelial cells: a role for c‐flip and implications for anoikis. J Cell Biol 2001; 152: 633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Panka DJ, Mier JW. Canstatin inhibits Akt activation and induces Fas‐dependent apoptosis in endothelial cells. J Biol Chem 2003; 278: 37632–6. [DOI] [PubMed] [Google Scholar]
- 20. Daniel S, Arvelo MB, Patel VI et al A20 protects endothelial cells from TNF‐, Fas‐, and NK‐mediated cell death by inhibiting caspase 8 activation. Blood 2004; 104: 2376–84. [DOI] [PubMed] [Google Scholar]
- 21. Joussen AM, Poulaki V, Mitsiades N et al Suppression of Fas‐FasL‐induced endothelial cell apoptosis prevents diabetic blood‐retinal barrier breakdown in a model of streptozotocin‐induced diabetes. FASEB J 2003; 17: 76–8. [DOI] [PubMed] [Google Scholar]
- 22. Liao H, Xu J, Huang J. FasL/Fas pathway is involved in dengue virus induced apoptosis of the vascular endothelial cells. J Med Virol 2010; 82: 1392–9. [DOI] [PubMed] [Google Scholar]
- 23. Koide N, Morikawa A, Tumurkhuu G et al Lipopolysaccharide and interferon‐gamma enhance Fas‐mediated cell death in mouse vascular endothelial cells via augmentation of Fas expression. Clin Exp Immunol 2007; 150: 553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Darland DC, D'Amore PA. TGF beta is required for the formation of capillary‐like structures in three‐dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis 2001; 4: 11–20. [DOI] [PubMed] [Google Scholar]
- 25. Hirschi KK, Rohovsky SA, D'Amore PA. PDGF, TGF‐beta, and heterotypic cell‐cell interactions mediate endothelial cell‐induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 1998; 141: 805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang L, Nishihara H, Kano MR. Pericyte‐coverage of human tumor vasculature and nanoparticle permeability. Biol Pharm Bull 2012; 35: 761–6. [DOI] [PubMed] [Google Scholar]
- 27. Krammer PH. CD95′s deadly mission in the immune system. Nature 2000; 407: 789–95. [DOI] [PubMed] [Google Scholar]
- 28. Kumar S. Caspase function in programmed cell death. Cell Death Differ 2006; 14: 32–43. [DOI] [PubMed] [Google Scholar]
- 29. Dudley AC, Thomas D, Best J, Jenkins A. A VEGF/JAK2/STAT5 axis may partially mediate endothelial cell tolerance to hypoxia. Biochem J 2005; 390: 427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soriano P. Abnormal kidney development and hematological disorders in PDGF beta‐receptor mutant mice. Genes Dev 1994; 8: 1888–96. [DOI] [PubMed] [Google Scholar]
- 31. Zuliani C, Kleber S, Klussmann S et al Control of neuronal branching by the death receptor CD95 (Fas/Apo‐1). Cell Death Differ 2006; 13: 31–40. [DOI] [PubMed] [Google Scholar]
- 32. Lepple‐Wienhues A, Belka C, Laun T et al Stimulation of CD95 (Fas) blocks T lymphocyte calcium channels through sphingomyelinase and sphingolipids. Proc Natl Acad Sci USA 1999; 96: 13795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kano MR, Komuta Y, Iwata C et al Comparison of the effects of the kinase inhibitors imatinib, sorafenib, and transforming growth factor‐beta receptor inhibitor on extravasation of nanoparticles from neovasculature. Cancer Sci 2009; 100: 173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF‐A signaling and Bcl‐w expression. Blood 2011; 118: 2906–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Flow cytometric quantification of Fas expression in MS‐1 cells in monoculture or in coculture with 10T1/2 cells. (a) MS‐1 cells were selected by gating for PECAM1 positivity. The threshold for the gating was determined by comparing PECAM1 staining in MS‐1 cells (red) and 10T1/2 cells (light blue). (b) Using the threshold set in (a), MS‐1 cells in monoculture (red) or in coculture with 10T1/2 cells (blue) were gated. Two peaks in PECAM1 fluorescence were observed in the coculture condition: the major peak which coincided with the peak seen in monocultured MS‐1 cells, and a minor peak of lower fluorescence intensity resulting from the presence of 10T1/2 cells. (c) Comparison of Fas expression of the PECAM1 positive cells selected from (b) demonstrated a modest decrease in Fas fluorescence intensity in cocultured MS‐1 cells as opposed to MS‐1 cell in monoculture.
Fig. S2. TUNEL staining of xenografts revealed decreased apoptosis of MS‐1 cells in E+M condition xenografts. (a) Representative images of Terminal deoxynucleotidyl Transferase (TdT)‐mediated dUTP nick end labeling (TUNEL) staining (red) performed on E and E+M condition xenografts on day 7 together with staining for eGFP expressed in the injected MS‐1 cells (green). Inset: higher magnification of the area indicated by white dashed lines. White arrowheads indicate TUNEL positive MS‐1 cells. (b) Quantification of TUNEL positive vessels as shown in (a). TUNEL positive vessels were markedly reduced in the E+M condition xenografts. Scale bars = 100 μm. Data represented as mean ± SD in all graphs. *P < 0.05; Student's t‐test.
Fig. S3. Macroscopic evaluation of xenografts revealed that the presence of 10T1/2 cells or siRNA‐mediated knockdown of Fas in MS‐1 cells attenuated the severity of hemorrhage in vivo. (a) Representative macroscopic images of xenografts obtained on day 4. Black dotted lines and yellow arrows indicate tumor borders and severe hemorrhage, respectively. (b) The degree of hemorrhage was scored and summarized as a table. While all E condition xenografts presented with severe hemorrhage, the presence of 10T1/2 cells in the E+M condition prevented severe hemorrhage. The siRNA‐mediated knockdown of Fas in MS‐1 cells in the E‐FasKD condition showed an intermediate phenotype: not all xenografts presented with severe hemorrhage.


