Abstract
Sclerostin (SOST), a protein secreted from mature osteocytes in response to mechanical unloading and other stimuli, inhibits the osteogenic Wnt/β-catenin pathway in mesenchymal stem cells (MSCs) impeding their ability to differentiate into mineralizing osteoblasts.
Purpose
This review summarizes the crosstalk between adipose tissue and bone. It also reviews the origin, regulation, and role of SOST in osteogenesis and brings attention to an emerging role of this protein in the regulation of adipogenesis.
Recent Findings
Bone-derived molecules that drive MSC adipogenesis have not previously been identified, but recent findings suggest that SOST signaling may induce adipogenesis. In vivo SOST acts locally to induce changes in bone and, in vitro, increases adipogenesis in 3T3-L1 preadipocytes.
Summary
SOST is able to induce adipogenesis in certain preadipocytes, however bone-specific studies are needed to determine the effect of local SOST concentrations in healthy and disease models on bone marrow adipose tissue.
Keywords: Sclerostin, Adipogenesis, Bone Marrow Adipose Tissue, Fat, LRP, Wnt
Introduction
The skeleton, classically viewed as a structural element in vertebrates, is emerging as a key regulator of complex biological processes. Bone is now known to be a master regulator of multiple endocrine processes and plays a role in overall glucose metabolism, fertility, and the maintenance of the hematopoietic niche[1–5]. Cells in the bone marrow microenvironment and beyond are regulated by signals produced by osteocytes and osteoblasts (OBs) although the mechanisms are currently being identified. The bone marrow contains stem, progenitor, and multifunctional differentiated cell types of several different lineages which all work together to maintain a complex microenvironment influenced by endocrine, paracrine, and autocrine factors. In response to biochemical stimuli, mesenchymal stem cells (MSCs) differentiate into mature, functioning cells. In healthy bone marrow (BM), these MSCs can differentiate into chondrocytes, adipocytes (BMAs) or OBs (Fig.1)[6]. Bone marrow adipose tissue (MAT) is composed primarily of BMAs, but also contains other BM cells (e.g., immune cells, endothelial cells, fibroblasts, or OBs). MAT has recently been recognized as a distinct adipose depot important for, and responsive to metabolic status[7, 8]. Although much is known about regulation of white adipose tissue (WAT), researchers are only now starting to understand signaling pathways that regulate MAT. In addition to MAT, other adipose depots including WAT are likely regulated by skeletal factors; in fact, both intrinsic (genetic) and extrinsic factors resulting in changes in bone are often accompanied by a metabolic and adipose tissue phenotypes. Specific signals emanating from the bone and extending to local cells and to the periphery are slowly being uncovered (including osteocalcin) and new factors such as sclerostin (SOST) may add complexity to the organ- and tissue-specific regulation of adipogenesis both in local and distant tissues (MAT and WAT respectively) .
Figure 1.
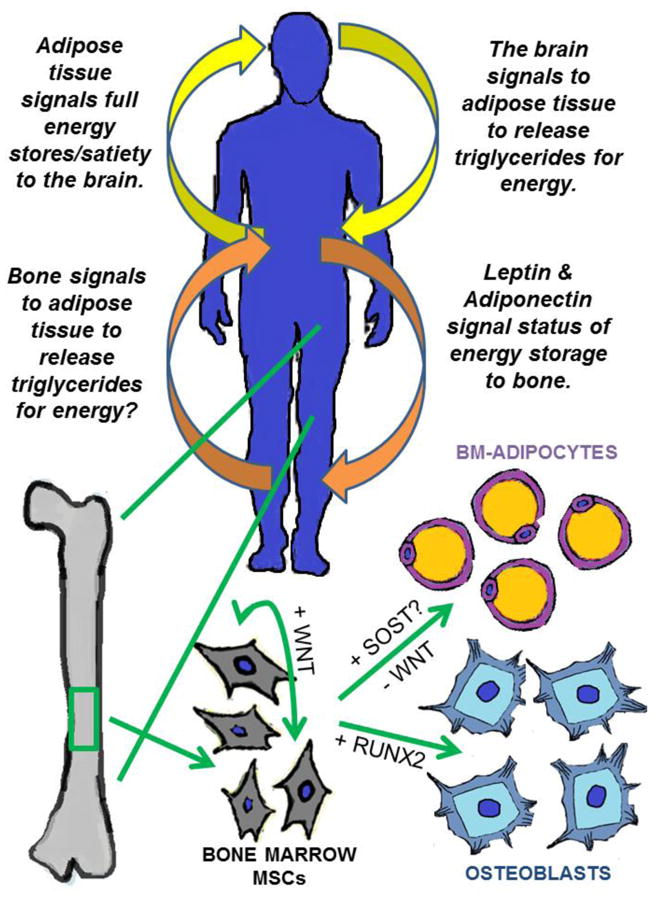
Signals between the brain, the body, and bone. The skeleton is emerging as a key regulator of complex biological processes, including the sending and receiving of endocrine signals. In response to biochemical stimuli, mesenchymal stem cells (MSCs) differentiate into mature, functioning cells. In healthy bone marrow (BM), MSCs differentiate into BM-adipocytes (BMAs) or osteoblasts (OBs) in response to the addition or removal of WNT signaling, respectively. WNT signaling induces RUNX2 expression driving OB differentiation, but inhibition of WNT signaling is required for the differentiation into adipocytes. Sclerostin (SOST), a WNT inhibitor, is emerging as a potential player in the differentiation of BM-MSCs, adding complexity to the regulation of bone marrow adipose tissue (MAT) in response to both adipose (energetic), and bone-derived signals.
Adipogenesis: Origins, Depots, and Induction
Energy storage and energy expenditure are tightly regulated and involve complex signaling networks between the brain and the periphery. In mammals, energy is stored as adipose tissue of which four main types have been characterized: white adipose tissue (WAT), brown adipose tissue (BAT), and bone marrow adipose tissue (MAT)[7, 9]. WAT and BAT arise from different progenitor cell populations, however, WAT can be induced toward browning and become “beige” adipose tissue, functionally similar to BAT in both phenotype and to a partial degree in thermogenic capabilities[10, 11]. The main cell type in all adipose tissues is the adipocyte, a cell primarily responsible for energy storage by way of lipid droplet formation in white tissue, and lipid droplet utilization for heat production in BAT. Adipocyte lipid droplets can quickly undergo lipolysis, releasing fatty acids from triglycerides for mobilization to meet systemic energy requirements[12]. In addition to the well characterized role in energy storage capacity, adipocytes also have endocrine functions, secreting hormones and signaling molecules involved in global energy metabolism known as adipokines. Leptin, a hormone synthesized and secreted from adipocytes, traditionally signals via its receptor (Lepr) to indicate satiety in the brain and full energy storage to various systems in the periphery. While circulating leptin levels are directly correlated with body fat percentage, a second adipose-derived hormone, adiponectin, displays an inverse relationship with this depot. Adiponectin, produced by WAT and MAT and is elevated in lean individuals and with calorie restriction[13], while extreme low levels of adiponectin are associated with risk of cardio-metabolic disease[14].
Adipose tissue resides in distinct depots in the mammalian body and the relative distribution of fat into these depots may affect overall health. Subcutaneous and visceral depots contain primarily WAT, while BAT is present during early, postnatal development. Small amounts of BAT persist into adulthood primarily above the clavicle and in the small of the back (subscapular region)[9]. MAT has been long characterized as a relatively inert part of the bone marrow, but recently has been recognized to have potentially diverse and important functions. Significantly, this depot begins to rapidly form at birth and makes up approximately 50-70% of the marrow space by adulthood[15, 16]. These cells appear to be different from WAT and BAT in terms of both gene expression and functional phenotype. While the true progenitor cell for bone marrow adipocytes (BMAs) remains in dispute, it is widely accepted that the progenitor arises within the marrow cavity and is likely common to both BMAs and OBs [17]. Recent work has shown that MAT is similar to WAT in that it contributes to adiponectin levels under various metabolic conditions, but distinct from WAT in that it responds differently to both calorie restriction(starvation/anorexia)[13] and treatment with insulin-sensitizing, peroxisome proliferator-activated receptor gamma (PPARγ) agonists[18].
In general, adipocytes are formed from different types of stem cells which first differentiate into preadipocytes resulting in the commitment of the cell down this specific lineage. Preadipocytes then proliferate to create a pool of preadipocytes from which mature adipocytes arise in response to biochemical stimuli. Many factors have been characterized as pro-adipogenic factors including transforming growth factor-β (TGF-β) family members (which activate SMAD transcription factors), and insulin signaling via its receptor. Both stimulate a cascade of transcriptional events involving early adipogenic factors such as PPARγ and CCAAT/enhancer–binding protein β and α (C/EBPβ and C/EBPα) to induce adipogenesis. Adipogenesis can be inhibited via TCF/LEF, which block increased transcription of C/EBPα and PPARγ[19].
Significantly, MSCs in the skeleton express leptin receptor (Ob-R or Lepr)[20], indicating they likely receive nutritional signals from various adipose depots. Conditional ablation of the long form of the Ob-R in vivo (Col3.6-Cre;Ob-Rbfl/fl) resulted in a significant increase in trabecular bone, suggesting that leptin signaling in early progenitors inhibits bone formation, and that MSCs themselves may directly respond to systemic energy status, independent from neural/brain signaling[21]. Importantly, Col2.3-Cre;Ob-Rbfl/fl mice, which result in conditional deletion of Lepr from osteoblasts, had no significant defects in osteogenesis. The same study investigated in vitro deletion of the leptin receptor in Ob-Rbfl/fl BM-MSCs via adenovirus Cre, revealing decreased mineralization capacity and increased adipogenesis in KO BM-MSCs, while BM-MSCs from leptin- and leptin receptor-deficient mice showed increased mineralization. Knockout of the leptin receptor in early mesenchymal progenitor cells in vivo (Col3.6-Cre;Ob-Rbfl/fl) yielded increased in vitro mineralization and adipogenesis[21]. These combined results suggest that leptin signaling is a key component during early lineage commitment and that sustained leptin signaling may help maintain “stemness” or early progenitor state.
Long bone-specific conditional deletion of Lepr (Prx1-Cre; Leprfl/fl) increased osteogenesis and decreased adipogenesis in these regions, while overall body mass and hematopoiesis remained unchanged[22]. This study also characterized Col2.3-Cre;Leprfl/fl mice and also reported no significant differences in bone parameters or adipogenesis[22], consistent with previously published results[21]. As expected, MSCs obtained from Prx1-Cre; Leprfl/fl femurs did not respond to leptin in vitro, while in comparison, wild-type MSCs yielded increased adipocytes and reduced OBs in response to leptin treatment. Significantly, this study also demonstrated that local action of leptin signaling is responsible for changes in bone due to high fat diet (HFD) administration, as Prx1-Cre;Leprfl/fl mice had increased bone parameters and decreased adipocytes in femur when compared to wild-type mice on HFD. They also showed increased fracture healing and increased osteogenesis after irradiation in Prx1-Cre;Leprfl/fl mice, suggesting that the Lepr acts locally to negatively regulate osteogenesis in states of bone and bone marrow damage [22]. These results indicate that Lepr is involved in the reciprocal regulation of BM-MSCs and their ability to differentiate into either OBs or adipocytes. The direct comparison with Col2.3-Cre;Leprfl/fl suggests that Lepr is not acting in OBs to regulate osteogenesis or adipogenesis. These data also suggest that the adipogenic differentiation of skeletal stem cells may require leptin signaling, linking the multipotent ability of BM-MSCs to energy availability and needs as indicated by adipose tissue. Adipose tissue also conveys information to bone indirectly via leptin signaling in the sympathetic nervous system. These signals appear to be largely anti-osteogenic, and are likely β-adrenergic receptor dependent[23].
Deletion of the parathyroid hormone receptor (PTH1R) in the long bones of mice via Prx1-Cre yielded opposite results compared to Prx1-Cre;Leprfl/fl. These mice exhibited reduced bone formation, increased bone resorption, and increased bone marrow adiposity[24]. Administration of PTH to wild-type mice significantly reduced MAT, a finding which has been confirmed in human male osteoporosis patients[24]. Although bone marrow progenitors are still under investigation, this recently published paper suggests that they are Pref1+/RANKL+[24], and identifies PTH as an additional factor which likely affects MSC differentiation and cell fate.
Bone as an Endocrine Organ
The link between metabolism, which includes nutritional stimuli and organismal energy availability, and the structural integrity of the skeleton has been a subject of interest for decades. The maintenance of skeletal function is energetically expensive, as both bone building by OBs and bone resorption by osteoclasts requires energy. In healthy humans, bone mineral density (BMD) is inversely correlated with both MAT and visceral fat levels but positively associated with subcutaneous fat. Interestingly, the relationship between bone and MAT is dynamic throughout development and during different metabolic disorders. During puberty, both BMD and MAT increase, but during aging MAT continues to increase while BMD decreases. In anorexic patients, both WAT and BMD severely decrease, but the MAT depot expands rapidly suggesting that MAT is regulated separately from other depots and that this energy store is preferentially salvaged during times of serious energy depletion[7]. The signals that stimulate this expansion are as yet uncharacterized, and whether they are derived from the brain, the bone, or both is unclear.
In vitro culture assays utilizing OBs suggest that OBs consume high levels of glucose and express glucose transporters Glut1, Glut3 and Glut4. Osteocyte- and osteoblast-specific deletion of Glut4 resulted in normal bone architecture, but increased peripheral fat and reduced insulin sensitivity[25]. This suggests that glucose utilization by the bone is a crucial component to healthy vertebrates and that its use impacts global energy usage and disposal.
Importantly, leptin deficiency results in high bone mass (in mice, humans, and sheep) as a result of increased bone formation, an effect which is recapitulated in neuron-specific deletion of the leptin receptor, but not an osteoblast-specific deletion[26]. This suggests hormonal signaling from the periphery to the brain to the bone, and is consistent with the idea that bone acquisition is tied to energy intake, signaling indirectly through central neural mechanisms[27]. The bone conversely signals to the brain and body to convey its energetic requirements, and consistent with this hypothesis, osteolineage cells have been shown to influence global energy metabolism in a number of studies. Importantly, the osteoblast- and osteocyte-derived osteocalcin, which functions locally and systemically[28], is a regulator of insulin sensitivity and secretion[2]. Indeed, in osteocalcin deficient mice, expression of Acyl CoA, UCP2, Pparγ, and adiponectin were all decreased, while adding exogenous osteocalcin to either islets or adipocytes in vitro increased the expression of both insulin and adiponectin respectively[28]. Importantly, some metabolic phenotypes generated in vivo via conditional removal of OBs could not be rescued with osteocalcin treatment, suggesting that additional bone-derived proteins may regulate adipose depot weight and energy expenditure/intake[29].
Wnt Signaling in the Bone Marrow Microenvironment
Multipotent cells such as bone marrow MSCs (BM-MSCs) respond to biochemical stimuli as they progress down different lineages and this process is largely controlled by canonical Wnt signaling. Wnt ligand molecules, a family of conserved, secreted glycoproteins, are critical in developmental patterning, tissue remodeling and the regulation of cellular proliferation and apoptosis. Canonical Wnt signaling includes the binding of Wnt ligands to frizzled receptors and/or co-receptors (LRP-4/5/6). This binding stimulates a cascade of events resulting in the accumulation of β-catenin in the cytoplasm and subsequent translocation to the nucleus where it activates genes important for cellular determination. In the OB, Wnt signaling leads to the expression of T-cell factor/lymphoid-enhancer (Tcf/Lef) family of transcription factors, which enhance expression of RUNX-2 and other osteogenic transcription factors and genes that induce OB differentiation and proliferation (C-MYC). Sustained Wnt signaling is also important in the maintenance of the pre-adipocyte state in MSCs and other adipocyte precursor cells. These combined results demonstrate that Wnt signaling is vital to the regulation of stem cell differentiation and may be abnormal in various pathogenic states (Fig. 1). Consistent with this hypothesis, transgenic mice overexpressing Wnt10b by bone cells (Ocn-Wnt10b) exhibit elevated numbers of OBs and increased bone formation and density[30], decreased bone marrow volume, and significantly less MAT[18]. Wnt signaling is also inhibited endogenously via the Dickkopf (DKK) family, and in bone DKK family members Dikkopf-1 (DKK1) and SOST have been specifically implicated in both BM maintenance and pathogenesis.
Sclerostin: Origins and Association with Human Disease
Osteocytes are the primary cell type found in bone and make up approximately 90% of the skeleton's cellular compartment. These terminally differentiated cells are derived from the formation of mineralized bone matrix over senescent OBs, and as the OBs become embedded, they begin to express osteocyte-specific genes. Significantly, genetic activation of β-catenin in osteocytes alone increases both cortical and trabecular bone, bone formation, and OB number[31] confirming that signals derived from the osteocyte can affect the bone marrow microenvironment. Osteocytes exclusively produce SOST, a potent Wnt antagonist. The bone formation process is modulated via SOST, which inhibits OB proliferation and differentiation. This protein is synthesized in the postnatal period and inhibits bone formation while also stimulating osteoclast formation and survival. Sost knock-out (SOST-KO) mice exhibit increased OB number, and high levels of cortical and trabecular bone[32].
The regulatory role of SOST in the equilibrium of osteogenesis makes it a therapeutic target for individuals with osteoporosis. Importantly, in the last 5-10 years studies have implicated polymorphisms in SOST as risk factors for osteoporosis[33, 34]. Significantly, a single T to C change in the regulatory region upstream of the SOST gene increases the risk of osteoporosis and is significantly associated with BMD in both Chinese and Caucasian populations[34, 35]. This single nucleotide variant abolishes binding of transcription factors C/EBPα and FOXA1 (modulators of ERα signaling) to the Sost/SOST gene. This long range enhancer and the variant therein provides a mechanistic link between SOST expression and osteoporosis, as well as the importance of estrogen signaling in the regulation of SOST expression and subsequent protein levels[34]. An anti-SOST molecule (romosozumab) has completed phase III clinical trials and has shown significant short term efficacy[36]. Treatment with the anti-SOST molecule in rodent models lead to increased bone volume as measured by a number of metrics (cortical thickness, trabecular volume, and trabecular thickness) [37, 38]. Our lab has also reproduced this finding with SOST neutralizing antibodies in mice in the tibia and vertebrae (unpublished data). In phase I, II, and III clinical trials, bone formation markers were increased upon treatment with anti-SOST antibodies, while lumbar spine and total hip BMD increased in a dose- and time-dependent manner[36, 38]. These studies demonstrate that anti-SOST therapy can be used to reduce the action of SOST during osteoporosis, leading to increased Wnt signaling and subsequent osteoblastogenesis.
Evidence of a role for SOST in adipogenesis
In humans, SOST increases with age[39] and older men have higher levels of circulating SOST than age matched women[39, 40]. Interestingly aging is associated with a decrease in bone formation and an increase in bone marrow adiposity[41–43], so it may be that the age-related increase in SOST regulates these phenotypes. Studies linking circulating SOST to adipose levels have consistently shown positive correlations with fat mass in men, but results are contradictory in women[40, 44, 45]. While specific adipose depots seem to have different relationships with bone, higher vertebral MAT has been reported in both male and female osteoporosis patients[42, 43]. This imbalance is hypothesized to be due to a shift in allocation of MSCs toward adipocyte formation and away from osteoblastogenesis.
Inhibition of canonical Wnt signaling has been implicated in the induction of adipogenesis, leading to an increase in the transcription of adipocyte-specific genes including PPARy. DKK1 has been shown to increase adipogenic differentiation of WAT stem cells[46]. In vitro studies have demonstrated the potential regulation of adipogenesis via DKK1 and SOST but the specific relationship between the osteocyte-produced SOST and BM-MSCs remains uncharacterized.
Initiation of preadipocyte differentiation in vivo is influenced by a tightly regulated balance of factors that control growth of existing adipocytes and differentiation of new adipocytes[19]. The fate of new adipocytes is heavily regulated by Wnt ligands and the expression of their receptors and co-receptors. Canonical Wnt signaling can be regulated by extracellular factors such as secreted Frizzled-related proteins, Wnt inhibitory factors, and DKK proteins. DKK proteins such as DKK1 inhibit Wnt signaling by binding to frizzled co-receptors LRP 5/6. Importantly, sustained Wnt signaling prevents differentiation of preadipocytes[47, 48], and transgenic expression of Wnt10b under Fabp4 promoter regulation results in reduced body fat content[49], suggesting that Wnt10b signaling in preadipocytes and adipocytes is a key regulator of the cellular transition from preadipocyte into mature adipocyte.
While sustained Wnt signaling in mouse preadipocytes has been linked to the maintenance of stemness and the preadipocyte state[48, 50], inhibition of this process is likely involved in terminal differentiation. DKK1 was identified as a potential inducer of adipogenesis in human microarray data and this result was confirmed in human subcutaneous preadipocytes via RT-PCR, which demonstrated increasing expression of the DKK1 gene and corresponding protein levels during adipocyte differentiation[51]. Dkk receptors are also expressed in adipocytes and become downregulated during differentiation, which could indicate autocrine signaling through WNT pathways. Importantly, this study showed that DKK1 gene expression and secreted protein were restricted to the stromal vascular fraction of human adipose tissue, and both were essentially undetected in mature subcutaneous and omental adipocytes, as well as mouse 3T3-L1 preadipocytes[51]. Ectopic expression of hDKK1 in 3T3-L1 cells inhibited Wnt signaling, and promoted adipogenesis in these cells as indicated by increased lipid accumulation and upregulation of the adipogenic markers Pparγ and Fatty acid binding protein 4 (Fabp4/Ap2)[51]. These results demonstrate that DKK1 promotes adipogenesis and suggests similar effects might be observed with other Wnt antagonists.
Importantly, SOST-KO mice have recently been characterized as having significantly less whole body fat, and smaller adipocytes. These changes were accompanied by improved glucose tolerance and enhanced insulin sensitivity. Inversely, mice with overexpression of SOST presented with excess adipose tissue and impaired glucose handling[52]. SOST has been shown to directly increase adipogenesis in mouse pre-adipocytes. Recombinant SOST enhanced the differentiation of 3T3-L1 mouse pre-adipocytes in a dose-responsive (2-20 ng/mL) manner[53]. The addition of SOST to adipocyte differentiation medium lead to an increase in intracellular lipid deposits after 5 days of treatment when compared to untreated differentiating cells. SOST treatment also increased adipocyte-specific gene expression of both Pparg and Cebpb while cell proliferation and cell death remained unchanged. Importantly, SOST treatment inhibited canonical Wnt3a activity in combined treatments, and also reduced transcriptional coactivator with PDZ motif (TAZ)-responsive transcriptional activity and gene expression. TAZ regulates the transcriptional activity of both RUNX2 and PPARγ via coactivation and corepression respectively, and thus may regulate MSC fate in conjunction with or separately from WNT/β-catenin signaling[54, 55]. Subsequent experiments with the addition of TAZ siRNA demonstrated an increase in lipid droplet formation and adipogenic gene expression levels similar to that of SOST treatment[53]. The authors suggest that these combined results connect SOST inhibition of WNT/TAZ to the increase in adipogenesis in 3T3-L1 cells.
Conclusions
Signals from both bone and adipose tissue are rapidly being uncovered as essential regulators of homeostasis and energy balance (Fig. 1). SOST, while traditionally characterized as a potent inhibitor of new bone formation, is being investigated for new roles in adipose development, and maintenance of the bone marrow microenvironment (BMM). Recently, MSC engraftment experiments with young and aged mice, demonstrated the importance of the BMM in regulating differentiation, with decreased osteoblastogenesis and increased adipogenesis in MSCs from young mice transplanted into old mice[56]. This indicates that bone health is likely tied to the makeup of the bone marrow milieu, and that changes observed in human patients during aging may be due to bone-derived signals such as SOST.
The BMM and the factors that regulate the delicate balance of cells therein, also play a role in metastasis and protection of cancer cells from treatments. Recent work suggests that bone metastasizing tumors likely interact with and are stimulated by various cells in the BMM. Specifically, bone marrow stromal cells have been shown to induce proliferation in multiple myeloma[57] and metastatic breast cancer cell lines[58], while pro-osteoblastic treatments have been used to successfully reduce tumor burden in bone[59, 60]. Very recently, studies suggest that mature bone marrow adipocytes may protect myeloma cells from chemotherapeutics[61] and that osteocyte-derived factors promote tumor cell proliferation[62]. Elevated levels of SOST have been detected in multiple myeloma patients and SOST has been shown to be increased in osteocytes directly exposed to tumor cells[62], suggesting that high SOST may contribute to conditions favorable to metastasis either directly or indirectly by regulating the cellular makeup of the bone marrow. Indeed, studies suggest that MAT contributes to systemic adiponectin levels[18], and that adiponectin levels may be related to myeloma susceptibility[63]. In mice, HFD may create permissible conditions for myeloma colonization of the bone marrow[64] and in humans, bone metastases more frequently occur in older patients who typically have increased bone marrow adiposity[65].
Although each of these pieces of data suggests a relationship between SOST, adipose tissue, and cancer invasion of bone, the direct signals and definitive roles of each contributing factor is as yet largely undefined. These reports demonstrate the importance of the microenvironment and bone-derived signals on energy metabolism and specifically adipose deposition. Additional studies are required to truly investigate the complex relationship between SOST and bone marrow adipose tissue as we seek to understand metastasis and uncover future disease treatments.
Acknowledgments
The authors’ work is supported by MMCRI Start-up funds, a pilot project Grant from NIH/NIGMS (P30GM106391), and the NIH/NIDDK (R24DK092759-01).
Footnotes
Compliance with Ethical Standards: Conflict of Interest: Heather Fairfield, Clifford J. Rosen, and Michaela R. Reagan each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Shao J, Wang Z, Yang T, et al. Bone Regulates Glucose Metabolism as an Endocrine Organ through Osteocalcin. Int J Endocrinol. 2015;2015:1–9. doi: 10.1155/2015/967673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fulzele K, Riddle RC, DiGirolamo DJ, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–19. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–124. doi: 10.1016/j.bbi.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reagan MR, Rosen CJ. Navigating the bone marrow niche: translational insights and cancer-driven dysfunction. Nat Rev Rheumatol. 2015 doi: 10.1038/nrrheum.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeMambro VE, Le PT, Guntur AR, et al. Igfbp2 Deletion in Ovariectomized Mice Enhances Energy Expenditure but Accelerates Bone Loss. Endocrinology. 2015;156:4129–4140. doi: 10.1210/en.2014-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 7.Fazeli PK, Horowitz MC, MacDougald OA, et al. Marrow fat and bone-new perspectives. J Clin Endocrinol Metab. 2013;98:935–945. doi: 10.1210/jc.2012-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheller EL, Rosen CJ. What's the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjørndal B, Burri L, Staalesen V, et al. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes. 2011;2011:490650. doi: 10.1155/2011/490650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalinovich AV, de Jong JMA, Cannon B, Nedergaard J. UCP1 in adipose tissues: two steps to full browning. Biochimie. 2017 doi: 10.1016/j.biochi.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Ishibashi J, Seale P. Medicine. Beige can be slimming. Science. 2010;328:1113–4. doi: 10.1126/science.1190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012;81:715–36. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- 13.Cawthorn WP, Scheller EL, Learman BS, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–75. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalamaga M, Karmaniolas K, Panagiotou A, et al. Low circulating adiponectin and resistin, but not leptin, levels are associated with multiple myeloma risk: a case-control study. Cancer Causes Control. 2009;20:193–9. doi: 10.1007/s10552-008-9233-7. [DOI] [PubMed] [Google Scholar]
- 15.Scheller EL, Troiano N, Vanhoutan JN, et al. Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Enzymol. 2014;537:123–39. doi: 10.1016/B978-0-12-411619-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol. 2001;22:1620–7. [PMC free article] [PubMed] [Google Scholar]
- 17•.Berry R, Rodeheffer MS, Rosen CJ, Horowitz MC. Adipose Tissue Residing Progenitors (Adipocyte Lineage Progenitors and Adipose Derived Stem Cells (ADSC) Curr Mol Biol reports. 2015;1:101–109. doi: 10.1007/s40610-015-0018-y. This is a comprehensive overview of the types of adipose tissue, how each of them functions, and what their similarities and differences are. Specifically, the lineage of each type of adipocyte is outlined in great detail, citing lineage tracing experiments and yielding evidence that bone marrow adipocytes are distinct from white adipocytes. Compilation of numerous findings in this review demonstrates that MSCs that give rise to osteoblasts and adipocytes are osterix-positive (neonatal) and both leptin receptor and nestin-positive (adult) determining that the majority of these two cell types arise from a common progenitor population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Sulston RJ, Learman BS, Zhang B, et al. Increased Circulating Adiponectin in Response to Thiazolidinediones: Investigating the Role of Bone Marrow Adipose Tissue. Front Endocrinol (Lausanne) 2016;7:128. doi: 10.3389/fendo.2016.00128. This paper utilizes a model published in 2007 with transgenic overexpression of Wnt10b in osteoblasts and osteocytes (Ocn-Wnt10b) which characterized increased bone (BMD, etc.) and decreased marrow space in these mice. The new paper by Sulston et al., shows direct evidence that (1) increased local WNT signaling leads to lower MAT and (2) that this signaling is able to partially restrict MAT expansion during treatment with TZD confirming that WNT signaling is a key regulator of MSC fate determination, but also that inhibition of this pathway must be required for normal MAT formation and expansion- stimulation of PPARγ in these cells is not enough. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDougald OA, Mandrup S. Adipogenesis: forces that tip the scales. Trends Endocrinol Metab. 13:5–11. doi: 10.1016/s1043-2760(01)00517-3. [DOI] [PubMed] [Google Scholar]
- 20.Zhou BO, Yue R, Murphy MM, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154–68. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheller EL, Song J, Dishowitz MI, et al. Leptin functions peripherally to regulate differentiation of mesenchymal progenitor cells. Stem Cells. 2010;28:1071–80. doi: 10.1002/stem.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Yue R, Zhou BO, Shimada IS, et al. Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell. 2016;18:782–96. doi: 10.1016/j.stem.2016.02.015. The leptin receptor (Lepr) was conditionally deleted from long bones during this study (Prx1-Cre;Lepr<fl/fl>) yielding animals with normal body mass. Limb bones from these animals had high bone parameters and reduced bone marrow adipose tissue demonstrating the importance of leptin signaling, and energetic requirements in the overall maintenance of the bone marrow microenvironment. [DOI] [PubMed] [Google Scholar]
- 23.Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 24.Fan Y, Hanai JI, Le PT, et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017;0:166–176. doi: 10.1016/j.cmet.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Frey JL, Wong GW, et al. Glucose Transporter-4 Facilitates Insulin-Stimulated Glucose Uptake in Osteoblasts. Endocrinology. 2016;157:4094–4103. doi: 10.1210/en.2016-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Y, Yadav VK, Suda N, et al. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci U S A. 2008;105:20529–33. doi: 10.1073/pnas.0808701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab. 2008;19:161–6. doi: 10.1016/j.tem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Yoshikawa Y, Kode A, Xu L, et al. Genetic evidence points to an osteocalcin-independent influence of osteoblasts on energy metabolism. J Bone Miner Res. 2011;26:2012–25. doi: 10.1002/jbmr.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett CN, Ouyang H, Ma YL, et al. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res. 2007;22:1924–32. doi: 10.1359/jbmr.070810. [DOI] [PubMed] [Google Scholar]
- 31.Tu X, Delgado-Calle J, Condon KW, et al. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc Natl Acad Sci U S A. 2015;112:E478–86. doi: 10.1073/pnas.1409857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Ominsky MS, Niu Q-T, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–9. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 33.Li WF, Hou SX, Yu B, et al. Genetics of osteoporosis: Accelerating pace in gene identification and validation. Hum Genet. 2010;127:249–285. doi: 10.1007/s00439-009-0773-z. [DOI] [PubMed] [Google Scholar]
- 34.Huang QY, Li GHY, Kung AWC. The -9247 T/C polymorphism in the SOST upstream regulatory region that potentially affects C/EBPalpha and FOXA1 binding is associated with osteoporosis. Bone. 2009;45:289–94. doi: 10.1016/j.bone.2009.03.676. [DOI] [PubMed] [Google Scholar]
- 35.Yerges LM, Klei L, Cauley JA, et al. High-Density Association Study of 383 Candidate Genes for Volumetric BMD at the Femoral Neck and Lumbar Spine Among Older Men. J Bone Miner Res. 2009;24:2039–2049. doi: 10.1359/jbmr.090524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 37.van Dinther M, Zhang J, Weidauer SE, et al. Anti-Sclerostin antibody inhibits internalization of Sclerostin and Sclerostin-mediated antagonism of Wnt/LRP6 signaling. PLoS One. 2013;8:e62295. doi: 10.1371/journal.pone.0062295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costa AG, Bilezikian JP. Sclerostin: therapeutic horizons based upon its actions. Curr Osteoporos Rep. 2012;10:64–72. doi: 10.1007/s11914-011-0089-5. [DOI] [PubMed] [Google Scholar]
- 39.Mödder UI, Hoey KA, Amin S, et al. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26:373–9. doi: 10.1002/jbmr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma YHV, Schwartz AV, Sigurdsson S, et al. Circulating sclerostin associated with vertebral bone marrow fat in older men but not women. J Clin Endocrinol Metab. 2014;99:E2584–90. doi: 10.1210/jc.2013-4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kügel H, Jung C, Schulte O, Heindel W. Age-and sex-specific differences in the 1 H-spectrum of vertebral bone marrow. J Magn Reson Imaging. 2001;268:263–268. doi: 10.1002/1522-2586(200102)13:2<263::aid-jmri1038>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 42.Griffith JF, Yeung DKW, Antonio GE, et al. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236:945–951. doi: 10.1148/radiol.2363041425. [DOI] [PubMed] [Google Scholar]
- 43.Griffith JF, Yeung DKW, Antonio GE, et al. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology. 2006;241:831–8. doi: 10.1148/radiol.2413051858. [DOI] [PubMed] [Google Scholar]
- 44.Sheng Z, Tong D, Ou Y, et al. Serum sclerostin levels were positively correlated with fat mass and bone mineral density in Central South Chinese postmenopausal women. Clin Endocrinol (Oxf) 2012;76:797–801. doi: 10.1111/j.1365-2265.2011.04315.x. [DOI] [PubMed] [Google Scholar]
- 45.Urano T, Shiraki M, Ouchi Y, Inoue S. Association of circulating sclerostin levels with fat mass and metabolic disease--related markers in Japanese postmenopausal women. J Clin Endocrinol Metab. 2012;97:E1473–7. doi: 10.1210/jc.2012-1218. [DOI] [PubMed] [Google Scholar]
- 46.Gustafson B, Smith U. The WNT inhibitor Dickkopf 1 and bone morphogenetic protein 4 rescue adipogenesis in hypertrophic obesity in humans. Diabetes. 2012;61:1217–24. doi: 10.2337/db11-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–3. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 48.Bennett CN, Ross SE, Longo KA, et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277:30998–1004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 49.Longo KA, Wright WS, Kang S, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279:35503–9. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- 50.Longo KA, Kennell JA, Ochocinska MJ, et al. Wnt Signaling Protects 3T3-L1 Preadipocytes from Apoptosis through Induction of Insulin-like Growth Factors. J Biol Chem. 2002;277:38239–38244. doi: 10.1074/jbc.M206402200. [DOI] [PubMed] [Google Scholar]
- 51.Christodoulides C, Laudes M, Cawthorn WP, et al. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J Cell Sci. 2006;119:2613–2620. doi: 10.1242/jcs.02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frey JL, Kim S, Li Z, et al. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. J Bone Miner Res. 2017;31:1–1. doi: 10.1002/jbmr.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Ukita M, Yamaguchi T, Ohata N, Tamura M. Sclerostin Enhances Adipocyte Differentiation in 3T3-L1 Cells. J Cell Biochem. 2015 doi: 10.1002/jcb.25432. n/a–n/a. This paper by Ukita et al. is the first direct examination of the effect of sclerostin on a preadipocyte. The authors demonstrate increased adipogenesis as evidenced by functional (oil red o) and genetic (qPCR) outputs, and suggest that the pro-adipogenic effect of SOST is via its traditional role in canonical WNT signaling inhibition. This is extremely promising work but doesn’t actually answer the question about the effect that SOST might be having in its local microenvironment. 3T3-L1 cells are pre-programmed as pre-adipocytes, similar to WAT. As demonstrated by the additional papers highlighted here, preadipocytes from WAT are distinct from bone marrow adipocytes, and thus bone-specific studies are still required to determine whether changing levels of sclerostin can affect the bone marrow adipose depot. [DOI] [PubMed] [Google Scholar]
- 54.Hong JH, Yaffe MB. TAZ: A beta-Catenin-like Molecule that Regulates Mesenchymal Stem Cell Differentiation. Cell Cycle. 2006;5:176–179. doi: 10.4161/cc.5.2.2362. [DOI] [PubMed] [Google Scholar]
- 55.Lei QY, Zhang H, Zhao B, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426–36. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh L, Brennan TA, Russell E, et al. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone. 2016;85:29–36. doi: 10.1016/j.bone.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang T, Lee YW, Rui YF, et al. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther. 2013;4:70. doi: 10.1186/scrt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yaccoby S, Wezeman MJ, Zangari M, et al. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91:192–9. [PMC free article] [PubMed] [Google Scholar]
- 60.Yaccoby S, Ling W, Zhan F, et al. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–11. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Z, Xu J, He J, et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget. 2015;6:34329–41. doi: 10.18632/oncotarget.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delgado-Calle J, Anderson J, Cregor MD, et al. Bidirectional Notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-15-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fowler JA, Lwin ST, Drake MT, et al. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood. 2011;118:5872–82. doi: 10.1182/blood-2011-01-330407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lwin ST, Olechnowicz SWZ, Fowler JA, Edwards CM. Diet-induced obesity promotes a myeloma-like condition in vivo. Leukemia. 2015;29:507–10. doi: 10.1038/leu.2014.295. [DOI] [PubMed] [Google Scholar]
- 65.Justesen J, Stenderup K, Ebbesen EN, et al. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–71. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]


