Abstract
Weekly administration of nanoparticle albumin‐bound paclitaxel (nab‐paclitaxel) has been shown to be a safe and effective treatment for metastatic breast cancer (MBC) in clinical studies. We conducted a multicenter, randomized, open‐label phase II study to compare the efficacy and safety of weekly nab‐paclitaxel and docetaxel in Japanese patients with human epidermal growth factor receptor 2‐negative MBC. The primary endpoint was progression‐free survival (PFS). Patients were randomized to receive nab‐paclitaxel (150 mg/m2 nab‐paclitaxel once per week for 3 of 4 weeks; n = 100) or docetaxel (75 mg/m2 docetaxel every 3 weeks; n = 100). The median PFS by independent radiologist assessment was 9.8 months (90% confidence interval [CI]: 8.5–11.2) for nab‐paclitaxel and 11.2 months (90% CI: 8.4–13.8) for docetaxel (hazard ratio: 1.25, P = 0.363), and the median overall survival was 42.4 months and 34.0 months, respectively. The overall response rate was 56.1% for nab‐paclitaxel and 52.5% for docetaxel. Adverse events in both treatment arms were similar to previous reports. Neutropenia was the most common adverse event in both arms, with 35.0% of patients in the nab‐paclitaxel arm and 89.0% in the docetaxel arm experiencing grade 4 neutropenia. Grade 3 peripheral sensory neuropathy occurred in 22.0% of patients in the nab‐paclitaxel and 5.0% in the docetaxel arm. In this study, although weekly nab‐paclitaxel 150 mg/m2 did not show superiority in PFS compared with docetaxel, efficacy outcomes were similar in patients treated with weekly nab‐paclitaxel and docetaxel.
Keywords: albumin‐bound paclitaxel, docetaxel, metastatic breast cancer, phase II, weekly regimen
Breast cancer is the most common cancer among American and Japanese women,1 with about one in eight (12%) women in the United States and one in 16 (6%) in Japan developing invasive breast cancer during their lifetime. In Japan, the predicted incidence of breast cancer is 90 000 (the most common cancer in women), with 14 000 breast cancer deaths (the fifth leading cause of death in women) in 2016.2
Taxane‐containing regimens were shown to improve overall survival (OS) in women with metastatic breast cancer (MBC) in a large systematic review,3 while concurrent combined use of anthracyclines and taxanes failed to prolong OS compared with single and sequential use of each drug.4, 5, 6 The single use of taxanes, such as paclitaxel and docetaxel, has thus become one of the first‐line regimens for MBC.7, 8
Weekly (qw) administration of paclitaxel in patients with MBC was more effective than administration every 3‐weeks (q3w) in terms of overall response rate (ORR), time to progression and OS (CALGB 9840).9 In an adjuvant setting, paclitaxel qw (hazard ratio [HR]: 1.27, P = 0.006) and docetaxel q3w (HR: 1.23, P = 0.02) were superior to paclitaxel q3w in terms of disease‐free survival, and OS was higher with paclitaxel qw (HR: 1.32, P = 0.01) compared with paclitaxel q3w.10 Based on this evidence, patients with MBC patients are often treated with paclitaxel qw or docetaxel q3w in clinical practice.
However, solvent‐based paclitaxel (sb‐paclitaxel) and docetaxel formulations have several limitations; both require solvents (Cremophor EL or polysorbate 80) to increase their solubility, and these solvents are associated with the development of hypersensitivity and decreased efficiency of drug delivery to the tumor.11, 12, 13 In contrast, nanoparticle albumin‐bound paclitaxel (nab‐paclitaxel) is a solvent‐free albumin‐bound form of paclitaxel, which enables it to be administered more quickly, without the need for steroids and anti‐histamine premedication to reduce solvent‐related hypersensitivity reactions. Compared with sb‐paclitaxel, nab‐paclitaxel demonstrated enhanced transport across endothelial cell monolayers and increased tumor delivery of paclitaxel.14, 15
In a phase III trial, nab‐paclitaxel q3w at a dose of 260 mg/m2 demonstrated a significantly higher ORR compared with sb‐paclitaxel in patients with MBC.16 Based on the results for sb‐paclitaxel, nab‐paclitaxel qw is also expected to have greater efficacy than nab‐paclitaxel q3w. Additionally, nab‐paclitaxel qw has been reported to allow a facilitate relative dose intensity, with improved antitumor activity compared with q3w.17, 18 Indeed, a phase II study demonstrated significantly longer progression‐free survival (PFS) with nab‐paclitaxel 150 mg/m2 qw as first‐line therapy compared with docetaxel 100 mg/m2 q3w, with a favorable safety profile in patients with MBC, most of whom were Caucasian.19 Nab‐paclitaxel (150 mg/m2) also showed favorable tolerability in a phase I study involving Japanese patients with MBC.20
Based on these results, we investigated further qw dosing schedules of nab‐paclitaxel in Japanese patients with MBC. Docetaxel 75 mg/m2 was determined to be a tolerable dose based on previous studies in Japanese breast cancer patients.21 We therefore aimed to compare the efficacy and safety profiles of nab‐paclitaxel 150 mg/m2 qw and docetaxel 75 mg/m2 in Japanese patients with MBC.
Materials and Methods
Study design
This multicenter, randomized, controlled, open‐label phase II study was conducted at 21 sites in Japan. The primary endpoint was PFS by independent radiologist assessment, which was defined as the time from enrollment until tumor progression or death from any cause, with censoring of patients who were lost to follow‐up. The secondary endpoints were ORR (proportion of patients who achieved a complete response [CR] or partial response [PR]), time to treatment failure (TTF; time from the date of enrollment to earliest date of last study drug administration, objective progressive disease [PD] or death) and OS. The disease control rate (proportion of patients who achieved CR, PR or stable disease) was also assessed. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and was approved by the ethical committee or review board of each institution. All patients provided written consent before their enrollment.
Patients
The main inclusion criteria were as follows: female sex; histologically or cytologically confirmed breast cancer; clinically confirmed MBC, with no history of chemotherapy after confirmation of metastasis; MBC without human epidermal growth factor receptor 2 overexpression determined by immunohistochemical staining or FISH (patients with immunohistochemical 3+ or 2+ and FISH‐positive results were excluded from the study); at least one measurable lesion defined by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 within 28 days prior to enrollment; Eastern Cooperative Oncology Group performance status (PS) of 0–1; retained organ functions; and no prior chemotherapy except for adjuvant/neoadjuvant chemotherapy. The main exclusion criteria were as follows: recurrence within 12 months after the last treatment with taxane therapy before/after surgery, or progression during taxane therapy prior to surgery; symptomatic or treatment‐requiring brain metastasis; pleural fluid; ascites; grade ≥2 pre‐existing peripheral neuropathy; or pericardial fluid requiring drainage.
Treatment
Patients were randomly assigned (1:1) via a centralized randomization system to receive either nab‐paclitaxel or docetaxel using a minimization allocation method. The randomization procedure was generated by EPS Corporation (Tokyo, Japan), independent of the sponsor. The randomization was stratified by prior received taxanes as adjuvant therapy (yes or no) and Eastern Cooperative Oncology Group PS (0 or 1). Nab‐paclitaxel at an initial dose of 150 mg/m2 was administered intravenously over 30 min, qw for 3 of 4 weeks. Dose reductions were permitted to manage toxicities such as neutropenia, thrombocytopenia, or non‐hematologic toxicities (Table S1). Patients requiring further dose reduction than 80 mg/m2 (level 3) were withdrawn from the study (Tables S1, S2). Dose‐interruption criteria on Days 8 and 15 are shown in Table S3. A new treatment cycle was postponed for a maximum of 21 days if one or more criteria for starting the next cycle were not met (Table S4). The postponed cycle or omitted dosing was resumed after confirming that the criteria to start the next cycle were met.
Docetaxel at a dose of 75 mg/m2 was injected intravenously over 1 h, q3w. Dose reductions to 60 and 50 mg/m2 were permitted, according to the same criteria described for nab‐paclitaxel above. Discontinuation, dose omission, and cycle resumption were determined according to the same criteria as for nab‐paclitaxel.
The cycles for both study drugs were repeated until disease progression, development of unacceptable toxicity, or withdrawal of consent.
Assessments
Tumor assessment was done at screening and every 6 weeks from randomization. Additional assessments were carried out if disease progression was suspected. Tumor responses were evaluated according to the RECIST version 1.0.22 Tumor assessments were carried out by the independent radiologist assessment (ICON Medical Imaging, PA, USA) and by the investigators.
Adverse events were recorded and graded according to the Common Terminology Criteria for Adverse Events version 3.0.
Statistical analysis
Efficacy data were analyzed for the full analysis set, defined as eligible patients who received the study drug at least once. Pre‐specified subgroup analyses of OS and PFS were performed to assess the effects of prior use of adjuvant taxanes and of triple‐negative breast cancer (TNBC). The primary endpoint was analyzed at a 5% significance level using the log‐rank test and Cox's proportional hazards model. Survival was analyzed using medians with 90% or 95% confidence intervals (CI), respectively, using Kaplan–Meier estimates. ORR was compared between the two treatment arms using Fisher's exact test. TTF and OS were analyzed as for PFS with 95% CI.
We assumed the median PFS for nab‐paclitaxel 150 mg/m2 qw was 11.8 months and that for docetaxel 75 mg/m2 was 7.5 months, based on the results of a previous phase II study by Gradishar et al.19 We calculated that 152 patients would be needed to demonstrate the superiority of nab‐paclitaxel 150 mg/m2 qw compared with docetaxel 75 mg/m2 q3w at a one‐sided 0.05 significance level and power of 80%. Assuming that 5% of patients might be excluded from the full analysis set, we estimated that we required to enroll 160 patients in the present study. During the recruitment process, we revised this estimate to 192 patients to account for the fact that the number of PD events judged by an independent radiologist assessment tends to be lower than that judged by the investigator, based on a previous study of 762 patients, of whom 635 were judged to have PD according to the investigators’ assessment, compared with only 521 according to the independent radiologist assessment.23 The number of needed events (121 events) was not modified.
Results
Patients
From November 2009 to December 2012, 200 patients were enrolled in this study, of whom 197 were assessed as eligible. Figure 1 shows the CONSORT flow diagram of the study. A total of 200 patients were allocated to the two treatment arms, with 100 patients in each. All 200 patients received at least one treatment and served as the safety analysis population. Three patients were excluded from the full analysis set because of protocol deviations, including two in the nab‐paclitaxel and one in the docetaxel arm. The major reasons for treatment discontinuation were disease progression (57.1% nab‐paclitaxel, 45.5% docetaxel) and adverse events (26.5% nab‐paclitaxel, 27.3% docetaxel) (Fig. 1). The cut‐off date for the primary endpoint was 31 January 2013, and the median follow‐up time was 23.0 months.
Figure 1.
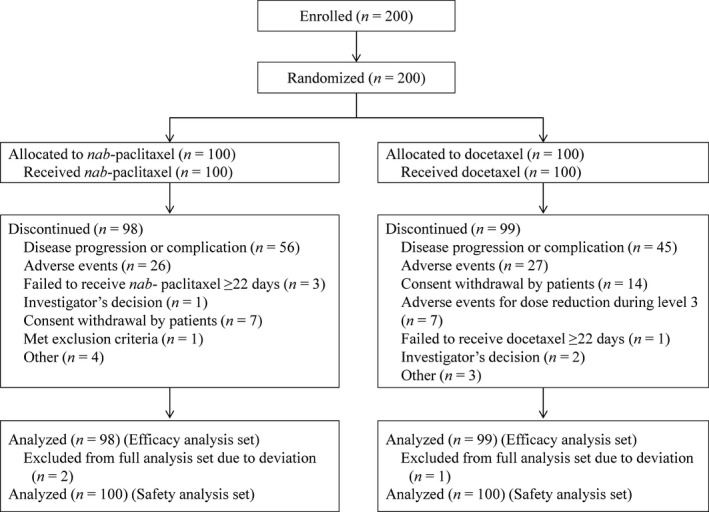
CONSORT flow diagram.
Patient characteristics (full analysis set) were balanced between the nab‐paclitaxel and docetaxel arms, respectively (Table 1), including median age (60 vs 58 years), age <65 years (76.5% vs 81.8%), ratio of TNBC (21.4% vs 15.2%), and prior receipt of taxanes as adjuvant chemotherapy (27.6% vs 30.3%).
Table 1.
Patient demographics and characteristics
| Nab‐paclitaxel (n = 98) | Docetaxel (n = 99) | P‐value† | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Sex | |||||
| Female | 98 | (100.0) | 99 | (100.0) | |
| Age (years) | |||||
| Median | 60.0 | 58.0 | |||
| Range [min, max] | [25, 74] | [33, 74] | |||
| ECOG performance status | |||||
| 0 | 87 | (88.8) | 86 | (86.9) | F: 0.828 |
| 1 | 11 | (11.2) | 13 | (13.1) | |
| Triple negative | |||||
| Yes | 21 | (21.4) | 15 | (15.2) | F: 0.274 |
| Estrogen receptor | |||||
| Negative | 23 | (23.5) | 16 | (16.2) | C: 0.198 |
| Positive | 75 | (76.5) | 83 | (83.8) | |
| Progesterone receptor | |||||
| Negative | 41 | (41.8) | 35 | (35.4) | C: 0.413 |
| Positive | 57 | (58.2) | 63 | (63.6) | |
| Unknown | 0 | (0.0) | 1 | (1.0) | |
| Organ metastases‡ | |||||
| Lung | 49 | (50.0) | 42 | (42.4) | |
| Bone | 44 | (44.9) | 53 | (53.5) | |
| Lymph node | 43 | (43.9) | 39 | (39.4) | |
| Liver | 37 | (37.8) | 46 | (46.5) | |
| Surgical history | |||||
| No | 29 | (29.6) | 34 | (34.3) | F: 0.542 |
| Yes | 69 | (70.4) | 65 | (65.7) | |
| Prior adjuvant therapy with taxanes | |||||
| Sb‐paclitaxel | 13 | (13.3) | 20 | (20.2) | F: 0.252 |
| Docetaxel | 14 | (14.3) | 10 | (10.1) | F: 0.392 |
Analysis set: full analysis set. †F, Fisher's exact test; C, χ2 test. ‡Multiple answers allowed. ECOG, Eastern Cooperative Oncology Group; sb, solvent‐based.
The median relative dose intensities for nab‐paclitaxel and docetaxel were 70.2% and 83.5%, respectively. The rates of second‐line therapy use were similar in both arms (94.9% nab‐paclitaxel vs 90.9% docetaxel).
Efficacy
The median PFS for nab‐paclitaxel and docetaxel by the independent radiologist assessment was 9.8 months (90% CI: 8.5–11.2) and 11.2 months (90% CI: 8.4–13.8) (HR: 1.25, P = 0.363), respectively (Fig. 2a). The median PFS by the investigator was 11.2 months (90% CI: 9.7–12.7) and 10.6 months (90% CI: 8.5–11.3) (HR: 0.81, P = 0.233), respectively (Fig. 2b). In the subgroup of patients who did not receive taxanes as adjuvant chemotherapy, the median PFS by the independent radiologist assessment was 10.9 months for nab‐paclitaxel (90% CI: 8.5–12.5) and 9.9 months (90% CI: 7.8–12.6) for docetaxel (HR: 1.05, P = 0.824). There was no significant difference in PFS between patients who had and had not received taxanes as adjuvant chemotherapy (Table 2). The median PFS in the subgroup of patients with TNBC, by the independent radiologist was 6.8 months for nab‐paclitaxel (95% CI: 5.3–6.9) and 7.0 months for docetaxel (95% CI: 2.9–9.6) (HR: 1.20, P = 0.624).
Figure 2.
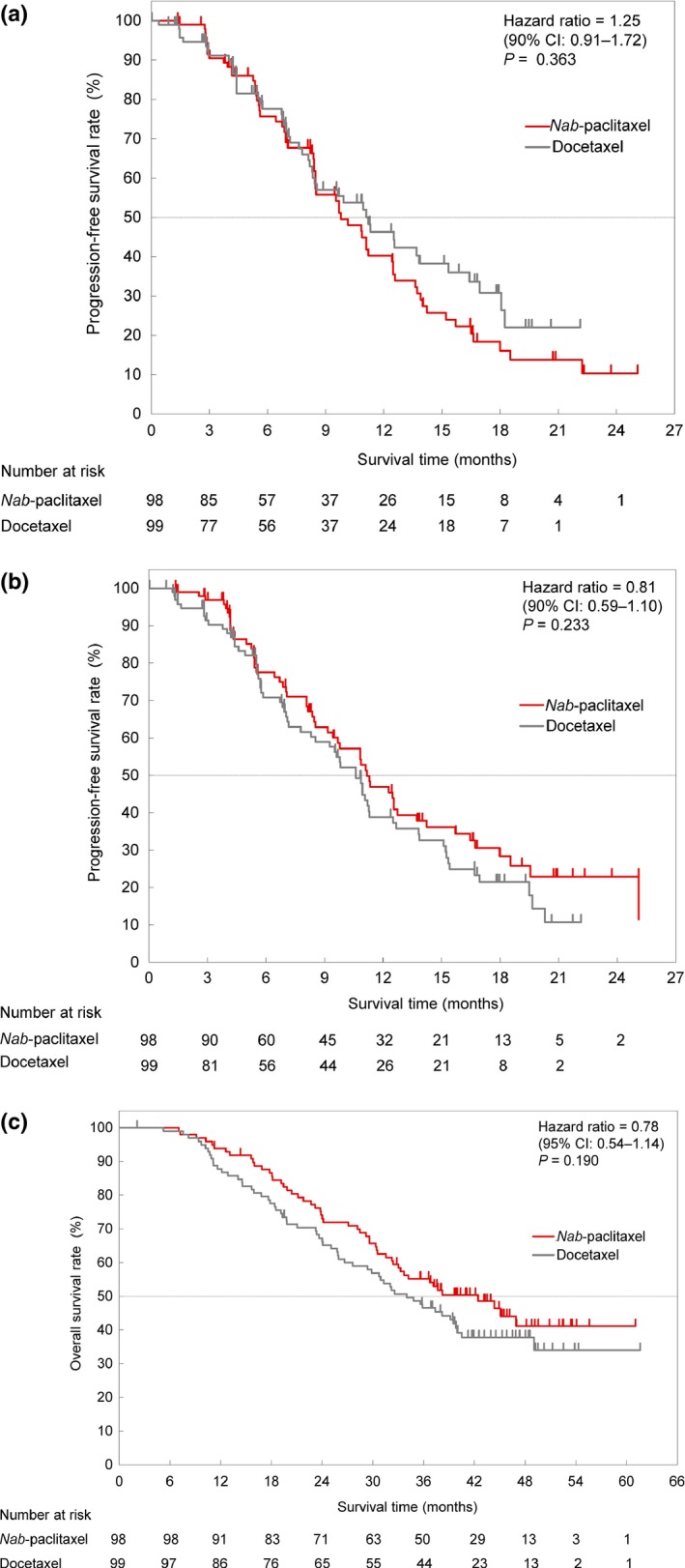
Kaplan–Meier plots of progression‐free survival by the independent radiologist assessment (a) and by investigators (b) Kaplan–Meier plots of overall survival (c). CI, confidence interval.
Table 2.
Stratified PFS assessed by independent radiologist and investigator
| Treatment | Patients | PFS, months | Hazard ratio† | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Group | n | Median | 90% CI | HR | 90% CI | |||
| Evaluated by independent radiologist | ||||||||
| Prior taxane therapy | No | Nab‐paclitaxel | 71 | 10.9 | 8.5, 12.5 | 1.05 | 0.72, 1.53 | 0.824 |
| Docetaxel | 71 | 9.9 | 7.8, 12.6 | |||||
| Yes | Nab‐paclitaxel | 27 | 8.4 | 6.9, 12.6 | 1.73 | 0.94, 3.17 | 0.133 | |
| Docetaxel | 28 | 15.3 | 11.2, 18.1 | |||||
| Evaluated by investigator | ||||||||
| Prior taxane therapy | No | Nab‐paclitaxel | 71 | 11.3 | 9.4, 14.2 | 0.73 | 0.51, 1.06 | 0.166 |
| Docetaxel | 71 | 9.8 | 7.8, 11.1 | |||||
| Yes | Nab‐paclitaxel | 27 | 9.8 | 8.4, 16.5 | 0.99 | 0.56, 1.75 | 0.983 | |
| Docetaxel | 28 | 12.5 | 9.3, 15.4 | |||||
Analysis set: full analysis set. †Cox's proportional hazards model was used. CI, confidence interval; HR, hazard ratio; n, number of patients; PFS, progression‐free survival.
The results for tumor response, ORR, and disease control rate are summarized in Table 3. There was no significant difference in ORR in the nab‐paclitaxel arm assessed by either the independent radiologists or investigators. The ORRs in TNBC patients were 52.4% and 46.7% in the nab‐paclitaxel and docetaxel arms, respectively (P = 1.000).
Table 3.
Best overall response
| Independent radiologist assessment | Investigator assessment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nab‐paclitaxel (n = 98) | Docetaxel (n = 99) | P‐value† | Nab‐paclitaxel (n = 98) | Docetaxel (n = 99) | P‐value† | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | |||
| CR | 1 | (1.0) | 1 | (1.0) | 0 | (0.0) | 0 | (0.0) | ||
| PR | 54 | (55.1) | 51 | (51.5) | 60 | (61.2) | 54 | (54.5) | ||
| SD | 36 | (36.7) | 34 | (34.3) | 35 | (35.7) | 34 | (34.3) | ||
| PD | 5 | (5.1) | 7 | (7.1) | 1 | (1.0) | 6 | (6.1) | ||
| NE | 2 | (2.0) | 6 | (6.1) | 2 | (2.0) | 5 | (5.1) | ||
| Overall response rate (CR + PR) | 55 | (56.1) | 52 | (52.5) | 0.669 | 60 | (61.2) | 54 | (54.5) | 0.388 |
| 95% CI (%) | (45.7, 66.1) | (42.2, 62.7) | (50.8, 70.9) | (44.2, 64.6) | ||||||
| Disease‐control rate (CR + PR + SD) | 91 | (92.9) | 86 | (86.9) | 0.238 | 95 | (96.9) | 88 | (88.9) | 0.049 |
| 95% CI (%) | (85.8, 97.1) | (78.6, 92.8) | (91.3, 99.4) | (81.0, 94.3) | ||||||
Analysis set: full analysis set. †Fisher's exact test. CI, confidence interval; CR, complete response; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
The median TTF in the nab‐paclitaxel and docetaxel arms was 7.0 months (95% CI: 5.6–8.5) and 5.7 months (4.6–7.2) (HR: 0.89, P = 0.414), respectively, as assessed by an independent radiologist assessment; and 8.1 months (95% CI: 6.4–9.2) and 5.7 months (95% CI: 5.2–8.1) (HR: 0.78, P = 0.117), as assessed by the investigators. The median OS for nab‐paclitaxel and docetaxel was 42.4 months (95% CI: 32.4–not reached) and 34.0 months (95% CI: 27.6–40.0) (HR: 0.78, P = 0.190), respectively (Fig. 2c), and the median OS in TNBC patients was 27.1 months (95% CI: 18.1–not reached) and 19.3 months (95% CI: 14.1–26.0) (HR: 0.56, P = 0.121).
Safety
All patients experienced some adverse events throughout the course of the study. The commonly reported adverse events (≥30% of patients) are summarized in Table 4. The most frequent adverse events in the nab‐paclitaxel arm were neutropenia (97.0%), leukocytopenia (96.0%), alopecia (95.0%), peripheral sensory neuropathy (88.0%), rash (61.0%) and nail disorder (57.0%), and those in the docetaxel arm were neutropenia (99.0%), leukocytopenia (99.0%), alopecia (91.0%), peripheral sensory neuropathy (69.0%), taste disturbance (67.0%), and nail disorder (57.0%). Anemia, peripheral sensory neuropathy, and rash occurred in at least 10% more patients in the nab‐paclitaxel arm compared with the docetaxel arm, while edema, loss of appetite, and taste disturbance occurred in at least 10% more patients in the docetaxel arm.
Table 4.
Adverse events occurring in ≥30% of patients in any treatment group
| Nab‐paclitaxel (n = 100) | Docetaxel (n = 100) | |||||||
|---|---|---|---|---|---|---|---|---|
| Any grade | ≥Grade 3 | Any grade | ≥Grade 3 | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Any adverse events | 100 | (100.0) | 88 | (88.0) | 100 | (100.0) | 99 | (99.0) |
| Hematologic toxicity | ||||||||
| Neutropenia | 97 | (97.0) | 78 | (78.0) | 99 | (99.0) | 98 | (98.0) |
| Leukopenia | 96 | (96.0) | 58 | (58.0) | 99 | (99.0) | 90 | (90.0) |
| Non‐hematotologic toxicity | ||||||||
| Alopecia | 95 | (95.0) | 0 | 91 | (91.0) | 0 | ||
| Peripheral sensory neuropthy | 88 | (88.0) | 22 | (22.0) | 69 | (69.0) | 5 | (5.0) |
| Rash | 61 | (61.0) | 1 | (1.0) | 50 | (50.0) | 0 | |
| Nail disorder | 57 | (57.0) | 0 | 57 | (57.0) | 0 | ||
| Anemia | 51 | (51.0) | 5 | (5.0) | 36 | (36.0) | 1 | (1.0) |
| Arthralgia | 47 | (47.0) | 1 | (1.0) | 40 | (40.0) | 0 | |
| Diarrhea | 44 | (44.0) | 0 | 48 | (48.0) | 2 | (2.0) | |
| Muscle pain | 44 | (44.0) | 1 | (1.0) | 46 | (46.0) | 0 | |
| Nausea | 43 | (43.0) | 1 | (1.0) | 44 | (44.0) | 0 | |
| Stomatitis | 42 | (42.0) | 1 | (1.0) | 34 | (34.0) | 0 | |
| Taste disturbance | 42 | (42.0) | 0 | 67 | (67.0) | 1 | (1.0) | |
| Edema | 40 | (40.0) | 3 | (3.0) | 51 | (51.0) | 4 | (4.0) |
| Malaise | 40 | (40.0) | 1 | (1.0) | 44 | (44.0) | 1 | (1.0) |
| Loss of appetite | 39 | (39.0) | 3 | (3.0) | 49 | (49.0) | 0 | |
| Nasopharyngitis | 38 | (38.0) | 0 | 36 | (36.0) | 0 | ||
| Fatigue | 33 | (33.0) | 1 | (1.0) | 31 | (31.0) | 0 | |
| Constipation | 25 | (25.0) | 1 | (1.0) | 30 | (30.0) | 0 | |
| Fever | 20 | (20.0) | 0 | 30 | (30.0) | 0 | ||
Analysis set: all treated patients.
Dose reductions were required in 65.3% in the nab‐paclitaxel arm and 56.6% in the docetaxel arm. The dose‐reduction rates due to neutropenia were 12.0% and 18.0%, and the incidences of grade 4 neutropenia were 35.0% and 89.0% in the nab‐paclitaxel and docetaxel arms, respectively. About 6.0% of nab‐paclitaxel and 33.0% of docetaxel patients received granulocyte colony‐stimulating factor (G‐CSF). Febrile neutropenia occurred in 1.0% of the nab‐paclitaxel arm and 8.0% of the docetaxel arm.
Peripheral sensory neuropathy occurred in 88.0% and 69.0% of the nab‐paclitaxel and docetaxel arms, respectively, with the most severe (grade 3) occurring in 22.0% and 5.0%, respectively. The dose reduction rate due to peripheral sensory neuropathy was 33.0% for nab‐paclitaxel and 7.0% for docetaxel.
Serious adverse events occurred in 17 patients (17.0%) in the nab‐paclitaxel arm and 14 (14.0%) in the docetaxel arm. Most of these serious adverse events were resolved or improved by adequate treatment or study treatment interruption. Adverse events leading to discontinuation occurred in 34.0% of the nab‐paclitaxel arm and 37.0% of the docetaxel arm and included peripheral sensory neuropathy (8.0%), edema (4.0%), facial palsy (3.0%), and macular edema (3.0%) in the nab‐paclitaxel arm, and edema (10.0%), peripheral sensory neuropathy (7.0%), peripheral edema (6.0%), and interstitial lung disease (4.0%) in the docetaxel arm. No treatment‐related deaths occurred in the present study.
Discussion
Previous studies have suggested that sb‐paclitaxel qw or docetaxel q3w is more effective than sb‐paclitaxel q3w for the treatment of breast cancer.9, 10 Although nab‐paclitaxel qw is already considered as standard therapy in patients with MBC in comparison with sb‐paclitaxel q3w, it should also be evaluated in comparison with sb‐paclitaxel qw or docetaxel q3w. Given that nab‐paclitaxel qw demonstrated dose dependent efficacy in a previous phase II study by Gradishar et al.,19 which showed longer PFS with nab‐paclitaxel qw than docetaxel q3w in Western countries, we investigated the efficacy of 150 mg/m2 nab‐paclitaxel qw. In contrast to the previous study, nab‐paclitaxel did not significantly prolong median PFS compared with docetaxel in Japanese patients, and failed to meet the primary endpoint of the study. In calculating our sample size, we estimated the median PFS (11.8 months for nab‐paclitaxel and 7.5 months for docetaxel) based on the results of the previous phase II study 19; however, PFS in the nab‐paclitaxel and docetaxel arms were actually 9.8 and 11.2 months, respectively. The patient characteristics in our study differed from those in the previous study; approximately 30% of patients in our study received adjuvant taxane therapy, while patients with prior adjuvant taxane therapy were not enrolled in the previous phase II study. Variation within our study population in terms of prior chemotherapy with taxanes (13.3% sb‐paclitaxel and 14.3% docetaxel in nab‐paclitaxel arm, and 20.2% sb‐paclitaxel and 10.1% docetaxel in docetaxel arm) may also have contributed to the discrepancy between our results and those of the previous study. On the other hand, our stratified analysis demonstrated that the median PFS was longer in patients without prior adjuvant taxane therapy compared with overall patients, similar to the previous phase II study.19
The median PFS in the docetaxel arm was longer than originally estimated, which might have been partly because of different dose levels between the two studies. Although the docetaxel dosage in the previous phase II study (100 mg/m2) was higher than in the present study (75 mg/m2), the median PFS in our docetaxel arm was longer than in reported in the previous study. The results of both the present and previous studies suggest that, although the anti‐tumor efficacy and toxicity of docetaxel are reported to be dose‐dependent in the range of 60–100 mg/m2, 75 mg/m2 appears to be a better starting dosage in terms of maintaining the relative dose intensity by managing adverse events, demonstrating efficacy in Japanese patients.24, 25, 26 In the present study, patients treated with nab‐paclitaxel had a longer median TTF (7.0 months vs 5.7 months) and median OS (42.4 months vs 34.0 months) compared with docetaxel, though the differences were not significant. Importantly, there was a discrepancy between the PD rates in the nab‐paclitaxel arm determined by investigators and those determined by independent radiologist assessment, possibly because investigators analyzed the images taking account of the clinical symptoms of each patient. Assessment of OS is considered to be more objective than PFS. The difference in relative dose intensities between the two treatment arms should also be noted; the median relative dose intensity in the nab‐paclitaxel arm was 70.2%, which was lower than that in the docetaxel arm (83.5%), and was also lower than that in the previous phase II study.27 In our study, the dose reduction rate in the nab‐paclitaxel arm was higher than in the docetaxel arm. The relative dose intensity of the standard 80 mg/m2 paclitaxel is reported more than 90% 28. Lower dose intensity might be one of the reasons for PFS not prolonged compared with docetaxel in this study, although treatment with nab‐paclitaxel 150 mg/m2 qw for multiple cycles was tolerable with dose interruptions or dose reductions, as reported in a phase I study in Japanese patients with MBC.20
Subgroup analysis of patients with TNBC in our study showed no significant difference in either PFS or ORR between the two treatment arms, though OS was longer in the nab‐paclitaxel arm compared with the docetaxel arm. The GeparSepto study29 showed that nab‐paclitaxel increased the pathological CR rate compared with sb‐paclitaxel, as part of sequential taxane–epirubicin/cyclophosphamide neoadjuvant treatment in patients with early breast cancer, implying remarkable efficacy in patients with TNBC. Furthermore, combinations of new immunotherapeutic agents and nab‐paclitaxel have recently been tested in patients with TNBC.30, 31, 32 More immunotherapeutic agents are expected to become available, and nab‐paclitaxel may thus play a role as a basis for the development of new therapies.
The safety and tolerability profiles of nab‐paclitaxel in the present study were similar to those in Gradishar et al.'s phase II study.19 Docetaxel resulted in a much higher incidence of grade 4 neutropenia than nab‐paclitaxel, and more patients in the docetaxel arm required G‐CSF treatment. Febrile neutropenia also occurred more often in the docetaxel arm. Given that febrile neutropenia is risk factor for infection‐related morbidity and mortality, and that frequent G‐CSF use may decrease patient quality of life during treatment, nab‐paclitaxel qw may be preferable, especially for elderly or less fit patients. Although the incidence of grade 3 peripheral sensory neuropathy was higher in the nab‐paclitaxel arm, this was comparable to previous studies in other countries.19
Macular edema has been reported in patients treated with taxanes. In this study, macular edema occurred in nine patients (9.0%), and only in the nab‐paclitaxel arm. Careful monitoring of subjective symptoms, such as decreased visual acuity, and appropriate adjustment of the study drug, including dose reduction or interruption, are needed to prevent the symptoms from worsening towards irreversible macular damage.
This was the first randomized study to evaluate the weekly administration of 150 mg/m2 nab‐paclitaxel in Japanese patients with MBC. Our results suggest that nab‐paclitaxel qw shows similar efficacy to docetaxel q3w in Japanese patients. Safety was as expected for nab‐paclitaxel and docetaxel; no new profiles were observed, and the frequency of febrile neutropenia was higher in the docetaxel arm. A better understanding of the safety and efficacy profiles of each drug will allow personalized treatments to be proposed based on the individual patient's characteristics.
Disclosure Statement
This study was sponsored by Taiho Pharmaceutical Co., Ltd. The study was designed by the steering committee under the responsibility of Taiho Pharmaceutical Co., Ltd., study drugs were provided by Taiho Pharmaceutical Co., Ltd., and Taiho Pharmaceutical Co., Ltd. collected and analyzed the data and contributed to the interpretation of the study. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. KT received research funding from MSD, AstraZeneca, Pfizer, Chugai Pharmaceutical, Daiichi Sankyo, Ono Pharmaceutical, Eisai and Daiichi Sankyo. KI received research funding from Parexel, Puma Biotechnology, Novartis, GSK, Pfizer, Chugai Pharmaceutical, and Daiichi Sankyo. NM received honoraria from Chugai Pharmaceutical, AstraZeneca, Eisai, Kyowa Hakko Kirin, Taiho Pharmaceutical, and Sanofi. ST received research funding from Chugai Pharmaceutical. MK received honoraria from Chugai Pharmaceutical, Kyowa Hakko Kirin, AstraZeneca, Taiho Pharmaceutical, Novartis, and Pfizer, and research funding from Chugai Pharmaceutical. YT received honoraria from Kyowa Hakko Kirin, Taiho Pharmaceutical, Meiji Seika Pharma, and Chugai Pharmaceutical, manuscript fees from Kyowa Hakko Kirin, and research funding from Takeda Pharmaceutical, Eisai, Chugai Pharmaceutical, and Novartis. H Iwata received honoraria from Chugai Pharmaceutical, Eisai, AstraZeneca, Novartis, Daiichi Sankyo, Taiho Pharmaceutical, manuscript fees from Eisai and Chugai Pharmaceutical, and research funding from GSK, Daiichi Sankyo, Chugai Pharmaceutical, Nippon Kayaku, Novartis, Pfizer, AstraZeneca, Eisai, Eli Lilly, and MSD. NY received research funding from MSD, Pfizer, and AstraZeneca. TS received honoraria from Novartis, Kyowa Hakko Kirin, Taiho Pharmaceutical, Eisai, Ono Pharmaceutical, Chugai Pharmaceutical, AstraZeneca, and Mochida Pharmaceutical, and research funding from Taiho Pharmaceutical, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Nihon Mediphysics, and EPS. TN received honoraria from Chugai Pharmaceutical, Novartis, and AstraZeneca. TI received honoraria from Chugai Pharmaceutical, and research funding from Takeda Pharmaceutical and Kyowa Hakko Kirin. MS received honoraria from Taiho Pharmaceutical, and research funding from Eisai and Ono Pharmaceutical. NS, HA, and HY received honoraria from Taiho Pharmaceutical. H Ishiguro received research funding from Chugai Pharmaceutical and served as a consultant for Eli Lilly Japan. YF received research funding from Nippon Kayaku. KA, TT, SO, KY, and JW declare no conflicts of interest.
Abbreviations
- CI
confidence interval
- CR
complete response
- G‐CSF
granulocyte colony‐stimulating factor
- HER2
human epidermal growth factor receptor 2
- HR
hazard ratio
- MBC
metastatic breast cancer
- nab‐paclitaxel
nanoparticle albumin‐bound paclitaxel
- ORR
overall response rate
- OS
overall survival
- PD
progressive disease
- PFS
progression‐free survival
- PR
partial response
- PS
performance status
- RECIST
Response Evaluation Criteria in Solid Tumors
- sb‐paclitaxel
solvent‐based‐paclitaxel
- SD
stable disease
- TNBC
triple‐negative breast cancer
- TTF
time to treatment failure
- qw
once‐weekly administration
- q3w
every‐3‐week administration
Supporting information
Table S1. Dose reduction schema for nab‐paclitaxel and docetaxel.
Table S2. Dose reduction criteria for nab‐paclitaxel and docetaxel.
Table S3. Dose‐interruption criteria within treatment cycle.
Table S4. Criteria to be met before starting next cycle of nab‐paclitaxel or docetaxel.
Acknowledgments
We thank all the participating patients and their families, as well as the investigators and clinical research coordinators. We are also grateful to Yutaka Ariyoshi, Kazuo Tamura, and Hironobu Minami, who served as members of the Data and Safety Monitoring Committee, and N. Saijo, MD, for valuable advice as the external medical specialist. We also thank ASCA Corporation for medical writing of the manuscript, funded by Taiho Pharmaceutical Co., Ltd. This study was sponsored by Taiho Pharmaceutical Co., Ltd.
Cancer Sci 108 (2017) 987–994
Funding Information
This study was sponsored by Taiho Pharmaceutical Co., Ltd.
Trial register and registration number: JapicCTI‐090921
References
- 1. American Cancer Society . Learn about cancer. What are the key statistics about breast cancer? [Cited 01 Nov 2016.] Available from URL: http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics.
- 2. Cancer Information Service . Projected cancer statistics, 2016. [Cited 18 Aug 2016.] Available from URL: http://ganjoho.jp/en/public/statistics/short_pred.html.
- 3. Ghersi D, Wilcken N, Simes RJ. A systematic review of taxane‐containing regimens for metastatic breast cancer. Br J Cancer 2005; 93: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biganzoli L, Cufer T, Bruning P et al Doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first‐line chemotherapy in metastatic breast cancer: the European Organization for Research and Treatment of Cancer 10961 Multicenter Phase III Trial. J Clin Oncol 2002; 20: 3114–21. [DOI] [PubMed] [Google Scholar]
- 5. Sledge GW, Neuberg D, Bernardo P et al Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front‐line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). J Clin Oncol 2003; 21: 588–92. [DOI] [PubMed] [Google Scholar]
- 6. Katsumata N, Watanabe T, Minami H et al Phase III trial of doxorubicin plus cyclophosphamide (AC), docetaxel, and alternating AC and docetaxel as front‐line chemotherapy for metastatic breast cancer: Japan Clinical Oncology Group trial (JCOG9802). Ann Oncol 2009; 20: 1210–15. [DOI] [PubMed] [Google Scholar]
- 7. Cardoso F, Costa A, Norton L et al ESO‐ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Ann Oncol 2014; 25: 1871–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. NCCN . Clinical practice guidelines in oncology. Breast cancer, version 1. 2016.
- 9. Seidman AD, Berry D, Cirrincione C et al Randomized phase III trial of weekly compared with every‐3‐weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER‐2 overexpressors and random assignment to trastuzumab or not in HER‐2 nonoverexpressors: final results of Cancer and Leukemia Group B Protocol 9840. J Clin Oncol 2008; 26: 1642–9. [DOI] [PubMed] [Google Scholar]
- 10. Sparano JA, Wang M, Martino S et al Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 2008; 358: 1663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 2001; 37: 1590–8. [DOI] [PubMed] [Google Scholar]
- 12. Weiss RB, Donehower RC, Wiernik PH et al Hypersensitivity reactions from taxol. J Clin Oncol 1990; 8: 1263–8. [DOI] [PubMed] [Google Scholar]
- 13. Ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet 2003; 42: 665–85. [DOI] [PubMed] [Google Scholar]
- 14. Desai N, Trieu V, Yao Z et al Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor‐free, albumin‐bound paclitaxel, ABI‐007, compared with cremophor‐based paclitaxel. Clin Cancer Res 2006; 12: 1317–24. [DOI] [PubMed] [Google Scholar]
- 15. Cucinotto I, Fiorillo L, Gualtieri S et al Nanoparticle albumin bound paclitaxel in the treatment of human cancer: nanodelivery reaches prime‐time? J Drug Deliv 2013; 905091 https://doi.org/10.1155/2013/905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gradishar WJ, Tjulandin S, Davidson N et al Phase III trial of nanoparticle albumin‐bound paclitaxel compared with polyethylated castor oil‐based paclitaxel in women with breast cancer. J Clin Oncol 2005; 23: 7794–803. [DOI] [PubMed] [Google Scholar]
- 17. Nyman DW, Campbell KJ, Hersh E et al Phase I and pharmacokinetics trial of ABI‐007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol 2005; 23: 7785–93. [DOI] [PubMed] [Google Scholar]
- 18. Blum JL, Savin MA, Edelman G et al Phase II study of weekly albumin‐bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanes. Clin Breast Cancer 2007; 7: 850–6. [DOI] [PubMed] [Google Scholar]
- 19. Gradishar WJ, Krasnojon D, Cheporov S et al Significantly longer progression‐free survival with nab‐paclitaxel compared with docetaxel a first‐line therapy for metastatic breast cancer. J Clin Oncol 2009; 27: 3611–19. [DOI] [PubMed] [Google Scholar]
- 20. Ando M, Yonemori K, Katsumata N et al Phase I and pharmacokinetic study of nab‐paclitaxel, nanoparticle albumin‐bound paclitaxel, administered weekly to Japanese patients with solid tumors and metastatic breast cancer. Cancer Chemother Pharmacol 2012; 69: 457–65. [DOI] [PubMed] [Google Scholar]
- 21. Toi M, Nakamura S, Kuroi K et al Phase II study of preoperative sequential FEC and docetaxel predicts of pathological response and disease free survival. Breast Cancer Res Treat 2008; 110: 531–9. [DOI] [PubMed] [Google Scholar]
- 22. Therasse P, Arbuck SG, Eisenhauer EA et al New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 23. Cortes J, O'Shaughnessy J, Loesch D et al Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open‐label randomised study. Lancet 2011; 377: 914–23. [DOI] [PubMed] [Google Scholar]
- 24. Watanabe T, Kuranami M, Inoue K et al Comparison of an AC‐taxane versus AC‐free regimen and paclitaxel versus docetaxel in patients with lymph node‐positive breast cancer: final results of the National Surgical Adjuvant Study of Breast Cancer 02 trial, a randomized comparative phase 3 study. Cancer 2017; 123: 759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harvey V, Mouridsen H, Semiglazov V et al Phase III trial comparing three doses of docetaxel for second‐line treatment of advanced breast cancer. J Clin Oncol 2006; 24: 4963–70. [DOI] [PubMed] [Google Scholar]
- 26. Kenmotsu H, Tanigawara Y. Pharmacokinetics, dynamics and toxicity of docetaxel: why the Japanese dose differs from the Western dose. Cancer Sci 2015; 106: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gradishar WJ, Krasnojon D, Cheporov S et al Phase II trial of nab‐paclitaxel compared with docetaxel as first‐line chemotherapy in patients with metastatic breast cancer: final analysis of overall survival. Clin Breast Cancer 2012; 12: 313–21. [DOI] [PubMed] [Google Scholar]
- 28. Taguchi T, Aihara T, Takatsuka Y et al Phase II study of weekly paclitaxel for docetaxel‐resistant metastatic breast cancer in Japan. Breast J 2004; 10: 509–13. [DOI] [PubMed] [Google Scholar]
- 29. Untch M, Jackisch C, Schneeweiss A et al Nab‐paclitaxel versus solvent‐based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto‐GBG 69): a randomised, phase 3 trial. Lancet Oncol 2016; 17: 345–56. [DOI] [PubMed] [Google Scholar]
- 30. New York University School of Medicine . Phase II study of pembrolizumab and nab‐paclitaxel in HER‐2 negative metastatic breast cancer. [Cited 27 May 2016.] Available from URL: https://clinicaltrials.gov/ct2/show/NCT02752685?term=Pembrolizumab+Nab-Paclitaxel+Breast+Cancer&rank=1.
- 31. Hoffmann‐La Roche . A study of atezolizumab in combination with nab‐paclitaxel compared with placebo with nab‐paclitaxel for participants with previously untreated metastatic triple negative breast cancer (IMpassion130). [Cited 22 Feb 2016.] Available from URL: https://clinicaltrials.gov/ct2/show/NCT02425891?term=NCT02425891&rank=1. NLM identifier: NCT02425891.
- 32. Adams S, Robinson JD, Hamilton EP et al Phase Ib trial of atezolizumab in combination with nab‐paclitaxel in patients with metastatic triple‐negative breast cancer (mTNBC). J Clin Oncol 2016; 34 (suppl; abstr 1009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Dose reduction schema for nab‐paclitaxel and docetaxel.
Table S2. Dose reduction criteria for nab‐paclitaxel and docetaxel.
Table S3. Dose‐interruption criteria within treatment cycle.
Table S4. Criteria to be met before starting next cycle of nab‐paclitaxel or docetaxel.


