Abstract
The tumor suppressor p53 gene is frequently mutated in human cancers, and the p53 protein suppresses cancer. However, the mechanism behind the p53‐mediated tumor suppression is still unclear. Recently, the mitochondria‐eating protein (Mieap) was identified as a p53‐inducible protein. Mieap induces the accumulation of lysosomal proteins within mitochondria (Mieap‐induced accumulation of lysosome‐like organelles within mitochondria, or MALM) in response to mitochondrial damage, and eliminates the oxidized mitochondrial proteins to repair unhealthy mitochondria. Furthermore, Mieap also induces vacuole‐like structures (Mieap‐induced vacuole, or MIV) to eat and degrade unhealthy mitochondria. Therefore, Mieap controls mitochondrial quality by repairing or eliminating unhealthy mitochondria by MALM or MIV, respectively. This mechanism is not mediated by canonical autophagy. Mieap‐deficient ApcMin/+ mice show strikingly high rates of intestinal tumor development as well as advanced‐grade adenomas and adenocarcinomas. The p53/Mieap/BCL2 interacting protein 3 mitochondrial quality control pathway is frequently inactivated in human colorectal cancers. Defects in Mieap‐regulated mitochondrial quality control lead to accumulation of unhealthy mitochondria in cancer cells. Cancer‐specific unhealthy mitochondria could contribute to cancer development and aggressiveness through mitochondrial reactive oxygen species and altered metabolism. Mieap‐regulated mitochondrial quality control is a newly discovered function of p53 that plays a critical role in tumor suppression.
Keywords: Autophagy, cancer metabolism, mitochondrial quality control, reactive oxygen species, tumor suppressor p53
Abbreviations
- BNIP3
BCL2 interacting protein 3
- KD
knockdown
- LAMP
lysosomal‐associated membrane protein
- MALM
Mieap‐induced accumulation of lysosome‐like organelles within mitochondria
- Mieap
mitochondria‐eating protein
- MIV
Mieap‐induced vacuole
- ROS
reactive oxygen species
- UVRAG
UV radiation resistance associated gene
The tumor suppressor p53 was first discovered in 1979 as an oncoprotein.1, 2, 3, 4, 5, 6 Since then, a number of studies have been carried out to clarify the function of p53, resulting in a tremendous number of reports. Currently, the following are believed to be essential for p53: (i) it is a transcription factor that activates the transcription of its target genes by binding to specific sequences;7 (ii) cell cycle arrest, apoptosis, DNA repair, and anti‐angiogenesis are the core functions in its tumor suppression;8 and (iii) it is frequently mutated in a broad range of human cancers.9 However, recent studies in murine models have clearly shown that cell cycle arrest and apoptosis are not required for tumor suppression.10, 11 These observations suggested that there is still a missing piece in the mechanism of p53‐dependent tumor suppression. Therefore, the importance of p53 in cancer suppression is well established, but its mechanism is still unclear.
As p53 is a transcription factor that regulates a large number of genes, the identification and characterization of these target genes are critically important for understanding its functions.12, 13 So far, a number of p53‐target genes have been identified and characterized by us, as well as other groups, as shown in Figure 1. It is known that p53AIP1,14 p53RDL1 (UNC5B),15 Bax,16 Noxa,17 Puma,18, 19 and UNC5A20 are apoptosis inducers, whereas Netrin‐1 is an apoptosis inhibitor.21 In addition, p21WAF1,22 14‐3‐3sigma,23 and Reprimo24 are cell cycle regulators, and p53R2 (RRM2B),25 XPC,26 and GADD4527 are involved in DNA repair. BAI1,28 TSP1,29 and SEMA3F30 regulate anti‐angiogenesis, whereas p53DINP1 (TP53INP1)31 and MDM232 are positive and negative regulators of p53, respectively. Both TIGAR33 and GLS234 are involved in metabolism and ALDH435 has anti‐oxidant activity. Thus, p53 regulates a huge number of cellular functions through transcriptional activation of its target genes. However, the mechanisms behind the regulation of these multiple functions to suppress cancer, and which targets and/or functions are the most critical for p53 suppression of tumors, are still unclear.
Figure 1.

Tumor suppressor p53 regulates a large number of functions through transcriptional activation of its target genes as a transcription factor. Apoptosis, cell cycle arrest, DNA repair, and anti‐angiogenesis are believed to be the core functions for p53‐mediated tumor suppression. Mieap is a p53‐target gene, and the function is involved in mitochondrial quality control.
Mieap (the mitochondria‐eating protein) was identified as a p53‐target gene, and has been found to be frequently inactivated in human cancer cell lines through promoter methylation, implying the role of Mieap in tumor suppression.36 Surprisingly, Mieap was also found to be involved in mitochondrial quality control (Fig. 1).36, 37 Mieap‐regulated mitochondrial quality control is frequently inactivated in human cancers. This leads to a striking and specific accumulation of unhealthy mitochondria in cancer cells, and the cancer‐specific unhealthy mitochondria generate high levels of ROS. Mitochondrial ROS and abnormal metabolism caused by cancer‐specific unhealthy mitochondria probably contribute to cancer development and progression. Mieap‐regulated mitochondrial quality control is likely one of the new mechanisms for p53 tumor suppression.
New mechanisms for mitochondrial quality control
Mieap controls mitochondrial quality by repairing or eliminating unhealthy mitochondria through MALM or MIV, respectively (Fig. 2).36, 37 In response to mitochondrial damage, lysosomal proteins are accumulated in the mitochondria in a Mieap‐dependent manner. This phenomenon is generally considered as mitochondrial autophagy or mitophagy. However, during this phenomenon, the destruction of mitochondrial structure does not occur in spite of the accumulation of lysosomal proteins.36 In addition, autophagosomes and autolysosomes are not observed through electron microscopic analysis. At least four lysosomal proteins (LAMP1, LAMP2, cathepsin B, and cathepsin D) have been detected within the mitochondria by immuno‐electron microscopic analysis, while two lysosomal proteins (cathepsin B and cathepsin D) were shown to be within the mitochondria through a proteinase K protection assay. In brief, in this assay, isolated mitochondria are subjected to proteinase K digestion. The proteins within the mitochondria are protected from degradation. Therefore, after the treatment, the result is rapidly and easily confirmed by Western blot analysis.36 Therefore, this function has been denoted as MALM.
Figure 2.
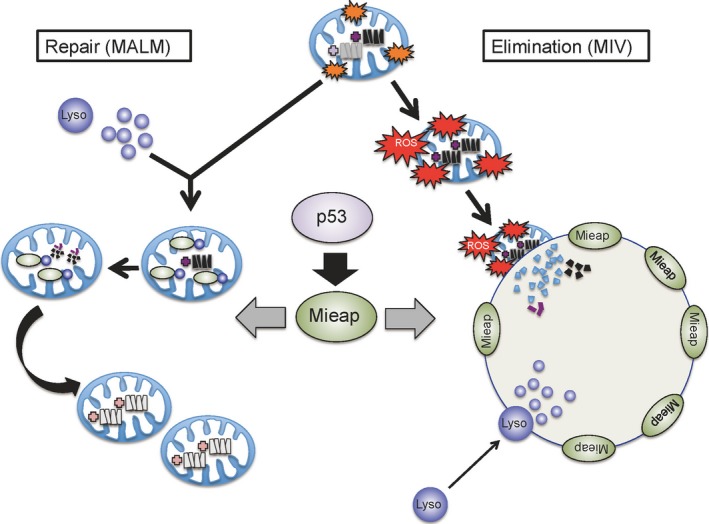
Mitochondria‐eating protein (Mieap)‐regulated mitochondrial quality control. In response to mitochondrial damage, p53‐activated Mieap induces accumulation of lysosomal proteins within mitochondria to eliminate the oxidized mitochondrial proteins (MALM). The MALM repairs unhealthy mitochondria. Mieap also induces large vacuole‐like structures (MIV) to eat and degrade very dangerous and unhealthy mitochondria producing high levels of reactive oxygen species (ROS). Therefore, p53/Mieap maintains mitochondrial integrity by repairing or eliminating unhealthy mitochondria by MALM or MIV, respectively. Lyso, lysosome.
One possible role of MALM is the elimination of oxidized mitochondrial proteins, because oxidized proteins are accumulated in the mitochondria of MALM‐deficient cells.36 In addition, mitochondrial ROS levels are known to be higher in MALM‐deficient cells.36 These observations suggest that MALM plays a role in the elimination of oxidized mitochondrial proteins to maintain the mitochondrial integrity. Consistent with this hypothesis, MALM has also been reported to improve ATP synthesis activity and decrease mitochondrial ROS generation, thus promoting the health of mitochondria (Fig. 3).36
Figure 3.
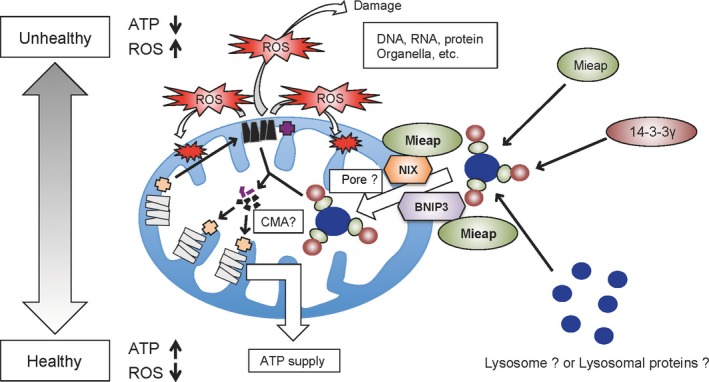
Hypothetical model for the mitochondria‐eating protein (Mieap)‐induced accumulation of lysosome‐like organelles within mitochondria (MALM) mechanism. Unhealthy mitochondria produce high level of reactive oxygen species (ROS). Mitochondrial ROS induces the interaction of Mieap and two mitochondrial outer‐membrane proteins, BCL2 interacting protein 3 (BNIP3) and NIX. The interaction of Mieap, BNIP3, and NIX leads to the formation of a pore, through which lysosomal proteins or lysosome‐like organelles accumulate within mitochondria. 14‐3‐3γ interacts with Mieap in cytosol, and translocates into the mitochondria, which mediates the elimination of the oxidized mitochondrial proteins in the mitochondria. Therefore, MALM maintains the healthy status of the mitochondria, indicating an increase of ATP synthesis activity and a decrease of ROS generation. CMA, chaperone‐mediated autophagy.
Both BNIP3 and NIX mediate the induction of MALM by interacting with Mieap at the mitochondrial outer membrane in response to mitochondrial damage, mitochondrial ROS generation, and/or hypoxia (Fig. 3).38 The interaction of Mieap, BNIP3, and NIX induces the formation of a pore in the mitochondrial double membrane, which mediates the translocation of lysosomal proteins from the cytosol to the intramitochondrial region.38 Interestingly, the pore formed by the interaction of the three proteins is not associated with cell death.38
In contrast, 14‐3‐3γ was also identified as a Mieap‐interacting protein, but its role was found to be critically different from that of BNIP3 and NIX.39 It was found that 14‐3‐3γ interacts with Mieap in the cytosol, and then translocates into the mitochondria.39 It then mediates the lysosomal degradation of the oxidized mitochondrial proteins within the mitochondria (Fig. 3).39
Mieap‐induced vacuoles are vacuole‐like structures (Fig. 4).37 They ingest and degrade unhealthy mitochondria by accumulating lysosomes.37 Mieap‐induced vacuoles can be produced by the overexpression of Mieap in various cancer cell lines, but not in normal cell lines. This is due to the difference in the mitochondrial ROS levels between cancer cells and normal cells.37 Reactive oxygen species scavengers (ebselen and N‐acetylcysteine) efficiently inhibit MALM induction and degradation of mitochondria by MIV.37 Therefore, high levels of mitochondrial ROS may play a critical role in targeting unhealthy mitochondria by MALM and MIV.
Figure 4.
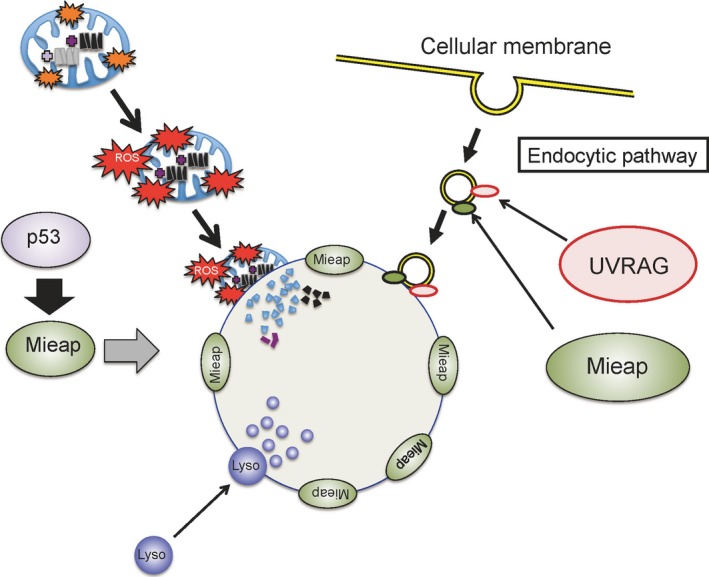
Hypothetical model for the mitochondria‐eating protein (Mieap)‐induced vacuole (MIV) mechanism. Severely damaged and dangerous mitochondria produce very high levels of reactive oxygen species (ROS), which are extremely toxic to the cell. These ROS induce the generation of MIVs. The MIVs uptake and degrade the dangerous mitochondria to maintain cellular homeostasis. UV radiation resistance associated gene (UVRAG) protein mediates the MIV formation by interacting with Mieap. The endocytic pathway also plays a critical role in MIV formation. Lyso, lysosome.
We found that UVRAG40 mediated the formation of MIV (2016) (Fig. 4). It was previously reported that UVRAG regulates the maturation of endosomes and autolysosomes.41, 42 The formation of MIVs is inhibited by phosphatidylinositol 3‐kinase inhibitors, including 3MA and LY294002.37 Therefore, the endocytic pathway may be involved in the generation of MIVs (Fig. 4). The UVRAG gene has been reported to be mutated in the cancer cells of a small proportion (3%–5%) of colorectal cancer patients.43
When MALM is inhibited, MIV generation is induced through endogenous Mieap (Fig. 5).37 This implies that the highly dangerous and unhealthy mitochondria that MALM is unable to repair produces high levels of ROS, and are thus eliminated by MIV. Because the knockdown of p53 and/or Mieap in A549 cells completely inhibited the induction of both MALM and MIV, the mechanisms of MALM and MIV are probably strictly regulated by the p53/Mieap‐regulatory pathway (Fig. 5).37 These facts strongly suggest that p53 maintains mitochondrial integrity through transcriptional activation of Mieap, which regulates the mitochondrial quality control.
Figure 5.
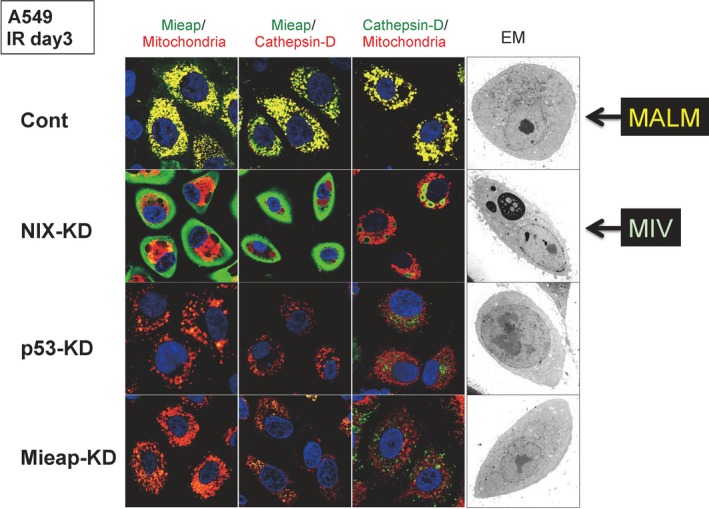
Interplay between mitochondria‐eating protein (Mieap)‐induced accumulation of lysosome‐like organelles within mitochondria (MALM) and Mieap‐induced vacuoles (MIV): repair? Or elimination? Four cancer cell lines derived from A549 (lung cancer cell line), control, NIX‐knockdown (KD), p53‐KD, and Mieap‐KD, were irradiated by ionizing radiation (IR). 3 days after IR, immunostaining experiments were carried out with anti‐Mieap antibody (Mieap), anti‐cathepsin D antibody (Cathepsin‐D), and DsRed‐mito (Mitochondria). MALM occurred in the control cells, in which the colocalization of Mieap, lysosome, and mitochondria is observed (yellow signals). When MALM is inhibited by NIX‐KD, MIVs appear, eat, and degrade the mitochondria in the NIX‐KD cells. Both MALM and MIV are cancelled in the p53‐KD and Mieap‐KD cells. Therefore, MALM and MIV are closely related to each other when making a decision about repair or elimination of unhealthy mitochondria. Both MALM and MIV are strictly regulated by the p53/Mieap pathway. Reproduced from Kitamura et al.37 with permission from. EM, electron microscopy.
In general, quality control of the mitochondria is regulated by the canonical autophagy of mitochondria, known as “mitophagy”.44 In this mechanism, the damaged mitochondria are sequestered by double‐membraned autophagosomes; the autophagosomes containing the damaged mitochondria then fuse to lysosomes and turn into autolysosomes in order to degrade the autophagosomal content. Therefore, double‐membraned autophagosomes are essential for canonical mitophagy. Additionally, the process is very rapid and is completed within a few hours.45, 46 In contrast, in Mieap‐regulated mitochondrial quality control, autophagosomes and autolysosomes are not involved in MALM or MIV.36, 37 These processes are relatively slow and continue for 24–72 h.36, 37 Furthermore, Parkin and Pink1 have been established to play a pivotal role in mitophagy.47, 48, 49, 50 However, they are not involved in MALM or MIV. These facts clearly suggest that Mieap‐regulated mitochondrial quality control is critically different from the canonical autophagy of mitochondria, mitophagy.
Mieap‐deficient colorectal cancer mouse model
In order to clarify the in vivo role of Mieap in tumorigenesis, we developed a strain of Mieap KO mice. Mieap KO mice were born normally and were able to grow after birth. Using these Mieap KO mice, we generated Mieap‐deficient ApcMin/+ mice.51 ApcMin/+ mice develop multiple benign tumors in the small intestine, and are therefore a murine intestinal tumor model.52, 53 Mieap‐deficient ApcMin/+ mice showed a much shorter lifetime compared to the Mieap‐WT ApcMin/+ mice.51 This was due to substantially higher number and size of intestinal tumors in Mieap‐deficient ApcMin/+ mice.51 Moreover, intestinal tumors in the Mieap‐deficient ApcMin/+ mice showed more advanced grades of adenomas and adenocarcinomas than Mieap‐WT ApcMin/+ mice.51 These results clearly suggest that Mieap deficiency promotes cancer development and malignancy in vivo.
Interestingly, the mitochondria in cancer cells of Mieap‐deficient ApcMin/+ mice were morphologically abnormal, suggesting that Mieap deficiency leads to the accumulation of unhealthy mitochondria in cancer cells, thus causing increased oxidative stress in Mieap‐deficient tumors.51
The results from the studies on Mieap‐deficient ApcMin/+ mice suggest that the inactivation of the Mieap‐regulated mitochondrial quality control leads to accumulation of unhealthy mitochondria and increased mitochondrial ROS generation, which probably promotes cancer development and aggressiveness in vivo.
Mieap‐regulated mitochondrial quality control is frequently inactivated in human colorectal cancer
The promoter for the Mieap gene is frequently methylated in various human cancer cell lines, resulting in the loss of Mieap expression in cancer cell lines.36 To evaluate the status of the methylation of Mieap promoters in primary cancer tissues, we examined primary cancer samples from 57 colorectal cancer patients.54 We observed promoter methylation of Mieap in only 5 out of 57 patients (9%). In contrast, the promoter methylation of BNIP3 was found in 28 out of 57 patients (47%). p53 Mutation was found in nearly 50% of colorectal cancer tissues that did not show methylation of the Mieap and BNIP3 promoters. These results indicate that the p53/Mieap/BNIP3‐regulated mitochondrial quality control pathway is inactivated in more than 70% of colorectal cancer patients.54 Therefore, BNIP3 is probably the most important target for inactivation of Mieap‐regulated mitochondrial quality control in human colorectal cancers.
As a BH3‐only protein that belongs to the Bcl‐2 family, BNIP3 is believed to play a critical role in necrosis‐like cell death under hypoxic conditions by causing the opening of mitochondrial permeability transition pores.55, 56 However, our recent study clearly showed that BNIP3 mediates MALM through pore formation by interacting with Mieap and NIX, and that these pores are not mitochondrial permeability transition pores.38 Knockdown of BNIP3‐knockdown (KD) in LS174T colorectal cancer cell lines severely impaired mitochondrial localization of Mieap, resulting in the inhibition of MALM induction.54 The MALM‐deficient cancer cells (p53‐KD, Mieap‐KD, and BNIP3‐KD cancer cells) accumulated unhealthy mitochondria and these cancer‐specific unhealthy mitochondria produced high levels of ROS under hypoxia.54 Interestingly, BNIP3‐KD colorectal cancer cells as well as p53‐KD and Mieap‐KD cells showed a striking enhancement of migratory and invasive activities in hypoxic conditions.54 The enhanced migration and invasive activities of these cancer cells were dependent on increased generation of mitochondrial ROS.54
These observations from human colorectal cancer tissues clearly support our hypothesis that the Mieap‐regulated mitochondrial quality control is frequently inactivated in human cancers in vivo, leading to the accumulation of unhealthy mitochondria producing high levels of ROS. These cancer‐specific unhealthy mitochondria could greatly contribute to cancer development and aggressiveness through oxidative stress and abnormal metabolism (Fig. 6).
Figure 6.
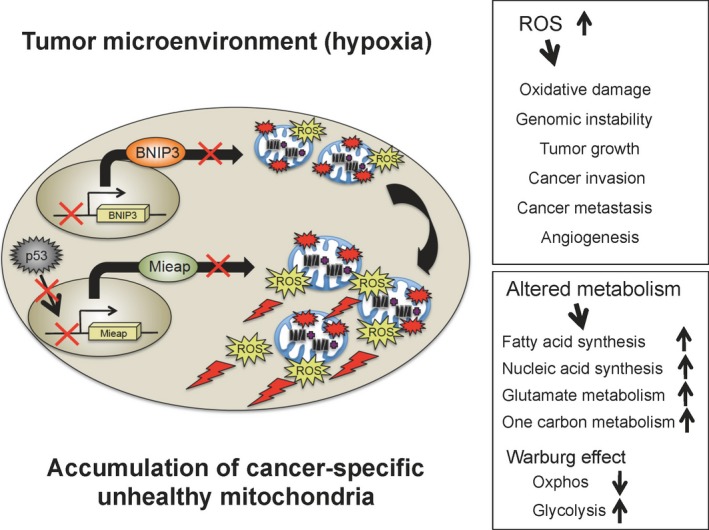
Cancer‐specific unhealthy mitochondria in the hypoxic tumor microenvironment function as a driving force for cancer development and progression. In human cancer, mitochondria‐eating protein (Mieap)‐regulated mitochondrial quality control is frequently inactivated by p53 mutations and/or Mieap/BCL2 interacting protein 3 (BNIP3) promoter methylation. This leads to the accumulation of cancer specific unhealthy mitochondria in the hypoxic tumor microenvironment. Cancer‐specific unhealthy mitochondria produce high levels of ROS. Mitochondrial ROS induce oxidative damage and genomic instability, and promote tumor growth, cancer invasion, metastasis, and tumor angiogenesis. Cancer specific unhealthy mitochondria also causes altered metabolism including activation of fatty acid synthesis, nucleic acid synthesis, glutamate metabolism, and one carbon metabolism, defective oxidative phosphorylation, and upregulation of glycolysis. Therefore, mitochondrial reactive oxygen species (ROS) and altered metabolism caused by cancer‐specific unhealthy mitochondria could greatly contribute to cancer development and progression.
Implications and future directions
Discovery of Mieap as a p53‐target gene opens up a new avenue for exploration of the mechanism behind p53 tumor suppression, in which mitochondrial quality control plays a critical role in tumor suppression. Inactivation of Mieap‐regulated mitochondrial quality control results in the accumulation of unhealthy mitochondria in cancer cells. These cancer‐specific unhealthy mitochondria produce high levels of ROS and cause abnormal metabolism, both of which would be beneficial for cancer progression. The Mieap‐regulated mitochondrial quality control is inactivated in human gastric cancers, breast cancers, and pancreatic cancers (2016). Mieap‐deficiency also promotes cancer development, aggressiveness, and malignancy in mouse models of gastric cancer (Tsuneki, Nakamura, and Arakawa, 2016). Therefore, p53/Mieap‐regulated mitochondrial quality control plays an important role as a universal tumor suppressor in a broad range of human cancers.
Nearly 38 years after its discovery, the full mechanism for p53 tumor suppression remains unknown. Emerging evidence is suggesting that metabolic regulation and cellular redox control are additional core functions for this mechanism.57, 58 Mieap‐regulated mitochondrial quality control is involved in both of these functions, because inactivation of the Mieap pathway in cancer cells results in the accumulation of unhealthy mitochondria, causing abnormal metabolism and increased oxidative stress. Therefore, the maintenance of mitochondrial integrity by p53 seems to be critical for the suppression of cancer development and progression.
In fact, many p53‐target genes are known to be involved in the maintenance of mitochondrial integrity. p53R2 (RRM2B) was initially identified as a ribonucleotide reductase that supplies dNTPs for DNA repair.25 However, germ‐line mutations of p53R2 were found in many patients with mitochondrial diseases, suggesting that p53R2 is essential for the supply of dNTPs for mitochondrial DNA synthesis.59 This implies that p53 also controls mitochondrial DNA synthesis through p53R2.59 In p53‐mutated cancers, the concentration of dNTPs is dysregulated, leading to high rates of mutation of mitochondrial DNA and increased mitochondrial ROS generation. Aldehyde dehydrogenase 4 is an antioxidant in the mitochondria that effectively scavenges mitochondrial ROS.35 Glutaminase 2 also functions as an antioxidant protein in the mitochondria, catalyzing the hydrolysis of glutamine to glutamate.34 CABC1 60 encodes coenzyme Q10 and SCO2 61 catalyzes the synthesis of cytochrome c oxidase 2, both of which are key components in the oxidative phosphorylation chain. Based on these facts, we propose a hypothesis that tumor suppressor p53 is the guardian of the mitochondria.
Mutation of p53 and/or Mieap/BNIP3 promoter methylation leads to the accumulation of unhealthy mitochondria in cancer cells (Fig. 6). “Unhealthy mitochondria” are still functional, but produce lower levels of ATP and higher levels of ROS. They are not equivalent to “abnormal mitochondria” that are severely damaged, pathological, and non‐functional, and fail to produce ATP due to dissipation of the mitochondrial membrane potential. Abnormal mitochondria are eliminated through Parkin/Pink1 pathway‐mediated canonical autophagy (mitophagy).44, 47, 48, 49, 50 Using electron microscopy, we further confirmed that unhealthy mitochondria in cancer cells are round and enlarged, and have a very poor cristae structure (2016). This may reflect the roles of these mitochondria in cancer metabolism and redox status. Therefore, we define these mitochondria as “cancer‐specific unhealthy mitochondria.”
We observed that cancer‐specific unhealthy mitochondria are accumulated in nearly 100% of cancer cells in the tumor microenvironment, and that this phenomenon is common in a broad range of human cancers (Nakamura, Tsuneki, and Arakawa, 2016). Cancer‐specific unhealthy mitochondria produce high levels of ROS under the in vivo hypoxic tumor microenvironment (Fig. 6).62 The elevated mitochondrial ROS causes oxidative damage to the DNA, RNA, proteins, and lipids.63 This induces genomic instability. The mitochondrial ROS contribute to tumor growth, epithelial–mesenchymal transition, cancer invasion, cancer metastasis, and tumor angiogenesis through the activation of hypoxia‐inducible factor‐1, nuclear factor‐κB, MMPs, AKT, Erk1/2, and JNK (Fig. 6).64, 65, 66, 67, 68, 69, 70, 71, 72 Cancer‐specific unhealthy mitochondria also cause abnormalities in the metabolism, such as activation of fatty acid synthesis, nucleic acid synthesis, glutamate metabolism, one carbon metabolism, defective oxidative phosphorylation, upregulation of glycolysis, and reduction in activity of the TCA cycle (Fig. 6).73, 74, 75, 76 We speculate that cancer‐specific unhealthy mitochondria function as a driving force for cancer development and progression. Further comprehensive and careful investigation on cancer‐specific unhealthy mitochondria would help in identification and characterization of molecules, signaling pathways, and metabolites that compose cancer's Achilles heel. For instance, an antibody against a molecule(s) localized in cancer‐specific unhealthy mitochondria could be used to detect cancer cells during pathological diagnosis, thus acting as a novel biomarker(s). Inhibitors against oncogenic signaling pathways or oncometabolites derived from cancer‐specific unhealthy mitochondria would be useful as new anticancer drugs. Therefore, the development of a method to target cancer‐specific unhealthy mitochondria could provide new strategies for the prevention, diagnosis, and therapy of a broad range of human cancers.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
We thank all of the members of Arakawa's Laboratory for their pivotal contributions to this work, who are largely responsible for discovery of the Mieap function. This work was supported in part by the Japan Society for the Promotion of Science (KAKENHI Grant Nos. 24240117 to H.A., 22501021 to Y.N., 23659178 to H.A., 25430124 to Y.N., and 25670169 to H.A.), the Ministry of Health, Labor and Welfare of Japan for the Practical Research for Innovative Cancer (H26‐practical‐general‐001 to H.A.), the National Cancer Center Research and Development Fund (Grant No. 23‐B‐7 to H.A.), and the Japan Agency for Medical Research and Development (AMED Grant No. 15ck0106006h0002 to H.A.).
Cancer Sci 108 (2017) 809–817
Funding Information
AMED, (Grant/Award Number: ‘15ck0106006h0002‘) KAKENHI, (Grant/Award Number: ‘22501021‘,’23659178‘,’24240117‘,’25430124‘,’25670169‘)
References
- 1. Lane DP, Crawford LV. T antigen is bound to a host protein in SV40‐transformed cells. Nature 1979; 278: 261–3. [DOI] [PubMed] [Google Scholar]
- 2. Linzer DI, Levine AJ. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40‐transformed cells and uninfected embryonal carcinoma cells. Cell 1979; 17: 43–52. [DOI] [PubMed] [Google Scholar]
- 3. Kress M, May E, Cassingena R, May P. Simian virus 40‐transformed cells express new species of proteins precipitated by anti‐simian virus 40 tumor serum. J Virol 1979; 31: 472–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melero JA, Stitt DT, Mangel WF, Carroll RB. Identification of new polypeptide species [48‐55K] immunoprecipitated by antiserum to purified large T antigen and present in SV40‐infected and transformed cells. Virology 1979; 93: 466–80. [DOI] [PubMed] [Google Scholar]
- 5. Smith AE, Smith R, Paucha E. Characterization of different tumor antigens present in cells transformed by simian virus 40. Cell 1979; 18: 335–46. [DOI] [PubMed] [Google Scholar]
- 6. DeLeo AB, Jay G, Appella E, Dubois GC, Law LW, Old LJ. Detection of a transformation‐related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci USA 1979; 76: 2420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Menendez D, Inga A, Resnick MA. The expanding universe of p53 targets. Nat Rev Cancer 2009; 9: 724–37. [DOI] [PubMed] [Google Scholar]
- 8. Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000; 408: 307–10. [DOI] [PubMed] [Google Scholar]
- 9. Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science 1991; 253: 49–53. [DOI] [PubMed] [Google Scholar]
- 10. Li T, Kon N, Jiang L et al Tumor suppression in the absence of p53‐mediated cell‐cycle arrest, apoptosis, and senescence. Cell 2012; 149: 1269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valente LJ, Gray DH, Michalak EM et al p53 efficiently suppresses tumor development in the complete absence of its cell‐cycle inhibitory and apoptotic effectors p21, Puma, and Noxa. Cell Rep 2013; 3: 1339–45. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura Y. Identification of p53‐target genes and their functional analysis. Cancer Sci 2004; 95: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53‐mediated tumour suppression. Nat Rev Cancer 2014; 14: 359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oda K, Arakawa H, Tanaka T et al p53AIP1, a potential mediator of p53‐dependent apoptosis, and its regulation by Ser‐46‐phosphorylated p53. Cell 2000; 102: 849–62. [DOI] [PubMed] [Google Scholar]
- 15. Tanikawa C, Matsuda K, Fukuda S, Nakamura Y, Arakawa H. p53RDL1 regulates p53‐dependent apoptosis. Nat Cell Biol 2003; 5: 216–23. [DOI] [PubMed] [Google Scholar]
- 16. Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995; 80: 293–9. [DOI] [PubMed] [Google Scholar]
- 17. Oda E, Ohki R, Murasawa H et al Noxa, aBH3‐only member of the Bcl‐2 family and candidate mediator of p53‐induced apoptosis. Science 2000; 288: 1053–8. [DOI] [PubMed] [Google Scholar]
- 18. Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 2001; 7: 673–82. [DOI] [PubMed] [Google Scholar]
- 19. Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 2001; 7: 683–94. [DOI] [PubMed] [Google Scholar]
- 20. Miyamoto Y, Futamura M, Kitamura N, Nakamura Y, Baba H, Arakawa H. Identification of UNC5A as a novel transcriptional target of tumor suppressor p53 and a regulator of apoptosis. Int J Oncol 2010; 36: 1253–60. [DOI] [PubMed] [Google Scholar]
- 21. Arakawa H. Netrin‐1 and its receptors in tumorigenesis. Nat Rev Cancer 2004; 4: 978–87. [DOI] [PubMed] [Google Scholar]
- 22. el‐Deiry WS, Tokino T, Velculescu VE et al WAF1, a potential mediator of p53 tumor suppression. Cell 1993; 75: 817–25. [DOI] [PubMed] [Google Scholar]
- 23. Hermeking H, Lengauer C, Polyak K et al 14‐3‐3sigma is a p53‐regulated inhibitor of G2/M progression. Mol Cell 1997; 1: 3–11. [DOI] [PubMed] [Google Scholar]
- 24. Ohki R, Nemoto J, Murasawa H et al Reprimo, a new candidate mediator of the p53‐mediated cell cycle arrest at the G2 phase. J Biol Chem 2000; 275: 22627–30. [DOI] [PubMed] [Google Scholar]
- 25. Tanaka H, Arakawa H, Yamaguchi T et al A ribonucleotide reductase gene involved in a p53‐dependent cell‐cycle checkpoint for DNA damage. Nature 2000; 404: 42–9. [DOI] [PubMed] [Google Scholar]
- 26. Adimoolam S, Ford JM. p53 and DNA damage‐inducible expression of the xeroderma pigmentosum group C gene. Proc Natl Acad Sci USA 2002; 99: 12985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kastan MB, Zhan Q, el‐Deiry WS et al A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia‐telangiectasia. Cell 1992; 71: 587–97. [DOI] [PubMed] [Google Scholar]
- 28. Nishimori H, Shiratsuchi T, Urano T et al A novel brain‐specific p53‐target gene, BAI1, containing thrombospondin type 1 repeats inhibits experimental angiogenesis. Oncogene 1997; 15: 2145–50. [DOI] [PubMed] [Google Scholar]
- 29. Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin‐1. Science 1994; 265: 1582–4. [DOI] [PubMed] [Google Scholar]
- 30. Futamura M, Kamino H, Miyamoto Y et al Possible role of semaphoring 3F, a candidate tumor suppressor gene at 3p21.3, in p53‐regulated tumor angiogenesis suppression. Cancer Res 2007; 67: 1451–60. [DOI] [PubMed] [Google Scholar]
- 31. Okamura S, Arakawa H, Tanaka T et al p53DINP1, a p53‐inducible gene, regulates p53‐dependent apoptosis. Mol Cell 2001; 8: 85–94. [DOI] [PubMed] [Google Scholar]
- 32. Barak Y, Juven T, Haffner R, Oren M. mdm2 expression is induced by wild type p53 activity. EMBO J 1993; 12: 461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bensaad K, Tsuruta A, Selak MA et al TIGAR, a p53‐inducible regulator of glycolysis and apoptosis. Cell 2006; 126: 107–20. [DOI] [PubMed] [Google Scholar]
- 34. Suzuki S, Tanaka T, Poyurovsky MV et al Phosphate‐activated glutaminase (GLS2), a p53‐inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci USA 2010; 107: 7461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoon KA, Nakamura Y, Arakawa H. Identification of ALDH4 as a p53‐inducible gene and its protective role in cellular stresses. J Hum Genet 2004; 49: 134–40. [DOI] [PubMed] [Google Scholar]
- 36. Miyamoto Y, Kitamura N, Nakamura Y et al Possible existence of lysosome‐like organelle within mitochondria and its role in mitochondrial quality control. PLoS One 2011; 6: e16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kitamura N, Nakamura Y, Miyamoto Y et al Mieap, a p53‐inducible protein, controls mitochondrial quality by repairing or eliminating unhealthy mitochondria. PLoS One 2011; 6: e16060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakamura Y, Kitamura N, Shinogi D et al BNIP3 and NIX mediates Mieap‐induced accumulation of lysosomal proteins within mitochondria. PLoS One 2012; 7: e30767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miyamoto T, Kitamura N, Ono M et al Identification of 14‐3‐3gamma as a Mieap‐interacting protein and its role in mitochondrial quality control. Sci Rep 2012; 2: 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liang C, Feng P, Ku B et al Autophagic and tumor suppressor activity of a novel Beclin1‐binding protein UVRAG. Nat Cell Biol 2006; 8: 688–99. [DOI] [PubMed] [Google Scholar]
- 41. Liang C, Lee JS, Inn KS et al Beclin1‐binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol 2008; 10: 776–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3‐kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 2008; 19: 5360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. He S, Zhao Z, Yang Y et al Truncating mutation in the autophagy gene UVRAG confers oncogenic properties and chemosensitivity in colorectal cancers. Nat Commun 2015; 6: 7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 2011; 12: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mizushima N. Autophagy: process and function. Genes Dev 2007; 21: 2861–73. [DOI] [PubMed] [Google Scholar]
- 46. Kim I, Rodriguez‐Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 2007; 462: 245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 2008; 183: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Narendra D, Jin SM, Tanaka A et al PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 2010; 8: e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kawajiri S, Saiki S, Sato S et al PINK1 is recruited to mitochondria with parkin and associated with LC3 in mitophagy. FEBS Lett 2010; 584: 1073–9. [DOI] [PubMed] [Google Scholar]
- 50. Matsuda N, Sato S, Shiba K et al PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 2010; 189: 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tsuneki M, Nakamura Y, Kinjo T, Nakanishi R, Arakawa H. Mieap suppresses murine intestinal tumor via its mitochondrial quality control. Sci Rep 2015; 5: 12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer 2001; 1: 55–67. [DOI] [PubMed] [Google Scholar]
- 53. Fodde R, Smits R. Disease model: familial adenomatous polyposis. Trends Mol Med 2001; 7: 369–73. [DOI] [PubMed] [Google Scholar]
- 54. Kamino H, Nakamura Y, Tsuneki M et al Mieap‐regulated mitochondrial quality control is frequently inactivated in human colorectal cancer. Oncogenesis 2016; 4: e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vande Velde C, Cizeau J, Dubik D et al BNIP3 and genetic control of necrosis‐like cell death through the mitochondrial permeability transition pore. Mol Cell Biol 2000; 20: 5454–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ 2009; 16: 939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Berkers CR, Maddocks OD, Chenung EC, Mor I, Vousden KH. Metabolic regulation by p53 family members. Cell Metab 2013; 18: 617–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Humpton TJ, Vousden KH. Regulation of cellular metabolism and hypoxia by p53. Cold Spring Harb Perspect Med 2016; 6: a026146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bourdon A, Minai L, Serre V et al Mutation of RRM2B, encoding p53‐controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat Genet 2007; 39: 776–80. [DOI] [PubMed] [Google Scholar]
- 60. Iiizumi M, Arakawa H, Mori T, Ando T, Nakamura Y. Isolation of a novel gene, CABC1, encoding a mitochondrial protein that is highly homologous to yeast activity of bc1 complex. Cancer Res 2002; 62: 1246–50. [PubMed] [Google Scholar]
- 61. Matoba S, Kang JG, Patino WD et al p53 regulates mitochondrial respiration. Science 2006; 312: 1650–3. [DOI] [PubMed] [Google Scholar]
- 62. Guzy RD, Hoyos B, Robin E et al Mitochondrial complex III is required for hypoxia‐induced ROS production and cellular oxygen sensing. Cell Metab 2005; 1: 401–8. [DOI] [PubMed] [Google Scholar]
- 63. Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014; 24: R453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Giannoni E, Parri M, Chiarugi P. EMT and oxidative stress: a bidirectional interplay affecting tumor malignancy. Antioxid Redox Signal 2012; 16: 1248–63. [DOI] [PubMed] [Google Scholar]
- 65. Radisky DC, Levy DD, Littlepage LE et al Rac1b and reactive oxygen species mediate MMP‐3‐induced EMT and genomic instability. Nature 2005; 436: 123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cannito S, Novo E, Compagnone A et al Redox mechanisms switch on hypoxia‐dependent epithelial‐mesenchymal transition in cancer cells. Carcinogenesis 2008; 29: 2267–78. [DOI] [PubMed] [Google Scholar]
- 67. Klimova T, Chandel NS. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ 2008; 15: 660–6. [DOI] [PubMed] [Google Scholar]
- 68. Lin X, David CA, Donnelly JB et al A chemical genomics screen highlights the essential role of mitochondria in HIF‐1 regulation. Proc Natl Acad Sci USA 2008; 105: 174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF‐kappa B transcription factor and HIV‐1. EMBO J 1991; 10: 2247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinase. Free Radic Biol Med 2004; 37: 768–84. [DOI] [PubMed] [Google Scholar]
- 71. Weinberg F, Chandel NS. Reactive oxygen species‐dependent signaling regulates cancer. Cell Mol Life Sci 2009; 66: 3363–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Weinberg F, Hamanaka R, Wheaton WW et al Mitochondrial metabolism and ROS generation are essential for Kras‐mediated tumorigenicity. Proc Natl Acad Sci USA 2010; 107: 8788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hirayama A, Kami K, Sugimoto M et al Quantitative metabolome profiling of colon and stomach caner microenvironment by capillary electrophoresis time‐of‐flight mass spectrometry. Cancer Res 2009; 69: 4918–25. [DOI] [PubMed] [Google Scholar]
- 74. DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008; 7: 11–20. [DOI] [PubMed] [Google Scholar]
- 75. Beloribi‐Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016; 5: e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yang M, Vousden KH. Serine and one‐carbon metabolism in cancer. Nat Rev Cancer 2016; 16: 650–62. [DOI] [PubMed] [Google Scholar]


