Abstract
Identifying environmental influences on inhibitory control (IC) may help promote positive behavioral and social adjustment. Although chronic stress is known to predict lower IC, the immediate effects of acute stress are unknown. The parasympathetic nervous system (PNS) may be a mechanism of the stress-IC link, given its psychophysiological regulatory role and connections to prefrontal brain regions critical to IC. We used a focused assessment of IC (the stop-signal task) to test whether an acute social stressor (the Trier Social Stress Test) affected participants’ pre- to post-IC performance (n = 58), compared to a control manipulation (n = 31). High frequency heart-rate variability was used as an index of PNS activity in response to the manipulation. Results indicated that stress impaired IC performance, blocking the practice effects observed in control participants. We also investigated the associations between PNS activity and IC; higher resting PNS activity predicted better pre-manipulation IC, and greater PNS stressor reactivity protected against the negative effects of stress on IC. Together, these results are the first to document the immediate effects of acute stress on IC and a phenotypic marker (PNS reactivity to stressors) of susceptibility to stress-induced IC impairment. This study suggests a new way to identify situations in which individuals are likely to exhibit IC vulnerability and related consequences such as impulsivity and risk taking behavior. Targeting PNS regulation may represent a novel target for IC-focused interventions.
Keywords: Inhibitory Control, Stress, Parasympathetic Nervous System, Self-Regulation
Inhibitory control (IC), the ability to stop a prepotent response, allows individuals to flexibly meet environmental demands instead of relying on impulsive response tendencies (Diamond, 2013). Identifying environmental influences on IC is important because impairment is implicated in negative outcomes, such as substance use (Iacono, Malone, & McGue, 2008) and psychopathology (Wright, Lipsyc, Dupuis, Thayapararajah, & Schachar, 2014). Chronic stress has been associated with IC impairment and disruption of underlying neurobiology (Mani, Mullainathan, Shafir, & Zhao, 2013; Mika et al., 2012). However, the immediate effects of acute stress are poorly understood, despite the plausible link between IC impairment and accumulation of stressful experiences. Delineating impacts of acute stress could offer insight into the chronic stress–IC link and contexts in which individuals are susceptible to IC lapses and impulsive behavior.
The autonomic nervous system (ANS) may be a key underlying mechanism in the acute stress-IC link given its regulatory role across emotional, cognitive, and physiological domains (Thayer, Åhs, Fredrikson, Sollers, & Wager, 2012). The parasympathetic nervous system (PNS), one ANS branch, is of primary importance because of its fast-acting regulation of heart rate via the vagus nerve, allowing flexible psychophysiological responses (Thayer et al., 2012). Notably, PNS function is critical for modulating arousal demands in reaction to acute stress and is also linked to cognitive function (Yim, Quas, Rush, Granger, & Skoluda, 2015; Thayer, Hansen Saus-Rose, & Johnsen, 2009). Specifically, the prefrontal brain regions (e.g., medial prefrontal cortex; anterior cingulate cortex) critical for IC performance also control limbic regions that regulate PNS activity. Accordingly, we suggest that shared neural systems could help explain both a resting PNS-IC link and an acute stress-IC link (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004; Thayer et al., 2012; Verbruggen & Logan, 2008; Graziano & Derefinko, 2013).
Specifically, preliminary evidence suggests associations between resting PNS activity and performance on some (Hovland et al., 2012; Beaumont et al., 2012), but not all (Capuana, Dywan, Tays, & Segalowitz, 2012) IC-related measures. However, extant paradigms have confounded multiple cognitive processes (e.g., emotion processing, working memory) with IC and are not tied to a specific neural system. Given that brain regions underlying “pure” IC are well established and linked to PNS regulation, an investigation of the link between baseline PNS activity and a focused measure of IC, such as stop signal reaction time (SSRT), is needed. The SSRT, assessed during the stop signal paradigm, is a particularly rigorous measure for examining individual differences in IC because of the adaptive nature of the allowable response window, which ensures that all participants maintain approximately 50% accuracy. Specifically, by ‘holding’ participants at 50% accuracy, speed/accuracy trade-off strategies become less relevant. The SSRT has been advocated for as the most suitable laboratory paradigm for IC research (Seli, Cheyne, Smilek, 2012; Verbruggen & Logan, 2008).
Top-down regulation of the limbic system by prefrontal regions is commonly used to explain the IC-PNS link, but evidence also suggests a bidirectional relationship, with limbic regions contributing bottom-up arousal demands (Blair & Ursache, 2011; Park & Thayer, 2014). Consistent with such bottom-up arousal demands, exposure to acute stress may lead to subsequent impairment of prefrontal cortex function, and consequently lower IC performance.
Although there has been no research to date examining the immediate consequences of acute stress on IC, two studies have examined delayed (10–30 mins) effects of acute stress, with the goal of understanding possible effects of cortisol, which peaks approximately twenty minutes post-stressor (Dickerson & Kemeny, 2004). These studies document conflicting results, with one reporting higher post-stressor versus post-control IC (assessed via SSRT; Schwabe, Hoffken, Tegenthoff, & Wolf, 2013) while another (using a Go/No-Go task) reported slower Go Trial reaction time, and no effects on IC accuracy (Scholz et al., 2013). Neither study examined how individual differences in cortisol linked to IC, but Schwabe and colleagues (2013) found that pharmacologically blocking the effects of cortisol nulled the effects of acute stress. However, both of these studies had substantial limitations including small sample sizes (11 – 18 per group) and no assessment of pre-stressor IC. Related research in the working memory domain demonstrated that acute stress impaired n-back task reaction time and accuracy performance, with effects of acute stress diminishing over time (Schoofs, Preuss, & Wolf, 2008). Taken together, such results suggest that the immediate aftermath of an acute stressor may negatively impact performance on demanding executive function tasks. However, research using larger sample sizes that incorporates pre/post assessments and investigations of putative underlying neurobiological mechanisms is needed to further characterize this phenomenon.
In the present study, we specifically examined the extent to which lower PNS regulation in response to acute stress might result in subsequent IC impairment. Prior research using social-evaluative stressor tasks (i.e. the Trier Social Stressor Task; TSST) documents PNS engagement during the task, suggesting arousal regulation consistent with the demands of presenting a well-regulated speech to socially-threatening judges (Yim et al., 2015). Given such findings, it could be hypothesized that participants who demonstrate more flexible PNS engagement to the TSST (i.e. higher PNS) would be better able to manage increased post-stressor arousal demands during an IC task. However, because there has been no research to date examining the immediate effects of an arousal manipulation via acute stress on IC, the extent to which PNS activity could account for a relationship between acute stress and IC impairment is unknown.
Here, we conducted the first assessment of the immediate effects of acute stress on IC with the goal of examining the effects of PNS activity as an explanatory mechanism underlying an acute-stress and IC link. Specifically, a repeated measures design was used to assess the extent to which a social-evaluative stressor (the TSST) affected a rigorous index of IC (SSRT; Aron, Robbins, & Poldrack, 2014). We hypothesized that acute stress (vs. control) would impair IC. We further expected that (a) higher resting PNS activity would predict better pre-manipulation IC, and (b) for stressor participants, higher (vs. lower) PNS activity during acute stress would indicate resilience to the stressor and relatively better post-manipulation IC.
Method
Participants
Participants were 97 undergraduate students at the University of Oregon (50 [53.2%] female, age M = 20.09, SD = 3.86) recruited from the Department of Psychology human subjects pool who received course credit for participation1. Participants identified as 57.4% non-Hispanic Caucasian race/ethnicity, 14.9% Hispanic, 10.6% Asian, 3.2% Black, and 13.9% other. Three participants declined to report their age, gender, or ethnicity, and age data from an additional 17 were unavailable due to errors in data collection. We assigned participants to the stressor condition at a ratio of 2:1 to have greater statistical power to quantify individual differences in PNS activity within the stressor condition. A target sample size of 100 was linked to the recruitment goal of 70 participants in the stressor condition, based on previous individual difference research investigating PNS activity and cognitive performance (Beaumont et al., 2012). All participants provided informed consent in accordance with the University of Oregon Institutional Review Board.
Procedure
Before coming to the lab, participants provided informed consent and completed brief online questionnaires that included requests for demographic information. Upon arrival to the lab, participants provided additional consent for the lab portion of the study, which began between noon and 4:00 p.m. to control for diurnal variation in high-frequency heart rate variability (HF-HRV) and cortisol concentrations (Yamasaki et al., 1996). During consent, participants were told that in addition to playing games on a computer, they would be asked to either read magazines or speak in front of a panel of judges. Following consent, participants were asked to wear a Polar Heart Rate monitor for the duration of the experiment (Polar Electro Inc., Lake Success, NY). Participants then completed a brief in-lab questionnaire that is used to assess mental and physical health diagnoses and medication use. Baseline heart rate and HF-HRV were obtained from a four-minute period during which participants were seated. Participants then completed two ~5-minute blocks of the stop-signal task, followed by either the stressor or control manipulation. Following this manipulation, participants completed two additional ~5-minute blocks of the stop-signal task.
Cortisol was measured to confirm a successful stressor manipulation between groups. Saliva samples (2mL) were collected at three time points during the experiment: baseline (following the cardiovascular recording session); immediately after the experimental manipulation (i.e., stressor or control); and after the post-manipulation stop-signal task (15 minutes post-stressor). All samples were collected via passive drool method, placed on ice during the experiment, and transferred to a −35°C freezer following the experimental session.
Measures
Stressor and control task
The Trier Social Stress Test (TSST), a well-validated social-evaluative stressor, was used to elicit a stress response (Kirschbaum et al., 1993). Participants were given 5 minutes to prepare a speech about the ideal job they planned to pursue after they graduated from college. After this 5-minute period, participants were escorted into another room where they had 5 minutes to give the speech to a neutral panel of judges, after which they completed mental subtraction out loud for 5 minutes. If participants asked questions of the judges or were silent for more than 20 seconds, the panelists prompted participants to continue using a series of neutral responses. Participants who complete the TSST are expected to show larger cortisol responses as well as higher heart rate and reduced HF-HRV compared with those who complete the control task.
For the control task, participants were instructed to quietly read magazines for a total of 15 minutes. Participants were instructed to sit for the first 5 minutes and then to walk into another room and stand for the next 10 minutes in order to control for orthostatic challenge (i.e., sitting, walking, and standing) on heart rate physiology across conditions.
Cortisol samples were thawed to room temperature, vortexed, centrifuged (10 min @ 3500 rpm), and aliquoted into polypropylene containers. Samples were refrozen before being sent to the University of Trier Biochemical Laboratory in Trier, Germany for assay. Samples were assayed in duplicate using DELFIA (time-resolved fluorescence immunoassay). Intra-assay coefficients of variation (CVs) averaged 5.4%. Samples > 2nmol/L (n=10) were reanalyzed if CVs were > 15%. Cortisol data were natural log transformed to normalize variable distribution. Outliers > 2.5 SDs from the mean within each condition were winsorized (1–2 per time point). Area-under-the-curve with respect to ground (AUCg; Pruessner et al., 2003) was calculated as an index of cortisol response.
Stop-signal task
The stop-signal task (SST) is a widely used tool to measure inhibitory control that requires the inhibition of a prepotent response (Verbruggen & Logan, 2008). For the Go trials, participants were asked to respond as quickly and as accurately as possible to a visual stimulus (“X” or “O”) displayed in the center of the screen with their right and left index fingers. For the Stop trials, participants were instructed to inhibit their response to the visual stimulus following a sound played at a variable delay (stop-signal delay; SSD). Each run of the SST consisted of one practice block and two experimental blocks. The SSD was adjusted following two objective 1-up/1-down tracking procedures that increased or decreased the SSD by 50 ms for each accurate and inaccurate response, respectively, in order to obtain similar accuracy (~50%) across participants. Each practice block consisted of 32 trials (69% Go, 31% Stop). To continue to the experimental blocks, the participant had to establish a mean reaction time of less than 750 ms and inhibit their response on at least 20% of the practice Stop trials. Following the practice block were two experimental blocks, each consisting of 128 trials (75% Go, 25% Stop). Total time for each run was approximately 12 minutes. The SST was completed on Lenovo ThinkPads using E-Prime Software (Psychology Software Tools Inc., Pittsburgh, PA, USA).
The main dependent measure of the SST is the stop-signal reaction time (SSRT); lower SSRT indicates better inhibitory control. SSRT was calculated using the quantile method (Congdon et al., 2012). A quantile measure of reaction time (RT) on correct Go trials was calculated by rank ordering correct Go RTs and selecting the RT associated with the proportion of incorrect No-Go trials (i.e., failed inhibition). An estimate of SSRT was then obtained by subtracting the average SSD from this quantile RT. The resulting SSRT represents the average time for an individual to successfully inhibit a response approximately 50% of the time (Congdon et al., 2012).
PNS activity: HF-HRV
PNS activity was assessed by monitoring HF-HRV. Participants wore a Polar Heart Rate Monitor (Model RS800CX, USA) and Polar Wearlink heart rate chest band transmitter. Cardiovascular activity was recorded at baseline, during all blocks of the SST and during all phases of the TSST. Cardiovascular data were visually inspected for artifacts by at least two research assistants, who removed offending segments and scored the data using Kubios HRV software (Tarvainen, Niskanen, Lipponen, Ranta-Aho, & Karjalainen, 2009). Discrepancies were investigated by a third researcher, who resolved any conflicts in the scoring. With Kubios software, 1-minute increments of heart rate were submitted to frequency-domain calculations of HF-HRV. An autoregressive model was used to estimate high-frequency power within the 0.15–0.4 Hz band of variability, which has been found to be a relatively clean index of PNS activity (Bernston et al., 1997). Kubios’ normalized values for these autoregressive estimates were used to control for the usual positive skew in HF-HRV values. To examine PNS reactivity, HF-HRV during the stressor was regressed on baseline HF-HRV, producing unstandardized residual scores representative of the change in PNS activity relative to baseline. This method for modeling change in PNS activation was chosen a priori because it allows for testing the effects of stress-related PNS activity beyond the hypothesized effects of resting PNS activity on IC.
Additional measures, not reported here, include self-report questionnaires of emotion, early life stress, and eating behaviors, which were collected to investigate other research questions of interest.
Data Analysis Plan
Behavioral (RTs, SSRT) and heart rate data were examined for outliers and incomplete data. For behavioral data from the SST, eight participants were excluded from all analyses due to missing pre-stress SSRT data (five for failing to follow task instructions, three due to computer failure). An additional eight participants were missing only post-stress SSRT data (four due to time constraints, four due to computer failure) and so were excluded from relevant analyses. For heart rate data, 12 participants were excluded: two because of discomfort with Polar Watch and 10 because a clean, robust heart rate recording was lacking.
Descriptive statistics and analyses were conducted in SPSS (Version 22.0). Although age and gender differences in heart rate physiology have been reported in previous research (Yamasaki et al., 1996), they were not correlated to any experimental variables of interest in this undergraduate sample and thus were not included in inferential statistics. Repeated-measures ANOVAs were used to examine the hypothesized Time × Condition interaction effects of the acute stressor on inhibitory control performance and the physiological stress responses. Linear regressions were used to examine the extent to which resting HF-HRV and HF-HRV reactivity to the stressor predict IC performance (pre-stressor and change over time).
Results
Acute Stressor Manipulation
We examined between-groups changes in the cardiovascular response and cortisol response to stress to confirm that the TSST induced the expected response. Repeated-measures ANOVA revealed significant between-groups differences in HR, F(1, 74) = 19.60, p <0.001, η2 = 0.21, but not in HF-HRV, F(1, 74) = 0.17, p > 0.05. Confirming the stressor manipulation, cortisol concentrations increased in the stress condition (AUCg M = 2.78, SD = 1.38) compared to control (AUCg M = 1.94, SD = 1.38; F(1,83) = 7.02, p = 0.010, η2 = 0.078).
Effects of Acute Stressor on IC performance
Descriptive statistics for pre- and post-SSRT are presented in Table 1 for the entire sample and by condition. A repeated-measures ANOVA tested the hypothesis that participants experiencing the acute stressor would demonstrate lower IC performance relative to those experiencing a control condition. Results indicated a significant main effect of time, F(1, 79) = 12.92, p < .001, partial η2 = .14, such that, across conditions, there was reduction in SSRT (improved IC performance) from pre (M = 194.91 ms, SD = 4.76 ms) to post (M = 177.40 ms, SD = 4.69 ms; Figure 1). In addition, there was a significant Time × Condition effect, F(1, 79) = 5.76, p < .05, partial η2 = .07. Follow-up pairwise comparisons indicated that participants in the control condition experienced a significant reduction in SSRT pre to post (M = −29.21 ms, SD = 7.81 ms), F(1, 79) = 14.00, p < .001, partial η2 = .15, indicative of improved IC performance consistent with practice effects, while participants in the acute stress condition exhibited no significant SSRT reduction (M = −5.82 ms, SD = 5.83 ms), F(1, 79) = 1.00, p > .05; Figure 1.).
Table 1.
Means (SDs) of of study variables
| Stress Condition | Control Condition | Full Sample | |
|---|---|---|---|
| SSRT, pre- (ms) | 193.3 (38.5) | 199 (48.6) | 195.2 (41.9) |
| SSRT, post- (ms) | 183.5 (42.9) | 172.5 (35.3) | 179.7 (40.6) |
| ΔSSRT (% change) | −0.94 (24.17) | −10.9 (20.18) | −4.35 (23.24) |
| HRV, baseline (n.u.) | 38.09 (15.09) | 45.5 (14.98) | 40.37 (15.35) |
| ΔHRV (n.u.) | −0.63 (8.99) | 3.03 (11.2) | 0.51 (9.8) |
| Cortisol, baseline (log-transformed nmol/L) | 1.51 (0.72) | 1.18 (0.7) | 1.4 (0.72) |
| Cortisol, AUCG | 2.78 (1.38) | 1.94 (1.38) | 2.5 (1.43) |
Figure 1.
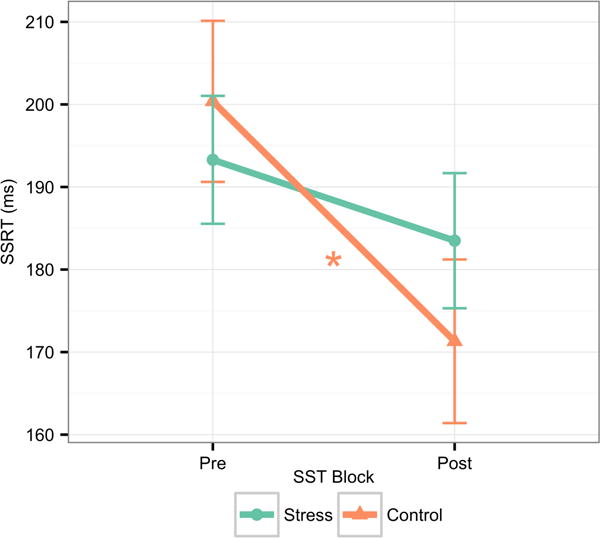
Change in SSRT across blocks by condition. An acute social stressor blocks the IC task practice effects observed in the control condition (F(1, 79) = 5.76, p < .05, partial η2 = .07). Error bars represent 95% confidence
Note: * p < .001
Relationship Between HF-HRV and Baseline IC Performance
A Pearson’s correlation across all participants assessed the presence of an association between resting HF-HRV and baseline (“pre”) IC as measured by SSRT. This analysis indicated a significant negative correlation (r = −.23, p < .05) such that individuals with higher resting HF-HRV exhibited shorter baseline SSRT scores, suggesting better IC performance (Figure 2).
Figure 2.

Baseline HF-HRV predicts better SSRT. Baseline HF-HRV predicts better SSRT during pre-manipulation SST (Pearson’s r = −0.23, p < .05). Participants with higher HF-HRV at baseline, indicative of more robust resting PNS activity, exhibited better IC (i.e., shorter SSRTs) at baseline.
Note: * p < .05
Individual differences in HF-HRV relevant to the stressor and IC performance
We investigated the relationship between change in HF-HRV during the manipulation relative to baseline and SSRT percent change pre- to post-manipulation among participants in the stress condition to establish whether individual differences in PNS activity during the stressor were related to IC change. Individuals who exhibited increased HF-HRV during the stressor—indicative of a buffered physiological response—had faster post-manipulation SSRTs, indicating better IC (Pearson’s r = −.35, p < .05; see Figure 3). There was no significant relationship between HF-HRV during the control manipulation and SSRT (Pearson’s r = .20, p > .05. These correlations were determined to differ significantly from each other using Fisher’s r to Z transformation and associated Z-test (Z = −2.17, p < .05). Notably, these effects were specific to HF-HRV; cortisol response was examined in a similar set of analyses to ensure that individual differences were related specifically to the fast-acting PNS system and not to the slower-acting HPA system. No significant relationships between IC performance and cortisol were found.
Figure 3.
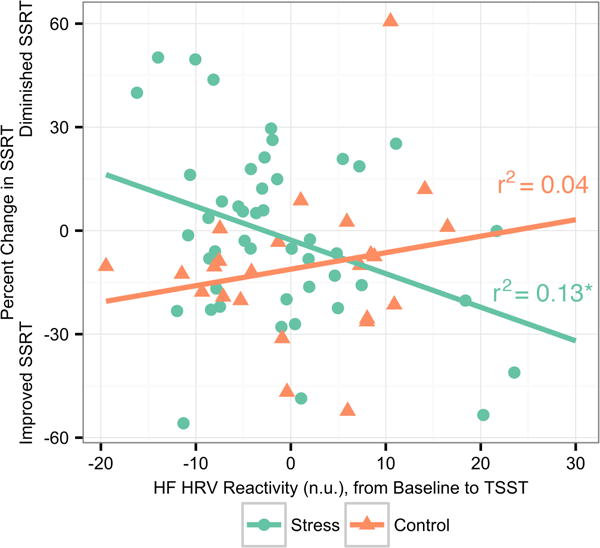
Change in HF-HRV predicts SSRT change in Stress but not Control condition. HF-HRV reactivity due to manipulation predicts percent change in SSRT from pre- to post-manipulation in Stress but not Control (Fisher’s r to Z transformation; Z = −2.30, p < .05). In the Stress condition, increased reactivity to the stressor (i.e., a larger decline in HF-HRV) was associated with diminished SSRT performance from pre- to post-manipulation. No relationship between HF-HRV reactivity and SSRT performance change was found in the Control condition.
Note: * p < .01
Discussion
The present study demonstrated that acute stress impairs IC compared to a non-stressful control condition. Specifically, control participants exhibited a significant reduction in SSRT, consistent with commonly reported task practice effects (Manuel et al., 2013); however, stress blocked this effect, preventing pre- to post-manipulation IC improvement in stress-exposed participants. These results are the first to demonstrate immediate impacts of acute stress on IC and are consistent with findings that accumulated stressful events (i.e., chronic stress) impair IC. For example, animal research documents that exposure to chronic stress impairs IC over time (Mika et al., 2012). Similarly, seasonal farmers exhibit lower IC under pre-harvest financial stress as opposed to post-harvest security (Mani et al., 2013). Incorporating the contextual role of acute stress into current models of IC may help scientists better understand individual differences in IC and identify situations that induce IC vulnerability.
These findings highlight the relevance of PNS activity to IC at baseline and in response to stress. Increased resting PNS activity predicted better pre-manipulation IC, consistent with the theory that individuals with better prefrontal brain function can more flexibly allocate resources to regulate limbic system activity and execute goal-directed behavior. Additionally, PNS reactivity to stress was linked to individual differences in IC impairment. This finding challenges conclusions from a previous chronic stress study that found physiological indices (i.e., blood pressure) to be unrelated to IC impairment (Mani et al., 2013). We theorize that individuals who exhibit exaggerated arousal and are less able to regulate their heart rate physiology in response to an acute stressor experience subsequent performance decrements on prefrontal-dependent tasks due to cognitive load, consistent with bottom-up arousal demands on IC-critical, prefrontal regions (Park & Thayer, 2014). An alternate explanation could be that individuals who have higher top-down prefrontal cortex control are both able to exhibit more flexible PNS regulation in response to acute stress as well as higher IC performance following acute stress. Although less ‘mechanistic’, this explanation would still suggest that HF-HRV responsivity may be a useful biomarker associated with IC resilience to acute stress.
Although no prior research to date has examined the immediate effects of acute stress on IC, it is important to place the present findings in the context of previous research that has examined the delayed effects of acute stress on inhibitory control. One previous study (small sample size, no pre-stress assessment of IC) found that at 30 minutes post-stressor, individuals in the acute stress (versus control) group exhibited higher IC. Interestingly, this effect was eliminated by the administration of mineralocorticoid receptor (MR) blocking drugs, which the authors suggest indicated that the release of glucocorticoids and subsequent binding at MRs was responsible for improved IC, 30 minutes post-stressor. If such results replicate in larger samples, it is a thought-provoking possibility that differential effects of acute stress may exist immediately post-stressor versus at a 30 minute delay. In order to better examine such questions, systematic research is needed to investigate the effects of acute stress in identical laboratory conditions across a variety of delay periods. We also argue for the use of repeated measures designs to ensure that post-stressor differences are not due to spurious pre-stressor baseline difference in IC.
Subsequent investigations should also examine the role of individual differences in candidate biological systems (e.g., PNS, HPA) to examine the extent to which individual differences in both stress reactivity and recovery are linked to cognitive performance generally, and IC specifically. In the present study, we found that individual differences in PNS responsivity to stress a significant portion of the variance in IC impairment immediately following acute stress, with no associations with cortisol reactivity. Prior research examining the possibility of delayed post-stressor IC enhancement has not mapped a similar link of individual differences in biological system responsivity to IC.
Questions remain regarding causal mechanisms of IC impairment and interplay between related biological systems following acute stress exposure. Concurrent assessment of other biological systems (e.g., neural activation, sympathetic nervous system activity) along with investigations across acute stressors of variable type and intensity would offer valuable insights. In future research that is sufficiently powered, it may also be valuable to examine the effects of potential moderators such as gender, age, and race/ethnicity on the acute stress-IC association given the relevance of such variables to PNS function and stress reactivity in previous research (Hill et al., 2015; Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004). Future research on the role of individual differences in variables that affect biological measures of acute stress reactivity and/or IC (e.g. chronic stress, early life adversity, substance use, mental disorders and sleeping patterns) would be highly informative for our understanding of the generalizability of results and the extent to which differential sensitivity to acute stress may contribute to disruptions in IC.
The ability of acute stress to alter IC has substantial implications for understanding IC lapses, such as those implicated in risk-taking (e.g. substance use) behavior (López-Caneda, Holguín, Cadaveira, Corral, & Doallo, 2014). PNS reactivity to stressors may serve as a phenotypic marker of compromised IC following acute stress and indicate susceptibility to impulsive behavior. Future research should examine the extent to which acute IC impairment predicts real-world risk-taking behavior. These findings also have intervention implications: Targeting PNS regulation could buffer individuals from the immediate cognitive effects of acute stress.
Acknowledgments
The authors thank Cheryl Mikkola for editorial assistance and Dr. Andrea Gierens at the Biochemical Labor at the Biochemical Laboratory, Psychobiology, University of Trier for support regarding cortisol assay procedures. Leslie E. Roos received support from HHS-2014-ACF-ACYF-CA-0803. Elliot Berkman received support from NIH grants R01 AG048840, R21 CA175241 and P50 DA035763. Philip A. Fisher received support from NIH grants R01 HD075716 and P50 DA035763.
Footnotes
Author Contributions
L. E. Roos developed the study concept and all authors contributed to study design. Data collection was performed by L.E. Roos, K. Faraday, and K. Hyslop. Data analysis and interpretation was conducted by L. E. Roos, and E. L. Knight under the supervision of E. Berkman, and P. A. Fisher. L. E. Roos, E. L. Knight, K. G. Beauchamp, K. Faraday, and K. Hyslop drafted the manuscript, with E. Berkman, and P. A. Fisher providing critical revisions. All authors approved of the final manuscript for submission.
Data from additional participants (n = 23) with self-reported mental health diagnoses, neurological conditions, and/or psychotropic medication use were not included in this study, given known associations between these diagnoses and medication use and PNS activity (Beauchaine, 2001; Lotufo, Valiengo, Benseñor, & Brunoni, 2012; Kemp & Quintana, 2013). Due to constraints associated with university human subjects pool recruitment guidelines, they could not be excluded from participation and thus were removed prior to data analysis.
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: One decade on. Trends in Cognitive Sciences. 2014;18(4):177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Development and psychopathology. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beaumont A, Burton AR, Lemon J, Bennett BK, Lloyd A, Vollmer-Conna U. Reduced cardiac vagal modulation impacts on cognitive performance in chronic fatigue syndrome. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Van Der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Blair C, Ursache A. A bidirectional model of executive functions and self-regulation. In: Vohs KD, Baumeister RF, editors. Handbook of self-regulation: Research, theory, and applications. 2nd. New York, NY: Guilford Press; 2011. pp. 300–320. [Google Scholar]
- Capuana LJ, Dywan J, Tays WJ, Segalowitz SJ. Cardiac workload and inhibitory control in younger and older adults. Biological Psychology. 2012;90(1):60–70. doi: 10.1016/j.biopsycho.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Canli T, Poldrack RA. Measurement and reliability of response inhibition. Frontiers in Psychology. 2012;3(37):1–10. doi: 10.3389/fpsyg.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annual Review of Psychology. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological bulletin. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Hill LK, Hu DD, Koenig J, Sollers JJ, III, Kapuku G, Wang X, Thayer JF. Ethnic differences in resting heart rate variability: a systematic review and meta-analysis. Psychosomatic medicine. 2015;77(1):16. doi: 10.1097/PSY.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovland A, Pallesen S, Hammar Å, Hansen AL, Thayer JF, Tarvainen MP, Nordhus IH. The relationships among heart rate variability, executive functions, and clinical variables in patients with panic disorder. International Journal of Psychophysiology. 2012;86(3):269–275. doi: 10.1016/j.ijpsycho.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS. The relationship between mental and physical health: insights from the study of heart rate variability. International Journal of Psychophysiology. 2013;89(3):288–296. doi: 10.1016/j.ijpsycho.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: the impact of age and gender. International journal of behavioral medicine. 2004;11(2):116–121. doi: 10.1207/s15327558ijbm1102_8. [DOI] [PubMed] [Google Scholar]
- López-Caneda E, Holguín SR, Cadaveira F, Corral M, Doallo S. Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: a review. Alcohol and alcoholism. 2014;49(2):173–181. doi: 10.1093/alcalc/agt168. [DOI] [PubMed] [Google Scholar]
- Lotufo PA, Valiengo L, Benseñor IM, Brunoni AR. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia. 2012;53(2):272–282. doi: 10.1111/j.1528-1167.2011.03361.x. [DOI] [PubMed] [Google Scholar]
- Mani A, Mullainathan S, Shafir E, Zhao J. Poverty impedes cognitive function. Science. 2013;341(6149):976–980. doi: 10.1126/science.1238041. [DOI] [PubMed] [Google Scholar]
- Manuel AL, Bernasconi F, Spierer L. Plastic modifications within inhibitory control networks induced by practicing a stop-signal task: An electrical neuroimaging study. Cortex. 2013;49(4):1141–1147. doi: 10.1016/j.cortex.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Mika A, Mazur GJ, Hoffman AN, Talboom JS, Bimonte-Nelson HA, Sanabria F, Conrad CD. Chronic stress impairs prefrontal cortex-dependent response inhibition and spatial working memory. Behavioral Neuroscience. 2012;126(5):605–619. doi: 10.1037/a0029642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G, Thayer JF. From the heart to the mind: Cardiac vagal tone modulates top-down and bottom-up visual perception and attention to emotional stimuli. Frontiers in Psychology. 2014;5(278) doi: 10.3389/fpsyg.2014.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Scholz U, La Marca R, Nater UM, Aberle I, Ehlert U, Hornung R, Kliegel M. Go no-go performance under psychosocial stress: Beneficial effects of implementation intentions. Neurobiology of learning and memory. 2009;91(1):89–92. doi: 10.1016/j.nlm.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Preuß D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. 2008;33(5):643–653. doi: 10.1016/j.psyneuen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Höffken O, Tegenthoff M, Wolf OT. Stress-induced enhancement of response inhibition depends on mineralocorticoid receptor activation. Psychoneuroendocrinology. 2013;38(10):2319–2326. doi: 10.1016/j.psyneuen.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Seli P, Cheyne JA, Smilek D. Attention failures versus misplaced diligence: Separating attention lapses from speed–accuracy trade-offs. Consciousness and cognition. 2012;21(1):277–291. doi: 10.1016/j.concog.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV—a software for advanced heart rate variability analysis. In: Sloten J, Verdonck P, Nyussen M, Haueisen J, Magjarevic R, editors. 4th European Conference of the International Federation for Medical and Biological Engineering. IFMBE Proceedings. Vol. 22. Berlin, Heidelberg, Germany: Springer; 2009. pp. 1022–1025. [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37(2):141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan G. Response inhibition in the stop-signal paradigm. Trends in Cognitive Sciences. 2008;12(11):418–424. doi: 10.1016/j.tics.2008.07.005. http://doi.org/10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends in cognitive sciences. 2008;12(11):418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L, Lipszyc J, Dupuis A, Thayapararajah SW, Schachar R. Response inhibition and psychopathology: A meta-analysis of go/no-go task performance. Journal of Abnormal Psychology. 2014;123(2):429–439. doi: 10.1037/a0036295. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Kodama M, Matsuhisa M, Kishimoto M, Ozaki H, Tani A, Kamada T. Diurnal heart rate variability in healthy subjects: Effects of aging and sex difference. American Journal of Physiology-Heart and Circulatory Physiology. 1996;271(1):H303–H310. doi: 10.1152/ajpheart.1996.271.1.H303. [DOI] [PubMed] [Google Scholar]
- Yim IS, Quas JA, Rush EB, Granger DA, Skoluda N. Experimental manipulation of the Trier Social Stress Test-Modified (TSST-M) to vary arousal across development. Psychoneuroendocrinology. 2015;57:61–71. doi: 10.1016/j.psyneuen.2015.03.021. [DOI] [PubMed] [Google Scholar]


