Abstract
The penile and clitorial anatomy of four species of Talpid moles (broad-footed, star-nosed, hairy-tailed, and Japanese shrew moles) were investigated to define penile and clitoral anatomy and to examine the relationship of the clitoral anatomy with the presence or absence of ovotestes. The ovotestis contains ovarian tissue and glandular tissue resembling fetal testicular tissue and can produce androgens. The ovotestis is present in star-nosed and hairy-tailed moles, but not in broad-footed and Japanese shrew moles. Using histology, 3D reconstruction, and morphometric analysis, sexual dimorphism was examined in regard to a nine feature masculine trait score that included perineal appendage length (prepuce), anogenital distance, and presence/absence of bone.
The presence/absence of ovotestes was discordant in all four mole species for sex differentiation features. For many sex differentiation features, discordance with ovotestes was observed in at least one mole species. The degree of concordance with ovotestes was highest for hairy-tailed moles and lowest for broad-footed moles. In relationship to phylogenetic clade, sex differentiation features also did not correlate with the similarity/divergence of the features and presence/absence of ovotestes. Hairy-tailed and Japanese shrew moles reside in separated clades, but they exhibit a high degree of congruence. Broad-footed and hairy-tailed moles reside within the same clade but had one of the lowest correlations in features and presence/absence of ovotestes. Thus, phylogenetic affinity and the presence/absence of ovotestes are poor predictors for most sex differentiation features within mole external genitalia.
Keywords: Moles, external genitalia, penis, clitoris, prepuce
Introduction
Most eutherian mammals possess external genitalia that are sexually dimorphic. Males have a penis that is traversed to near the tip by a urethra, as well as a scrotum that encloses the testes. The typical clitoris is usually markedly smaller than the penis with the urethra exiting near the clitoris, not at its tip. In addition, the clitoris is associated with a vaginal opening (at least during the breeding season). Moles, ring-tailed lemurs (Lemur catta) and spotted hyenas (Crocuta crocuta) are extreme examples of masculinization of female external genitalia (Matthews, 1935; Drea and Weil, 2008; Cunha et al., 2014). For these species the terms penile clitoris and peniform clitoris have been used to describe their external genitalia. For spotted hyenas, and perhaps also the ring-tailed lemurs, these descriptive terms are fully justified, but not for moles (Sinclair et al., 2016b). Female moles have external genitalia that, upon visual examination, are similar in size and shape to that of the male. In fact, it can be difficult for the casual observer to distinguish between the sexes during the non-breeding season when the vaginal opening is “closed”.
External genitalia of European moles (Talpa europaea) were first described by Wood-Jones and Matthews (Matthews, 1935; Wood-Jones, 1914). The prominent perineal appendage in adult female moles was designated as clitoris or peniform clitoris by these investigators, while the prominent perineal appendage in adult male moles was designated as penis. These designations were based solely upon gross observations and not upon histology. This early terminology of mole external genitalia was adapted and perpetuated by subsequent investigators that have used additional terms to describe female mole external genitalia, namely: penile clitoris, peniform clitoris, phallus, phallic-like clitoris and female urinary papilla (Sinclair et al., 2016b). Recently, we employed histology to re-examine the anatomy of external genitalia of the North American broad-footed mole (Scapanus latimanus). Histology is particularly useful for distinguishing the components of male and female external genitalia such as prepuce, penis and clitoris, which each have a distinctive and unique histologic signature. By histological examination the perineal appendages of male and female broad-footed moles are the prepuce, which are covered externally with a hair-bearing epidermis and are completely devoid of erectile bodies and other penile/clitoral-specific features. The penis of the broad-footed mole lies deep within the preputial space and is an “internal organ” in the resting state. Likewise, the clitoris of the broad-footed mole is also an “internal organ” defined by a U-shaped clitoral epithelial lamina (Sinclair et al., 2016b) similar to that seen in mice (Weiss et al., 2012). Our new terminology clearly applies to the four species of moles examined in the current paper: (a) broad-footed moles (Scapanus latimanus), (b) star-nosed moles (Condylura cristata), (c) hairy-tailed moles (Parascalops breweri) and (d) Japanese shrew moles (Urotrichus talpoides).
Several, but not all, mole species having a “penile clitoris” also possess ovotestes containing an ovarian interstitial gland (Carmona et al., 2008; Mossman and Duke, 1973; Whitworth et al., 1999; Rubenstein et al., 2003; Matthews, 1935; Jiménez et al., 1988; Jiménez et al., 1993; Sánchez et al., 1996). In adult Spanish (Talpa occidentalis) and European moles (Talpa europaea), circulating levels of testosterone correlate with ovarian interstitial gland weight, with higher concentrations of testosterone present in sexually mature moles during the non-breeding season when the ovarian interstitial gland was largest (Jiménez et al., 1993; Zurita et al., 2003; Whitworth et al., 1999). The presence of ovotestes and its production of androgens are in accord with the possibility that the masculinized female external genitalia in these species are the result of androgens produced by the developing ovarian interstitial gland (Zurita et al., 2003; Whitworth et al., 1999) or the placenta. Applying our new terminology, we now have examined the external genitalia of broad-footed, star-nosed, hairy-tailed and Japanese shrew moles and commented on the presence or absence of ovotestes. Our study has confirmed that the perineal elevation in male and female broad-footed, star-nosed, hairy-tailed and Japanese shrew moles is prepuce. The penis and clitoris of these 4 species of moles are internally situated organs exhibiting a unique distinctive morphology. A prominent female prepuce is observed in mole species with or without ovotestes.
Methods
Animals
Observations reported in this paper were made on adult male and female moles obtained in both the breeding and non-breeding seasons. Immature animals were identified and excluded from this study. The numbers of animals examined are presented in Table 1.
Table 1.
Analysis of various mole species
| Species of Mole: | Breeding State at Collection: | Number of Adults Investigated: | |
|---|---|---|---|
| Males | Females | ||
|
| |||
| Broad-footed Mole (Scapanus latimanus) | Breeding | 2 | 3 |
|
| |||
| Non-Breeding | 5 | 5 | |
|
| |||
| Star-nosed Mole (Condylura cristata) | Non-Breeding | 4 | 5 |
|
| |||
| Hairy-tailed Mole (Parascalops breweri) | Non-Breeding | 2 | 2 |
|
| |||
| Japanese shrew Mole (Urotrichus talpoides) | Breeding | 2 | 2 |
Broad-footed mole (Scapanus latimanus)
Live broad-footed moles were obtained from golf courses in San Francisco and Moraga, California between 2008 and 2010. Moles were euthanized using 0.05 mL of Euthasol (Virbac Animal Health Inc.) diluted with saline. Animals were either perfused with 4% paraformaldehyde before dissection, or the lower torso was cut open to expose the reproductive organs and immersed in fixative.
Star-nosed mole (Condylura cristata) & Hairy-tailed mole (Parascalops breweri)
Star-nosed and hairy-tailed moles were trapped in Emporium, PA by Dr. Kenneth Catania of Vanderbilt University and Dr. Diana Bautista of University of California, Berkeley. All animals were caught in June or July, during the non-breeding season, and females did not display a perforate vagina in either species (Eadie and Hamilton, 1956; Eadie 1939). Moles were either euthanized and fixed immediately or were housed at Vanderbilt University for 1–3 months before euthanasia and fixed in 4% paraformaldehyde.
Japanese shrew mole (Urotrichus talpoides)
Japanese shrew moles were collected in Kagamisu, Miyazaki, Japan in March 2008 by Dr. Akio Shinohara from the University of Miyazaki, Japan. The animals were determined to be in a reproductively active state based on the perforate vagina in the females, the presence of developing sperm in the male testis and fully developed sperm in the epididymes.
After fixation and transfer to 70% ethanol the internal and external genitalia were photographed in situ after the hair surrounding the external genitalia had been plucked or trimmed under a dissecting microscope to allow a clear view of the external genitalia. Prepuce length was measured twice for each animal using electronic calipers to the nearest 0.01mm, from the tip of the phallus to where the mid portion of the structure connected with the body (Fig. 1), and the mean value for each individual was used for all further analysis. Anogenital distance (AGD) was also measured using electronic calipers from the center of the anus to the middle of the prepuce at the point where it connected to the body, and the mean value for each individual was used for all further analysis (Fig. 1). A reliable hairy-tailed mole AGD was not possible due to tail and immediate anal area being removed prior to shipment to our lab, the surrounding genital area and internal reproductive organs remained intact and undisturbed. Dissected external genitalia were embedded in paraffin, and 10μm sections were stained with hematoxylin and eosin. Three-dimensional reconstruction was carried out as described previously (Sinclair et al., 2016b). Morphometric analysis of internal and external features of the prepuce, penis and clitoris was carried out by determining the distance from the distal tip of the prepuce to the structure of interest.
Figure 1.
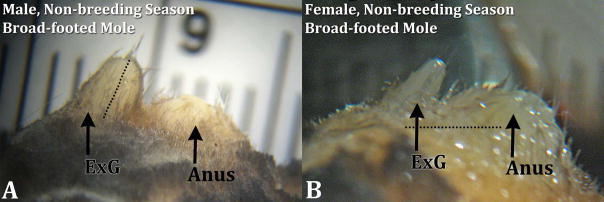
Side views of male (A) and female (B) broad-footed mole (Scapanus latimanus) external genitalia (ExG) from the non-breeding season. The size of male and female external genitalia is remarkably similar. The dotted line in the male image (A) depicts the measurement taken for prepuce length; the horizontal dotted line in the female image (B) depicts the measure taken for anogenital distance. For both images the cranial aspect is to the left.
Tfm mice
Adult XTfm/Y (N=3) and wild-type male (N=4) and female C57Bl/6 (N=4) mice were euthanized. The perineal region was treated with Nair © to remove hair on and around the perineal appendage (external prepuce). Side view photographs were taken with a digital camera with millimeter rule in the background. The photographs were used to measure the height to which the external prepuce projects above the body surface.
Results
Broad-footed moles
As described previously (Sinclair et al., 2016b), the penis of broad-footed moles resides within the preputial space, and is not attached or tethered to the prepuce in adults, except where the inner preputial epithelium reflects onto the penile surface deep within the preputial space. Thus, the penis is mobile and presumably capable of extending beyond the preputial meatus during mating and urination (Fig. 2). The penile urethra opens into the preputial space ~400μm from the distal tip of the penis. In contrast, the clitoris of the broad-footed mole is ventrally tethered to surrounding stromal tissue in proximal regions where the clitoris is represented as an inverted U-shaped epithelial lamina (Figs. 3C & 4B). Distally the epithelium of the clitoris is in contact with the epithelium of the prepuce, thus at no point does the clitoris lie within the preputial space unattached to the prepuce (Fig. 3).
Figure 2.
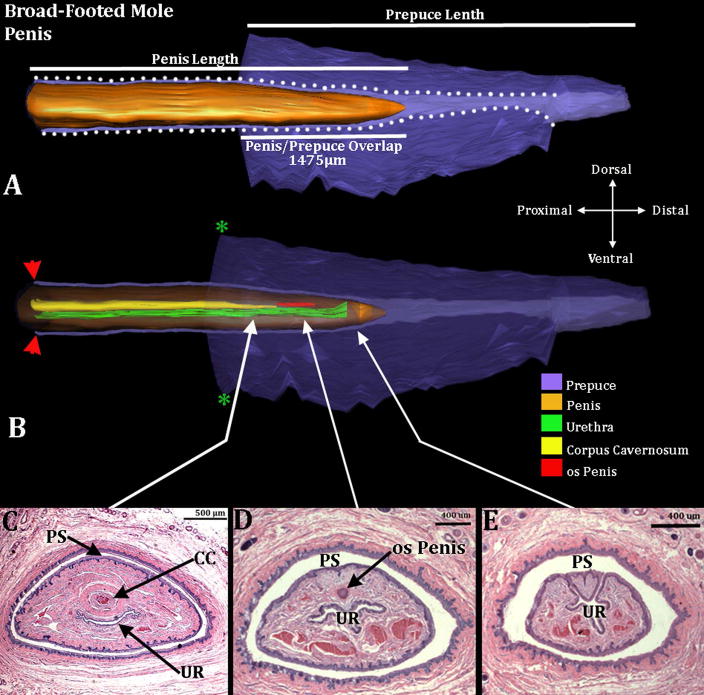
Three-dimensional reconstruction of the prepuce and penis of an adult broad-footed mole (Scapanus latimanus) with transverse sections depicting morphology at different locations within the penis. Three-dimensional reconstruction (A) the prepuce is semi-transparent with the penis opaque orange demonstrating the shape and the position of penis relative to the prepuce. The mole penis at rest is an “internal” organ housed within the preputial space (lighter purple region bordered by white dotted lines). Three white lines are labeled denoting the length of the external prepuce, the length of the penis and the length the penis/prepuce overlap. Three dimensional reconstruction (B) the prepuce and penis are rendered semi-transparent to show the internal morphology of the penis. The perineal appendage (prepuce) merges with the body surface (green asterisks). Red arrowheads denote the reflection of the inner preputial epithelium onto the surface of the penis. The distal end of the urethra (green) is the urethral meatus where urine/semen exits from the penis. Three transverse section images (C–E) depict the internal morphology of the penis from proximal to distal at differing morphological points. CC=Corpus Cavernosum, PS=Preputial Space, UR= Urethra. Note the morphometric measures mentioned in the text.
Figure 3.
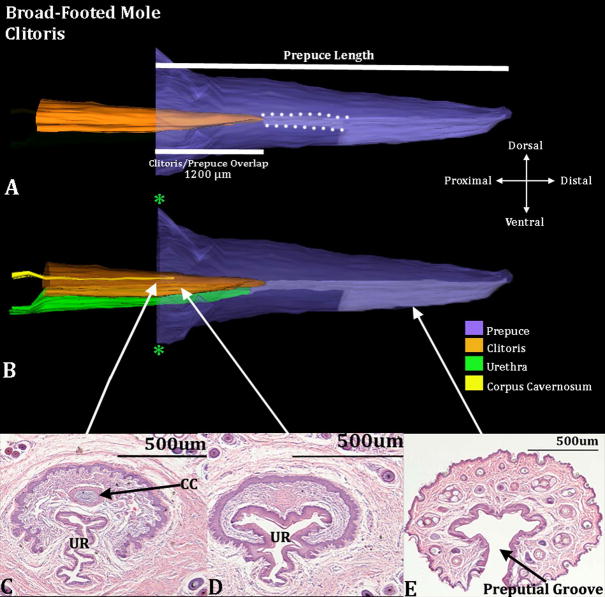
Three-dimensional reconstruction of the prepuce and clitoris of an adult broad-footed mole (Scapanus latimanus) with transverse sections depicting morphology at different locations within the clitoris. Three-dimensional reconstruction (A) the prepuce is semi-transparent with the clitoris opaque orange demonstrating the shape and the position of clitoris relative to the prepuce. The mole clitoris is housed within the prepuce (in purple); lighter purple depicts the preputial grove distally and when bordered by white dotted lines depicts the preputial space. The 2 white lines denote the prepuce length and the length of the clitoris/prepuce overlap. Three-dimensional reconstruction (B) the prepuce and clitoris are rendered semi-transparent to show the internal morphology of the clitoris. The perineal appendage (prepuce) merges with the body surface (green asterisks). Three transverse H&E section images (C–E) depict the morphology of the clitoris and prepuce at differing morphological points from proximal to distal. CC=Corpus Cavernosum, UR=Urethra. Note the morphometric measures mentioned in the text.
Figure 4.
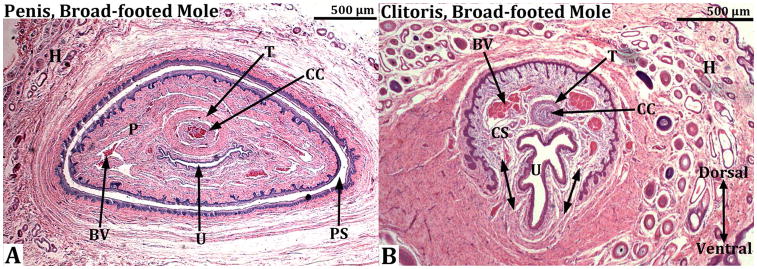
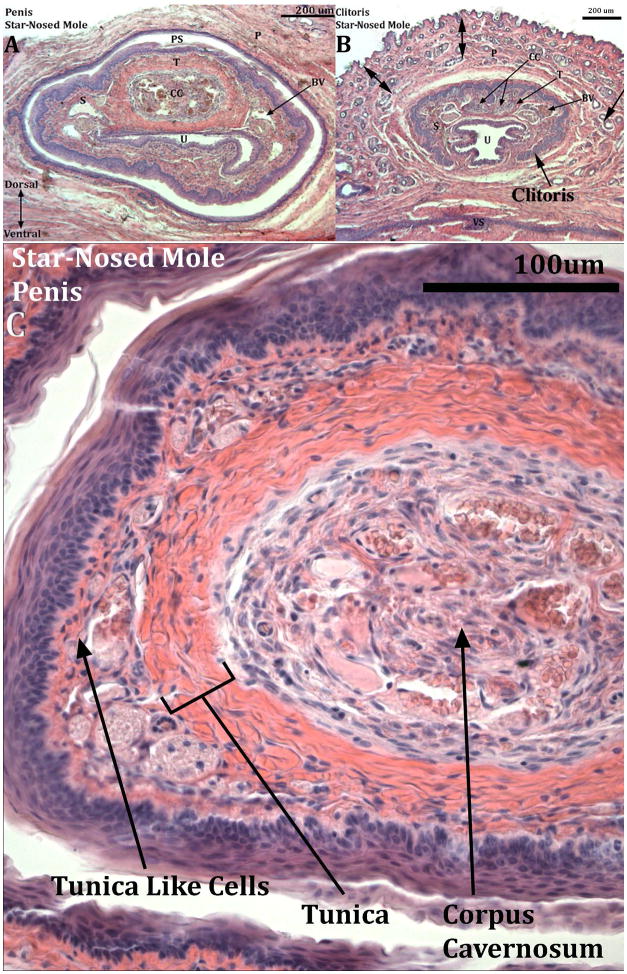
Transverse sections through the penis (A) and clitoris (B) of an adult broad-footed mole (Scapanus latimanus). In (A) note that the penis (P) resides within the preputial space (PS) and contains the urethra (U), the corpus cavernosum (CC) surrounded by the tunica albuginea (T), and blood-filled spaces (BV). The os penis is not present at the level of this section. In (B) the U-shaped clitoral lamina partially surrounds the urethra (U). Residing within the space defined by the clitoral lamina is the corpus cavernosum (CC) surrounded by the tunica albuginea (T) and blood filled spaces (BV). Note that clitoral stroma (CS) within the U-shaped clitoral lamina is in continuity ventrally with the surrounding stroma (double-headed arrows). Finally, the substance of the preputial wall contains hair follicles (H) in both (A) and (B). This image was taken from Sinclair et al., 2016b.
Prepuce Length
Female broad-footed moles possess a prominent hair-bearing prepuce similar in size and shape to the male prepuce (Fig. 1). Length of the adult female prepuce was not significantly different from that of adult males (Mean ± St.Dev. male=2.635 ±0.389mm, female=2.747 ±0.331mm) (p=0.406). In an attempt to control for body size as a confounding factor, prepuce length was normalized to either body weight or body length. When normalized to body weight, adult female prepuce length was slightly longer than adult male preputial length (p=0.038). When normalized to body length there was no difference in preputial length between males and females (p=0.844).
Penile/Preputial & Clitoral/Preputial Overlap
Penile/preputial or clitoral/preputial overlap denotes that portion of the penis or clitoris that projects beyond the body surface in the resting state and thus is situated within the male or female prepuce (Figs. 2A & 3A). Previous studies have measured the length of the prepuce and defined this as penile/clitoral length. A measure of penile/preputial or clitoral/preputial overlap reveals the extent to which the penis and clitoris are superimposed and associated with the external hair-bearing prepuce. This measure was obtained by counting serial sections from the distal tip of the penis or clitoris to the point where the external prepuce became contiguous with the body wall (green asterisks in figures 2B & 3B). Clitoral/preputial overlap in the broad-footed mole is on average 1200μm (St.Dev. =147) (Fig. 3), while penile/preputial overlap is 1475μm (St.Dev. =177) (Fig. 2). Thus, the portion of the penis or clitoris that projects beyond the body surface is not significantly different between the sexes (p=0.110).
Additionally the percent of penile/preputial or clitoral/preputial overlap was calculated relative to total preputial length. By this convention penile/preputial overlap represents less than half of total preputial length (41.07%, St.Dev. =0.12%) in adult male broad-footed moles (Fig. 2), while in female broad-footed moles clitoral/preputial overlap relative to total preputial length was 43.15% (St.Dev. =8.48%) (Fig. 3). The difference between males and females was not significant (p=0.761).
Internal Penile/Clitoral Composition
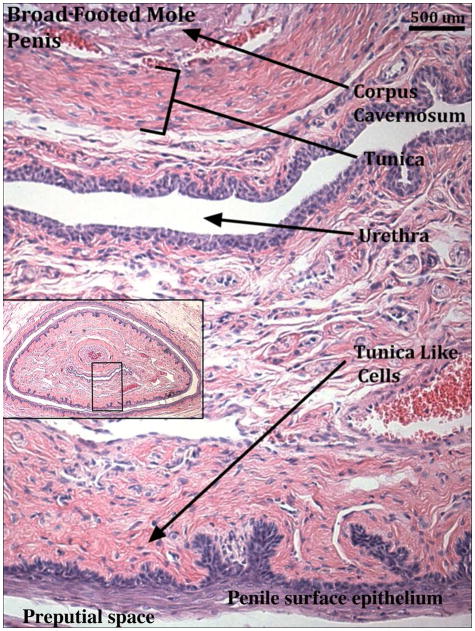
Broad-footed male moles possess an os penis (os penis mean length=450μm, St.Dev. =141), while females lack an os clitoris (Figs. 2–3). The penis and clitoris both possess a corpus cavernosum surrounded by a tunica (Figs. 2–4). In males the distal tip of the corpus cavernosum ends approximately 700μm from the distal tip of the penis (St.Dev. =141μm), while in females the corpus cavernosum ends 363μm from the distal tip of the clitoris (St.Dev. =95μm). The male urethra is completely enclosed within penile stroma and opens into the preputial space ~400μm from the tip of the penis (Fig. 2). In contrast, the female urethra is situated about 1/4 ventral to the clitoral epithelial lamina throughout its length but never fully resides within the space defined by the clitoral lamina (Figs. 3–4). A corpus spongiosum (human terminology) or corpora cavernosa urethrae (mouse terminology), erectile bodies associated with the urethra, do not exist in either male or female broad-footed moles. However, a complex network of blood vessels is present throughout the length of the penis and clitoris with males having a somewhat greater amount of blood vessels. We call this diffuse network of blood vessels the corpus cavernosum glandis of male and female moles (Fig. 4). Close examination of the skin of the penis revealed a dermis exceptionally rich in collagen fibers surrounding the corpus cavernosum glandis (Fig. 5). This dense band of stroma, lying just deep to the penile epithelium, may function in a manner similar to the tunica albuginea allowing for blood to fill these blood spaces and aid in rigidity of erection.
Figure 5.
Transverse H&E stained section of the adult broad-footed mole (Scapanus latimanus) penis displaying the band of tunica-like cells lying just deep to the penile epithelium. Black box in the inset image represents location of the magnified region in the penis.
Masculine Traits Score
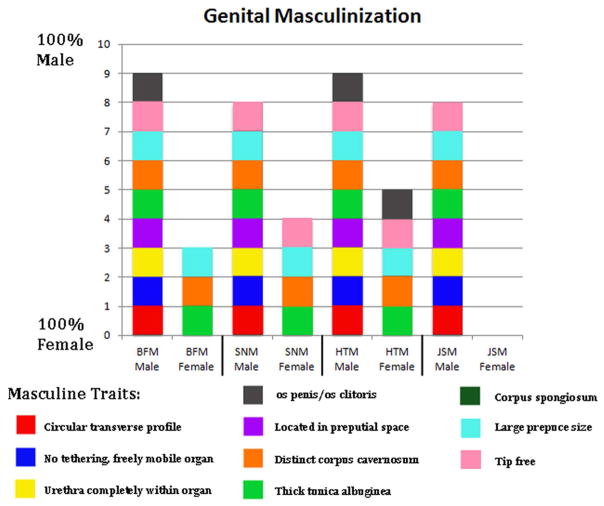
Nine homologous mole penile/clitoral morphologic features were used to construct a masculine traits score. The penis of the broad-footed mole possesses all 9 typical male features. The clitoris of this species possesses 3 out of the 9 typical male features, namely: (a) large prepuce size, (b) a distinct corpus cavernosum, and (c) the thick tunica surrounding the corpus cavernosum (Fig. 6).
Figure 6.
Graph depicting the level of genital “masculinization” according to the 9 defined morphological traits. BFM=broad-footed mole (Scapanus latimanus), SNM=star-nosed mole (Condylura cristata), HTM=hairy-tailed mole (Parascalops breweri), JSM=Japanese shrew mole (Urotrichus talpoides).
Star-nosed moles
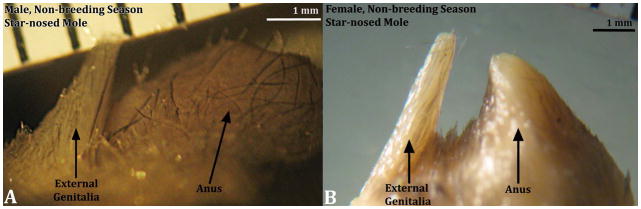
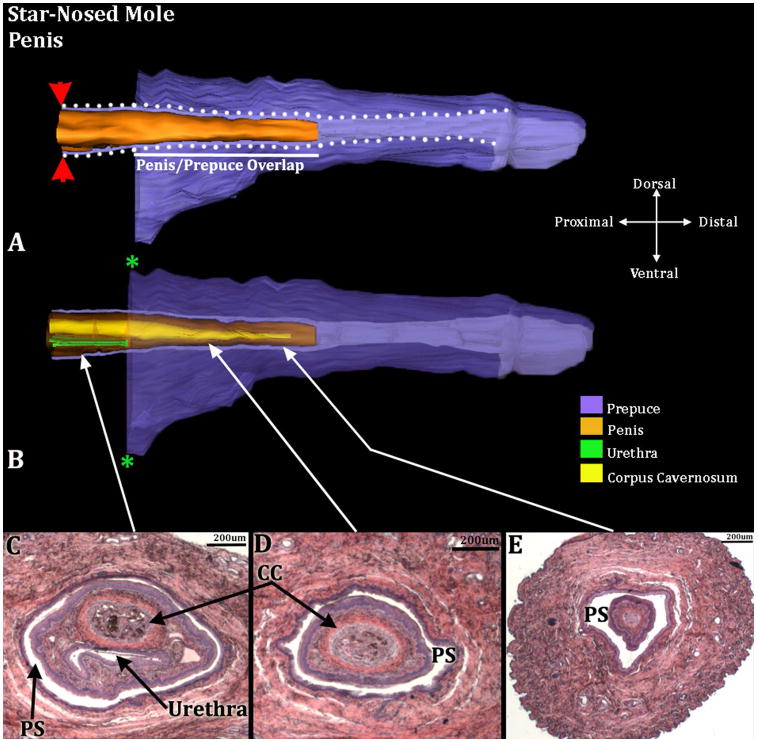
The perineal appendages of male and female star-nosed moles consist of a hair-bearing prepuce devoid of erectile bodies and other penile/clitoral features, as is the case for broad-footed moles (Fig. 7). Internally, the penis resides within the preputial space. Penile surface epithelium reflects onto the inner preputial epithelium deep within the preputial space, and thus the penis is freely mobile within the preputial space (Fig. 8). The penile urethra of the star-nosed mole does not extend to the distal aspect of the penis, but instead opens into the preputial space 1050μm from the distal tip of the penis. Note the absence of urethra in figures 8D & E, indicating that the penile urethra does not extend to the tip of the penis.
Figure 7.
Side views of male (A) and female (B) star-nosed mole (Condylura cristata) external genitalia from the non-breeding season. The size of male and female external genitalia is remarkably similar. For both images the cranial aspect is to the left.
Figure 8.
Three-dimensional reconstruction of the prepuce and penis of an adult star-nosed mole (Condylura cristata) with transverse section images depicting morphology at different locations within the penis. Three-dimensional reconstruction (A) the prepuce is semi-transparent with the penis opaque orange demonstrating the shape and the position of penis relative to the prepuce. The mole penis at rest is an “internal” organ housed within the preputial space (lighter purple region bordered by white dotted lines). The white line denotes the length of the penis/prepuce overlap. Red arrowheads denote the reflection of the inner preputial epithelium onto the surface of the penis. Three dimensional reconstruction (B) the prepuce and penis are rendered semi-transparent to show the internal morphology of the penis. The perineal appendage (prepuce) merges with the body surface (green asterisks). The distal end of the urethra (green) is the urethral meatus where urine/semen exits from the penis. Three transverse H&E section images (C–E) depict the internal morphology of the penis from proximal to distal at differing morphological points. CC=Corpus Cavernosum, PS=Preputial Space. Note the morphometric measures mentioned in the text.
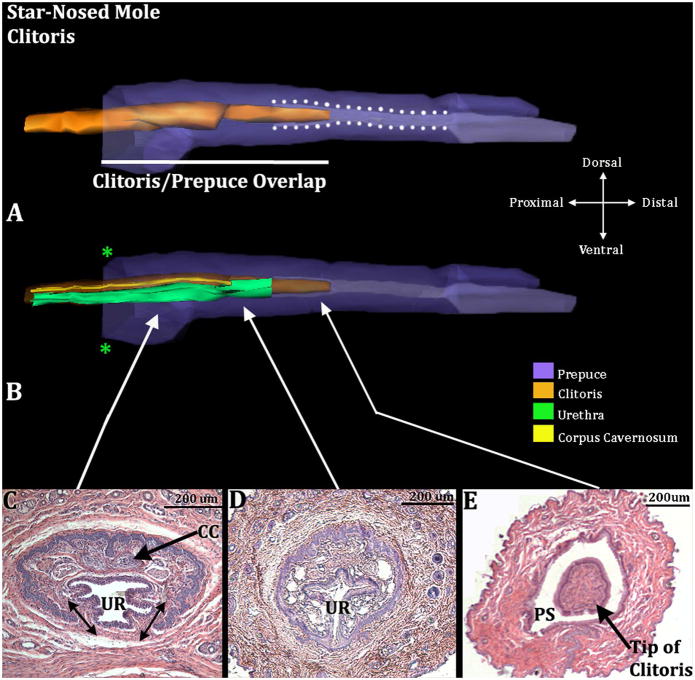
The proximal portion of the U-shaped clitoris is tethered ventrally as a result of confluence of clitoral stroma with ventral stroma (doubled headed arrows in Figs. 9C & 10B). However, the distal portion of the clitoris is completely surrounded by epithelium and projects into the preputial space (Fig. 9A & E). Thus, the tip of the clitoris is un-tethered for approximately 417.5μm (SD=159). Proximally the clitoris is defined by the U-shaped clitoral epithelial lamina similar to that observed in mice (Weiss et al., 2012) and other mole species (Fig. 9C & 10B) (Sinclair et al., 2016b). The female urethra of star-nosed moles is situated mostly within the confines of the U-shaped clitoral epithelial lamina (Fig. 9C–D & 10B).
Figure 9.
Three-dimensional reconstruction of the prepuce and clitoris of an adult star-nosed mole (Condylura cristata) with transverse sections depicting morphology at different locations within the clitoris. Three-dimensional reconstruction (A) the prepuce is semi-transparent with the clitoris opaque orange demonstrating the shape and the position of clitoris relative to the prepuce. The clitoris is housed within the prepuce (in purple); lighter purple depicts the preputial grove distally and when bordered by white dotted lines depicts the preputial space. The white line denotes the length of the clitoris/prepuce overlap. Three-dimensional reconstruction (B) the prepuce and clitoris are rendered semi-transparent to show the internal morphology of the clitoris. The perineal appendage (prepuce) merges with the body surface (green asterisks). Three transverse H&E section images (C–E) depict the morphology of the clitoris and prepuce at differing morphological points from proximal to distal. Note, in C, the clitoral stroma within the U-shaped clitoral lamina is in continuity ventrally with the surrounding stroma (double-headed arrows). CC=Corpus Cavernosum, PS=Preputial Space, UR= Urethra. Note the tip of the clitoris is free and resides within the preputial space (A&E). Morphometric measures are mentioned in the text.
Figure 10.
Transverse H&E stained sections of an adult star-nosed mole (Condylura cristata) penis (A & C) and clitoris (B). Letters for identification of pertinent structures are: BV (blood vessel), CC (corpus cavernosum), P (prepuce), PS (preputial space), S (shaft of clitoris or penis), T (tunica), U (urethra), VS (closed vaginal seam). Dorsal double-headed arrows in (B) = region of hair follicles of the female prepuce. The penis in (C) is a high magnification photo depicting the band of tunica-like cells lying just deep to the penile epithelium.
Prepuce Length
Female star-nosed moles possess a prominent hair-bearing prepuce similar to that of the male (Fig. 7). However, prepuce length was significantly greater in males versus females (Mean ± St.Dev. male=5.350 ±0.626mm, female=3.622 ±0.514mm, p=0.0026).
Penile/Preputial & Clitoral/Preputial Overlap
Penile/preputial or clitoral/preputial overlap was measured the same way as for broad-footed moles. Clitoral/preputial overlap of the star-nosed mole is on average 2232μm (St.Dev. =416) (Fig. 9), while penile/preputial overlap is 2005μm (St.Dev. =219) (Fig. 8). Thus, the portion of the penis or clitoris that projects beyond the body surface is not significantly different between the sexes (p=0.512).
Percent of penile/preputial or clitoral/preputial overlap was also calculated relative to preputial length. By this convention penile/preputial overlap represents less than half of total preputial length (46.57%, St.Dev. =11.89%) in male star-nosed moles (Fig. 8), while in female star-nosed moles clitoral/preputial overlap relative to total preputial length was 66.95% (St.Dev. =9.49%) (Fig. 9). This difference was statistically significant (p=0.036).
Internal Penile/Clitoral Composition
Neither male nor female star-nosed moles possess an os penis or os clitoris. Both sexes have a corpus cavernosum surrounded by a tunica. However, similar to the broad-footed mole, the tunica and corpus cavernosum are larger in males than in females (Figs. 8, 9, 10). In males the distal tip of the corpus cavernosum ends at approximately 180μm from the distal tip of the penis (St.Dev. =72), while in females the corpus cavernosum ends 228μm from the distal tip of the clitoris (St.Dev. =55). The male urethra resides completely within penile stroma (Fig. 8C) and opens into the preputial space ~1050μm from the tip of the penis (Fig. 8B). In contrast, the female urethra is situated almost completely within the confines of the U-shaped clitoral lamina throughout most of its length (Fig. 9 & 10B). Corpus spongiosum (human terminology) or corpus cavernosum urethrae (mouse terminology) were absent in both male and female star-nosed moles. However, similar to the broad-footed mole, there is a diffuse network of blood vessels throughout the length of the penis and clitoris, designated as corpus cavernosum glandis. Males have a greater amount of this network of blood vessels than females (Fig. 10). Close examination of the skin of the penis revealed a dermis exceptionally rich in collagen fibers surrounding the corpus cavernosum glandis (Fig. 10C) lying just deep to the penile surface epithelium in star-nosed moles, which may function in a similar manner to the tunica albuginea.
Masculine Traits Score
The star-nosed mole penis possesses 8 out of the 9 common masculine characteristics. It only lacks the os penis. In contrast, the clitoris of the star-nosed mole possesses 4 out of the 9 masculine characteristics, displaying (a) large prepuce size, (b) a freely mobile distal tip of the clitoris, (c) a distinct corpus cavernosum, and (d) a thick tunica that surrounds the corpus cavernosum (Fig. 6).
Hairy-tailed moles
The penis resides within the preputial space and is not attached to the prepuce in adults except proximally where the inner preputial epithelium reflects onto the penile surface (Fig. 11). The penile urethra opens into the preputial space ~450μm from the distal tip of the penis. In females the stroma within the U-shaped clitoris is confluent with ventral stroma, and thus the U-shaped clitoris is tethered ventrally (Fig. 12C), even though the distal tip of the clitoris is “free” (not attached to the prepuce) and lies within the preputial space (Fig. 12A & E). Indeed, the clitoral tip is un-tethered for 400μm and 260μm in the two specimens available for examination.
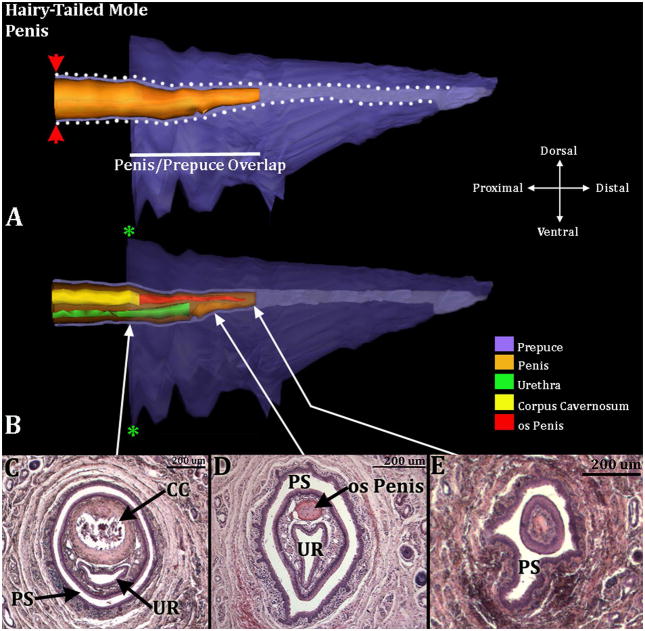
Figure 11.
Three-dimensional reconstruction of the prepuce and penis of an adult hairy-tailed mole (Parascalops breweri) with transverse section images depicting morphology at different locations within the penis. Three-dimensional reconstruction (A) the prepuce is semi-transparent with the penis opaque orange demonstrating the shape and the position of penis relative to the prepuce. The mole penis at rest is an “internal” organ housed within the preputial space (lighter purple region bordered by white dotted lines). The white line denotes the length of the penis/prepuce overlap. The red arrowheads denote the reflection of the inner preputial epithelium onto the surface of the penis. Three dimensional reconstruction (B) the prepuce and penis are rendered semi-transparent to show the internal morphology of the penis. The perineal appendage (prepuce) merges with the body surface (green asterisks). The distal end of the urethra (green) is the urethral meatus where urine/semen exits from the penis. Three transverse H&E section images (C–E) depict the internal morphology of the penis from proximal to distal at differing morphological points. CC=Corpus Cavernosum, PS=Preputial Space, UR= Urethra. Note the morphometric measures mentioned in the text.
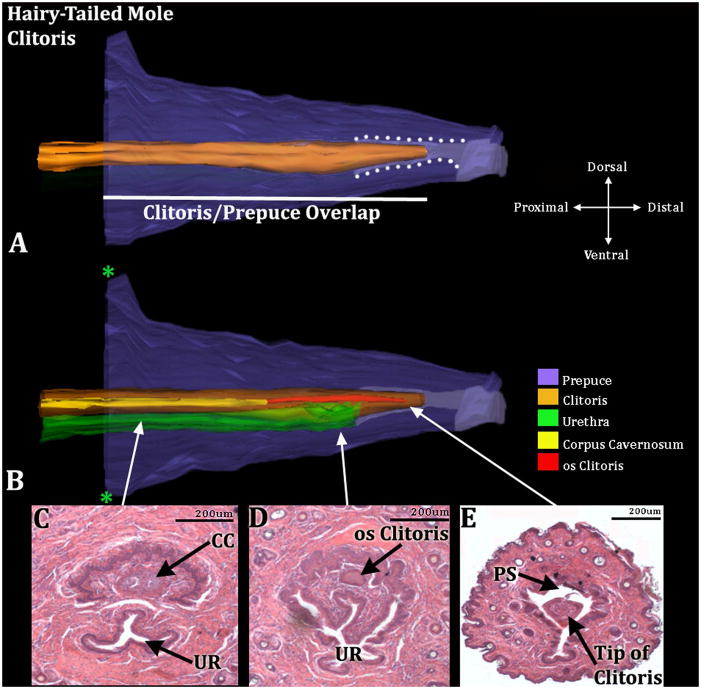
Figure 12.
Three-dimensional reconstruction of the prepuce and clitoris of an adult hairy-tailed mole (Parascalops breweri) with transverse sections depicting morphology at different locations within the clitoris. Three-dimensional reconstruction (A) the prepuce is semi-transparent with the clitoris opaque orange demonstrating the shape and the position of clitoris relative to the prepuce. The mole clitoris is housed within the prepuce (in purple); lighter purple depicts the preputial grove distally and when bordered by white dotted lines depicts the preputial space. The white line denotes the length of the clitoris/prepuce overlap. Three-dimensional reconstruction (B) the prepuce and clitoris are rendered semi-transparent to show the internal morphology of the clitoris. The perineal appendage (prepuce) merges with the body surface (green asterisks). Three transverse H&E section images (C–E) depict the morphology of the clitoris and prepuce at differing morphological points from proximal to distal. CC=Corpus Cavernosum, PS=Preputial Space, UR= Urethra. Note the tip of the clitoris is free and resides within the preputial space (A&E). Morphometric measures are mentioned in the text.
Prepuce Length
Female hairy-tailed moles possess a prominent prepuce similar in size and shape to that of the male (Figs. 11 & 12). Prepuce length was similar in males (2.55mm & 2.60mm) and females (2.65mm & 2.57mm). Only 2 male and 2 female hairy-tailed moles could be obtained for this study.
Penile/Preputial & Clitoral/Preputial Overlap
Clitoral/preputial overlap in the hairy-tailed female mole is noticeably longer at 2200μm & 2150μm (Fig. 12), than penile/preputial overlap at 1100μm & 1180μm in length (Fig. 11). Calculation of the amount of penile/preputial or clitoral/preputial overlap relative to total preputial length indicated that penile/preputial overlap represents less than half of total preputial length 43.14% & 45.38% (Fig. 11) in hairy-tailed male moles, while in female hairy-tailed moles clitoral/preputial overlap relative to total preputial length was much greater in amount at 83.02% & 83.66% (Fig. 12).
Internal Penile/Clitoral Composition
Both male (n=2) and female (n=2) hairy-tailed moles possess bone within the penis and clitoris, respectively, which lies distal to the corpus cavernosum. The os penis is 650μm & 720μm in length and the os clitoris is 600μm & 750μm (Figs. 11 & 12). Both sexes also possess a corpus cavernosum surrounded by a tunica, which is larger in males than in females. In males the distal tip of the corpus cavernosum ends at approximately 700μm & 790μm from the distal tip of the penis, while in females it ends 900μm & 750μm from the distal tip of the clitoris. The penile urethra resides completely within penile stroma in males (Fig. 11C & D) and opens into the preputial space ~450μm from the tip of the penis. The female urethra is situated only about 1/4 within the clitoris (clitoral tissue defined as the tissue within the confines of U-shaped clitoral epithelial lamina) (Figs. 12C & D). Erectile bodies comparable to the corpus spongiosum (human terminology) and corpus cavernosum urethrae (mouse terminology) were not observed in male and female hairy-tailed moles. However, similar to the broad-footed and star-nosed moles, there is a network of blood vessels throughout the length of the penis and clitoris, termed the corpus cavernosum glandis, which is larger in males versus females.
Masculine Traits Score
The penis of the hairy-tailed mole possesses all 9 of the typical masculine characteristics. The clitoris of the species is clearly different internally from the penis but does possess 5 out of the 9 masculine characteristics displaying (a) a large prepuce size, (b) a freely mobile distal tip of the clitoris, (c) an os clitoris, (d) a distinct corpus cavernosum, and (e) a thick tunica that surrounds the corpus cavernosum (Fig. 6).
Japanese shrew moles
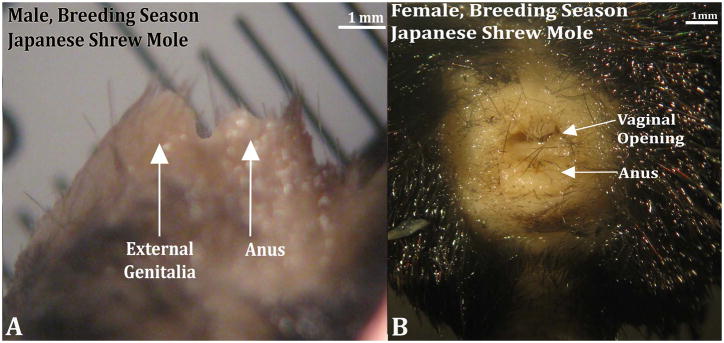
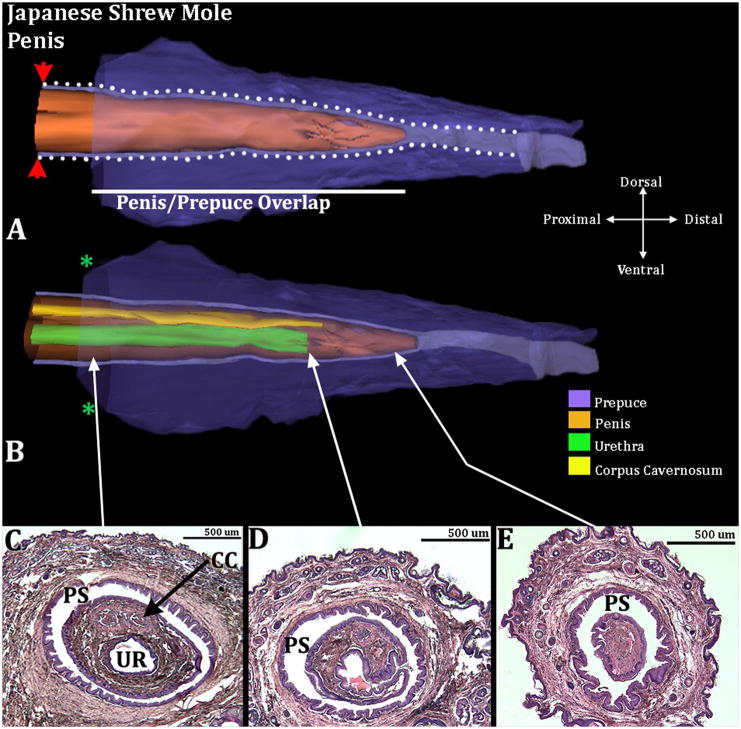
Male Japanese shrew moles have a distinct perineal appendage, which consists of a hair-bearing prepuce (Fig. 13). The penis resides within the preputial space and is not attached to the prepuce in the adult male except proximally where the inner preputial epithelium reflects onto the penile surface (Fig. 14). The penile urethral opens into the preputial space 350 and 475μm micrometers from the tip of the penis (Fig. 14B). In females, the internally situated clitoris is not attached to the prepuce and is an immobile organ due to ventral confluence of clitoral stroma with surrounding stromal tissue (Fig. 15). Only 2 male and 2 female Japanese shrew moles were available for this study.
Figure 13.
Japanese shrew mole (Urotrichus talpoides) male and female external genitalia from the breeding season. Note the female (B) lacks a prominent perineal elevation but does possess a vaginal opening. Cranial aspect is to the left in the male (A) and to the top in the female (B).
Figure 14.
Three-dimensional reconstruction of the prepuce and penis of an adult Japanese shrew mole (Urotrichus talpoides) with transverse section images depicting morphology at different locations within the penis. Three-dimensional reconstruction (A) the prepuce is semi-transparent with the penis opaque orange demonstrating the shape and the position of penis relative to the prepuce. The mole penis at rest is an “internal” organ housed within the preputial space (lighter purple region bordered by white dotted lines). The white line denotes the length of the penis/prepuce overlap. The red arrowheads denote the reflection of the inner preputial epithelium onto the surface of the penis. Three dimensional reconstruction (B) the prepuce and penis are rendered semi-transparent to show the internal morphology of the penis. The perineal appendage (prepuce) merges with the body surface (green asterisks). Three transverse H&E section images (C–E) depict the internal morphology of the penis from proximal to distal at differing morphological points. CC=Corpus Cavernosum, PS=Preputial Space, UR= Urethra. Note the morphometric measures mentioned in the text.
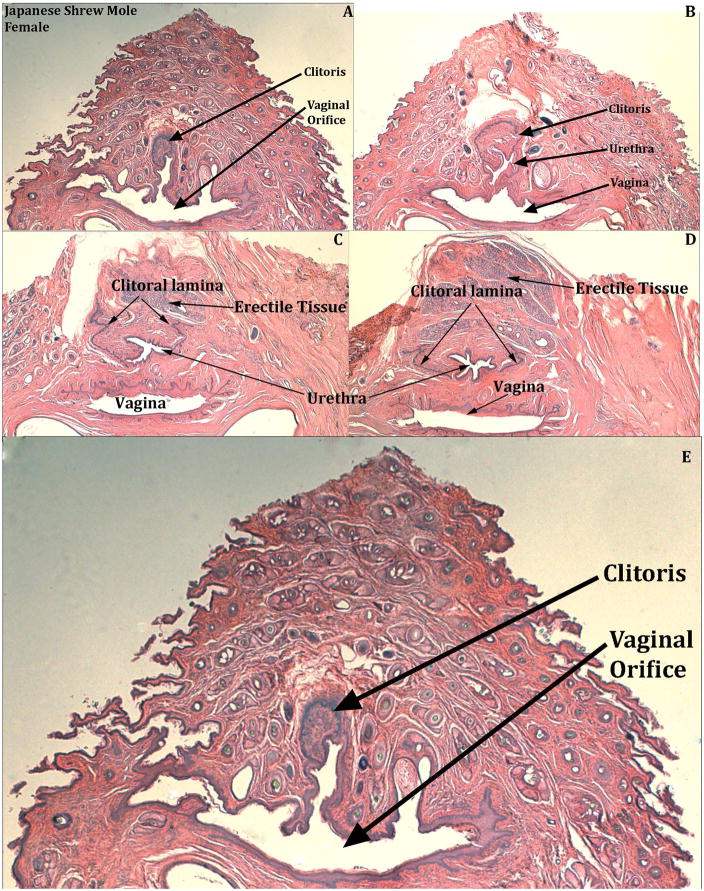
Figure 15.
Transverse H&E stained sections of adult Japanese shrew mole (Urotrichus talpoides) female genitalia from the breeding season identifying the distal beginning of the clitoris and the connection of the urethra to the vaginal vestibule (E). Sections (A–D) progress distal to proximal from left to right. In (A & E) note that the U-shaped clitoral lamina is fused to the urethra, which opens into the vaginal orifice. In (B) the urethra is separate from the vagina. In (C & D) the U-shaped clitoral lamina is represented merely as its right and left pieces that flank the urethra and dorsal to the urethra is diffuse erectile tissue.
Prepuce Length
Adult male Japanese shrew moles possess a prominent hair-bearing prepuce lacking erectile tissue and other penis-specific elements. Male preputial length was 2.10mm & 2.45mm (n=2). Adult female Japanese shrew moles do not have a protruding perineal appendage (Fig. 13). Therefore, a preputial length measurement was not applicable.
Penile/Preputial Overlap
Penile/preputial overlap of the Japanese shrew mole is 1480μm & 1690μm (n=2). Percent penile/preputial overlap relative to total preputial length was calculated at 70.48% & 68.98% (Fig. 14).
Internal Penile/Clitoral Composition
Japanese shrew moles do not have an os penis. The two males examined have a distinct corpus cavernosum surrounded by a tunica, which ends distally 260μm & 300μm from the distal tip of the penis. The penile urethra exits ventrally into the preputial space 350μm & 475μm from the distal tip of the penis (Fig. 14B & D). The vascular network throughout the penis is not as extensive as that seen in male or female broad-footed moles and therefore is not deemed to be a corpus cavernosum glandis in this species. In female Japanese shrew moles there are rudimentary erectile structures dorsal to the urethra (Fig. 15C & D). The urethra opens into the vaginal introitus but proximally the urethra separates from the vagina and is partially enclosed by the parentheses-shaped epithelial lamina (Fig. 15).
Masculine Traits Score
The penis of Japanese shrew moles possesses 8 out of the 9 masculine characteristics lacking only the os penis. The female external genitalia of this species possesses 0 out of the 9 masculine characteristics making it, by these measures, fully feminized external genitalia (Fig. 6).
Anogenital Distance
Anogenital distance was measured twice for each animal, and the mean value used. No significant difference in AGD was observed between male and female broad-footed moles (Mean ± St.Dev. male=2.454 ±0.474mm, female=2.259 ±0.655mm) (p=0.384). Other scientists have normalized AGD to body weight or body length to compare males to females. There was no significant difference in AGD between male and female broad-footed moles when analyzed by body weight (p=0.401) or body length (p=0.407). Similarly, there was no significant difference in AGD between male and female star-nosed moles (Mean ± St.Dev. male=2.410 ±0.079mm, female=2.297 ±0.119mm, p=0.219). In female Japanese shrew moles distance from the anus to the vaginal opening was 1.25mm & 1.45mm (n=2). In males the average AGD is longer (2.45mm & 2.75mm, n=2).
Preputial length in XTfm/Y mice
Length of the perineal elevation (prepuce) was 3.33mm (St.Dev. =0.28, N=3) in feminized XTfm/Y mice, 3.15mm in wild-type (X+/X+) female mice (St.Dev. =0.06, N=4) and 5.54mm in wild-type (X+/Y) male mice (St.Dev. =0.28, N=4). Thus, for XTfm/Y mice and wild-type female mice lengths of the perineal elevation (prepuce) were not significantly different. Length of the preputial elevation in wild-type males was significantly longer than that of XTfm/Y (p=0.0002) and wild-type (X+/X+) female mice (p<0.0001) (Fig. 16).
Figure 16.
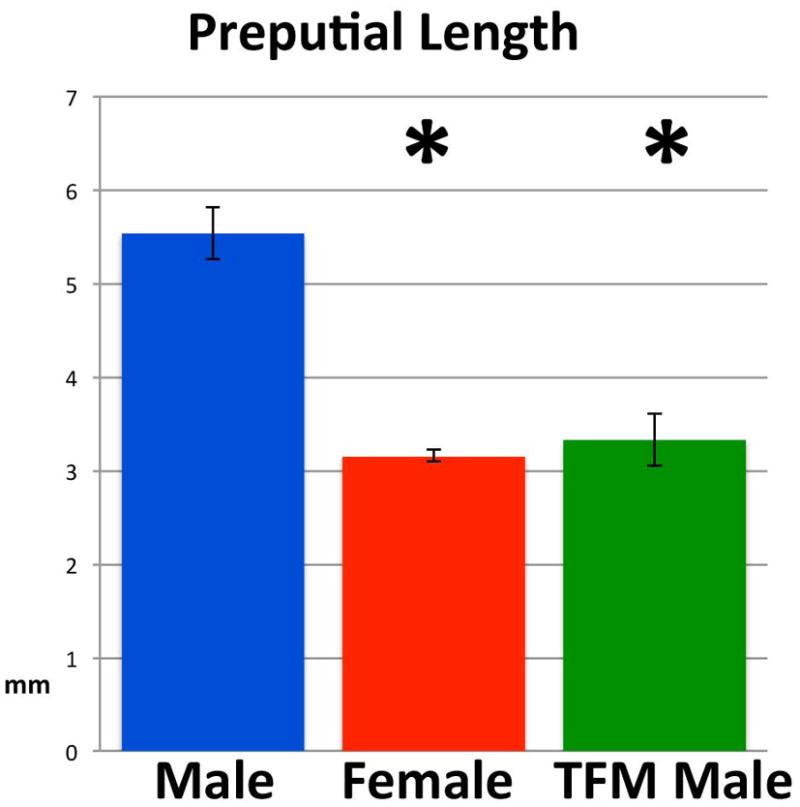
Length of the perineal appendage (prepuce) of wild-type male and female C57Bl/6 mice and XTfm/Y mice (on a C57BL/6 background). * signifies a statistically significant difference from wild-type male preputial length.
Discussion
In a previous paper (Sinclair et al., 2016b) we demonstrated that the perineal appendage of male and female broad-footed moles is the prepuce and not the penis or clitoris, which are both “internal organs” with a histologic signature distinctly different from the prepuce. Histology of external genitalia of male and female broad-footed moles has provided objective anatomical criteria for defining prepuce, penis and clitoris. Accordingly, histological examination confirms our earlier findings, and thus the prominent perineal appendage in all 4 mole species examined (broad-footed, star-nosed, hairy-tailed and Japanese shrew moles) consists of a hair-bearing prepuce devoid of penile or clitoral histologic signatures. Accordingly, the old descriptive terms for perineal appendages of male and female moles (peniform clitoris, penile clitoris, phallus, penis, clitoris and phallus-like clitoris) certainly do not apply for these species and may not apply for European moles as well. Thus, the new terminology described in Sinclair et al. has been verified and will be used in the present paper (Sinclair et al., 2016b).
Three-dimensional reconstruction and morphometric analysis have been employed to obtain an accurate description of the patterning of elements constituting the external genitalia of the 4 mole species examined. Penile/preputial and clitoral/preputial overlap determined by morphometric and by 3- dimensional reconstruction emphasize the point that the penis and clitoris, each with their unique histologic signature, are internal organs residing within (penis) or deep (clitoris) to the preputial space, even though in female star-nosed and hairy-tailed moles the distal aspect of the clitoris projects freely into the preputial space. Morphometric analysis of histologic sections also demonstrates that, in all 4 mole species, the penile urethra does not open at the tip of the penis. Instead the urethral meatus is situated a considerable distance proximal to the tip of the penis, similar to that of the mouse (Rodríguez et al., 2011).
For most mammalian species, external genitalia are sexually dimorphic with the penis substantially larger than the clitoris. The difference in size and morphology of male and female external genitalia has been attributed to androgen action in the case of males and the absence thereof in females (Yamada et al., 2003). However, females of several mammalian species exhibit profoundly masculinized external genitalia, notably the spotted hyena, several species of moles and several primates (spider monkeys, howler monkeys, chimpanzee and ring-tailed lemurs) (Wislocki, 1936; Drea and Weil, 2008; Cunha et al., 2014). The fossa (Cryptoprocta ferox), a cat-like, carnivorous mammal endemic to Madagascar, is a special case in so far as masculinization of female external genitalia is seen in juveniles, but disappears in adult females (Hawkins et al., 2002). The question concerning the mechanism of masculinization of female external genitalia is generally approached as an issue of androgen action, with exploration for a source of androgens, and if possible (rarely), with experimental studies of the effects of various forms of “androgen blockade/deprivation”. While female fetuses of spotted hyenas are exposed to endogenous androgens (Licht et al., 1998), formation and growth of the pendulous penile clitoris of the spotted hyena is androgen-independent even though development of certain internal phallic structures is androgen-dependent (Cunha et al., 2014). Transient masculinization of the clitoris of Fossa fossana is not associated with elevated female androgen levels (Hawkins et al., 2002). For mice (and perhaps also other rodents) length of the perineal appendage (prepuce) of females is ~57% that of males. The substantial size of the wild-type female perineal appendage (prepuce) is not significantly different from that of androgen-insensitive XTfm/Y mice. These data suggest that the basal size (length) of the mouse perineal appendage (prepuce) in females is androgen-independent, and the incremental increase in size of the male mouse perineal appendage (prepuce) is androgen-dependent. This idea is consistent with regulation of anogenital distance in mice, which is affected by intrauterine position, but cannot be reduced in females below a certain point as a result of prenatal treatment with an anti-androgen (vom Saal and Bronson, 1978; Vom Saal, 1978; Mitchell et al., 2015). Thus, there is precedent for the idea that certain aspects of male and female external genitalia may be androgen-independent (Cunha et al., 2014).
Sexual dimorphism of external genitalia within the four species of moles examined can be assessed in a variety of ways: (a) length/size of the perineal appendage (preputial length), (b) AGD, (c) presence or absence of an os penis/os clitoris, or (d) masculine trait score. The presumption is that masculine features in males and/or females are elicited during pre- and postnatal development by androgens whose source could be the testes, fetal or maternal ovotestes, placenta or adrenal gland. For female moles, masculinization has been previously attributed to androgens derived from the ovotestes present in several European mole species (Talpa romana, T. occidentalis, and T. europaea), Condylura cristata (star-nosed moles), Mogera wogura (Japanese mole), and Neurotrichus gibbsii (American shrew mole) (Zurita et al., 2003; Jiménez et al., 1993; Sánchez et al., 1996; Barrionuevo et al., 2004; Matthews, 1935; Whitworth et al., 1999; Rubenstein et al., 2003; Carmona et al., 2008). Broad-footed (Scapanus latimanus) and Japanese shrew (Urotrichus talpoides) moles do not have ovotestes, which has also been reported for the eastern mole (Scalopus aquaticus) and coast mole (Scapanus orarius) (Rubenstein et al., 2003; Carmona et al., 2008) In 1935 Matthews speculated that in Talpa europaea the “interstitial gland (of ovotestis) was not only homologous with the testis, but that it also produced male hormones which affect the general metabolism and form of the animal” (Matthews, 1935). This speculation was subsequently confirmed in reports of Leydig cells and the production of testosterone in the interstitial gland of ovotestes of female moles (Talpa occidentalis and Talpa europaea) (Jiménez et al., 1993; Whitworth et al., 1999; Zurita et al., 2003). Leydig cells within ovotestes of Talpa occidentalis were first recognized morphologically at 5 days postpartum (Barrionuevo et al., 2004) even though at this stage serum testosterone is barely detectable (<0.020ng/ml), but rises to 0.5 to 0.8ng/ml at 30 days postnatal when serum testosterone levels in female Talpa occidentalis equal or exceed that in males of the same age (Zurita et al., 2003). Subsequent studies have correlated circulating testosterone levels with weight/volume of the interstitial gland in Talpa occidentalis and Talpa europaea and have demonstrated that ovarian weight and serum testosterone are highest in the non-breeding versus the breeding season in female Talpa occidentalis (Jiménez et al., 1993; Whitworth et al., 1999; Zurita et al., 2003). The postnatal rise in serum testosterone levels in female Talpa eruopaea, Talpa occidentalis, and Talpa romana is associated with the development of epididymes associated with the ovarian bursa near the interstitial gland (Zurita et al., 2003; Jiménez et al., 1993; Sánchez et al., 1996; Barrionuevo et al., 2004). Female epididymes in Talpa occidentalis persist and grow throughout juvenile and adult life (Zurita et al, 2003).
Now, with at least 4 (a-d above) ways of assessing sexual dimorphism of mole external genitalia, we can explore the relationship between ovotestes and sexual dimorphism of mole external genitalia. For all four species of moles examined in this paper, the correlation is discordant between the presence/absence of ovotestes and the status of (a) preputial length, (b) AGD, (c) presence or absence of an os penis/os clitoris, or (d) masculine trait score. In Table 2, entries indicated in green are indicative of concurrence between presence or absence of ovotestes and the morphologic feature indicated. Entries in red are indicative of a lack of consistency between the presence or absence of ovotestes and the morphologic feature. As can be seen, for each morphologic feature there is at least one mole species that is inconsistent with androgen production by the ovotestes.
Table 2.
A comparison of morphological features of external genitalia in four species of mole Scapanus latimanus, Condylura cristata, Parascalops breweri, and Urotrichus talpoides.
| Species | Prepuce length | AGD | Bone | Masculine trait score | Ovotestes |
|---|---|---|---|---|---|
| Broad-footed | M=F | M=F | M= + | M=9 | No |
| F= − | F=3 | ||||
| Star-nosed | M>F | M=F | M= − | M=8 | Yes |
| F= − | F=4 | ||||
| Hairy-tailed | M=F | NA | M= + | M=9 | Yes |
| F= + | F=5 | ||||
| Japanese shrew | M>F | M>F | M= − | M=8 | No |
| F= − | F=0 |
M= Male, F=Female, NA=Not Applicable (data not available), AGD= anogenital distance
Broad-footed mole males and hairy-tailed male and female moles have bone (os penis or os clitoris) within external genitalia. From studies in rats and mice, bone formation is considered to be androgen-dependent, at least in some fashion. The os penis arises from a mesenchymal condensation that ossifies in neonatal male mice and rats (Murakami, 1984; Murakami and Mizuno, 1986; Murakami, 1986; 1987; Glucksmann et al., 1976). Surprisingly, an os penis and an os clitoris is present in male and female wild-type mice, respectively (Weiss et al., 2012; Glucksmann et al., 1976), as well as in androgen-insensitive XTfm/Y mice (Yang et al., 2010; Murakami, 1987). This suggests that initial formation of the os penis and os clitoris as well as its ossification is androgen-independent. However, the os penis of adult male mice is approximately 6.5 times longer than the os clitoris (Weiss et al., 2012). Thus, postnatal growth of bone within external genitalia is androgen-dependent (Glucksmann and Cherry, 1972; Glucksmann et al., 1976). Indeed, neonatal anti-androgen treatment or neonatal castration inhibits the increase in size and calcification of the os penis, while neonatal treatment of females with androgens stimulates growth of the os clitoris in rats and mice (Glucksmann et al., 1976; Glucksmann and Cherry, 1972). We conclude that a prominent os penis or os clitoris is indicative of androgen action. In developing males the source of androgen is the testes, which are known to produce androgens during fetal, neonatal, and adult periods (Bloch, 1967; Bloch et al., 1971; Feldman and Bloch, 1978).
A key observation is the absence of an os penis and os clitoris in male and female star-nosed (having ovotestes) and Japanese shrew moles (lacking ovotestes). Given that males of these two mole species have functioning testes and develop normal male internal genitalia, the absence of bone formation in external genitalia of male star-nosed and Japanese shrew moles may be explained by failure of formation of the bone precursor, which as discussed above is an androgen-independent event (at least in mice). Absence of the bone precursor could account for absence of an os penis and os clitoris in star-nosed and Japanese shrew moles, thus making androgens (whether derived from testes or ovotestes) irrelevant for this feature. The European mole (Talpa europaea) has an ovotestis and possesses an os penis but the status of an os clitoris in the females is unknown (Schultz et al., 2016). The status of the os penis and os clitoris in other species of European moles (Talpa occidentalis and Talpa romana) having ovotestes has not been reported (Zurita et al., 2003; Matthews, 1935; Beolchini et al., 2000). However, if absence of bone in external genitalia of Japanese shrew moles is discarded from Table 2, then the status of all 4 external genitalia features in hairy-tailed and Japanese shrew moles is concordant with the presence/absence of ovotestes.
Anogenital distance is a metric that increases from birth to adulthood as the animal grows in size. Sex differences in AGD can be recognized at birth in several species including rats and mice. Postnatally, male and female AGDs increase, but they maintain a sex difference throughout life. AGD is increased in males in response to androgens derived from fetal and neonatal testes, implying early sensitivity of the perineal region to androgens (Callegari et al., 1987; Hotchkiss et al., 2007b; Vandenbergh and Huggett, 1995; Godet, 1946; Hotchkiss and Vandenbergh, 2005; Hotchkiss et al., 2007a). Indeed, AGD is increased in female mice whose intrauterine position is between two male fetuses, implying exquisite sensitivity of the developing perineum to androgens (vom Saal et al., 1990). Sensitivity of the perineal region to androgens is maintained into adulthood, as AGD remains responsive to androgens in adult rats (Mitchell et al., 2015) suggesting that final AGD is a function of androgen exposure from prenatal to adult stages.
Anogenital distance was measured in three mole species and has also been reported for the European mole (Matthews, 1935). The presence or absence of ovotestes versus AGD is not concordant across the 3 mole species in Table 2. Given the absence of ovotestes in broad-footed female moles, the expectation was that AGD would be greater in males versus females. However, AGD in broad-footed moles was not significantly different in males and females. Either female broad-footed moles are exposed to androgens during development via a source other than maternal or fetal ovotestes, or AGD is not a reliable indicator of androgen action during development in this mole species. Thus, for these 3 mole species (Table 2) the correlation between the presence or absence of ovotestes and AGD is not consistent.
Sexual dimorphism in length of the perineal appendage (prepuce) is another potentially androgen-regulated feature. For preputial length, the correlation between this parameter and the presence or absence of ovotestes is also poor. Given the absence of ovotestes in female broad-footed moles, the expectation was that prepuce length should be greater in males versus females. However, prepuce length was not statistically different in both sexes. Conversely, in star-nosed female moles, which lack ovotestes, the expectation was that prepuce length should be equal in males versus females, but instead prepuce length was greater in males versus females. This lack of correspondence between the presence or absence of ovotestes as a potential source of androgens and preputial length raises the question of whether preputial length is androgen-independent. This idea is supported by data from spotted hyenas and analysis of size of the perineal appendage (prepuce) of XTfm/Y mice as discussed above. Length of the prepuce (like AGD) is a metric that increases proportionally as the animal grows, and thus is presumably subject to growth regulation from birth to adulthood. The substantial growth of the female mouse perineal appendage (prepuce) to adult size is not associated with other androgen-dependent features such as retention and development of Wolffian derivatives or female prostate, suggesting that androgen levels in female mouse embryos/neonates is sub-optimal for development of male internal genitalia, whether mode of androgen exposure is via local diffusion from the testes (Wolffian duct) or via systemic androgens. Thus, we must entertain the idea that preputial length in female moles may also be androgen-independent.
Rats and mice actually have two prepuces (Blaschko et al., 2013; Sinclair et al., 2016a). The prominent perineal appendage of rats and mice is the external prepuce. The prepuce of moles appears to be homologous with the external prepuce of rats and mice in so far as, in all three groups, this structure creates a voluminous space housing the penis. The internal prepuce of rats and mice is a flap of skin integral to the penis, a configuration similar to that of humans (Blaschko et al., 2013; Sinclair et al., 2016a). We have recently speculated that the external prepuce is protective for the penis, insuring cleanliness and protecting this sensitive organ (Sinclair et al., 2016b). This arrangement appears to be appropriate for mammals built low to the ground such as rats, mice, moles and other species.
In this paper we established a masculine trait score based upon the presence in males and the absence in females of nine morphological features (See Fig. 6). Using this metric, broad-footed and hairy-tailed male moles exhibit all 9 masculine features, whereas male star-nosed and Japanese shrew moles exhibit 8 of the 9 masculine features, only lacking an os penis. Female moles exhibit a range of masculine trait scores: zero for female Japanese shrew moles (complete feminization) and varying degrees of masculinization for female broad-footed moles (scoring 3), female star-nosed moles (scoring 4), and female hairy-tailed moles (scoring 5). Masculine trait scores of 4 and 5 in female star-nosed and hairy-tailed moles and zero in female Japanese shrew moles correlates with the presence of ovotestes in female star-nosed and hairy-tailed moles and the absence of ovotestes in female Japanese shrew moles. However, broad-footed female moles have a masculine trait score of 3, but lack ovotestes. The interpretation of masculine trait score in female broad-footed moles is two-fold: (a) either there is an alternate source of androgens, or (b) the feature in question (large prepuce size as well as a corpus cavernosum with a tunica albuginea) are androgen-independent. The possibility that prepuce length is at least partially androgen-independent has been discussed above. Clearly, masculine trait score is a complex metric involving many individual features, which may differ in sensitivity to androgens as well as in their “androgen-programming window of sensitivity”.
In summary, there is a lack of concordance across all 4 mole species between the four morphologic features examined and the presence/absence of ovotestes. For each feature there is discordance in at least one mole species.
The dogma of sex differentiation enunciated by Jost is that androgens play a central role in masculine development. In the presence of androgens, the male pattern of internal and external genitalia emerges, while in the absence of androgens the female pattern develops (Jost, 1953; 1965). While this idea is correct and applicable to development of external genitalia of humans and many other species, other mechanisms are possible in so far as certain aspects of development of external genitalia have been shown to be androgen-independent. For example, the female spotted hyena (Crocuta crocuta) has a pendulous penile clitoris similar in size to the penis. Treatment of fetal spotted hyenas with an anti-androgenic cocktail (flutamide plus finasteride) has minimal effects on formation and growth in length of the penis and clitoris (Drea et al., 1998; Cunha et al., 2005). Likewise, gonadectomy of prepubertal hyena pups has minimal effect on length of the adult penis and clitoris (Glickman et al., 1998). Thus, growth in length of the penis and clitoris of the spotted hyena is androgen-independent. Even though male perineal appendages and AGD are slightly larger in males versus females, the considerable size of perineal appendages (prepuce) in female rats, mice, and moles may also be androgen-independent as discussed above. Given the incomplete nature of the endocrine literature in moles, it is difficult to infer whether baseline size of the female prepuce is androgen-dependent or androgen-independent, even though preputial size in adult female mice appears to be androgen-independent based upon examination of androgen receptor mutant mice.
Comparing the four species of moles examined in this paper, the degree of concordance with ovotestes is highest for hairy-tailed moles and lowest for broad-footed moles. Indeed the hairy-tailed mole is the one species exhibiting complete concordance with ovotestes even though AGD measurements were not possible (Table 2). Focusing on bone within the external genitalia, it is likely that there are events at play other than androgen status. A key observation is the absence of an os penis in male star-nosed and Japanese shrew moles as well as the absence of an os clitoris in female star-nosed, which have ovotestes. However, if absence of bone in external genitalia of Japanese shrew moles is due to failure of formation of the bone precursor as discussed above, then the absence of an os penis in Japanese shrew moles can be discarded from Table 2. Accordingly, the status of external genitalia features in hairy-tailed and Japanese shrew moles would be concordant with the presence/absence of ovotestes.
Prepuce length is the feature with the greatest discordance with the presence or absence of ovotestes, raising the possibility that prepuce length is not androgen-regulated in female broad-footed and star-nosed moles as discussed above. We cannot exclude the possibility that androgens derived from the ovotestes may regulate prepuce length in hairy-tailed moles, while reduced prepuce length in female Japanese shrew moles may be attributed to an absence of ovotestes.
Finally, formation and growth regulation of the mole perineal appendage (prepuce) may be either androgen-dependent or androgen-independent and may vary on a species basis. The precedent for this idea is exemplified in hyaenids in which brown hyenas, striped hyenas and aardwolfs exhibit “standard” sexual dimorphism of external genitalia (Wells, 1968; Ewer, 1973), whereas female spotted hyenas exhibit profoundly masculinized external genitalia with a pendulous penile clitoris of similar size to that of the penis (Cunha et al., 2014). Thus, regulation of development and growth of external genitalia (size as well as internal features) can be radically different between species within the same phylogenetic group. Shinohara et al have shown that the 12 talpid genera they investigated can be resolved into 7 clades (Shinohara et al., 2003). Hairy-tailed (Parascalops breweri) and broad-footed (Scapanus latimanus) moles reside in the highly fossorial North American clade. Star-nosed moles (Condylura cristata) reside in the aquatic/fossorial North American clade, and Japanese shrew moles (Urotrichus talpoides) reside in the semi-fossorial Japanese clade. Accordingly, the expectation is that species within the same clade would exhibit similar features (presence or absence of ovotestes, prepuce length, AGD, bone and/or masculine trait score), while species in divergent clades may be expected to exhibit differences in these features in the females. Accordingly, broad-footed and hairy-tailed mole females (within the same clade) would be expected to exhibit similar features. This is not the case. Hairy-tailed moles have ovotestes, broad-footed moles do not, and there are major discrepancies in prepuce length (perineal elevation), penis/clitoris length, and masculine trait score in these two species (Table 2). Interestingly, the hairy-tailed mole female has an os clitoris, a free distal tip of the clitoris, and an ovotestes, while the closely related broad-footed mole female lacks all 3 features. Perhaps the loss of the ovotestes in the broad-footed mole female is linked with the loss of the other 2 masculine features. Hairy-tailed and Japanese shrew moles reside in separate clades, and would be expected to show a higher degree of divergence in features, which is the case. Broad-footed and Japanese shrew mole females, residing in separate clades, both lack ovotestes and yet also show a high degree of divergence of features. Star-nosed and broad-footed moles, also in different clades, exhibit a moderate degree of congruence of features. Thus, phylogenetic affinity is a poor predictor for most features within mole external genitalia.
Acknowledgments
This research was supported by NSF grant IOS-0920793 and NIH grant RO1 DK0581050.
Literature Cited
- Barrionuevo FJ, Zurita F, Burgos M, Jiménez R. Testis-like development of gonads in female moles. New insights on mammalian gonad organogenesis. Developmental biology. 2004;268:39–52. doi: 10.1016/j.ydbio.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Beolchini F, Rebecchi L, Capanna E, Bertolani R. Female gonad of moles, genus Talpa (Insectivora, mammalia): ovary or ovotestis? J Exp Zool. 2000;286:745–754. [PubMed] [Google Scholar]
- Blaschko SD, Mahawong P, Ferretti M, Cunha TJ, Sinclair A, Wang H, Schlomer BJ, Risbridger G, Baskin LS, Cunha GR. Analysis of the effect of estrogen/androgen perturbation on penile development in transgenic and diethylstilbestrol-treated mice. Anatomical record. 2013;296:1127–1141. doi: 10.1002/ar.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch E. The conversion of 7-3-H-pregnenolone and 4-14-C-progesterone to testosterone and androstenedione by mammalian fetal testes in vitro. Steroids. 1967;9:415–430. doi: 10.1016/0039-128x(67)90029-3. [DOI] [PubMed] [Google Scholar]
- Bloch E, Lew M, Klein M. Studies on the inhibition of fetal androgen formation: testosterone synthesis by fetal and newborn mouse testes in vitro. Endocrinology. 1971;88:41–46. doi: 10.1210/endo-88-1-41. [DOI] [PubMed] [Google Scholar]
- Callegari C, Everett S, Ross M, Brasel JA. Anogenital ratio: measure of fetal virilization in premature and full-term newborn infants. J Pediatr. 1987;111:240–243. doi: 10.1016/s0022-3476(87)80075-6. [DOI] [PubMed] [Google Scholar]
- Carmona FD, Motokawa M, Tokita M, Tsuchiya K, Jiménez R, Sánchez-Villagra MR. The evolution of female mole ovotestes evidences high plasticity of mammalian gonad development. J Exp Zool B Mol Dev Evol. 2008;310:259–266. doi: 10.1002/jez.b.21209. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Place NJ, Baskin L, Conley A, Weldele M, Cunha TJ, Wang YZ, Cao M, Glickman SE. The ontogeny of the urogenital system of the spotted hyena (Crocuta crocuta Erxleben) Biology of reproduction. 2005;73:554–564. doi: 10.1095/biolreprod.105.041129. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Risbridger G, Wang H, Place NJ, Grimace M, Cunha TJ, Weldele M, Conley AJ, Bar cellos D, Agawam S, Baraga A, Drea C, Sitter PK, Corsica EM, McPhaul MJ, Hammond GL, Baskin LS, Glickman SE. Development of the External Genitalia: Perspectives from the Spotted Hyena (Crocuta crocuta) Differentiation; research in biological diversity. 2014;87:4–22. doi: 10.1016/j.diff.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drea CM, Weil A. External genital morphology of the ring-tailed lemur (Lemur catta): females are naturally “masculinized”. J Morphol. 2008;269:451–463. doi: 10.1002/jmor.10594. [DOI] [PubMed] [Google Scholar]
- Drea CM, Weldele ML, Forger NG, Corsica EM, Frank LG, Licht P, Glickman SE. Androgens and masculinization of genitalia in the spotted hyaena (Crocuta crocuta). 2. Effects of prenatal anti-androgens. J Reprod Fertil. 1998;113:117–127. doi: 10.1530/jrf.0.1130117. [DOI] [PubMed] [Google Scholar]
- Eadie WR. A Contribution to the Biology of Parascalops breweri. Journal of Mammalogy. 1939;20:150–173. [Google Scholar]
- Eadie WR, Hamilton WJ. Notes on reproduction in the star-nosed mole. J Mammal. 1956;37:223–231. [Google Scholar]
- Ewer RF. The Carnivores. Cornell University Press; Ithaca, New York: 1973. [Google Scholar]
- Feldman SC, Bloch E. Developmental pattern of testosterone synthesis by fetal rat testes in response to luteinizing hormone. Endocrinology. 1978;102:999–1007. doi: 10.1210/endo-102-4-999. [DOI] [PubMed] [Google Scholar]
- Glickman SE, Corsica EM, Frank LG, Licht P, Weldele ML, Drea CM. Androgens and masculinization of genitalia in the spotted hyaena (Crocuta crocuta). 3. Effects of juvenile gonadectomy. Journal of reproduction and fertility. 1998;113:129–135. doi: 10.1530/jrf.0.1130129. [DOI] [PubMed] [Google Scholar]
- Glucksmann A, Cherry CP. The hormonal induction of an os clitoridis in the neonatal and adult rat. Journal of anatomy. 1972;112:223–231. [PMC free article] [PubMed] [Google Scholar]
- Glucksmann A, Ooka-Souda S, Miura-Yasugi E, Mizuno T. The effect of neonatal treatment of male mice with antiandrogens and of females with androgens on the development of the os penis and os clitoridis. Journal of anatomy. 1976;121:363–370. [PMC free article] [PubMed] [Google Scholar]
- Godet R. Biologie Experimentale - Modifications de lorganogenese des voies urogenitales des embryons de Taupe (Talpa-Europaea L) par action du propionate de testosterone. Comptes Rendus Hebdomadaires Des Seances De L Academie Des Sciences. 1946;222:1526–1527. [Google Scholar]
- Hawkins CE, Dallas JF, Fowler PA, Woodroffe R, Racey PA. Transient masculinization in the fossa, Cryptoprocta ferox (Carnivora, Viverridae) Biology of reproduction. 2002;66:610–615. doi: 10.1095/biolreprod66.3.610. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Furr J, Makynen EA, Ankley GT, Gray LE., Jr In utero exposure to the environmental androgen trenbolone masculinizes female Sprague-Dawley rats. Toxicology letters. 2007a;174:31–41. doi: 10.1016/j.toxlet.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Lambright CS, Ostby JS, Parks-Saldutti L, Vandenbergh JG, Gray LE., Jr Prenatal testosterone exposure permanently masculinizes anogenital distance, nipple development, and reproductive tract morphology in female Sprague-Dawley rats. Toxicological sciences : an official journal of the Society of Toxicology. 2007b;96:335–345. doi: 10.1093/toxsci/kfm002. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Vandenbergh JG. The anogenital distance index of mice (Mus musculus domesticus): an analysis. Contemporary topics in laboratory animal science / American Association for Laboratory Animal Science. 2005;44:46–48. [PubMed] [Google Scholar]
- Jiménez R, Burgos M, Caballero L, Dıaz de la Guardia R. Sex reversal in a wild population of Talpa occidentalis (insectivora, mammalia. Gen Res Camb. 1988;52:135–140. [Google Scholar]
- Jiménez R, Burgos M, Sánchez A, Sinclair AH, Alarcon FJ, Marin JJ, Ortega E, Diaz de la Guardia R. Fertile females of the mole Talpa occidentalis are phenotypic intersexes with ovotestes. Development. 1993;118:1303–1311. doi: 10.1242/dev.118.4.1303. [DOI] [PubMed] [Google Scholar]
- Jost A. Problems of fetal endocrinology: The gonadal and hypophyseal hormones. Rec Prog Horm Res. 1953;8:379–418. [Google Scholar]
- Jost A. Gonadal hormones in the sex differentiation of the mammalian fetus. In: Urpsrung RL, DeHaan H, editors. Organogenesis Holt, Rinehart and Winston. New York: 1965. pp. 611–628. [Google Scholar]
- Licht P, Hayes T, Tsai P, Cunha G, Kim H, Golbus M, Hayward S, Martin MC, Jaffe RB, Glickman SE. Androgens and masculinization of genitalia in the spotted hyaena (Crocuta crocuta). 1. Urogenital morphology and placental androgen production during fetal life. J Reprod Fertile. 1998;113:105–116. doi: 10.1530/jrf.0.1130105. [DOI] [PubMed] [Google Scholar]
- Matthews LH. The estrous cycle and intersexuality in the female mole (Talpa europea Linn) Proc Zool Soc London, Series B. 1935;230:347–383. [Google Scholar]
- Mitchell RT, Mungall W, McKinnell C, Sharpe RM, Cruickshanks L, Milne L, Smith LB. Anogenital distance plasticity in adulthood: implications for its use as a biomarker of fetal androgen action. Endocrinology. 2015;156:24–31. doi: 10.1210/en.2014-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman HW, Duke KL. Comparative morphology of the mammalian ovary. University of Wisconsin Press; London: 1973. [Google Scholar]
- Murakami R. Histogenesis of the os penis and os clitoridis in rats. Development, Growth and Differentiatiion. 1984;26:419–426. doi: 10.1111/j.1440-169X.1984.00419.x. [DOI] [PubMed] [Google Scholar]
- Murakami R. Development of the os penis in genital tubercles cultured beneath the renal capsule of adult rats. Journal of anatomy. 1986;149:11–20. [PMC free article] [PubMed] [Google Scholar]
- Murakami R. A histological study of the development of the penis of wild-type and androgen-insensitive mice. Journal of anatomy. 1987;153:223–231. [PMC free article] [PubMed] [Google Scholar]
- Murakami R, Mizuno T. Proximal-distal sequence of development of the skeletal tissues in the penis of rat and the inductive effect of epithelium. Journal of embryology and experimental morphology. 1986;92:133–143. [PubMed] [Google Scholar]
- Rodríguez E, Jr, Weiss DA, Yang JH, Menshenina J, Ferretti M, Cunha TJ, Barcellos D, Chan LY, Risbridger G, Cunha GR, Baskin LS. New insights on the morphology of adult mouse penis. Biology of reproduction. 2011;85:1216–1221. doi: 10.1095/biolreprod.111.091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein NM, Cunha GR, Wang YZ, Campbell KL, Conley AJ, Catania KC, Glickman SE, Place NJ. Variation in ovarian morphology in four species of New World moles with a peniform clitoris. Reproduction. 2003;126:713–719. doi: 10.1530/rep.0.1260713. [DOI] [PubMed] [Google Scholar]
- Sánchez A, Bullejos M, Burgos M, Hera C, Stamatopoulos C, Diaz De la Guardia R, Jiménez R. Females of four mole species of genus Talpa (insectivora, mammalia) are true hermaphrodites with ovotestes. Molecular reproduction and development. 1996;44:289–294. doi: 10.1002/(SICI)1098-2795(199607)44:3<289::AID-MRD2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Schultz NG, Lough-Stevens M, Abreu E, Orr T, Dean MD. The baculum was gained and lost multiple times during mammalian evolution. Integrative and Comparative Biology. 2016 doi: 10.1093/icb/icw034. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara A, Campbell KL, Suzuki H. Molecular phylogenetic relationships of moles, shrew moles, and desmans from the new and old worlds. Molecular phylogenetics and evolution. 2003;27:247–258. doi: 10.1016/s1055-7903(02)00416-5. [DOI] [PubMed] [Google Scholar]
- Sinclair AW, Cao M, Baskin L, Cunha GR. Diethylstilbestrol-Induced Mouse Hypospadias: “Window of Susceptibility”. Differentiation; research in biological diversity. 2016a;91:1–18. doi: 10.1016/j.diff.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AW, Glickman SE, Baskin L, Cunha GR. Anatomy of mole external genitalia: Setting the record straight. Anatomical record. 2016b;299:385–399. doi: 10.1002/ar.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh JG, Huggett CL. The anogenital distance index, a predictor of the intrauterine position effects on reproduction in female house mice. Lab Anim Sci. 1995;45:567–573. [PubMed] [Google Scholar]
- Vom Saal FS. Cyproterone acetate exposure during gestation in mice retards fetal growth. Physiology & behavior. 1978;21:515–517. doi: 10.1016/0031-9384(78)90122-1. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Bronson FH. In utero proximity of female mouse fetuses to males: effect on reproductive performance during later life. Biology of reproduction. 1978;19:842–853. doi: 10.1095/biolreprod19.4.842. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Quadagno DM, Even MD, Keisler LW, Keisler DH, Khan S. Paradoxical effects of maternal stress on fetal steroids and postnatal reproductive traits in female mice from different intrauterine positions. Biology of reproduction. 1990;43:751–761. doi: 10.1095/biolreprod43.5.751. [DOI] [PubMed] [Google Scholar]
- Weiss DA, Rodríguez E, Jr, Cunha T, Menshenina J, Barcellos D, Chan LY, Risbridger G, Baskin L, Cunha G. Morphology of the external genitalia of the adult male and female mice as an endpoint of sex differentiation. Molecular and cellular endocrinology. 2012;354:94–102. doi: 10.1016/j.mce.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells ME. A comparison of the reproductive tracts of corcuta crocuta, hyaena hyaena and proteles cryostats. East Africa Wildlife Journal. 1968;6:63–70. [Google Scholar]
- Whitworth DJ, Licht P, Racey PA, Glickman SE. Testis-like steroid genesis in the ovotestis of the European mole, Talpa europaea. Biology of reproduction. 1999;60:413–418. doi: 10.1095/biolreprod60.2.413. [DOI] [PubMed] [Google Scholar]
- Wislocki GB. The external genitalia of the simian primates. Human Biology. 1936;8:309–347. [Google Scholar]
- Wood-Jones F. Some phases in the reproductive history of the female Mole (Talpa europea); Proceedings of the Zoological Society of London???; 1914. pp. 191–216. [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation; research in biological diversity. 2003;71:445–460. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
- Yang JH, Menshenina J, Cunha GR, Place N, Baskin LS. Morphology of mouse external genitalia: implications for a role of estrogen in sexual dimorphism of the mouse genital tubercle. The Journal of urology. 2010;184:1604–1609. doi: 10.1016/j.juro.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita F, Barrionuevo FJ, Bert P, Ortega E, Burgos M, Jiménez R. Abnormal sex-duct development in female moles: the role of anti-Mullein hormone and testosterone. The International journal of developmental biology. 2003;47:451–458. [PubMed] [Google Scholar]