Abstract
Background
As transfusion in the elderly patients has increased over the last decades, and with the aim of improving blood policy, post-transfusion red blood cell alloimmunisation, a delayed serological transfusion reaction, was investigated in patients 80 years old or over.
Materials and methods
For every adverse reaction to a transfusion, a report is sent to the French haemovigilance database. All cases of red blood cell alloimmunisation reported in the haemovigilance database were collected, and an analysis was performed on those cases in transfused patients 80 years old or over.
Results
There were 11,625 reports of red blood cell alloimmunisation from 1 January, 2008 to 31 December, 2013, of which 3,617 (31.1%) occurred in patients 80 years old or over. Among this subgroup, red blood cell concentrates were the most frequently involved blood component (3,482 reports, 96.3%). Red blood cell alloimmunisation after transfusion of platelet concentrates was also notified (132 reports, 3.7%). Anti-KEL1 was the most frequent antibody (874 reports, 24.2%). The imputability of the blood component was certain in 2,340 cases (64.7%) and probable in 1,078 (29.8%). In 2013, the incidence of red blood cell alloimmunisation was 4.14 per 1,000 transfused patients aged 80 years old or over.
Discussion
In a 6-year national survey in which 40,570 reports were made, there were 3,617 cases of red blood cell alloimmunisation in transfused recipients of 80 years old or over. This delayed serological transfusion reaction is not rare. Red blood cell concentrates were predominantly involved, but cases caused by platelet concentrates were also described. In order to prevent alloimmunisation in the elderly, several factors must be evaluated before transfusing matched red blood cell concentrates: the patient’s age, pathology and its outcome, the type of transfusion support (chronic or not), life expectancy, and blood product availability.
Keywords: alloimmunisation, red blood cell, elderly, transfusion
Introduction
Transfusion in elderly patients has increased over the last decades. In a first French study, conducted between March and December 1997, of 3,206 blood product recipients, 1,816 (57%) were over 65 years old (969 females and 847 males)1. Nine years later, a second study found that the mean age of transfused patients was 63.9 years (median 70.0 years), and that 23.7% of transfused patients were 80 years old or over2 (per 100,000 population, 18.9 between 70 and 79 years, 24.6 between 80 and 89 years and 38.0 over 90 years). Another 5 years later, a third study found the same median age, but that the proportion of transfused patients 80 years old or over had increased to 28.7%3.
The same evolution was observed in England. From data obtained during two 14-day periods in October 1999 and June 2000, Wells et al.4 saw that the mean age of transfused patients was 62.7 years (median 67 years), and that per 100,000 population, red blood cells (RBC) were received by 15,087 patients of 65 years or over vs 2,184 in those less than 65 years old. In a second study, with data collected during two 14-day periods in May and October 2004, Wallis et al.5 found that the mean age of transfusion recipients had increased to 63.6 years.
In their study of 335 transfused patients, Dzick and Medeiros6 observed that RBC alloimmunisation did not decline with age. In military veterans, Tormey et al.7 found 577 RBC alloantibodies in 443 transfused patients, of whom 418 were male (94.4%). The prevalence of RBC alloantibodies was 2.4% (443 among 18,750 transfusion records). In these male veterans, the most frequent specificity of RBC alloantibodies was anti-KEL1 (118/540; 21.9%) followed by anti-RH3 (105/540; 19.4%). The risk of RBC alloimmunisation increased in multi-transfused elderly patients. A study of RBC alloimmunisation was performed on 272 patients with myelodysplastic syndrome or chronic myelomonocytic leukaemia and chronic transfusion support8. The median age was 74 years and these patients received a median of 33 RBC components. Forty-two (15%) acquired 81 RBC alloantibodies with predominantly anti-KEL1 specificity (26/81; 32%) and anti-RH3 (19/81; 23%).
In their survey, Cobain et al.9 noted that RBC components were the most frequently transfused blood product in older patients.
In France, RBC alloimmunisation, a delayed serological transfusion reaction, is consistently reported in the national haemovigilance database of the French National Agency for Medicine and Health Products Safety (ANSM). RBC antibody screening is now mandatory 1 to 3 months after the transfusion of at least one RBC concentrate during an inpatient hospital stay or at home. In a study conducted from 2007 to 2009 in a regional setting10, among 2,169 adverse event reports in transfused patients of 80 years old or over, 240 (11.1%) were cases of post-transfusion alloimmunisation, affecting 150 females (62.5%) and 90 males (37.5%). RBC components were the most incriminated blood products (233 reports; 97.1%). Alloimmunisation against RH blood group antigens was the most frequently observed reaction (75 reports), and predominantly showed anti-RH3 specificity (52 reports). This regional study was expanded to a national scale and extended over a longer period in order to confirm that RBC alloimmunisation in elderly transfused patients is not a rare event. The findings of this survey are reported here.
Materials and methods
For each adverse reaction to a transfusion, a report is sent to the national haemovigilance system of the ANSM. From among all the adverse reaction reports collected in the database system, we analysed post-transfusion acquired RBC alloimmunisation in patients of 80 years old or over. The incidence of RBC alloimmunisation, the specificity of the RBC alloantibodies, the blood component involved and its imputability were evaluated. The χ2 test was used for the statistical analysis.
Results
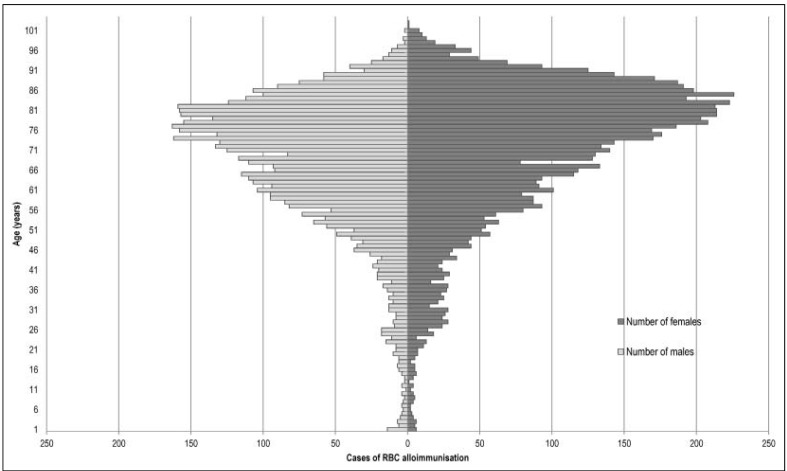
From 1 January 2008 to 31 December 2013, there were 40,570 notifications of adverse reactions in transfused patients, among which 11,625 reports (28.7%) were of RBC alloimmunisation. Of these 11,625 cases of RBC alloimmunisation, 3,617 (31.1%) involved transfused patients of 80 years old or more, including 2,435 females (67.3%) and 1,182 males (32.7%). From 2008 to 2013, an increase in RBC alloimmunisation was observed, from 1,574 cases in 2008 (including 433 in recipients 80 years old or over) to 2,185 in 2013 (including 707 in recipients 80 years old or over) (Table I). Furthermore, the cases of RBC alloimmunisation increased with age (Figure 1).
Table I.
RBC alloimmunisation notifications in France from 2008 to 2013.
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| RBC Abs | Other | RBC Abs | Other | RBC Abs | Other | RBC Abs | Other | RBC Abs | Other | RBC Abs | Other | RBC Abs | Other | |
| N. of reports | 1,574 | 4,963 | 1,715 | 5,367 | 1,827 | 4,862 | 2,118 | 4,637 | 2,206 | 4,489 | 2,185 | 4,627 | 11,628 | 28,945 |
| N. of reports in patients ≥80 years old | 433 | 940 | 534 | 1,011 | 549 | 921 | 672 | 944 | 722 | 964 | 707 | 1,032 | 3,617 | 5,812 |
RBC: red blood cell; Abs: antibodies.
Figure 1.
RBC alloimmunisation after transfusion: pyramid-shaped diagram representing the population by age-groups.
RBC: red blood cell.
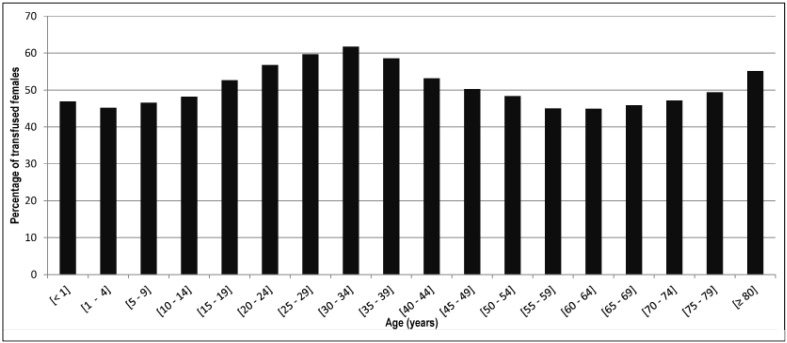
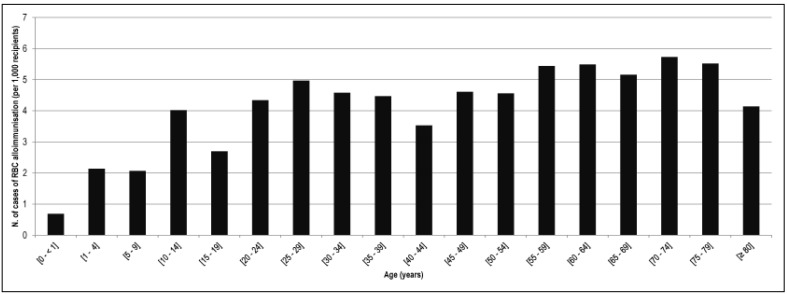
For year 2013, supplementary data were available and analysed. From 1 January, 2013 to 31 December, 2013, 473,886 patients were transfused in France. Between the ages of 15 and 49 years, the most transfused patients were females (Figure 2). Similar results were obtained in the 170,658 patients of 80 years old or over: 94,100 females (55.1%) vs 76,558 males (44.9%). This difference was statistically significant (p<0.01). Among the transfused patients, the highest incidence of the RBC alloimmunisation (over 5.0 cases per 1,000 transfused patients) was seen in patients of 55 to 79 years old (Figure 3). In transfused patients of 80 years old or over, the incidence decreased to 4.14 cases per 1,000 patients. There were 707 reported cases of post-transfusion RBC alloimmunisation, vs 1,478 in patients 79 years old or under (Table I). This difference was statistically significant (p<0.01).
Figure 2.
Percentage of transfused females by age range in 2013.
Figure 3.
Incidence of RBC alloimmunisation observed per 1,000 recipients by age range in 2013.
RBC: red blood cell.
Prior RBC alloimmunisation was notified in only 298 cases (8.2%), whereas in 3,150 cases (87.1%) there has been no prior alloimmunisation. Of the patients with prior alloimmunisation, 220 were females (73.8%) and 78 males (26.2%) (Table II). RBC antibodies with one specificity (251 cases) and two specificities (45 cases) were predominantly observed in female patients, in 184 (73.3%) and 35 (77.7%) cases, respectively.
Table II.
Number and percentage of prior RBC alloimmunisations observed in all transfused patients: divided by the number of RBC antibody specificities and by sex.
| Prior RBC alloimmunisation | Sex | |||
|---|---|---|---|---|
|
| ||||
| N. of prior antibodies | Female | Male | % female | Total |
| 1 | 184 | 67 | 73.3 | 251 |
| 2 | 35 | 10 | 77.7 | 45 |
| ≥3 | 1 | 1 | 50.0 | 2 |
|
| ||||
| Total | 220 | 78 | 73.8 | 298 |
RBC: red blood cell.
RBC concentrates were the most frequently involved blood component (3,482 reports; 96.3%). Platelet concentrates were implicated in only 132 cases (3.7%). The mean delay between the implicated blood transfusion and the diagnosis of new RBC alloantibodies was 193.6±417.7 days.
The most frequently detected RBC antibody was anti-KEL1 (874 reports; 24.2%) followed by anti-RH3 (777 reports; 21.5%) and anti-JK1 (572 reports; 15.8%). For other RH blood group antigens, anti-RH4 was notified in 229 reports (6.3%), anti-RH2 in 189 (5.2%), anti-RH1 in 172 (4.8%) and anti-RH5 in 44 (1.2%). A combination of two or more RBC alloantibodies was notified in 587 reports (16.2%).
The imputability of the transfused blood component was considered as certain in 2,340 reports (64.7%) because of the known presence of the involved RBC antigen(s) during the transfusion. It was considered as probable in 1,078 case (29.8%), when the presence of the antigen was unknown, but no other source of alloimmunisation was found. The imputability was possible in 161 cases (4.5%) and unlikely or excluded in 35 (0.9%). Lastly, the imputability could be not evaluated in three cases.
Discussion
The number of RBC alloimmunisation reports increased between 2008 and 2012 (Table I), and this delayed serological transfusion reaction was predominantly observed in female patients (67.3%). Several factors could explain these results. Firstly, in France, the life expectancy of females is longer than that of males. Secondly, transfused patients were mainly 70 years old and over2–3. Among 473,886 recipients in 2013, 275,080 were over 70 years old (58.0%), and 170,658 were 80 years old or over (36.0%). More than 50.0% of transfused patients between 15 and 49 years old were females and women also predominated among the transfused patients 80 years old or over (55.1%) (Figure 2). Longer life expectancy in females and higher age of transfused patients could explain the elevated prevalence of transfusion in females (55.1%) among patients 80 years old or over in 2013. Thirdly, from birth to 50 years old, women who are transfused must be given RH (RH2, RH3, RH4, and RH5) and KEL (KEL1) matched RBC concentrates. Above the age of 50 years, a male or female patient without a RBC antibody may be transfused with RH KEL (RH2, RH3, RH4, RH5, and KEL1) non-matched RBC concentrates. The prevalence of RBC antibodies is higher in female recipients. In a study of 55,350 transfusion recipients including 29,580 females and 25,770 males, Spielmann and Seidl11 found 322 RBC antibodies in females (1.09%) vs 121 in males (0.47%). As regards the clinically significant RBC antibodies that are mostly involved in immediate (KEL and JK) and delayed (RH, FY and MNS) transfusion reactions, Saverimuttu et al.12 observed that the risk of these anitbodies increases with the age of the recipient and is higher in females.
In our study, RBC alloimmunisation in recipients prior to the reported transfusion reactions was infrequent (8.2%). This observation confirms that patients of 80 years old or over can acquire new RBC alloantibodies after transfusion and that, despite their age, the ability of their immune system to produce these antibodies remains preserved, as reported by Dizk and Medeiros6. Nevertheless, due to antibody evanescence, a prior RBC alloimmunisation caused by a pregnancy (30 or more years previously) in female patients cannot be formally excluded by a negative RBC alloantibody screening at the time of the incriminated transfusion. The same caveat applies to male and female patients who were previously transfused. In our study, prior RBC alloimmunisation was predominantly observed in female recipients (Table II).
The most frequently involved blood products were RBC concentrates (96.3% of cases). Cobain et al.9 observed that RBC products are the most transfused blood component in older patients. However, RBC alloimmunisation can also develop after a platelet concentrate transfusion in patients 80 years old or over, albeit with a lower incidence (3.7% in our study). The volume of residual RBC in platelet concentrates is variable13, and RBC-derived microparticles may be involved14. Nevertheless, despite the low residual volume of RBC in platelet blood products, elderly patients are able to produce alloantibodies to these RBC.
The period between the transfusion and detection of RBC alloimmunisation in patients was long, being a mean of 194.3 days, despite the fact that it is now mandatory in France to perform a RBC antibody screen between 1 to 3 months after at least one RBC concentrate transfusion. It is not registered in the haemovigilance system whether RBC alloimmunisation is detected at that control screening or later, just before a new transfusion. The lack of this information means that a conclusion cannot be reached on the issue of delayed alloimmunisation. Schonewille et al.15 recently showed that more antibodies are found after a long period, but that some immunisations are only detectable after a short period. Safety surely commands that we combine a control test some weeks after transfusion and another screen before each new RBC transfusion.
Anti-KEL1 alone or associated with other specificities was the alloantibody most frequently notified in the post-transfusion RBC alloimmunisation reports (24.2%) followed by anti-RH3 (21.5%). In a study of male military veterans, Tormey et al.6 also observed that anti-KEL1 (21.9%) and anti-RH3 (19.4%) were the most frequent RBC alloantibodies. The incidence of anti-JK1 was lower (3.7%) in their study than in ours (15.8%), but our study population was different, as it included both female and male transfusion recipients.
Conclusions
Our 6-year national survey in France shows that post-transfusion RBC alloimmunisation in elderly patients is not a rare delayed serological transfusion reaction, and that RBC concentrates are the most frequently involved blood component.
Prevention of RBC alloimmunisation in elderly patients needs to be improved. Nevertheless, several factors must be considered when using matched RBC concentrates: the patient’s age, pathology and its outcome, the type of transfusion support (chronic or not), the patient’s life expectancy, and blood product availability.
Footnotes
Authorship contributions
PM designed the study, analysed the data and wrote the paper. GB collected and analysed the data. JYP and
The Authors declare no conflicts of interest.
References
- 1.Mathoulin-Pélissier S, Salmi LR, Verret C, Demoures B for the RECEPT investigators. Blood transfusion in a random sample of hospitals in France. Transfusion. 2000;40:1140–6. doi: 10.1046/j.1537-2995.2000.40091140.x. [DOI] [PubMed] [Google Scholar]
- 2.Quaranta JF, Berthier F, Courbil R, et al. [Who uses blood? Results of a cross-sectional and nationwide survey]. Transfus Clin Biol. 2009;16:21–9. [In French.] [Google Scholar]
- 3.Rapport de l’enquête nationale: Un jour donné 2011 Etablissement français du sang. 2013. [Accessed on 15/04/2016]. Available at: https://www.dondusang.net/rewrite/article/5702/efs/actualites-nationales/utilisation-des-produits-sanguins:l-efs-a-realise-une-etude-nationale.htm?idRubrique=899.
- 4.Wells AW, Mounter PJ, Chapman CE, et al. Where does blood go? Prospective observational study of red cell transfusion in north England. BMJ. 2002;325:1–4. doi: 10.1136/bmj.325.7368.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallis JP, Wells AW, Chapman CE on behalf of the Northern Regional Transfusion. Changing indications for red cell transfusion from 2000 to 2004 in the North of England. Transfus Med. 2006;16:411–7. doi: 10.1111/j.1365-3148.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 6.Dzik WH, Medeiros LJ. Age and erythrocyte alloimmunization. Vox Sang. 1986;51:73. doi: 10.1111/j.1423-0410.1986.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 7.Tormey CA, Fisk J, Stack G. Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion. 2008;48:2069–76. doi: 10.1111/j.1537-2995.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- 8.Sanz C, Nomdedeu M, Belkaid M, et al. Red blood cell alloimmunization in transfused patients with myelodysplastic syndrom or chronic myelomonocytic leukaemia. Transfusion. 2013;53:710–5. doi: 10.1111/j.1537-2995.2012.03819.x. [DOI] [PubMed] [Google Scholar]
- 9.Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfus Med. 2007;17:1–15. doi: 10.1111/j.1365-3148.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- 10.Moncharmont P, Meyer F. [Appearance of anti-red cell antibodies in 80 years old and over transfused patients: result of 3 years haemovigilance survey]. Transfus Clin Biol. 2010;17:249–53. doi: 10.1016/j.tracli.2010.08.002. [In French.] [DOI] [PubMed] [Google Scholar]
- 11.Spielmann W, Seidl S. Prevalence of irregular red cell antibodies and their significance in blood transfusion and antenatal care. Vox Sang. 1974;26:551–9. doi: 10.1111/j.1423-0410.1974.tb02731.x. [DOI] [PubMed] [Google Scholar]
- 12.Saverimuttu J, Greenfield T, Rotenko I, et al. Implications for urgent transfusion of uncrossmatched blood in the emergency department: The prevalence of clinically significant red cell antibodies within different patients group. Emerg Med. 2003;15:239–43. doi: 10.1046/j.1442-2026.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 13.Lozano M, Cid J. The clinical implications of platelet transfusions associated with ABO or Rh(D) incompatibility. Transfus Med Rev. 2003;17:57–68. doi: 10.1053/tmrv.2003.50003. [DOI] [PubMed] [Google Scholar]
- 14.Kitazawa J, Nollet K, Morioka H, et al. Non-D Rh antibodies appearing after apheresis platelet transfusion: stimulation by red cells or microparticules? Vox Sang. 2011;100:395–400. doi: 10.1111/j.1423-0410.2010.01435.x. [DOI] [PubMed] [Google Scholar]
- 15.Schonewille H, Honohan A, van der Watering LMG, et al. Incidence of alloantibody formation after ABO-D or extended matched red blood cell transfusions: a randomized trial (MATCH study) Transfusion. 2016;56:311–20. doi: 10.1111/trf.13347. [DOI] [PubMed] [Google Scholar]