Abstract
Purpose
Combining the mTOR inhibitor ridaforolimus and the anti-IGFR antibody dalotuzumab demonstrated antitumor activity, including partial responses, in estrogen receptor (ER)-positive advanced breast cancer, especially in high proliferation tumors (Ki67 > 15%).
Methods
This randomized, multicenter, international, phase II study enrolled postmenopausal women with advanced ER-positive breast cancer previously treated with a nonsteroidal aromatase inhibitor (NCT01234857). Patients were randomized to either oral ridaforolimus 30 mg daily for 5 of 7 days (once daily [qd] × 5 days/ week) plus intravenous dalotuzumab 10 mg/kg/week or oral exemestane 25 mg/day, and stratified by Ki67 status. Due to a high incidence of stomatitis in the ridaforolimus– dalotuzumab group, two sequential, nonrandomized, reduced-dose cohorts were explored with ridaforolimus 20 and 10 mg qd × 5 days/week. The primary endpoint was progression-free survival (PFS).
Results
Median PFS was 21.4 weeks for ridaforolimus 30 mg qd × 5 days/week plus dalotuzumab 10 mg/kg (n = 29) and 24.3 weeks for exemestane (n = 33; hazard ratio = 1.00; P = 0.5). Overall survival and objective response rates were similar between treatment arms. The incidence of drug-related, nonserious, and serious adverse events was higher with ridaforolimus/dalotuzumab (any ridaforolimus dose) than with exemestane. Lowering the ridaforolimus dose reduced the incidence of grade 3 stomatitis, but overall toxicity remained higher than acceptable at all doses without improved efficacy.
Conclusions
The combination of ridaforolimus plus dalo-tuzumab was no more effective than exemestane in patients with advanced ER-positive breast cancer, and the incidence of adverse events was higher. Therefore, the combination is not being further pursued.
Keywords: Ridaforolimus, mTOR, Dalotuzumab, IGF1R, Breast cancer
Introduction
Endocrine therapy is the preferred treatment for estrogen receptor (ER)-positive advanced breast cancer in postmenopausal women [1]. However, most patients will eventually develop progressive disease because of the development of resistance [2]. Chemotherapy may be initiated after failure of endocrine approaches [1], but tolerability is an issue for many patients. New treatments with efficacy against ER-positive tumors, or that restore sensitivity to previously effective hormone therapies, are urgently needed.
Development of endocrine resistance has been linked to aberrant activation of the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway in a variety of cancers, including breast cancer [3, 4]. mTOR is a regulator of multiple signaling pathways, including insulin-like growth factor 1 receptor (IGF1R), and is involved in the control of cellular growth, proliferation, metabolism, and angiogenesis [5, 6]. An mTOR inhibitor has been shown to be effective in patients with ER-positive breast cancer when combined with an aromatase inhibitor [7].
Ridaforolimus (MK-8669), an analog of rapamycin, is a potent and selective mTOR inhibitor that has demonstrated good tolerability and modest antitumor activity as monotherapy in patients with solid tumors [8–12]. However, the efficacy of mTOR inhibitors as single agents is limited by feedback upregulation of PI3K/AKT signaling [13–15]. This effect is mediated by insulin receptor substrate 1 (IRS1), an adaptor protein that propagates IGF1R signaling by facilitating receptor-mediated activation of PI3K. Activation of p70 S6 kinase by mTOR complex 1 (mTORC1) results in inhibitory phosphorylation and degradation of IRS1 and, consequently, reduced activation of PI3K/AKT signaling. Conversely, inhibition of mTORC1 relieves p70 S6 kinase-mediated inhibition of IRS1, thereby promoting PI3K-mediated activation of AKT and downstream survival signaling [15]. Inhibiting IGF1R in conjunction with mTORC1 inhibition should abrogate this feedback activation of IRS1, potentially leading to more effective antitumor activity.
This hypothesis has been evaluated in preclinical studies of combination therapy with mTOR and IGF1R inhibitors [15], including ridaforolimus [16] in combination with the anti-IGF1R monoclonal antibody dalotuzumab (MK-0646) [17], which demonstrated additive/synergistic antitumor activity in mouse xenograft experiments and colorectal cancer cell lines [16]. A phase I trial of patients with solid malignancies showed ridaforolimus plus dalotuzumab to be a tolerable combination with antitumor activity in patients with ER-positive breast cancer and identified the recommended dose for phase II trials as ridaforolimus 30 mg/day × 5 days/week (5 out of every 7 days) plus dalotuzumab 10 mg/kg/week [16].
The present phase II study was undertaken to determine whether the combination of ridaforolimus and dalotuzumab would improve patient outcomes compared with the steroidal aromatase inhibitor exemestane in postmenopausal patients with ER-positive breast cancer progressing after treatment with a nonsteroidal aromatase inhibitor. It also assessed the combination's effect in the subset of patients with high proliferative, luminal B-like disease, as measured by a high Ki67 labeling index [18].
Methods
Study design and patients
This was a randomized, multicenter, open-label phase II study originally intended to incorporate a 2-part design to evaluate ridaforolimus plus dalotuzumab compared with exemestane (part A), or compared with ridaforolimus or dalotuzumab monotherapy (part B), in women with ER-positive breast cancer. (ClinicalTrials.gov identifier: NCT01234857; EudraCT Number: 2010-019867-13; Protocol Number PN041). However, part B was not initiated due to excess toxicity and lack of activity improvement observed in part A. The trial was conducted at 50 trial centers (4 Belgium, 2 Canada, 3 Denmark, 2 France; 1 Germany; 1 Israel; 1 Italy, 4 South Korea, 6 Spain, 1 Sweden, 4 Taiwan; and 21 United States). Written informed consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Independent ethics committees reviewed and approved the protocol and applicable amendments for each institution.
Eligible postmenopausal women had histologically confirmed ER-positive (≥1% tumor cells positive by immunohistochemistry) and human epidermal growth factor receptor 2 (HER2)-negative (negative by fluorescence in situ hybridization or <3+ by immunohistochemistry) metastatic or locally advanced breast cancer. Post-menopausal status was defined as prior bilateral oophorectomy or 12 months since last menstrual period with no prior hysterectomy (patients aged >45 years also required biochemical evidence of postmenopausal status), or biochemical evidence of postmenopausal status for patients aged >60 years with prior hysterectomy and without bilateral oophorectomy. Entry into the trial required disease recurrence or progression after prior treatment with a nonsteroidal aromatase inhibitor (anastrozole or letrozole) in the adjuvant or metastatic setting. All patients had at least one measurable metastatic lesion according to Response Evaluation Criteria In Solid Tumors (RECIST) v1.1 [19], Eastern Cooperative Oncology Group (ECOG) performance status ≤1, adequate organ function, and an archival tumor specimen of acceptable quality and quantity for central Ki67 determination. Patients receiving any concurrent systemic tumor therapy or that had received previous treatment with rapamycin or rapamycin analogs, IGF1R inhibitors, PI3K inhibitors, or other experimental agents targeting the PI3K/AKT/mTOR pathway were excluded.
Treatments
In part A, patients received oral ridaforolimus 30 mg administered on days 1–5, 8–12, 15–19, and 22–26 (once daily [qd] × 5 days/week) plus intravenous dalotuzumab 10 mg/kg/week on days 1, 8, 15, and 22 or exemestane 25 mg/day orally.
Patients were stratified by high or low tumor proliferation, based on the Ki67 labeling index. Low proliferation was defined as having a Ki67 labeling index <15%, and high proliferation was defined as having a Ki67 labeling index ≥15%. The luminal B subpopulation is distinguished from luminal A by a Ki67 labeling index of greater than 13.25%, ER expression, and absence of HER2 overexpression [18, 20, 21].
Accrual of patients in part A to ridaforolimus 30 mg plus dalotuzumab or exemestane was stopped after the first 62 randomized patients because of a higher-than-expected rate of stomatitis in the combination arm. To investigate lower and potentially more tolerable doses of ridaforolimus, sequentially enrolled single-arm cohorts were treated with ridaforolimus 20 mg and then ridaforolimus 10 mg in combination with dalotuzumab 10 mg/kg/week.
Endpoints and assessments
The primary objective was to assess the safety and tolerability of ridaforolimus and dalotuzumab in the all-patients-as-treated population. The primary efficacy endpoint was progression-free survival (PFS) in the intention-to-treat (ITT) population and a subset of patients with high proliferation breast tumors. PFS was defined as the time from randomization to progressive disease or death, whichever occurred first. Bidimensional diagnostic anatomic imaging (using computerized tomography or magnetic resonance imaging scans) was used to measure disease status at baseline and every 8 weeks during treatment and analyzed by independent central review, with local investigator review as supportive analysis. Bone scans were required for patients with skeletal metastases. Tumor responses were measured using enhanced RECIST v1.1 criteria [19].
Secondary efficacy end points included objective response rate (ORR) and overall survival (OS) in the ITT population. The additional nonrandomized lower dose ridaforolimus combination arms were analyzed using the same study end points as the randomized arms of the trial.
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Serious AEs were defined as any life-threatening AE, or AE resulting in death, persistent or significant disability, hospitalization, new type of cancer, overdose, or other events according to medical judgment. Adverse events known to occur with rapamycin analogs and selected for clinical interest were hyperglycemia, stomatitis, infusion-related reaction, and pneumonitis.
Statistical analysis
Part A was designed to randomly assign approximately 140 patients in a 1:1 ratio to either ridaforolimus 30 mg in combination with dalotuzumab or exemestane monotherapy. Part A was event driven, with a target of 90 PFS events, giving 67% power to detect a hazard ratio (HR) of 0.56 for ridaforolimus 30 mg plus dalotuzumab relative to exemestane, corresponding to an 80% improvement in median PFS (from 4.5 to 8.1 months).
An interim efficacy analysis was initially planned for approximately 4 weeks prior to the projected completion of enrollment for part A. If ridaforolimus plus dalotuzumab combination therapy demonstrated superiority compared with exemestane, either at interim analysis or at the final analysis of part A, enrollment would continue into part B of the trial. Superiority at interim analysis was defined as an HR for PFS of 0.73 based on 45 events, with a 70% confidence interval (CI) <1.
Part B, if opened, would randomly assign approximately 212 patients to ridaforolimus plus dalotuzumab, ridaforolimus monotherapy, or dalotuzumab monotherapy. Part B was to be event driven, with a target of 102 PFS events giving a power of 80% to detect an HR of 0.56, corresponding to an 80% improvement in median PFS.
Efficacy analysis was performed on the ITT population. PFS and OS were estimated using the Kaplan–Meier method with significance determined by the Cox proportional hazards model. The ORR was compared using the Miettinen and Nurminen method. Due to the early termination of the trial, no inferential testing was performed, and P values provided are for descriptive purposes only.
Results
Demographics and disease characteristics
In total, 115 postmenopausal women with locally advanced or metastatic ER-positive breast cancer were enrolled (Table 1). For part A, 29 patients were initially enrolled into the ridaforolimus 30 mg plus dalotuzumab arm and 33 into the exemestane arm. However, because of higher-than-expected rates of toxicity, enrollment was stopped. To explore lower and potentially more tolerable doses, subsequent patients were enrolled in a ridaforolimus 10 mg plus dalotuzumab arm (n = 26) and a ridaforolimus 20 mg plus dalotuzumab arm (n = 27). The overall median age was 62 years (range 33–87 years). Baseline characteristics were generally balanced across treatment groups, except that more patients in the exemestane arm (85%) had an ECOG performance status of 0 than patients in the ridaforolimus combination arms (58–67% across doses), and a slightly higher percentage of patients in the ridaforolimus combination arms (70–77% across doses) received prior radiation therapy compared with those in the exemestane arm (55%).
Table 1. Patient characteristics.
| n (%)a | RIDA 10 mg + DALO n = 26 | RIDA 20 mg + DALO n = 27 | RIDA 30 mg + DALO n = 29 | Exemestane n = 33 | Total N = 115 |
|---|---|---|---|---|---|
| Median age, years (range) | 63.5 (37–87) | 60 (34–77) | 60 (33–80) | 62 (40–82) | 62 (33–87) |
| Race | |||||
| White | 23 (88.5) | 26 (96.3) | 23 (79.3) | 26 (78.8) | 98 (85.2) |
| Asian | 1 (3.8) | 1 (3.7) | 5 (17.2) | 5 (15.2) | 12 (10.4) |
| Black or African American | 1 (3.8) | 0 | 1 (3.4) | 1 (3.0) | 3 (2.6) |
| Multiracial | 1 (3.8) | 0 | 0 | 1 (3.0) | 2 (1.7) |
| Centrally assessed Ki67 status | |||||
| Low | 8 (30.8) | 6 (22.2) | 8 (27.6) | 6 (18.2) | 28 (24.3) |
| High | 18 (69.2) | 21 (77.8) | 21 (72.4) | 27 (81.8) | 87 (75.7) |
| ECOG performance status | |||||
| 0 | 15 (57.7) | 18 (66.7) | 17 (58.6) | 28 (84.8) | 78 (67.8) |
| 1 | 10 (38.5) | 9 (33.3) | 12 (41.4) | 5 (15.2) | 36 (31.3) |
| 2 | 1 (3.8) | 0 | 0 | 0 | 1 (0.9) |
| Prior radiation | 20 (76.9) | 19 (70.4) | 22 (75.9) | 18 (54.5) | 79 (68.7) |
| Prior surgery | 22 (84.6) | 21 (77.8) | 24 (82.8) | 25 (75.8) | 92 (80.0) |
| Prior line of therapy | |||||
| Neoadjuvant | 6 (23.1) | 6 (22.2) | 5 (17.2) | 4 (12.1) | 21 (18.3) |
| Adjuvant | 7 (26.9) | 9 (33.3) | 10 (34.5) | 12 (36.4) | 38 (33.0) |
| First-line | 13 (50.0) | 11 (40.7) | 13 (44.8) | 16 (48.5) | 53 (46.1) |
| Second-line | 0 | 1 (3.7) | 1 (3.4) | 0 | 2 (1.7) |
| Not available | 0 | 0 | 0 | 1 (3.0) | 1 (0.9) |
DALO dalotuzumab, ECOG Eastern Cooperative Oncology Group, RIDA ridaforolimus
Unless otherwise specified
Patient disposition
Overall, the majority of patients discontinued the study because of progressive disease (59%), followed by AEs (20%)—particularly in the ridaforolimus 30 mg plus dalotuzumab arm—withdrawal of consent (10%) and physician decision (10%) (Table 2).
Table 2. Patient disposition.
| n (%) | RIDA 10 mg + DALO n = 26 | RIDA 20 mg + DALO n = 27 | RIDA 30 mg + DALO n = 29 | Exemestane n = 33 | Total N = 115 |
|---|---|---|---|---|---|
| Discontinued | 26 (100) | 27 (100) | 29 (100) | 33 (100) | 115 (100) |
| Adverse event | 3 (11.5) | 5 (18.5) | 9 (31.0) | 6 (18.2) | 23 (20.0) |
| Physician decision | 6 (23.1) | 2 (7.4) | 2 (6.9) | 1 (3.0) | 11 (9.6) |
| Progressive disease | 13 (50.0) | 20 (74.1) | 15 (51.7) | 20 (60.6) | 68 (59.1) |
| Protocol violation | 0 | 0 | 0 | 1 (3.0) | 1 (0.9) |
| Patient decision | 4 (15.4) | 0 | 3 (10.3) | 5 (15.2) | 12 (10.4) |
Each patient is counted once for trial disposition based on the latest corresponding disposition record
DALO dalotuzumab, RIDA ridaforolimus
Safety
All patients receiving combination therapy experienced ≥1 AE. The most commonly reported AEs in patients receiving ridaforolimus 30 mg plus dalotuzumab were stomatitis (89.7%), nausea (37.9%), fatigue (34.5%), decreased appetite (34.5%), hyperglycemia (31.0%), and dysgeusia (31.0%) (Table 3). The type and frequency of AEs were similar in the ridaforolimus 10 mg plus dalotuzumab and ridaforolimus 20 mg plus dalotuzumab combination arms compared with the ridaforolimus 30 mg plus dalotuzumab arm. Although the number of drug-related AEs and serious AEs was comparable across ridaforolimus dose regimens, and the number of patients who discontinued therapy because of an AE or drug-related AE appeared to decline with reduced ridaforolimus dose, the rate of stomatitis remained high (Table 3). The most common grade 3 AEs were stomatitis and hyperglycemia and occurred in all the ridaforolimus plus dalotuzumab arms. No patients receiving exemestane experienced stomatitis.
Table 3. Adverse event summary.
| n (%) | RIDA 10 mg = DALO n = 26 | RIDA 20 mg + DALO n = 27 | RIDA 30 mg = DALO n = 29 | Exemestane n = 33 |
|---|---|---|---|---|
| With ≥1 AE | 26 (100.0) | 27 (100.0) | 29 (100.0) | 31 (93.9) |
| Stomatitis | 21 (80.8) | 22 (81.5) | 26 (89.7) | 0 (0) |
| Nausea | 6 (23.1) | 8 (29.6) | 11 (37.9) | 3 (9.1) |
| Fatigue | 11 (42.3) | 11 (40.7) | 10 (34.5) | 7 (21.2) |
| Decreased appetite | 4 (15.4) | 11 (40.7) | 10 (34.5) | 0 (0) |
| Diarrhea | 8 (30.8) | 5 (18.5) | 9 (31.0) | 2 (6.1) |
| Dysgeusia | 8 (30.8) | 9 (33.3) | 9 (31.0) | 0 (0) |
| Hyperglycemia | 9 (34.6) | 6 (22.2) | 9 (31.0) | 0 (0) |
| Epistaxis | 4 (15.4) | 9 (33.3) | 7 (24.1) | 0 (0) |
| With drug-related AEs | 25 (96.2) | 27 (100.0) | 29 (100.0) | 22 (66.7) |
| Stomatitis | 21 (80.8) | 22 (81.5) | 26 (89.7) | 0 (0) |
| Fatigue | 11 (42.3) | 11 (40.7) | 10 (34.5) | 7 (21.2) |
| Dysgeusia | 8 (30.8) | 9 (33.3) | 9 (31.0) | 0 (0) |
| Decreased appetite | 4 (15.4) | 11 (40.7) | 10 (34.5) | 0 (0) |
| Nausea | 6 (23.1) | 8 (29.6) | 11 (37.9) | 3 (9.1) |
| Hyperglycemia | 9 (34.6) | 6 (22.2) | 9 (31.0) | 0 (0) |
| Diarrhea | 8 (30.8) | 5 (18.5) | 9 (31.0) | 2 (6.1) |
| Epistaxis | 4 (15.4) | 9 (33.3) | 7 (24.1) | 0 (0) |
| With serious AEs | 7 (26.9) | 7 (25.9) | 9 (31.0) | 5 (15.2) |
| With serious drug-related AEs | 4 (15.4) | 1 (3.7) | 5 (17.2) | 0 |
| Died | 0 | 1 (3.7) | 1 (3.4) | 1 (3.0) |
| Discontinued because of AE | 4 (15.4) | 6 (22.2) | 10 (34.5) | 1 (3.0) |
| Discontinued because of drug-related AE | 3 (11.5) | 4 (14.8) | 9 (31.0) | 1 (3.0) |
| Discontinued because of a serious AE | 2 (7.7) | 3 (11.1) | 2 (6.9) | 0 |
| Discontinued because of a serious drug-related AE | 2 (7.7) | 1 (3.7) | 1 (3.4) | 0 |
| AEs of clinical interest by grade | ||||
| Stomatitis | 22 (84.6) | 22 (81.5) | 26 (89.7) | 1 (3.0) |
| Grade 1 | 5 (19.2) | 1 (3.7) | 4 (13.8) | 1 (3.0) |
| Grade 2 | 15 (57.7) | 9 (33.3) | 12 (41.4) | 0 |
| Grade 3 | 2 (7.7) | 12 (44.4) | 10 (34.5) | 0 |
| Infusion-related reaction | 1 (3.8) | 3 (11.1) | 5 (17.2) | 0 |
| Grade 1 | 0 | 0 | 3 (10.3) | 0 |
| Grade 2 | 1 (3.8) | 3 (11.1) | 2 (6.9) | 0 |
| Hyperglycemia | 9 (34.6) | 8 (29.6) | 11 (37.9) | 1 (3.0) |
| Grade 1 | 0 | 3 (11.1) | 0 | 1 (3.0) |
| Grade 2 | 3 (11.5) | 4 (14.8) | 6 (20.7) | 0 |
| Grade 3 | 6 (23.1) | 1 (3.7) | 5 (17.2) | 0 |
| Pneumonitis | 0 | 1 (3.7) | 0 | 0 |
| Grade 2 | 0 | 1 (3.7) | 0 | 0 |
AE adverse event, DALO dalotuzumab, RIDA ridaforolimus
Discontinuations due to AEs and drug-related AEs for the ridaforolimus 30-, 20-, and 10-mg plus dalotuzumab combination therapy arms were 34.5 and 31.0%; 22.2 and 14.8%; and 15.4 and 11.5%, respectively. The incidence of drug-related AEs, serious AEs, and discontinuations due to an AE was higher among patients receiving ridaforolimus plus dalotuzumab compared with those receiving exemestane (Table 3).
Three deaths occurred during the study period: 1 from hepatic failure (unrelated to treatment) of a patient receiving ridaforolimus 20 mg plus dalotuzumab, 1 from malignant neoplasm progression (unrelated to treatment) of a patient receiving exemestane, and 1 from treatment-related meningism and pneumonia of a patient in the ridaforolimus 30 mg plus dalotuzumab combination arm.
Efficacy
Kaplan–Meier analyses of PFS for the ITT population (n = 115) based on independent radiologist review are shown in Fig. 1. The median PFS was 21.4 weeks in the ridaforolimus 30 mg plus dalotuzumab arm and 24.3 weeks in the exemestane arm (HR 1.00; 95% CI 0.46–2.19; P = 0.5; Fig. 1a). The median PFS for the ridaforolimus 10 mg plus dalotuzumab and 20 mg plus dalotuzumab combination arm was 23.3 and 25.7 weeks, respectively (Fig. 1b). Based on local investigator evaluation, median PFS was 16.0 weeks in the ridaforolimus 30 mg plus dalotuzumab combination arm and 15.4 weeks in the exemestane arm (HR 0.99; 95% CI 0.53–1.87; P = 0.492). The median PFS in the ridaforolimus 10- and 20-mg combination arm was 16.1 and 15.9 weeks, respectively.
Fig. 1.
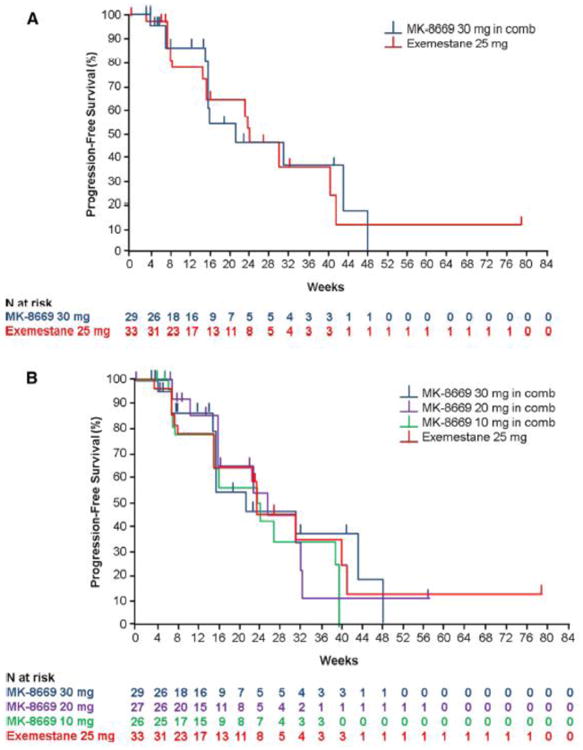
Kaplan-Meier estimate of progression-free survival by central radiologic review for the randomized arms of the study, ridaforolimus 30 mg qd × 5/week + dalotuzumab (n = 29) versus exemestane (n = 33) (a), and all 3 ridaforolimus + dalotuzumab dosing cohorts and exemestane (b)
In total, 72% of patients in the ridaforolimus 30-mg arm (21/29), 78% of those in the ridaforolimus 20-mg arm (21/27), and 69% of those in the ridaforolimus 10-mg arm (18/26) had high proliferation breast tumors with a Ki67 labeling index ≥15% (Table 1). Based on independent radiologist review, the median PFS in these patient populations was 15.9 weeks (95% CI 15.7–31.3 weeks), 22.7 weeks (95% CI 16.0–31.1 weeks), and 23.8 weeks (95% CI 16.0–39.6 weeks), respectively. The median PFS in patients in the exemestane arm with high proliferation breast tumors (82%, 27/33) was 23.6 weeks (95% CI 14.7–40.3 weeks) but was not statistically different from the ridaforolimus 30 mg plus dalotuzumab arm (HR 1.07; 95% CI 0.47–2.47; P = 0.565). Based on local investigator review, the median PFS in this patient population was 16.0 weeks (95% CI 15.1–21.4 weeks), 13.9 weeks (95% CI 8.1–22.3 weeks), and 16.1 weeks (95% CI 15.6–39.7 weeks) for the ridaforolimus 30-, 20-, and 10-mg treatment arms, respectively. The median PFS for patients receiving exemestane was 14.7 weeks (95% CI 8.1–25.0 weeks), but was not statistically different from the ridaforolimus 30-mg arm (HR 0.92; 95% CI 0.46–1.87; P = 0.414). Overall survival was similar between the 2 arms, with a median OS of 24.7 months for patients in the ridaforolimus 30 mg plus dalotuzumab arm and 24.9 months in the exemestane arm (HR 1.28; 95% CI 0.65–2.49; P = 0.763) (Fig. 2).
Fig. 2. Overall survival for randomized population, ridaforolimus 30 mg + Dalotuzumab (n = 29) versus exemestane (n = 33).
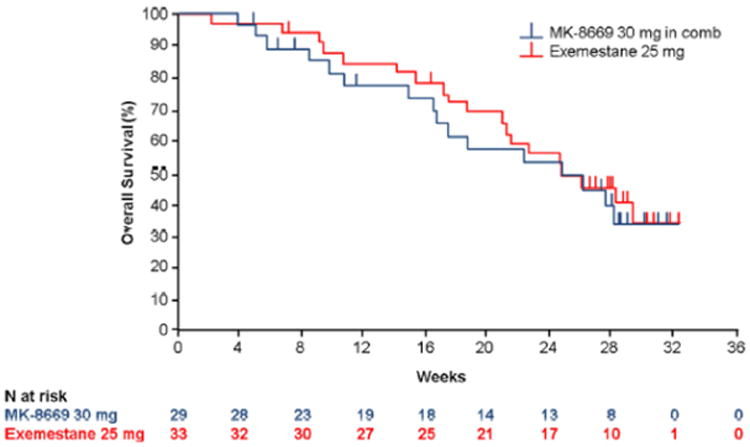
Table 4 lists the best overall tumor response by both independent central review and local investigator review. There were no complete responses in any group by either independent central or local investigator review. By independent review, there was 1 partial response (PR; 3.4%) in the ridaforolimus 30 mg combination therapy arm and none in the exemestane arm (P = 0.128). There were no PRs in the lower dose ridaforolimus arms by independent review. By local investigator review, there were 2 PRs (ORR 6.9%) in the ridaforolimus 30 mg plus dalotuzumab arm (ORR 3.0%), 1 PR in both the ridaforolimus 20 mg plus dalotuzumab (ORR 3.7%) and the ridaforolimus 10 mg plus dalotuzumab arms (ORR 3.8%), and 1 PR in the exemestane arm (P = 0.207).
Table 4. Summary of best overall tumor response.
| n (%) | RIDA 10 mg + DALO n = 26 | RIDA 20 mg + DALO n = 27 | RIDA 30 mg + DALO n = 29 | Exemestane n = 33 |
|---|---|---|---|---|
| Central radiology | ||||
| CR | 0 | 0 | 0 | 0 |
| PR | 0 | 0 | 1 (3.4) | 0 |
| SD | 10 (38.5) | 12 (44.4) | 7 (24.1) | 12 (36.4) |
| PD | 5 (19.2) | 2 (7.4) | 4 (13.8) | 7 (21.2) |
| NE | 1 (3.8) | 2 (7.4) | 4 (13.8) | 3 (9.1) |
| Local investigator | ||||
| CR | 0 | 0 | 0 | 0 |
| PR | 1 (3.8) | 1 (3.7) | 2 (6.9) | 1 (3.0) |
| SD | 18 (69.2) | 16 (59.3) | 19 (65.5) | 16 (48.5) |
| PD | 6 (23.1) | 9 (33.3) | 6 (20.7) | 13 (39.4) |
| NE | 0 | 0 | 0 | 0 |
Only confirmed responses are included
CR complete response, DALO dalotuzumab, NE not evaluable, PD progressive disease, PR partial response, RIDA ridaforolimus, SD stable disease
Discussion
This randomized, open-label, phase II study tested the addition of the anti-IGF1R antibody dalotuzumab to the mTOR inhibitor ridaforolimus for the treatment of post-menopausal patients with advanced ER-positive breast cancer. The results did not demonstrate any meaningful improvement in the PFS, OS, or ORR over exemestane. The primary objective was not met, and the study protocol was amended to investigate lower doses of ridaforolimus because of a high rate of toxicity in the original ridaforolimus 30 mg plus dalotuzumab treatment arm.
In patients receiving ridaforolimus 30 mg plus dalotuzumab, the median PFS was similar to that for exemestane monotherapy, based on both independent radiologist review and investigator evaluation. The OS and ORR were also similar between the randomized treatment arms, indicating that the 2 treatments have similar clinical efficacy in ER-positive, HER2-negative advanced breast cancer. The analysis of PFS in patients with high Ki67 labeling index did not show significant benefit for the ridaforolimus plus dalotuzumab combination compared with exemestane monotherapy.
The efficacy findings of the ridaforolimus plus dalotuzumab combination are in contrast to those observed in the phase I trial of the combination, which reported 3 PRs out of 11 patients (27%) with high proliferation ER-positive breast cancer [16]. Similarly, PFS was lower in this study than in the phase I trial (23.8–15.9 weeks vs >6 months). The PFS reported for both arms of the trial was also somewhat lower than that reported for the everolimus plus exemestane combination (approximately 8 months) in the BOLERO-2 trial, although a little higher than the 3 months reported for exemestane monotherapy in that trial [7, 22].
The most frequently reported drug-related AEs for patients receiving ridaforolimus 30 mg plus dalotuzumab were stomatitis, fatigue, dysgeusia, decreased appetite, nausea, hyperglycemia, and diarrhea, which are all expected AEs of ridaforolimus as an mTOR inhibitor. However, the very high levels of grade 3 stomatitis reported for the ridaforolimus 30 mg plus dalotuzumab treatment arm were unexpected. To investigate lower and potentially more tolerable doses of ridaforolimus, sequentially enrolled single-arm cohorts were treated with either ridaforolimus 20 or 10 mg in combination with dalotuzumab 10 mg/kg/week. The toxicity profile of these lower doses was generally similar to the 30 mg dose. Stomatitis, the most common AE associated with mTOR inhibitors and the primary toxicity concern at the 30 mg qd × 5 days/week dose, was also evident at lower ridaforolimus doses, though there appeared to be fewer patients with grade 3 stomatitis at the ridaforolimus 10 mg dose. Management strategies for patients undergoing treatment with mTOR inhibitors who develop this condition include patient education to aid early detection and topical management strategies or use of dose adjustments [23]. The incidence of serious AEs was similar across all ridaforolimus doses; however, there appeared to be a dose-related decrease in the number of patients who discontinued because of an AE (10 mg, 15.4%; 20 mg, 22.2%; 30 mg, 34.5%). This and the tendency toward a reduction in serious AEs suggest that there may be some dose-related toxicity associated with ridaforolimus, despite the similar incidence of other drug-related AEs and serious AEs across doses.
Overall, the incidence of drug-related, nonserious, and serious AEs was higher in patients treated with ridaforolimus combination therapy (any dose) than in patients receiving exemestane monotherapy. It is notable, however, that PFS was prolonged among patients with high-proliferation breast tumors treated with lower doses of ridaforolimus compared with the higher dose, and this suggests that patients treated with lower doses remained on therapy longer because of less toxicity and could be an indication of activity.
This study was discontinued early because of a higher-than-expected stomatitis rate in the ridaforolimus group. It is important to note the discordance in toxicity reported in the phase I trial compared with the present study that may be due to many factors. Phase I centers are specialized in developing new therapies and may be more familiar with monitoring and managing toxicities. At the time that the study was launched, it is conceivable that many of the participating centers would have had limited experience with mTOR inhibitor therapy. In the case of mTOR inhibitors, it has been shown that patient education, preventive measures, and early treatment with mouthwashes and/or lidocaine preparations help limit stomatitis and decrease dose interruptions [24, 25]. Potentially, the lack of familiarity of these centers with these measures may have resulted in higher toxicity and may partly explain the discrepancy in tolerability between phase I and phase II. Also, the lack of concordance of activity observed between the phase I study and the current study could be the result of patient selection bias or limited exposure to the experimental therapy.
Although the study was prematurely discontinued and the efficacy results considered inconclusive, the partial responses observed in this study suggest that the combination of ridaforolimus plus dalotuzumab does have anti-tumor activity in ER-positive breast cancer that has progressed after endocrine therapy, and this provides a rationale for further investigation in combination with endocrine therapy.
Another trial compared the combination of ridaforolimus 30 mg and exemestane 25 mg daily with ridaforolimus 10 mg/day, dalotuzumab 10 mg/kg/week, and exemestane 25 mg/day in 80 postmenopausal patients with high-proliferation, ER-positive breast cancer (NCT01605396). However, the study did not demonstrate superiority of the triple-combination arm compared with the ridaforolimus plus exemestane arm [26]. Based on this finding, further development of ridaforolimus and dalotuzumab with or without an aromatase inhibitor is not being pursued.
In summary, the present trial did not demonstrate its primary hypothesis that combination of ridaforolimus plus dalotuzumab would improve PFS over exemestane monotherapy in patients with ER-positive advanced breast cancer who have already developed progressive disease on previous endocrine therapy. The combination had similar efficacy to exemestane, although the recommended phase II dose, based on a phase I trial in patients with solid tumors, proved to be too toxic. Lowering the dose of ridaforolimus resulted in a modest reduction in the incidence of severe stomatitis, although the overall incidence of AEs remained high. Other combinations of mTOR/IGFR inhibitors are associated with similar tolerability issues [27, 28]; however, the antitumor activity suggested in this study indicates that this might be a sound strategy if tolerability concerns can be addressed.
Acknowledgments
Medical writing and editorial assistance was provided by Tim Ibbotson, PhD, of ApotheCom (Yardley, Pennsylvania, USA), with funding provided by Merck & Co., Inc., Kenil-worth, New Jersey, USA.
Funding: This work was supported by a grant from Merck & Co., Inc.
Footnotes
Aspects of this study have been previously presented at the following congresses: 2013 San Antonio Breast Cancer Symposium (SABCS); December 10-14, 2013; San Antonio, TX, USA and 2013 ASCO Annual Meeting; June 3-7, 2011; Chicago, IL, USA.
Compliance with ethical standards: Informed consent: Written informed consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Independent ethics committees reviewed and approved the protocol and applicable amendments for each institution.
Conflict of interest: AA has served on advisory boards for Bayer, Roche, and Lilly. JC has received personal fees for lectures and consulting from Roche, Celgene, Novartis, and Eisai. ME and ART received grants from Merck & Co., Inc., for the conduct of this study. HSR has received research support from Merck & Co., Inc., and Novartis. SE, CG, EI, MBJ, DM, and KP are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., and may hold stock or stock options in the company. The other authors have nothing to disclose.
References
- 1.Breast Cancer V1. National Comprehensive Cancer Network, Inc; 2014. [Accessed February 2, 2017]. The NCCN Clinical Practice Guidelines in Oncology™. 2014. http://www.nccn.org/ [Google Scholar]
- 2.Germano S, O'Driscoll L. Breast cancer: understanding sensitivity and resistance to chemotherapy and targeted therapies to aid in personalised medicine. Curr Cancer Drug Targets. 2009;9:398–418. doi: 10.2174/156800909788166529. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SR. Clinical efforts to combine endocrine agents with targeted therapies against epidermal growth factor receptor/human epidermal growth factor receptor 2 and mammalian target of rapamycin in breast cancer. Clin Cancer Res. 2006;12:1061s–1068s. doi: 10.1158/1078-0432.CCR-05-2125. [DOI] [PubMed] [Google Scholar]
- 4.Burstein HJ. Novel agents and future directions for refractory breast cancer. Semin Oncol. 2011;38(Suppl 2):S17–S24. doi: 10.1053/j.seminoncol.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 6.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elit L. Drug evaluation: AP-23573—an mTOR inhibitor for the treatment of cancer. IDrugs. 2006;9:636–644. [PubMed] [Google Scholar]
- 9.Rivera VM, Squillace RM, Miller D, et al. Ridaforolimus (AP23573; MK-8669), a potent mTOR inhibitor, has broad antitumor activity and can be optimally administered using intermittent dosing regimens. Mol Cancer Ther. 2011;10:1059–1071. doi: 10.1158/1535-7163.MCT-10-0792. [DOI] [PubMed] [Google Scholar]
- 10.Mita MM, Poplin E, Britten CD, et al. Phase I/IIa trial of the mammalian target of rapamycin inhibitor ridaforolimus (AP23573; MK-8669) administered orally in patients with refractory or advanced malignancies and sarcoma. Ann Oncol. 2013;24:1104–1111. doi: 10.1093/annonc/mds602. [DOI] [PubMed] [Google Scholar]
- 11.Chawla SP, Staddon AP, Baker LH, et al. Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas. J Clin Oncol. 2012;30:78–84. doi: 10.1200/JCO.2011.35.6329. [DOI] [PubMed] [Google Scholar]
- 12.Colombo N, McMeekin DS, Schwartz PE, et al. Ridaforolimus as a single agent in advanced endometrial cancer: results of a single-arm, phase 2 trial. Br J Cancer. 2013;108:1021–1026. doi: 10.1038/bjc.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 14.Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiCosimo S, Sathyanarayanan S, Bendell JC, et al. Combination of the mTOR inhibitor ridaforolimus and the anti-IGF1R monoclonal antibody dalotuzumab: preclinical characterization and phase I clinical trial. Clin Cancer Res. 2015;21:49–59. doi: 10.1158/1078-0432.CCR-14-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broussas M, Dupont J, Gonzalez A, et al. Molecular mechanisms involved in activity of h7C10, a humanized monoclonal antibody, to IGF-1 receptor. Int J Cancer. 2009;124:2281–2293. doi: 10.1002/ijc.24186. [DOI] [PubMed] [Google Scholar]
- 18.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yardley DA, Noguchi S, Pritchard KI, et al. Everolimus plus exemestane in postmenopausal patients with HR breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30:870–884. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yardley DA. Adverse event management of mTOR inhibitors during treatment of hormone receptor-positive advanced breast cancer: considerations for oncologists. Clin Breast Cancer. 2014;14:297–308. doi: 10.1016/j.clbc.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Boers-Doets CB, Raber-Durlacher JE, Treister NS, et al. Mammalian target of rapamycin inhibitor-associated stomatitis. Future Oncol. 2013;9:1883–1892. doi: 10.2217/fon.13.141. [DOI] [PubMed] [Google Scholar]
- 25.Seiler S, Kosse J, Loibl S, et al. Adverse event management of oral mucositis in patients with breast cancer. Breast Care. 2014;9:232–237. doi: 10.1159/000366246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rugo HS, Tredan O, Ro J, et al. Results from the phase 2 trial of ridaforolimus, dalotuzumab, and exemestane compared to ridaforolimus and exemestane in advanced breast cancer. Cancer Res. 2015;75:D5–1. doi: 10.1007/s10549-017-4375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quek R, Wang Q, Morgan JA, et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res. 2011;17:871–879. doi: 10.1158/1078-0432.CCR-10-2621. [DOI] [PubMed] [Google Scholar]
- 28.Sclafani F, Kim TY, Cunningham D, et al. A randomized phase II/III Study of dalotuzumab in combination with cetuximab and irinotecan in chemorefractory, KRAS wild-type, metastatic colorectal cancer. J Natl Cancer Inst. 2015;107:djv258. doi: 10.1093/jnci/djv258. [DOI] [PubMed] [Google Scholar]


