Abstract
Snowmelt, surface run-off or stormwater releases in urban environments can result in significant discharges of particulate matter-bound polycyclic aromatic hydrocarbons (PAHs) into aquatic environments. Recently, more specific activities such as road tunnel washing have been identified as contributing to contaminant load to surface waters. However, knowledge of PAH accessibility in particulate matter (PM) of urban origin that may ultimately be released into urban surface waters is limited. In the present study, we evaluated the accessibility of PAHs associated with seven distinct (suspended) particulate matter samples collected from different urban sources. Laboratory-based infinite sink extractions with silicone rubber (SR) as extractor phase demonstrated a similar pattern of PAH accessibility for most PM samples. Substantially higher accessible fractions were observed for the less hydrophobic PAHs (between 40 and 80 % of total concentrations) compared with those measured for the most hydrophobic PAHs (< 5 % of total concentrations). When focusing on PAHs bound to PM from tunnel wash waters, first-order desorption rates for PAHs with logKow > 5.5 were found in line with those commonly found for slowly or very slowly desorbing sediment-associated contaminants. PAHs with logKow < 5.5 were found at higher desorbing rates. The addition of detergents did not influence the extractability of lighter PAHs but increased desorption rates for the heavier PAHs, potentially contributing to increasing the toxicity of tunnel wash waters when surfactants are used. Implications of total and accessible PAH concentrations measured in our urban PM samples are discussed in a context of management of PAH and PM emission to the surrounding aquatic environment. Although only fully assessing PAHs in this work, further study should consider other contaminants such as OPAHs, which were also detected in all PM samples.
Graphical abstract
INTRODUCTION
Urban aquatic environments often represent a sink for particulate matter (PM), dirt and dust, or eroded surface particulate originating from roads and other paved and constructed areas. The sorption of hydrophobic organic contaminants to this PM can strongly affect their fate, distribution and ecotoxicological risk in the aquatic environment1–3. Urban run-off has been identified as an important source of polycyclic aromatic hydrocarbons (PAHs) to the aquatic environment4. The type of organic matter (OM) present in soils, sediment, or run-off waters from urban areas affects the extent of sorption of compounds such as PAHs, and ultimately their availability for transfer to the water column or into organisms. Exceptionally strong (ad)sorption to combustion-derived carbonaceous materials such as black carbon, soot or kerogen is responsible for organic carbon-water distribution coefficients significantly higher than those predicted from equilibrium partitioning between amorphous regions of OM and water2. Implications of this phenomenon are lower than expected and variable sediment-to-biota bioaccumulation factors, and limited potential for microbial biodegradation2. PAHs emitted together with this OM have been shown to be strongly bound, resulting in low pore water concentrations in sediments, a low proportion of chemicals in sediment available for partitioning, and slow to very slow desorption rates5–7. The risk posed by PM-associated contaminants in urban environments can be better assessed by estimating freely dissolved concentrations using passive sampling8–11, estimating the accessible concentration of contaminants12, or estimating contaminant desorption rates13.
Direct release through surface run-off and sewer and storm water networks following heavy rainfall represents an important pathway for this urban PM to the aquatic environment (e.g. rivers, lakes, or coastal areas)14. However in recent years, other more specific activities have been identified as potentially significant sources of PAHs to the urban aquatic environment. PM accumulated in snow over a long period of time during winter can be released during snowmelt into peak events15. In Oslo (Norway), trials of a snow-melting plant built on a barge using seawater to melt and process excess snow removed from urban areas are under way. Typical seasonal processing of hundreds of thousands of cubic meters of snow has the potential to discharge urban PM into fjord waters. Despite the ability of the plant to remove a significant proportion of suspended particulate matter (SPM) through sedimentation tanks and filters, the PM content of the effluent waters can still reach hundreds of milligrams per liter. Another sporadic yet significant source of PM-associated contaminants that has received increased attention in recent years is tunnel washing16. PM concentrations up to 23 g L−1 have been measured in wash waters from tunnels in Norway while PAH concentrations as high as 49 μg L−1 have been measured previously in tunnel wash waters17. Large volumes of water are used (ca 60 – 100 L water per meter in a two tube tunnel (two-lanes/tube) and depending on traffic density, tunnels may require washing up to 12 times a year. Tunnel wash waters containing detergents are commonly discharged into storm water retention basins. However, the majority of the Norwegian tunnels (~1000) do not have any treatment of the tunnel wash water, and are directly discharged into the local environment.
Data on PAH availability or accessibility in actual urban particulate matter that may reach aquatic environments remain scarce. Therefore, the present study aimed to assess the accessibility of PAHs present in seven road-impacted PM samples that would likely be discharged into the aquatic environment. These samples included (i) SPM from tunnel wash waters from two different tunnels in Norway, (ii) PM samples collected from mobile road sweepers collecting settled dust from the road prior to tunnel washing, (iii) SPM from the effluent waters of a snow-melting barge, (iv) SPM from the River Alna, an urban river in Oslo, and (v) marine sediments collected in the vicinity of the outlet pipe where tunnel wash water and seepage water from the Oslofjord sub-sea tunnel are discharged. The primary objective was to evaluate the rapidly desorbable or accessible concentration of PAHs in these PM samples using SR passive samplers. A secondary objective was to evaluate the effect of the detergent used for tunnel wash on PAH desorption from tunnel wash water SPM samples. During processing, several samples analyzed for oxygenated PAHs (OPAHs). Finally, implications of observed PAH accessibility are discussed for these particulate sources of contamination for the surrounding aquatic environments.
MATERIAL AND METHODS
Solvent and standards
An Elgastat Maxima HPLC deionization option 3 ultrapure water system was used to obtain ultrapure water. HPLC-grade dichloromethane and pentane were from Rathburn. HPLC-grade methanol was from Riedel-de-Haen, and HPLC-grade cyclohexane was from J.T. Baker. Sodium azide was obtained from Sigma-Aldrich. Standards for PAHs and their deuterated homologues from Chiron were of analytical-grade with purities of >99 % for PAHs and >99.5 % for deuterated PAHs. A full list of PAHs studied here and their respective logKow values can be found in Table SI-3. For OPAHs, standards with purity of 97% or higher were obtained from CDN Isotopes (Pointe-Claire, Quebec, Canada), Chiron (Trondheim, Norway) and Sigma-Aldrich, (St. Louis, MO).
Silicone rubber
AlteSil™ SR sheets purchased from Altec Products Ltd were initially Soxhlet extracted with ethyl acetate for 24 hours to remove oligomers and other impurities. Sheets of silicone rubber were then left to dry before being cut into pieces (mean of 4.67 g, RSD of 1.8 %, n = 81). A final clean of these passive samplers was undertaken by soaking in methanol for two consecutive periods of 24 hours. The samplers were then left to dry before storage in a clean glass jar until use. Five samplers were kept as preparation blanks.
Particulate matter sample collection
Seven urban PM samples collected from various road-impacted environments in and around Oslo (Norway) were evaluated in this study (Table 1 and further detail in SI). Samples were taken at different times of the year, but all will be expected to be impacted through traffic, road-associated and urban activities, and may ultimately reach the aquatic environment. The (S)PM samples include those from tunnel washing (Nordby and Granfoss tunnels), from mobile road sweepers (also from Nordby and Granfoss tunnels), from the Alna River, a snow-melting plant in Oslo, and a marine sediment sample from the Oslofjord. All samples were placed in clean glass jars and frozen at −20 °C upon collection.
Table 1.
Description of the seven particulate matter samples obtained from in and around Oslo (Norway)
| Sample ID | TUN1 | TUN2 | SWE1 | SWE2 | SPM | SNOW | SED |
|---|---|---|---|---|---|---|---|
| Sample type | SPM from tunnel wash waters | PM from mobile road sweepers | River SPM | SPM from effluent | Bottom sediment | ||
| Location | Nordby tunnel | Granfoss tunnel | Nordby tunnel | Granfoss tunnel | Alna River | Snow-melting plant | Oslo fjord |
| Sampling procedure | CFCa | CFCa | Grab | Grab | Sediment trap | CFCa | Grab |
| TOCb (%) | 9.4 | 8.4 | 1.3 | 1.0 | 4.7 | 8.6 | 0.20d |
| RCc (%) | 3.8 | 3.1 | 0.78 | 0.37 | 3.0 | 4.0 | 0.19d |
| ΣPAHse (μg g−1 dw) | 6.7 | 5.6 | 0.81 | 0.67 | 26 | 6.0 | 2.3 |
CFC: continuous- flow centrifugation;
total organic carbon content measured with Rock-Eval;
Refractory carbon content measured with Rock-Eval;
unreliable results due to low TOC value of the sediment;
Sum of 16 US EPA PAHs
Laboratory batch experiments
Non-exhaustive extractions were set-up to quantify the rapidly desorbing (or accessible) concentration of PAHs in the different PM samples. Depending on the material (coarse or fine), 3–16 g wet weight of PM was placed in 50 mL centrifuge tubes (previously cleaned in the furnace at 550 °C), and capped with aluminium-lined lids. Higher samples masses were used for the coarser materials because of the lower organic carbon contents. Samples were thawed and homogenized prior to sub-sampling, and ultrapure water (40 mL) was added to each tube. To limit PAH biodegradation during the test, sodium azide (NaN3) was added as biocide (final concentration of 100 g L−1). The volume of the SR was selected to ensure the extraction was not limited by the capacity of the passive sampler18. Centrifuge tubes were placed on an orbital shaker at 150 rpm (19–22 °C), and tubes were manually rotated daily. After two weeks, passive samplers were removed from solution with clean tweezers, rinsed with ultrapure water and wiped with a clean tissue before being placed in clean glass jars for solvent extraction. Visual inspection showed that minimal amounts of particles were left on the surface of the silicone samplers following retrieval from the centrifuge tubes. New clean passive samplers were placed in the centrifuge tubes for two additional weeks since four weeks were expected to be sufficient to measure the accessible PAH concentration in these PM samples18. Passive samplers from each centrifuge extraction tube were pooled for analysis. For selected assays, this manipulation was repeated with a third SR sampler and samplers analysed separately to evaluate how exhaustive the extraction procedure was. Extractions were prepared in triplicate for fine PM samples, while five replicates were used for the coarser PM samples.
For tunnel wash water samples (samples TUN1 and TUN2), extractions were performed as above, and also undertaken in the presence of different concentrations of detergent. The detergent TK 601 (Teknisk Kjemisk Produksjon AS, Norway), a commonly used detergent for tunnel washing was added at concentration levels of 0.5, 3 and 6 % (L/L). The detergent is composed of 1–4 % trisodium nitrilotriacetate, 1–5 % 2(2-butoxy ethoxy) ethanol, 1–5 % ethoxylated C12-C14 alcohols, and 1–5 % ethoxylated C9-C11 alcohols.
SR and particulate matter extraction
Passive samplers were extracted and analysed for PAHs. Passive samplers were cleaned in the laboratory by rinsing with ultrapure water and then dried using a clean paper tissue before soaking overnight in pentane (150 mL). Surrogate standards for PAHs (d8-naphthalene, d10-biphenyl, d8-acenaphthylene, d8-dibenzothiophene, d10-pyrene, d12-benz[a]anthracene and d12-perylene) were added during the first extraction step. This step was repeated with fresh pentane for another 12 hours. Extracts were combined and reduced under a stream of nitrogen and diluted by 50 % with dichloromethane for further clean-up by gel permeation chromatography with dichloromethane as mobile phase19. Extracts were further reduced to 50–100 μL before PAH analysis by GC/MS. SR samplers spiked with known amounts of PAHs were used to check recoveries for our extraction procedure. The first pentane extraction allowed the recovery on average of 96 % of PAHs (93–97 %) while the second extraction allowed the recovery of 4 % on average of PAHs (2.8–4.7 %). The third extraction resulted in proportion of PAHs recovered in the range 0.12–0.63 % indicating that the pentane-based PAH extraction was quantitative. Spiked SR samplers (180–270 ng sampler−1 of individual PAHs) analysed with every batch of SR analysis were used to evaluate Interbatch variability in extraction and analysis of PAHs from SR. The median of absolute deviations for individual PAHs between the measured concentrations in the spiked sample and the mean values from all measurements undertaken from this batch of spiked samplers was 9.1 % (range of 1.3–30 %).
Results of our participation to the Quasimeme DE-13 passive sampler intercomparison exercise for which a total of 21 labs reported data showed our measurements are robust (Z-scores for PAH concentrations measured in field-exposed silicone rubber passive sampler were ~0 for 4 compounds, < 1 for 6 compounds, < 2 for 2 substances and < 3 for one compound).
For the measurement of total PAH concentrations in PM samples, an extraction of 1–2 g PM was undertaken with dichloromethane (20 mL) in an ultrasonic bath (Branson 5510, Frequency: 40 kHz) for 120 min. Surrogate standards for PAHs (d8-naphthalene, d10-biphenyl, d8-acenaphthylene, d8-dibenzothiophene, d10-pyrene, d12-benz[a]anthracene and d12-perylene) were added during the first extraction step. This step was repeated with fresh dichloromethane (20 mL) for 60 min. Extracts were combined and traces of water removed with Na2SO4. Extracts were further reduced and cleaned-up by gel permeation chromatography. Extracts were reduced using nitrogen before GC/MS analysis. A certified reference material, SRM-1944 (NIST) was analysed together with the samples. The median of deviations between measured concentrations and certified values for all PAHs of interest was 15 % (range of 0 to 61 %).
GC/MS analysis
Analysis was on a Agilent 7890A gas chromatograph (GC) linked to an Agilent 5975c inert XL EI/CI mass spectrometer (MS) operated in single ion monitoring mode (SIM) with electron impact ionisation (70 keV). Separation was on a DB-5MS column (30 m, 0.25 mm i.d. and 0.25 μm film thickness, Agilent JW Scientific) following a pulsed splitless injection (1 μL injection, pulse pressure 20 psi for 1.2 min, injector temperature of 300 °C). The helium carrier gas flow was set to 1.2 mL min−1. The GC oven temperature programme started with a step at 60 °C (held for 2 min) before an increase to 250 °C (at the rate 7 °C min−1), followed by an increase to 310 °C (at the rate of 15 °C min−1) with this temperature held for 5 min. Temperatures for the ion source, quadrupole and transfer line were set to 230, 150 and 280 °C, respectively. The relative response of surrogate standards and 7-point calibration curves were used for the quantification. A deviation of the qualifier/quantifier ion response under 20 % was used for identification. Limits of detection for SR sampler extracts and for particulate matter samples were 5 ng sampler−1 and 2 ng g−1 dry weight PM (except for naphthalene wih a value of 10 ng g−1).
Supporting parameters
Grain size distribution was obtained using laser granulometry (Figure SI-1). Rock-Eval analysis was conducted to estimate the total organic carbon (TOC) content of the PM samples and to provide further information on the nature of the organic carbon (Table SI-2).
RESULTS AND DISCUSSION
Total PAH concentrations
Total PAH concentrations varied by up to three orders of magnitude between the different particulate samples (Tables 1 and SI-3). The sum of 16 US EPA PAHs ranged from 0.67 μg g−1 for a mobile road sweeper sample to a maximum of 26 μg g−1 for the River Alna SPM. PAH concentrations in Samples TUN1 and TUN2 (SPM from tunnel wash waters) were very similar despite being from different tunnels where key factors such as traffic, driving speed, type of vehicle or time interval since last wash may affect PAH content of the PM. As described in SI, the only major difference between the two tunnels is the cleaning frequency of the Granfoss tunnel twice as high as that of the Nordby tunnel. PAH concentrations in PM samples from the two mobile road sweepers were also comparable, but a factor of 5–10 below those found for TUN1 and TUN2. This is not surprising since sweeper samples were coarser (Figure SI-1) and had correspondingly lower TOC content (Table SI-2). When total PAH concentrations are normalized to TOC content, no major differences between tunnel washing samples can be seen (Table SI-4). Incidentally, TOC-normalized concentrations in SPM from the snow-melting plant are also comparable to tunnel wash samples. PAH concentrations for the Alna River SPM remain higher than those from tunnel washing. On a TOC-normalized basis, Oslofjord sediments are generally present at the highest PAH concentrations, particularly for the lighter PAHs. However this may be the result of the low and uncertain organic carbon content of that sediment sample.
PAH levels measured in SPM from tunnel wash waters in our study are of similar order of magnitude as concentrations reported previously20–22. Indeed, Oda et al. (2001)21 found concentrations of individual PAHs in the range of 0.14–1.1 μg g−1 for road soil sampled inside a tunnel with 30 000 cars/day usage. However, they measured higher PAH concentrations (0.2–6.9 μg g−1) in tunnel dust collected from the guard rail, most as a result of sampling of finer (soot) particles with higher concentrations. PAH concentrations in fine road dust (< 2 μm) measured by Rogge et al. (1993)20 were slightly higher (0.16–9.4 μg g−1). PAH concentrations in the Alna River SPM sample (25.7 μg g−1) are relatively high when compared with PAH concentrations measured in SPM from rivers with strong urban influence23–25. Fluoranthene and pyrene concentrations in the Alna River SPM (5 and 4.1 μg g−1, respectively) were one order of magnitude higher than the average of concentrations found by Zhou et al. (2008)25 in SPM in rivers in the United Kingdom. Stout et al. (2004)23 reported that PAH concentrations in 96 % of 280 bed sediment samples from urban-influenced environments were below 20 μg g−1. The specific release of snow-associated PM during snowmelt could be responsible for the high PAH concentrations measured bound to the Alna River SPM.
PAH diagnostic ratios
Diagnostic ratios of PAHs can be used to trace the possible source of PAH contamination26–28. Selected diagnostic ratios are plotted on Figure SI-2 and represent some of the most commonly used PAH diagnostic ratios. First, it is interesting to note that most of these ratios are able to separate samples RIV and SED (Alna River SPM and Oslofjord bottom sediments) from the other PM samples. All four samples from the tunnel washing (from tunnel wash waters and road sweepers) are generally grouped together for most PAH diagnostic ratios (Table SI-2). This is perhaps not surprising since PAH contamination in tunnel and snowmelter samples may be less weathered than for the river SPM and fjord sediments. While traffic intensity is low for the Oslofjord tunnel (that impact the fjord sediment sample), the proportion of heavy vehicles and is high compared with the other two tunnels and this could affect PAHs profiles.
The fluoranthene/(fluoranthene+pyrene) ratios (FL/(FL+PYR)) between 0.33 and 0.38 are slightly lower than expected for road dust and samples from road tunnels27. However, this ratio may be lower if PAH input also included sources such as used engine oil or diesel oil, diesel combustion which show FL/(FL+PYR) ratio 0.30–0.3927. The low indeno[1,2,3-cd]pyrene/(indeno[1,2,3-cd]pyrene+benzo[ghi]perylene) ratios (IP/(IP+BghiP)) for tunnel samples TUN1, TUN2, SWE1 and SWE2 (0.15–0.20) could be indicative of gasoline combustion and are lower than those found previously for tunnel samples27. Ratios of benz[a]anthracene/(benzo[a]anthracene+chrysene) (BaA/(BaA+CHRY)) > 0.35 may be representative of vehicular emission28. Tunnel samples showed ratios in the range 0.33–0.35 (Figure SI-2). Sample SED shows a much lower BaA/(BaA+CHRY) ratio indicating a possible petrogenic source of contamination, possibly related to bitumen in asphalt or car tires. The benzo[a]pyrene/benzo[ghi]perylene ratios (BaP/BghiP) for tunnel samples (0.16–0.38) would be indicative of non-traffic related sources according to Katsoyiannis et al. (2007)29. Ratios of anthracene over phenanthrene (ANT/(ANT+PHE)) below 0.1 for tunnel samples and SNW (from the snowmeltingfacility) are indicative of traffic-related PAH emissions26. A benzo[a]pyrene/(benzo[a]pyrene+benzo[e]pyene) ratio (BaP/(BaP+BeP)) of 0.5 is expected for freshly emitted particles while ratios lower than 0.5 would be indicative of ageing as a result of preferential photolysis of BaP28. A ratio slightly closer to 0.5 can be seen for the SPM from the Alna River indicating input of freshly emitted particles. For all other PM samples, ratios are much lower than 0.5. These diagnostic ratios generally show that PAHs present in these PM samples do not originate from single emission sources, but instead are the result of multiple traffic and road-related sources. Diagnostic ratios appear to efficiently group and distinguish tunnel PM samples from other samples.
PAH accessibility
Non-exhaustive extractions were conducted using SR. All passive sampler-PM exposures, except for the Oslofjord sediment, resulted in PAH concentrations well above quantification limits. We expected that extraction conditions resulted in sufficient capacity of the passive samplers to extract over 90 % (see Supporting information) of the accessible fraction of PAHs able to desorb within the experimental time frame18. In addition, depletive conditions were assured by exchanging passive samplers after two weeks. For Sample TUN1, SR samplers were used in three consecutive exposures and were analysed separately. The largest amounts of individual PAHs with logKow < 5.5 were extracted during the first SR exposure (on average 63 %) with decreasing amounts absorbed over the second (23 %) and third exposures (14 %). For more hydrophobic PAHs, this was not the case. On average, the first to last extractions of more hydrophobic PAHs (log Kow> 5.5) were 20, 45, 35 %, respectively.
The proportion of PAH extracted with SR from PM presented in Table SI-5 and shown graphically on Figure 1 were calculated as the ratio of the PAH mass extracted by SR per gram of PM (dry weight) ove the total PAH concentration in PM measured by exhaustive extraction. These ranged from a few percent for the highest molecular weight PAHs (e.g. benzo[ghi]perylene) to values approaching 80 % for acenaphthene and fluorene in TUN1. Notably, most PM samples (TUN1, TUN2, SWE1, SWE2, RIV and SNW) show a similar PAH extractability. For sample SED, the Oslofjord bottom sediments, passive sampler extractions demonstrated a very low PAH accessibility (see Table SI-5). Relative standard deviations (% RSD) of the accessible concentration measurements ranged from 1.7 to 72 % across PAHs and PM samples (n = 3 or 5). The average of RSDs calculated for each PAH for each PM sample were 38, 13, 20, 10, 14, 18 and 15 % for TUN1, TUN2, SWE1, SWE2, and SNW, respectively. For acenaphthene and fluorene, the fraction extracted is between 50 and 80 % of the total concentrations for most PM samples. The accessible concentration of dibenzothiophene, phenanthrene, and anthracene amounts to approximately half of the total PM concentrations. For fluoranthene, the extractable fraction can vary from 22 % for SPM from the Alna River to 64 % for particles from Nordby tunnel wash water. The extractable fraction for benz[a]anthracene and pyrene is between 16 and 50 %. Finally for higher molecular weight PAHs, the accessible concentration reduced to below 15 % of the total PM concentrations. No more than 5 % of benzo[ghi]perylene could be extracted from any of the samples. Overall these results generally indicate a substantial accessibility of lower molecular weight PAHs in most of these road-impacted PM. The desorbable concentration appears generally lower for naphthalene and acenaphthylene than for other PAHs with logKow > 5.5. The rapidly desorbing fraction of these volatile substances may have been reduced as a result of particle ageing, volatilization following particle emission or desorption into water following their release into washwaters. With increasing compound’s hydrophobicity, the extractable concentration of PAHs decreases, reaching values below 15 % of the total concentration for the most hydrophobic PAHs (logKow > 6.0). Smedes et al. (2012)18 found accessible concentrations of PAHs in sediment a factor of 2 to 10 lower than total PAH concentrations, in agreement with our results. Our results are also in agreement with the strong PAH sorption observed in soot-water systems30, 31. Jonker and Koelmans (2001)30 found organic carbon-water distribution coefficients (logKoc) in the range of 6.24–8.89 for PAHs in traffic soot. They also estimated that the fraction of PAHs unavailable for partitioning/equilibrium distribution in this traffic soot-water system ranged from 50 % for phenanthrene to 85 % for benzo[ghi]perylene. This is consistent with our results. For the low MW PAHs, data from successive extractions of sample TUN1 indicate that the desorbing fraction generally becomes exhausted. In these conditions, the fraction obtained with two successive sampler extractions provides acceptable estimates (86 % on average for the lower MW PAHs of the three consecutive extractions) of the fraction of these particle-bound compounds that is available for partitioning. Since such depletion is not observed for the higher MW PAHs, it is possible that in TUN1, the operationally-defined rapidly desorbing fraction is small and the measurement we undertake here is mostly of PAHs in the slowly or very slowly desorbing fraction13, 31, 32. According to Poot et al. (2014)6, the percentage of refractory carbon in sample TUN1 would tend to support this.
Figure 1.
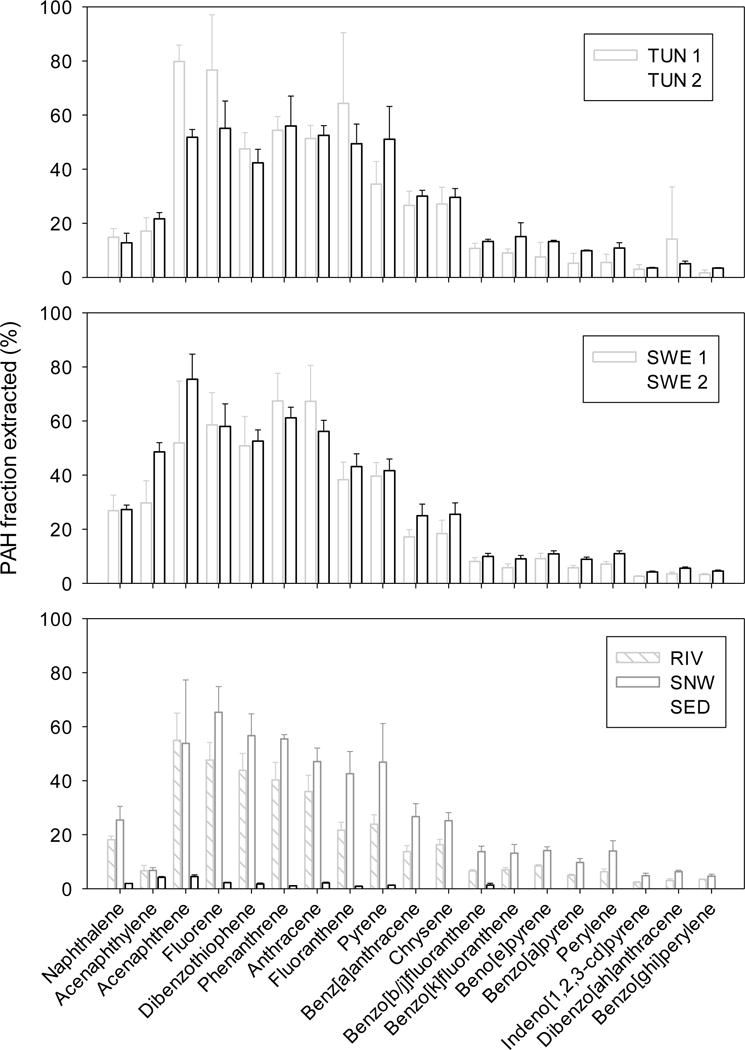
Fraction of PAHs extracted with two consecutive SR extractions from seven PM samples from various road-impacted aquatic environments (including for TUN1). Note that for Sample SED (Oslofjord sediment), masses absorbed into silicone for PAHs with logKow > 5.5 that were below LOQ are not shown. For fluoranthene, only data from duplicate extractions are used.
We must mention procedural artefacts that can influence our results. First, continuous-flow centrifugation (CFC) may not allow the sampling of the finest particles in suspension (e.g. < 1 μm). SPM samples from tunnel wash waters or from the snow-melting floating plant may not include the entire finest fraction of suspended particles. It is also possible that a PAH loss from the rapidly desorbing fraction occurs during sampling with CFC. When comparing PAH accessibility in samples from tunnel wash waters with those from mobile road sweepers, only minor differences in accessibility can be observed. Significantly more accessible naphthalene and acenaphthylene (and acenaphthene for Sample SWE2) is seen in Samples SWE1 and SWE2 from mobile road sweepers compared with respective samples from tunnel washwaters. The lower accessibility in tunnel washwater PM samples may be due to near instantaneous PAH desorption following the release of particles into washwaters.
Influence of tunnel wash detergent on PAH extractability
TUN1 and TUN2 were also subjected to non-exhaustive extraction in the presence of a detergent (TK 601) often used during tunnel wash operations in Norway. Accessible concentrations of PAHs obtained in the presence of TK 601 (volume ratios of 0.005, 0.03 and 0.06) relative to those measured without detergent are presented in Figure 2 (and in Tables SI-6 and SI-7). A ratio above 1 indicates an increase in PAH extractability with use of detergent. We calculated relative percent differences (RPD) based on duplicate measurements of accessible PAH concentrations in presence of detergent. These RPDs were low and in the range of 0.2–35 % (median of 6.6) and 1.6–38 % (median of 15 %) for samples TUN1 and TUN2, respectively across the three detergent concentrations (Tables SI-6 and SI-7). For TUN1, the dispersion of the data appears significantly lower for the extractions in the presence of detergent (Table SI-6). For TUN2, data dispersion in absence or presence of detergent is similar. The use of detergent does not seem to influence the extractability of lighter PAHs (i.e. from naphthalene to pyrene), with ratios between 0.60 and 1.18 (and an average of 1.0 and 0.96 for TUN1 and TUN2, respectively for PAHs with logKow < 5.5). For the more hydrophobic PAHs, the addition of detergent resulted in increased extractability. This increase was more pronounced for the more hydrophobic PAHs, reaching an increase of a factor of 8 for benzo[ghi]perylene for TUN1 when using 3 % of TK 601. However, no clear pattern of relative increase in PAH extractability can be seen for the different detergent concentrations. For TUN1, the 3 % concentrations appeared to provide the maximum enhancement in extractability. For sample TUN2, the two higher detergent concentration levels (3 and 6 %) resulted in similar PAH extractability, but clearly higher than those obtained with 0.5 % detergent.
Figure 2.
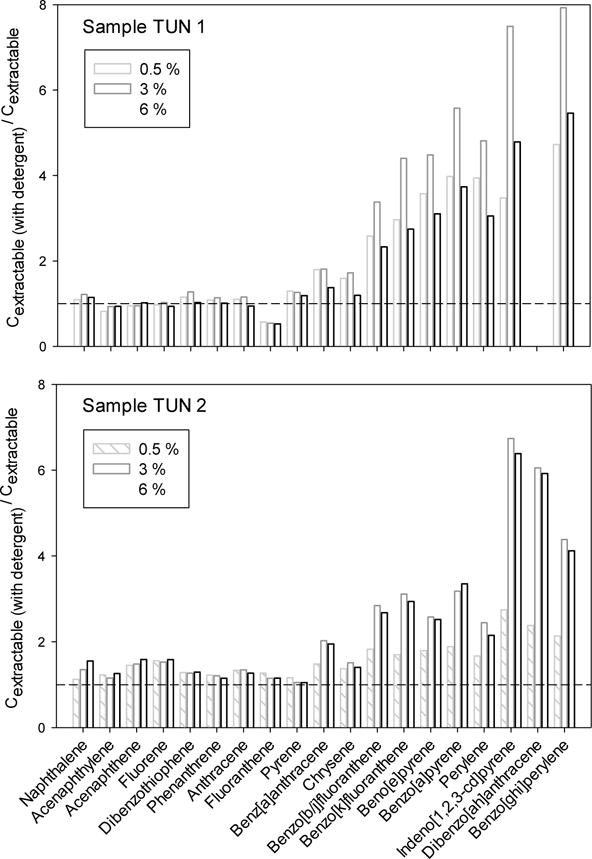
Accessible PAH concentration estimated using SR in the presence of 0.5, 3 and 6 % (v/v) of detergent relative to extractions without detergent
It is generally well-known that the addition of surfactants to soil and sediment slurries can promote the desorption of hydrophobic contaminants such as PAHs from soils and sediments33, 34. Allan et al. (2007)33 showed that hydropropyl-β-cyclodextrin (HPCD) addition to contaminated soil slurries was able to enhance the fraction of PAHs available to degrading bacteria in these soils. These conclusions were supported by a study by Zhu and Aitken (2010)34 who used other types of surfactants. Such enhancements were observed for all PAHs (except for naphthalene) in their study. We only see an enhancement for the higher MW PAHs. The contaminant mobilization effect of TK 601 does not appear to be significant for the lighter PAHs. Cuypers et al. (2002)35 demonstrated that the use of TritonX-100 surfactant for the extraction of PAHs from two sediments did not result in appreciably different residual sediment concentrations for three-ring PAHs, from those obtained through either Tenax® extraction, HPCD or through biodegradation. TritonX-100 was, however, able to enhance the extractability of 4–6 ring PAHs considerably. This is in agreement with our data. More recently, tests involving the use of an infinite absorptive sink (e.g. SR) and a mobilizing agent (cyclodextrin or digestive fluids) have been proposed for the measurement of bioaccessibility and applied to PAH in wood or fuel soot36, 37. The detergent in our study or mobilizing agent likely enhances the solubility of the most hydrophobic PAHs in water and facilitates the transfer into the silicone. It is also possible that the detergent has an impact on the physico-chemical characteristics of the PM samples, enabling a PAH fraction to desorb that would otherwise not be available for partitioning into water.
The results from extractions with addition of detergent have some procedural implications. The addition of detergent tends to increase the release of the more hydrophobic PAHs. At the same time it does not influence low molecular weight PAH amounts absorbed by SR samplers. These results confirm that the volume of SR used in this experiment was sufficient to achieve depletive conditions. The low accessibility of more hydrophobic PAHs in these PM samples is not an artefact of the extraction test using SR.
Apparent first-order PAH desorption rates
For TUN1, passive samplers from the first, second and third exposures were extracted separately. It is therefore possible to calculate apparent first-order PAH desorption rates (kd) for the 6 week-long assays conducted without and with 6 % detergent. Apparent desorption curves with first-order kinetics were generally good with R2 in the range 0.69 to 1.00 (with the mean of R2 across all substances and treatments > 0.90). Examples of desorption curves with and without the presence of detergent (6 %) are presented in Figure SI-3 for selected PAHs across a range of logKow values. Mean logkd (n = 2 and 3 for experiment with and without detergent) are reported in Table SI-8 and presented in Figure 3. As expected, kd values appear to decrease with increase PAH molecular weight or hydrophobicity38. The logarithm of desorption rates (h−1) ranged from −2.47 for fluorene to −4.4 for benzo[ghi]perylene. Desorption rates for the more hydrophobic PAHs correspond generally well with literature values of slow to very slow desorption rates of PAHs from sediments or from industrial sites6, 39. For naphthalene and acenaphthylene, desorption rates were lower than expected (Table SI-8). This could indicate, as mentioned previously, earlier losses of these volatile substances from the rapidly desorbing pool of the PM due to ageing. PAH desorption curves obtained using Tenax® extractions13, 32, 40 can generally be modelled through an initial rapid desorption stage followed by periods with slow and very slow desorption rates with the rapidly desorbing stage expected to be complete after 30 hours40. This means the desorption rates we report may be a mixture of rapid and slow desorption rates. At the same time, Jonker et al. (2005)32 found that the rapidly-desorbing fraction of PAHs in traffic or oil soot were extremely low. This is supported by the high refractory carbon content of the tunnel wash SPM (Table SI-2). Barnier et al. (2014)39 also found small rapidly-desorbing fractions for PAHs in industrial soils. In addition, the size of the slowly desorbing fraction relative to the rapidly desorbing fraction tends to increase with increasing compound’s hydrophobicity13. This implies that the effect of the mixture of desorption rates will tend to have less effect on the more hydrophobic PAHs. Poot et al. (2014)6 demonstrated that the size of the very slowly-desorbing PAH fraction in European riverbed sediments increases with increasing amount of residual carbon as measured by Rock-Eval. The proportion of Rock-Eval-measured RC in TUN1 was in the range of values by Poot et al. (2014)6, indicating that much of the PAHs are likely present in the slowly to very slowly-desorbing pool of contaminants.
Figure 3.
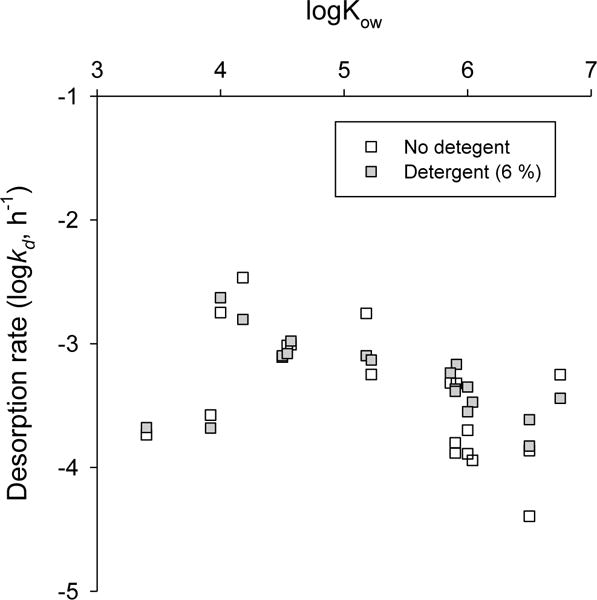
Logarithm of PAH desorption rates for tunnel wash PM, TUN1 (h−1) in the presence and absence of detergent
Adding the detergent at a 6 % concentration level did not appear to result in increased desorption rates for the least hydrophobic PAHs (see Table SI-8). For more hydrophobic PAHs (from benz[a]anthracene to benzo[ghi]perylene), desorption rates in the presence of detergent are consistently higher than those obtained with water only. Bueno-Montes et al. (2011)41 also found that adding the surfactant Brij 35 to already-bioremediated soil suspensions was able to increase the fraction of desorbing PAHs.
Implications
Different procedures exist for the measurement of contaminant availability and accessibility in soils and sediments. These include the estimation of freely dissolved concentrations, of contaminant desorption rates, or the estimation of an accessible fraction/concentration9, 12, 18, 40. It becomes apparent from our data that the optimal extraction time for the estimation of an accessible concentration is somewhat operational. It is likely to vary with sediment characteristics and the way the extraction is conducted. In our case, a four to six-week long extraction of tunnel wash water SPM appears to result in the extraction of a proportion of the more hydrophobic PAHs in the slowly-desorbing fraction that cannot be considered directly available for partitioning. The rapidly-desorbing concentration for these substances is likely to be very low and this is supported by the high % RC measured by Rock-Eval6.
We evaluated the accessibility of PAHs in a range of urban particulate matter that may ultimately find their way into the aquatic environment. Despite the varied sources and methods for obtaining these PM samples, similar trends in accessible concentrations of PAHs could be observed, and this information can be helpful to those in charge of urban water management. A large proportion of less hydrophobic PAHs is likely capable of desorption when such PM is released into urban aquatic environments. For the more hydrophobic compounds, only a very small proportion will be available for partitioning into the dissolved phase. According to our kd estimates, months, if not years will be needed for desorption of 99 % of sorbed higher MW PAHs. The use of detergent during tunnel washing may affect the accessibility of the more hydrophobic particle-bound PAHs particularly when wash waters are transferred to retention ponds where the detergent-containing waters are kept for periods of weeks before discharge into the environment.
One interesting question that we may be able to answer is whether particle emission into rivers through stormwater discharge or snowmelt has the potential to increase freely dissolved concentrations in urban rivers. We have undertaken some rudimentary modelling to evaluate to what extent PAH accessibility measured in PM in the present study can affect freely dissolved concentrations when this PM is emitted to rivers (see supporting information). Modelling based on accessible concentrations shows that PM release resulting in a SPM content in the Alna River of 45–480 mg L−1 (actual range measured in 2003, Table SI-9) has the potential to increase freely dissolved concentrations by a factor of 10–1000. While this assumes that the accessible concentration measured here can desorb near instantaneously, it may not be the case since most of the desorbing hydrophobic PAHs are likely in the slowly or very slowly desorbing fraction6. Similar modelling based instead on desorption rates measured here (for tunnel wash PM) and a PM residence time of 10 hours in the river demonstrates that lower fluctuations in freely dissolved concentrations of the more hydrophobic PAHs may be expected upon release of this PM into the river Alna. For a relatively short urban river such as the Alna (16 km long), the release of urban PM into the river may not be a crucial driver of the freely dissolved concentration of PAHs. For such a transfer of PAHs to be possible, a higher activity of PAHs on the particulate matter than in water is needed to ensure PAH desorption can occur. In our experiment, we assume the driving force for desorption was maximal while under environmental conditions it will not be. More accurate in situ measurements and modelling is required to evaluate this.
However, this work has only considered several PAHs. Many more substances including other polycyclic aromatic substances (e.g. OPAHs) are present in urban PM are likely to find their way into water42, 43. Preliminary analyses of OPAHs in PM material from this study show seven different OPAHs (Table SI-10), with at least 5 OPAHs present in each sample. Anthraquinone was typically seen at or near the highest concentrations (41–191 ng g−1 dw), which is consistent with other OPAH data in river water44 and urban stream sediments45. Further work should therefore aim to evaluate the release of these chemicals in urban aquatic environments. Since the organic carbon in most of the PM samples evaluated here consisted of a substantial amount of refractory carbon as measured with Rock-Eval, the emission of this PM into rivers will provide an additional sorption phase with strong sorption capacity, particularly for planar substances3. This may ultimately act to reduce freely dissolved concentrations of contaminants in waters to which this PM is emitted.
Supplementary Material
Acknowledgments
We acknowledge the Norwegian Public Roads Administration NPRA for funding this study (the R&D programme Nordic Road Water (NORWAT, www.vegvesen.no/norwat). We would like to thank Kjersti Wike Kronvall and Turid Hertel-Aas from the NPRA for their input throughout this project. Sigurd Øxnevad (NIVA) is thanked for providing the Oslofjord. Our gratitude also goes to NCC for enabling the collection and use of a SPM sample from the effluent of their snow-melting plant. Harry Veld (Deltares, NL) is thanked for Rock-Eval analyses.
Footnotes
SUPPORTING INFORMATION
Details of particulate matter sampling and characterisation (Tables SI-1 and 2; Figure SI-1), total PAH concentrations (Tables SI-3 and 4), PAH diagnostic ratios (Figure SI-2), accessible PAH concentrations in the absence/presence of detergent (Tables SI-5, 6 and 7), PAH desorption rates (Figure SI-3 and Table 8), modeling of the influence of PM release on freely dissolved riverine concentrations of PAHs (Figure SI-4 and Table SI-9) and preliminary accessible OPAH concentrations (Table SI-10).
References
- 1.Warren N, Allan I, Carter J, House W, Parker A. Pesticides and other micro-organic contaminants in freshwater sedimentary environments—a review. Applied Geochemistry. 2003;18(2):159–194. [Google Scholar]
- 2.Cornelissen G, Gustafsson Ö, Bucheli TD, Jonker MT, Koelmans AA, van Noort PC. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environmental Science & Technology. 2005;39(18):6881–6895. doi: 10.1021/es050191b. [DOI] [PubMed] [Google Scholar]
- 3.Koelmans AA, Jonker MT, Cornelissen G, Bucheli TD, Van Noort P, Gustafsson Ö. Black carbon: the reverse of its dark side. Chemosphere. 2006;63(3):365–377. doi: 10.1016/j.chemosphere.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman EJ, Mills GL, Latimer JS, Quinn JG. Urban runoff as a source of polycyclic aromatic hydrocarbons to coastal waters. Environmental science & technology. 1984;18(8):580–587. doi: 10.1021/es00126a003. [DOI] [PubMed] [Google Scholar]
- 5.Jonker MT, van der Heijden SA, Kotte M, Smedes F. Quantifying the Effects of Temperature and Salinity on Partitioning of Hydrophobic Organic Chemicals to Silicone Rubber Passive Samplers. Environmental science & technology. 2015 doi: 10.1021/acs.est.5b00286. [DOI] [PubMed] [Google Scholar]
- 6.Poot A, Jonker M, Gillissen F, Koelmans AA. Explaining PAH desorption from sediments using Rock Eval analysis. Environmental Pollution. 2014;193:247–253. doi: 10.1016/j.envpol.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 7.Accardi-Dey A, Gschwend PM. Reinterpreting literature sorption data considering both absorption into organic carbon and adsorption onto black carbon. Environmental science & technology. 2003;37(1):99–106. doi: 10.1021/es020569v. [DOI] [PubMed] [Google Scholar]
- 8.Booij K, Hoedemaker JR, Bakker JF. Dissolved PCBs, PAHs, and HCB in pore waters and overlying waters of contaminated harbor sediments. Environmental Science & Technology. 2003;37(18):4213–4220. doi: 10.1021/es034147c. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg MS, Chapman PM, Allan IJ, Anderson KA, Apitz SE, Beegan C, Bridges TS, Brown SS, Cargill JG, McCulloch MC. Passive sampling methods for contaminated sediments: Risk assessment and management. Integrated environmental assessment and management. 2014;10(2):224–236. doi: 10.1002/ieam.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allan IJ, Ruus A, Schaanning MT, Macrae KJ, Næs K. Measuring nonpolar organic contaminant partitioning in three Norwegian sediments using polyethylene passive samplers. Science of the Total Environment. 2012;423:125–131. doi: 10.1016/j.scitotenv.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Ruus A, Allan IJ, Øxnevad S, Schaanning MT, Borgå K, Bakke T, Næs K. In vivo bioaccumulation of contaminants from historically polluted sediments—relation to bioavailability estimates. Science of the Total Environment. 2013;442:336–343. doi: 10.1016/j.scitotenv.2012.10.060. [DOI] [PubMed] [Google Scholar]
- 12.Reichenberg F, Mayer P. Two complementary sides of bioavailability: accessibility and chemical activity of organic contaminants in sediments and soils. Environmental Toxicology and Chemistry. 2006;25(5):1239–1245. doi: 10.1897/05-458r.1. [DOI] [PubMed] [Google Scholar]
- 13.Cornelissen G, van Noort P, Govers HA. Desorption kinetics of chlorobenzenes, polycyclic aromatic hydrocarbons, and polychlorinated biphenyls: sediment extraction with Tenax® and effects of contact time and solute hydrophobicity. Environmental Toxicology and Chemistry. 1997;16(7):1351–1357. [Google Scholar]
- 14.Taebi A, Droste RL. Pollution loads in urban runoff and sanitary wastewater. Science of the total environment. 2004;327(1):175–184. doi: 10.1016/j.scitotenv.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Westerlund C, Viklander M. Transport of total suspended solids during snowmelt–influence by road salt, temperature and surface slope. Water, air, and soil pollution. 2008;192(1–4):3–10. [Google Scholar]
- 16.Meland S, Borgstrøm R, Heier LS, Rosseland BO, Lindholm O, Salbu B. Chemical and ecological effects of contaminated tunnel wash water runoff to a small Norwegian stream. Science of the Total Environment. 2010;408(19):4107–4117. doi: 10.1016/j.scitotenv.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Meland S. Management of road runoff among European National Road Administrations - Compliance with the Water Framework Directive?. 12th Urban Environment Symposium; Oslo (Norway). 1–3 June 2015; Submitted. [Google Scholar]
- 18.Smedes F, van Vliet LA, Booij K. Multi-ratio equilibrium passive sampling method to estimate accessible and pore water concentrations of polycyclic aromatic hydrocarbons and polychlorinated biphenyls in sediment. Environmental science & technology. 2012;47(1):510–517. doi: 10.1021/es3040945. [DOI] [PubMed] [Google Scholar]
- 19.Allan IJ, Harman C, Ranneklev SB, Thomas KV, Grung M. Passive sampling for target and nontarget analyses of moderately polar and nonpolar substances in water. Environmental Toxicology and Chemistry. 2013;32(8):1718–1726. doi: 10.1002/etc.2260. [DOI] [PubMed] [Google Scholar]
- 20.Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BR. Sources of fine organic aerosol. 2. Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks. Environmental science & technology. 1993;27(4):636–651. [Google Scholar]
- 21.Oda J, Nomura S, Yasuhara A, Shibamoto T. Mobile sources of atmospheric polycyclic aromatic hydrocarbons in a roadway tunnel. Atmospheric Environment. 2001;35(28):4819–4827. [Google Scholar]
- 22.Göbel P, Dierkes C, Coldewey W. Storm water runoff concentration matrix for urban areas. Journal of contaminant hydrology. 2007;91(1):26–42. doi: 10.1016/j.jconhyd.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Stout SA, Uhler AD, Emsbo-Mattingly SD. Comparative evaluation of background anthropogenic hydrocarbons in surficial sediments from nine urban waterways. Environmental science & technology. 2004;38(11):2987–2994. doi: 10.1021/es040327q. [DOI] [PubMed] [Google Scholar]
- 24.Cailleaud K, Forget-Leray J, Souissi S, Hilde D, LeMenach K, Budzinski H. Seasonal variations of hydrophobic organic contaminant concentrations in the water-column of the Seine Estuary and their transfer to a planktonic species Eurytemora affinis (Calanoida, copepoda). Part 1: PCBs and PAHs. Chemosphere. 2007;70(2):270–280. doi: 10.1016/j.chemosphere.2007.05.095. [DOI] [PubMed] [Google Scholar]
- 25.Zhou JL, Fileman TW, Evans S, Donkin P, Llewellyn C, Readman JW, Mantoura RFC, Rowland SJ. Fluoranthene and pyrene in the suspended particulate matter and surface sediments of the Humber Estuary, UK. Marine Pollution Bulletin. 1998;36(8):587–597. [Google Scholar]
- 26.Dvorská A, Lammel G, Klánová J. Use of diagnostic ratios for studying source apportionment and reactivity of ambient polycyclic aromatic hydrocarbons over Central Europe. Atmospheric Environment. 2011;45(2):420–427. [Google Scholar]
- 27.Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S. PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry. 2002;33(4):489–515. [Google Scholar]
- 28.Tobiszewski M, Namieśnik J. PAH diagnostic ratios for the identification of pollution emission sources. Environmental Pollution. 2012;162:110–119. doi: 10.1016/j.envpol.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Katsoyiannis A, Terzi E, Cai QY. On the use of PAH molecular diagnostic ratios in sewage sludge for the understanding of the PAH sources. Is this use appropriate? Chemosphere. 2007;69(8):1337–1339. doi: 10.1016/j.chemosphere.2007.05.084. [DOI] [PubMed] [Google Scholar]
- 30.Jonker MT, Koelmans AA. Polyoxymethylene solid phase extraction as a partitioning method for hydrophobic organic chemicals in sediment and soot. Environmental science & technology. 2001;35(18):3742–3748. doi: 10.1021/es0100470. [DOI] [PubMed] [Google Scholar]
- 31.Jonker MT, Koelmans AA. Extraction of polycyclic aromatic hydrocarbons from soot and sediment: solvent evaluation and implications for sorption mechanism. Environmental science & technology. 2002;36(19):4107–4113. doi: 10.1021/es0103290. [DOI] [PubMed] [Google Scholar]
- 32.Jonker MT, Hawthorne SB, Koelmans AA. Extremely slowly desorbing polycyclic aromatic hydrocarbons from soot and soot-like materials: evidence by supercritical fluid extraction. Environmental science & technology. 2005;39(20):7889–7895. doi: 10.1021/es0505191. [DOI] [PubMed] [Google Scholar]
- 33.Allan IJ, Semple KT, Hare R, Reid BJ. Cyclodextrin enhanced biodegradation of polycyclic aromatic hydrocarbons and phenols in contaminated soil slurries. Environmental science & technology. 2007;41(15):5498–5504. doi: 10.1021/es0704939. [DOI] [PubMed] [Google Scholar]
- 34.Zhu H, Aitken MD. Surfactant-enhanced desorption and biodegradation of polycyclic aromatic hydrocarbons in contaminated soil. Environmental science & technology. 2010;44(19):7260–7265. doi: 10.1021/es100112a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuypers C, Pancras T, Grotenhuis T, Rulkens W. The estimation of PAH bioavailability in contaminated sediments using hydroxypropyl-β-cyclodextrin and Triton X-100 extraction techniques. Chemosphere. 2002;46(8):1235–1245. doi: 10.1016/s0045-6535(01)00199-0. [DOI] [PubMed] [Google Scholar]
- 36.Gouliarmou V, Mayer P. Sorptive bioaccessibility extraction (SBE) of soils: combining a mobilization medium with an absorption sink. Environmental science & technology. 2012;46(19):10682–10689. doi: 10.1021/es301515s. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Pignatello JJ, Tao S, Xing B. Bioacessibility of PAHs in Fuel Soot Assessed by an in Vitro Digestive Model: Effect of Including an Absorptive Sink. Environmental science & technology. 2015;49(6):3905–3912. doi: 10.1021/es505898v. [DOI] [PubMed] [Google Scholar]
- 38.van Noort P, Poot A, Koelmans AA. Analysis of organic contaminant desorption kinetic data for sediments and soils: Implications for the Tenax extraction time for the determination of bioavailable concentrations. Science of The Total Environment. 2014;490:235–238. doi: 10.1016/j.scitotenv.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Barnier C, Ouvrard S, Robin C, Morel JL. Desorption kinetics of PAHs from aged industrial soils for availability assessment. Science of The Total Environment. 2014;470:639–645. doi: 10.1016/j.scitotenv.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 40.Cornelissen G, Rigterink H, ten Hulscher DE, Vrind BA, van Noort P. A simple Tenax® extraction method to determine the availability of sediment‐sorbed organic compounds. Environmental Toxicology and Chemistry. 2001;20(4):706–711. [PubMed] [Google Scholar]
- 41.Bueno-Montes M, Springael D, Ortega-Calvo JJ. Effect of a nonionic surfactant on biodegradation of slowly desorbing PAHs in contaminated soils. Environmental science & technology. 2011;45(7):3019–3026. doi: 10.1021/es1035706. [DOI] [PubMed] [Google Scholar]
- 42.Forsberg ND, O’Connell SG, Allan SE, Anderson KA. Passive sampling coupled to ultraviolet irradiation: A useful analytical approach for studying oxygenated polycyclic aromatic hydrocarbon formation in bioavailable mixtures. Environmental toxicology and chemistry. 2014;33(1):177–181. doi: 10.1002/etc.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Layshock JA, Wilson G, Anderson KA. Ketone and quinone‐substituted polycyclic aromatic hydrocarbons in mussel tissue, sediment, urban dust, and diesel particulate matrices. Environmental Toxicology and Chemistry. 2010;29(11):2450–2460. doi: 10.1002/etc.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connell SG, McCartney MA, Paulik LB, Allan SE, Tidwell LG, Wilson G, Anderson KA. Improvements in pollutant monitoring: Optimizing silicone for co-deployment with polyethylene passive sampling devices. Environmental Pollution. 2014;193:71–78. doi: 10.1016/j.envpol.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Witter AE, Nguyen MH. Determination of oxygen, nitrogen, and sulfur-containing polycyclic aromatic hydrocarbons (PAHs) in urban stream sediments. Environmental Pollution. 2016;209:186–196. doi: 10.1016/j.envpol.2015.10.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


