Abstract
Outcomes of patients with gastric cancer who exhibit positive peritoneal lavage cytology findings (CY +) vary by diagnostic methods because of quantitative and qualitative cancer cell diversity. This study sought to establish practical diagnostic criteria for performing curative resections, based on peritoneal lavage cytology findings in gastric cancer patients. We enrolled 1028 patients with gastric cancer who underwent R0/1 (n = 911) or R2 (n = 117) resections and analyzed relationships between cancer cell findings in peritoneal lavage fluid and clinicopathological factors in the R0/1 group. We found 68 patients with CY + status. Receiver operating characteristic analyses and multivariate analyses showed that the presence of ≥1 signet ring cell, ≥5 cell clusters or ≥50 isolated cancer cells in peritoneal lavage fluid predicted poor prognoses in the 68 CY + patients. High‐risk CY + group patients with at least one of the above predictors had the highest hazard ratio (HR = 3.28, P < 0.001). The remaining (low‐risk) patients had a survival curve similar to that of patients with a normal cytology. The high‐risk CY + patients who underwent R1 resection had poor prognoses despite no macroscopic peritoneal metastasis (2% 5‐year survival)—equivalent to that of patients who underwent R2 resection. The CY + criteria defined in this study could help identify candidates for curative resection as an initial therapy for gastric cancer.
Keywords: Cytology, gastric cancer, lavage, peritoneal, signet‐ring cell
Intraoperative peritoneal lavage (PL) cytology is a useful method for detecting peritoneal dissemination in the absence of macroscopically visible metastatic tumors. Positive PL cytology (CY+) is an indicator of M1 disease in gastric cancer, as was first described in the TNM classification of the International Union Against Cancer, 7th Edition, in 2009.1 The Society of American Gastrointestinal and Endoscopic Surgeons recommends performing PL cytology at the time of staging laparoscopy;2 the utility of the cytological findings has increased with the prevalence of preoperative staging laparoscopy.
Patients who are found to be CY+ by the conventional method have a median survival period measured only in months, even after undergoing macroscopically curative surgical resections.3, 4, 5 Conventional cytological diagnosis of lavage fluid simply involves microscopic observation of Papanicolaou‐stained slides, and can be performed at most institutions capable of providing intraoperative rapid cytological diagnosis to determine whether a surgical resection should be continued. However, the sensitivity of detecting tumor cells floating in PL fluid has been limited by the difficulty of distinguishing a few tumor cells from a larger number of non‐tumor cells, including mesothelial cells or macrophages, and has been based only on morphological observation. Therefore, other methods, including immunocytochemistry (ICC) and reverse transcriptase‐polymerase chain reaction (RT‐PCR), have been developed and used to detect small numbers of tumor cells in lavage fluid with higher sensitivity. These techniques are more useful for detecting small numbers of tumor cells in lavage fluid than conventional cytodiagnosis. However, outcomes of CY+ patients varied by each modified method to detect small numbers of tumor cells described above. For example, the 5‐year survival rate after curative resection among patients with a tumor depth of T3/T4 was 0% when evaluated by conventional cytology,3, 4, 5 8–44% by ICC cytology,6, 7, 8, 9 and 40% by RT‐PCR.10 These earlier results raise the question of whether ICC cytology and RT‐PCR could provide improved indications for surgical resection.
The diagnostic criterion for CY+ status is the identification of at least one tumor cell; this criterion was established based on morphological findings consisting of only qualitative factors—cellular and structural atypia, such as the formation of cancer cell clusters. However, effusion materials, such as pleural effusion and ascites, can include tumor cells and reflect the level of tumor cell dissemination. Consequently, quantitative factors should be considered when predicting patient outcome, and not just the presence or absence of tumor cells. In other words, cytological criteria should be established based on not only qualitative aspects, but also quantitative aspects, to predict patient outcomes.
This study aimed to construct new objective criteria based on both qualitative and quantitative examinations of conventional PL specimens, which could predict patient outcomes and indicate whether surgical resection should be performed as initial therapy in patients with gastric cancer.
Materials and Methods
Patients and pathological specimens
The records of 1028 consecutive patients with gastric cancer treated between October 1992 and July 2009 were selected from a prospectively maintained database at the National Cancer Center Hospital East, Japan. These patients underwent gastrectomies and PL cytological examinations during their surgeries, and each was pathologically diagnosed with pT3 or T4 gastric cancer. Of the 1028 patients, 911 and 117 underwent R0/1 and R2 resections, respectively, according to TNM Residual Tumor (R) classification. The R2 resection group included patients who had unresectable metastases and needed palliative resection for stenosis or bleeding of primary tumors. The R0/1 group (n = 911) was examined in this study to establish new cytological criteria of PL cytology. The remaining 117 patients who had undergone R2 resections were examined only to compare them with the prognoses of patients in the R0/1group, using new cytological criteria proposed in this study. Gastrectomies and systematic D2 regional lymph node dissections were performed when potentially curative resections were considered possible, even for patients with stage IV disease. Surgical treatment of gastric cancer with potentially resectable distant metastases is controversial and not standard therapy. The patients with stage IV disease on whom we performed R0/1 resections were strictly selected before surgery and have had good outcomes.11, 12 These patients with stage IV disease was included to investigate the influence of CY+ status on outcomes in the R0/1 group compared with other predictors of poor prognosis. Patients who had received neoadjuvant therapies were excluded. Various clinicopathological parameters recorded were reviewed. Staging was classified by the TNM classification of the International Union Against Cancer, 7th Edition.1 Overall survival was measured from the date of surgical resection until the date of death. The Institutional Review Board of the National Cancer Centre approved this study protocol (Registration No. 2014‐055).
Cytopathology
We performed PL cytology with a consistent procedure; results were reported within an hour from the start of the operation. In principle, patients diagnosed as CY+ underwent R1 resection with curative intent if no other distant metastasis was present.
The cytological diagnostic procedures were as follows: immediately after the opening of the abdominal cavity and before any manipulation of the tumor, 100 mL of physiological saline was instilled into the pelvic cavity and the 50 mL of lavage fluid was aspirated. When ascites was present, the 50 mL ascites was collected without lavage. The amounts of collected fluid were thus about the same for all patients in this study. The fluid thus obtained was centrifuged at room temperature. Extracted samples were then smeared onto the two glass slides and fixed with 95% methanol, stained with Papanicolaou, and prepared for microscopic examination. Cytological diagnoses were performed promptly during the surgeries. In this study, the same slides were reviewed retrospectively again by three cytologists: a certified cytopathologist (S.F.) of the Japanese Society of Clinical Cytology (JSCC), a certified cytotechnologist (S.Y.) of the JSCC and the International Academy of Cytology, and a cytopathological fellow (E.H.). Atypical cytologies, including irregularly shaped nuclei, ratio of nucleus to cytoplasm (N/C ratio), increased nuclear chromatin, anisonucleosis (notably different nuclei sizes), signet ring cells, intracytoplasmic luminae, and cell cluster formations (three‐dimensional [3D] aggregation of more than 3 cancer cells) were evaluated in this study.
Statistical analysis
An analysis of overall survival (OS) between subgroups was performed using the Kaplan–Meier method; differences were compared using the log‐rank test. The optimal cut‐off values of quantitative factors in PL fluid for discriminating 2‐year patient survival were defined using receiver operating characteristic (ROC) analyses. To further evaluate the discrimination performance of patient survival, we calculated the area under the curve and compared correlated ROC curves under nonparametric assumptions. A Cox‐regression model was used for univariate and multivariate analyses. All variables were used for univariate and multivariate analyses; variables for a multivariate analysis were selected using a backward stepwise approach for factors of which P < 0.05 in univariate analyses. For multiple subgroup OS analyses, hazard ratios (HR) and 95% confidence intervals (CI) within each subgroup were summarized and displayed in a forest plot using the Cox regression model. Clinical characteristics of the two groups were compared using chi‐square, Fisher exact, or Mann–Whitney U tests. All reported P‐values were two‐sided; P < 0.05 was considered significant. All statistical analyses were performed using IBM SPSS Statistics, v18, for Windows (IBM Corporation, Armonk, NY, USA).
Results
Clinicopathological characteristics of the R0/1 group
The 911 (88.6%) patients who underwent R0/1 resection with curative intent were included in the survival analyses to establish new diagnostic criteria based on PL cytology. Their median follow‐up period was 62.3 months (range: 1.0–184.2 months). Of these 911 patients, 139 (15.3%) had been pathologically diagnosed with stage IV disease without considering their CY results; of these patients, 37 (26.6%) had disseminated metastasis directly adjacent to the peritoneum or retroperitoneum of the stomach, 20 (14.4%) had liver metastasis, and 87 (62.6%) had non‐regional lymph node metastasis that was categorized as distant metastasis according to the TNM classification. R0/1 resections that included metastatic sites were performed for all the patients, even those who were ultimately diagnosed as having stage IV disease.
Peritoneal lavage fluid was collected and examined intra‐operatively in all 911 patients, of whom 68 (7.5%) exhibited definitive cancer cells or atypical cells that were highly suspected of being adenocarcinoma cells both qualitatively and quantitatively, and were classified as CY+.
Comparison of clinicopathological characteristics by PL cytology results (normal cytology or CY+) revealed that CY+ patients had more advanced pathological characteristics, such as infiltrative growth pattern of the Borrmann's classification (Type 4), larger primary tumors, pT4a/b tumor depth, lymph node metastasis and distant metastasis (Table S1).
Atypical cytology in PL fluid
Atypical cytology in cancer cells was evaluated in detail through microscopic analyses of slides from 68 CY+ patients. Microscopic findings associated with atypical cytology included irregularly shaped nuclei (present or absent; Fig. 1a), N/C ratio (<0.8 or ≥0.8; Fig. 1b), increased nuclear chromatin (present or absent; Fig. 1c), anisonucleosis (<1.5 or ≥1.5; Fig. 1d), signet ring cells (present or absent; Fig. 1e), intracytoplasmic lumina (present or absent; Fig. 1f), and cell clusters (present or absent; Fig. 1g/h). Signet ring cells have characteristic ring‐shaped nuclei due to intra‐cytoplasmic mucin and exhibit independent morphology; therefore detailed nuclear findings (such as irregular shape, N/C ratio, nuclear chromatin and anisonucleosis) were not examined in the 10 patients with only signet ring cells. Patients who were considered CY+ according to the above definitions of atypical cytology had significantly poorer outcomes than patients with no cancer cells in their PL fluid, among both the 911 patients in the R0/1 group and the 772 patients with no macroscopic distant metastasis (Fig. S1).
Figure 1.
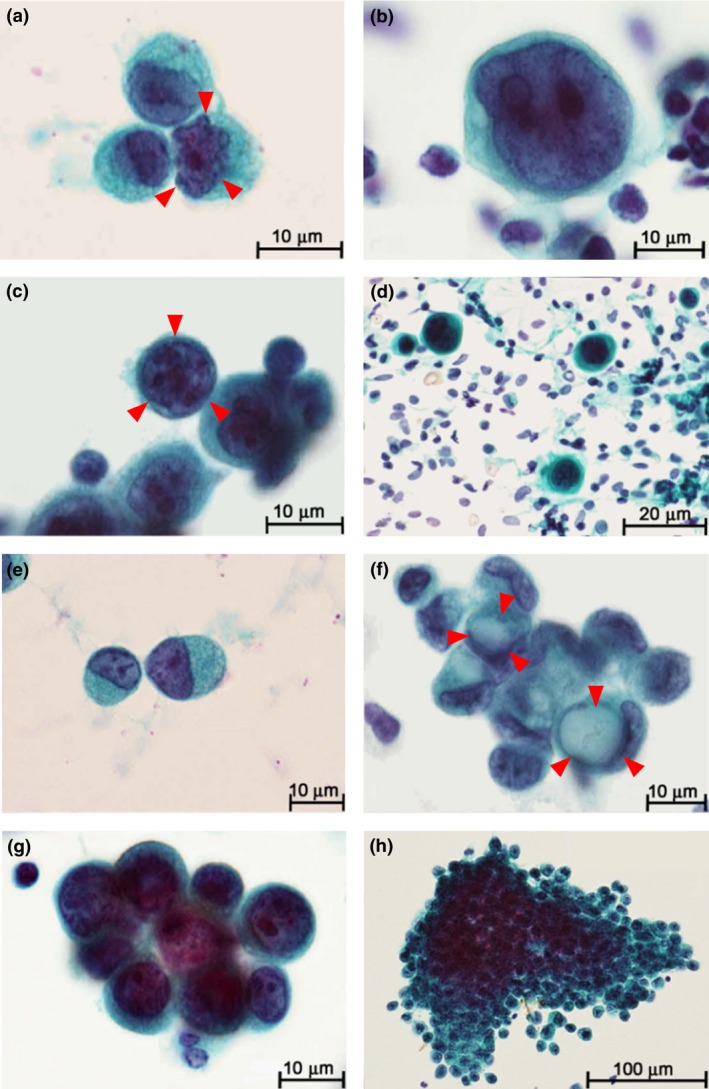
Gastric cancer cells in peritoneal lavage fluid. (a) Nuclear irregularity of gastric adenocarcinoma cell (arrows). (b) Ratio of nucleus to cytoplasm (N/C) ≥0.8. (c) Increased nuclear chromatin stained with hematoxylin (arrows). (d) Anisonucleosis defined by remarkable differences (≥1.5 times) in nuclear sizes. (e) Signet ring cell. (f) Intracytoplasmic lumen (arrows). (g/h) Formation of cell clusters defined by the 3‐dimensional (3D) aggregation of ≥3 cancer cells.
We focused on both qualitative and quantitative factors for our cytological criteria and divided the patients into four subgroups according to their total number of cancer cells: 1–49, 50–199, 200–999, and ≥1000 and the total number of cell clusters: 0, 1–4, 5–10, and ≥11, so as to be almost equal about the numbers of patients. The stepwise decrease in prognostic value was observed according to the step‐wise increase in numbers of cancer cells; the HRs of the ≥50 cancer‐cell subgroups were significantly higher than the normal cytology subgroup (Fig. 2a). The HRs of the ≥5 cell‐cluster subgroups were also significantly higher than the normal cytology subgroup; notably, the HR of the 0 cell‐cluster subgroup compared with that of the normal cytology subgroup was higher than that of the 1–4 cell‐cluster subgroup (with the normal cytology subgroup used as reference; 0 cell cluster: HR = 2.48, P = 0.001; 1–4 cell clusters: HR = 1.42, P = 0.274; Fig. 2b). Among the cell cluster 0 subgroup, 8 of 17 patients (47.1%) possessed ≥50 isolated cancer cells such as signet ring cells in PL fluid, which suggests that this factor increases HR. In addition, the optimal cut‐off value of the total number of cancer cells identified by the ROC curve analysis was 41.5 among the 68 CY+ patients (Fig. S2a). As none of these patients’ PL specimens had 42–49 cancer cells, we determined that the value was 50. The ROC curve for cell cluster numbers was based on the same CY+ patients, minus eight patients who had ≥50 isolated cancer cells but no cell clusters (i.e., n = 60). The optimal cut‐off number of cell clusters was 4.5 (Fig. S2b). These two cut‐off values indicated patients with favorable and unfavorable survival outcomes.
Figure 2.
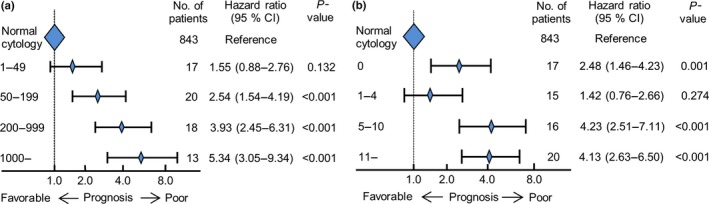
Hazard ratio ranges with 95% confidence intervals for overall survival, by (a) total number of cancer cells; and (b) total number of cell clusters in peritoneal lavage fluid.
Classification of CY+ patients
Univariate Cox regression analyses were performed to examine the relationship between qualitative and quantitative factors associated with atypical cytology and the outcomes of the 68 CY+ patients. Three factors were associated with OS: signet ring cells, ≥50 cancer cells, and ≥5 cell clusters (Table 1). A multivariate analysis revealed that two factors—the presence of any signet ring cells (CY‐S) and ≥5 cell clusters (CY‐C)—were independently correlated with poor outcome (signet ring cell: HR: 7.19, 95% CI: 1.9–26.8, P = 0.003; ≥5 cell clusters: HR: 2.97, 95% CI: 1.57–5.61, P = 0.001). The outcomes of both the CY‐S and CY‐C subgroups were significantly poorer than those of the CY‐S−/CY‐C− subgroup (CY‐S: P = 0.004; CY‐C: P = 0.001; Fig. 3a,b). Thus, the factors described in Table 1 were examined for 24 patients with negative results for both CY‐S and CY‐C. Among these patients, the presence of ≥50 isolated cancer cells was significantly associated with shorter OS (Fig. 3c, P = 0.002). Only 4 of 24 patients (16.7%) had ≥50 isolated cancer cells in their PL fluid (CY‐I); all of them had recurrence and died of gastric cancer within 2 years (Fig. 3c). Based on their outcomes, we defined the CY‐I subgroup as high‐risk CY+. Although the HRs for the CY‐S, CY‐C, and CY‐I subgroups were significantly higher than that of the normal cytology subgroup, no significant difference in outcome was observed for the CY‐S−/CY‐C−/CY‐I− (CY‐N) subgroup and the normal cytology subgroup (Fig. S3).
Table 1.
Hazards analysis of atypical cytology in 68 CY+ patients
| Atypical cytology | No. of patients (%) n = 68 | Univariate analysis* | Multivariate analysis* | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P‐value* | Hazard ratio (95% CI) | P‐value | ||
| Irregular shape of a nucleus† | |||||
| Absent | 50 (86.2) | Reference | |||
| Present | 8 (13.8) | 1.01 (0.45–2.26) | 0.974 | ||
| N/C ratio† | |||||
| 0.8> | 26 (44.8) | Reference | |||
| 0.8≤ | 32 (55.2) | 1.29 (0.73–2.27) | 0.375 | ||
| Increased nuclear chromatin† | |||||
| Absent | 26 (44.8) | Reference | |||
| Present | 32 (55.2) | 1.22 (0.69–2.15) | 0.497 | ||
| Anisonucleosis†, ‡ | |||||
| Absent | 39 (67.2) | Reference | |||
| Present | 19 (32.8) | 1.22 (0.68–2.20) | 0.509 | ||
| Signet ring cell | |||||
| Absent | 58 (85.3) | Reference | Reference | ||
| Present | 10 (14.7) | 2.79 (1.35–5.76) | 0.004 | 7.19 (1.93–26.78) | 0.003 |
| Intracytoplasmic lumina | |||||
| Absent | 47 (69.1) | Reference | |||
| Present | 21 (30.9) | 0.96 (0.54–1.71) | 0.898 | ||
| Cell cluster§ | |||||
| Absent | 17 (25.0) | Reference | |||
| Present | 51 (75.0) | 1.17 (0.64–2.13) | 0.613 | ||
| Total number of cancer cells | |||||
| 50> | 17 (25.0) | Reference | |||
| 50≤ | 51 (75.0) | 2.29 (1.20–4.37) | 0.012 | ||
| Number of cell clusters§ | |||||
| 5> | 32 (47.1) | Reference | Reference | ||
| 5≤ | 36 (52.9) | 2.34 (1.37–4.00) | 0.001 | 2.97 (1.57–5.61) | 0.001 |
*A Cox‐regression model was used for univariate and multivariate analyses. †Ten patients with only signet ring cells were not examined for detailed nuclear findings. ‡A remarkable difference in size of nuclei (<1.5 or ≥1.5). §Cell cluster formation was defined by the three‐dimensional (3D) aggregation of more than 3 cancer cells. CI, confidence interval; CY+, positive peritoneal lavage cytology; N/C ratio, ratio of nucleus to cytoplasm.
Figure 3.
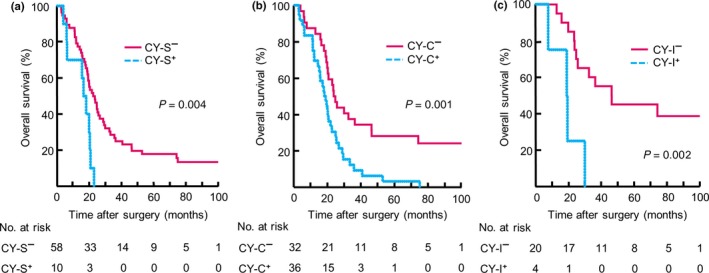
(a) Kaplan–Meier curves for signet ring cell present (CY‐S+) and absent (CY‐S−) subgroups in 68 patients with positive peritoneal lavage cytology findings (CY+) (b) Kaplan–Meier curves for ≥5 cell clusters (CY‐C+) and cell clusters <5 (CY‐C−) subgroups among 68 CY+ patients (c) Kaplan–Meier curves for ≥50 isolated cancer cells (CY‐I+) and isolated cancer cells <50 (CY‐I−) subgroups in 24 CY+ patients without either CY‐S+ or CY‐C+. Numbers of patients at risk are shown below each curve.
Diagnostic algorithm for CY+ findings
We considered each subgroup with at least one of CY‐S, CY‐C or CY‐I as a high‐risk CY+ subgroup (CYHigh); the CYHigh subgroup had poor outcomes, equivalent to those of patients with M1 disease. The remaining subgroup (CY‐N) was classified as a low‐risk CY+ subgroup (CYLow). A diagnostic algorithm for PL cytology was then proposed based on this subclassification (Fig. 4a). Initially, samples were screened for the presence of factors such as CY‐S, CY‐C, and CY‐I, and samples exhibiting at least one of these factors were classified as CYHigh. The remaining CY‐N subgroup was classified as CYLow. There were no significant differences between patients with CYHigh and CYLow regarding clinicopathological factors (Table S2). The OS curves for the normal cytology, CYLow and CYHigh groups are shown in Figure 4(b). In a multivariate Cox regression analysis, CYHigh factor was an independent predictor of poor prognosis, with the highest HR of all examined factors (HR: 3.14, 95% CI: 2.29–4.31, P < 0.001; Table 2), whereas CYLow had an HR similar to the normal cytology group in univariate analysis (HR: 1.14, 95% CI: 0.64–2.03, P = 0.646). Of the 48 patients with CYHigh findings, 47 patients (97.9%) experienced recurrences. The most frequent first recurrence site was the peritoneum (n = 34), followed by the lymph nodes (n = 10). The optimal cut‐off value of the tumor size identified by the ROC curve analysis was 8.05 (Fig. S4).
Figure 4.
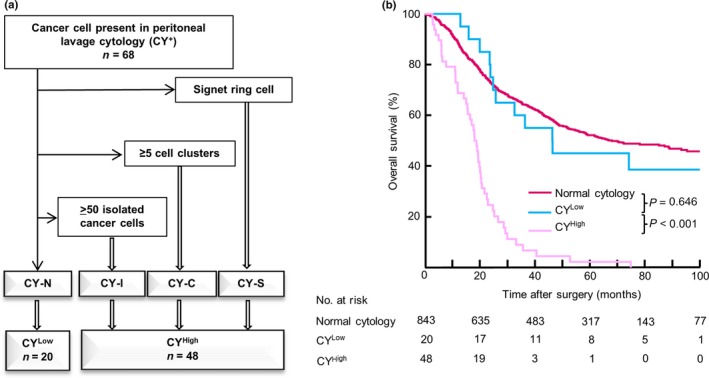
(a) Diagnostic algorithm for peritoneal lavage cytology (b) Kaplan–Meier curves for normal cytology, and for CYLow and CYHigh subgroups among 911 patients with gastric cancer. Numbers of patients at risk are shown below each curve.
Table 2.
Hazards analysis of pathological prognostic factors in 911 patients underwent R0/1 resection
| No. of patients (%) n = 911 | Univariate analysis* | Multivariate analysis* | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | ||
| Borrmann classification | |||||
| Non‐Type 4 | 774 (85.0) | Reference | Reference | ||
| Type 4† | 137 (15.0) | 2.34 (1.88–2.90) | <0.001 | 1.88 (1.45–2.42) | <0.001 |
| Tumor size‡ | |||||
| 8.0> | 582 (63.9) | Reference | Reference | ||
| 8.0≤ | 329 (36.1) | 1.95 (1.63–2.34) | <0.001 | 1.33 (1.08–1.63) | 0.007 |
| Lauren classification | |||||
| Intestinal type | 368 (40.4) | Reference | Reference | ||
| Diffuse type | 543 (59.6) | 1.54 (1.27–1.87) | <0.001 | 1.24 (1.01–1.52) | 0.042 |
| Tumor depth§ | |||||
| T3 | 376 (41.3) | Reference | Reference | ||
| T4a | 483 (53.0) | 2.12 (1.73–2.60) | <0.001 | ||
| T4b | 52 (5.7) | 2.86 (1.97–4.15) | <0.001 | 1.54 (1.08–2.20) | 0.018 |
| Lymph‐node metastasis§ | |||||
| N0/1 | 366 (40.2) | Reference | Reference | ||
| N2/3 | 458 (50.3) | 2.48 (2.00–3.07) | <0.001 | ||
| Distant metastasis | 87 (9.5) | 4.95 (3.69–6.64) | <0.001 | 2.25 (1.73–2.93) | <0.001 |
| Lymphatic invasion | |||||
| Absent | 267 (29.3) | Reference | Reference | ||
| Present | 644 (70.7) | 1.84 (1.48–2.29) | <0.001 | 1.49 (1.18–1.87) | 0.001 |
| Venous invasion | |||||
| Absent | 152 (16.7) | Reference | Reference | ||
| Present | 759 (83.3) | 1.43 (1.11–1.84) | 0.006 | 1.48 (1.14–1.94) | 0.004 |
| Liver metastasis | |||||
| Absent | 891 (97.8) | Reference | Reference | ||
| Present | 20 (2.2) | 1.75 (1.05–2.93) | 0.033 | 1.93 (1.12–3.32) | 0.019 |
| Peritoneal metastasis | |||||
| Absent | 874 (95.9) | Reference | Reference | ||
| Present | 37 (4.1) | 2.08 (1.43–3.34) | <0.001 | 1.64 (1.11–2.41) | 0.013 |
| Peritoneal lavage cytology¶ | |||||
| Negative | 843 (92.5) | Reference | Reference | ||
| CYLow | 20 (2.2) | 1.14 (0.64–2.03) | 0.646 | ||
| CYHigh | 48 (5.3) | 4.47 (3.28–6.10) | <0.001 | 3.14 (2.29–4.31) | <0.001 |
*A Cox‐regression model was used for univariate and multivariate analyses. †Infiltrative growth pattern of the Borrmann's classification. ‡The cut‐off value of a tumor size influencing on poor prognosis was defined by receiver operating characteristic curve (Fig. S4). §TNM classification of International Union Against Cancer (UICC) 7th edition. ¶Among positive peritoneal lavage (CY+), the subgroup with presence of a signet ring cell (CY‐S), ≥5 cell clusters (CY‐C), or ≥50 isolated cancer cells (CY‐I) in peritoneal lavage fluid were defined as CYHigh and the remaining subgroup was defined as CYLow. CI, confidence interval.
Prognostic effect of CYHigh status with no macroscopic distant metastasis
Prognostic analysis was performed for 772 patients with no macroscopic distant metastasis to examine applicability of our algorithm to the patient population. Kaplan–Meier curves for the normal cytology, CYLow and CYHigh groups among the 772 patients are shown in Figure S5; a log rank test between CYHigh versus CYLow was also performed to measure the influence of CY+ status on patient outcome (P < 0.001). A multivariate Cox regression analysis in patients with no macroscopic distant metastasis showed CYHigh to have the highest HR of all examined factors (HR: 2.95, 95% CI: 2.03–4.30, P < 0.001, Table S3), which attests to the prognostic implications of CYHigh and shows this cytology algorithm is also applicable to the 772 patients with no macroscopic distant metastasis.
R1 resection for CYHigh patients with no macroscopic peritoneal metastasis
According to our algorithm, 54 (5.3%) out of the 1028 patients enrolled in the current study were CYHigh patients with no macroscopic peritoneal metastasis (P‐CYHigh; Fig. S6). We investigated the influence of R1 and R2 resections on the outcomes of these P‐CYHigh patients. A comparison of clinicopathological characteristics among P‐CYHigh patients according to their residual tumor classification status revealed that the R2 resection group was significantly older and had higher percentages of distant lymph node metastasis and liver metastasis than the R1 resection group (Table S4). The OS curve for the 54 P‐CYHigh patients is shown in Figure 5. The 2‐year and 5‐year survival rates of the 43 patients who underwent R1 resection with curative intent were 25.2% and 2.4%, respectively, which did not significantly differ from the 2‐year and 5‐year survival rates of the 11 patients who underwent R2 resection (27.2% and 0.0%, respectively; P = 0.284).
Figure 5.
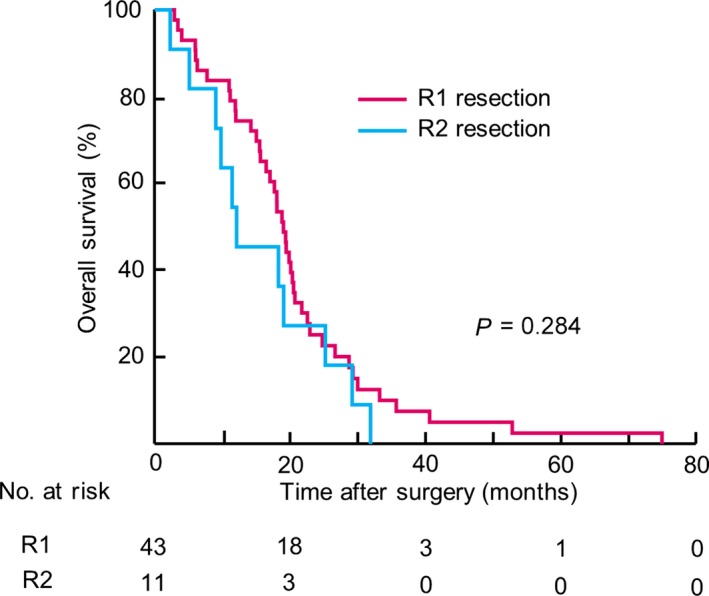
Kaplan–Meier curves for patients’ residual tumor classifications among 54 patients with CYHigh and without macroscopic peritoneal metastasis (P‐CYHigh). Numbers of patients at risk are shown below each curve.
Discussion
We propose a new algorithm to select candidates for curative gastric cancer resection. CYHigh status strongly predicted poor prognosis, with the highest hazard ratio among clinicopathological factors, including TNM factors. The survival curve of P‐CYHigh patients who underwent R1 resection was similar to that of the R2 resection group (Fig. 5), whereas the curve for CYLow patients, whose cancer cells were found in lavage fluid but did not meet the CYHigh criteria, was similar to that of the normal cytology group (Fig. 4b). Therefore, our algorithm could help discern patients likely to benefit from R1 resection.
This study focused on the conventional method used at most institutions to enable quick intraoperative reporting. Conventional cytological diagnoses are based on qualitative morphological findings, but not on quantitative factors; the diagnosis is reported as either the presence (positive) or absence (negative) of cancer cells. However, the quantity of tumor cells in lavage fluid is highly variable for gastric cancer, and sensitivity for tumor cells among the various methods, including conventional cytology, ICC and RT‐PCR, is inconsistent. Use of a tumor‐specific antibody with ICC may characterize undetermined cancer cells from noncancerous cells in conventional cytology and can enable higher detection rates;6 RT‐PCR for mRNA of tumor cell‐specific genes is a sensitive and specific method for even very small numbers of tumor cells, and is reportedly superior to other methods for objective measurement of peritoneal micrometastasis and prediction of peritoneal recurrence.13, 14 However, the predictive effect of detecting small numbers of tumor cells in peritoneal fluid is unclear.15, 16 Small amounts of tumor mRNA are known not to affect prognosis because the highly sensitive detection of clinically insignificant tumor cells are unlikely to predict metastatic tumors.16 In contrast, the current study showed that CYHigh status (as defined in this study), using the conventional cytology, is a strong independent predictor of a poor survival outcome, even after R1 resection. The criteria for a CYHigh may be useful for excluding patients with a small number of cancer cells present in the lavage fluid who might benefit from surgical resection.
The cytopathological diagnosis should be conducted by a standardized procedure in all institutions. Our conventional cytological diagnosis of lavage fluid is simple, and can be performed at most institutions capable of providing rapid intraoperative cytological diagnosis to determine whether a surgical resection should be continued. However, this study was so small cohort and underpowered, so procedure standardization and our proposed CY+ criteria require further validation through a multicenter study or a larger cohort, as a next step.
Among the criteria of CYHigh, CY‐S was a qualitative diagnostic criterion in the present study. The presence of a signet ring cell is easy to detect and can be considered as an objective criterion because of the specific features of these cells, such as their non‐cohesiveness and the presence of intracytoplasmic mucin that push the nucleus to the periphery of the cell. Signet ring cells were mostly formed from a large number of isolated tumor cells in peritoneal fluid. Among patients with CY‐S, 7 patients (70%) had >500 isolated signet ring cells in peritoneal fluid. The histological subtype that includes signet ring cells is associated with poor prognosis among patients with gastric cancer, with tumor cells that exhibit a high affinity for lymphatic tissue.17 Piessen et al. showed patients with signet ring cells to have more lymph node metastases than patients without signet ring cells and a higher rate of peritoneal recurrence.17 Three‐dimensional (3D) aggregates composed of cohesive cells forming ≥5 cell clusters in peritoneal fluid (CY‐C) were also shown to independently predict poor prognosis. These results revealed that both qualitative and quantitative examinations are important in PL cytology. Previous experimental data showed the significance of the formation of cell clusters in intraperitoneal cancer metastasis.18 These 3D aggregates of tumor cells, called multicellular spheroids, have been shown to be resistant to anoikis and apoptosis, including those induced by chemotherapy, in ovarian cancer;19, 20, 21 a cell line that formed cell clusters also had a superior migratory and invasive capacity compared with a cell line that did not form multicellular spheroids.20 Similar results have been reported for the gastric cancer cell line MKN 45.22 In a clinical study, the existence of carcinoma cell clusters in PL cytology was correlated with poor outcome, similar to gastric cancer patients with macroscopic peritoneal metastasis.23 These results suggest that the formation of cancer cell clusters might be a first step in gaining a survival advantage in peritoneal fluid. For patients without CY‐S or CY‐C in their PL cytology specimen, at least 50 isolated tumor cells (defined as CY‐I) were required to predict a poor prognosis, similar to CY‐S or CY‐C described above.
Staging laparoscopies greatly benefit patients with gastric cancer in terms of formulating individualized therapeutic plans; PL cytology as part of these procedures can provide diagnostic guidance. The utility of PL cytology increased with the prevalence of preoperative staging laparoscopy. Our PL cytology had been performed intraoperatively before 2009, even though staging laparoscopy was not then performed in our institution. This additional research purpose, in which we believe we have succeeded, was to construct appropriate diagnostic criteria for PL cytology performed during staging laparoscopies, which improve decisions for optimal therapy for patients with gastric cancer.
As the 911 patients in this study had undergone surgery between 1992 and 2009, they differ from patients who undergo perioperative chemotherapy or radiotherapy as the current standard therapy. Further analysis is necessary to verify the diagnostic criteria apply to the current population of the patients with gastric cancer who undergo the standard therapy. As an additional study, Kaplan–Meier curves according to our proposed criteria among 260 patients who underwent adjuvant therapy were drawn and a log‐rank test showed a significant gap between CYHigh patients and CYLow patients (Fig. S7, P < 0.001). This result implies that CYHigh patients should avoid gastrectomy followed by adjuvant chemotherapy, and instead undergo intensive, individualized therapy.
In conclusion, the present study revealed that candidates for curative resection as an initial therapy for gastric cancer can be accurately selected based on a diagnosis of CYHigh or CYLow status, using conventional PL cytology and our proposed algorithm consisting of three critical factors (CY‐S, CY‐C and CY‐I). The criteria generated by the objective analyses in the present study could potentially become actual diagnostic criteria for conventional PL cytology in patients with gastric cancer.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting information
Fig. S1. Kaplan–Meier curves for normal cytology and positive peritoneal lavage cytology findings (CY+) subgroups among (a) the 911 patients in the R0/1 group and (b) the 772 patients with no macroscopic distant metastasis.
Fig. S2. Optimal cutoff values determined using a receiver operating characteristics (ROC) curves: (a) ROC curve for the total number of cancer cells (b) ROC curve for the total number of cell clusters.
Fig. S3. Hazard ratio ranges with 95% confidence intervals for overall survival by CY factors, including presence of any signet ring cells (CY‐S), ≥5 cell clusters (CY‐C), ≥50 isolated cancer cells (CY‐I) and the remaining subgroup (CY‐N).
Fig. S4. Optimal cutoff value determined using a receiver operating characteristics (ROC) curve for the influence of tumor size on poor prognosis.
Fig. S5. Kaplan‐Meier curves for the normal cytology, CYLow and CYHigh groups among the 772 patients with no macroscopic distant metastasis.
Fig. S6. Flow diagram to select patients with CYHigh and without macroscopic peritoneal metastasis (P‐CYHigh) out of the 1028 patients who underwent gastrectomies.
Fig. S7. Kaplan‐Meier curves for normal cytology, CYLow and CYHigh subgroups in the 260 patients who underwent adjuvant chemotherapy.
Table S1. Comparison of clinicopathological characteristics of the 911 patients in R0/1 group by peritoneal lavage cytology results (Normal cytology versus CY+).
Table S2. Comparison of clinicopathological factors of the CY+ patients by the proposed algorithm (CYHigh versus CYLow).
Table S3. Hazards analysis of pathological prognostic factors in the 772 patients with no macroscopic distant metastasis.
Table S4. Comparison of clinicopathological characteristics among P‐CYHigh patients according to their residual tumor classification status (R1 vs R2).
Cancer Sci 108 (2017) 978–986
Funding Information
No sources of funding were declared for this study.
References
- 1. Sobin L, Gospondarowicz M, Wittekind C. TNM Classification of Malignant Tumours, 7th edn Hoboken, NJ: Wiley‐Blackwell, 2009. [Google Scholar]
- 2. Hori Y. Diagnostic laparoscopy guidelines: this guideline was prepared by the SAGES Guidelines Committee and reviewed and approved by the Board of Governors of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), November 2007. Surg Endosc 2008; 22: 1353–83. [DOI] [PubMed] [Google Scholar]
- 3. Bando E, Yonemura Y, Takeshita Y et al Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg 1999; 178: 256–62. [DOI] [PubMed] [Google Scholar]
- 4. Kodera Y, Yamamura Y, Shimizu Y et al Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol 1999; 72: 60–5. [DOI] [PubMed] [Google Scholar]
- 5. Bentrem D, Wilton A, Mazumdar M, Brennan M, Coit D. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol 2005; 12: 347–53. [DOI] [PubMed] [Google Scholar]
- 6. Benevolo M, Mottolese M, Cosimelli M et al Diagnostic and prognostic value of peritoneal immunocytology in gastric cancer. J Clin Oncol 1998; 16: 3406–11. [DOI] [PubMed] [Google Scholar]
- 7. Nekarda H, Gess C, Stark M et al Immunocytochemically detected free peritoneal tumour cells (FPTC) are a strong prognostic factor in gastric carcinoma. Br J Cancer 1999; 79: 611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenberg R, Nekarda H, Bauer P, Schenck U, Hoefler H, Siewert JR. Free peritoneal tumour cells are an independent prognostic factor in curatively resected stage IB gastric carcinoma. Br J Surg 2006; 93: 325–31. [DOI] [PubMed] [Google Scholar]
- 9. de Manzoni G, Verlato G, Di Leo A et al Peritoneal cytology does not increase the prognostic information provided by TNM in gastric cancer. World J Surg 2006; 30: 579–84. [DOI] [PubMed] [Google Scholar]
- 10. Katsuragi K, Yashiro M, Sawada T, Osaka H, Ohira M, Hirakawa K. Prognostic impact of PCR‐based identification of isolated tumour cells in the peritoneal lavage fluid of gastric cancer patients who underwent a curative R0 resection. Br J Cancer 2007; 97: 550–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hioki M, Gotohda N, Konishi M, Nakagohri T, Takahashi S, Kinoshita T. Predictive factors improving survival after gastrectomy in gastric cancer patients with peritoneal carcinomatosis. World J Surg 2010; 34: 555–62. [DOI] [PubMed] [Google Scholar]
- 12. Kinoshita T, Kinoshita T, Saiura A, Esaki M, Sakamoto H, Yamanaka T. Multicentre analysis of long‐term outcome after surgical resection for gastric cancer liver metastases. Br J Surg 2015; 102: 102–7. [DOI] [PubMed] [Google Scholar]
- 13. Kodera Y, Nakanishi H, Ito S et al Quantitative detection of disseminated free cancer cells in peritoneal washes with real‐time reverse transcriptase‐polymerase chain reaction: a sensitive predictor of outcome for patients with gastric carcinoma. Ann Surg 2002; 235: 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaneko K, Yano M, Yamano T et al Detection of peritoneal micrometastases of gastric carcinoma with green fluorescent protein and carcinoembryonic antigen promoter. Cancer Res 2001; 61: 5570–4. [PubMed] [Google Scholar]
- 15. Wong J, Coit D. Detection of gastric cancer peritoneal metastases by peritoneal lavage: current limitations and future perspectives. Surgery 2012; 152: 1–4. [DOI] [PubMed] [Google Scholar]
- 16. Fujiwara Y, Doki Y, Taniguchi H et al Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer 2007; 10: 197–204. [DOI] [PubMed] [Google Scholar]
- 17. Piessen G, Messager M, Leteurtre E, Jean‐Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg 2009; 250: 878–87. [DOI] [PubMed] [Google Scholar]
- 18. Sodek KL, Murphy KJ, Brown TJ, Ringuette MJ. Cell‐cell and cell‐matrix dynamics in intraperitoneal cancer metastasis. Cancer Metastasis Rev 2012; 31: 397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Desoize B, Jardillier J. Multicellular resistance: a paradigm for clinical resistance? Crit Rev Oncol Hematol 2000; 36: 193–207. [DOI] [PubMed] [Google Scholar]
- 20. Sodek KL, Ringuette MJ, Brown TJ. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int J Cancer 2009; 124: 2060–70. [DOI] [PubMed] [Google Scholar]
- 21. Santini MT, Rainaldi G, Indovina PL. Apoptosis, cell adhesion and the extracellular matrix in the three‐dimensional growth of multicellular tumor spheroids. Crit Rev Oncol Hematol 2000; 36: 75–87. [DOI] [PubMed] [Google Scholar]
- 22. Liu J, Ma L, Xu J et al Spheroid body‐forming cells in the human gastric cancer cell line MKN‐45 possess cancer stem cell properties. Int J Oncol 2013; 42: 453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Majima T, Ichikura T, Mochizuki H. Prognostic significance of the cytologic features of free cancer cells in the peritoneal cavity of patients with gastric cancer. Surg Today 2002; 32: 35–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Kaplan–Meier curves for normal cytology and positive peritoneal lavage cytology findings (CY+) subgroups among (a) the 911 patients in the R0/1 group and (b) the 772 patients with no macroscopic distant metastasis.
Fig. S2. Optimal cutoff values determined using a receiver operating characteristics (ROC) curves: (a) ROC curve for the total number of cancer cells (b) ROC curve for the total number of cell clusters.
Fig. S3. Hazard ratio ranges with 95% confidence intervals for overall survival by CY factors, including presence of any signet ring cells (CY‐S), ≥5 cell clusters (CY‐C), ≥50 isolated cancer cells (CY‐I) and the remaining subgroup (CY‐N).
Fig. S4. Optimal cutoff value determined using a receiver operating characteristics (ROC) curve for the influence of tumor size on poor prognosis.
Fig. S5. Kaplan‐Meier curves for the normal cytology, CYLow and CYHigh groups among the 772 patients with no macroscopic distant metastasis.
Fig. S6. Flow diagram to select patients with CYHigh and without macroscopic peritoneal metastasis (P‐CYHigh) out of the 1028 patients who underwent gastrectomies.
Fig. S7. Kaplan‐Meier curves for normal cytology, CYLow and CYHigh subgroups in the 260 patients who underwent adjuvant chemotherapy.
Table S1. Comparison of clinicopathological characteristics of the 911 patients in R0/1 group by peritoneal lavage cytology results (Normal cytology versus CY+).
Table S2. Comparison of clinicopathological factors of the CY+ patients by the proposed algorithm (CYHigh versus CYLow).
Table S3. Hazards analysis of pathological prognostic factors in the 772 patients with no macroscopic distant metastasis.
Table S4. Comparison of clinicopathological characteristics among P‐CYHigh patients according to their residual tumor classification status (R1 vs R2).


