Abstract
Background
Total body water (V) is an imprecise metric for normalization of dialytic urea clearance (Kt). This poses a risk of early mortality/technique failure (TF). We examined differences in the distribution of peritoneal Kt/V when V was calculated with actual weight (AW), ideal weight (IW), and adjusted weight (ADW). We also examined the associations of these Kt/V measurements, Kt/body surface area (BSA), and non-normalized Kt with mortality and TF.
Methods
This is a retrospective cohort study of 534 incident peritoneal dialysis (PD) patients from the Dialysis Morbidity and Mortality Study Wave 2 linked with United States Renal Data System through 2010. Using Cox-proportional hazard models, we examined the relationship of several normalization strategies for peritoneal urea clearance, including Kt/VAW, Kt/VIW, Kt/VADW, Kt/BSA, and non-normalized Kt, with the outcomes of mortality and TF. Harrell’s c-statistics were used to assess the relative predictive ability of clearance metrics for mortality and TF. The distributions of Kt/VAW, KT/VIW, and KT/VADW were compared within and between body mass index (BMI) strata.
Results
Median patient age: 59 (54% male; 72% white; 91% continuous ambulatory PD [CAPD]). Median 24-hour urine volume: 700 mL; median estimated glomerular filtration rate (eGFR) at initiation: 7.15 mL/min/1.73 m2. Technique failure and transplant-censored mortality at 5 years: 37%. Death and transplant-censored TF at 5 years: 60%. There were no significant differences in initial eGFR and 24-hour urine volume across BMI strata. There were statistically significant differences in each Kt/V calculation within the underweight, overweight, and obese strata. After adjustment, there were no significant differences in the hazard ratios (HRs) for TF/mortality for each clearance calculation. Harrell’s c-statistics for mortality for each clearance calculation were 0.78, and for TF, 0.60 – 0.61.
Conclusions
Peritoneal urea clearances are sensitive to subtle changes in the estimation of V. However, there were no detectable significant associations of Kt/VAW, Kt/VIW, Kt/VADW, Kt/BSA, or Kt with TF or mortality.
Keywords: Peritoneal dialysis, technique failure, mortality, urea clearance, adjusted weight, ideal weight, normalizaton of urea clearance, Kt/V
Dialytic urea clearance (Kt/V), a metric of urea clearance, is commonly used to measure the adequacy of peritoneal dialysis (PD). The importance of the achieved Kt/V to the overall effectiveness of PD is debated due to a dearth of evidence supporting a graded linear association between higher Kt/Vperitoneal and improved survival (1–4). The absence of an association might be attributable to shortcomings in the calculation of V, an estimate of total body water (TBW) throughout which urea distributes. The Watson formula is commonly used to estimate V in PD (5). This formula, derived in a healthy cohort, overestimates and underestimates TBW in obese and underweight patients, respectively (6). The inaccuracy likely results from its poor representation of body composition, as it assumes no inter-individual variability in the fat to fat-free mass ratio. Thus, at extremes of body mass index (BMI), biased estimation of V might lead to under- or over-delivery of dialysis, which might increase technique failure (TF) and negatively influence patient survival.
In this study, we contrasted Kt/V when calculating V using actual weight (AW), ideal weight (IW), and adjusted weight (ADW). We used IW, the weight for a given sex and height associated with longest survival, to attenuate underestimation and overestimation of V in underweight and obese patients, respectively (7). Adjusted weight, an intermediate between IW and AW, was used to lessen overestimation of V, particularly in the obese. We hypothesized that calculating V in these ways would reveal significant differences in the distribution of achieved Kt/V across and within BMI strata. Further, we hypothesized that by comparing Kt normalized to each of these calculations of V and to body surface area (BSA), the associations between Ktperitoneal and TF and patient survival would differ.
METHODS
STUDY DESIGN AND COHORT CHARACTERISTICS
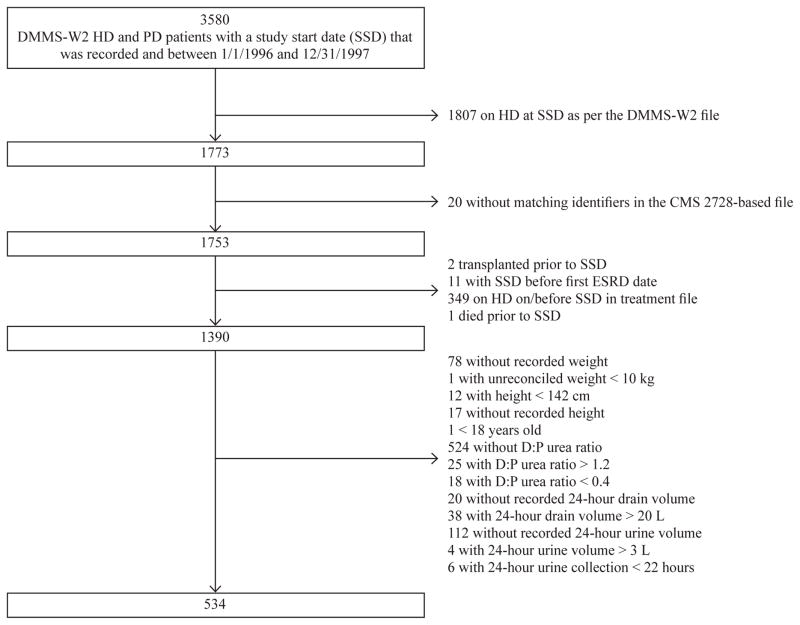
We assessed the association of achieved PD urea clearance with the outcomes of death and TF when clearance (Kt) is normalized to 3 different estimates of TBW (VAW, VIW, and VADW), to BSA, and without normalization. We used data from the Dialysis Morbidity and Mortality Study, Wave 2 (DMMS-W2), a prospective study by the United States Renal Data System (USRDS). The DMMS-W2 randomly sampled 799 dialysis units between 1 January 1996 and 31 December 1997. It included all incident PD patients, plus a 20% random sample of incident hemodialysis (HD) patients. It excluded patients < 18 years of age or with a prior kidney transplant. The date of study entry and time of baseline data collection was 60 days after the first date of end-stage renal disease (ESRD). Medical information for each subject was abstracted using medical, dialysis, and facility records, as well as patient-provided history. Quality-of-life questionnaires were also administered (8). After restricting to PD patients, the final cohort was assembled by excluding those without data on 24-hour urine volume (UV), with incomplete data for calculation of Ktperitoneal, or with data out of established ranges (see Figure 1).
Figure 1.
This demonstrates the assembly of the cohort. Patients were excluded for various reasons including unreliable data with regard to time of study entry or outcome occurrence, missing or implausible data in fields necessary for calculation of peritoneal urea clearance, and missing or implausible data for 24–hour urine volume.
CALCULATION AND NORMALIZATION OF DIALYSIS DOSE
Weekly peritoneal urea clearance was defined as Ktperitoneal and calculated at the time of study entry as dialysate urea/plasma urea X 24 hour dialysis drain volume X 7. The ratio of dialysate urea to plasma urea (D:P ratio) was considered plausible only if ≥ 0.4 or ≤ 1.2. Outliers were excluded. The lower bound of the D:P ratio was based on a model of urea kinetics in a low membrane transporter with some allowance for error (9). The upper bound was justified because urea will ultimately equilibrate between the plasma and dialysate (ratio 1.0) with allowance for error (10). Weekly peritoneal urea clearance was normalized by dividing by V (Watson formula) and BSA (Dubois formula), respectively (5,10). The weight variable in the Watson formula was computed using AW (recorded at study entry), IW (Devine formula), and ADW (7,11). Adjusted weight assumes a fixed percentage of the difference between AW and IW is attributable to fat-free mass (38% for men; 32% for women) (11).
OUTCOME ASSESSMENT AND CENSORING EVENTS
Mortality was ascertained using USRDS data through 2010. Technique failure was defined as the first occurrence of at least 60 consecutive days on HD during the study period. First date of transplant was a censoring event for each analysis. Death and TF were treated as censoring events in the TF and mortality outcome analyses, respectively.
COVARIATES
Baseline characteristics were obtained at entry into the DMMS-W2. If demographic variables were missing, they were abstracted from the Medical Evidence Form in USRDS. In clinical practice, residual renal urea clearance (Kt/Vresidual) is subtracted from the targeted total urea clearance goal (Kt/Vtotal) to determine the required contribution of Kt/Vperitoneal. Residual renal function (RRF) has an independent significant association with mortality outcomes in PD and is an important confounder of the relationship between Ktperitoneal and mortality (1). The DMMS-W2 study captured markers of RRF, including 24-hour UV and 24-hour urine urea nitrogen (UUN) clearance, on a voluntary basis. Among patients with complete data for Kt/Vperitoneal, 24-hour UUN clearance was approximately 65% missing and thus not used in the primary analysis. Because 24-hour UV correlates well with 24-hour UUN clearance and independently associates with mortality, it was used as a marker of RRF in the primary analysis. Estimated glomerular filtration rate (eGFR) was calculated via the 4-variable modification of diet in renal disease (MDRD) equation with the serum creatinine from the time of dialysis initiation (12).
STATISTICAL ANALYSIS
Continuous variables were summarized with means or medians and categorical variables with proportions. The Student’s t-test or Wilcoxon rank-sum test was used to compare continuous variables and the chi-square test, categorical variables across groups. The Kruskal-Wallis test was used to compare cal- with each respective normalization strategy culated Ktperitoneal and RRF metrics across BMI categories. Correlation coefficients were calculated using the Spearman method.
Cox proportional hazards models were used to assess the relationships of mortality and TF with peritoneal Kt, Kt/VAW, value of ≤ 0.2 Kt/VIW, Kt/VADW, and Kt/BSA. Covariates with a pfor the unadjusted associations with the outcome of interest were included in the initial multivariable models. Backwards elimination was used to fit parsimonious final multivariable models. Covariates were retained within the multivariable models if their p values were ≤ 0.05, they were confounders of the relationship between exposure and outcome (i.e. ≥ 15% change in the coefficient of the variable of interest), or they had previously been shown to be confounders. Sex, age, and race were forced into the final multivariable models regardless of significance. We also evaluated the interaction and both BMI and gender. The hazard ratios between Ktperitoneal were expressed per 1 standard deviation (HR) for Ktperitoneal for comparison across different normalization approaches. We used Harrell’s c-statistics, the time-to-event analogue of the area under the receiver-operator characteristic curve, to compare each final multivariable model’s ability to predict the outcomes of mortality and TF (13). Model assumptions were assessed using Schoenfeld residuals.
In a sensitivity analysis, we imputed additional surrogates of RRF, including 24-hour UUN clearance, total Kt/V (peritoneal + residual), and weekly UUN clearance. We also imputed other covariates with missing values (missing proportion ranging from 0.2 to 6.5%). We used the method of imputation by chained equations (Stata ICE command) (14,15). This is a multivariable approach using the conditional distribution of each covariate, given other predictor variables, to impute the variables with missing values, one by one, iteratively. We implemented the imputation process 10 times, to create 10 datasets with complete data. We then fit Cox proportional hazards models for each set and combined the results incorporating the variability between imputations according to the combination rules by Rubin et al. (Stata MI Estimate command) (14–16). All analyses were conducted with Stata, version 12.0 (College Station, TX, USA).
RESULTS
Baseline patient characteristics are summarized in Table 1. Median age was 59; 54% were male; 72% white; 48% diabetic; 91% were using CAPD. Approximately 5% of the cohort was underweight (BMI < 18.5 kg/m2) and 24% was obese (BMI > 30 kg/m2). The median 24-hour UV was 700 mL and median eGFR prior to initiation of dialysis was 7.15 mL/min/1.73 m2.
TABLE 1.
Baseline Characteristics of 534 Incident ESRD Patients on PD*
| Covariate | Value |
|---|---|
| Age at start of study | 59 (45–68) |
| Men | 54% |
| Race | |
| White | 72% |
| Black | 21% |
| Other | 7% |
| Weight (kg) | 73.8 (54.5–86.8) |
| Height (cm) | 170 (160–178) |
| BMI (kg/m2) | |
| <18.5 | 5% |
| 18.5–25 | 39% |
| 25–30 | 32% |
| >30 | 24% |
| BSA (m2) | 1.84 |
| PD modality | |
| CAPD | 91% |
| CCPD | 9% |
| Number of PD patients at the dialysis facility (1996) | 41 (26–86) |
| eGFR at initiation of PD (mL/min/1.73 m2) | 7.15 (5.67–9.58) |
| Systolic BP (mmHg) | 143.6±19.47 |
| Diastolic BP (mmHg) | 81.1±12.05 |
| 24-hour urine volume (mL) | 700 (400–1,150) |
| Serum albumin (g/dL) | 3.5 (3.15–3.80) |
| Serum phosphorus (mmol/L) | 5.2 (4.4–6.3) |
| Serum bicarbonate (meq/L) | 24 (20–27) |
| Hemoglobin (g/L) | 10.7 (9.6–12.1) |
| Diabetes | 48% |
| Peripheral vascular disease | 16% |
| Cardiovascular disease | 43% |
| Ability to ambulate independently | 95% |
| Ability to bathe/dress independently | 72% |
| Living alone | 15% |
| Completed high school | 75% |
| Primary cause of renal disease | |
| Diabetes | 44% |
| Hypertension | 22% |
| Primary glomerulonephritis | 11% |
| Other | 23% |
| Vactual | 37.8 (31.8–43.9) |
| Videal | 35.1 (28.8–40.3) |
| Vadjusted | 35.7 (29.7–41.8) |
| D:P ratio | 0.9 (0.80–1.0) |
| 24-hour dialysate volume (L) | 8 (8–10) |
| Ktperitoneal (L/week) | 58.7 (52.4–66.3) |
| Kt/Vactual - peritoneal | 1.6 (1.3–1.8) |
| Kt/Videal - peritoneal | 1.8 (1.5–2.0) |
| Kt/Vadjusted - peritoneal | 1.7 (1.4–1.9) |
| Kt/BSA | 32.1 (27.9–36.6) |
| Creatinine/urea averaged clearance (L/week/1.73 m2); n=177 | 43.8 (25.6–63.0) |
| Creatinine clearance (L/week/1.73 m2); n=183 | 55.09 (31.19–82.08) |
| Urea clearance (L/week/1.73 m2); n=181 | 29.6 (16.18–44.6) |
| Kt/V actual-residual; n=181 | 0.8 (0.5–1.3) |
| Kt/V actual-total; n=181 | 2.35 (1.96–2.80) |
ESRD = end-stage renal disease; PD = peritoneal dialysis; BMI = body mass index; BSA = body surface area; CAPD = continuous ambulatory PD; CCPD = continuous cycling PD; eGFR = estimated glomerular filtration rate; BP = blood pressure; V = total body water; D:P ratio = ratio of solute concentrations in dialysate and plasma; Kt = dialytic urea clearance.
Median (interquartile range) for continuous variables; percentage for categorical variables.
The median calculated Ktperitoneal was 58.7 L/week. The following were the median calculated peritoneal urea clearances for each normalization approach: Kt/Vactual 1.6; Kt/Videal 1.8, Kt/Vadjusted 1.7; Kt/BSA 32.1. Table 2 shows median (interquartile range [IQR]) clearances by BMI category. With the exception of patients with normal BMI, the pairwise differences within each BMI category between each V-normalized clearance were statistically significant. The Kt was statistically significantly different across BMI categories, except between the normal and overweight patients, with a trend towards increased Ktperitoneal with greater BMI.
TABLE 2.
Median (IQR) Achieved Peritoneal Clearance by BMI Category
| BMI (kg/m2) | Ktperitoneal | Kt/Vactual -peritoneal | Kt/Videal -peritoneal | Kt/Vadjusted-peritoneal | Kt/BSA peritoneal |
|---|---|---|---|---|---|
| Underweight <18.5 (n=27) |
47.85 (42.6–55.5)a | 1.75 (1.56–2.06)b | 1.61 (1.41–1.98)b | 1.65 (1.47–2.01)b | 32.06 (29.52–37.67) |
| Normal 18.5–25 (n=207) |
58.59 (51.80–66.41)a | 1.73 (1.44–1.97) | 1.74 (1.42–1.98)b | 1.72 (1.44–1.99)b | 34.59 (29.74–38.96) |
| Overweight 25–30 (n=172) |
57.79 (51.85–64.70)a | 1.54 (1.27–1.77)b | 1.70 (1.41–2.03)b | 1.64(1.38–1.93)b | 31.04 (26.82–35.28) |
| Obese >30 (n=128) |
61.69 (56.35–67.77)a | 1.42 (1.20–1.61)b | 1.83 (1.57–2.16)b | 1.66 (1.43–1.95)b | 30.07 (27.06–33.86) |
IQR = interquartile range; BMI = body mass index; Kt = dialytic urea clearance; V = total body water; BSA = body surface area.
p value <0.01 for Kruskal Wallis test for between-BMI comparison.
p value <0.001 for paired t-test between each respective Kt/V values within BMI categories.
Among those who had 24-hour urinary clearance measured, the median creatinine/urea averaged clearance was 43.8 L/week/1.73 m2 (n = 177); median urea clearance, 29.6 L/week/1.73 m2 (n = 181); and median creatinine clearance, 55.1 L/week/1.73 m2 (n = 183). Of the181 patients who had 24-hour UUN clearance, the median (IQR) residual and total Kt/V were 0.8 (0.5 – 1.3) and 2.35 (1.96 – 2.80), respectively. The correlation coefficient for 24-hour UUN clearance and 24-hour UV was 0.61 (p < 0.001) and for 24-hour creatinine/urea averaged clearance and 24-hour UV, r = 0.51 (p < 0.001). There was no statistically significant difference between 24-hour UV and eGFR at dialysis initiation between BMI categories.
ASSOCIATION OF NON-NORMALIZED AND NORMALIZED PERITONEAL CLEARANCE WITH MORTALITY
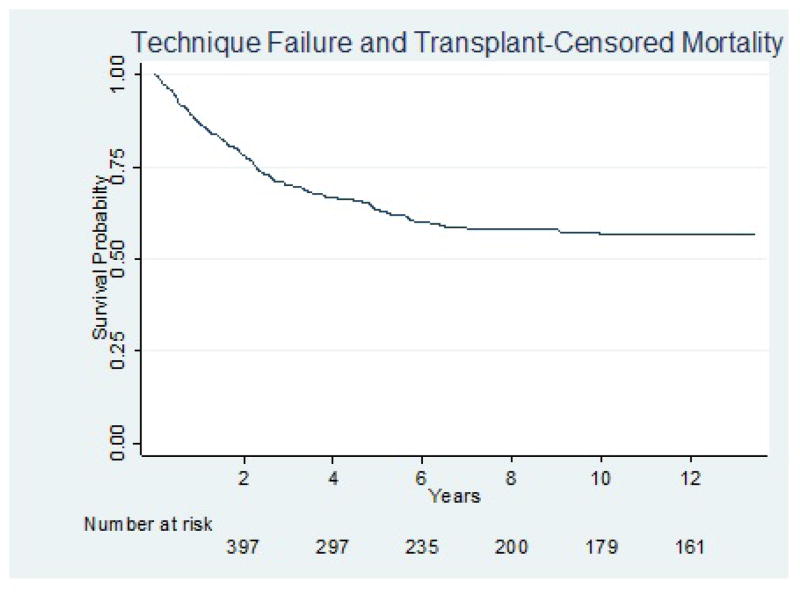
After censoring for first occurrence of TF or transplant, 1-year, 5-year, and 10-year mortality were 13%, 37%, and 43%, respectively (Figure 2). In both unadjusted and multivariable analyses, there were no significant associations between any metric of Ktperitoneal and death. In the unadjusted analysis, compared to patients with a normal BMI, underweight patients had a higher hazard of death, while overweight and obese patients had similar lower hazards of death, but none of these associations were statistically significant. Neither BMI nor gender modified the association between any metric of Ktperitoneal and death (p value for interaction > 0.05 in both cases). Body mass index did not significantly associate with death after adjustment. Table 3 demonstrates both unadjusted and final multivariable models for each metric of peritoneal clearance. The Harrell’s c-statistics were nearly identical for each adjusted model, indicating similar predictive ability across various metrics of Ktperitoneal. Although these models have moderate predictive ability (0.78), Ktperitoneal did not contribute significantly. Characteristics that were significantly associated with death in the multivariable analyses included older age, lower serum albumin, presence of diabetes, failure to graduate from high school, living alone, and inability to ambulate independently. The eGFR at initiation of dialysis was consistently associated with an increased hazard of death at higher values. Twenty-four-hour UV was significantly associated with death at lower values in unadjusted analysis but lost significance in multivariable analysis. None of the studied metrics of Ktperitoneal were significantly associated with death in either unadjusted or multivariable analyses adjusting for 24-hour UV. After multiple imputation of missing data and adjustment for 24-hour UUN clearance, there was still no significant association between any metric of Ktperitoneal and death.
Figure 2.
Kaplan Meier curve for mortality. Patients were censored at first occurrence of TF or kidney transplant. TF = technique failure.
TABLE 3.
Unadjusted and Adjusted Models of Mortality
| Covariate | Unadjusted HR (95% CI)a |
Model 1 HR (95% CI)b |
Model 2 HR (95% CI)b |
Model 3 HR (95% CI)b |
Model 4 HR (95% CI)b |
Model 5 HR (95% CI)b |
|---|---|---|---|---|---|---|
| Age | 1.05 (1.04–1.06) | 1.04 (1.03–1.05) | 1.04 (1.03–1.05) | 1.04 (1.03–1.05) | 1.04 (1.03–1.05) | 1.04 (1.03–1.05) |
| Sex (female vs male) | 1.02 (0.77–1.33) | 0.94 (0.69–1.24) | 0.88 (0.64–1.21) | 0.87 (0.61–1.22) | 0.87 (0.44–1.06) | 0.91 (0.67–1.23) |
| Race | ||||||
| White | Ref | Ref | Ref | Ref | Ref | Ref |
| Black | 0.60 (0.41–0.88) | 0.68 (0.44–1.05) | 0.69 (0.44–1.07) | 0.68(0.44–1.05) | 0.68 (0.44–1.06) | 0.68 (0.44–1.07) |
| Other | 0.85 (0.52–1.40) | 0.73 (0.42–1.28) | 0.72 (0.41–1.26) | 0.72 (0.41–1.25) | 0.72 (0.41–1.25) | 0.72 (0.41–1.26) |
| 24–hour urine volume (L) | 0.69 (0.54–0.88) | 0.78 (0.60–1.02) | 0.80 (0.60–1.06) | 0.80 (0.60–1.03) | 0.79 (0.60–1.04) | 0.79 (0.60–1.05) |
| Serum albumin (g/dL) | 0.42 (0.34–0.53) | 0.49 (0.37–0.65) | 0.50 (0.37–0.66) | 0.49 (0.37–0.65) | 0.49 (0.37–0.66) | 0.49 (0.37–0.66) |
| Diabetes (yes vs no) | 2.59 (1.95–3.44) | 1.89 (1.37–2.61) | 1.91 (1.37–2.64) | 1.90 (1.37–2.61) | 1.90 (1.38–2.62) | 1.90 (1.38–2.62) |
| eGFR at initiation of PD (mL/min/1.73 m2) | 1.12 (1.09–1.15) | 1.08 (1.05–1.12) | 1.09 (1.05–1.12) | 1.09 (1.05–1.13) | 1.09 (1.05–1.13) | 1.08 (1.05–1.12) |
| Education level (more than 12 years vs not) | 0.47 (0.35–0.62) | 0.62 (0.45–0.84) | 0.63 (0.46–0.86) | 0.62 (0.45–0.86) | 0.63 (0.46–0.86) | 0.62 (0.46–0.86) |
| Living status (lives alone vs with others) | 1.93 (1.38–2.70) | 1.78 (1.23–2.59) | 1.79 (1.23–2.60) | 1.80 (1.24–2.61) | 1.79 (1.23–2.60) | 1.79 (1.23–2.60) |
| Ambulation status (ambulation with limitation vs not) | 3.72 (2.34–5.91) | 1.91 (1.15–3.19) | 1.97 (1.17–3.31) | 1.99 (1.18–3.37) | 1.99 (1.18–3.35) | 1.97 (1.17–3.30) |
| * Kt | 0.51 (0.1– 4.0) | 2.34 (0.21–26.7) | ||||
| * Kt/Vactual –peritoneal | 2.29 (0.38–13.9) | 2.50 (0.25–25.2) | ||||
| * Kt/Videal –peritoneal | 1.37 (0.23– 8.2) | 2.41 (0.20–28.4) | ||||
| * Kt/Vadjusted–residual | 1.80 (0.28–11.3) | 2.64 (0.22–32.0) | ||||
| * Kt/BSA peritoneal | 1.18 (0.14–10.0) | 2.41 (0.2–29.4) | ||||
| Harrell’s c–statistic | 0.78 | 0.78 | 0.78 | 0.78 | 0.78 | |
HR = hazard ratio; CI = confidence interval; eGFR = estimated glomerular filtration rate; PD = peritoneal dialysis; Kt = dialytic urea clearance; V = total body water; BSA = body surface area.
Hazard ratios for clearance metrics are expressed per 1 standard deviation.
This is the HR for the univariable association between each covariate and mortality.
This is the adjusted HR for the association between each covariate and mortality within the multi–variable model.
ASSOCIATION OF NON-NORMALIZED AND NORMALIZED PERITONEAL CLEARANCE WITH TECHNIQUE FAILURE
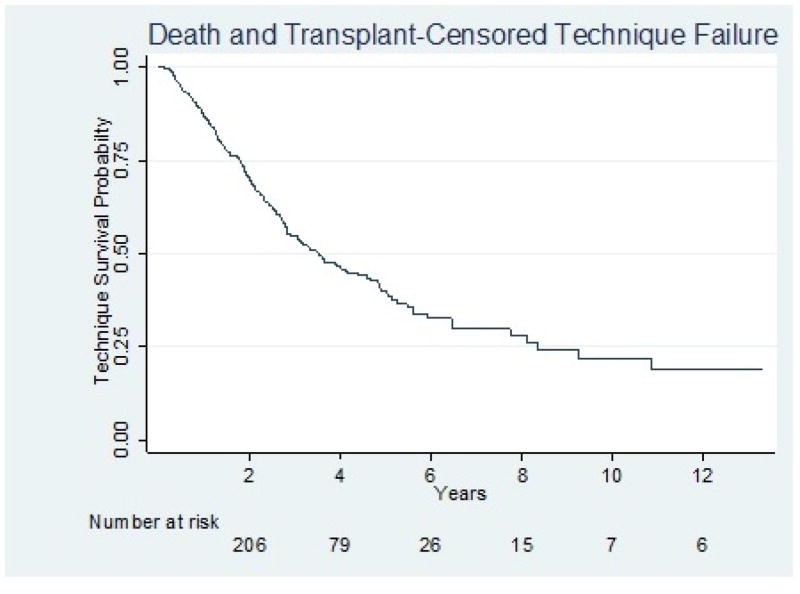
After censoring for first occurrence of death or transplant, 1-year, 5-year, and 10-year TF were 13%, 60%, and 78%, respectively (Figure 3). There was no significant association between any metric of Ktperitoneal and TF in either unadjusted or multivariable analyses. Compared to patients with a normal BMI, underweight, overweight, and obese patients had a higher hazard for TF in unadjusted analysis with a significant HR for obese vs normal BMI (HR 1.52; 95% confidence interval [CI] 1.07 –2.17). However, BMI did not meet criteria for incorporation into the multivariable models and failed to modify the association between Ktperitoneal and TF. In a sensitivity analysis, BMI was forced into the model with no improvement in model fit. Table 4 shows the both unadjusted and multivariable analyses of each metric of peritoneal clearance. The Harrell’s c-statistics were between 0.60 and 0.61 for all the multivariable models, indicating similar predictive abilities across various metrics of Ktperitoneal and modest predictive abilities of the models overall. Characteristics that were significantly associated with TF in unadjusted analysis include black race, lower eGFR at dialysis initiation, and inability to dress/bathe oneself. In multivariable analysis, only the inability to dress/bathe remained statistically significant. As with the mortality outcome, there was no significant association between any metric of Ktperitoneal and TF, in either unadjusted or multi-variable analyses adjusting for 24-hour UV. After multiple imputation of missing data and adjusting for 24-hour UUN clearance, there was still no significant association between any metric of Ktperitoneal and TF.
Figure 3.
Kaplan Meier curve for TF. Patients were censored at first occurrence of death or kidney transplant. TF = technique failure.
TABLE 4.
Unadjusted and Adjusted Models of Technique Failure
| Covariate | Unadjusted HR (95% CI)a |
Model 1 HR (95% CI)b |
Model 2 HR (95% CI)b |
Model 3 HR (95% CI)b |
Model 4 HR (95% CI)b |
Model 5 HR (95% CI)b |
Model 6 HR HR (95% CI)b |
|---|---|---|---|---|---|---|---|
| Age | 0.99 (0.99–1.00) | 1.00 (0.98–1.01) | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) | 0.99 (0.97–1.01) |
| Sex (female vs male) | 0.82 (0.62–1.08) | 0.77 (0.55–1.09) | 0.82 (0.58–1.15) | 0.93 (0.64–1.34) | 0.90 (0.61–1.32) | 0.90 (0.63–1.35) | 0.86 (0.61–1.20) |
| Race | |||||||
| White | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Black | 1.56 (1.15–2.12) | 1.43 (0.97–2.12) | 1.32 (0.90–1.93) | 1.30 (0.89–1.91) | 1.31 (0.90–1.93) | 1.31 (0.89–1.92) | 1.31 (0.90–1.92) |
| Other | 0.69 (0.40–1.21) | 0.62 (0.31–1.21) | 0.71 (0.37–1.37) | 0.70 (0.36–1.35) | 0.71 (0.36–1.36) | 0.70 (0.36–1.36) | 0.70 (0.36–1.36) |
| eGFR at initiation of PD (mL/min/1.73 m2) | 0.96 (0.91–0.99) | 0.97 (0.91–1.02) | |||||
| 24–hour urine volume (L) | 0.96 (0.76–1.21) | 1.06 (0.79–1.43) | |||||
| Ability to dress and bathe (unable vs able to bathe independently) | 1.55 (1.09–2.21) | 1.57 (1.09–2.27) | 1.65 (1.16–2.37) | 1.66 (1.16–2.38) | 1.66 (1.16–2.38) | 1.66 (1.16–2.38) | 1.66 (1.16–2.38) |
| * Kt | 1.44 (0.17– 11.9) | 0.26 (0.02– 3.9) | |||||
| * Kt/Vactual-peritoneal | 0.23 (0.03–1.47) | 0.10 (0.01–1.38) | |||||
| * Kt/Videal-peritoneal | 0.60 (0.09–3.93) | 0.21 (0.01–3.19) | |||||
| * Kt/Vadjusted-peritoneal | 0.36 (0.05–2.47) | 0.13 (0.01– 2.01) | |||||
| * Kt/BSAperitoneal | 0.39 (0.04–3.37) | (0.01– 2.10) | |||||
| Harrell’s c–statistic | 0.59 | 0.60 | 0.61 | 0.60 | 0.60 | 0.60 | |
HR = hazard ratio; CI = confidence interval; eGFR = estimated glomerular filtration rate; PD = peritoneal dialysis; Kt = dialytic urea clearance.
Hazard ratios for clearance metrics are expressed per 1 standard deviation.
This is the HR for the univariable association between each covariate and technique failure.
This is the adjusted HR for the association between each covariate and technique failure within the multivariable model.
ACHIEVED V-NORMALIZED PERITONEAL CLEARANCE ACROSS BMI STRATA
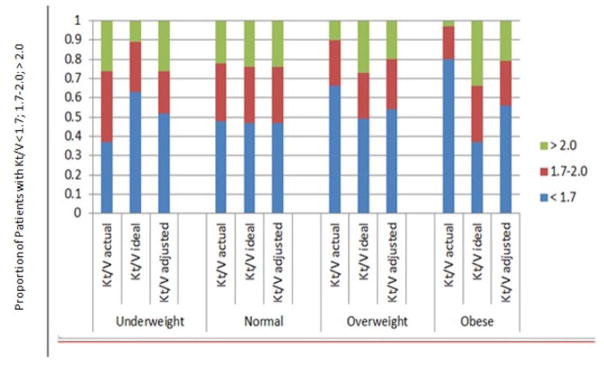
Figure 4 demonstrates the relative proportions of patients within each BMI stratum who achieved Kt/V of < 1.7, 1.7 – 2.0, and > 2.0 when AW, IW, and ADW were used. Within the underweight, overweight, and obese strata, the pairwise differences between each strategy of Kt/V were statistically significant. Among obese patients, approximately 80% had a peritoneal Kt/VAW of < 1.7, but calculating V using IW decreased this percentage to 37%. When using ADW to calculate V, 56% of obese patients had a peritoneal Kt/V < 1.7.
Figure 4.
The relative proportions of patients within each BMI stratum who achieved peritoneal Kt/V of < 1.7, 1.7–2.0, and > 2.0 when AW, IW, and ADW were used. BMI = body mass index; Kt = dialytic urea clearance; AW = actual weight; IW = ideal weight; ADW = adjusted weight.
DISCUSSION
This large cohort study of incident US PD patients supports the findings of numerous previous studies that also did not detect a significant relationship between Ktperitoneal and hard clinical outcomes. While the various metrics (AW, ID, ADW) applied in this study were hypothesized to mitigate biased estimations of V, particularly in the obese and underweight populations, we did not detect a significant association between non-normalized Ktperitoneal or metrics of normalized Ktperitoneal and either mortality or TF, even after multivariable adjustment and sensitivity analyses. Fried et al., suggested that Kt/VAW was associated with mortality and hospitalization more closely than Kt/VIW (17). Based on a low representation of underweight patients, they did not infer whether there is an appropriate BMI-specific approach to calculating V. They recognized that an intermediate metric between IW and AW might be useful for obese patients (17). One possible option, ADW, has not been applied to a PD population previously. However, we could not demonstrate that normalizing to VADW was more closely associated with outcomes in the obese than either Kt/VIW or Kt/VAW.
We also investigated BSA as a normalization metric. Normalization to BSA is well studied in HD but not in PD (18–22). Body surface area is hypothesized to be a better metric because it approximates metabolic rate (23–26). Also, the V:BSA ratio differs by gender. Body surface area alters dose-mortality relationships in women and small children on HD when compared to V-normalized Kt and was proposed as an explanation for why women suffered greater mortality than men in the lower clearance arm of the HEMO study (19,20). The Kt/BSA was not significantly associated with mortality or TF in our study.
Although Ktperitoneal was not associated significantly with mortality or TF, our predictive model performed well, especially for mortality (c-statistic, 0.78). Age, serum albumin, diabetes, eGFR, education level, living status, and ambulation status were all significantly associated with mortality in multivariable models. Higher eGFR was associated with poorer survival, perhaps due to unmeasured confounders related to both initiating dialysis at a higher eGFR and adverse outcomes, such as recurrent severe volume overload, refractory hyperkalemia, or lower creatinine generation, which might be indicative of poorer nutritional status and low muscle mass (27,28).
Although we detected no difference in outcomes across normalization metrics, our study demonstrates striking variations in the distributions of 3 different V-normalized Ktperitoneal values within BMI strata. The current Kidney Disease Outcomes Quality Initiative’s (KDOQI) guidelines suggest a target total Kt/V of 1.7 (29). Our results show that calculating V in different ways substantially affects the proportion of patients who move below and above the recommended target, especially within the extreme BMI stratum. In the case of the obese, 80% did not meet the 1.7 Kt/V target when AW was used to calculate V, but 37% did with IW. Given that our data support previous studies that fail to show a significant association between increased Ktperitoneal and hard outcomes, it raises the question of whether a single Ktperitoneal target for patients of all body habitus types is an appropriate benchmark for overall PD adequacy. Use of a single target Kt/V with AW will likely label many patients, particularly the obese, as having inadequate PD, especially when RRF is lost.
Obesity has been paradoxically associated with improved mortality in HD (30–32). Observational data suggest that obesity might also be protective in PD (33–35). We showed a non-significant trend towards better survival in overweight and obese patient compared to patients with a normal BMI, while underweight patients showed an increased hazard of death in unadjusted analysis. There was a significantly increased hazard for TF in the obese compared to those with normal BMI, but not after multi-variable adjustment. Historically, recruitment and retention of obese PD patients has been difficult due to the pervasive theory that adequate Ktperitoneal is unattainable (30,36,37). Our study did not show statistically significant differences in median Kt/V values between BMI groups but rather differences within groups when different V metrics were used and this was most profound with obesity. Thus, the potential survival benefit of obesity and the lack of a consistent association between Ktperitoneal and outcomes further emphasizes the need to better define adequacy targets for PD that consider BMI.
Our study is limited by its retrospective nature and lack of time-updated data on Ktperitoneal values, prescription alterations, and changes in RRF, potentially limiting detection of an association between Ktperitoneal and outcomes. Also, the tight correlation between normalization metrics and lack of influenced sub-information about how calculated Ktperitoneal sequent clinical care may have reduced our ability to explore the relative strengths and weaknesses of the metrics. Our cohort was limited to patients with complete, plausible data for variables required to calculate normalized Ktperitoneal. Patients with incomplete data appeared to be more sedentary and from smaller dialysis programs. While a concern for selection bias, the unadjusted mortality and TF rates were not significantly altered when patients with missing data were included. Approximately 65% of patients had no 24-hour UUN clearance. As this was an optional data field and patients missing this covariate had 24-hour UV recorded, we believe it was unlikely to be systematically missing with respect to attributes related to outcomes. The variable for independent dressing/bathing was missing for 30% of subjects, but caused no significant change in the point estimates of the primary exposure variable. All other model covariates had a small amount of missing data (0.6% to 6.5%). After multiple imputation and adjustment for UUN clearance in sensitivity analyses, results were robust. Further, we recognize that there are intrinsic difficulties with studying Kt/V since Kt and V are not independent. A higher Kt is often prescribed due to a higher V. Also, patients who are low transporters tend to be less volume-expanded than high transporters. This is relevant since volume expansion is associated with worse clinical outcomes (38,39). To circumvent this challenge, we conducted a sensitivity analysis in which Kt and V were inserted into models separately while also adjusting for UV but not ultrafiltration volume, as we did not have access to it. In unadjusted analysis, VAW and VADW were significantly associated with mortality (HR 0.98, p = 0.02; HR 0.98, p = 0.04, respectively). However, these were not significant after multivariable adjustment. Finally, the study cohort utilized CAPD as the dominant dialysis modality as compared to the prevalent use of continuous cycling peritoneal dialysis (CCPD) today. In addition, in the mid-1990s, total clearance targets were higher (2.0 vs 1.7 now); peritonitis rates higher; and obesity less prevalent (40,41). However, given the broad representation of dialysis dose and BMI in this cohort, the observations remain highly generalizable to present day.
In summary, in a cohort of incident PD patients, we demonstrated that urea clearance values are highly sensitive to subtle changes in the calculation of V. This is particularly true in obese patients and might be of further importance when RRF is lost. Despite significant changes in the absolute clearance values, calculating V with IW and ADW failed to strengthen the association with TF or mortality. These observations are timely given the anticipated growth of PD under the Medicare Prospective Payment System and the institution of a minimum total Kt/V target of 1.7 by the ESRD Quality Incentive Program (42). Further studies are necessary to optimize clearance targets at extreme BMIs and to explore whether or not failing to achieve current targets is a risk for TF or reduced quality of life.
Footnotes
DISCLOSURES
Funding for this investigation was provided by the National Research Service Award Institutional Pre-doctoral Training Grant T32 DK 007785. The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government. The authors have no conflicts of interest to declare.
References
- 1.Bargman JM, Thorpe KE, Churchill DN, Group CPDS. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. JASN. 2001;12(10):2158–62. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 2.Lo WK, Ho YW, Li CS, Wong KS, Chan TM, Yu AW, et al. Effect of Kt/V on survival and clinical outcome in CAPD patients in a randomized prospective study. Kidney Int. 2003;64(2):649–56. doi: 10.1046/j.1523-1755.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 3.Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. JASN. 2002;13(5):1307–20. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 4.Lo WK, Lui SL, Chan TM, Li FK, Lam MF, Tse KC, et al. Minimal and optimal peritoneal Kt/V targets: results of an anuric peritoneal dialysis patient’s survival analysis. Kidney Int. 2005;67(5):2032–8. doi: 10.1111/j.1523-1755.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- 5.Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33(1):27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- 6.Johansson AC, Samuelsson O, Attman PO, Bosaeus I, Haraldsson B. Limitations in anthropometric calculations of total body water in patients on peritoneal dialysis. JASN. 2001;12(3):568–73. doi: 10.1681/ASN.V123568. [DOI] [PubMed] [Google Scholar]
- 7.Pai MP, Paloucek FP. The origin of the “ideal” body weight equations. Ann Pharmacother. 2000;34(9):1066–9. doi: 10.1345/aph.19381. [DOI] [PubMed] [Google Scholar]
- 8.USRDS Dialysis Morbidity and Mortality (Wave 2) 1997 [Online] Available from: https://www.usrds.org/download/1997/ch04.pdf.
- 9.Twardowski Z. Peritoneal equilibration test. Perit Dial Int. 1987;7:138. doi: 10.3747/pdi.2008.00262. [DOI] [PubMed] [Google Scholar]
- 10.Dubois D. A formula to estimate the approximate surface area if height and weight be known. Arch Int Med. 1916;17:863– 71. [PubMed] [Google Scholar]
- 11.Krenitsky J. Adjusted body weight, pro: evidence to support the use of adjusted body weight in calculating calorie requirements. Nutr Clin Pract. 2005;20(4):468–73. doi: 10.1177/0115426505020004468. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 13.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Royston P. Multiple imputation of missing values. Stata J. 2004;4(3):227–41. [Google Scholar]
- 15.Royston P. Multiple imputation of missing data: update. Stata J. 2005;5(2):188–201. [Google Scholar]
- 16.Rubin DB. Inference and missing data (with discussion) Biometrika. 1976;63:581–92. [Google Scholar]
- 17.Fried L, Hebah N, Finkelstein F, Piraino B. Association of Kt/V and creati-nine clearance with outcomes in anuric peritoneal dialysis patients. Am J Kidney Dis. 2008;52(6):1122–30. doi: 10.1053/j.ajkd.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Daugirdas JT, Depner TA, Greene T, Kuhlmann MK, Levin NW, Chertow GM, et al. Surface-area-normalized Kt/V: a method of rescaling dialysis dose to body surface area—implications for different-size patients by gender. Semin Dial. 2008;21(5):415–21. doi: 10.1111/j.1525-139X.2008.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daugirdas JT, Greene T, Chertow GM, Depner TA. Can rescaling dose of dialysis to body surface area in the HEMO study explain the different responses to dose in women versus men? CJASN. 2010;5(9):1628–36. doi: 10.2215/CJN.02350310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daugirdas JT, Hanna MG, Becker-Cohen R, Langman CB. Dose of dialysis based on body surface area is markedly less in younger children than in older adolescents. CJASN. 2010;5(5):821–7. doi: 10.2215/CJN.08171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez SP, Kapke A, Port FK, Wolfe RA, Saran R, Pearson J, et al. Dialysis dose scaled to body surface area and size-adjusted, sex-specific patient mortality. CJASN. 2012;7(12):1977–87. doi: 10.2215/CJN.00390112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spalding EM, Chandna SM, Davenport A, Farrington K. Kt/V underestimates the hemodialysis dose in women and small men. Kidney Int. 2008;74(3):348–55. doi: 10.1038/ki.2008.185. [DOI] [PubMed] [Google Scholar]
- 23.Basile C, Vernaglione L, Lomonte C, Bellizzi V, Libutti P, Teutonico A, et al. Comparison of alternative methods for scaling dialysis dose. Nephrol Dial Transplant. 2010;25(4):1232–9. doi: 10.1093/ndt/gfp603. [DOI] [PubMed] [Google Scholar]
- 24.Singer MA, Morton AR. Mouse to elephant: biological scaling and Kt/V. Am J Kidney Dis. 2000;35(2):306–9. doi: 10.1016/s0272-6386(00)70341-6. [DOI] [PubMed] [Google Scholar]
- 25.Daugirdas JT, Levin NW, Kotanko P, Depner TA, Kuhlmann MK, Chertow GM, et al. Comparison of proposed alternative methods for rescaling dialysis dose: resting energy expenditure, high metabolic rate organ mass, liver size, and body surface area. Semin Dial. 2008;21(5):377–84. doi: 10.1111/j.1525-139X.2008.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morton AR, Singer MA. The problem with Kt/V: dialysis dose should be normalized to metabolic rate not volume. Semin Dial. 2007;20(1):12–5. doi: 10.1111/j.1525-139X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 27.Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Ramkumar N, Pappas LM, Cheung AK. Impact of timing of initiation of dialysis on mortality. JASN. 2003;14(9):2305–12. doi: 10.1097/01.asn.0000080184.67406.11. [DOI] [PubMed] [Google Scholar]
- 28.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–19. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 29.Work-Group PDA. National Kidney Foundation Kidney Disease Outcomes Quality Initiative—2006 Updates. 2006 [Online] Available from: http://www.kidney.org/professionals/kdoqi/guideline_uphd_pd_va/pd_wg.htm.
- 30.Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85(11):991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalantar-Zadeh K, Streja E, Molnar MZ, Lukowsky LR, Krishnan M, Kovesdy CP, et al. Mortality prediction by surrogates of body composition: an examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am J Epidemiol. 2012;175(8):793–803. doi: 10.1093/aje/kwr384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis. 2014;56(4):415–25. doi: 10.1016/j.pcad.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzamaloukas AH, Bunting D. A 100-kg man on peritoneal dialysis (PD) with a borderline Kt/V: to PD or not to PD. Perit Dial Int. 2003;23(2):200–7. [PubMed] [Google Scholar]
- 31.Nolph KD, Jensen RA, Khanna R, Twardowski ZJ. Weight limitations for weekly urea clearances using various exchange volumes in continuous ambulatory peritoneal dialysis. Perit Dial Int. 1994;14(3):261–4. [PubMed] [Google Scholar]
- 32.Shibagaki Y, Faber MD, Divine G, Shetty A. Feasibility of adequate solute clearance in obese patients on peritoneal dialysis: a cross-sectional study. Am J Kidney Dis. 2002;40(6):1295–300. doi: 10.1053/ajkd.2002.36904. [DOI] [PubMed] [Google Scholar]
- 33.McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. JASN. 2003;14(11):2894–901. doi: 10.1097/01.asn.0000091587.55159.5f. [DOI] [PubMed] [Google Scholar]
- 34.Stack AG, Murthy BV, Molony DA. Survival differences between peritoneal dialysis and hemodialysis among “large” ESRD patients in the United States. Kidney Int. 2004;65(6):2398–408. doi: 10.1111/j.1523-1755.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 35.Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int. 2003;64(5):1838–44. doi: 10.1046/j.1523-1755.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 36.de Mutsert R, Grootendorst DC, Boeschoten EW, Dekker FW, Krediet RT. Is obesity associated with a survival advantage in patients starting peritoneal dialysis? Contrib Nephrol. 2009;163:124–31. doi: 10.1159/000223790. [DOI] [PubMed] [Google Scholar]
- 37.Nolph KD, Jensen RA, Khanna R, Twardowski ZJ. Weight limitations for weekly urea clearances using various exchange volumes in continuous ambulatory peritoneal dialysis. Perit Dial Int. 1994;14(3):261–4. [PubMed] [Google Scholar]
- 38.Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Page D. Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. The Canada-USA (CANUSA) Peritoneal Dialysis Study Group. JASN. 1998;9(7):1285–92. doi: 10.1681/ASN.V971285. [DOI] [PubMed] [Google Scholar]
- 39.Wang T, Heimburger O, Waniewski J, Bergstrom J, Lindholm B. Increased peritoneal permeability is associated with decreased fluid and small-solute removal and higher mortality in CAPD patients. Nephrol Dial Transplant. 1998;13(5):1242–9. doi: 10.1093/ndt/13.5.1242. [DOI] [PubMed] [Google Scholar]
- 40.Bender FH, Bernardini J, Piraino B. Prevention of infectious complications in peritoneal dialysis: best demonstrated practices. Kidney Int Suppl. 2006;(103):S44–54. doi: 10.1038/sj.ki.5001915. [DOI] [PubMed] [Google Scholar]
- 41.Burkart JM, Schreiber M, Korbet SM, Churchill DN, Hamburger RJ, Moran J, et al. Solute clearance approach to adequacy of peritoneal dialysis. Perit Dial Int. 1996;16(5):457–70. [PubMed] [Google Scholar]
- 42.Centers for Medicare and Medicaid Services National Provider Call. End-Stage Renal Disease Quality Incentive Program, Payment Year 2015 Final Rule. 2013 [Online] Available from: http://www.cms.gov/Outreach-and-Education/Outreach/NPC/Downloads/2013-03-13-NPC-ESRD-Presentation.pdf.