Abstract
Background
To evaluate men, with lower urinary tract symptoms and newly elevated serum prostate specific antigen (PSA) to determine whether a three-week course of ciprofloxacin antibiotics lowers serum PSA levels and affects recommendations for prostate biopsy.
Methods
A prospective, controlled, single-center prospective trial of 177 men with a newly elevated PSA and lower urinary tract symptoms was conducted. Patients were randomized to three weeks of ciprofloxacin or observation. After three weeks, patients PSA levels and derivatives were repeated. At the end of 3 weeks, all patients underwent TRUS guided systematic 12-core prostate biopsies regardless of the final PSA value.
Results
Of 177 men who completed the study, 88 were in the treatment and 89 in the observation group. 46.5% of treatment and %18 of control groups patients PSA levels had decreased after 3 weeks and a significant PSA reduction was observed in the treatment group compare to control group (p: 0.035) but no significant prostate cancer detection rates were observed between the groups (p: 0.418). Also, in the treatment group prostate cancer detection rate was significantly higher in patients whom PSA levels were decreased (p: 0.011).
Conclusion
This study has shown that, use empirical antibiotic treatment decreased the PSA levels but did not have any effect on prostate cancer detection. In addition, prostate cancer detection rates were found to be higher in patients with reduced PSA levels after treatment. Therefore, it may not be safe to rule out biopsies in patients who achieve a satisfactory PSA response to antibiotics.
Keywords: Antibiotherapy, BPH, Prostate cancer, PSA
Abbreviations: fPSA, free prostate-specific antigen; IPSS, International Prostate Symptom Score; Qmax, maximum flow rates; PCa, prostate cancer; PSA, prostate-specific antigen; PSAD, prostate-specific antigen density; TRUS, transrectal ultrasonography
1. Introduction
The use of, prostate-specific antigen (PSA) as a serum marker has revolutionized prostate cancer (PCa) diagnosis1 and has resulted in changes that include an increase in the number of prostate biopsies performed. However, screening for PCa is one of the most controversial topics in urological literature.2 Some authors argue that the use of current American Urological Association guidelines may lead to a significant number of men with aggressive PCa being missed.3 By contrast, a Cochrane review that was published in 2013 has determined that PSA screening is associated with an increased diagnosis of PCa, but no benefit was observed on overall survival.4
There is no consensus on how to manage high PSA levels that have occasionally been detected during PSA screening, because PSA levels can increase for several reasons, including trauma, ejaculation, and rectal and urethral procedures. In addition, numerous noncancerous etiologies can cause elevated PSA levels, such as benign prostatic hyperplasia, inflammation, and infection.5, 6 Most urologists make decisions on the basis of their training and experience. Some of them, in daily practice, use antibiotics to reduce high PSA values. After a course of antibiotics, the PSA measurement is repeated and if it remains elevated, biopsy is recommended. If it significantly decreases, a biopsy may be avoided.
Several studies have shown that receiving antibiotic treatment prior to deciding to have a biopsy can reduce PSA values to normal levels, and biopsy can be avoided.7, 8 However, empiric antibiotic use in this setting is associated with drug-related side effects9, promotion of microbial resistance,10 and an increased rate of sepsis after prostate biopsy.11 Furthermore, high occurrence of Gleason scores ≥7 PCa (17%) at low PSA levels (≤2 ng/mL) shows that, the decrease in PSA should not be undertaken.12
In this prospective and controlled study, we tried to investigate the effect of antibiotics on total PSA (tPSA) and free PSA (fPSA) levels in patients with high PSA levels. The PSA ratios during and at the end of antibiotic treatment were measured; the cancer detection rates were investigated and compared with the control group.
2. Patients and methods
The study was conducted between June 2014 and November 2016 on 177 patients who had been referred to Okmeydanı Training and Research Hospital outpatient department. The study was approved by the local ethics committee, and informed consent was obtained from all participants. Patients with lower urinary tract symptoms and shown to have a PSA level higher than 2.5 ng/mL and a palpably normal digital rectal examination were included in the study.
In all cases, detailed history was taken, and physical examinations were performed. International Prostate Symptom Score (IPSS) assessments were performed, and urine samples for urine analysis and urine culture were taken. Blood samples were taken for measurement of creatinine and blood urea nitrogen levels. Digital rectal examination was conducted, and KUB was taken for all patients. The urinary system was examined with urinary system ultrasound, and postvoid residual urine was measured. Prostate volume (PV) was measured with transrectal ultrasonography (TRUS) (GE Health_ Lociq 200 Pro). In addition, maximum flow rates (Qmax) of all cases were assessed with uroflowmetry.
Patients who had urinary infection, chronic kidney disease, bladder tumor, prostate tumor, neurogenic bladder, urethral stenosis, history of 5-alpha reductase inhibitor treatment, bladder calculi, having signs of acute or chronic prostatitis, and also patients who had a history of prostate surgery or prostate needle biopsy were excluded. In addition, those who had acute urinary system infection, hypersensitivity to quinolones, urinary retention, and who had recent digital examination history as well as cases with urethral catheter, which could have effects on serum PSA levels, were excluded.
Determination of tPSA and fPSA levels was repeated twice in each visit to prevent laboratory errors. The tPSA and fPSA analyses were conducted using the test “total and free prostate-specific antigen” (Roche Diagnostics, Cobas 6000) on a Modular E-Module of Roche Diagnostics, USA. All measurements were done in a central laboratory in blinded fashion and according to the manufacturer’s instructions in a central laboratory.
Patients were randomized systematically into two groups according to the order of admission. Those in the first group were given 500 mg oral ciprofloxacin twice a day for 21 days. The second control group received no treatment. Just after the termination of antibiotic treatment, all patients were reevaluated using the same parameters. At the end of 3 weeks, all patients underwent TRUS-guided systematic 12-core prostate biopsies regardless of the final PSA value.
TRUS-guided prostate biopsies were performed with the patient in the left decubitus position, using a biplanar 7.5-MHz transrectal ultrasound probe. Prior to the procedure, local anesthesia with periprostatic nerve blockade was done. With an 18-gauge needle, 12 core prostate biopsies were taken, and specimens were examined in the pathology department of our hospital.
Mean, standard deviation, median, and percentage values were used for descriptive statistics. The distribution of variables was checked with Kolmogorov–Smirnov test. Mann–Whitney U test was used for the comparison of quantitative data. Wilcoxon signed-rank test was used for the repeated measurement analysis. Chi-square test was used for the comparison of the comparison of qualitative data. SPSS 22.0 was used for statistical analysis. A p value less than 0.05 was considered statistically significant.
3. Results
A total of 177 patients participated in the study. The control group had a mean age of 58.9 ± 9.5 years, and the treatment group had a mean age of 60.2 ± 7.1 (P = 0.255). There were no differences between the two groups in terms of age, tPSA, fPSA, %f/t PSA (percent-free PSA), prostate-specific antigen density (PSAD), PV, Qmax, and IPSS (Table 1).
Table 1.
Comparison of groups at randomization
| Control group |
Treatment group |
P | |||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | ||
| No. of patients | 89 | 88 | |||
| Age (yr) | 58.9 ± 9.5 | 57.0 | 60.2 ± 7.1 | 58.4 | 0.255a) |
| Prostate volume (mL) | 26.4 ± 5.8 | 26.0 | 31.3 ± 7.8 | 30.0 | 0.112a) |
| Qmax (mL/s) | 10.3 ± 3.2 | 10.0 | 10.3 ± 3.2 | 12.0 | 0.164a) |
| IPSS | 17.6 ± 3.6 | 18.0 | 17.0 ± 4.0 | 18.0 | 0.388a) |
| PSA (ng/mL) | 6.4 ± 5.2 | 4.5 | 6.1 ± 2.9 | 4.7 | 0.294a) |
| fPSA (ng/mL) | 1.2 ± 1.0 | 1.0 | 1.4 ± 0.8 | 1.2 | 0.154a) |
| Percent free PSA (%) | 25.3 ± 22.9 | 18.3 | 25.4 ± 14.3 | 22.1 | 0.175a) |
| PSAD (ng/mL2) | 0.24 ± 0.03 | 0.23 | 0.16 ± 0.02 | 0.15 | 0.108a) |
fPSA, free PSA; IPSS, International Prostate Symptom Score; percent free PSA, % f/t PSA; PSAD, prostate-specific antigen density; Qmax, maximum flow rate; SD, standard deviation.
Mann–Whitney U test.
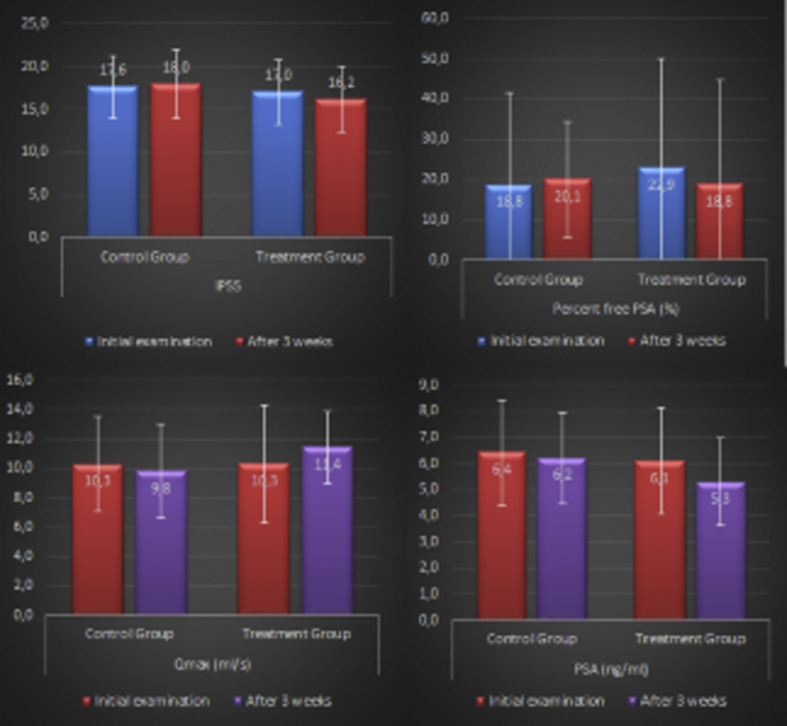
The mean ± standard deviation values of the initial PSA in the treatment and nontreatment groups were 6.1 ± 2.9 ng/mL and 6.4 ± 2.2 ng/mL, respectively (P = 0.294). After 3 weeks of antibiotic treatment, the mean of the final PSA in the treatment group decreased to 5.3 ± 2.6 ng/mL, and significant change was observed between initial versus final PSA levels (P = 0.035). In the control group after a 3-week period, the mean PSA level was measured (6.2 ± 1.9 ng/mL), and it was determined that the PSA reduction in the control group was not significant (P = 0.118). When comparing the mean PSA reductions between the two groups, PSA reduction was significant (P = 0.022). As for the mean change in PSA level from baseline to biopsy, antibiotic treatment decreased PSA levels in 46.5% of patients, whereas 15% of controls showed a decrease in PSA levels.
When we compared the patients prior to randomization, there were no significant differences in terms of PSAD levels (P = 0.115). PSAD levels decreased from 0.194 ng/mL2 to 0.169 ng/mL2 in the treatment group after the antibiotic treatment, and decreased from 0.246 ng/mL2 to 0.238 ng/mL2 in the control group (P = 0.122). The reduction in PSAD after 3 weeks in the treatment group was not significant (P = 0.115) (Fig. 1).
Fig 1.
Comparison of groups after 3 weeks.
The comparison of initial and final levels of fPSA revealed a significant difference in control group patients; in the treatment group, no significant reduction was observed in percent fPSA values after 3 weeks (P = 0.115). There was no statistically significant improvement in IPSS and Qmax with the antibiotic treatment. No difference was observed in the control group, as expected (Table 2).
Table 2.
Comparison of groups after 3 weeks
| Control group |
Treatment group |
P | |||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | ||
| IPSS | |||||
| Initial examination | 17.6 ± 3.6 | 18.0 | 17.0 ± 4.0 | 18.0 | 0.388a) |
| After 3 wk | 17.9 ± 3.8 | 18.0 | 16.2 ± 3.8 | 18.0 | 0.120a) |
| P value | 0.115b) | 0.112b) | |||
| PSA (ng/mL) | |||||
| Initial examination | 6.4 ± 2,2 | 6.1 | 6.1 ± 2.9 | 6.2 | 0.294a) |
| After 3 wk | 6.2 ± 1.9 | 5.9 | 5.3 ± 2.6 | 5.2 | 0.042a) |
| P value | 0.118b) | 0.035b) | |||
| fPSA (ng/mL) | |||||
| Initial examination | 1.2 ± 1.0 | 1.0 | 1.4 ± 0.8 | 1.3 | 0.154a) |
| After 3 wk | 1.3 ± 0.9 | 1.1 | 1.0 ± 0.9 | 0.9 | 0.083a) |
| P value | 0.920b) | 0.070b) | |||
| Percent free PSA (%) | |||||
| Initial examination | 18.8 ± 9.2 | 17.3 | 22.9 ± 11.3 | 19.1 | 0.175a) |
| After 3 wk | 20.1 ± 10.3 | 16.2 | 18.8 ± 7.9 | 17.3 | 0.069a) |
| p value | 0.478b) | 0.115b) | |||
| Qmax(mL/s) | |||||
| Initial examination | 10.3 ± 3.2 | 10.0 | 10.6 ±3.2 | 12.1 | 0.164a) |
| After 3 wk | 9.8 ± 4.0 | 9.0 | 11.4 ± 2.4 | 12.8 | 0.117a) |
| P value | 0.459b) | 0.126b) | |||
| PSAD (ng/mL2) | |||||
| Initial examination | 0.246 ± 0.03 | 0.231 | 0.194 ± 0.02 | 0.150 | 0.115a) |
| After 3 wk | 0.238 ± 0.02 | 0.222 | 0.169 ± 0.01 | 0.178 | 0.122a) |
| P value | 0.255b) | 0.119b) | |||
fPSA, free PSA; IPSS, International Prostate Symptom Score; percent free PSA, % f/t PSA; PSAD, prostate-specific antigen density; Qmax, maximum flow rate; SD, standard deviation.
Bold represents significant P values.
Mann–Whitney U test.
Wilcoxon signed-rank test.
Overall, PCa was detected in 40 of 177 (22.5 %) patients who had PSA levels ≥2.5 ng/mL and 30 of 113 (26.5%) of patients who had PSA levels ≥4 ng/mL. In the control group, 22 of 89 (24%) men were diagnosed with PCa, whereas 18 of 88 patients (21.5%) in the antibiotic group were diagnosed with cancer (P = 0.718). In addition, as a result of pathologic examination, there was no difference between the two groups in terms of Gleason scores (Table 3).
Table 3.
Comparison of Prostate carcinoma detection rates
| Control group |
Treatment group |
P | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | |||
| Gleason score | 6.5 ± 0.5 | 6.5 | 6.6 ± 0.5 | 7.0 | 0.598a) | |
| Overall PCa ratios | (−) | 67 | 70 | 0.418b) | ||
| (+) | 22 (24.7%) | 18 (20.4%) | ||||
| Gleason score | 6.5 ± 0.5 | 6.5 | 6.1 ± 0.5 | 6.2 | 0.374a) | |
| Patients with decreased PSA levels | (−) | 10 | 29 | 0.759b) | ||
| (+) | 4 28.5% | 12 | 29.2% | |||
| Gleason score | 6.6 ± 0.5 | 6.5 | 7.2 ± 0.5 | 6.8 | 0.698a) | |
| Patients with elevated or unchanged PSA levels | (−) | 57 | 41 | 0.003ba) | ||
| (+) | 18 24.7% | 6 | 12.7% | |||
(−), benign pathology; (+), prostate carcinoma (PCa); PSA, prostate-specific antigen; SD, standard deviation.
Bold represent significant P value.
Mann–Whitney U test.
Chi-square test.
In the control group, 75 of 89 (84%) patients had elevated PSA levels and PCa was detected in 18 (24%) of those patients. Fourteen of 89 (15.7%) patients had decreased PSA levels, and PCa was detected in four patients (28.5%). Moreover, three of 89 (3%) patients had a mean PSA reduction of >50%, and two of those patients were found to have PCa on biopsy.
In the treatment group, 41 of 88 patients had decreased PSA levels and PCa was detected in 12 patients (13.6%). Regarding the degree of PSA level decrease, in the antibiotic group, five of 88 (5.6%) patients had a reduction of >50% with four patients having negative biopsies. Ten (11%) patients had a reduction between 25% and 50%, and two of these patients had PCa. In 26 (29.5%) patients, the PSA levels were reduced <25%, and PCa was detected in three of them. In 47 patients, the PSA levels were increased by 1–5% or were unchanged.
The overall PCa detection rate was 20.4% in the treatment group. PCa was detected in 6.8% of patients with elevated PSA levels and in 13.6% of patients with decreased PSA levels. When the two groups were compared according to the cancer detection rates, it was observed that the cancer detection rates were significantly higher in patients with decreased PSA levels after 3 weeks of empiric antibiotic treatment (P = 0.011). There were no significant differences between the groups in terms of Gleason scores (Table 4).
Table 4.
Comparison of Prostate carcinoma detection rates according to PSA change in treatment group
| Patients with elevated PSA levels |
Patients with decreased PSA levels |
P | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | |||
| Gleason score | 6.6 ± 0.5 | 6.5 | 7.2 ± 0.5 | 6.8 | 0.698a) | |
| Treatment group | (−) | 41 | 29 | 0.011b) | ||
| (+) | 6 (6.8%) | 12 | 13.6% | |||
(−), benign pathology; (+), prostate carcinoma; PSA, prostate-specific antigen; SD, standard deviation.
Bold represent significant P value.
Mann–Whitney U test.
Chi-square test.
4. Discussion
The purpose of this controlled study was to analyze the effect of antibiotic treatment on PSA and PSA derivatives to investigate if a relevant PSA reduction will be observed, and also to investigate whether empiric antibiotic treatment had any effect on pathology results.
Declines in PSA levels ranging from 7.1% to 43% after antibiotic treatment have been reported.13 Kyung et al14 had given short-term antibiotic treatment to chronic nonbacterial prostatitis patients with elevated PSA levels, and they observed that mean PSA and PSAD significantly decreased after treatment. In 2008, Serretta15 reported that, after antibiotic treatment, 60% of patients showed a decrease in their mean PSA levels and added that no cancer was detected if PSA decreased below 4 ng/mL or more than 70% in asymptomatic patients with newly elevated PSA levels.
There is currently no evidence-based information on whether the decrease in PSA levels is caused by antibiotic or by natural variation of the PSA.16 Zhang et al17 reported that a physiological fluctuation of 10–20% was observed in the PSA levels of normal men while screening was conducted. In our controlled trial, 46.5% of patients receiving antibiotic treatment showed a reduction in mean PSA levels, but in the control group only 15% of patients showed a decrease in mean PSA levels. Similar to the studies of Kyung et al and Serretta et al, in our study after antibiotic treatment PSA levels showed a significant decrease compared to those in the control group. The reduction in PSA levels after antibiotic therapy was not associated with the diagnosis of PCa, but we did not investigate the relation between chronic prostatitis and PSA level changes.
PSA is still the cornerstone of PCa screening and diagnosis in current clinical practice. Inaccuracy of PSA is partly attributable to the influence of a number of genetic, clinical (infection or inflammation), and biological factors modifying PSA blood levels. Results of a large multicenter trial suggested the use of serum total PSA greater than 4 ng/mL as a threshold for performing prostate biopsies.18 Unfortunately, using a single value for men of all ages results in the exclusion of an unacceptably high number of patients with clinically significant early-stage disease, as approximately 20% to 50% of clinically significant organ confined PCa occurs in men with serum total PSA of less than 4 ng/mL.19, 20 In this study, 25% (10 patients) of patients who had PCa with Gleason scores ≥6 would have been missed if the threshold PSA level of ≥4 ng/mL had been used.
To increase the detection rate of PCa, PSAD and percent-free PSA were used. Studies in men with PSA between 4 ng/ml and 10 ng/mL indicate a PCa risk of less than 8% with a percent-free PSA greater than 25%, versus a greater than 56% risk with a percent-free PSA less than 10%.21, 22 PSAD is found by dividing the PSA level by the PV, with densities greater than 0.15 ng/mL2 more suggestive of PCa.23 We did not observe any significant decreases in PSAD level outcomes regardless of whether the patients received antibiotics. In our trial, the reduction in free PSA levels in the antibiotic group was greater than the decrease in total PSA levels, resulting in a decrease in the percent-free PSA, but this was not statistically significant. In addition, the PCa detection rate did not show a significant change between the patients whose percent-free PSA decreased after 3 weeks of ciprofloxacin treatment.
The use of empiric antibiotics for elevated PSA levels may result in side effects and a potentially increased risk of infection after biopsy. Akduman et al24 evaluated the effect of long-term fluoroquinolone treatment prior to the prostate biopsy in terms of postprocedure sepsis in 558 patients, and they concluded that long-term fluoroquinolone use to prevent unnecessary prostate biopsy may result in postbiopsy sepsis caused by fluoroquinolone-resistant microorganisms. In another trial (2008), Feliciano et al11 evaluated the records of 1,293 patients who underwent prostate biopsy because of elevated PSA and found that individuals who received 3 weeks of fluoroquinolone-based antibiotic treatment prior to biopsy had a significantly higher incidence of postbiopsy sepsis (5.4% vs. 1.7%, P = 0.05), and all patients in whom bacteria were identified in cultures harbored a fluoroquinolone-resistant organism. In our study, we did not diagnose any sepsis or urinary infection; only one patient had prolonged dysuria, but no bacteria were identified in the culture.
There are no data to confirm that it is safe to defer biopsy in patients who exhibit decreased PSA levels. In their study, Toktas et al25 evaluated the possible effects of antibiotic treatment on PSA ≥4 ng/mL in asymptomatic patients. They observed that 23% of patients had mean PSA levels that were reduced after treatment; in addition, none of the patients had PSA level that fell below 2.5 ng/mL, and those who showed a mean decrease of 57% had a diagnosis of PCa after treatment. However, after antibiotic therapy only the mean PSA levels of nine patients were reduced by 57% to below 2.5 ng/mL, and these data were not evaluated in terms of statistics. In our study, after treatment only 5.6% of patients whose mean PSA levels were reduced ≥50% and only three patients whose pathology results were negative had mean PSA levels below 2.5 ng/mL. In the observation group, however, the mean PSA levels of two patients were reduced by 50% to below 2.5 ng/mL, and PCa was detected in these patients.
The total incidence of PCa between the antibiotic and control groups has been examined in many studies. Saribacak and coworkers26 compared the PCa ratios between antibiotic treatment group and control group patients with elevated PSA levels. They found that there was no statistically significant difference between the groups in terms of pathology results. Similarly, we detected PCa rates in 21.5% of the antibiotic group and 24% of the control group and found no difference in PCa detection. We also compared the PCa detection rates in terms of changes in PSA levels after antibiotic treatment and observed that PCa detection rate was higher in patients whose PSA levels were decreased after treatment. This suggests that treatment with antibiotics is not the most appropriate approach for decreasing PSA levels and minimizing the number of prostate biopsies.
There are several limitations to the present study. First, the study examined a single antibiotic agent given to the patient for a certain length of time. It is possible that another type of antibiotic could result in different outcomes in PSA level changes and biopsy findings. Second, we believe that stronger data will be obtained with a larger patient population and longer period of antibiotic therapy. Third, we did not compare the changes in PSA levels among the patients with the subtypes of pathology results such as benign prostate hyperplasia and chronic prostatitis and high prostatic intraepithelial neoplasia.
5. Conclusion
This study has shown that empirical antibiotic treatment decreased the PSA levels but did not have any effect on PCa detection. In addition, PCa detection rates were higher in patients whose PSA levels decreased after treatment. Therefore, it may not be safe to rule out biopsies in patients who achieve a satisfactory PSA response to antibiotics.
5.1. Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
None declared.
References
- 1.Stamey T.A., Yang N., Hay A.R., McNeal J.E., Freiha F.S., Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 2.Loeb S. Guideline of guidelines: prostate cancer screening. BJU Int. 2014;114:323. doi: 10.1111/bju.12854. [DOI] [PubMed] [Google Scholar]
- 3.Auffenberg G.B., Meeks J.J. Application of the 2013 American Urological Association early detection of prostate cancer guideline: who will we miss? World J Urol. 2014;32:959. doi: 10.1007/s00345-014-1341-2. [DOI] [PubMed] [Google Scholar]
- 4.Hayes J.H., Barry M.J. Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA. 2014;311:1143. doi: 10.1001/jama.2014.2085. [DOI] [PubMed] [Google Scholar]
- 5.Schaeffer A.J., Wu S.C., Tennenberg A.M., Kahn J.B. Treatment of chronic bacterial prostatitis with levofloxacin and ciprofloxacin lowers serum prostate specific antigen. J Urol. 2005;174:161–164. doi: 10.1097/01.ju.0000162017.24965.2b. [DOI] [PubMed] [Google Scholar]
- 6.Schaeffer A.J., Landis J.R., Knauss J.S., Propert K.J., Alexander R.B., Litwin M.S. Demographic and clinical characteristics of men with chronic prostatitis: the national institutes of health chronic prostatitis cohort study. J Urol. 2002;168:593–598. [PubMed] [Google Scholar]
- 7.Bulbul M.A., Wazzan W., Hijaz A., Shaar A. The effect of antibiotics on elevated serum prostate specific antigen inpatients with urinary symptoms and negative digital rectal examination: A pilot study. J Med Liban. 2002;50:23–25. [PubMed] [Google Scholar]
- 8.Bozeman C.B., Carver B.S., Eastham J.A., Venable D.D. Treatment of chronic prostatitis lowers serum prostate specific antigen. J Urol. 2002;167:1723–1726. [PubMed] [Google Scholar]
- 9.Owens R.C., Jr., Ambrose P.G. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(Suppl 2):144–157. doi: 10.1086/428055. [DOI] [PubMed] [Google Scholar]
- 10.Kahlmeter G., Menday P., Cars O. Non-hospital antimicrobial usage and resistance in community-acquired Escherichia coli urinary tract infection. J Antimicrob Chemother. 2003;52:1005–1010. doi: 10.1093/jac/dkg488. [DOI] [PubMed] [Google Scholar]
- 11.Feliciano J., Teper E., Ferrandino M., Macchia R.J., Blank W., Grunberger I. The incidence of fluoroquinolone resistant infections after prostate biopsy – are fluoroquinolones still effective prophylaxis? J Urol. 2008;179:952–955. doi: 10.1016/j.juro.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 12.Eggener S.E., Large M.C., Gerber G.S., Pettus J., Yossepowitch O., Smith N.D. Empiric antibiotics for an elevated prostate-specific antigen (PSA) level: a randomised, prospective, controlled multi-institutional trial. BJU Int. 2013;112:925. doi: 10.1111/bju.12241. [DOI] [PubMed] [Google Scholar]
- 13.Karazanashvili G., ManagadzeL Prostate-specific antigen (PSA) value change after antibacterial therapy of prostate inflammation, as a diagnostic method for prostate cancer screening in cases of PSA value within 4-10 ng/ml and nonsuspicious results of digital rectal examination. Eur Urol. 2001 May;39(5):538–543. doi: 10.1159/000052500. [DOI] [PubMed] [Google Scholar]
- 14.Kyung Y.S., Lee H.C., Kim H.J. Changes in serum prostate-specific antigen after treatment with antibiotics in patients with lower urinary tract symptoms/benign prostatic hyperplasia with prostatitis. Int Neurourol J. 2010 Aug;14(2):100–104. doi: 10.5213/inj.2010.14.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serretta V., Catanese A., Daricello G., Liotta R., Allegro R., Martorana A. PSA reduction (after antibiotics) permits to avoid or postpone prostate biopsy in selected patients. Prostate Cancer Prostatic Dis. 2008;11(2):148–152. doi: 10.1038/sj.pcan.4500996. [DOI] [PubMed] [Google Scholar]
- 16.Ehdaie B., Poon B.Y., Sjoberg D.D., Recabal P., Laudone V., Touijer K. Variation in serum prostate-specific antigen levels in men with prostate cancer managed with active surveillance. BJU Int. 2016 Oct;118(4):535–540. doi: 10.1111/bju.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L., Loblaw A., Klotz L. Modeling prostate specific antigen kinetics in patients on active surveillance. J Urol. 2006 Oct;176:1392–1397. doi: 10.1016/j.juro.2006.06.103. [DOI] [PubMed] [Google Scholar]
- 18.Catalona W.J., Richie J.P., Ahmann F.R. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: Results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–1290. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 19.Stenman U.H., Hakama M., Knekt P. Serum concentrations of prostate specific antigen and its complex with alpha 1-antichymotrypsinbefore diagnosis of prostate cancer. Lancet. 1994;344:1594–1598. doi: 10.1016/s0140-6736(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 20.Gann P.H., Hennekens C.H., Stampfer M.J. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273:289–294. [PubMed] [Google Scholar]
- 21.Van Cangh P.J., De Nayer P., De Vischer L. Free to total prostate-specific antigen (PSA) ratio improves the discrimination between prostate cancer and benign prostatic hyperplasia (BPH) in the diagnostic gray zone of 1.8 to 10 ng/mL total PSA. Urology. 1996;48:67–70. doi: 10.1016/s0090-4295(96)00613-9. [DOI] [PubMed] [Google Scholar]
- 22.Vashi A.R., Wojno K.J., Henricks W. Determination of the “reflex range” and appropriate cut points for percent free prostate-specific antigen in 413 men referred for prostatic evaluation using the AxSYM system. Urology. 1997;49:19–27. doi: 10.1016/S0090-4295(96)00511-0. [DOI] [PubMed] [Google Scholar]
- 23.Benson M.C., Whang I.S., Pantuck A. Prostate specific antigen density: A means of distinguishing benign prostatic hypertrophy and prostate cancer. J Urol. 1992;147:815–816. doi: 10.1016/s0022-5347(17)37393-7. [DOI] [PubMed] [Google Scholar]
- 24.Akduman B., Akduman D., Tokgöz H., Erol B., Türker T., Ayoğlu F. Long-term fluoroquinolone use before the prostate biopsy may increase the risk of sepsis caused by resistant microorganisms. Urology. 2011 Aug;78(2):250–255. doi: 10.1016/j.urology.2011.02.065. [DOI] [PubMed] [Google Scholar]
- 25.Toktas G., Demiray M., Erkan E., Kocaaslan R., Yucetas U., Unluer S.E. The effect of antibiotherapy on prostate-specific antigen levels and prostate biopsy results in patients with levels 2.5 to 10 ng/mL. JEndourol. 2013 Aug;27(8):1061–1067. doi: 10.1089/end.2013.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saribacak A., Yilmaz H., Ciftci S., Ustuner M., Ozkan L., Ozkan T.A. The role of empiric antibiotic treatment in preventing unnecessary prostate biopsies in asymptomatic patients with PSA levels between 4 and 10 ng/ml. Int J ClinExp Med. 2014 Aug 15;7(8):2230–2235. [PMC free article] [PubMed] [Google Scholar]