Abstract
Overexpression of programmed death‐1 (PD‐1) ligands contributes to an immunosuppressive microenvironment. Nivolumab is a PD‐1‐blocking antibody that inhibits the PD‐1 pathway and showed good efficacy in several types of malignancy. This phase II study examined the efficacy and safety of nivolumab in 17 Japanese patients with refractory/relapsed classical Hodgkin lymphoma previously treated with brentuximab vedotin. Sixteen patients were included in efficacy analyses and 17 in safety analyses. The primary endpoint was the centrally assessed objective response rate (ORR). The study was commenced in March 2015. We report data obtained at a cutoff of 16 March 2016, at which time 11 patients were still receiving nivolumab. The median (range) duration of treatment and follow‐up were 7.0 (1.4–10.6) months and 9.8 (6.0–11.1) months, respectively. All 17 patients had previously received brentuximab vedotin. The ORR was 81.3% (95% confidence interval [CI]: 54.4–96.0%; 13/16 patients), with complete remission and partial remission in 4 and 9 patients, respectively. The overall survival (OS) and progression‐free survival (PFS) rates at 6 months were 100 and 60.0% (95% CI: 31.8–79.7%), respectively; the median OS and PFS were not reached. The most common adverse events (AE) were pyrexia (41.2%), pruritus (35.3%), rash (35.3%) and hypothyroidism (29.4%). Four patients (23.5%) experienced grade 3 or 4 AE, but most AE were of grade 1 or 2. In conclusion, nivolumab is a potentially effective and tolerable treatment option for Japanese patients with relapsed/refractory classical Hodgkin lymphoma previously treated with brentuximab vedotin.
Keywords: Hodgkin lymphoma, immunotherapy, Japanese, nivolumab, programmed death‐1
First‐line standard treatments for classical Hodgkin lymphoma (cHL) include chemotherapy and radiotherapy, which have been reported to be curative in approximately 80% of patients.1, 2 However, some patients experience disease relapse or have refractory disease. Eligible patients may undergo autologous stem cell transplantation (ASCT) after salvage chemotherapy. Patients with relapsed or refractory cHL after ASCT have a poor prognosis and the currently available conventional cytotoxic treatments are often ineffective.3
Several treatment strategies have been developed to improve the prognosis of relapsed or refractory cHL, including antibody–drug conjugates. One of these is brentuximab vedotin, which comprises a chimeric monoclonal antibody targeting CD30 linked to an antimicrotubule agent (vedotin).4, 5 Several clinical trials of brentuximab vedotin have been conducted, and it is now available in several countries, including Japan, for the treatment of relapsed or refractory CD30‐positive cHL. Brentuximab vedotin was associated with objective response rates (ORR) of 75% and 67% in a multinational phase II study6 and a Japanese phase I/II study,7 respectively. However, patients who experience disease progression after brentuximab vedotin usually have a poor prognosis. Indeed, median progression‐free survival (PFS) and overall survival (OS) associated with the subsequent treatment following brentuximab vedotin are 3.5 and 25.2 months, respectively.8 These findings highlight the need for alternative treatment options for cHL.
The programmed death 1 (PD‐1) pathway is an intracellular signaling pathway that regulates the response of activated T cells. Some cancers exploit this pathway to evade anti‐tumor immune responses. Accordingly, PD‐1–blocking antibodies have been developed for the treatment of solid tumors.9, 10, 11 Recent studies have also revealed the expression of PD‐1 ligands in many cases of cHL as a result of amplification of 9p24.1, and that PD‐1 ligand overexpression contributes to an immunosuppressive microenvironment and worse prognosis.12, 13, 14, 15, 16
Nivolumab, a human IgG4 anti‐PD‐1 monoclonal antibody, acts as an immune checkpoint inhibitor and blocks the signal that prevents activated T cells from targeting cancer cells. A phase I study (CheckMate 039) conducted in the United States showed that nivolumab was associated with good efficacy in heavily pretreated patients with relapsed or refractory cHL, with an ORR of 87%.17 We report the results of a multicenter phase II study, which was designed to examine the efficacy and safety of nivolumab in Japanese patients with relapsed or refractory cHL previously treated with brentuximab vedotin.
Patients and Methods
Ethics
This study was conducted in accordance with the Helsinki Declaration, Good Clinical Practice and relevant Japanese regulations. The study was performed at seven sites in Japan, and was approved by the institutional review board at each site. All patients provided written informed consent. This study was registered with the Japan Pharmaceutical Information Center (identifier: JapicCTI‐142755).
Patients
This study enrolled patients aged ≥20 years with a histopathological diagnosis of cHL. Patients with a history of treatment with ASCT, patients for whom ASCT was not indicated, and patients who refused to receive treatment with ASCT were eligible. Patients who were previously treated with brentuximab vedotin or were clinically unqualified for treatment with brentuximab vedotin even if they had not received brentuximab vedotin were eligible. Major inclusion criteria were a detectable lesion on fluorodeoxyglucose positron emission tomography (FDG‐PET) and at least one lesion with a longest diameter of ≥15 mm on computed tomography (CT) or magnetic resonance imaging (MRI), Eastern Cooperative Oncology Group performance status (PS) of 0–1, life expectancy of ≥3 months, neutrophil count ≥750/mm3, platelet count ≥50 000/mm3, hemoglobin ≥8.0 g/dL, aspartate aminotransferase and alanine aminotransferase ≤3.0× the institution's upper limit of normal (ULN), total bilirubin ≤2.0× the institution's ULN, and creatinine ≤1.5 mg/dL or creatinine clearance ≥40 mL/min (according to the Cockcroft–Gault equation). Major exclusion criteria were nodular lymphocyte‐predominant Hodgkin lymphoma, central nervous system involvement, concurrent or history of chronic autoimmune disease, a current or past history of interstitial lung disease or pulmonary fibrosis, concurrent diverticulitis or symptomatic gastrointestinal ulcerative disease requiring treatment, a history of organ allograft or allogeneic hematopoietic stem cell transplantation, a history of prior treatment with therapeutic antibodies or pharmacotherapies for regulation of T‐cells.
Study design and treatments
Patients received their first dose of nivolumab (3 mg/kg) on Day 1 of the treatment phase, and subsequent doses were to be administered on Day 1 of each 14‐day cycle. Nivolumab was to be continued if the treatment continuation criteria were met, and the trial was to continue until all patients discontinued treatment in the event of progressive disease (PD), an unacceptable adverse event (AE) or other clinically relevant reasons. However, after the first confirmation of PD or if the patient had not received a dose of nivolumab within the last 6 weeks for specific reasons or for ≥6 weeks in the case of steroid tapering after the treatment of drug‐related AE, administration of nivolumab could be continued if continuation was deemed appropriate by the investigator and the patient agreed with this decision.
During treatment, CT or MRI were to be performed in cycles 4, 8, 12, 18, 24, 32, 40, 48 and 61, and every 13 cycles thereafter. FDG‐PET was to be performed on Day 15 in cycles 8, 12 and 24. Patients who discontinued for reasons other than PD and whose response was complete remission (CR)/partial remission (PR)/SD at discontinuation were to undergo diagnostic imaging every 8–12 weeks, for as long as possible, until starting the next treatment for cHL or confirmation of PD or relapse.
Study measures and endpoints
The primary endpoint was the ORR assessed by a central review committee in accordance with the Revised International Working Group criteria for malignant lymphoma.18 The secondary endpoints included the investigator‐assessed ORR, CR rate, PR rate, duration of response, time to response, PFS, OS, disappearance rate of B symptoms, median time to disappearance of B symptoms, occurrence rate of B symptoms, rate of change in the sum of the products of the greatest diameters of the target lesion, and the maximum rate of change in the sum of the products of the greatest diameters of the target lesion. We also evaluated safety endpoints, including AE, laboratory tests, vital signs, body weight, 12‐lead electrocardiograph, chest X‐ray and PS. AE were classified and graded according to the Common Terminology Criteria for Adverse Events version 4.0. Anti‐nivolumab antibodies were determined using an electrochemiluminescence immunoassay.
Data analysis
For the purpose of this study, nivolumab was deemed to be effective if the lower bound of the 95% confidence interval (CI) calculated using the Clopper–Pearson method was above the threshold response rate of 20%. Assuming a response rate of 60%, a sample size of 15 patients was sufficient to provide a power of 90.5% with a one‐sided significance level of 2.5%. Efficacy and safety data were analyzed using the efficacy and safety analysis sets, respectively. The efficacy analysis set was defined as all patients with cHL (as confirmed by the central pathological review committee or by the pathologists at the study sites) who received at least one dose of nivolumab. The safety analysis set was defined as all patients enrolled in the study who received at least one dose of nivolumab. The 95% CI for response rates was calculated using the Clopper–Pearson method. PFS and OS were estimated using the Kaplan–Meier method. All statistical analyses were performed using the SAS (version 9.3; SAS Institute, Cary, NC, USA).
Results
Patients
The study was started on 18 March 2015, and the data cutoff for these analyses was 16 March 2016. Seventeen patients were enrolled and received nivolumab. One of these patients was diagnosed with B‐cell lymphoma, unclassifiable, with features intermediate between diffuse large B‐cell lymphoma (DLBCL) and cHL (intermediate DLBCL/cHL) by a central pathological review committee and was excluded from the efficacy analyses, but was included in the safety analyses. The baseline characteristics of patients and their prior treatments are summarized in Table 1. The disease subtype at diagnosis (study site) was nodular sclerosis in 8 patients (47.1%), mixed cellularity in 6 (35.3%), lymphocyte depleted in 2 (11.8%), and unclassified in 1 (5.9%). The median age of the patients was 63 years (range 29–83). All of the patients had received prior chemotherapy with a median of 3 (range 2–5) regimens. All of the patients had received brentuximab vedotin, and 5 (29.4%) had previously undergone ASCT. The best overall response (BOR) to brentuximab vedotin was CR in 2 patients, PR in 5, SD in 4 and PD in 5, and was not evaluated in 1 patient.
Table 1.
Baseline characteristics and prior treatments (safety analysis set, N = 17)
| Value | ||
|---|---|---|
| Sex, n (%) | Male | 13 (76.5) |
| Female | 4 (23.5) | |
| Age, n (%) | Median (range) | 63.0 (29‐83) |
| <65 years | 9 (52.9) | |
| ≥65 years | 8 (47.1) | |
| Time since diagnosis, months | Median (range) | 24.0 (8.9‐89.0) |
| ECOG PS, n (%) | 0 | 8 (47.1) |
| 1 | 9 (52.9) | |
| Disease subtype, n (%) | Nodular sclerosis | 8 (47.1) |
| Lymphocyte rich | 0 (0.0) | |
| Mixed cellularity | 6 (35.3) | |
| Lymphocyte depleted | 2 (11.8) | |
| Unclassified | 1 (5.9) | |
| Disease stage at study enrolment, n (%) | II | 4 (23.5) |
| III | 5 (29.4) | |
| IV | 8 (47.1) | |
| B symptoms, n (%) | Absent | 12 (70.6) |
| Present | 5 (29.4) | |
| Relapse or refractory† (to most recent therapy), n (%) | Relapse | 1 (5.9) |
| Refractory | 16 (94.1) | |
| Number of prior chemotherapy regimens | Median (range) | 3 (2–5) |
| Prior brentuximab vedotin | 17 (100.0) | |
| BOR to brentuximab vedotin, n (%) | CR | 2 (11.8) |
| PR | 5 (29.4) | |
| SD | 4 (23.5) | |
| PD | 5 (29.4) | |
| Not evaluated | 1 (5.9) | |
| Prior ASCT, n (%) | 5 (29.4) | |
| BOR to ASCT, n (%) | CR | 3 (60.0) |
| PR | 1 (20.0) | |
| SD | 1 (20.0) | |
| Prior radiotherapy, n (%) | 9 (52.9) |
Data are presented as the n (%), unless otherwise indicated. ASCT, autologous stem cell transplantation; BOR, best overall response; CR, complete remission; ECOG PS, Eastern Cooperative Oncology Group performance status; PD, progressive disease; PR, partial remission; SD, stable disease. †Relapse indicates best response of complete remission to the most recent prior therapy, and refractory indicates best response of partial remission, stable disease, or progressive disease to the most recent prior therapy.
At the time of data cutoff, 11 patients were still receiving nivolumab and 6 patients had discontinued treatment. Reasons for treatment discontinuation were AE (2 patients, including 1 patient with interstitial lung disease), PD (2 patients) and no dose of nivolumab for more than 6 weeks after the nearest time point of dosing (2 patients). The median (range) number of doses, number of cycles, duration of treatment and relative dose intensity were 15 (4–23), 16 (4–24), 7.0 months (1.4–10.6 months) and 91.7% (79.7–101.0%), respectively.
Tumor responses and survival
In the efficacy analysis set (n = 16), the centrally assessed ORR was 81.3% (95% CI 54.4–96.0%; 13/16 patients; Table 2), comprising CR in 4 patients (25.0%) and PR in 9 patients (56.3%). The median time to response was 8.0 weeks (range 7.1–35.9 weeks). Interestingly, the BOR in the patient with intermediate DLBCL/cHL was CR, and this patient was still receiving nivolumab at the data cutoff. Of the 4 patients whose BOR to previous brentuximab vedotin treatment was PD, the BOR induced by nivolumab was CR in 2 patients and PR in 2 patients. Because the follow‐up duration was short, the median duration of response could not be calculated.
Table 2.
Tumor responses and survival rates (efficacy analysis set, N = 16)
| Centrally assessed | Investigator assessed | |
|---|---|---|
| Tumor response | ||
| CR, n (%) | 4 (25.0) | 3 (18.8) |
| PR, n (%) | 9 (56.3) | 7 (43.8) |
| SD, n (%) | 1 (6.3) | 3 (18.8) |
| PD, n (%) | 1 (6.3) | 3 (18.8) |
| Not evaluable | 1 (6.3) | 0 (0.0) |
| ORR (CR + PR), % (95% CI) | 81.3 (54.4–96.0) | 62.5 (35.4–84.8) |
| Progression‐free survival (centrally assessed) | ||
| Rate at 6 months, % (95% CI) | 60.0 (31.8–79.7) | |
| Median (range), months | NR (0.0†–11.0†) | |
| Overall survival | ||
| Rate at 6 months, % | 100 | |
| Time to response (centrally assessed) | ||
| Responders, n (%) | 13 | |
| Median (range), weeks | 8.0 (7.1–35.9) | |
| Duration of response (centrally assessed) | ||
| Ongoing responders‡, n (%) | 9 (69.2) | |
| Median (range), months | NR (0.0†–9.2†) | |
CI, confidence interval; CR, complete remission; NR, not reached; ORR, objective response rate; PD, progressive disease; PR, partial remission; SD, stable disease. †Censored value. ‡Responders still showing a response at the time of data cutoff.
The tumor response of CR or PR was sustained in 9 out of 13 patients at the data cutoff; the remaining 4 patients experienced PD (Fig. 1). The tumor response of PR in 2 patients was sustained after discontinuation of nivolumab administration. Another patient achieved PR after discontinuation of nivolumab administration. Five patients were treated beyond progression at the data cutoff. Five patients had B symptoms at baseline (Table 1). In the 4 patients included in the efficacy analysis set, the B symptoms disappeared after administration of nivolumab in all 4 patients. The median time to disappearance of B symptoms was 8.1 weeks (range 2.3–8.1 weeks). B symptoms did not occur during treatment with nivolumab in any of the 12 patients without these symptoms at baseline.
Figure 1.
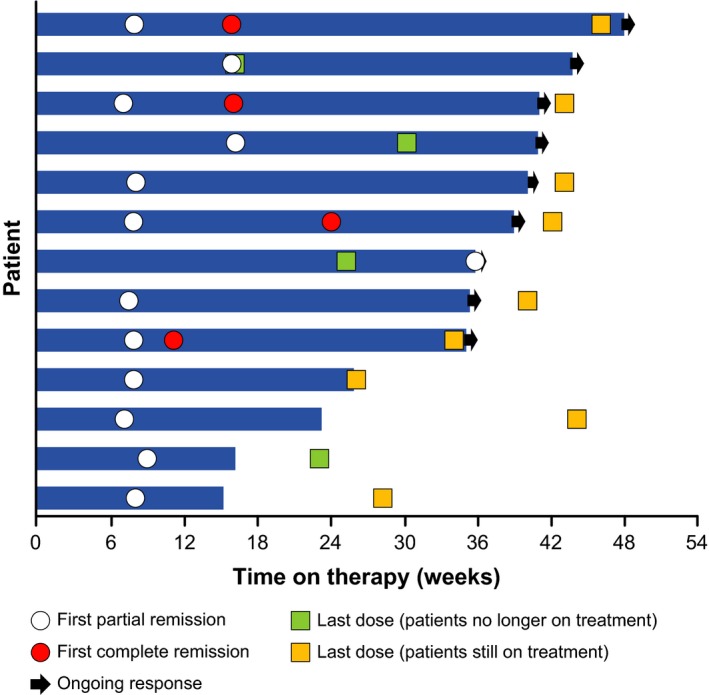
Tumor responses in individual patients classified according to the Revised Response Criteria for Malignant Lymphoma (evaluable patients, N = 13; efficacy data as assessed by a central review committee).
Figure 2 shows Kaplan–Meier plots of PFS for a median follow‐up of 9.8 months (range 6.0–11.1 months). Because the follow‐up duration was short, the median PFS and OS were not reached at the time of the database cutoff. The PFS and OS rates at 6 months were 60.0% (95% CI 31.8–79.7%) and 100%, respectively (Table 2).
Figure 2.
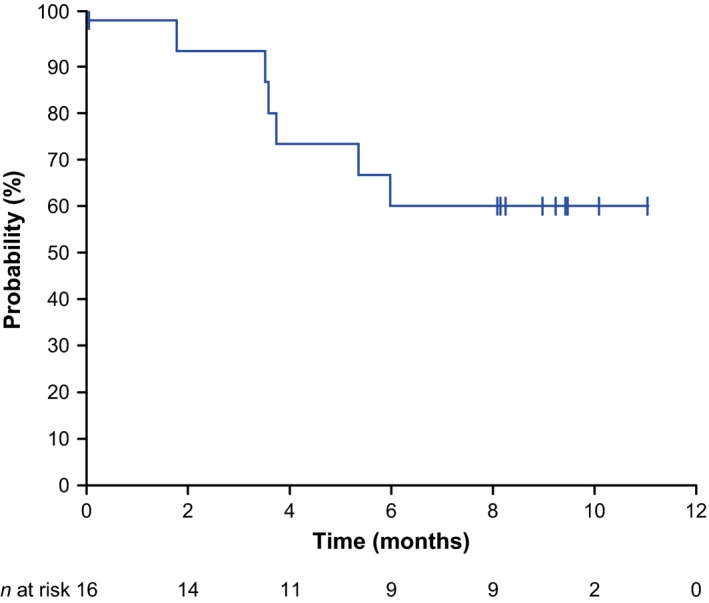
Kaplan–Meier plots of progression‐free survival (efficacy analysis set, N = 16; efficacy data as assessed by a central review committee).
Tumor dimensions
Figure 3(a) shows a waterfall plot of tumor size, as assessed by the central review committee, in the 16 patients included in the efficacy analysis set. As indicated in this figure, the tumor shrank in 14 patients (87.5%), but not in 1 patient. The tumor size could not be assessed in 1 patient. Figure 3(b) shows the change in the sum of the diameters of the target lesion, as assessed by the central review committee. This figure shows that the tumor shrank in almost all of the patients who received nivolumab, and this shrinkage continued long term.
Figure 3.
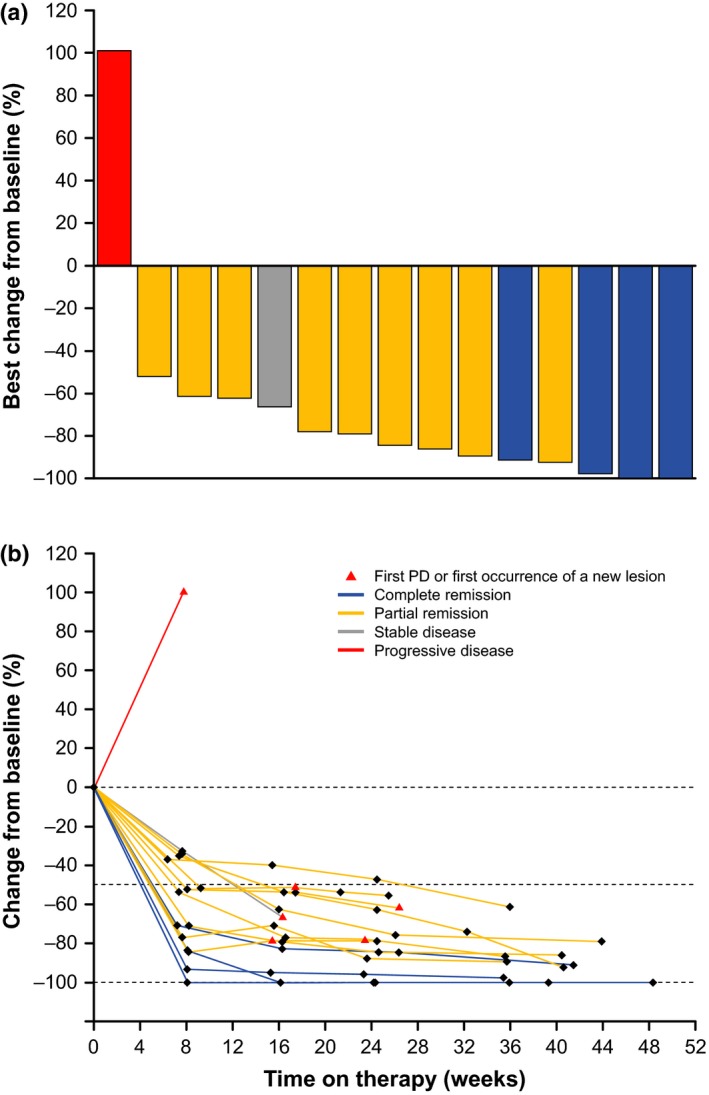
Waterfall plot (a) and transition (b) of the maximum rate of change in the sum of the products of the diameters of the target lesion (evaluable patients, N = 15; efficacy data as assessed by a central review committee). Blue = complete remission; grey = stable disease; red = progressive disease; yellow = partial remission. PD, progressive disease.
Anti‐nivolumab antibody detection
The anti‐nivolumab antibody test at baseline was positive in 1 patient; however, none of the patients had received nivolumab before the study. None of the remaining 16 patients were positive for anti‐nivolumab antibodies at any time during the administration of nivolumab.
Safety
Table 3 shows the AE in the safety analysis set, which comprised all 17 treated patients. All of the patients experienced 1 or more AE, the most common AE being pyrexia (7 [41.2%] of 17 patients), pruritus (6 [35.3%]), rash (6 [35.3%]) and hypothyroidism (5 [29.4%]). The majority of these were grade 1 or 2. Four patients (23.5%) experienced grade 3 or 4 AE, which included anemia, lymphopenia, thrombocytopenia, pyrexia, hepatic function abnormal, pneumonia, hyponatremia, fulminant type 1 diabetes mellitus, interstitial lung disease and rash (each 1 patient, 5.9%). There were no deaths.
Table 3.
Adverse events with an incidence of ≥10% and immune‐related adverse events (safety analysis set, N = 17)
| All Grade | Grade 3–4 | |||
|---|---|---|---|---|
| n | % | n | % | |
| AEs in ≥10% of patients | ||||
| Overall | 17 | 100 | 4 | 23.5 |
| Pyrexia | 7 | 41.2 | 1 | 5.9 |
| Pruritus | 6 | 35.3 | ||
| Rash | 6 | 35.3 | 1 | 5.9 |
| Hypothyroidism | 5 | 29.4 | ||
| Headache | 4 | 23.5 | ||
| Fatigue | 3 | 17.6 | ||
| Nasopharyngitis | 3 | 17.6 | ||
| Back pain | 2 | 11.8 | ||
| Cataract | 2 | 11.8 | ||
| Constipation | 2 | 11.8 | ||
| Dermal cyst | 2 | 11.8 | ||
| Dermatitis acneiform | 2 | 11.8 | ||
| Diarrhea | 2 | 11.8 | ||
| Dizziness | 2 | 11.8 | ||
| Hepatic function abnormal | 2 | 11.8 | 1 | 5.9 |
| Hyponatremia | 2 | 11.8 | 1 | 5.9 |
| Malaise | 2 | 11.8 | ||
| Myalgia | 2 | 11.8 | ||
| Edema | 2 | 11.8 | ||
| Upper respiratory tract inflammation | 2 | 11.8 | ||
| Categories of immune‐related AE | ||||
| Skin disorders | 8 | 47.1 | 1 | 5.9 |
| Endocrine disorders | 6 | 35.3 | 1 | 5.9 |
| Gastrointestinal disorders | 3 | 17.6 | 0 | 0 |
| Hepatic disorders | 2 | 11.8 | 0 | 0 |
| Pulmonary disorders | 1 | 5.9 | 1 | 5.9 |
| Hypersensitivity and infusion reactions | 1 | 5.9 | 0 | 0 |
| Renal disorders | 0 | 0 | 0 | 0 |
AE, adverse event.
Six serious AE (pyrexia, hepatic function abnormal, hyponatremia, fulminant type 1 diabetes mellitus, interstitial lung disease and rash [each 1 patient, 5.9%]) occurred in 3 patients. All of the serious AE were judged to be drug‐related. Of these, interstitial lung disease and rash led to treatment discontinuation. Nivolumab was also discontinued in 1 patient owing to grade 2 peripheral neuropathy; this patient experienced peripheral neuropathy in the previous treatment with brentuximab vedotin, which was not classified as serious.
Most immune‐related AE reported were of grade 1 or 2. Grade 3 or 4 immune‐related AE were rash (skin disorder), fulminant type 1 diabetes mellitus (endocrine disorder) and interstitial lung disease (pulmonary disorder) in 1 patient each.
The treatment was temporarily withdrawn with a delay in subsequent doses in 7 patients (41.2%) because of AE (lymphopenia, thrombocytopenia, enterocolitis, fatigue, pyrexia, hepatic function abnormal, bronchitis, nasopharyngitis, pharyngitis, pneumonia, lung infection, hyponatremia, dermal cyst and rash [1 patient each, 5.9%]).
Discussion
Here, we report the results of a multicenter phase II trial of nivolumab in Japanese patients with relapsed or refractory cHL who had previously received brentuximab vedotin. The centrally assessed ORR was 81.3%, with CR or PR in 4 and 9 patients, respectively. Of these, 9 patients continued to show a tumor response at the data cutoff.
The response rate and other efficacy outcomes determined in this trial were generally comparable with those of two prior studies (CheckMate 03917 and CheckMate 20519) in the USA, Canada and Europe. In CheckMate 039, a phase I study of nivolumab in 23 patients with relapsed or refractory cHL,17 the ORR was 87% (95% CI: 66–97%), with CR in 4 patients and PR in 16 patients; the remaining 3 patients had SD. The phase II CheckMate 205 study19 enrolled 80 patients with cHL who had received ASCT and brentuximab vedotin. The ORR was 66% (95% CI: 54.8–76.4%), with CR and PR rates of 9 and 58%, respectively. Meanwhile, 62% of patients showed a continuing response at the data cutoff. Our results and those of prior studies (CheckMate 039, CheckMate205) suggest that nivolumab is an effective treatment option for patients with relapsed or refractory cHL. The cHL was the nodular sclerosis type in 22 out of 23 patients enrolled in CheckMate 039, while patients enrolled in the present Japanese study had various histopathologic subtypes. The present results imply that nivolumab is effective in various subtypes of cHL. Our results are also notable because nearly half of the patients were elderly, with older patients often having a poor prognosis or being intolerant to conventional chemotherapy regimens.20, 21
Considering that nivolumab is likely to be used in patients who experience disease progression despite treatment with brentuximab vedotin, it is also important to assess the outcomes of nivolumab in these patients. Of the 4 patients whose BOR to brentuximab vedotin was PD, the BOR to nivolumab was CR or PR in 2 patients each. Therefore, nivolumab may be effective in patients whose disease is refractory to brentuximab vedotin.
Because the majority of patients were still receiving nivolumab without disease progression at the data cutoff, it was not possible to calculate the median PFS, OS, or duration of response. The median duration of treatment was 7.0 months at the data cutoff. Future analyses are needed to estimate the median PFS, OS or duration of response.
In the present study, nivolumab had an acceptable safety profile. Although AE occurred in all patients, most AE were of grade 1 or 2 and were manageable. Although the frequency of grade ≥3 AE was not high, 10 grade ≥3 AE occurred in 4 patients (23.5%) and 6 AE in 3 patients (17.6%) were considered serious (pyrexia, hepatic function abnormal, hyponatremia, fulminant type 1 diabetes mellitus, interstitial lung disease and rash). Two patients who experienced serious AE (interstitial lung disease or rash) discontinued treatment. Most of the serious AE were considered to be immune‐related AE. Most immune‐related AE in the present study were manageable through the administration of corticosteroids. However, the treatment of fulminant type 1 diabetes mellitus required long‐term insulin replacement therapy.22 Fulminant type 1 diabetes mellitus was not reported in CheckMate 039 and CheckMate 205; however, fulminant type 1 diabetes mellitus has been reported in several patients (with melanoma, lung cancer and renal cell carcinoma) treated with nivolumab or pembrolizumab,23, 24, 25, 26 and might be related to inappropriate activation of T cells. Therefore, physicians should be aware of the risk of this AE in some patients, such as those with a family history of type 1 diabetes mellitus or a human leukocyte antigen haplotype associated with fulminant type 1 diabetes mellitus. Interstitial lung disease is another serious AE that occurred in our study. Pneumonitis was reported in 2 (3%) patients (1 grade 2 and 1 grade 3) in CheckMate 205. Both cases were judged to be drug‐related and both resolved with corticosteroid treatment. Physicians should be vigilant for these AE, which are characteristic of anti‐PD‐1 antibody therapy.
The overall safety profile of nivolumab in the present study was consistent with those reported in CheckMate 039 and CheckMate 205. Considering these findings as well as the lack of clinically meaningful changes in vital signs, physical findings and laboratory tests, nivolumab is likely to be tolerable in patients with relapsed or refractory cHL.
Brentuximab vedotin is regarded as an effective drug for treating cHL, and it was associated with high ORR in several studies.6, 7 For example, ORR of 75 and 67% were reported in a multinational phase II study6 and in a phase I/II study in Japan,7 respectively. However, the median PFS was relatively short, being 5.6 months in the multinational study and 11.1 months in the Japanese study, and a sizeable proportion of patients in both studies experienced disease relapse or had refractory disease. Considering these relatively unfavorable outcomes, there is a clear need for developing alternative treatments for cHL.
Several limitations of this study warrant mention. First, we were unable to determine the median PFS and median OS based on the available data at the data cutoff. However, this is unsurprising, because most patients were still receiving nivolumab at the data cutoff, and their response was maintained at this time. Future analyses will be conducted once additional data are available. Another possible limitation is the relatively small number of patients. However, the relatively low prevalence of cHL in Japan may make it difficult to enroll a larger number of patients. Finally, this study had an open‐label, non‐randomized design, but this is common in phase II studies in this setting. Randomized controlled trials and studies enrolling patients after fewer treatment regimens (e.g. before brentuximab vedotin or ASCT) may be valuable to help clarify when nivolumab should be administered in the treatment of cHL.
In conclusion, the present results indicate that nivolumab is a potentially effective treatment option for Japanese patients with relapsed or refractory cHL previously treated with brentuximab vedotin, and has an acceptable safety profile. Nivolumab was also effective in patients with a variety of cHL subtypes. Further investigation is warranted to determine the exact role of nivolumab in the treatment of cHL.
Disclosure Statement
D. Maruyama has received honoraria from Takeda Pharmaceutical and Janssen Pharmaceutical. K. Hatake has received honoraria from Ono Pharmaceutical and Kyowa Hakko Kirin, and research funding from Takeda Pharmaceutical. T. Kinoshita has received research funding from Ono Pharmaceutical. K. Ando has received research funding from Renascience, Bristol‐Myers Squibb K.K. and Kyowa Hakko Kirin. Y. Shirasugi has received honoraria from Bristol‐Myers Squibb K.K. and Novartis Pharma K.K. K. Tobinai has received honoraria from Takeda Pharmaceutical and research funding from Takeda Pharmaceutical, Ono Pharmaceutical and MSD K.K. All other authors have no conflicts of interest to declare.
Acknowledgments
The authors thank all of the patients who participated in this study, and their families, as well as all investigators, physicians, nurses and clinical research coordinators who helped with this study. The authors are grateful to pathologists Koichi Ohshima (Kurume University Hospital, Fukuoka) and Jun‐Ichi Tamaru (Saitama Medical Center, Saitama) for their support in the Central Pathological Review Committee; and to Kazuo Tamura (Fukuoka University Hospital, Fukuoka), Kunihiro Tsukasaki (National Cancer Center Hospital East, Chiba), Kenichi Ishizawa (Yamagata University Faculty of Medicine, Yamagata), Terufumi Kato (Kanagawa Cancer Center, Yokohama) and Masashi Takahashi (Yujinkai Yujin‐Yamazaki Hospital, Shiga) for reviewing the clinical data as members of the Efficacy and Safety Evaluation Committee. This study was sponsored by Ono Pharmaceutical. The authors thank Nicholas D. Smith of Edanz Group Japan K.K. for medical writing support, which was funded by Ono Pharmaceutical.
Cancer Sci 108 (2017) 1007–1012
Funding Information
Ono Pharmaceutical Co., Ltd.
Clinical trial registration number: JapicCTI‐142755 (Japan Pharmaceutical Information Center)
References
- 1. Makita S, Maruyama D, Maeshima AM et al Clinical features and outcomes of 139 Japanese patients with Hodgkin lymphoma. Int J Hematol 2016; 104: 233–44. [DOI] [PubMed] [Google Scholar]
- 2. Ogura M, Itoh K, Kinoshita T et al Phase II study of ABVd therapy for newly diagnosed clinical stage II–IV Hodgkin lymphoma: Japan Clinical Oncology Group study (JCOG 9305). Int J Hematol 2010; 92: 713–24. [DOI] [PubMed] [Google Scholar]
- 3. Kewalramani T, Nimer SD, Zelenetz AD et al Progressive disease following autologous transplantation in patients with chemosensitive relapsed or primary refractory Hodgkin's disease or aggressive non‐Hodgkin's lymphoma. Bone Marrow Transplant 2003; 32: 673–9. [DOI] [PubMed] [Google Scholar]
- 4. Alley SC, Okeley NM, Senter PD. Antibody‐drug conjugates: Targeted drug delivery for cancer. Curr Opin Chem Biol 2010; 14: 529–37. [DOI] [PubMed] [Google Scholar]
- 5. Alperovich A, Younes A. Targeting CD30 using brentuximab vedotin in the treatment of Hodgkin lymphoma. Cancer J 2016; 22: 23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Younes A, Gopal AK, Smith SE et al Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol 2012; 30: 2183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ogura M, Tobinai K, Hatake K et al Phase I/II study of brentuximab vedotin in Japanese patients with relapsed or refractory CD30‐positive Hodgkin's lymphoma or systemic anaplastic large‐cell lymphoma. Cancer Sci 2014; 105: 840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheah CY, Chihara D, Horowitz S et al Patients with classical Hodgkin lymphoma experiencing disease progression after treatment with brentuximab vedotin have poor outcomes. Ann Oncol 2016; 27: 1317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Homet Moreno B, Ribas A. Anti‐programmed cell death protein‐1/ligand‐1 therapy in different cancers. Br J Cancer 2015; 112: 1421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jezeršek Novaković B. Checkpoint inhibitors in Hodgkin's lymphoma. Eur J Haematol 2016; 96: 335–43. [DOI] [PubMed] [Google Scholar]
- 11. Sunshine J, Taube JM. PD‐1/PD‐L1 inhibitors. Curr Opin Pharmacol 2015; 23: 32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen BJ, Chapuy B, Ouyang J et al PD‐L1 expression is characteristic of a subset of aggressive B‐cell lymphomas and virus‐associated malignancies. Clin Cancer Res 2013; 19: 3462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Green MR, Monti S, Rodig SJ et al Integrative analysis reveals selective 9p24.1 amplification, increased PD‐1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B‐cell lymphoma. Blood 2010; 116: 3268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paydas S, Bağir E, Seydaoglu G, Ercolak V, Ergin M. Programmed death‐1 (PD‐1), programmed death‐ligand 1 (PD‐L1), and EBV‐encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol 2015; 94: 1545–52. [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto R, Nishikori M, Kitawaki T et al PD‐1‐PD‐1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood 2008; 111: 3220–4. [DOI] [PubMed] [Google Scholar]
- 16. Roemer MG, Advani RH, Ligon AH et al PD‐L1 and PD‐L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 2016; 34: 2690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ansell SM, Lesokhin AM, Borrello I et al PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015; 372: 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheson BD, Pfistner B, Juweid ME et al Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–86. [DOI] [PubMed] [Google Scholar]
- 19. Younes A, Santoro A, Shipp M et al Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem‐cell transplantation and brentuximab vedotin: A multicentre, multicohort, single‐arm phase 2 trial. Lancet Oncol 2016; 17: 1283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Böll B, Görgen H, Fuchs M et al ABVD in older patients with early‐stage Hodgkin lymphoma treated within the German Hodgkin Study Group HD10 and HD11 trials. J Clin Oncol 2013; 31: 1522–9. [DOI] [PubMed] [Google Scholar]
- 21. Stamatoullas A, Brice P, Bouabdallah R et al Outcome of patients older than 60 years with classical Hodgkin lymphoma treated with front line ABVD chemotherapy: Frequent pulmonary events suggest limiting the use of bleomycin in the elderly. Br J Haematol 2015; 170: 179–84. [DOI] [PubMed] [Google Scholar]
- 22. Munakata W, Ohashi K, Yamauchi N, Tobinai K. Fulminant type I diabetes mellitus associated with nivolumab in a patient with relapsed classical Hodgkin lymphoma. Int J Hematol 2017; 105: 383–6. [DOI] [PubMed] [Google Scholar]
- 23. Hughes J, Vudattu N, Sznol M et al Precipitation of autoimmune diabetes with anti‐PD‐1 immunotherapy. Diabetes Care 2015; 38: e55–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin‐Liberal J, Furness AJ, Joshi K, Peggs KS, Quezada SA, Larkin J. Anti‐programmed cell death‐1 therapy and insulin‐dependent diabetes: A case report. Cancer Immunol Immunother 2015; 64: 765–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyoshi Y, Ogawa O, Oyama Y. Nivolumab, an anti‐programmed cell death‐1 antibody, induces fulminant type 1 diabetes. Tohoku J Exp Med 2016; 239: 155–8. [DOI] [PubMed] [Google Scholar]
- 26. Okamoto M, Okamoto M, Gotoh K et al Fulminant type 1 diabetes mellitus with anti‐programmed cell death‐1 therapy. J Diabetes Investig 2016; 7: 915–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


