Abstract
Background
To evaluate the relationship between postoperative prostate-specific antigen (PSA) levels and biochemical recurrence (BCR) after radical prostatectomy, especially in patients with positive surgical margins (PSMs).
Materials and methods
A total of 144 patients who underwent radical prostatectomies performed by a single surgeon without any neoadjuvant or adjuvant treatment were analyzed. Differences in clinicopathological factors were compared by surgical margin status, and the relationship between postoperative PSA level and BCR in patients with PSMs was evaluated.
Results
Fifty of the 144 patients (34.7%) had PSMs. Of these, 74% experienced BCR. The negative surgical margins and PSMs groups differed significantly in terms of PSA level at diagnosis, clinical T stage, and risk group by the cancer of the prostate risk assessment score (P = 0.002, P = 0.002, and P = 0.004, respectively). Also, the nadir PSA level, tumor volume, and BCR rate differed between the two groups (P = 0.007, P = 0.015, and P = 0.005, respectively) On Kaplan–Meier analysis, BCR-free survival was better in the negative surgical margins than the PSMs group (64.1 vs. 55.4 months, log-rank test, P = 0.011). BCR-free survival did not differ significantly in PSMs patients according to whether PSA level was or was not detectable at 1 month postoperatively. However, BCR-free survival improved when the nadir PSA level was undetectable (compared to detectable) in PSMs patients (64.3 vs. 26.1 months, log-rank test, P < 0.001). In PSMs patients belonging to the high risk group by cancer of the prostate risk assessment score, BCR-free survival was significantly better when the PSA level attained the nadir within 3 months, compared to > 6 months, postoperatively (64.2 vs. 29.5 months, log-rank test, P = 0.022).
Conclusion
If PSA is detectable in PSMs patients until 1 month after operation, cautious observation may be possible. If the nadir is attained within 3 months postoperatively in high-risk patients with PSMs, better BCR-free survival may be expected.
Keywords: Positive Surgical Margins, Prostatectomy, Prostate-Specific Antigen, Recurrence
1. Introduction
Radical prostatectomy is a valuable option for clinically localized prostate cancer.1 However, about one-third of patients undergoing radical prostatectomy will experience biochemical recurrence (BCR), defined as an elevation in prostate-specific antigen (PSA) level.2, 3, 4, 5 Positive surgical margins (PSMs) are unfavorable pathological findings including seminal vesicle invasion and extraprostatic extension, and associated with BCR. PSMs suggest that some cancer cells remain in the surgical bed. However, PSMs are affected by remnant normal tissue, inadequate surgical skill, and iatrogenic incision.6, 7 Regardless of the cause, PSMs affect postoperative PSA levels; patients with PSMs may have detectable levels despite the apparent success of prostatectomy.8 However, some patients with PSMs experience undetectable PSA levels during serial checking, without any adjuvant treatment. Thus, the interpretation of PSMs as primary treatment failure remains controversial.8, 9 When PSMs occur after prostatectomy, it is important to know which patients will benefit from additional treatment. Not all patients with PSMs or low but detectable PSA levels after prostatectomy benefit from adjuvant therapy.9, 10
Many studies have explored the associations among PSMs, the PSA level, and BCR. We explored how the postoperative PSA level was related to BCR, especially limited to the patients with PSMs, after evaluating the clinical characteristics of them. Kaplan–Meier analysis was used to focus principally on the relationship between postoperative PSA status and BCR in patients with PSMs.
2. Materials and methods
A total of 144 patients who underwent radical prostatectomy from 2008 to 2014 were reviewed retrospectively. All patients were operated upon by a single surgeon; no patient received any adjuvant or neoadjuvant therapy. Medical records were retrieved after the work was approved by the Institutional Review Board of Inje University Busan Paik Hospital. Postoperative PSA levels were initially checked 1 month after surgery, followed by serial evaluation. The nadir PSA was defined as lowest PSA during the observation period and detectable PSA was defined as PSA > 0.01 ng/mL. BCR was defined as two consecutive PSA levels ≥ 0.2 ng/mL at any time postoperatively. All patients were divided into negative surgical margins (NSMs) and PSMs groups.
Preoperative and postoperative variables were compared between the groups. Age, PSA level at diagnosis, Gleason score (from biopsy specimens), and clinical T stage, were the preoperative variables included. Three risk groups (low, intermediate, and high) were calculated by cancer of the prostate risk assessment (CAPRA) scores and also included in preoperative variables.11 Postoperative 1 month PSA, nadir PSA levels, the time from surgery to attainment of the PSA nadir, total tumor volume (reported by the pathologist), BCR rate, and the time from surgery to BCR were analyzed as postoperative variables. In the PSMs group, PSA recurrence was analyzed by the postoperative 1 month PSA level (detectable vs. undetectable), nadir PSA level (detectable vs. undetectable), and the time to the nadir PSA level (in 3-month intervals).
SPSS software, version 20 (IBM, United States), was used for all analyses, and the significance level was set to P = 0.05. Data are presented as means with standard deviations. Analysis of variance was used to compare numerical values including age, PSA level, prostate volume, and the times to the nadir PSA level and BCR rate. Categorical variables were compared with the aid of the Chi-square test. Kaplan–Meier survival curves of BCR were drawn, and the log rank test was used to compare the two groups.
3. Results
Table 1 shows the clinicopathological variables by surgical margin status. The PSA level at diagnosis differed significantly between the groups, being higher in the PSMs group (P = 0.002). The NSMs group contained significantly more patients of clinical stage ≤ T2 and at low risk by CAPRA score. Of the postoperative variables, the nadir PSA level was lower in the NSMs than the PSMs group (mean 0.014 ng/mL and 0.019 ng/mL, respectively; P = 0.007). Additionally, the tumor volume of the PSMs group was about twice that of the NSM group (mean 33.1 vs. 15.7%, P = 0.015).
Table 1.
Preoperative and postoperative variables according to surgical margin status
| Variablesa | NSM group (n = 94) | PSM group (n = 50) | P |
|---|---|---|---|
| Preoperative variables | |||
| Age (yr) | 67.3 (± 6.7) | 64.6 (±6.5) | 0.654 |
| PSA at diagnosis (ng/mL) | 10.5 (± 6.7) | 16.3 (± 11.4) | 0.002 |
| Prostate volume (g) | 35.5 (± 18.6) | 36.4 (± 16.1) | 0.859 |
| GS at diagnosis | 0.225 | ||
| ≤ 6 | 40 (42.5) | 14 (28) | |
| 7 | 37 (39.4) | 24 (48) | |
| ≥ 8 | 17 (18.1) | 12 (24) | |
| Clinical T stage | 0.002 | ||
| ≤ T2 | 84 (89.4) | 34 (68) | |
| > T2 | 10 (10.6) | 16 (32) | |
| CAPRA score risk group | 0.004 | ||
| Low | 39 (41.5) | 9 (18) | |
| Intermediate | 32 (34) | 17 (34) | |
| High | 23 (24.5) | 24 (48) | |
| Postoperative variables | |||
| Postop. PSA (ng/mL) | 0.960 (±0.186) | 0.140 (±0.209) | 0.422 |
| Nadir PSA (ng/mL) Period until nadir PSA (mo) |
0.014 (±0.016) 4.3 (±2.9) |
0.019 (±0.027) 4.2 (±3.2) |
0.007 0.842 |
| Tumor volume (%) | 15.7 (±16.7) | 33.1 (±21.9) | 0.015 |
| No. of biochemical recurrence | 8 (8.5) | 37 (74) | 0.005 |
| Period until BCR (mo) | 32.7 (±20.4) | 35.2 (±22.6) | 0.497 |
BCR, biochemical recurrence; CAPRA, cancer of the prostate risk assessment; GS, Gleason score; NSM, negative surgical margin; PSA, prostate-specific antigen; PSM, positive surgical margin.
Variables are presented as mean and standard deviation or number (%).
BCR was more common in the PSMs than the NSMs group (74 vs 8.5%, P = 0.005). However, the postoperative 1-month PSA level did not differ significantly by surgical margin status, being in fact higher in the NSMs group. The time to BCR did not differ significantly between the two groups.
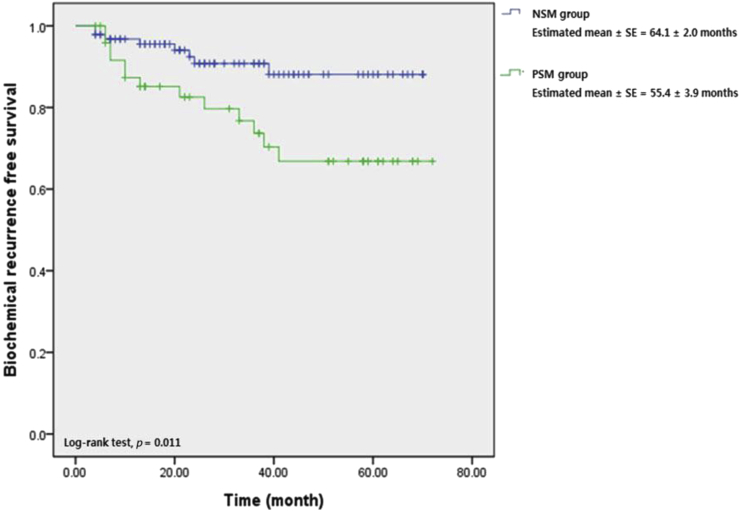
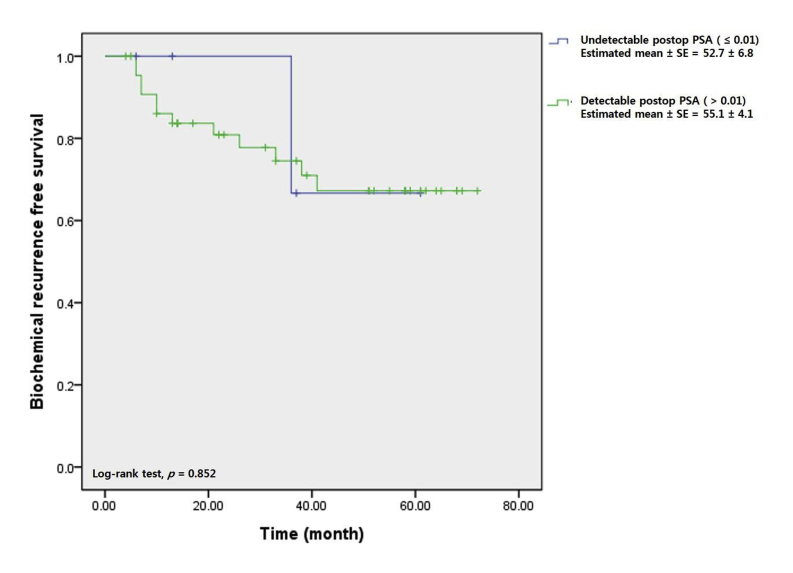
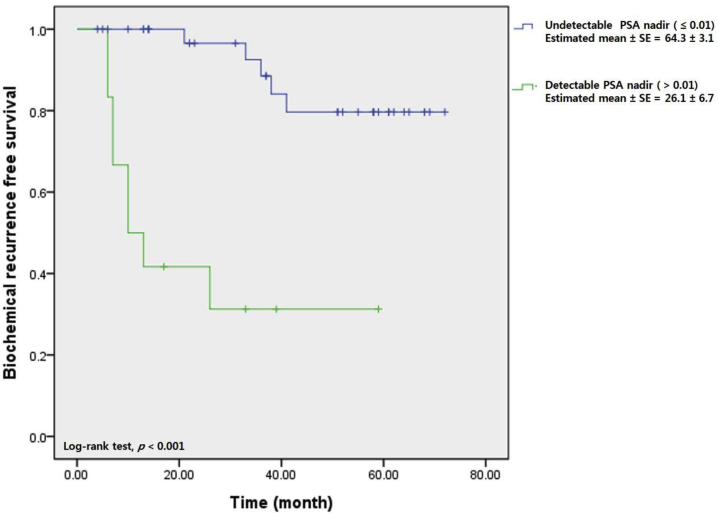
On Kaplan–Meier analysis, the estimated mean BCR-free survival periods were 55.4 ± 3.9 months and 64.1 ± 2.0 months in the PSMs and NSMs groups, respectively, with statistical significance (log rank test, P = 0.011; Fig. 1). Fig. 2 shows that BCR-free survival in the PSMs group did not differ significantly between those in whom PSA was and was not detectable 1 month postoperatively (55.1 ± 4.1 months vs. 52.7 ± 6.8 months, log rank test, P = 0.852). However, when the PSA nadir was undetectable in the PSMs group, BCR-free survival was superior to that of patients in whom the PSA nadir was detectable (64.3 ± 3.1 months vs. 26.1 ± 6.7 months, log rank test, P = 0.001) (Fig. 3).
Fig. 1.
Biochemical recurrence free survival curve stratified by surgical margin status. NSM, negative surgical margin; PSM, positive surgical margin.
Fig. 2.
Biochemical recurrence free survival curve stratified by postoperative prostate-specific antigen (PSA) level (estimated within 1month postoperatively) in positive surgical margin group.
Fig. 3.
Biochemical recurrence free survival curve stratified by prostate-specific antigen (PSA) nadir in positive surgical margin group.
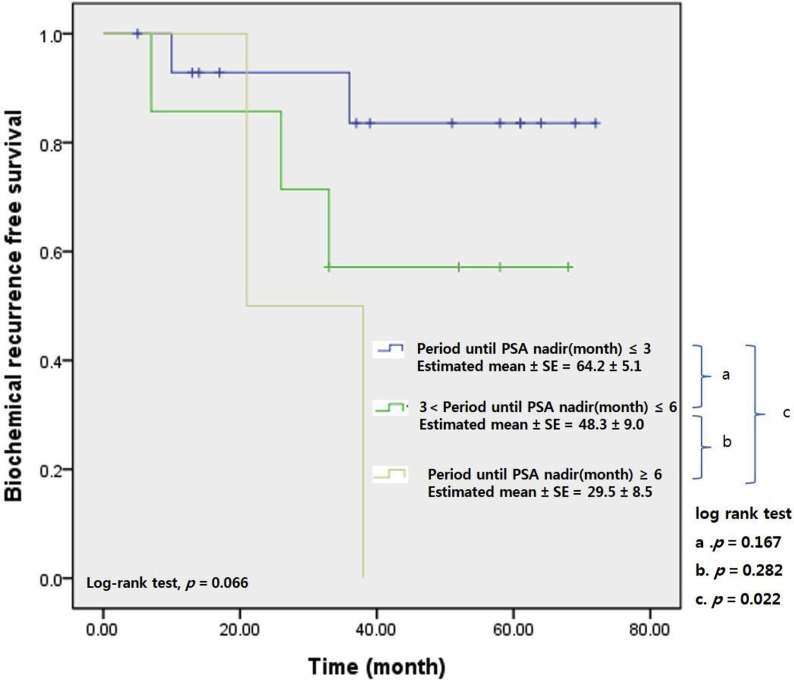
Fig. 4 shows the correlations between BCR-free survival and the time to the PSA nadir in high risk patients with PSMs. A total of 24 patients of PSMs were classified high risk group by CAPRA score. Among them, 15 patients (62.5%) attained the PSA nadir within 3 months postoperatively. In addition, seven patients (29.2%) attained the PSA nadir from 3 months to 6 months after surgery. Two patients (8.3%) attained the PSA nadir after 6 months postoperatively. Survival differed significantly between those in whom the PSA nadir was attained within 3 months of surgery and those in whom the nadir was not attained within 6 months of surgery (64.2 ± 5.1 vs. 29.5 ± 8.5 months, log rank test, P = 0.022).
Fig. 4.
Biochemical recurrence-free survival curve stratified by period until prostate-specific antigen (PSA) nadir (3 months interval) in high risk patients by cancer of the prostate risk assessment (CAPRA) risk stratification among patients with positive surgical margin.
4. Discussion
PSMs are one cause of BCR after radical prostatectomy and elevations in PSA level may be seen even after complete removal of the prostate gland in patients with PSMs. PSMs may be caused not only by incomplete cancer excision, but also by inadvertent capsular incision during surgery, artifacts associated with tissue processing, and remnant normal prostatic tissue.6, 12 The incidence of PSMs is 10–60%.13, 14, 15 Our incidence was 34.7% in a single-surgeon series, comparable to previous data.
A large retrospective analysis found that PSMs were significantly associated with a higher preoperative PSA level, a greater body mass index, more advanced pathological stage, a higher Gleason score, greater tumor volume, and a smaller prostate volume.16 Another Asian report also found that a PSMs group had a higher preoperative PSA level, a lower prostate weight, a higher pathological T stage, and a higher Gleason score.15 Likewise, other reports have consistently found that adverse clinicopathological features (tumor stage, higher Gleason score, higher preoperative PSA level, and greater tumor volume) were associated with PSMs after radical prostatectomy.16, 17, 18, 19, 20 Similarly, we show here that unfavorable clinicopathological factors were frequently more associated with PSMs than NSMs groups with significance. The PSMs group had higher preoperative PSA levels, clinical T stages, risk stratification level by CAPRA scores (P = 0.002, P = 0.002, and P = 0.004, respectively); and greater tumor volumes (P = 0.015). The postoperative PSA nadir was also significantly higher in the PSMs group (P = 0.007). However, the time to attainment of the PSA nadir did not differ between the PSMs and NSMs groups (4.3 months and 4.2 months, respectively, P = 0.842).
PSMs clearly increase the BCR, but not necessarily cancer-specific mortality. Most previous studies found that PSMs predicted BCR.17, 21, 22, 23 However, BCR correlated poorly with both prostate cancer-specific mortality (PCSM) and overall mortality. Boorjian et al16 found that PSMs increased the risk of BCR and the need for salvage treatment but were not independently associated with cancer-specific death or overall mortality. A recent report of 15-year follow up data also found that PSMs did not significantly predict PCSM.24 One study exploring the interaction between PSA level and PCSM found that the Gleason grade and pathological stage were stronger predictors of PCSM than PSMs.25 A limitation of our present study is that we lack data on mortality, but the BCR incidence differed significantly between the PSMs and NSMs groups (74% vs 8.5%, P = 0.005). Also, the mean BCR-free survival period was longer in the NSMs group than the PSMs group (55.4 ± 3.9 months vs. 64.1 ± 2.0 months, log rank test, P = 0.011).
Serum PSA is made by prostatic epithelial cells and has a half-life of 3.15 days; thus, after seven half-lives, the PSA level declines to 0.78% of the initial value. As such, PSA should become undetectable within 1 month after radical prostatectomy.26, 27 Persistently detectable, or rising, PSA levels after radical prostatectomy indicate residual cancer or recurrence.28 However, some studies found that patients exhibiting detectable (and stable) PSA after radical prostatectomy often did not experience clinical progression or a progressive rise in PSA level.10, 29 Indeed, most patients with detectable (and stable) PSA levels after surgery had stable disease.29
In the present study, we focused principally on the relationship between the postoperative 1-month PSA level and BCR in the patients with PSMs. The postoperative 1-month PSA level did not differ by surgical margin status (P = 0.422). Kaplan–Meier analysis showed that the BCR-free survival of PSMs patients was similar in those in whom PSA was and was not detectable postoperative 1-month (55.1 ± 4.1 months vs. 52.7 ± 6.8 months, log rank test, P = 0.852). Thus, postoperative 1-month PSA levels of the patients with PSMs did not seem to affect either BCR or BCR-free survival. This shows that PSA levels should be serially measured postoperatively, even in patients with PSMs for decision about adjuvant therapy. The pattern of PSA change is more important than one-time measurements, as indicated in previous studies.29, 30 The present study showed that the attainment (or not) of the PSA nadir (undetectable) affected BCR in patients with PSMs. Fig. 3 shows that the BCR-free survival of PSMs group was highest in cases with undetectable PSA nadirs (64.3 ± 3.1 months vs. 26.1 ± 6.7 months, log rank test, P = 0.001). Thus, in the patients with PSMs, attainment of an undetectable PSA nadir was important for BCR-free survival.
The patients classified with high risk are at an increased risk of recurrence and need for adjuvant therapy.9, 30 However, there is no consensus for the optimal time about adjuvant treatment of men with high risk. Most guidelines recommend PSA follow-up at 3-month intervals after surgery for high-risk patients.31, 32 We thought that the risk of recurrence was high and the possibility of considering adjuvant therapy was also high in patients with high risk, especially among the patients with PSMs. The authors therefore analyzed the difference in BCR over time to PSA nadir in patients at high risk among patients with PSMs. In the patients with PSMs belonging to the high risk group, BCR-free survival was better when the PSA nadir was attained within 3 months (compared to > 6 months) after prostatectomy; the mean BCR-free survival period of the former group was 64.2 months (Fig. 4). We expect that this result will be used to define the period of time over which PSA levels should be measured for a decision about adjuvant therapy in high risk patients with PSMs.
If urologists encounter PSMs after radical prostatectomy, and PSA is detectable, adjuvant therapy is strongly advised. However, neither the best therapy, nor the timing thereof, is clear. Especially, when considering radiation therapy, many factors influence long-term effectiveness and the development of adverse effects. The European Organisation for Research and Treatment of Cancer (EORTC) trial 22911 showed that immediate postoperative irradiation improved the clinical progression-free survival of patients with PSMs; however, late adverse effects were significantly more frequent in the irradiation than the wait-and-see group.33 Irradiation of all patients with PSMs or detectable PSA could lead to overtreatment, increasing the incidence of treatment-related toxicities.10, 20, 29, 33, 34 Therefore, commonly accepted guidelines recommend that if adjuvant therapy is planned, clinicians must consider not only unfavorable pathological features, but also PSA kinetics (including the PSA doubling time), and imaging data.31, 32
We suggest that serial PSA level checks, documentation of the nadir, and other clinicopathological factors, allow appropriate treatment options to be chosen after radical prostatectomy. This is the case even if PSA does not become immediately undetectable in the patients with PSMs after radical prostatectomy. Although we studied only a small retrospective series, we hope that our work can contribute to data interpretation, and the formulation of treatment guidelines for PSMs.
Conflict of interest
All authors have no conflicts of interest to declare.
References
- 1.Pound C.R., Partin A.W., Eisenberger M.A., Chan D.W., Pearson J.D., Walsh P.C. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 2.Pound C.R., Partin A.W., Epstein J.I., Walsh P.C. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395–406. doi: 10.1016/s0094-0143(05)70386-4. [DOI] [PubMed] [Google Scholar]
- 3.Roehl K.A., Han M., Ramos C.G., Antenor J.A., Catalona W.J. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: Long-term results. J Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 4.Zincke H., Oesterling J.E., Blute M.L., Bergstralh E.J., Myers R.P., Barrett D.M. Long-term (15 years) results after radical prostatectomy for clinically localized (stage T2c or lower) prostate cancer. J Urol. 1994;152:1850–1857. doi: 10.1016/s0022-5347(17)32399-6. [DOI] [PubMed] [Google Scholar]
- 5.Catalona W.J., Smith D.S. 5-year tumor recurrence rates after anatomical radical retropubic prostatectomy for prostate cancer. J Urol. 1994;152:1837–1842. doi: 10.1016/s0022-5347(17)32397-2. [DOI] [PubMed] [Google Scholar]
- 6.Wieder J.A., Soloway M.S. Incidence, etiology, location, prevention and treatment of positive surgical margins after radical prostatectomy for prostate cancer. J Urol. 1998;160:299–315. [PubMed] [Google Scholar]
- 7.Eastham J.A., Kattan M.W., Riedel E., Begg C.B., Wheeler T.M., Gerigk C. Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens. J Urol. 2003;170:2292–2295. doi: 10.1097/01.ju.0000091100.83725.51. [DOI] [PubMed] [Google Scholar]
- 8.Ohori M., Wheeler T.M., Kattan M.W., Goto Y., Scardino P.T. Prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 1995;154:1818–1824. [PubMed] [Google Scholar]
- 9.Collette L., van Poppel H., Bolla M., van Cangh P., Vekemans K., Da Pozzo L. Patients at high risk of progression after radical prostatectomy: Do they all benefit from immediate post-operative irradiation? (EORTC trial 22911) Eur J Cancer. 2005;41:2662–2672. doi: 10.1016/j.ejca.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Koulikov D., Mohler M.C., Mehedint D.C., Attwood K., Wilding G.E., Mohler J.L. Low detectable prostate specific antigen after radical prostatectomy–treat or watch? J Urol. 2014;192:1390–1396. doi: 10.1016/j.juro.2014.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooperberg M.R., Pasta D.J., Elkin E.P., Litwin M.S., Latini D.M., Du Chane J. The University of California, San Francisco Cancer of the Prostate Risk Assessment Score: A straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173:1938–1942. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein J.I. Incidence and significance of positive margins in radical prostatectomy specimens. Urol Clin North Am. 1996;23:651–663. doi: 10.1016/s0094-0143(05)70343-8. [DOI] [PubMed] [Google Scholar]
- 13.Mauermann J., Fradet V., Lacombe L., Dujardin T., Tiguert R., Tetu B. The impact of solitary and multiple positive surgical margins on hard clinical end points in 1712 adjuvant treatment-naive pT2-4 N0 radical prostatectomy patients. Eur Urol. 2013;64:19–25. doi: 10.1016/j.eururo.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Ploussard G., Agamy M.A., Alenda O., Allory Y., Mouracade P., Vordos D. Impact of positive surgical margins on prostate-specific antigen failure after radical prostatectomy in adjuvant treatment-naive patients. BJU Int. 2011;107:1748–1754. doi: 10.1111/j.1464-410X.2010.09728.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee J.W., Ryu J.H., Kim Y.B., Yang S.O., Lee J.K., Jung T.Y. Do positive surgical margins predict biochemical recurrence in all patients without adjuvant therapy after radical prostatectomy? Korean J Urol. 2013;54:510–515. doi: 10.4111/kju.2013.54.8.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boorjian S.A., Karnes R.J., Crispen P.L., Carlson R.E., Rangel L.J., Bergstralh E.J. The impact of positive surgical margins on mortality following radical prostatectomy during the prostate specific antigen era. J Urol. 2010;183:1003–1009. doi: 10.1016/j.juro.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 17.Alkhateeb S., Alibhai S., Fleshner N., Finelli A., Jewett M., Zlotta A. Impact of positive surgical margins after radical prostatectomy differs by disease risk group. J Urol. 2010;183:145–150. doi: 10.1016/j.juro.2009.08.132. [DOI] [PubMed] [Google Scholar]
- 18.Chang S.S., Cookson M.S. Impact of positive surgical margins after radical prostatectomy. Urology. 2006;68:249–252. doi: 10.1016/j.urology.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 19.Grossfeld G.D., Chang J.J., Broering J.M., Miller D.P., Yu J., Flanders S.C. Impact of positive surgical margins on prostate cancer recurrence and the use of secondary cancer treatment: Data from the CaPSURE database. J Urol. 2000;163:1171–1177. quiz 1295. [PubMed] [Google Scholar]
- 20.Vis A.N., Schroder F.H., van der Kwast T.H. The actual value of the surgical margin status as a predictor of disease progression in men with early prostate cancer. Eur Urol. 2006;50:258–265. doi: 10.1016/j.eururo.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 21.Corcoran N.M., Hovens C.M., Metcalfe C., Hong M.K., Pedersen J., Casey R.G. Positive surgical margins are a risk factor for significant biochemical recurrence only in intermediate-risk disease. BJU Int. 2012;110:821–827. doi: 10.1111/j.1464-410X.2011.10868.x. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto K., Masumori N., Takei F., Fukuta F., Takahashi A., Itoh N. Prognostic value of surgical margin status for biochemical recurrence following radical prostatectomy. Jpn J Clin Oncol. 2008;38:31–35. doi: 10.1093/jjco/hym135. [DOI] [PubMed] [Google Scholar]
- 23.Oh J.J., Hong S.K., Byun S.S., Choe G., Lee S.E. Prognostic significance of positive surgical margins after radical prostatectomy among pT2 and pT3a prostate cancer. Urol Oncol. 2013;31:595–600. doi: 10.1016/j.urolonc.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Eggener S.E., Scardino P.T., Walsh P.C., Han M., Partin A.W., Trock B.J. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185:869–875. doi: 10.1016/j.juro.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalfin H.J., Dinizo M., Trock B.J., Feng Z., Partin A.W., Walsh P.C. Impact of surgical margin status on prostate-cancer-specific mortality. BJU Int. 2012;110:1684–1689. doi: 10.1111/j.1464-410X.2012.11371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stamey T.A., Kabalin J.N., McNeal J.E., Johnstone I.M., Freiha F., Redwine E.A. Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. II. Radical prostatectomy treated patients. J Urol. 1989;141:1076–1083. doi: 10.1016/s0022-5347(17)41175-x. [DOI] [PubMed] [Google Scholar]
- 27.Partin A.W., Oesterling J.E. The clinical usefulness of prostate specific antigen: Update 1994. J Urol. 1994;152:1358–1368. doi: 10.1016/s0022-5347(17)32422-9. [DOI] [PubMed] [Google Scholar]
- 28.Naito S. Evaluation and management of prostate-specific antigen recurrence after radical prostatectomy for localized prostate cancer. Jpn J Clin Oncol. 2005;35:365–374. doi: 10.1093/jjco/hyi113. [DOI] [PubMed] [Google Scholar]
- 29.Shinghal R., Yemoto C., McNeal J.E., Brooks J.D. Biochemical recurrence without PSA progression characterizes a subset of patients after radical prostatectomy. Prostate-specific antigen. Urology. 2003;61:380–385. doi: 10.1016/s0090-4295(02)02254-9. [DOI] [PubMed] [Google Scholar]
- 30.Leibovici D., Spiess P.E., Agarwal P.K., Tu S.M., Pettaway C.A., Hitzhusen K. Prostate cancer progression in the presence of undetectable or low serum prostate-specific antigen level. Cancer. 2007;109:198–204. doi: 10.1002/cncr.22372. [DOI] [PubMed] [Google Scholar]
- 31.Cornford P., Bellmunt J., Bolla M., Briers E., De Santis M., Gross T. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–642. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Mohler J.L., Armstrong A.J., Bahnson R.R., D'Amico A.V., Davis B.J., Eastham J.A. Prostate cancer, version 1. 2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 33.Bolla M., van Poppel H., Tombal B., Vekemans K., Da Pozzo L., de Reijke T.M. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018–2027. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 34.Swindle P., Eastham J.A., Ohori M., Kattan M.W., Wheeler T., Maru N. Do margins matter? the prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2008;179:S47–S51. doi: 10.1016/j.juro.2008.03.137. [DOI] [PubMed] [Google Scholar]