Abstract
Promising antitumor activities of nivolumab, a fully humanized IgG4 inhibitor antibody against the programmed death‐1 protein, were suggested in previous phase 1 studies. The present phase 2, single‐arm study (JAPIC‐CTI #111681) evaluated the antitumor activities of nivolumab and explored its predictive correlates in advanced melanoma patients at 11 sites in Japan. Intravenous nivolumab 2 mg/kg was given repeatedly at 3‐week intervals to 35 of 37 patients enrolled from December 2011 to May 2012 until they experienced unacceptable toxicity, disease progression, or complete response. Primary endpoint was objective response rate. Serum levels of immune modulators were assessed at multiple time points. As of 21 October 2014, median response duration, median progression‐free survival, and median overall survival were 463 days, 169 days, and 18.0 months, respectively. The overall response rate and 1‐ and 2‐year survival rates were 28.6%, 54.3%, and 42.9%, respectively. Thirteen patients remained alive at the end of the observation period and no deaths were drug related. Grade 3–4 drug‐related adverse events were observed in 31.4% of patients. Pretreatment serum interferon‐γ, and interleukin‐6 and ‐10 levels were significantly higher in the patients with objective tumor responses than in those with tumor progression. In conclusion, giving repeated i.v. nivolumab had potent and durable antitumor effects and a manageable safety profile in advanced melanoma patients, strongly suggesting the usefulness of nivolumab for advanced melanoma and the usefulness of pretreatment serum cytokine profiles as correlates for predicting treatment efficacy.
Keywords: Antibody therapy, biomarker, check‐point inhibitor, clinical trial, programmed cell death‐1
As most cancer cells have huge numbers of genetic alterations that directly or indirectly provide plenty of tumor‐associated antigens that can be recognized by host immune cells,1, 2 they need to be capable of escaping elimination by the host immune system.3 Consequently, blockade of the mechanisms involved in these escape systems could lead to promotion of effective immune responses against cancer. This hypothesis was proven for the first time in 2010 in a double‐blind, phase 3 clinical trial in cancer patients.4 Specifically, the study showed that treatment with an antibody against cytotoxic T‐lymphocyte antigen 4 (CTLA4), an immune checkpoint receptor involved in T‐cell suppression through B7 ligation, significantly improved the survival of patients with metastatic melanoma. These results have warranted the development of other types of checkpoint inhibitors, including anti‐programmed death‐1 (PD‐1) antibodies.
PD‐1 is another immune checkpoint receptor that is expressed on activated T cells5 and provides inhibitory signals toward T‐cell activation when engaged by its ligands, PD‐1 ligand 1 (PD‐L1) and PD‐1 ligand 2 (PD‐L2). Physiological activation of the PD‐1 system mediates immune tolerance and mitigates collateral tissue damage.6, 7 The PD‐l pathway has been suggested to play important roles in the escape mechanisms of tumor cells, and blockade of the PD‐1 system was reported to significantly suppress tumor growth in multiple mouse tumor models.8 Based on the information regarding PD‐1 blockade described above, nivolumab (ONO‐4538/BMS‐936558/MDX‐1106) was developed as a potential anticancer drug. Nivolumab is a fully humanized monoclonal IgG4 antibody antagonistic to PD‐1 with high affinity (KD: 2.6 nM/L) and has no other Fc function: antibody‐dependent cellular cytotoxicity and complement‐dependent cytotoxicity. Safety profiles and promising antitumor activities in phase 1 clinical trials have been reported in patients with multiple types of cancer, including melanoma, non‐small cell lung cancer, and renal cell cancer.9
To evaluate the potency of repeated i.v. injections of nivolumab, we carried out a phase 2, single‐arm study in advanced melanoma patients. We also examined the immunological profiles of the patients to identify characteristic factors that might be used as predictive correlates for antitumor responses with nivolumab treatment.
Materials and Methods
Study design and enrolled patients
The present study was a phase 2 trial sponsored by (ONO Pharmaceutical Ltd, Osaka, Osaka, Japan). The primary objective of this multiple‐site, open‐label, single‐arm study was to assess the overall response rate (ORR) to nivolumab (ONO‐4538/BMS‐936558), a fully humanized IgG4‐antagonizing monoclonal antibody against PD‐1, in Japanese patients with malignant melanoma. Secondary objectives included overall survival (OS), progression‐free survival (PFS), immune‐related ORR defined by proposed immune‐related Response Evaluation Criteria in Solid Tumors (RECIST) criteria,10 safety (adverse events), pharmacokinetic parameters, and potential biomarkers.
Patients were determined to be eligible if they had recurrent or unresectable stage III or IV melanoma with a history of dacarbazine treatment failure. Other eligibility criteria included age ≥20 years, and Eastern Cooperative Oncology Group performance status score of 0 or 1. Key exclusion criteria were melanomas with primary tumors in the esophagus or rectum, or with tumors in the brain or meninges (primary/metastatic).
This trial was approved by the local ethics committees of all participating centers, and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent before enrolment. Nivolumab 2 mg/kg was given as an i.v. infusion every 3 weeks in each 6‐week treatment cycle. Responses were assessed by the investigators after each treatment cycle solely to determine whether the patients could receive the next cycle of treatment. Clinical responses were assessed according to RECIST version 1.1 (RECIST 1.1) and immune‐related RECIST, and the severities of adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE 4.0, http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm). Patients received treatment unless they had a confirmed complete response (CR), immune‐related CR, or immune‐related progressive disease, unacceptable adverse effects, or had withdrawn their consent. Patients with immune‐related progressive disease could receive a treatment cycle beyond the apparent initial disease progression only if they were clinically stable.
Tumor responses, the primary endpoint of the trial, were assessed by an independent radiology review committee (IRC) consisting of three experts in a control office independent from the local trial sites according to the RECIST 1.1 criteria and designated “confirmed tumor responses”. Only confirmed tumor responses were taken into account when examining the antitumor efficacy of the treatment.
Assessment of immunological characteristics of patients to identify potential biomarkers
Venous blood was drawn from the patients at multiple time points, including just before giving nivolumab, and analyzed for peripheral blood lymphocyte (PBL) surface markers and for serum cytokine levels using the Human Cytokine Antibody Array (Procarta Cytokine Assay Kit Human 18‐plex; Affymetrix, Santa Clara, CA, USA). Results were analyzed for any significant correlations with the outcomes of nivolumab treatment. The measured surface markers on PBL included cluster of differentiation (CD) 3, CD4, CD8, CD14, CD25, CD45RA, CD127, PD‐1, PD‐L1, and C‐C chemokine receptor (CCR) 7. Regarding PD‐1, both the expression and occupancy were measured using the methods reported previously.11 The panel of measured cytokines included interleukin (IL)‐6, IL‐7, IL‐8, IL‐10, IL‐12p70, IL‐22, IL‐23p19, interferon (IFN)‐α2, IFN‐γ, tumor necrosis factor (TNF)‐β, monocyte chemoattractant protein‐1, stromal cell‐derived factor 1A, interferon gamma‐induced protein 10 (IP10), monokine induced by interferon gamma (MIG), granulocyte‐macrophage colony‐stimulating factor, platelet‐derived growth factor, hepatocyte growth factor, and vesicular endothelial growth factor‐A.
Statistical analysis
Primary endpoint of the study was the ORR assessed by the IRC. In the study, the expected ORR was set at 30.0%, the threshold ORR was assumed to be 12.5%, and the number of patients required to ensure a statistical power of at least 80.0% by the binomial test (normal approximation) at a one‐sided significance level of 5.0% was calculated to be 29. Considering that approximately 15% of patients would drop out and thus not be evaluated, 35 patients were planned for enrolment.
The primary analysis population for the efficacy endpoints was the full analysis set defined as the group of patients who received at least one dose of nivolumab and met the major eligibility criteria. The analysis population for the safety endpoints was the safety analysis set, defined as the group of patients who have received at least one dose of nivolumab.
We calculated the ORR assessed by the IRC and its two‐sided 90% Wilson confidence interval (CI). We also calculated the median and two‐sided 90% CI for OS and PFS by the Kaplan–Meier method. We tabulated the incidence rates of adverse events reported during the treatment phase. P‐values for biomarker assessments were determined by a t‐test. P‐values for differences in biomarker measurements between pre‐dose and post‐dose were determined by a paired t‐test. Statistical analyses for the examined factors were carried out using SAS ver. 9.2 or later (SAS Institute Inc., Cary, NC, USA). However, statistical analyses for biomarkers were carried out using EXSUS ver. 8.1 (CAC Croit Corporation, Tokyo, Japan), based on SAS ver. 9.4 TS1M (SAS Institute Inc.).
Results
Patients and treatment
Thirty‐seven melanoma patients were enrolled in the trial from December 2011 to May 2012. Of these, 35 (94.6%) patients received nivolumab. Two enrolled patients were not treated because they did not meet the study eligibility criteria before treatment (i.e. one who was human T‐cell leukemia virus type 1‐positive and one who refused to provide consent). As shown in Table 1, all patients were of Asian ethnic origin and had received chemotherapy before entry into the study. BRAF and NRAS mutations were found in seven and two of 21 examined patients, respectively.
Table 1.
Baseline characteristics of patients in the present study
| Characteristic | ||
|---|---|---|
| Age (years) | ||
| Median (range) | 64 (28–79) | |
| <65 | 19 (54.3%) | |
| ≥65 | 16 (45.7%) | |
| Sex | ||
| Male | 12 (34.3%) | |
| Female | 23 (65.7%) | |
| Ethnic origin | ||
| Asian | 35 (100%) | |
| ECOG PS | ||
| 0 | 27 (77.1%) | |
| 1 | 8 (22.9%) | |
| Disease status | ||
| Unresectable Stage IIIC | 2 (5.7%) | |
| Unresectable Stage IV | 9 (25.7%) | |
| Recurrence | 24 (68.6%) | |
| CNS metastasis | 1 (2.9%) | |
| Type of previous treatment | ||
| Chemotherapy | 35 (100.0%) | |
| Molecularly targeted drug | 1 (2.9%) | |
| Immunotherapy | 25 (71.4%) | |
| No. previous systemic treatments | ||
| 1 | 12 (34.3%) | |
| 2 | 13 (37.1%) | |
| >2 | 10 (28.6%) | |
| Previous radiotherapy | 10 (28.6%) | |
| Disease classification (%) | ||
| SSM: superficial spreading melanoma | 8 (22.9%) | |
| NM: nodular melanoma | 5 (14.3%) | |
| ALM: acral lentiginous melanoma | 9 (25.7%) | |
| Unknown (8)/Mucosal (4)/Other (1) | 13 (37.1%) | |
| BRAF and NRAS mutation status | BRAF | NRAS |
| Mutations | 7 (20%) | 2 (5.7%) |
| Wild‐type | 14 (40%) | 19 (54.3%) |
| Unknown | 14 (40%) | 14 (40%) |
| Metastasis stage | ||
| M1c | 20 (57.1%) | |
| M0, M1a, or M1b | 15 (42.9%) | |
Data are n (%) unless otherwise stated. CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status.
Patients received a median of 7.0 doses (interquartile range [IQR]: 4–16 doses) of nivolumab with a median treatment duration of 5.3 months (95% CI: 2.8–7.9 months). Median follow up for IRC‐assessed responses was 7.3 months (IQR: 4–19.6 months) and the median follow up for OS was 18 months (IQR: 6–26.3 months). Among the reasons for discontinuation, disease progression was the most common. After discontinuation of nivolumab treatment, seven (20.0%) patients received subsequent systemic cancer treatment, most commonly dacarbazine. Among the seven patients identified as having BRAF mutations, one patient showed partial response (PR) with nivolumab.
Tumor responses and survival (clinical responses)
According to the IRC, one (2.9%) and nine (25.7%) patients achieved CR and PR, respectively. Meanwhile, 13 (37.1%) and 11 (31.4%) patients were evaluated to have stable disease and progressive disease, respectively. Overall response rate was 28.6% (10/35 patients) (Table 2; Fig. 1a). As of 21 October 2014, median duration of response (Fig. 1b,c), median PFS (Fig. 2a), and median OS (Fig. 2b) were 463.0 days, 169.0 days, and 18.0 months, respectively. The 1‐ and 2‐year survival rates were 54.3% and 42.9%, respectively. Thirteen patients remained alive at the end of the observation. The ORR according to the proposed immune‐related RECIST criteria was 34.3% (12/35 patients). For various reasons, some patients with objective responses continued to respond even after cessation of nivolumab treatment (Fig. 1c).
Table 2.
Best responses to treatment
| N = 35 | |
|---|---|
| Best overall response | |
| Complete response | 1 (2.9%) |
| Partial response | 9 (25.7%) |
| Stable disease | 13 (37.1%) |
| Progressive disease | 11 (31.4%) |
| Not evaluated | 1 (2.9%) |
| Objective response | |
| Percentage of patients (95% CI) | 28.6 (17.9–42.3) |
Data are based on the central review assessment according to the Response Evaluation Criteria in Solid Tumors version 1.1 criteria on 21 October 2014.
Figure 1.
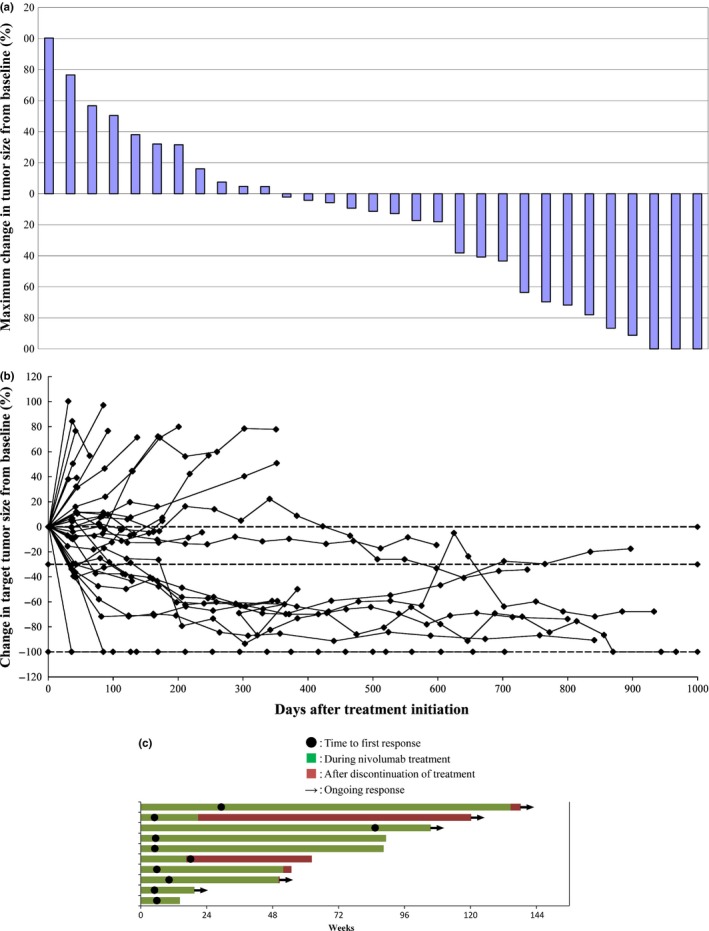
Antitumor activities associated with nivolumab treatment. (a) Maximum reductions in tumor size and other changes. Based on findings of the independent radiology review committee (IRC) assessment in October 2014, we included patients with complete target lesion data, a baseline assessment, and at least one on‐treatment assessment before progression or the start of subsequent treatment (n = 31). The line at −30% indicates the threshold for an objective response according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) criteria. Two of the three patients who showed a 100% reduction in the size of target lesions had non‐target lesions determined to be non‐complete response (CR) and non‐progressive disease. Thus, only one patient was judged to be CR. (b) Changes in target tumor sizes over time. Based on the IRC assessment in October 2014, we included patients with an evaluable response (n = 34) who had a baseline assessment and at least one on‐treatment tumor assessment. Tumor burden was measured as the sum of the longest diameters of the target lesions. (c) Times to and durations of responses (Swimmers plots). The plots show the times to first response and durations of objective response found in patients with partial or complete response (responders) treated with nivolumab according to the RECIST 1.1 criteria.
Figure 2.
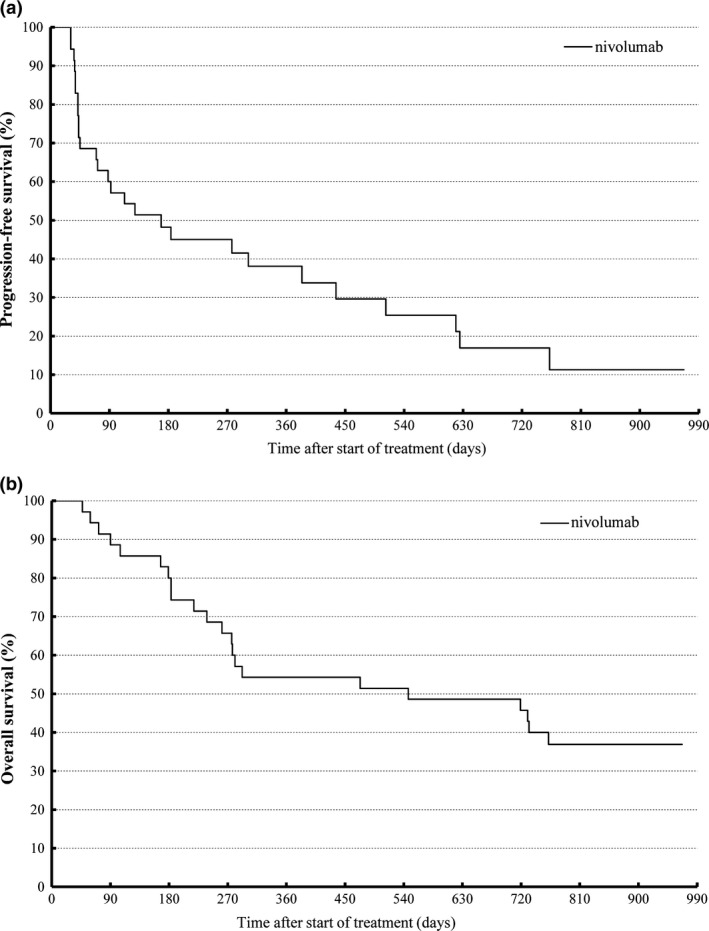
Kaplan–Meier analysis of patient survival. (a) Progression‐free survival (PFS). (b) Overall survival. PFS was based on the assessment by the independent radiology review committee.
Adverse events (safety profile)
Grade 3–4 drug‐related adverse events were observed in 31.4% of patients (Table 3), but there were no drug‐related deaths. Adverse events included diarrhea (grade 3; one patient), nausea (grade 3; one patient), and liver disorders (grade 3 elevation of liver enzyme levels; two patients). One patient experienced grade 2 interstitial lung disease and dropped out from the trial according to the protocol.12 As consolidation of the lung did not improve after cessation of nivolumab treatment, the patient was treated with i.v. steroid. This steroid treatment was successful, with no obvious tumor progression thereafter (Fig. 3).
Table 3.
Treatment‐related adverse events that occurred in at least 5% of all treated patients (excluding laboratory tests)
| Any grade | Grade 3–4a | |
|---|---|---|
| Category | ||
| Drug‐related AE | ||
| Any grade | 85.7% | |
| Grade 3–4 | 31.4% | |
| Endocrine disorders | ||
| Hypothyroidism | 5 (14.3%) | |
| Gastrointestinal disorders | ||
| Diarrhea | 4 (11.4%) | 1 (2.9%) |
| Nausea | 3 (8.6%) | 1 (2.9%) |
| Stomatitis | 2 (5.7%) | |
| General disorders | ||
| Fatigue | 5 (14.3%) | |
| Malaise | 2 (5.7%) | |
| Hepatobiliary disorders | ||
| Liver disorders | 2 (5.7%) | 2 (5.7%) |
| Musculoskeletal and connective tissue disorders | ||
| Muscle spasms | 2 (5.7%) | |
| Nervous system disorders | ||
| Dysgeusia | 2 (5.7%) | |
| Peripheral neuropathy | 2 (5.7%) | |
| Skin and subcutaneous tissue disorders | ||
| Leukoderma | 6 (17.1%) | |
| Pruritus | 11 (31.4%) | |
| Rash | 2 (5.7%) | |
| Rash maculopapular | 2 (5.7%) | |
| Seborrheic dermatitis | 2 (5.7%) | |
| Skin hypopigmentation | 4 (11.4%) | |
All were grade 3. Data are number of events (%), and include all events reported between the first dose and 28 days after the last dose of nivolumab. Grade 3–4 treatment‐related adverse events (excluding laboratory tests) reported in <5% of patients included pneumonia, hyponatremia, and psoriatic arthropathy reported in one patient (2.9%) each.
Figure 3.
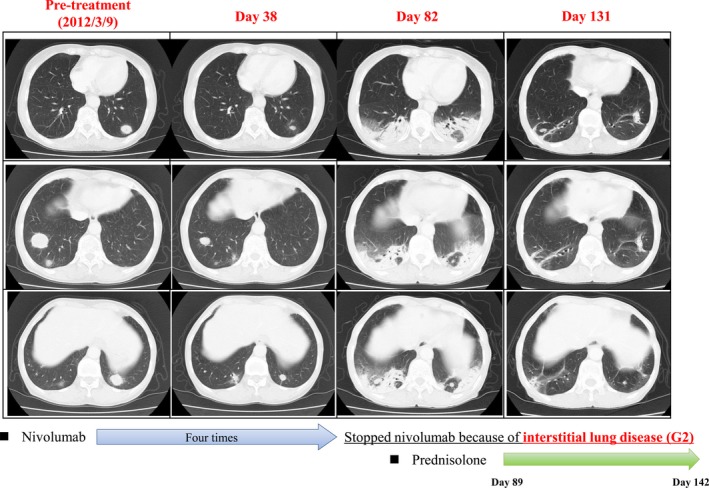
Computed tomography (CT) images of one patient with an objective response (PR) and interstitial lung disease with nivolumab treatment. The patient who had metastatic lung tumors showed a partial response (PR). After four cycles of nivolumab treatment, the patient showed consolidation in a CT scan. This interstitial lung disease was successfully treated by stopping nivolumab treatment and giving i.v. steroid. The tumors did not regrow after the lung disease had resolved. (Some of the images were presented in a previous report by Nakashima and colleagues.12)
Fluctuation of immunological parameters with nivolumab treatment
Figure 4 shows the data for immunological parameters in all treated patients before and after the start of nivolumab treatment. Numbers of all lymphocytes, CD3+ cells, CD4+ cells, and CD8+ cells were significantly lower at 3 days after the start of treatment than before treatment. Ratios of PD‐1+ cells among CD3+, CD4+, and CD8+ cells in peripheral blood mononuclear cells were significantly lower on day 3 and remained at similar levels on day 43 compared with the corresponding ratios before treatment. Serum concentrations of MIG (chemokine [C‐X‐C motif] ligand [CXCL] 9) and IP10 on day 43 were significantly higher compared with concentrations prior to treatment.
Figure 4.
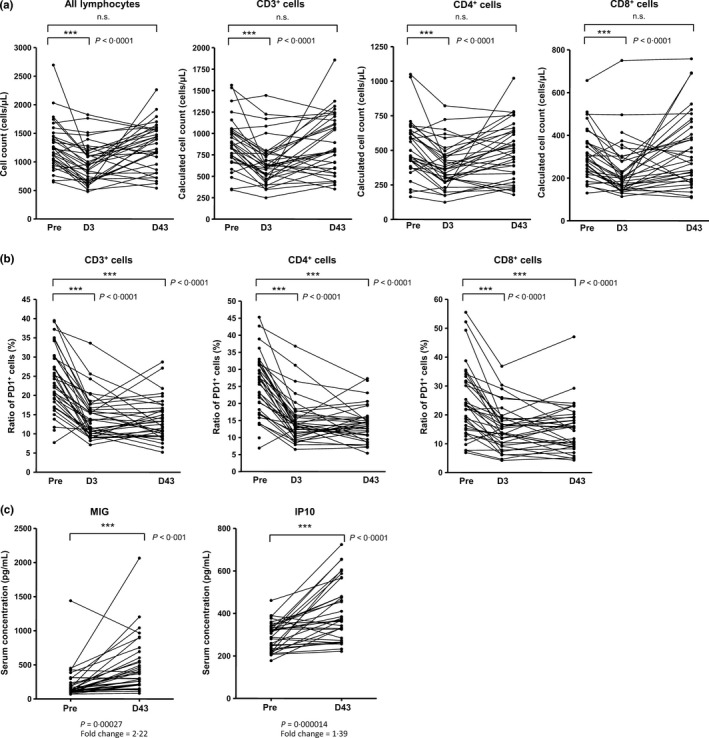
Changes in immunological factors over time associated with giving nivolumab. (a) Lymphocyte counts. The lymphocyte counts and the percentage of lymphocytes that were CD3+, CD4+, and CD8+ were measured at pretreatment, day 3, and day 43. Numbers of CD3+, CD4+, and CD8+ cells were calculated from the percentages of lymphocytes that were CD3+, CD3+CD4+, and CD3+CD8+ (as measured by flow cytometry) and the numbers of lymphocytes. P‐values were calculated by a paired t‐test. (b) Ratios of PD, programmed death (PD)‐1+ cells. Percentage of PD‐1‐expressing CD3+, CD3+CD4+, and CD3+CD8+ T cells at pretreatment, day 3, and day 43 were measured by flow cytometry. P‐values were calculated with a paired t‐test. (c) Cytokine levels in serum. Serum levels of monokine induced by interferon gamma and interferon gamma‐induced protein 10 at pretreatment and day 43 are shown. P‐values were calculated with a paired t‐test. IP10, interferon gamma‐induced protein 10; MIG, monokine induced by interferon gamma.
Potential biomarkers
To search for potential biomarkers that could be useful to predict the responses to nivolumab treatment before therapy, we examined the concentrations of a panel of cytokines in serum samples from the patients immediately before nivolumab treatment and compared these levels between patients with CR or PR (responders) and patients with progressive disease (non‐responders) (Table S1; Fig. 5a). We found that the IFN‐γ, IL‐6, and IL‐10 levels in the responders were significantly higher than those in the non‐responders (P < 0.0001, P = 0.0007 and P < 0.0001 respectively). Furthermore, the concentrations of these cytokines were significantly correlated with one another (Fig. 5b).
Figure 5.
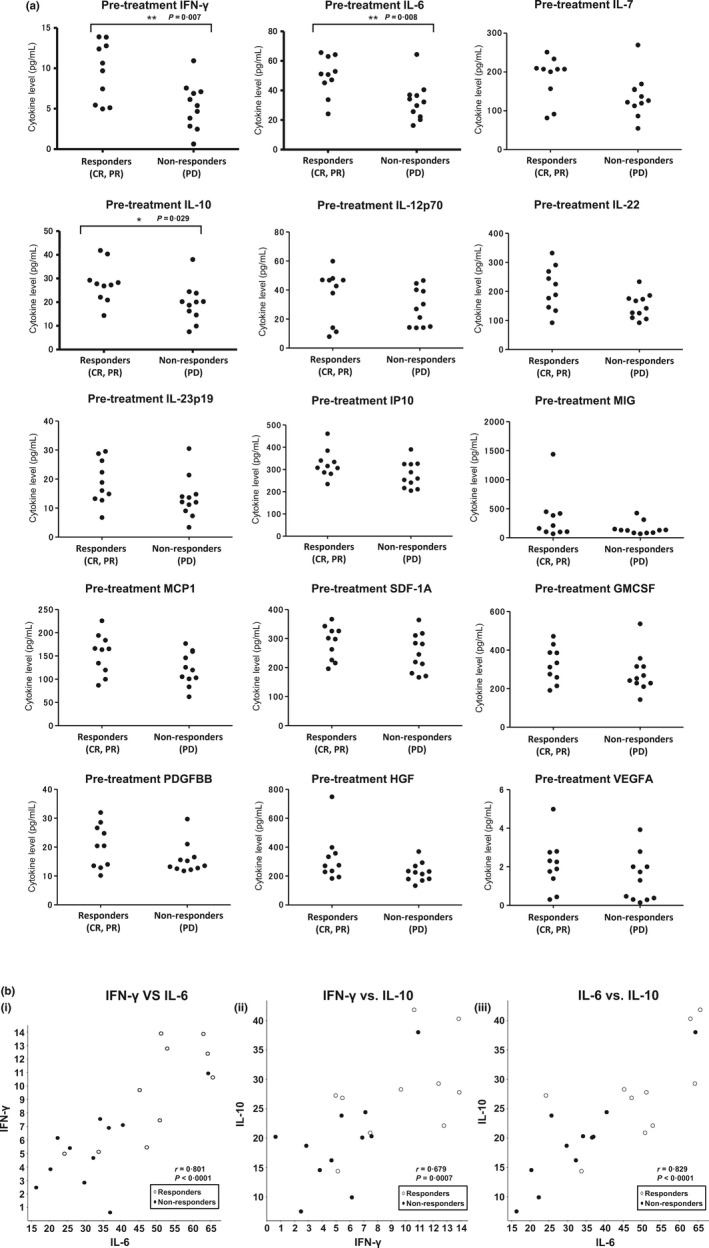
Relationships between serum cytokine levels before treatment and antitumor responses associated with nivolumab treatment. (a) Serum levels of cytokines before treatment were plotted for patients with complete or partial response (responders) and patients with progressive disease (PD; non‐responders). P‐values were determined with a t‐test. SDF‐1A, stromal cell‐derived factor‐1A; GMCSF, granulocyte‐macrophage colony‐stimulating factor; HGF, hepatocyte growth factor; IFN, interferon; IL, interleukin; IP10, interferon gamma‐induced protein 10; MCP1, Monocyte Chemotactic Protein‐1; MIG, monokine induced by interferon gamma; PDGF, platelet‐derived growth factor; VEGFA, vesicular endothelial growth factor‐A. (b) Correlation coefficients for the levels of the cytokines of interest. Cytokine levels before treatment were plotted for responders and non‐responders. (i) IFN‐γ vs IL‐6; (ii) INF‐γ vs IL‐10; (iii) IL‐6 vs IL‐10, and vesicular endothelial growth factor‐A.
Discussion
We conducted a phase 2 clinical trial of nivolumab monotherapy for treatment of metastatic melanoma in Japan. The results of the trial showed that nivolumab treatment is associated with significant antitumor responses and manageable safety profiles in patients with metastatic melanoma, including tumors of mucosal origin. These results are similar to the previously reported clinical studies in the USA and Western countries on the mostly Caucasian patients who had lower percentages of mucosal melanoma.13 The antitumor responses were mostly durable in the patients with objective responses (CR and PR) upon continuation of the treatment cycles. Ratios of PD‐1+ cells among CD3+, CD4+, and CD8+ cells were significantly lower during the treatment cycles for at least 43 days, compared with the ratios before treatment. Furthermore, nivolumab could successfully bind to PD‐1 expressed on these cells in a continuous manner for a prolonged time with the dosage and treatment cycles used in the present trial (data not shown). These findings suggest that nivolumab had the ability to inhibit the suppression of T‐cell functions for as long as the treatment was continued. As shown in Figure 1c, the tumors in some responders remained controlled even after treatment discontinuation for reasons other than achieving CR (adverse events and reversal of initial consent). This phenomenon was not fully expected, because the inhibition of effector phase suppression is not supposed to be achieved after clearance of nivolumab from these patients.14 This observation might indicate that long‐lasting antitumor immune responses can be induced, at least in some patients, as a result of successful modulation of the effector functions for a limited time period. In theory, successful induction of effective antitumor immune responses can cause death of tumor cells, and these dying cells can release new and abundant tumor antigens for a new round of effective immune responses. Further examination of the long‐term effects of immune induction with nivolumab therapy after discontinuation of the treatment would be useful for the development of more durable immunotherapy regimens.
Regarding the adverse events observed in this trial, the most prominent was interstitial lung disease (organizing pneumonia), which was also shown to be associated with nivolumab treatment in another study.15 We found that the interstitial lung disease could be successfully managed with appropriate treatment consisting of discontinuation of nivolumab and initiation of steroid therapy, as described in our previous case report.12 Contrary to our concerns, the antitumor responses of this patient were not impaired, at least not immediately after resolution of the lung disease. Although the adverse events experienced in this phase 2 trial were less frequent and severe compared with those observed after CTLA‐4 treatment,4, 16 any type of autoimmune response can trigger serious dysfunctions in a variety of organs. Therefore, the condition of patients treated with nivolumab needs to be closely monitored to detect any adverse events, which might deviate sharply from those observed with conventional cytotoxic drugs, in the early stages.
To assess the short‐term influence of nivolumab treatment on the immune system of patients, we examined the fluctuations of immunological parameters associated with its administration. Nivolumab treatment was associated with immediate and significant decreases in peripheral blood CD3+, CD4+, and CD8+ T‐cell counts and serum MIG (CXCL9) and IP10 (CXCL10) levels. All of these observations can be explained by the activation of T cells existing in the bloodstream upon nivolumab treatment. Specifically, the activated T‐cell subsets could secrete IFN‐γ and induce CXCL9 and CXCL10. This promoted adhesion of activated T cells existing in the bloodstream to endothelial cells by binding to the cell surface chemokine receptor CXCR3 in preparation for further immigration from the bloodstream to the tissues.17, 18 These hypothetical mechanisms for the immediate fluctuations could be examined in more detail using the blood samples taken from the patients at earlier time points after the treatment in future clinical trials.
As nivolumab has been shown to have potent antitumor activities in certain percentages of patients with multiple cancer types, identification of biomarkers that can predict clinical responses to nivolumab prior to start of treatment appears to be very important for further development of this drug. PD‐L1 expression in tumor cells in tissue samples has been suggested as a logical and promising candidate.9, 19 However, the reliability of this assessment is still controversial.20 One reason for the difficulties associated with estimating PD‐L1 expression in tumor samples is its fluctuation in response to the tumor microenvironment. It has been demonstrated that tumor cells express PD‐L1 not only constitutively with oncogenic transformation, but also adaptively upon immunological stimulation including inflammatory cytokines.8 Furthermore, multiple reports have shown recently that chemotherapy might influence the PD‐L1 expression of the tumor cells as well.21, 22, 23 Thus, the discovery of other reliable predictive biomarkers is anxiously awaited.
In the present trial, we found that serum IFN‐γ, IL‐6, and IL‐10 levels were significantly higher in patients with objective tumor responses than in those with tumor progression. As the levels of these cytokines were significantly correlated with one another, their collective elevation might reflect the spontaneous initiation of immune responses and suppression at the effector phase at the same time. Therefore, IFN‐γ and IL‐6 might represent the existence of spontaneous antitumor immune responses at the tumor sites, and IL‐10 might represent the existence of tumor evasion systems to suppress T‐cell activation. As the elevated levels of serum IL‐10 could be reduced after successful treatment with nivolumab, we examined the IL‐10 levels in the serum samples obtained after the treatment. As shown in Figure S1a, the post‐treatment serum IL‐10 levels on day 43 decreased numerically (fold change of the averages was 0.859 in responder) but had no statistical significance (P = 0.19) in the responders. In non‐responders, the post‐treatment serum IL‐10 levels on day 43 increased numerically (fold change of the averages was 1.179 in non‐responders) but had no statistical significance either (P = 0.15). Thus, at this point, we do not have evidence to say that serum IL‐10 levels of the responders would decrease after successful therapy. We also analyzed the difference in the levels of serum IL‐10 on day 43 between responders and non‐responders (Fig. S1b) as we did for the serum obtained before the treatment (Fig. 5a). They did not show a significant difference. These findings might correctively imply that the level of IL‐10 would be affected by successful nivolumab treatment. Further evaluation would be warranted regarding the levels of these cytokines with possible ones suggested by other investigators24 in future clinical trials. These studies would be necessary to provide more definitive evidence to prove that these factors can be truly accurate predictive markers for antitumor responses to nivolumab. They might also provide the evidence to understand the mechanism of cytokine elevation.
The results of the present study strongly suggest the usefulness of nivolumab in treating advanced melanoma patients. Based on the results of the present study, nivolumab was approved for the treatment of advanced melanoma in Japan for the first time. Evaluation of the pretreatment cytokine profiles in the peripheral blood serum would be useful to select appropriate patients for nivolumab treatment.
Disclosure Statement
Honoraria was received from Ono Pharmaceutical (N Yamazaki, Y Kiyohara, H Uhara, T Takenouchi, H Tahara), Takeda Pharmaceutical (N Yamazaki), Bristol‐Myers Squibb (N Yamazaki, Y Kiyohara, H Uhara), Chugai Pharmaceutical (N Yamazaki, Y Kiyohara, H Uhara, T Takenouchi), and Boehringer Ingelheim (N Yamazaki). Research funding was received from Ono Pharmaceutical (N Yamazaki, Y Kiyohara, Y Fujisawa, H Minami), Bristol‐Myers Squibb (N Yamazaki, Y Kiyohara, H Minami), Chugai Pharmaceutical (Y Kiyohara, H Minami), MSD (Y Kiyohara, Y Fujisawa), Glaxo Smith Kline (Y Kiyohara), Novartis Pharma (N Yamazaki, H Minami), and Sanofi (H Tahara). H Iizuka, J Uehara, F Otsuka, T Isei, K Iwatsuki, H Uchi, and H Ihn have no conflict of interest to declare. This study was funded by ONO Pharmaceutical, Japan, including the editing service provided by ASCA Corporation, Japan. The study was planned, conducted, and analyzed independently by the authors.
Supporting information
Table S1. Cytokine and chemokine measurements.
Fig. S1. IL‐10 levels of responders and non‐responders. (a) IL‐10 levels before and after nivolumab treatment. (b) IL‐10 levels after nivolumab treatment (43 days after treatment).
Acknowledgments
The authors are grateful to the patients and their families and the investigators, nurses, and staff members who participated in this study. We would like to thank the late Dr Moroi for his support in the conduct of the study and his valuable comments regarding the structure and content of this manuscript. The work was funded by ONO Pharmaceutical Co., Ltd, Japan. We also acknowledge the statistical support of Mr Eiichiro Morishima (Ono Pharmaceutical Co. Ltd, Osaka, Japan).
Cancer Sci 108 (2017) 1022–1031
Funding Information
ONO Pharmaceutical Co., Ltd, Japan.
References
- 1. Alexandrov LB, Nik‐Zainal S, Wedge DC et al Signatures of mutational processes in human cancer. Nature 2013; 500: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348: 69–74. [DOI] [PubMed] [Google Scholar]
- 3. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science 2011; 331: 1565–70. [DOI] [PubMed] [Google Scholar]
- 4. Hodi FS, O'Day SJ, McDermott DF et al Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992; 11: 3887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keir ME, Liang SC, Guleria I et al Tissue expression of PD‐L1 mediates peripheral T cell tolerance. J Exp Med 2006; 203: 883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fife BT, Pauken KE. The role of the PD‐1 pathway in autoimmunity and peripheral tolerance. Ann NY Acad Sci 2011; 1217: 45–59. [DOI] [PubMed] [Google Scholar]
- 8. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Topalian SL, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolchok JD, Hoos A, O'Day S et al Guidelines for the evaluation of immune therapy activity in solid tumors: immune‐related response criteria. Clin Cancer Res 2009; 15: 7412–20. [DOI] [PubMed] [Google Scholar]
- 11. Brahmer JR, Drake CG, Wollner I et al Phase I study of single‐agent anti‐programmed death‐1 (MDX‐1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakashima K, Naito T, Omori S et al Organizing pneumonia induced by nivolumab in a patient with metastatic melanoma. J Thorac Oncol 2016; 11: 432–3. [DOI] [PubMed] [Google Scholar]
- 13. Topalian SL, Sznol M, McDermott DF et al Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. Clin Oncol 2014; 32: 1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ribas A. Tumor immunotherapy directed at PD‐1. N Engl J Med 2012; 366: 2517–9. [DOI] [PubMed] [Google Scholar]
- 15. Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti‐PD‐1‐related pneumonitis during cancer immunotherapy. N Engl J Med 2015; 373: 288–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348: 56–61. [DOI] [PubMed] [Google Scholar]
- 17. Weng Y, Siciliano SJ, Waldburger KE et al Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. J Biol Chem 1998; 273: 18288–91. [DOI] [PubMed] [Google Scholar]
- 18. Booth V, Keizer DW, Kamphuis MB, Clark‐Lewis I, Sykes BD. The CXCR3 binding chemokine IP‐10/CXCL10: structure and receptor interactions. Biochemistry 2002; 41: 10418–25. [DOI] [PubMed] [Google Scholar]
- 19. Garon EB, Rizvi NA, Hui R et al Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015; 372: 2018–28. [DOI] [PubMed] [Google Scholar]
- 20. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanaka K, Miyata H, Sugimura K et al Negative influence of programmed death‐1‐ligands on the survival of esophageal cancer patients treated with chemotherapy. Cancer Sci 2016; 107: 726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fujita Y, Yagishita S, Hagiwara K et al The clinical relevance of the miR‐197/CKS1B/STAT3‐mediated PD‐L1 network in chemoresistant non‐small‐cell lung cancer. Mol Ther 2015; 23: 717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu S, Tao Z, Hai B et al miR‐424(322) reverses chemoresistance via T‐cell immune response activation by blocking the PD‐L1 immune checkpoint. Nat Commun 2016; 7: 11406 https://doi.org/10.1038/ncomms11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nonomura Y, Otsuka A, Nakashima C et al Peripheral blood Th9 cells are a possible pharmacodynamic biomarker of nivolumab treatment efficacy in metastatic melanoma patients. Oncoimmunology 2016; 5(12): e1248327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cytokine and chemokine measurements.
Fig. S1. IL‐10 levels of responders and non‐responders. (a) IL‐10 levels before and after nivolumab treatment. (b) IL‐10 levels after nivolumab treatment (43 days after treatment).


