Summary
OCV‐C02 is a peptide vaccine consisting of two peptide epitopes derived from ring finger protein 43 (RNF43) and translocase of outer mitochondrial membrane 34 (TOMM34). This Phase 1 study assessed the safety, preliminary efficacy and immunological responses following OCV‐C02 administration in patients with advanced or relapsed colorectal cancer who were intolerant or refractory to standard chemotherapy. Primary endpoint was any occurrence of dose‐limiting toxicity (DLT) during cycle 1. Secondary endpoints were treatment‐emergent adverse events, efficacy and immunological responses. Efficacy was evaluated based on overall response rate, disease control rate, time to treatment failure and overall survival. Immunological responses were evaluated by measuring CTL, delayed‐type hypersensitivity (DTH) and regulatory T cells (Tregs). Twenty‐four patients who were HLA‐A*24:02‐positive were enrolled and grouped into four cohorts of six patients each: cohorts 1, 2, 3, and 4 which received s.c. OCV‐C02 (emulsifying agent: Montanide™ ISA 51 VG) 0.3, 1, 3, and 6 mg/body, respectively. After cycle 1, patients who were eligible and willing to continue vaccination proceeded to the extended treatment period. No DLT occurred in cycle 1 and no major safety problems were reported throughout the trial. One patient in cohort 2, three patients in cohort 3 and two patients in cohort 4 achieved stable disease. CTL and DTH responses following vaccination were also observed across the four cohorts. OCV‐C02 at 0.3 to 6 mg/body was found to be safe and well tolerated. Trial registrations: JAPIC clinical trials registry (ID: JapicCTI‐132075) and ClinicalTrials.Gov (ID: NCT01801930).
Keywords: Colorectal cancer, OCV‐C02, peptide vaccine, RNF43, TOMM34
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer‐related death worldwide, with an estimated 1.4 million new cases and approximately 700 000 CRC‐related deaths in 2012.1, 2, 3 Evidence‐based treatment modalities for CRC patients include surgery, radiotherapy and chemotherapy. Early stages of CRC are highly curable by surgery, with 5‐year survival rates of approximately 60–90%.4 In contrast, in most patients with unresectable advanced or metastatic colorectal cancer, the prognosis is usually poor and the 5‐year survival rate is 12.5%.5, 6 Systemic chemotherapy including 5‐fluorouracil, irinotecan and oxaliplatin are usually indicated, either in combination or sequential regimens, for these patients to reduce tumor‐related symptoms and prolong median overall survival (OS).7, 8 However, the disease is mostly incurable and often progressive as a result of chemoresistance, and chemotherapy is often associated with toxic side‐effects that affect patient's quality of life.7, 9 These treatment limitations have driven the development of alternative treatment strategies with improved efficacy, safety and tolerability.10
Recently, cancer immunotherapy is gaining much attention as another treatment option for CRC. In addition, advances in genomic‐based technologies have largely contributed to the advancement in immunotherapy through the identification and characterization of over 70 tumor‐associated antigens (TAA) and their peptide epitopes including several for CRC.11 Understanding the oncogenic roles of these molecules in cancer growth and their high immunogenicity has driven the development of peptide‐based vaccines as emerging new anti‐cancer therapy.11, 12, 13 When presented on human leukocyte antigen (HLA) molecules, the antigenic peptides are recognized by specific CTL triggering the immune system to eliminate cancer cells and generate antigen‐specific immune cells that promote immune‐mediated cancer regression.9
OCV‐C02 is a peptide vaccine, which consists of ring finger protein 43 (RNF43)‐721 and translocase of outer mitochondrial membrane 34 (TOMM34)‐299 peptides that were derived from two TAA expressed in CRC, RNF43 and TOMM34, respectively.14, 15, 16 It is HLA‐A*24:02‐restricted, whereby this HLA class I allele has been reported to have higher frequency in certain ethnic populations including Japanese.17, 18, 19 RNF43 is not expressed in normal adult tissues and is found at low levels in fetal kidney and lung.14, 20 TOMM34 is expressed abundantly in the testis and ovary with low or no expression in other normal organs.15, 20 In CRC, the expression of each of the TAA was found to be upregulated in approximately 80% of the cancerous tissues as compared with the corresponding non‐cancerous tissues.14, 15, 16 Preclinical studies have demonstrated that the inhibition of RNF43 and TOMM34 genes markedly reduced CRC growth, which suggested that these two genes promote the growth of CRC.14, 15, 16 These two peptides are currently undergoing several clinical studies and were found to induce CTL response in CRC patients.12, 16, 21 OCV‐C02, which is a combination of these two peptides, is expected to demonstrate specific cytotoxic effect against CRC and would be clinically beneficial to patients, especially those who do not respond well to other treatment options.
The present study was conducted to assess the safety and tolerability of OCV‐C02 through the evaluation of dose‐limiting toxicity (DLT) in patients with unresectable metastatic CRC who were refractory or intolerant to standard chemotherapy. In addition, preliminary efficacy of and immunological responses to OCV‐C02 were also evaluated.
Materials and Methods
Study design
This study was an open‐label, non‐randomized, dose‐escalation phase 1 trial conducted at four centers in Japan from March 2013 to December 2015, in accordance with the ethical principles of the Declaration of Helsinki, Pharmaceutical Affairs Law, Ordinance on Good Clinical Practice, relevant notifications, and trial protocol that was approved by the institutional review board of each trial site. All patients provided written informed consent prior to the start of the study. Those who were eligible and willing to continue with treatment provided written informed consent before proceeding to cycle 2 and subsequent cycles in the extended treatment period.
The study consisted of a screening period to screen for eligible patients and treatment period that contained a treatment cycle of 28 days, which was repeated every 4 weeks until disease progression or emergence of unacceptable toxicity that included DLT or DLT‐equivalent treatment‐emergent adverse events (TEAE). The trial included four cohorts of six patients each: cohorts 1, 2, 3, and 4, which received 0.3, 1, 3, and 6 mg of OCV‐C02, respectively. These four dose levels were carried out in an escalating manner. At each dose level, safety was confirmed in each patient before proceeding to the next patient and escalation to the next dose level was carried out if no more than one patient developed a DLT during the preceding dose.
Patient eligibility
Patients aged ≥20 years with advanced or relapsed histologically confirmed colorectal adenocarcinoma without indication for surgical resection, who were refractory or intolerant to standard chemotherapy, and positive for HLA‐A*24:02 were eligible to participate in this trial. Patients were required to have at least 3 months of life expectancy, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 1, and adequate hematological (white blood cell [WBC] count ≥3000/μL, neutrophil count ≥1500/μL, hemoglobin ≥9.0 g/dL, and platelet count ≥75 000/μL), hepatic (aspartate aminotransferase [AST] and alanine transaminase [ALT] values ≤5‐fold the upper limit of normal [ULN] and total bilirubin value ≤1.5‐fold ULN set by the trial sites) and renal (serum creatinine ≤1.5‐fold the ULN set by the trial sites) functions. Patients were excluded if they had received cancer treatments including administration of TAA, transfusion of lymphocytes or dendritic cells, systemic corticosteroids, or immunological‐related drugs within 4 weeks before starting OCV‐C02 administration and unapproved drugs in Japan within 4 weeks prior to enrolment in this trial or requiring puncture drainage procedures for pleural fluid, ascites or pericardial fluid. Exclusion criteria also included those who were having concomitant malignancy, had or suspected to have complications of interstitial lung disease, cardiac failure, diabetes mellitus, hypertension, autoimmune diseases, central nervous system (CNS) metastasis of colorectal cancer, mental disorder, active infection or positive HIV antibody test, allergic reaction to any components of OCV‐C02, pregnant, lactating or had any other reasons judged as inappropriate to be enrolled in the present study.
HLA‐A genotyping was carried out at a contract laboratory, LSI Medience Corporation (Tokyo, Japan), using polymerase chain reaction sequence‐specific primer (PCR‐SSP) and PCR sequence‐based typing (PCR‐SBT) methods to select patients who were positive for HLA‐A*24:02 genotype.
Peptides
OCV‐C02 consists of two peptides, RNF43‐721 (NSQPVWLCL) and TOMM34‐299 (KLRQEVKQNL). The peptides were synthesized by American Peptide Company Inc. (Sunnyvale, CA, USA) according to a standard solid‐phase synthesis method and purified by reverse‐phase HPLC.15, 18
Study treatment
In each treatment cycle, OCV‐C02 was given s.c. in the inguinal or axillary region once per week for 4 consecutive weeks (days 1, 8, 15, and 22) and examination was carried out up to Day 29. The first cycle (cycle 1) was the main evaluation of the study, whereby any occurrence of DLT was examined from days 1 to 29. Then, patients who wished to continue with the treatment, were not categorized under any of the discontinuation criteria and had provided their written informed consent were permitted to proceed to the extended treatment period (cycles 2 and thereafter until meeting the termination criteria) using the same dosing schedule as that for cycle 1. Those who terminated the trial after cycle 1 were subjected to post‐treatment assessments 28 days after the final dose.
Prior to vaccination, the lyophilized formulation of OCV‐C02 was dissolved in physiological saline and combined with an emulsifying agent containing Montanide™ ISA 51 VG (Seppic Inc., Paris, France) at a volume ratio of 1:1. One milliliter of the vaccine contained 3 mg of each peptide. Patients in cohorts 1, 2, 3, and 4 received 0.1, 0.33, 1, and 2 mL (1 mL × 2 injection sites) of OCV‐C02, respectively.
Study outcomes
Evaluation criteria of the study were safety, efficacy and exploratory. As the safety endpoints, the primary endpoint was the assessment of DLT in cycle 1 and the secondary endpoints were TEAE and ECOG PS. For efficacy, the endpoints were overall response rate, disease control rate, time to treatment failure and OS. Immunological responses, which were RNF43‐ and TOMM34‐derived peptide‐specific CTL response, delayed‐type hypersensitivity (DTH) response, and percentage of regulatory T cells (Tregs), were evaluated as the exploratory endpoints.
Dose‐limiting toxicity
Dose‐limiting toxicity was defined as the emergence of the following TEAE from days 1 to 29 of cycle 1, in which a causal relationship with OCV‐C02 could not be ruled out: Grade 3 or higher non‐hematological toxicities except for injection site reaction and laboratory abnormalities that lasted for <7 days without clinical symptoms, and hematological toxicities of ≥grade 3 febrile neutropenia, ≥grade 4 anemia, ≥grade 4 platelet count decrease and ≥grade 4 neutropenia or lymphocytopenia that lasted for ≥7 days. During DLT evaluation, if no more than one of six patients developed a DLT, the trial was allowed to proceed to the next dose level. If a DLT was observed in two or more of six patients, discussion with medical advisor was required on whether or not to discontinue the trial. In addition, the occurrence of DLT‐equivalent TEAE, which was defined as DLT that occurred during the extended treatment period (in cycle 2 and thereafter), was evaluated.
Treatment‐emergent adverse events
The occurrence of any TEAE was assessed and the severity was evaluated based on the Common Terminology Criteria for Adverse Events (CTCAE) 4.0 (Japanese version).
Efficacy
Efficacy was assessed by examining the changes from baseline (during screening) in the sizes of tumor lesions using computed tomography (CT) scan between days 21 and 29 of cycle 1 and the following even‐numbered cycles for those who participated in the extended treatment, and within 7 days at trial discontinuation whenever the condition of the patients permitted. Tumor response was evaluated in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) guideline version 1.1. Overall response rate was evaluated for each cycle in which lesions were measured and disease control rate was determined. Time to treatment failure and OS were analyzed using the Kaplan–Meier method. Survival outcomes were investigated at least 1 year after the start of OCV‐C02 administration.
Immunological responses
Cytotoxic T‐lymphocyte responses against RNF43‐ and TOMM34‐derived peptides were measured using Enzyme‐Linked ImmunoSpot (ELISpot) and intracellular cytokine staining (ICS) assays. Blood samples (40 mL for ELISpot and 30 mL for ICS) were taken at baseline, on day 29 of cycle 1 and subsequent even‐numbered cycles and, whenever possible, 28 days after the final dose for those who terminated the trial after cycle 1. The assays were conducted by LSI Medience Corporation. Materials and methods for ELISpot and ICS assays are described in Doc. S1.
For DTH response evaluation, 50 μL OCV‐C02, prepared by dissolving OCV‐C02 preparations for injection with 10 mL saline solution, was given intradermally to patients in the central part of the flexor side of the forearm. Skin redness and induration were examined 48 h after administration at baseline and between days 22 and 27 of cycle 1 and subsequent even‐numbered cycles.
For measurement of Tregs, 10 mL blood samples were collected at baseline and on day 29 of cycle 1 and subsequent even‐numbered cycles. The percentage of Tregs was measured as percentage of CD4+ CD25+ FoxP3+ T cells out of total CD4 + T cells in cryopreserved peripheral blood mononuclear cells by flow cytometry at LSI Medience Corporation.
Statistical analysis
All patients who received OCV‐C02 were included in the safety and efficacy analyses. All statistical analyses were done using SAS software (version 9.2) (SAS Institute, Cary, NC, USA).
Results
Of the 59 patients who provided their consent, 24 were eligible to enter the study and the remaining 35 patients were regarded as screen failure. Reasons for screen failure were as follows: 24 patients were HLA‐A*24:02‐negative, two patients did not meet the criteria of organ function, seven patients were not able to take OCV‐C02 within 21 days after giving informed consent, one patient fell under the exclusion criteria and one patient withdrew consent. Demographic and baseline characteristics of patients who received OCV‐C02 treatment are summarized in Table 1. Overall, the median age was 62 (range 36–77) years. All 24 patients had one or more metastases, mostly in the lung, lymph nodes and liver. Most of them had surgical history and had undergone more than three chemotherapy regimens. The dose and number of vaccinations for each patient are shown in Table 2. All 24 patients completed cycle 1. Of the 24 patients, 16 met the inclusion criteria of the extended treatment and provided consent to enter the subsequent cycles. Of these, six patients completed cycle 2 and proceeded to cycle 3 wherein three completed the cycle and moved on to the subsequent one. However, none of the three patients completed cycle 4. Trial discontinuation at each cycle was because of clear progression of the primary disease, except for one discontinuation in cohort 2 during cycle 2 as a result of the occurrence of grade 4 hyponatremia.
Table 1.
Demographic and baseline characteristics of patients
| Total N = 24 | |
|---|---|
| Sex, N (%) | |
| Male | 16 (66.7) |
| Female | 8 (33.3) |
| Median age, years (range) | 62 (36–77) |
| ECOG PS, N (%) | |
| 0 | 14 (58.3) |
| 1 | 10 (41.7) |
| Pathological diagnosis, N (%) | |
| Tubular adenocarcinoma | 21 (87.5) |
| Poorly differentiated adenocarcinoma | 1 (4.2) |
| Adenocarcinoma, NOS | 2 (8.3) |
| Location of primary lesion, N (%) | |
| Right‐sided colon | 4 (16.7) |
| Left‐sided colon | 6 (25.0) |
| Rectum | 14 (58.3) |
| Metastasis, N (%) | |
| Lung | 21 (87.5) |
| Lymph nodes | 18 (75.0) |
| Liver | 16 (66.7) |
| Peritoneal | 3 (12.5) |
| Bone | 1 (4.2) |
| Pleural | 1 (4.2) |
| Adrenal gland | 1 (4.2) |
| No. colorectal cancer chemotherapy regimens, N (%) | |
| ≤3 regimens | 7 (29.2) |
| >3 regimens | 17 (70.8) |
| Prior chemotherapy regimens/agents, N (%) | |
| Fluoropyrimidines | 24 (100) |
| Irinotecan | 24 (100) |
| Oxaliplatin | 24 (100) |
| Angiogenesis inhibitor | 21 (87.5) |
| Anti‐EGFR monoclonal antibody | 9 (37.5) |
| Regorafenib | 8 (33.3) |
| TAS‐102 | 4 (16.7) |
| Colorectal cancer surgical history, N (%) | |
| Yes | 21 (87.5) |
| No | 3 (12.5) |
| Colorectal cancer radiotherapy history, N (%) | |
| Yes | 7 (29.2) |
| No | 17 (70.8) |
Angiogenesis inhibitor, bevacizumab/ramucirumab; anti‐EGFR monoclonal antibody, cetuximab/panitumumab; ECOG PS, Eastern Cooperative Oncology Group Performance status; EGFR, epidermal growth factor receptor; NOS, not otherwise specified.
Table 2.
Listing of CTL responses, percentage of regulatory T cells, lymphocyte count, injection site reaction, efficacy and post‐treatment therapy for each patient
| Patient no. | Dose (mg/body) | No. vaccinations | CTL responses | Tregs (%) | Baseline lymphocyte count (/mm3) | Occurrence of injection site reaction (Yes/No) | OR | TTF (months) | OS (months) | Post‐treatment therapy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ELISpot | ICS | |||||||||||||||||
| RNF43 | TOMM34 | RNF43 | TOMM34 | |||||||||||||||
| B | C1 | B | C1 | B | C1 | B | C1 | B | C1 | |||||||||
| 1 | 0.3 | 8 | 0 | 0 | 0 | 0 | 0 | 0.002 | 0 | 0 | 0.50 | 1.81 | 1420.4 | No | PD | 1.9 | 3.6 | Radiotherapy |
| 2 | 0.3 | 8 | 64 | 0 | 0 | 0 | 0.005 | 0 | 0.007 | 0 | 2.36 | 2.57 | 2053.2 | No | PD | 2.1 | 7.5 | Regorafenib |
| 3 | 0.3 | 8 | 0 | 20 | 0 | 0 | 0 | 0.003 | 0 | 0.006 | 1.34 | 2.05 | 1937.5 | No | PD | 1.8 | 8.2 | S‐1, calcium folinate, bevacizumab |
| 4 | 0.3 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0.006 | 0 | 1.04 | 1.51 | 1301.5 | Yes | PD | 0.9 | 8.6 | Bevacizumab, thermotherapy, capecitabine, oxaliplatin |
| 5 | 0.3 | 8 | 0 | 191 | 21 | 11 | 0 | 0.001 | 0 | 0 | 1.31 | 2.64 | 1956.0 | No | PD | 1.8 | 9.3 | Radiotherapy |
| 6 | 0.3 | 4 | 0 | 0 | 0 | 0 | 0.002 | 0.01 | 0 | 0 | 1.36 | 0.75 | 1392.4 | No | PD | 0.7 | 2.5 | – |
| 7 | 1 | 8 | 0 | 44 | 22 | 31 | 0.063 | 0.062 | 0 | 0.006 | 4.70 | 3.16 | 1341.6 | No | PD | 0.7 | 17.1 | Regorafenib |
| 8 | 1 | 4 | 0 | 41 | 13 | 126 | 0.038 | 0.117 | 0.009 | 0.011 | 3.13 | 3.21 | 1958.5 | Yes | PD | 0.9 | 16.6 | UFT, calcium folinate, bevacizumab |
| 9 | 1 | 8 | 12 | 0 | 6 | 31 | 0.018 | 0.043 | 0 | 0.012 | 6.62 | 5.67 | 1237.3 | No | SD | 2.3 | 6.5 | Regorafenib |
| 10 | 1 | 4 | 13 | 0 | 8 | 0 | 0.03 | 0.019 | 0 | 0.005 | 1.91 | 1.93 | 984.0 | No | PD | 0.9 | Censored | Regorafenib, cetuximab, panitumumab |
| 11 | 1 | 4 | 0 | NA | 0 | NA | 0.465 | NA | 0 | NA | 4.65 | NA | 830.8 | No | PD | 0.9 | 3.0 | – |
| 12 | 1 | 4 | 9 | 14 | 29 | 0 | 0.025 | 0.075 | 0 | 0.004 | 4.32 | 3.76 | 1992.0 | No | PD | 0.9 | 1.3 | – |
| 13 | 3 | 11 | 0 | 24 | 0 | 0 | 0.005 | 0.029 | 0.005 | 0.013 | 1.81 | 3.09 | 2198.7 | Yes | PD | 1.7 | 11.1 | Regorafenib |
| 14 | 3 | 7 | 0 | 0 | 0 | 11 | 0.002 | 0.027 | 0 | 0 | 2.81 | 1.79 | 1054.0 | Yes | PD | 1.8 | 4.0 | Regorafenib |
| 15 | 3 | 11 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0.016 | 2.75 | 4.85 | 1072.1 | Yes | SD | 2.8 | 9.3 | Radiotherapy, 5‐FU |
| 16 | 3 | 14 | 1 | 86 | 0 | 17 | 0.011 | 0 | 0.005 | 0 | 2.86 | 2.80 | 1477.0 | No | SD | 3.5 | 15.5 | BBI608 |
| 17 | 3 | 15 | 174 | 0 | 24 | 52 | 0 | 0.001 | 0 | 0 | 3.04 | 8.15 | 1555.2 | No | SD | 3.6 | 7.5 | – |
| 18 | 3 | 4 | 0 | 435 | 26 | 0 | 0 | 0.032 | 0 | 0.001 | 3.63 | 2.32 | 1467.9 | No | PD | 0.9 | 4.5 | – |
| 19 | 6 | 7 | 5 | 3 | 2 | 18 | 0 | 0 | 0.001 | 0 | 2.87 | 3.13 | 732.1 | No | PD | 0.9 | 3.2 | – |
| 20 | 6 | 7 | 11 | 0 | 3 | 10 | 0 | 0 | 0.001 | 0.003 | 3.08 | 2.54 | 1181.3 | No | PD | 1.8 | Censored | S‐1, calcium folinate, bevacizumab |
| 21 | 6 | 6 | 0 | 9 | 0 | 3 | 0 | 0 | 0 | 0 | 4.39 | 3.64 | 1490.6 | No | PD | 1.4 | 4.8 | TAS‐102 |
| 22 | 6 | 10 | NA | 60 | NA | 3 | 0 | 0 | 0 | 0 | NA | 4.70 | 1144.0 | No | SD | 2.8 | 11.9 | – |
| 23 | 6 | 3 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 717.4 | No | PD | 0.7 | 2.8 | TAS‐102 |
| 24 | 6 | 15 | 4 | 47 | 6 | 0 | 0 | 0 | 0 | 0 | 3.23 | 2.45 | 2439.6 | Yes | SD | 3.9 | 10.0 | Nintedanib |
B, baseline; C1, cycle 1; CTL, cytotoxic T lymphocyte; ELISpot, Enzyme‐linked Immunospot; 5‐FU, fluorouracil; ICS, intracellular cytokine staining; NA, not available; OR, overall response; OS, overall survival; PD, progressive disease; RNF43, ring finger protein 43; S‐1, tegafur/gimeracil/oteracil; SD, stable disease; TOMM34, translocase of outer mitochondrial membrane 34; Tregs, regulatory T cells; TTF, time to treatment failure; UFT, tegafur/uracil; –, no post‐treatment therapy.
DLT and other safety outcomes
No DLT were reported in any of the patients by day 29 of cycle 1. During the extended treatment period, no DLT‐equivalent TEAE was observed except for one grade 4 case of hyponatremia. Although the event was considered to be associated with aggravated adrenal metastasis from a malignant tumor, the causal relationship with OCV‐C02 could not be ruled out. It was resolved later with sodium chloride injection 10%.
During the entire trial period, all 24 patients experienced at least one TEAE and 15 of them had at least one potentially drug‐related TEAE. However, more than half of the TEAE were of grade 1 or 2 in severity. The most frequently reported events included injection site reaction and vomiting (25% each), decreased appetite (20.8%), pyrexia (16.7%), and cancer pain, anemia, hypertension, nausea, back pain, proteinuria, malaise, elevated AST, elevated gamma‐glutamyl transferase (GGT) and elevated alkaline phosphatase (ALP) (12.5% each). All the events of injection site reaction were considered to be potentially related to OCV‐C02. Table 2 shows the patients who had injection site reaction. Eleven patients were reported to have ≥grade 3 TEAE including grade 3 events of ALP elevation, bilirubin elevation and diabetes mellitus, and grade 4 events of GGT elevation and hyponatremia. Except for diabetes mellitus and hyponatremia, which were observed in one patient each in cohort 2, other ≥grade 3 TEAE were considered not to be drug‐related. Other than that, there were no grade 5 TEAE, drug‐related serious TEAE and TEAE‐related deaths reported in this trial. Drug‐related TEAE observed throughout the trial are summarized in Table 3. One patient in cohort 2 died 18 days after the final dose in cycle 1 as a result of rapid progression of cancer including hepatic failure; therefore, the causal relationship with OCV‐C02 was ruled out. In addition, there were no significant changes in clinical laboratory values and no significant worsening in ECOG PS from baseline except for three, three, one, and two patients in cohorts 1, 2, 3, and 4, respectively, mostly because of disease progression. Vital sign‐related TEAE were mostly ≤grade 2 in severity. There were also no significant changes or abnormalities in bodyweight, 12‐lead electrocardiogram and chest X‐ray test.
Table 3.
Incidences of drug‐related adverse events according to Common Terminology Criteria for Adverse Events grades
| Treatment‐related adverse events | Cohort 1 0.3 mg/body | Cohort 2 1 mg/body | Cohort 3 3 mg/body | Cohort 4 6 mg/body | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 6 | N = 6 | N = 6 | N = 6 | N = 24 | ||||||
| All | ≥ G3 | All | ≥ G3 | All | ≥ G3 | All | ≥ G3 | All | ≥ G3 | |
| Gastrointestinal disorders | ||||||||||
| Nausea | 1 | 1 | ||||||||
| Vomiting | 1 | 1 | ||||||||
| General disorders and administration site condition | ||||||||||
| Pyrexia | 1 | 1 | 2 | |||||||
| Fatigue | 1 | 1 | ||||||||
| Injection site reaction | 1 | 1 | 3 | 1 | 6 | |||||
| Injection site induration | 1 | 1 | ||||||||
| Injection site erythema | 1 | 1 | ||||||||
| Induration | 1 | 1 | ||||||||
| Skin and s.c. tissue disorders | ||||||||||
| Pruritus | 2 | 2 | ||||||||
| Metabolism and nutrition disorders | ||||||||||
| Diabetes mellitus | 1 | 1 | 1 | 1 | ||||||
| Hyponatremia | 1 | 1 | 1 | 1 | ||||||
| Renal and urinary disorders | ||||||||||
| Proteinuria | 1 | 1 | 2 | |||||||
| Investigations | ||||||||||
| Eosinophil count increased | 1 | 1 | ||||||||
| Lymphocyte count decreased | 1 | 1 | ||||||||
| Vascular disorders | ||||||||||
| Hypertension | 1 | 1 | ||||||||
| Blood and lymphatic system disorders | ||||||||||
| Anemia | 1 | 1 | ||||||||
| Injury, poisoning and procedural complications | ||||||||||
| Infusion‐related reaction | 1 | 1 | ||||||||
Drug‐related adverse events were reported using Medical Dictionary for Regulatory Activities (MedDRA) version 18.0 and graded using National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0 (Japanese version). All, total incidences of treatment‐emergent adverse events (TEAE) (grades 1 to 4); ≥ G3, total incidences of ≥Grade 3 TEAE.
Efficacy
All 24 patients were assessed for tumor response. Overall, six patients achieved stable disease (SD) and the remaining patients had progressive disease (PD). Among the six SD cases, five were from the higher‐dose cohorts 3 and 4 as compared to one from the lower‐dose cohort 2. The overall response and disease control rates of the entire trial are shown in Table 4. One patient from cohort 4 showed a reduction (>0% but <10%) in the diameter of target lesion during cycles 1 and 2. Median time to treatment failure and median OS are shown in the Kaplan–Meier plots in Figures 1 and 2, respectively. Median times to treatment failure were 1.8, 0.9, 2.3, and 1.6 months and the median OS times were 7.9, 11.6, 8.4, and 7.4 months for cohorts 1, 2, 3, and 4, respectively. Individual data for overall response, time to treatment failure, overall survival and post‐treatment therapies are shown in Table 2.
Table 4.
Overall response and disease control rates
| Cohort 1 0.3 mg/body N = 6 | Cohort 2 1.0 mg/body N = 6 | Cohort 3 3.0 mg/body N = 6 | Cohort 4 6.0 mg/body N = 6 | Total N = 24 | |
|---|---|---|---|---|---|
| Stable disease (SD), N (%) | 0 (0.0) | 1 (16.7) | 3 (50.0) | 2 (33.3) | 6 (25.0) |
| Progressive disease (PD), N (%) | 6 (100.0) | 5 (83.3) | 3 (50.0) | 4 (66.7) | 18 (75.0) |
| Disease control rate (CR, PR or SD), N (%) | 0 (0.0) | 1 (16.7) | 3 (50.0) | 2 (33.3) | 6 (25.0) |
CR, complete response; PR, partial response.
Figure 1.
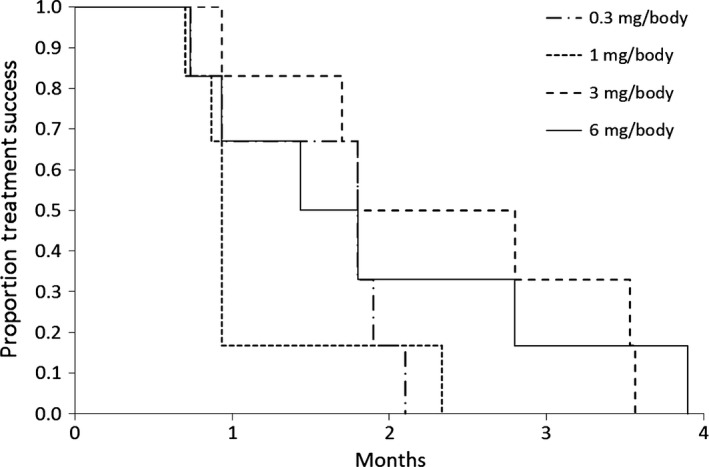
Time to treatment failure. Median time (months) to treatment failure of 24 patients with advanced or relapsed colorectal cancer treated with OCV‐C02 at doses of 0.3, 1, 3, and 6 mg/body.
Figure 2.
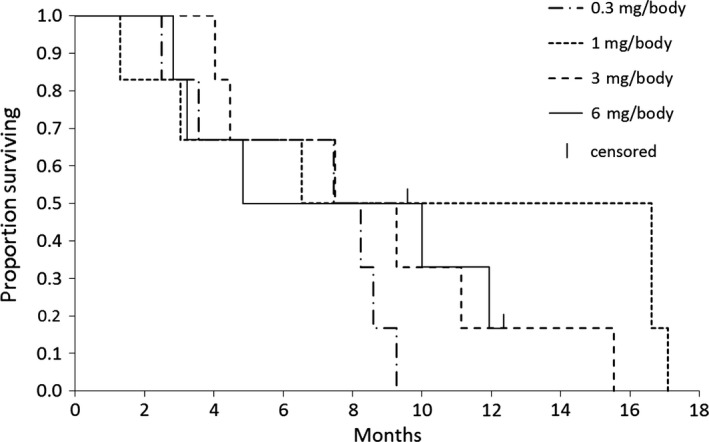
Overall survival. Median overall survival (months) of 24 patients with advanced or relapsed colorectal cancer treated with OCV‐C02 at doses of 0.3, 1, 3, and 6 mg/body. One patient each in cohort 2 (1 mg/body) and cohort 4 (6 mg/body) were treated as censored cases.
Immunological responses
The results of CTL responses measured by ELISpot and ICS assays, and DTH reactions (i.e. redness and induration) in cycles 1 and 2 are summarized in Table 5. With regard to Tregs, increase in Tregs was observed in 11 patients in cycle 1 and six patients in cycle 2, as compared with baseline level. No patients completed cycle 4; therefore, no immunological response data were obtained. Baseline and post‐cycle 1 CTL responses and Tregs (%) for each patient are shown in Table 2.
Table 5.
Positive cytotoxic T lymphocyte and delayed‐type hypersensitivity responses against RNF43‐derived and TOMM34‐derived peptides
| Cycle 1 | Cycle 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Cohort 1 0.3 mg/body | Cohort 2 1.0 mg/body | Cohort 3 3.0 mg/body | Cohort 4 6.0 mg/body | Cohort 1 0.3 mg/body | Cohort 2 1.0 mg/body | Cohort 3 3.0 mg/body | Cohort 4 6.0 mg/body | |
| No. patients (patient with positive response/total patients assessed) | ||||||||
| CTL response (ELISpot assay) | ||||||||
| RNF43 | 2/6 | 3/5 | 3/6 | 2/4 | 0/2 | 0/1 | 3/5 | 1/2 |
| TOMM34 | 0/6 | 3/5 | 3/6 | 3/4 | 0/2 | 0/1 | 1/5 | 0/2 |
| (ICS assay) | ||||||||
| RNF43 | 4/6 | 3/5 | 4/6 | 0/5 | 0/2 | 1/1 | 3/5 | 0/3 |
| TOMM34 | 1/6 | 5/5 | 3/6 | 1/5 | 0/2 | 1/1 | 3/5 | 1/3 |
| DTH reaction | ||||||||
| Redness | 3/6 | 2/6 | 5/6 | 3/5 | 1/3 | 1/2 | 5/5 | 1/3 |
| Induration | 1/6 | 1/6 | 1/6 | 0/5 | 0/3 | 0/2 | 0/5 | 0/3 |
CTL, cytotoxic T lymphocyte; DTH, delayed‐type hypersensitivity; ELISpot, Enzyme‐Linked ImmunoSpot; ICS, intracellular cytokine staining; RNF43, ring finger protein 43; TOMM34, translocase of outer mitochondrial membrane 34.
Discussion
The results of this trial showed that OCV‐C02 at the designated dose levels was safe in patients with advanced or relapsed CRC who were refractory or intolerant to standard chemotherapy. There were no DLT during the main evaluation period of cycle 1 and no evidence of major safety problems in any of the cohorts throughout the study. The most commonly observed TEAE included injection site reaction, vomiting, decreased appetite and pyrexia, and more than 50% of the incidences were mild or moderate (grade 1 or 2) in severity. Also, there was no significant deviation from baseline value/condition in other safety variables that were assessed in the present study. This is consistent with the safety outcomes that were reported in other clinical studies of peptide vaccines that are currently under development, and TEAE observed here were similar to those reported in the other studies.12, 16, 20, 21, 22 In addition, serious adverse reactions as a result of peptide vaccine therapies are very rare.9 Thus, it can be suggested that OCV‐C02 at 0.3–6 mg/body has a well‐tolerable profile with no major toxic effects.
Twenty five percent of the patients across cohorts 2–4 had SD. Median time to treatment failure and median OS time ranged from approximately 1–2 months and 8– 12 months, respectively. From the current preliminary efficacy analysis, although it may be difficult to clearly conclude whether or not tumor response was dose‐related, it was observed that the higher‐dose cohorts (cohorts 3 and 4) showed relatively better outcomes than the lower‐dose cohorts (cohorts 1 and 2) in terms of disease control rate in cycle 1, disease control rate in best overall response, reduction of target lesion, and time to treatment failure. As described in the ‘Guidance for Industry on Clinical Considerations for Therapeutic Cancer Vaccines’ by the US Food and Drug Administration (FDA),23 unlike traditional cytotoxic cancer treatment, cancer vaccine generally requires 2–3 months to develop antitumor immune response against the tumor. Therefore, delayed antitumor response should be expected for peptide vaccine. Considering this, three patients, patients no. 7, 13, and 19, were judged by the investigating physicians to be eligible to continue an additional treatment cycle even after they showed the first PD. However, all three remained at PD at the end of the cycle; hence, they were discontinued from the study. In contrast, the median OS for all four cohorts of OCV‐C02 was higher than the median OS observed in several global phase 3 trials of current treatments for similar criteria of metastatic refractory CRC patients, such as the CORRECT, CONCUR and RECOURSE studies.24, 25, 26
The results of RNF43‐ and TOMM34‐derived peptide‐specific CTL and DTH responses across the cohorts indicated that an immune response was triggered by OCV‐C02. In this vaccination approach, peptide epitopes derived from TAA associate with the respective HLA molecules activating CTL to eliminate tumor cells directly or indirectly through the secretion of cytokines such as interferon‐gamma (IFN‐γ) and tumor necrosis factor‐alpha (TNF‐α).27 Several studies involving the peptide epitopes of RNF43 and TOMM34 have shown that both are effective in inducing CTL.12, 16, 21 The exploratory analysis of this study showed no clear correlation between immunological responses and dose. However, CTL response might be a useful biomarker of efficacy for patients receiving peptide vaccination.12, 20 Lymphocyte counts prior to vaccination have been reported to be well correlated with OS of advanced cancer patients because lymphocytes are absolutely required for vaccine‐mediated immune boosting;28 thus, it is an important criterion for patient selection for peptide vaccination. However, in the current study, there was no limitation on the lymphocyte count during patient enrolment. Nevertheless, the clinical laboratory data collected during screening showed that of the 24 patients enrolled, 20 patients had a baseline lymphocyte count of more than 1000/mm3, and the remaining four patients (i.e. patient nos 10, 11, 19, and 23) had lymphocyte counts of less than 1000/mm3. Except for patient no. 10, whose case was a censored case, patient nos 11, 19, and 23 showed lower than median OS time of their respective cohorts. There were also reports suggesting the possibility of local site injection reaction as a good predictive marker for clinical effectiveness of peptide vaccine.12, 20, 29 A total of six patients (patient nos 4, 8, 13, 14, 15, and 24) showed OCV‐C02‐related injection site reaction, with OS of 8.6, 16.6, 11.1, 4, 9.3, and 10 months, respectively. Five (patient nos 4, 8, 13, 15, and 24) out of the six patients showed a relatively higher OS trend as compared to some other patients who did not have injection site reaction, which suggests that further investigations would be meaningful.
Numerous TAA from various cancers have been used for the development of peptide vaccines and a number of them have entered clinical trials. Also, some studies have involved the possibility of a multi‐modal treatment combination of peptide vaccines with other cancer therapies including chemotherapeutic agents and Programmed cell death 1 (PD‐1) blockade for improved immune response.16, 20, 21, 30 In spite of these efforts, none of the HLA‐restricted peptide vaccines under investigation has yet been approved for commercial use as a result of limited success at inducing clinical tumor regressions, despite reliable induction of T‐cell responses, and most of the current clinical trials showed limited efficacy. Therefore, a strong need remains for safe and efficacious peptide vaccines in the treatment of various cancer types/stages, including advanced CRC. Several studies have reported the results of peptide vaccines containing a combination of RNF43 and TOMM34 antigens for the treatment of CRC; however, those studies are physician‐initiated trials for research purposes. The current study was a company‐initiated trial for the purpose of new drug registration. In addition, patients did not show clinically significant adverse events or toxic effects; this observation suggested that OCV‐C02 may be considered safe for long‐term administration as compared with standard chemotherapies that are usually feasible for a shorter dosing schedule because of accompanying toxicity and/or drug resistance. Moreover, OCV‐C02 combines two CRC‐specific TAA, RNF43 and TOMM34, that are crucial to sustain growth of the cancer, which may allow CTL response to occur in almost all patients who received the vaccination because both TAA are highly expressed in CRC. As shown in the results of ELISpot and ICS assays for the main evaluation period of cycle 1, almost half of the total patients across the cohorts showed CTL response against either one or both peptides. Combining peptide epitopes derived from more than one TAA may be more advantageous because it may enhance CTL induction and circumvent the potential of antigen escape which has been observed when all the peptide epitopes were derived from a single TAA.27 Also, it was reported in other studies that patients showing CTL response to multiple peptides had better median survival time and 1‐year survival rate as compared to cytotoxic chemotherapy.16, 27 In addition, it was found that lower survival rates and more frequent metachronous metastases were observed in patients exhibiting higher RNF43 mRNA expression, and that RNF43 mRNA expression was significantly correlated with relapse after surgery.31 The superiority of OCV‐C02 in terms of median OS over that observed in studies of recent drugs is clinically meaningful, as the patients who participated in this study had been refractory to conventional chemotherapeutic agents/regimens including fluoropyrimidines, irinotecan and oxaliplatin, and some patients had been refractory to recent agents such as regorafenib and TAS‐102.
There were several limitations in the present study. The sample size of six patients in each cohort was small; hence, significant relationships with the data could not be concluded regarding efficacy. In addition, the treatment period was 4 weeks per cycle and considering not all the patients proceeded to cycle 4, the duration may be too short to evaluate the efficacy of OCV‐C02 because of the delayed effect of the cancer vaccine. Another limitation was that there was no specification in the inclusion criteria to include only patients who showed CTL response against both peptides of OCV‐C02. Patients who were enrolled in this study had positive CTL response towards either one or both peptides; therefore, this time, it may be difficult to obtain a more consistent antitumor effect and immunological responses. Future studies that include larger sample sizes, longer treatment periods and specification for patients to have CTL response against both RNF43‐ and TOMM34‐derived peptides may provide more precise outcomes.
In conclusion, OCV‐C02 at 0.3 to 6 mg/body exhibited a safe and well‐tolerated profile as well as tumor and immunological responses in recurrent or advanced stage CRC patients who were not responding well or were resistant to standard chemotherapeutic agents. OCV‐C02 could be a promising and clinically beneficial alternative for these patients who have lack of treatment options; therefore, further clinical studies are highly warranted.
Disclosure Statement
Yuh Sakata has received advisory fees and Satoru Iwasa has received research funding from Otsuka Pharmaceutical Co., Ltd. CK, YN and ELL are employees of Otsuka Pharmaceutical Co., Ltd. The other authors have no conflict of interest. RNF43‐721 and TOMM34‐299 peptides were patented by Oncotherapy Science, Inc. and licensed out to be developed by Otsuka Pharmaceutical Co. Ltd. The study was designed under the responsibility of and funded by Otsuka Pharmaceutical Co., Ltd (Tokyo, Japan). OCV‐C02 was provided by Otsuka Pharmaceutical Co., Ltd. Otsuka Pharmaceutical Co., Ltd collected and analyzed the data and contributed to the interpretation of the study. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Supporting information
Doc. S1. Materials and methods for Enzyme‐Linked ImmunoSpot (ELISpot) and intracellular cytokine staining (ICS) assays for the measurement of CTL response against RNF43‐ and TOMM34‐derived peptides.
Acknowledgments
The authors would like to thank the members of the Efficacy and Safety Data Committee, Dr Keisuke Aiba of The Tokyo Jikei University School of Medicine, Dr Atushi Sato of Hirosaki University Graduate School of Medicine, and Dr Hiroyuki Uetake of Tokyo Medical and Dental University for their advice on the evaluation of safety and efficacy, Mr Yoshimasa Isakari of Global Pharmaceutical Business, Otsuka Pharmaceutical Co., Ltd for conducting immunomonitoring analysis, and Mr Masaru Kamishohara and Mr Kentaro Ouchi of Medical Affairs, Otsuka Pharmaceutical Co., Ltd for their critical revision of the manuscript.
Cancer Sci 108 (2017) 1013–1021
Funding Information
The study was funded by Otsuka Pharmaceutical Co., Ltd.. OCV‐C02 was provided by Otsuka Pharmaceutical Co., Ltd.
References
- 1. Shapira S, Fokra A, Arber N, Kraus S. Peptides for diagnosis and treatment of colorectal cancer. Curr Med Chem 2014; 21: 2410–6. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 3. Kim JS, Kim YG, Park EJ et al Cell‐based immunotherapy for colorectal cancer with cytokine‐induced killer cells. Immune Netw 2016; 16: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moghimi‐Dehkordi B, Safaee A. An overview of colorectal cancer survival rates and prognosis in Asia. World J Gastrointest Oncol 2012; 4: 71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeSantis CE, Lin CC, Mariotto AB et al Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014; 64: 252–71. [DOI] [PubMed] [Google Scholar]
- 6. Pohlen U, Rieger H, Kunick‐Pohlen S, Berger G, Buhr HJ. Phase II study of regional chemotherapy using the hypoxic abdominal perfusion technique in advanced abdominal carcinoma. 5‐FU pharmacokinetics, complications and outcome. Anticancer Res 2007; 27: 667–74. [PubMed] [Google Scholar]
- 7. Foubert F, Matysiak‐Budnik T, Touchefeu Y. Options for metastatic colorectal cancer beyond the second line of treatment. Dig Liver Dis 2014; 46: 105–12. [DOI] [PubMed] [Google Scholar]
- 8. Marin JJ, Sanchez de Medina F, Castaño B et al Chemoprevention, chemotherapy, and chemoresistance in colorectal cancer. Drug Metab Rev 2012; 44: 148–72. [DOI] [PubMed] [Google Scholar]
- 9. Bartnik A, Nirmal AJ, Yang SY. Peptide vaccine therapy in colorectal cancer. Vaccines (Basel) 2012; 1: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kerr D. Clinical development of gene therapy for colorectal cancer. Nat Rev Cancer 2003; 3: 615–22. [DOI] [PubMed] [Google Scholar]
- 11. Stevanovic S. Identification of tumour‐associated T‐cell epitopes for vaccine development. Nat Rev Cancer 2002; 2: 514–20. [DOI] [PubMed] [Google Scholar]
- 12. Hazama S, Nakamura Y, Takenouchi H et al A phase I study of combination vaccine treatment of five therapeutic epitope‐peptides for metastatic colorectal cancer; safety, immunological response, and clinical outcome. J Transl Med 2014; 12: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamata Y, Kuhara A, Iwamoto T et al Identification of HLA class I‐binding peptides derived from unique cancer‐associated proteins by mass spectrometric analysis. Anticancer Res 2013; 33: 1853–9. [PubMed] [Google Scholar]
- 14. Yagyu R, Furukawa Y, Lin YM, Shimokawa T, Yamamura T, Nakamura Y. A novel oncoprotein RNF43 functions in an autocrine manner in colorectal cancer. Int J Oncol 2004; 25: 1343–8. [PubMed] [Google Scholar]
- 15. Shimokawa T, Matsushima S, Tsunoda T, Tahara H, Nakamura Y, Furukawa Y. Identification of TOMM34, which shows elevated expression in the majority of human colon cancers, as a novel drug target. Int J Oncol 2006; 29: 381–6. [PubMed] [Google Scholar]
- 16. Okuno K, Sugiura F, Hida JI et al Phase I clinical trial of a novel peptide vaccine in combination with UFT/LV for metastatic colorectal cancer. Exp Ther Med 2011; 2: 73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kubo RT, Sette A, Grey HM et al Definition of specific peptide motifs for four major HLA‐A alleles. J Immunol 1994; 152: 3913–24. [PubMed] [Google Scholar]
- 18. Uchida N, Tsunoda T, Wada S, Furukawa Y, Nakamura Y, Tahara H. Ring finger protein 43 as a new target for cancer immunotherapy. Clin Cancer Res 2004; 10: 8577–86. [DOI] [PubMed] [Google Scholar]
- 19. Baba T, Sato‐Matsushita M, Kanamoto A et al Phase I clinical trial of the vaccination for the patients with metastatic melanoma using gp100‐derived epitope peptide restricted to HLA‐A*2402. J Transl Med 2010; 8: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsushita N, Aruga A, Inoue Y, Kotera Y, Takeda K, Yamamoto M. Phase I clinical trial of a peptide vaccine combined with tegafur‐uracil plus leucovorin for treatment of advanced or recurrent colorectal cancer. Oncol Rep 2013; 29: 951–9. [DOI] [PubMed] [Google Scholar]
- 21. Hazama S, Nakamura Y, Tanaka H et al A phase ΙI study of five peptides combination with oxaliplatin‐based chemotherapy as a first‐line therapy for advanced colorectal cancer (FXV study). J Transl Med 2014; 12: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iwasa S, Yamada Y, Heike Y et al Phase I study of a new cancer vaccine of ten mixed peptides for advanced cancer patients. Cancer Sci 2016; 107: 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for Industry: Clinical Considerations for Therapeutic Cancer Vaccines. 2011. [Cited 1 Mar 2017] Available from URL: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/UCM278673.pdf
- 24. Grothey A, Van Cutsem E, Sobrero A et al Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013; 381: 303–12. [DOI] [PubMed] [Google Scholar]
- 25. Li J, Qin S, Xu R et al Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2015; 16: 619–29. [DOI] [PubMed] [Google Scholar]
- 26. Mayer RJ, Van Cutsem E, Falcone A et al Randomized trial of TAS‐102 for refractory metastatic colorectal cancer. N Engl J Med 2015; 372: 1909–19. [DOI] [PubMed] [Google Scholar]
- 27. Zhao L, Zhang M, Cong H. Advances in the study of HLA‐restricted epitope vaccines. Hum Vaccin Immunother 2013; 9: 2566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noguchi M, Mine T, Komatsu N et al Assessment of immunological biomarkers in patients with advanced cancer treated by personalized peptide vaccination. Cancer Biol Ther 2010; 10: 1266–79. [DOI] [PubMed] [Google Scholar]
- 29. Sato Y, Shomura H, Maeda Y et al Immunological evaluation of peptide vaccination for patients with gastric cancer based on pre‐existing cellular response to peptide. Cancer Sci 2003; 94: 802–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kleponis J, Skelton R, Zheng L. Fueling the engine and releasing the break: combinational therapy of cancer vaccines and immune checkpoint inhibitors. Cancer Biol Med 2015; 12: 201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loregger A, Grandl M, Mejías‐Luque R et al The E3 ligase RNF43 inhibits Wnt signaling downstream of mutated β‐catenin by sequestering TCF4 to the nuclear membrane. Sci Signal 2015; 8: ra90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Doc. S1. Materials and methods for Enzyme‐Linked ImmunoSpot (ELISpot) and intracellular cytokine staining (ICS) assays for the measurement of CTL response against RNF43‐ and TOMM34‐derived peptides.


