Abstract
The programmed death‐1/programmed death‐ligand 1 (PD‐L1) pathway is a negative feedback pathway that suppresses the activity of T cells. Previous studies reported that high PD‐L1 expression on tumor cells (TC) was associated with poor survival in patients with colorectal cancer; however, the prognostic evaluation of these studies was limited because they included patients at various disease stages. The purpose of the present study was to evaluate the relationship between PD‐L1 status in the immune microenvironment and the clinicopathological features of stage III colorectal cancer. Two hundred and thirty‐five patients were included in the analysis. PD‐L1 expression on TC and tumor‐infiltrating mononuclear cells (TIMC) was evaluated by immunohistochemistry. The median follow‐up of thisi study was 52.9 months. A total of 8.1% of stage III colorectal cancer showed high PD‐L1 expression on TC and 15.3% showed high PD‐L1 expression on TIMC. Patients with high PD‐L1 expression on TC had significantly shorter disease‐free survival (DFS) than patients with low expression (hazard ratio [HR] 2.36; 95% confidence interval [CI], 1.21–4.62; P = 0.012). In addition, patients with high PD‐L1 expression on TIMC were associated with longer DFS than patients with low expression (HR 0.40; 95% CI, 0.16–0.98; P = 0.046). These findings suggest that PD‐L1 expression status may be a new predictor of recurrence for stage III colorectal cancer patients and highlight the necessity of evaluating PD‐L1 expression on TC and TIMC separately in the tumor microenvironment.
Keywords: CD8‐positive T‐lymphocytes, colonic neoplasms, immune microenvironment, immunohistochemistry, tumor infiltrating mononuclear cells
Colorectal cancer is a major cause of cancer‐related death worldwide.1 In Japan, there were 124 921 new cases of colorectal cancer in 2011 and 48 485 deaths from the disease in 2014, respectively.2 Multimodality therapy consisting of surgery, chemotherapy and radiotherapy is the main treatment for operable cancer;3, 4, 5 however, the recurrence rate in patients with stage III colorectal cancer remains high.6
Previous studies have shown that molecular markers, including BRAF, KRAS and tumor mismatch repair (MMR) status, are useful for predicting recurrences in patients with stage III colorectal cancer.7, 8 In addition, the prognostic effect of the immune microenvironment has become increasingly recognized.9, 10, 11 The programmed death 1 (PD‐1)/programmed death 1‐ligand 1 (PD‐L1) signaling pathway is a negative feedback mechanism that suppresses the activity of T cells.12 PD‐L1 expression has been reported on tumor cells or tumor‐infiltrating immune cells in several malignancies, including colorectal cancer.13 Furthermore, it has been suggested that high PD‐L1 expression on tumor cells (TC) and/or tumor infiltrating immune cells is associated with prognosis across different tumor types, including esophageal cancer14, 15 and urothelial cancer.16 However, the prognostic value of PD‐L1 expression in patients with stage III colorectal cancer has not yet been established.
The aim of the present study was to evaluate the relationship between PD‐L1 status in the immune microenvironment and the clinicopathological features of stage III colorectal cancer.
Methods
Patients and samples
Formalin‐fixed paraffin‐embedded block specimens from surgical resection of the primary tumor were obtained from 318 patients with stage III colorectal cancer who underwent curative surgery and adjuvant chemotherapy at our institution between January 2009 and July 2012. We excluded 83 patients who received radiotherapy or chemoradiotherapy before surgery. A total of 235 patients were included in this study, and all of their samples were evaluable for immunohistochemistry (IHC). PD‐L1 expression on TC and tumor‐infiltrating mononuclear cells (TIMC) as well as the number of CD8 positive T cells were evaluated by IHC. TIMC were distinguished from TC by their morphological features following HE staining. Baseline clinicopathological characteristics and clinical outcome data were retrospectively collected from the Colorectal Cancer Database of the Department of Gastroenterological Surgery and the Pathological Diagnosis Database in the Department of Pathology. The Institutional Review Board of Toranomon Hospital approved the following data acquisition and tumor staining. The data cutoff date of clinical outcome for this analysis was July 2015.
Scoring for PD‐L1 expression
PD‐L1 expression was evaluated using an anti PD‐L1 rabbit monoclonal antibody (clone SP142; Spring Bioscience, Pleasanton, CA, USA). Two pathologists (N.I. and Y.M.) independently examined PD‐L1 expression. Specimens were scored as IHC low or high when <5% or ≥5% of cells were PD‐L1 positive, respectively. This criteria was validated in various types of cancer (Fig. 1a).17, 18, 19
Figure 1.
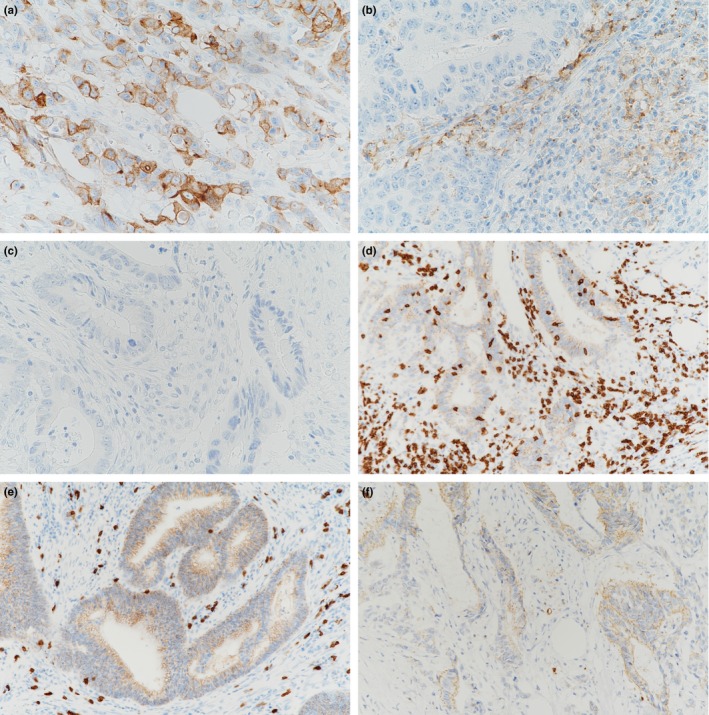
Representative photomicrographs of PD‐L1 expression on tumor cells (TC) and tumor infiltrating mononuclear cells (TIMC) (a), and CD8 positive T cell infiltration in the tumor microenvironment (b). High PD‐L1 expression on TC and low PD‐L1 expression on TIMC (a), low PD‐L1 expression on TC and high PD‐L1 expression on TIMC (b), and low PD‐L1 expression on TC and low PD‐L1 expression on TIMC (c). High CD8 expression in intratumoral and peritumoral cells is present (d), low CD8 expression in intratumoral cells and high CD8 expression in peritumoral cells (e), and low CD8 expression in intratumoral and peritumoral cells (f).
Scoring for CD8 positive T cell numbers
CD8 positive T cells were identified using an anti‐CD8 rabbit monoclonal antibody (clone SP57; Roche Tissue Diagnostics, Mannheim, Germany). Intratumoral CD8‐positive T cell (intra CD8) density, defined as CD8 cells that infiltrated into cancer nests, were scored IHC low or high when their mean number was <50 or ≥50, respectively. This criteria was validated in colorectal cancer.20, 21 Peritumoral CD8‐positive T cell (peri CD8) density, defined as CD8 cells that infiltrated into the cancer stroma or were distributed along the invasive margin of cancer, were scored IHC low or high when the mean number was <200 or ≥200, respectively. CD8‐positive T cell numbers were counted twice in a microscopic field at a magnification of ×200 (Fig. 1b).
Statistical analysis
The primary objective of the present study was to correlate the levels of PD‐L1 expression with disease‐free survival (DFS). DFS was defined as the duration between surgery and disease relapse, any cause of death before disease relapse, or the last follow‐up. Survival analysis was conducted using the Kaplan–Meier method and Cox proportional hazard regression. The multivariate Cox model included all variables with P < 0.10 in the univariate model. Fisher's exact test was used to assess the association of PD‐L1 expression with clinicopathological features. Statistical analyses were performed by SPSS software, version 23.0; IBM Corp, Armonk, NY, USA. Statistical significance was determined when as P < 0.05.
Results
Patients and tumor characteristics
A total of 235 patients were included in the analysis. A summary of patients and tumor characteristics is presented in Table 1.
Table 1.
Patient characteristics
| Number (%) | |
|---|---|
| Median age, years (range) | 63 (32–84) |
| Male/female | 140/95 (59.6/40.4) |
| Primary tumor site | |
| Right | 63 (26.8) |
| Left | 172 (73.2) |
| T status† | |
| T1 | 25 (10.6) |
| T2 | 28 (11.9) |
| T3 | 129 (54.9) |
| T4 | 53 (22.6) |
| Stage† | |
| IIIa | 49 (20.9) |
| IIIb | 141 (60.0) |
| IIIc | 45 (19.1) |
| Histological type | |
| Well‐moderately differentiated | 217 (92.3) |
| Poorly differentiated and mucinous | 18 (7.7) |
| Chemotherapy | |
| Fluoropyrimidine alone | 174 (74.0) |
| Fluoropyrimidine with oxaliplatin | 61 (26.0) |
†UICC TNM classification of malignant tumors, 7th edition.
PD‐L1 expression on tumor cells or tumor‐infiltrating mononuclear cells
High and low PD‐L1 expression on TC was observed in 19 (8.1%) and 216 patients (91.9%) out of 235 patients, respectively. High and low PD‐L1 expression on TIMC was observed in 36 (15.3%) and 199 patients (84.7%) out of 235 patients, respectively. No patients had high PD‐L1 expression on both TC and TIMC (Suppl. Table S1).
Association of PD‐L1 expression with disease‐free survival
The median follow‐up time of this study was 52.9 months (range 4.6–78.8). The total number of DFS events was 67. Kaplan–Meier survival analysis stratified by PD‐L1 expression is shown in Figure 2. Patients with high PD‐L1 expression on TC had a significantly shorter DFS than those with low expression (Fig. 2a), while patients with high PD‐L1 expression on TIMC showed the opposite result (Fig. 2b).
Figure 2.
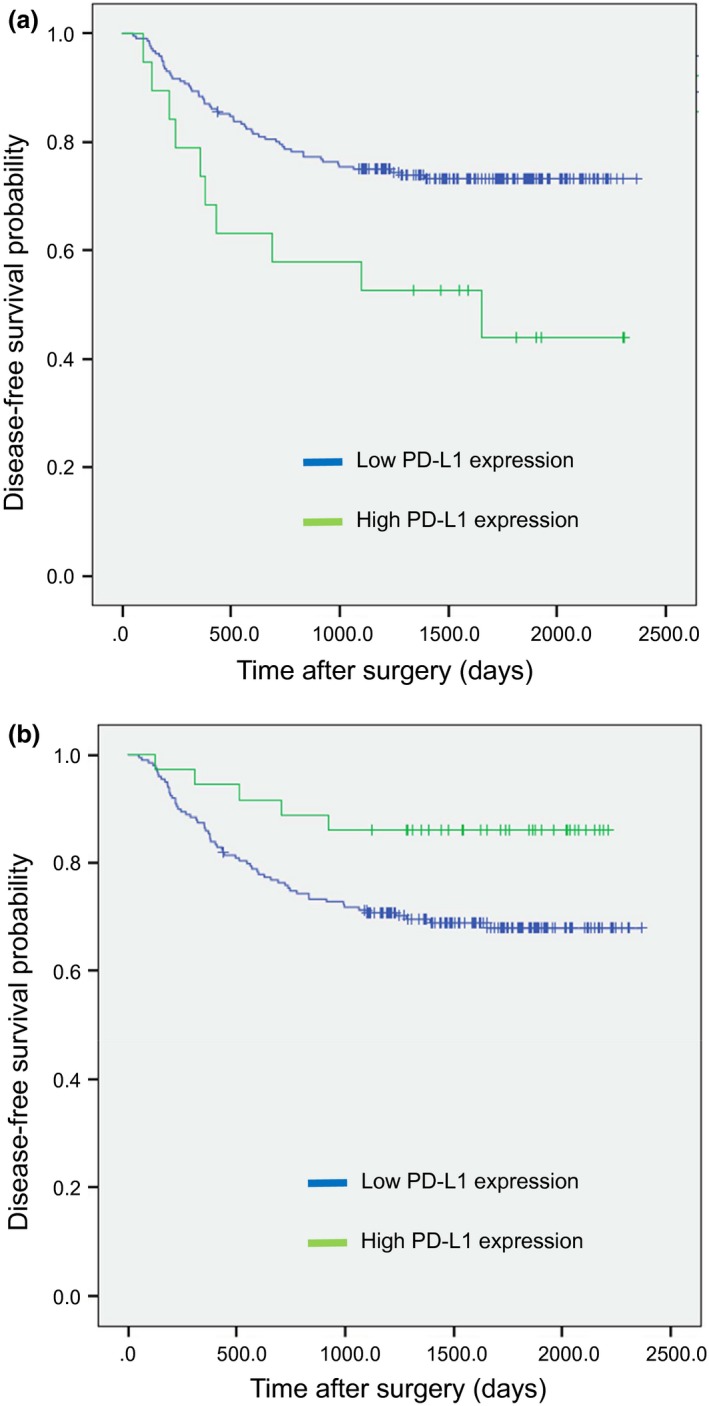
Kaplan–Meier survival analysis of disease‐free survival (DFS) stratified by PD‐L1 expression on tumor cells (TC) (a) and tumor infiltrating mononuclear cells (TIMC) (b).
Patients with a high PD‐L1 expression on TC had a significantly shorter DFS than those with a low expression in both univariate (hazard ratio [HR] 2.36; 95% confidence interval (CI): 1.21–4.62; P = 0.012] and multivariate analysis (HR 2.45; 95% CI: 1.24–4.85, P = 0.010). In contrast, patients with high PD‐L1 expression on TIMC had a significantly longer DFS than those with low expression in univariate analysis (HR 0.40; 95% CI: 0.16–0.98; P = 0.046) but not in multivariate analysis (HR 0.55; 95% CI: 0.23–1.52; P = 0.28) (Table 2).
Table 2.
Univariate and multivariate analyses of prognostic factors associated with DFS
| Univariate | Multivariate | |||
|---|---|---|---|---|
| DFS | DFS | |||
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Age, years (≤65 vs >65) | 0.988 (0.608–1.605) | 0.960 | ||
| Sex (male vs female) | 0.882 (0.537–1.447) | 0.619 | ||
| Right versus left | 0.854 (0.506–1.441) | 0.555 | ||
| wel versus por/muc | 2.804 (1.431–5.498) | 0.003 | 2.607 (1.325–5.131) | 0.006 |
| PD‐L1 expression (TC) low versus high | 2.361 (1.205–4.624) | 0.012 | 2.450 (1.239–4.847) | 0.010 |
| PD‐L1 expression (TIMC) low versus high | 0.395 (0.159–0.983) | 0.046 | 0.549 (0.233–1.518) | 0.277 |
| Intra CD8 low versus high | 0.352 (0.141–0.875) | 0.025 | 0.395 (0.156–1.004) | 0.051 |
| Peri CD8 low versus high | 0.932 (0.553–1.574) | 0.793 | ||
| Chemotherapy (without vs with oxaliplatin) | 1.382 (0.825–2.318) | 0.219 | ||
CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; intra CD8, intratumoral CD8‐positive T cell; peri CD8, peritumoral CD8‐positive T cell; por/muc, poorly differentiated/mucinous; TC, tumor cells; wel, well‐moderately differentiated; TIMC, tumor‐infiltrating mononuclear cells.
Association of PD‐L1 expression with clinicopathological features
As shown in Table 3), tumors with high PD‐L1 expression were associated with a more distant spread of lymph node metastasis (P = 0.008) and exhibited advanced TNM stage (P < 0.001). However, there was no relationship between PD‐L1 expression on TIMC and lymph node metastasis or TNM stage. Of note, tumors with high PD‐L1 expression on TIMC were significantly associated with increased intra CD8‐positive T cells (P < 0.001) (Table 3).
Table 3.
Association of clinicopathological features with PD‐L1 expression
| PD‐L1 expression (TCs)¶ | PD‐L1 expression (TIMCs)¶ | ||||||
|---|---|---|---|---|---|---|---|
| Low | High | P‐value | Low | High | P‐value | ||
| Age, years | ≤65 | 120 | 14 | 0.151 | 107 | 27 | 0.018 |
| >65 | 96 | 5 | 92 | 9 | |||
| Sex | Male | 128 | 12 | 0.811 | 119 | 21 | 0.856 |
| Female | 88 | 7 | 80 | 15 | |||
| Primary site | Right | 60 | 3 | 0.417 | 53 | 10 | 0.841 |
| Left | 156 | 16 | 146 | 26 | |||
| Histological type | wel† | 200 | 17 | 0.645 | 182 | 35 | 0.322 |
| por/muc† | 16 | 2 | 17 | 1 | |||
| T status‡ | T1 | 25 | 0 | 0.090 | 20 | 5 | 0.239 |
| T2 | 28 | 0 | 22 | 6 | |||
| T3 | 116 | 13 | 108 | 21 | |||
| T4 | 47 | 6 | 49 | 4 | |||
| N status‡ | N1 | 157 | 8 | 0.008 | 140 | 25 | 1.000 |
| N2 | 59 | 11 | 59 | 11 | |||
| Stage‡ | IIIa | 49 | 0 | <0.001 | 40 | 9 | 0.193 |
| IIIb | 132 | 9 | 117 | 24 | |||
| IIIc | 35 | 10 | 42 | 3 | |||
| Intra CD8 positive T cells¶ | Low | 181 | 14 | 0.334 | 175 | 20 | <0.001 |
| High | 35 | 5 | 24 | 16 | |||
| Peri CD8 positive T cells¶ | Low | 151 | 11 | 0.305 | 140 | 22 | 0.328 |
| High | 65 | 8 | 59 | 14 | |||
| Chemotherapy | FU alone§ | 162 | 12 | 0.279 | 146 | 28 | 0.682 |
| FU with Oxaliplatin | 54 | 7 | 53 | 8 | |||
†wel, well‐moderately differentiated, por/muc, poorly differentiated/mucinous; ‡UICC TNM classification of malignant tumors, 7th edition; §FU, fluoropyrimidine, ¶definition of TCs, TIMCs, intra CD8, and peri CD8 are shown in methods.
Association of CD8‐positive T cell density with disease‐free survival
Of 235 patients, 40 (17.0%) and 195 (83.0%) patients had high and low intra CD8‐positive T cell densities, respectively. Peri CD8‐positive T cell density was high in 73 patients (31.1%) and low in 162 patients (68.9%) (Suppl. Table S2).
Patients with high intra CD8‐positive T cell density had a significantly longer DFS than patients with low density in univariate analysis (HR 0.35; 95% CI: 0.14–0.88; P = 0.025) but not in multivariate analysis (Table 2). In addition, there was no correlation between peri CD8‐positive T cell density and DFS in both univariate and multivariate analyses (Table 2). Figure 3 shows the DFS Kaplan–Meier curve stratified by intra CD8‐positive T cell and PD‐L1 expression on TC (a), and by peri CD8‐positive T cell and PD‐L1 expression on TC (b). Patients with low PD‐L1 expression on TC and high intra CD8‐positive T cell numbers had the best prognosis (Fig. 3a), whereas patients with high PD‐L1 expression on TC and low peri CD8‐positive T cell numbers had the worst prognosis (Fig. 3b), although the relatively low number of patients in each group made it difficult to draw definitive conclusions.
Figure 3.
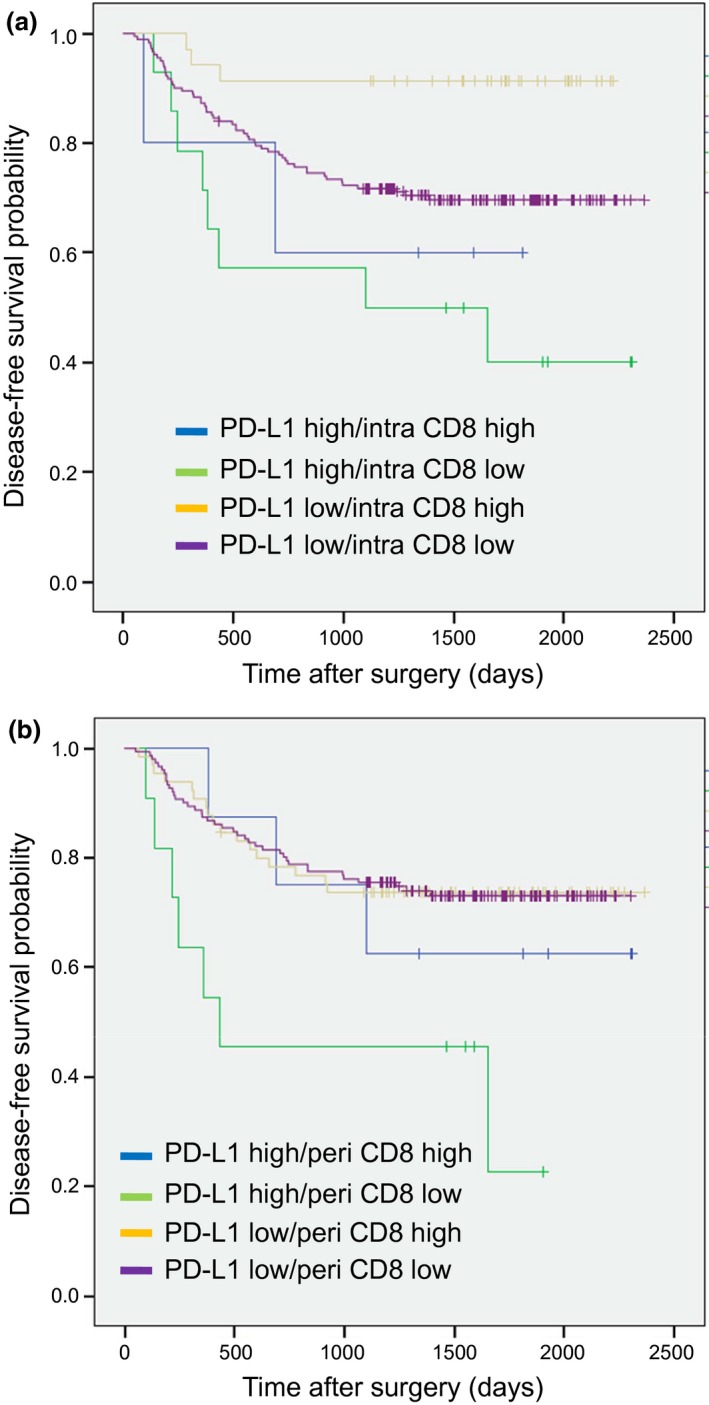
Kaplan–Meier survival analysis of disease‐free survival (DFS) stratified by intra CD8‐positive T cells/PD‐L1 expression on tumor cells (TC) (a), and peri CD8‐positive T cells/PD‐L1 expression on TC (b).
Discussion
The current study showed that high PD‐L1 expression on TC was significantly associated with a poor prognosis, whereas high PD‐L1 expression on TIMC was associated with a good prognosis. To the best of our knowledge, this is the first study to demonstrate the prognostic significance of PD‐L1 expression on stage III colorectal cancer.
PD‐L1 expression on TC may indicate immune evasion.12 Previous studies have reported that high PD‐L1 expression on TC is associated with advanced tumor stage and poor survival in patients with colorectal cancer.22, 23, 24 However, the prognostic evaluation of these studies has been limited because they included patients at various disease stages. In the current study, we focused on stage III colorectal cancer and demonstrated that high PD‐L1 expression on TC correlated with distant spread of lymph node metastasis, advanced tumor stage and poor prognosis. In addition, high infiltration of intra CD8 positive T cells was associated with longer DFS compared with those with low infiltration in the subset of low PD‐L1 expressing TC, but there was no DFS difference in subsets of high PD‐L1 expression (Fig. 3a). These results suggested that PD‐L1 expression on TC might make intra CD8 positive T cells inefficient, leading to stage progression and/or poor survival in patients with stage III colorectal cancer.
The function of PD‐L1 expression on TIMC has not been elucidated. The association of PD‐L1 expression on TIMC with tumor aggressiveness and prognosis has not been determined across various tumor types. Thompson et al.25 report that high PD‐L1 expression on TIMC is associated with poor prognosis in renal carcinoma patients, but the opposite has been reported in urothelial cancer patients.16 Here, we showed that high PD‐L1 expression on TIMC was not correlated with tumor aggressiveness but with longer survival, in contrast to PD‐L1 expression on TC. These findings highlight the need to evaluate these cell types separately within tumor microenvironments. In addition, we also showed the association of high PD‐L1 expression on TIMC with increased intra CD8 positive T cell infiltration. These findings suggest that PD‐L1 expression on TIMC may induce tumor antigen‐specific CD8‐positive T cells in tumor sites, which translates to a good prognosis in stage III colorectal cancer patients.
To date, several studies have reported that oncogenic pathway, such as BRAF, KRAS, phosphatidylinositol 3‐kinase, catalytic, alpha (PIK3CA) and MMR status, might be associated with prognosis in patients with colorectal cancer. However, no consistent association has been observed between those molecular markers and DFS in stage III colorectal cancer.8, 26, 27 In addition, the frequency of MMR deficient, which may be associated with PD‐L1 expression,28, 29 was less than 5% in stage III colorectal cancer patients,30 so that it may not influence our results.
Our study has several limitations. First, this study was a retrospective, single‐institution study; therefore, there was the potential for selection bias. Second, we evaluated PD‐L1 expression using only one antibody (SP142 clone). Previous study demonstrated the comparison of four different PD‐L1 IHC assays for lung cancer.31 They showed that the concordance rate of PD‐L1 positivity in all four assays was 50% (19 of the 38 cases).31 Importantly, SP142, the assay we used in our study, exhibited fewer stained tumor cells. Further investigation using other antibodies and cutoff values to validate our results is warranted. Third, we did not evaluate immune cell components in TIMC‐expressed PD‐L1 in our trial. It has become apparent that various myeloid cells express PD‐L1 in colorectal cancer,32 even though the prognostic value remains unclear. Further investigations are needed to identify the characteristics of PD‐L1‐expressed immune cells and reveal the function of PD‐L1 molecules in those cells.
Recently, a new therapeutic approach using immune checkpoint inhibitors has achieved breakthrough results across various tumor types.33, 34, 35, 36 In addition, immune microenvironment status, such as PD‐L1 expression on TC,37 TIMC17 and tumor infiltrating T cells, and MMR status38 may be a potential predictive biomarkers in cancer immunotherapy. We believe that our analysis might enhance the development of novel treatment approaches for stage III colorectal cancer.
In conclusion, we demonstrated that high PD‐L1 expression on TC negatively affected patient survival, whereas high PD‐L1 expression on TIMC was associated with a favorable prognosis of patients with stage III colorectal cancer. Further prospective investigation is warranted to verify these prognostic roles in stage III colorectal cancer patients.
Disclosure Statement
Y.M has received honoraria from Novartis and Kyowa Hakko Kirin, and his department received research funding from Chugai, Takeda and Taiho. T.T has received honoraria from Daiichi Sankyo and Kyowa Hakko Kirin, and his department received research funding from Chugai, Takeda, Taiho and Novartis. All remaining authors have no conflicts of interest to declare.
Supporting information
Table S1. The number of patients with high (H) and low (L) PD‐L1 expression on TC/TIMC.
Table S2. The number of patients with high (H) and low (L) intra/peri CD8 positive T cells.
Acknowledgments
The authors thank pathological technician T. Fukawa for excellent technical assistance, and Clinical Research Coordinator K. Nonogaki, E. Ozeki and H. Sato for data management.
Cancer Sci 108 (2017) 853–858
Funding Information
Research grant of Toranomon Hospital.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. The Editorial Board of the Cancer Statistics in Japan (ed.). Cancer Statistics in Japan – 2015. Available from URL: http://ganjoho.jp/data/reg_stat/statistics/brochure/2015/cancer_statistics_2015.pdf [Online; cited 13 July 2016.]
- 3. Andre T, Boni C, Mounedji‐Boudiaf L et al Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343–51. [DOI] [PubMed] [Google Scholar]
- 4. Yothers G, O'Connell MJ, Allegra CJ et al Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C‐07 trial, including survival and subset analyses. J Clin Oncol 2011; 29: 3768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sauer R, Becker H, Hohenberger W et al Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–40. [DOI] [PubMed] [Google Scholar]
- 6. Watanabe T, Itabashi M, Shimada Y et al Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20: 207–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zaanan A, Flejou JF, Emile JF et al Defective mismatch repair status as a prognostic biomarker of disease‐free survival in stage III colon cancer patients treated with adjuvant FOLFOX chemotherapy. Clin Cancer Res 2011; 17: 7470–8. [DOI] [PubMed] [Google Scholar]
- 8. Sinicrope FA, Shi Q, Smyrk TC et al Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology 2015; 148: 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pages F, Berger A, Camus M et al Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353: 2654–66. [DOI] [PubMed] [Google Scholar]
- 10. Huh JW, Lee JH, Kim HR. Prognostic significance of tumor‐infiltrating lymphocytes for patients with colorectal cancer. Arch Surg 2012; 147: 366–72. [DOI] [PubMed] [Google Scholar]
- 11. Prall F, Duhrkop T, Weirich V et al Prognostic role of CD8+ tumor‐infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol 2004; 35: 808–16. [DOI] [PubMed] [Google Scholar]
- 12. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gatalica Z, Snyder C, Maney T et al Programmed cell death 1 (PD‐1) and its ligand (PD‐L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev 2014; 23: 2965–70. [DOI] [PubMed] [Google Scholar]
- 14. Chen L, Deng H, Lu M et al B7‐H1 expression associates with tumor invasion and predicts patient's survival in human esophageal cancer. Int J Clin Exp Pathol 2014; 7: 6015–23. [PMC free article] [PubMed] [Google Scholar]
- 15. Loos M, Langer R, Schuster T et al Clinical significance of the costimulatory molecule B7‐H1 in Barrett carcinoma. Ann Thorac Surg 2011; 91: 1025–31. [DOI] [PubMed] [Google Scholar]
- 16. Bellmunt J, Mullane SA, Werner L et al Association of PD‐L1 expression on tumor‐infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol 2015; 26: 812–7. [DOI] [PubMed] [Google Scholar]
- 17. Herbst RS, Soria JC, Kowanetz M et al Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fehrenbacher L, Spira A, Ballinger M et al Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): A multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–46. [DOI] [PubMed] [Google Scholar]
- 19. Rosenberg JE, Hoffman‐Censits J, Powles T et al Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: A single‐arm, multicentre, phase 2 trial. Lancet 2016; 387: 1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naito Y, Saito K, Shiiba K et al CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998; 58: 3491–4. [PubMed] [Google Scholar]
- 21. Deschoolmeester V, Baay M, Van Marck E et al Tumor infiltrating lymphocytes: An intriguing player in the survival of colorectal cancer patients. BMC Immunol 2010; 11: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi SJ, Wang LJ, Wang GD et al B7‐H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS ONE 2013; 8: e76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song M, Chen D, Lu B et al PTEN loss increases PD‐L1 protein expression and affects the correlation between PD‐L1 expression and clinical parameters in colorectal cancer. PLoS ONE 2013; 8: e65821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang M, Li J, Wang D, Li S et al T‐cell infiltration and expressions of T lymphocyte co‐inhibitory B7‐H1 and B7‐H4 molecules among colorectal cancer patients in northeast China's Heilongjiang province. Tumour Biol 2014; 35: 55–60. [DOI] [PubMed] [Google Scholar]
- 25. Thompson RH, Gillett MD, Cheville JC et al Costimulatory B7‐H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA 2004; 101: 17174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roth AD, Tejpar S, Delorenzi M et al Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: Results of the translational study on the PETACC‐3, EORTC 40993, SAKK 60‐00 trial. J Clin Oncol 2010; 28: 466–74. [DOI] [PubMed] [Google Scholar]
- 27. Ogino S, Meyerhardt JA, Irahara N et al KRAS mutation in stage III colon cancer and clinical outcome following intergroup trial CALGB 89803. Clin Cancer Res 2009; 15: 7322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Masugi Y, Nishihara R, Yang J et al Tumour CD274 (PD‐L1) expression and T cells in colorectal cancer. Gut 2016; doi: 10.1136/gutjnl‐2016‐311421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenbaum MW, Bledsoe JR, Morales‐Oyarvide V, Huynh TG, Mino‐Kenudson M. PD‐L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor‐infiltrating lymphocytes. Mod Pathol 2016; 29: 1104–12. [DOI] [PubMed] [Google Scholar]
- 30. Yamanaka T, Oki E, Yamazaki K et al 12‐Gene Recurrence Score Assay Stratifies the Recurrence Risk in Stage II/III Colon Cancer With Surgery Alone: The SUNRISE Study. J Clin Oncol 2016; 34: 2906–13. [DOI] [PubMed] [Google Scholar]
- 31. Hirsch FR, McElhinny A, Stanforth D et al PD‐L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD‐L1 IHC Assay Comparison Project. J Thorac Oncol 2017; 12: 208–22. [DOI] [PubMed] [Google Scholar]
- 32. Llosa NJ, Cruise M, Tam A et al The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter‐inhibitory checkpoints. Cancer Discov 2015; 5: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Topalian SL, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Larkin J, Chiarion‐Sileni V, Gonzalez R et al Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus Docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ansell SM, Lesokhin AM, Borrello I et al PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015; 372: 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patel SP, Kurzrock R. PD‐L1 Expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 2015; 14: 847–56. [DOI] [PubMed] [Google Scholar]
- 38. Le DT, Uram JN, Wang H et al PD‐1 Blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015; 372: 2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The number of patients with high (H) and low (L) PD‐L1 expression on TC/TIMC.
Table S2. The number of patients with high (H) and low (L) intra/peri CD8 positive T cells.


