Abstract
Autoimmune haemolytic anaemia is an uncommon disorder to which paediatric haematology centres take a variety of diagnostic and therapeutic approaches. The Red Cell Working Group of the Italian Association of Paediatric Onco-haematology (Associazione Italiana di Ematologia ed Oncologia Pediatrica, AIEOP) developed this document in order to collate expert opinions on the management of newly diagnosed childhood autoimmune haemolytic anaemia.
The diagnostic process includes the direct and indirect antiglobulin tests; recommendations are given regarding further diagnostic tests, specifically in the cases that the direct and indirect antiglobulin tests are negative. Clear-cut definitions of clinical response are stated. Specific recommendations for treatment include: dosage of steroid therapy and tapering modality for warm autoimmune haemolytic anaemia; the choice of rituximab as first-line therapy for the rare primary transfusion-dependent cold autoimmune haemolytic anaemia; the indications for supportive therapy; the need for switching to second-line therapy. Each statement is provided with a score expressing the level of appropriateness and the agreement among participants.
Keywords: autoimmune haemolytic anaemia, child, DAT, diagnosis therapy
Introduction
Autoimmune haemolytic anaemia (AIHA) in childhood is an uncommon condition caused by the presence of auto-antibodies directed against antigens on the surface of red blood cells, leading to premature destruction of the cells1–3. The overall annual incidence is reported to be 1–3 cases/100,000 people and approximately 0.2 cases/1,000,000 individuals under 20 years of age4–8, although these figures are probably underestimates, partly because of the lack of understanding of the diagnostic tools. Nevertheless, AIHA is an important issue for many paediatricians, who have to deal with the diagnosis and treatment of critically ill patients in the absence of consistent support from the literature. On this background, the Red Cell Working Group of the Italian Association of Paediatric Onco-haematology (Associazione Italiana di Ematologia ed Oncologia Pediatrica, AIEOP) developed this document with the aim of providing shared recommendations for paediatricians.
These consensus recommendations are not intended as standards or fixed rules, but as an instrument to support paediatricians in the diagnostic work-up and initial treatment of newly diagnosed patients with AIHA.
Materials and methods
The design and methodology implemented to draw up these recommendations were similar to those adopted for the AIEOP Consensus Guidelines on childhood aplastic anaemia, congenital and acquired neutropenias, and immune thrombocytopenias9–11 using a procedure validated by the AIEOP board. In particular, representatives from 20 AIEOP centres and 10 other non-AIEOP centres participated in the AIHA Committee. Issues to be addressed in the Recommendations were identified by the whole Committee; each topic was developed by a subgroup in a single document, which included a brief description of the state-of-the-art knowledge, followed by specific recommendations.
In order to draw up the pre-guideline documents, the authors extracted evidence from the literature included in the Medline database (initially searched from January 1,1991 to December 31, 2015, and then updated in February 2016 during the compilation of the final draft). Search terms included: autoimmune haemolytic anaemia (AIHA), warm autoimmune haemolytic anaemia, warm antibodies, cold autoimmune haemolytic anaemia, direct antiglobulin test (DAT), indirect antiglobulin test (IAT), therapy, diagnosis, paediatric, children. The MEDLINE® search yielded 326 articles that were examined, 76 of which were selected as they offered data related to the topics of the present paper. The search was also extended to older papers, specifically retrieved following cited references, and to haematology textbooks. All the evidence collected was attributed a strength that was scored using the level of evidence criteria reported in Table I.
Table I.
Levels of evidence for studies evaluating the diagnosis and therapy of AIHA in children.
| Level of evidence | Study design |
|---|---|
| I (strongest) | Prospective randomised trial with high statistical value |
| II | Prospective randomised trial with lower statistical value |
| III | Non-randomised study with concurrent control group |
| IV | Non-randomised study with historical control group |
| V | Case report(s) with no control group |
Each draft was reviewed by the entire Committee and modified accordingly after exhaustive discussion. The Committee prepared statements that were then subjected to validation during the Consensus Conference, during which 44 participants scored the final items.
The strength of consensus was quantified on a 1–9 scale where 1 represented no consensus and 9 represented full consensus regarding the appropriateness and necessity of the practice. For each statement a mean score was calculated. Mean scores from 1 to 3 indicated an inappropriate practice, mean scores from 3.1 to 6.9 indicated a practice of uncertain appropriateness and mean scores from 7 to 9 signified an appropriate/necessary practice. The level of agreement among participants, indicating the rate of consensus, was also graded by evaluating the distribution of the standard deviations (SD) within each statement and then dividing the level of agreement into four categories:
strong agreement (variation more than 1 SD below the average of the variances, on a logarithmic scale);
moderate agreement (variation less than 1 SD below the average of the variances);
moderate disagreement (variation less than 1 SD above the average of the variances);
strong disagreement (variation more than 1 SD above the average of the variances).
Diagnosis of autoimmune haemolytic anaemia
Classification and initial evaluation
According to pathogenesis, AIHA can be defined as primary, with no other underlying condition (37%), post-infective (10%), or secondary (53%); in this last case, AIHA is part of a more complex disease, usually of immunological, infective or neoplastic nature1–4,12–25. AIHA can be classified based on both the thermal properties of the antibodies involved and the immunoglobulin class, as reported in Table II.
Table II.
Characteristics of the various forms of AIHA.
| Clinical form | Frequency (%) | DAT | Ig class | Thermal optimum (°C) | Avidity and ability to fix complement | Antigen specificity | Site of haemolysis |
|---|---|---|---|---|---|---|---|
| Warm antibodies | 60–70 | IgG+ or IgG+/C3d+ | IgG | 34–37 | −/+ | Anti-Rh | Extravascular |
| Cold antibodies | 20–25 | Neg. or C3d+ | IgM | 4–27 | +++ | Anti-I | Extravascular and intravascular |
| Cold paroxysmal haemoglobinuria | 6–12 | Neg. or C3d+ | IgG Biphasic |
Fixing 4–27 Lysis 34 37 |
+++ | Anti-P | Intravascular |
| Mixed AEA | < 5 | IgG+ or IgG+/C3d+ or C3d+ | IgG/IgM | IgG 34–37 IgM 4–27 |
++ | Anti-Rh Anti-I |
Extravascular and intravascular |
AIHA: autoimmune haemolytic anaemia; DAT: direct antiglobulin test; Ig: immunoglobulin; AEA: anti-erythrocyte autoantibodies; Neg.: negative.
The panel of experts outlined the importance of the initial evaluation, including history and physical examination, based on the first-level tests listed in Table III; the consensus on the appropriateness of these tests was 8.9-A. Whole blood count, together with reticulocyte count, haptoglobin, lactate dehydrogenase (LDH) and bilirubin are the parameters to define haemolytic anaemia. The reticulocyte count is typically high, as a consequence of increased erythropoiesis; nevertheless, in up to 39% of children, AIHA can be associated with initial reticulocytopenia1,4, due to antibodies against erythroid precursors, induction of apoptosis of bone marrow erythroblasts, or a concomitant viral infection, e.g. Parvovirus B1926–30. The search for auto-antibodies, which clarifies the immune mechanism involved in the haemolytic anaemia, is discussed below. Haemoglobinuria is found in the case of intravascular haemolysis and may be manifested be the emission of overtly dark urine. Table IV reports a list of additional tests, some of which may be appropriate, according to the patient’s particular situation, in order to identify underlying disorders in secondary forms of AIHA (8.3-B).
Table III.
First level tests.
| WBC count, red cell morphology on peripheral smear |
| Reticulocyte count |
| Indices of haemolysis (haptoglobin, indirect bilirubin, LDH) |
| DAT and IAT |
| Blood group |
| Liver and kidney function |
| Urinanalysis |
WBC: white blood cell; LDH: lactate dehydrogenase; DAT: direct antiglobulin test; IAT: indirect antiglobulin test.
Table IV.
Second-level tests.
| Extensive red blood cell typing in anticipation of possible transfusion |
| Further immune-haematological investigations: C3, C4, CH50 |
Auto-antibodies (ANA, anti DNA), antiphospholipid antibodies, RA test
|
| Hepatitis B and C, markers and HIV serology |
| Coagulation screen blood test |
| Serum total protein and protein electrophoresis |
| Immunoglobulin class quantification |
| C-reactive protein |
| EBV, CMV, Parvovirus B19, HSV serology |
| Other assessments for infectious diseases, as clinically appropriate |
C3: complement component 3; C4: complement component 4; CH50: total component activity; ANA: antinuclear antibodies; RA test: rheumatoid factor test; TG: thyroglobulin antibody; TPO: thyroid peroxidase antibody; HIV: human immunodeficiency virus; EBV: Epstein-Barr virus; CMV: cytomegalovirus; HSV: herpes simplex virus.
Bone marrow aspiration is not usually required: this investigation is only recommended in the presence of another associated cytopenia or persistent reticulocytopenia or on suspicion of neoplasia or myelodysplasia (8.7-B). The evaluation of bone marrow usually highlights maturing erythroid hyperplasia, sometimes with mild dyserythropoiesis and no abnormality of the remaining haematopoietic series1.
Immune-haematological diagnosis: the direct antiglobulin test
Laboratory diagnosis of AIHA is based on the demonstration of auto-antibodies adhering to autologous erythrocytes (the direct antiglobulin test [DAT] or direct Coombs’ test) and free in the serum (the indirect antiglobulin test [IAT] or indirect Coombs’ test)2.
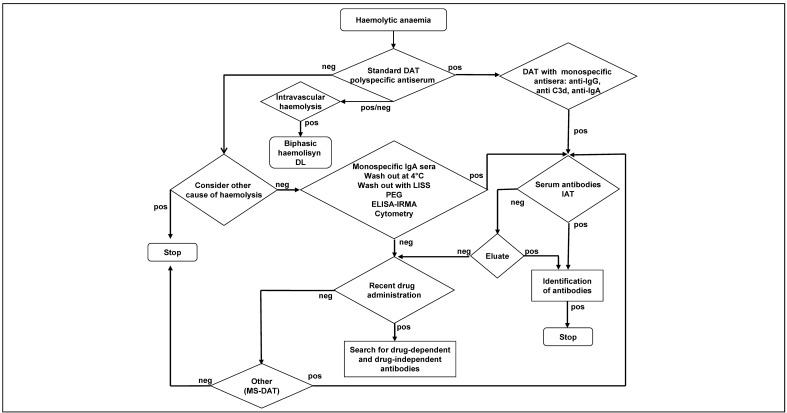
The DAT is performed first with polyspecific sera, capable of identifying both IgG and C3 on red blood cells, using various methods characterised by different sensitivities and specificities. If the screening DAT performed with polyspecific sera is positive, the use of monospecific anti-IgG and anti-C3 antiserum is recommended (8.8-A).
The search for antibodies adhering to red blood cells (DAT) must be combined with a search for irregular antibodies in the serum (IAT) and, if found, their identification (8.7-A).
The IAT can be positive even in the presence of auto-antibodies, detectable in 34% of cases of AIHA31–36, in addition to various other conditions of alloimmunisation (prior transfusion, pregnancy, etc.). Finally, in the case of a negative IAT, it is appropriate to repeat the search and the possible identification on the eluate of the red blood cells. If serum/eluate IAT are both negative, it is appropriate to consider a drug-induced AIHA (8.8-A).
In the case of “verified or suspected” AIHA with intravascular haemolysis, regardless of the result of the DAT (negative DAT and/or DAT positive for IgG and/or C3d), a search for the Donath-Landsteiner auto-antibody is recommended (8.8-A). This IgG auto-antibody, also known as biphasic haemolysin, cleaves complement at low temperatures and causes haemolysis at 37 °C and allows the diagnosis of paroxysmal cold haemoglobunuria.
Direct antiglobulin test-negative autoimmune haemolytic anaemia
Some cases of AIHA are not diagnosed by the commonly used DAT. The reported frequency of these DAT-negative AIHA is about 10%2,37. The diagnosis of DAT-negative AIHA, after the exclusion of other non-immune-mediated causes of haemolysis (listed in Table V), requires additional tests (8.7-B) with monospecific anti-IgA sera, since the content of anti-IgA immunoglobulin in polyspecific sera may be inadequate to recognise the presence of IgA antibodies2,38, and low ionic strength solutions (LISS) and/or polyethylene glycol (PEG) in order to detect low-affinity antibodies that can be washed out with routine methods35,39,40.
Table V.
Non-immune-mediated haemolytic anaemias.
| Congenital forms | Spherocytosis and other defects of the:
|
| Haemolytic anaemia from mechanical causes | Synthetic heart valves; march haemoglobinuria; cardiopulmonary bypass. |
| Haemolytic anaemia due to vascular injury | Microangiopathic anaemia; thrombotic thrombocytopenic purpura; haemolytic-uraemic syndrome; disseminated intravascular coagulation; arterio-venous malformations. |
| Haemolytic anaemia due to thermal damage | Extensive burns. |
| Haemolytic anaemia from chemical causes | Chemicals solvents; methyl chloride; lead; arsenic and hydrogen; snake venom. |
| Haemolytic anaemia due to infectious agents | Bacteria (Mycoplasma pneumoniae, Clostridium welchii); viruses (cytomegalovirus, herpes virus); protozoa (Plasmodium spp.). |
If the DAT is still negative, it is appropriate to use more sensitive methods, capable of detecting a smaller number of IgG adhering to red cells (8.3-B). These methods are: flow cytometry, able to detect even 30–40 molecules of IgG/red cell41, immunoenzymatic42–44 and immunoradiometric tests45, a complement-fixing antibody consumption test46, and tests after mitogenic stimulation in culture (MS-DAT)47.
“Warm” IgM autoimmune haemolytic anaemia
On rare occasions, haemolysis is due to atypical warm IgM antibodies, which are capable of inducing spontaneous red cell agglutination in vivo, resulting in more pronounced haemolysis and a very severe clinical picture48. Recognition of such antibodies may be difficult since the DAT may be positive for C3, or less frequently IgG and C3, so this form is often misdiagnosed as a cold or mixed AIHA. Positivity for C3 in a warm AIHA should lead to a suspicion of IgM involvement. Furthermore, some cases can be negative with the usual DAT. Therefore, a double DAT (dual direct antiglobulin test, DDAT), capable of revealing the presence of weak or non-agglutinating “warm” IgM auto-antibodies49, is recommended in severe cases with signs of in vivo agglutination (8.3-B).
Drug-induced autoimmune haemolytic anaemia
Drug-induced antibodies are “drug-independent” if they can be identified with the common techniques of antibody research (IAT), and “drug-dependent” if they need the presence of the drug in the reaction system (immune complex mechanism, DAT positive for complement), or pre-treatment of erythrocytes with drugs (hapten/neoantigen formation, DAT positive for IgG)2–16.
In the case of DAT-positive and serum/eluate IAT-negative AIHA, associated with recent administration of drugs, consideration of drug-induced AIHA is recommended (8.8-A).
A comprehensive summary of the diagnostic work up is reported in Figure 1.
Figure 1.
Diagnostic work up.
neg: negative; DAT: direct antiglobulin test; pos: positive; DL: biphasic haemolysins of Donath-Landsteiner; IAT: indirect antiglobulin test; LISS: low ionic strength; PEG: polyethylene glycol; ELISA: enzyme-linked immunosorbent assay; IRMA: immunoradiometric tests; MS-DAT: direct antiglobulin test after mitogenic stimulation.
Treatment of autoimmune haemolytic anaemia
Preliminary to the discussion of therapy, the response criteria were defined. A complete response was defined as the achievement of a haemoglobin (Hb) concentration greater than or equal to the lower normal limit for age, with no signs of haemolysis, i.e. normal reticulocyte count and bilirubin concentration (8.9-A). A partial response was defined as an increase of Hb of ≥2 g/dL, without the Hb concentration reaching a normal value for the patient’s age (8.5-B). No response was defined as an increase of Hb <2 g/dL and/or dependence on transfusions (8.4-B).
Warm autoimmune haemolytic anaemia
Pharmacological treatment
Steroids are the first-choice treatment in all cases of warm-type AIHA50–54. Initial treatment involves the use of oral prednisone at a dose of 1–2 mg/kg/day (8.6-B); in the case of poor compliance to oral administration, intravenous methylprednisolone can be used (0.8–1.6 mg/kg/day) (8.6-B); in severe cases, a higher initial dose may be indicated, i.e. intravenous methylprednisolone 1–2 mg/kg every 6–8 hours for 1–3 days (7.9-C). Routine use of high-dose steroids is not recommended (7.1-D).
Intravenous immunoglobulins have been used in AIHA in addition to steroids1,55–61. In a review of 73 cases of AIHA, Flores et al. concluded that treatment with intravenous immunoglobulins (0.4–0.5 g/kg for 5 days) was effective in 39.7% of patients, with a higher efficacy (54.5%) in children55. Intravenous immunoglobulins may, therefore, be indicated as adjunctive therapy to steroids, in more severe cases (7.8-B).
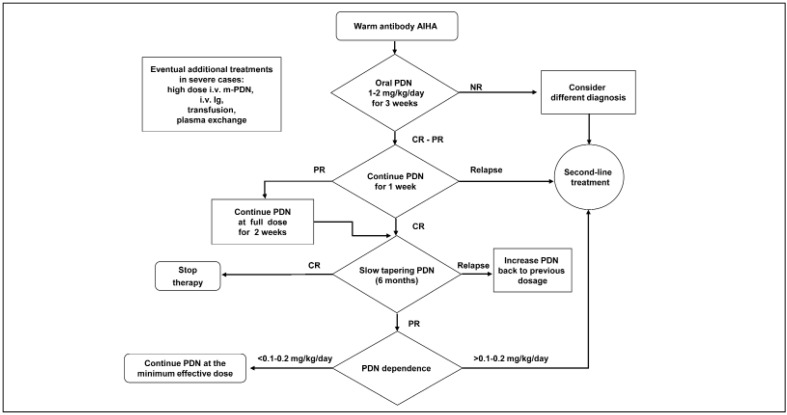
A comprehensive therapeutic algorithm is proposed in Figure 2. Steroid tapering should always be slow, in order to extend the treatment for at least 6 months (7.8-C): a gradual and sustained reduction of the steroid dose correlates with a lower incidence of relapse62.
Figure 2.
First-line therapy of warm antibody AIHA.
If steroid induces no response at 3 weeks, having excluded a different diagnosis, the patient is shifted to second line treatment (8.6-A). For responsive patients, initial steroid therapy should last at least 4 weeks (8.6-B); in the case of CR, steroid can be tapered (8.8-B); in the case of PR, the full dosage should be continued for 2 more weeks. In any case, after 6 weeks, steroid must be tapered (8.4-B). Tapering schedule: the full dosage is reduced by 25–50% over 4 weeks, thereafter, the reduction must be gradual, in order to extend the treatment for at least 6 months (7.8-C); if a relapse or exacerbation of the hemolysis is observed, during the tapering process, the dosage should be brought back at the previous level (8.2-C).
AHIA: autoimmune haemolytic anaemia; PDN: prednisone; Ig: immunoglobulin; NR: no response, CR: complete response, PR: partial response.
The indications to start a second-line therapy are no response to the first-line treatment (9-A), or steroid dependence, with a prednisone dosage of 0.1–0.2 mg/kg/day, the Consensus being uncertain on whether to fix the dosage threshold at 0.1 (5.3-D) or 0.2 (6.3-D) mg/kg/day.
Transfusion therapy
Transfusion of packed red blood cells is not a routine treatment for several reasons: often it is difficult to find red cell units matched to the recipient, both because of the auto-antibodies reacting with the donor’s red blood cells and because of the possible simultaneous presence of allo-antibodies. Auto- and allo-antibodies may be responsible for the destruction of transfused red cells, with exacerbation of the haemolytic process63. Transfusion should, therefore, be reserved to cases of very severe anaemia, in patients with impairment of vital signs (8.5-C). It is recommended that the patient’s blood samples are tested for a timely and complete definition of red cell phenotype and detection of possible allo-antibodies masked by auto-antibodies (8.5-B). Extensive red cell antigen typing is recommended with the goal of improving the transfusion performance; the typing should preferably be performed using molecular methods, including at least: C, c, D, E, e, K, Jka, Jkb, Fya, Fyb, S, and s (8.6-B). It is recommended that only quantities sufficient to improve symptoms (approximately 3–5 mL/kg) are transfused, in order to minimise the complications of overload and incompatibility (8.6-B). Packed red blood cells for transfusion in this context must be leucodepleted, preferably prior to storage (8.8-A). the transfusion must be performed slowly, under careful supervision and within the maximum time allowed of 4 hours (8.5-B).
Plasma exchange
Thorough systematic studies regarding plasma exchange in paediatric AIHA are lacking and clinical data are limited to case reports64–66. The rationale of using plasma exchange is to remove circulating immune complexes, complement activated components and circulating auto-antibodies. It is estimated that each cycle can remove up to 65% of the circulating auto-antibodies, so, it is frequently necessary to repeat the procedure.
Plasma exchange is an option that should be considered only for extremely severe cases of AIHA, with no response to either transfusion or pharmacological therapy (8.0-C). It should be taken into account as an alternative to splenectomy in the case of emergency (7.2-C). Given its indication for use in critically ill patients, it should be performed in centres with solid paediatric experience.
Cold autoimmune haemolytic anaemia
Cold antibody AIHA is usually secondary, mostly associated with bacterial or viral infections (Mycoplasma pneumoniae, Epstein-Barr virus, varicella, hepatitis C, rubella, parvovirus, mumps, cytomegalovirus)1; therapy is, therefore, based on control of the underlying disease. Useful measures include: keeping the patient warm, maintaining adequate hydration, and monitoring urine output in the presence of significant intravascular haemolysis. If red cell transfusion is required, it is advised that the erythrocytes are pre-heated during transfusion with a heat generator inside the tubing. Exchange transfusion can be a valid treatment option in the acute phase of AIHA because both the sensitised red blood cells and the circulating auto-antibodies can be removed simultaneously. Plasma exchange may be considered, with AIHA mediated by IgM having a better response to this procedure than that mediated by IgG, probably because of the different dimensions of the two types of molecules and the prevalent distribution of IgM within the circulation67.
Pharmacological treatment is indicated only for the rare, primary transfusion-dependent forms (8.2 -B). Steroids induce a limited clinical response in less than 15% of adults affected by primary forms of cold AIHA67,68. There is anecdotal evidence regarding beneficial effects of high-dose intravenous steroid therapy69–71. The recommendation to use steroids is of uncertain appropriateness (6.9-D) and the administration of alpha-interferon or immunosuppressants had disappointing results67,68,72,73. Rituximab, a humanised chimeric monoclonal antibody directed against the CD20 antigen expressed by B lymphocytes, proved to be effective and well-tolerated: two prospective trials in adults, although neither controlled nor randomised, showed that rituximab induced a response in 45–60% of cases74,75; this percentage rose with the addition of fludarabine76. Rituximab can, therefore, be considered the first choice of treatment for primary forms of cold AIHA (8.0-C). The average dose is 375 mg/m2/week for 4 weeks; the response is independent of age, previous treatment, and association with other drugs.
Conclusions
The present report represents a valuable effort to support paediatricians by providing a tool for streamlining diagnosis and managing first-line therapy of childhood AIHA. Notwithstanding the limitations of opinion-based recommendations, the Committee achieved a broad consensus on issues related to how to treat children newly diagnosed with AIHA, yielding a comprehensive review of all relevant clinical aspects.
Acknowledgements
*Collaborators, all members of the AIEOP AIHA Committee:
- Carlo Baronci (Department of Paediatric Haematology and Oncology, “Bambino Gesù” Paediatric Hospital, Rome);
- Anna M. Casadei (Department of Paediatrics, “La Sapienza” University, Rome);
- Maddalena Casale (Department of Women, Children and General and Specialised Surgery, Second University of Naples, Naples);
- Tommaso Casini (Paediatric Onco-Hematology, “Meyer” University Hospital, Florence);
- Giovanni Cazzaniga (Paediatric Clinic, University of Milan-Bicocca, “M. Tettamanti” Research Centre, Monza);
- Andrea Ciliberti (Paediatric Onco-haematology Unit “Casa Sollievo della Sofferenza” Hospital, IRCCS, San Giovanni Rotondo);
- Serelina Coluzzi (Immunohaematology and Transfusion Medicine Unit, Policlinico Umberto I, “La Sapienza” University, Rome);
- Paola Corti (Paediatric Department, University of Milan-Bicocca, San Gerardo Hospital, Monza);
- Elena Facchini (Department of Paediatrics, “Lalla Seràgnoli” Haematology-Oncology Unit, University of Bologna, Bologna);
- Fiorina Giona (Division of Haematology, Department of Cellular Biotechnologies and Haematology, “La Sapienza” University of Rome, Rome);
- Paola Giordano (“F. Vecchio” Paediatric Unit, University Hospital, Policlinico, Bari);
- Ilaria Lazzareschi (Division of Paediatric Oncology, Catholic University of Rome, Rome);
- Agostino Nocerino (Department of Paediatrics, “S. Maria della Misericordia” University Hospital, Udine);
- Paolo Perseghin (Transfusion Medicine Service, Apheresis Unit, “San Gerardo” Hospital, Monza);
- Daniela Peruccio (Immunohaematology, OIRM Sant’Anna, Turin);
- Angelamaria Petrone (Paediatrics, APSS Trento, Rovereto);
- Antonella Sau (Paediatric Onco-hematology Unit, Department of Haematology, P.O. “Spirito Santo”, Pescara);
- Raffaella Schirò (Paediatric Haematology, “V. Buzzi” Hospital, ICP, Milan);
- Fabio Tucci (Paediatric Onco-Haematology, “Meyer” University Hospital, Florence);
- Manuela Tumino (Paediatric Onco-haematology Clinic, University of Padua, Padua);
- Isabella Vasta (Paediatric Onco-haematology Unit, “Vito Fazi” Hospital, Lecce);
- Marco Zecca (Paediatric Haematology-Oncology and Research Laboratories, “Fondazione IRCCS Policlinico San Matteo”, Pavia).
We also thank Fabio Pellegrini, Massimiliano Copetti, and Andrea Fontana (Biostatistics Unit at the “Casa Sollievo della Sofferenza” Hospital, San Giovanni Rotondo) for their statistical analysis.
Footnotes
The Authors declare no conflicts of interest.
References
Where possible, “A” is added for studies conducted in adults, and “P” for those conducted in paediatric patients and the level of evidence (I–V), as described in Table I, is stated.
- 1.Ware RE. Autoimmune hemolytic anemia. In: Orkin SH, Nathan DG, Ginsburg D, et al., editors. Nathan and Oski’s Hematology of Infancy and Childhood. Philadelphia, PA: Saunders Elsevier; 2009. pp. 613–58. [Google Scholar]
- 2.Petz LD, Garratty G. Immune hemolytic anemias. New York: Churchill Livingstone; 2004. [Google Scholar]
- 3.Vaglio S, Arista MC, Perrone MP, et al. Autoimmune hemolytic anemia in childhood: serologic features in 100 cases. Transfusion. 2007;47:50–4. doi: 10.1111/j.1537-2995.2007.01062.x. (P-V) [DOI] [PubMed] [Google Scholar]
- 4.Aladjidi N, Leverger G, Leblanc T, et al. New insights into childhood autoimmune hemolytic anemia: a French national observational study of 265 children. Haematologica. 2011;96:655–63. doi: 10.3324/haematol.2010.036053. (P-III) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aladjidi N, Fernandes H, Leblanc T, et al. Evans syndrome in children: long-term outcome in a prospective French national observational cohort. Front Pediatr. 2015;3:79. doi: 10.3389/fped.2015.00079. (P-III) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habibi B, Homberg JC, Schaison G, Salmon C. Autoimmune hemolytic anemia in children. A review of 80 cases. Am J Med. 1974;56:61–9. doi: 10.1016/0002-9343(74)90751-7. (P-V) [DOI] [PubMed] [Google Scholar]
- 7.Sokol RJ, Hewitt S, Stamps BK, Hitchen PA. Autoimmune hemolysis in childhood and adolescence. Acta Haematol. 1984;72:245–57. doi: 10.1159/000206397. (P-V) [DOI] [PubMed] [Google Scholar]
- 8.Zuelzer WW, Mastrangelo R, Stulberg CS, et al. Autoimmune hemolytic anemia. Natural history and viral-immunologic interactions in childhood. Am J Med. 1970;49:80–93. doi: 10.1016/s0002-9343(70)80116-4. (P-V) [DOI] [PubMed] [Google Scholar]
- 9.Barone A, Lucarelli A, Onofrillo D, et al. Diagnosis and management of acquired aplastic anemia in childhood. Guidelines from the Marrow Failure Study Group of the Pediatric Haemato-Oncology Italian Association (AIEOP) Blood Cells Mol Dis. 2015;55:40–7. doi: 10.1016/j.bcmd.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Fioredda F, Calvillo M, Bonanomi S, et al. Neutropenia Committee of the Marrow Failure Syndrome Group of the AIEOP (Associazione Italiana Emato-Oncologia Pediatrica) Congenital and acquired neutropenias consensus guidelines on therapy and follow-up in childhood from the Neutropenia Committee of the Marrow Failure Syndrome Group of the AIEOP (Associazione Italiana Emato-Oncologia Pediatrica) Am J Hematol. 2012;87:238–43. doi: 10.1002/ajh.22242. [DOI] [PubMed] [Google Scholar]
- 11.De Mattia D, Del Vecchio GC, Russo G, et al. Management of chronic childhood immune thrombocytopenic purpura: AIEOP Consensus Guidelines. Acta Haematol. 2010;123:96–109. doi: 10.1159/000268855. [DOI] [PubMed] [Google Scholar]
- 12.Petz LD. Cold antibody autoimmune haemolytic anemias. Blood Rev. 2008;22:1–15. doi: 10.1016/j.blre.2007.08.002. (AP-V) [DOI] [PubMed] [Google Scholar]
- 13.Segel GB, Charles H, Packman CH. Hemolytic anemias resulting from extracellular factors-immune haemolytic anemias. In: Kliegman RM, Stanton BMD, St Geme J, et al., editors. Nelson Textbook of Pediatrics. Philadelphia, PA: Saunders Elsevier; 2011. [Google Scholar]
- 14.Schattner A. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine. 2005;23:3876–86. doi: 10.1016/j.vaccine.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Garratty G. Drug-induced immune hemolytic anemia. Hematol Am Soc Hematol Educ Program. 2009:73–9. doi: 10.1182/asheducation-2009.1.73. [DOI] [PubMed] [Google Scholar]
- 16.Giannouli S, Voulgarelis M, Ziakas PD, Tzioufas AG. Anaemia in systemic lupus erythematous: from pathophysiology to clinical assessment. Ann Rheum Dis. 2006;65:144–8. doi: 10.1136/ard.2005.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorelik M, Debski R, Frangoul H. Autoimmune hemolytic anemia with giant cell hepatitis: case report and review of the literature. J Pediatr Hematol Oncol. 2004;26:837–9. [PubMed] [Google Scholar]
- 18.Evans RS, Takahashi K, Duane RT, et al. Primary thrombo-cytopenic purpura and acquired hemolytic anemia; evidence for a common etiology. AMA Arch Intern Med. 1951;87:48–65. doi: 10.1001/archinte.1951.03810010058005. (AP-V) [DOI] [PubMed] [Google Scholar]
- 19.Wang WC. Evans syndrome in childhood: pathophysiology, clinical course and treatment. Am J Pediatr Hematol Oncol. 1988;10:330–8. doi: 10.1097/00043426-198824000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Pui CH, Wilimas J, Wang W. Evans syndrome in childhood. J Pediatr. 1980;97:754–8. doi: 10.1016/s0022-3476(80)80258-7. (P-V) [DOI] [PubMed] [Google Scholar]
- 21.Notarangelo LD, Fisher A, Geha RS, et al. Primary immunodeficiencies: 2009 update: the International Union of Immunological Societies (IUIS) Primary Immunodeficiency (PID) Expert Committee. J Allergy Clin Immunol. 2009;24:1161–78. [Google Scholar]
- 22.Chen FE, Owen I, Savage D, et al. Late onset haemolysis and red cell autoimmunisation after allogeneic bone marrow transplant. Bone Marrow Transplant. 1997;19:491–5. doi: 10.1038/sj.bmt.1700677. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien TA, Eastlund T, Peters C, et al. Autoimmune haemolytic anaemia complicating haematopoietic cell transplantation in paediatric patients: high incidence and significant mortality in unrelated donor transplants for non-malignant diseases. Br J Haematol. 2004;127:67–75. doi: 10.1111/j.1365-2141.2004.05138.x. (P-V) [DOI] [PubMed] [Google Scholar]
- 24.Sallah S, Wan JY, Hanrahan LR. Future development of lymphoproliferative disorders in patients with autoimmune hemolytic anemia. Clin Cancer Res. 2001;7:791–4. (A-IV) [PubMed] [Google Scholar]
- 25.Carapella de Luca E, Casadei AM, di Piero G, et al. Auto-immune haemolytic anaemia in childhood: follow-up in 29 cases. Vox Sang. 1979;36:13–20. doi: 10.1111/j.1423-0410.1979.tb04392.x. (P-V) [DOI] [PubMed] [Google Scholar]
- 26.Liesveld JL, Rowe JM, Lichtman MA. Variability of the erythropoietic response in autoimmune hemolytic anemia: analysis of 109 cases. Blood. 1987;69:820–6. (A-IV) [PubMed] [Google Scholar]
- 27.Jastaniah WA, Pritchard SL, Wu JK, Wadsworth LD. Hyperuricemia and reticulocytopenia in association with autoimmune hemolytic anemia in two children. Am J Clin Pathol. 2004;122:49–54. doi: 10.1309/8DXD-VJT9-UN60-YACT. (P-V) [DOI] [PubMed] [Google Scholar]
- 28.Van De Loosdrecht AA, Hendriks DW, Blom NR, et al. Excessive apoptosis of bone marrow erythroblasts in a patient with autoimmune haemolytic anaemia with reticulocytopenia. Br J Haematol. 2000;108:313–5. doi: 10.1046/j.1365-2141.2000.01867.x. (P-V) [DOI] [PubMed] [Google Scholar]
- 29.de Las Nieves López MA, Medina Perez MF, Gónzalez Hermoso C. Erythroblastopenia and parvovirus B19 infection in a healthy child. Haematologica. 2000;85:E07. (P-V) [PubMed] [Google Scholar]
- 30.Smith MA, Shah NS, Lobel JS. Parvovirus B19 infection associated with reticulocytopenia and chronic autoimmune hemolytic anemia. Am J Pediatr Hematol Oncol. 1989;11:167–9. (P-V) [PubMed] [Google Scholar]
- 31.Issitt PD, Pavone BG, Goldfinger D, et al. Anti-Wrb, and other autoantibodies responsible for positive direct antiglobulin tests in 150 individuals. Br J Haematol. 1976;34:5–18. doi: 10.1111/j.1365-2141.1976.tb00168.x. (A-V) [DOI] [PubMed] [Google Scholar]
- 32.Wallhermfechtel MA, Pohl BA, Chaplin H. Alloimmunization in patients with warm autoantibodies. A retrospective study employing three donor alloabsorptions to aid in antibody detection. Transfusion. 1984;24:482–5. doi: 10.1046/j.1537-2995.1984.24685066805.x. [DOI] [PubMed] [Google Scholar]
- 33.Laine ML, Beattie KM. Frequency of alloantibodies accompanying autoantibodies. Transfusion. 1985;25:545–6. doi: 10.1046/j.1537-2995.1985.25686071427.x. [DOI] [PubMed] [Google Scholar]
- 34.Leger RM, Garratty G. Evaluation of methods for detecting alloantibodies underlying warm autoantibodies. Transfusion. 1999;39:11–6. doi: 10.1046/j.1537-2995.1999.39199116889.x. [DOI] [PubMed] [Google Scholar]
- 35.Branch DR, Petz LD. Detecting alloantibodies in patients with autoantibodies. Transfusion. 1999;39:6–10. doi: 10.1046/j.1537-2995.1999.39199116888.x. [DOI] [PubMed] [Google Scholar]
- 36.Shirey RS, Boyd JS, Parwani AV, et al. Prophylactic antigen-matched donor blood for patients with warm autoantibodies: an algorithm for transfusion management. Transfusion. 2002;42:1435–41. doi: 10.1046/j.1537-2995.2002.00234.x. (A-V) [DOI] [PubMed] [Google Scholar]
- 37.Barcellini W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014;124:930–6. doi: 10.1182/blood-2014-06-583021. (A-III) [DOI] [PubMed] [Google Scholar]
- 38.McGann PT, McDade J, Mortier NA, et al. IgA-mediated autoimmune hemolytic anemia in an infant. Pediatr Blood Cancer. 2011;56:837–9. doi: 10.1002/pbc.22932. (P-V) [DOI] [PubMed] [Google Scholar]
- 39.Yamada C, Serrano-Rahman L, Vasovic LV, et al. Antibody identification using both automated solid-phase red cell adherence assay and a tube polyethylene glycol antiglobulin method. Transfusion. 2008;48:1693–8. doi: 10.1111/j.1537-2995.2008.01736.x. [DOI] [PubMed] [Google Scholar]
- 40.Winters JL, Richa EM, Bryant SC, et al. Polyethylene glycol antiglobulin tube versus gel microcolumn: influence on the incidence of delayed hemolytic transfusion reactions and delayed serologic transfusion reactions. Transfusion. 2010;50:1444–52. doi: 10.1111/j.1537-2995.2010.02609.x. [DOI] [PubMed] [Google Scholar]
- 41.Lin JS, Hao TC, Lyou JY, et al. Clinical application of a flow cytometric direct antiglobulin test. Transfusion. 2009;49:1335–46. doi: 10.1111/j.1537-2995.2009.02130.x. (AP-V) [DOI] [PubMed] [Google Scholar]
- 42.Leikola J, Perkins HA. Enzyme-linked antiglobulin test: an accurate and simple method to quantify red cell antibodies. Transfusion. 1980;20:138–44. doi: 10.1046/j.1537-2995.1980.20280169953.x. [DOI] [PubMed] [Google Scholar]
- 43.Kiruba R, Han P. Quantitation of red cell-bound immunoglobulin and complement using enzyme-linked antiglobulin consumption assay. Transfusion. 1988;28:519–24. doi: 10.1046/j.1537-2995.1988.28689059023.x. [DOI] [PubMed] [Google Scholar]
- 44.Bencomo AA, Diaz M, Alfonso Y, et al. Quantitation of red cell-bound IgG, IgA, and IgM in patients with autoimmune hemolytic anemia and blood donors by enzyme-linked immunosorbent assay. Immunohematology. 2003;19:47–53. [PubMed] [Google Scholar]
- 45.Jeje MO, Blajchman MA, Steeves K, et al. Quantitation of red cell-associated IgG using an immunoradiometric assay. Transfusion. 1984;24:473–6. doi: 10.1046/j.1537-2995.1984.24685066803.x. [DOI] [PubMed] [Google Scholar]
- 46.Gilliland BC, Leddy JP, Vaughan JH. The detection of cell-bound antibody on complement-coated human red cells. J Clin Invest. 1970;49:898–906. doi: 10.1172/JCI106309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barcellini W, Clerici G, Montesano R, et al. In vitro quantification of anti-red blood cell antibody production in idiopathic autoimmune haemolytic anaemia: effect of mitogen and cytokine stimulation. Br J Haematol. 2000;111:452–60. doi: 10.1046/j.1365-2141.2000.02380.x. (A-V) [DOI] [PubMed] [Google Scholar]
- 48.Arndt PA, Leger RM, Garratty G. Serologic findings in autoimmune hemolytic anemia associated with immunoglobulin M warm autoantibodies. Transfusion. 2009;49:235–42. doi: 10.1111/j.1537-2995.2008.01957.x. (AP-V) [DOI] [PubMed] [Google Scholar]
- 49.Bartolmäs T, Salama A. A dual antiglobulin test for the detection of weak or nonagglutinating immunoglobulin M warm autoantibodies. Transfusion. 2010;50:1131–4. doi: 10.1111/j.1537-2995.2009.02533.x. (A-V) [DOI] [PubMed] [Google Scholar]
- 50.Dameshek W, Rosenthal MC, Schwartz LI. The treatment of acquired hemolytic anemia with adrenocorticotrophic hormone (ACTH) N Engl J Med. 1950;244:117–27. doi: 10.1056/NEJM195101252440401. (A-V) [DOI] [PubMed] [Google Scholar]
- 51.Naithani R, Agrawal N, Mahapatra M, et al. Autoimmune hemolytic anemia in India: clinico-hematological spectrum of 79 cases. Hematology. 2006;11:73–6. doi: 10.1080/10245330500345587. (AP-V) [DOI] [PubMed] [Google Scholar]
- 52.Naithani R, Agrawal N, Mahapatra M, et al. Autoimmune hemolytic anemia in children. Pediatr Hematol Oncol. 2007;24:309–15. doi: 10.1080/08880010701360783. (P-V) [DOI] [PubMed] [Google Scholar]
- 53.Gupta V, Shukla J, Bhatia BD. Autoimmune hemolytic anemia. Ind J Ped. 2008;75:451–4. doi: 10.1007/s12098-008-0071-0. (P-V) [DOI] [PubMed] [Google Scholar]
- 54.Allgood JW, Chaplin H., Jr Idiopathic acquired autoimmune hemolytic anemia. A review of forty-seven cases treated from 1955 through 1965. Am J Med. 1967;43:254–7. doi: 10.1016/0002-9343(67)90168-4. (A-V) [DOI] [PubMed] [Google Scholar]
- 55.Flores G, Cunningham-Rundles C, Newland AC, Bussel JB. Efficacy of intravenous immunoglobulin in the treatment of autoimmune hemolytic anemia: results in 73 patients. Am J Hemathol. 1993;44:237–42. doi: 10.1002/ajh.2830440404. (AP-V) [DOI] [PubMed] [Google Scholar]
- 56.Pocecco M, Ventura A, Tamaro P, Longo F. High-dose IVIgG in autoimmune hemolytic anemia. J Pediatr. 1986;109:726. doi: 10.1016/s0022-3476(86)80252-9. (P-V) [DOI] [PubMed] [Google Scholar]
- 57.Bussel JB, Hilgartner MW. The use and mechanism of action of intravenous immunoglobulin in the treatment of immune haematologic disease. Br J Haematol. 1984;56:1–7. doi: 10.1111/j.1365-2141.1984.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 58.Wienblatt ME. Treatment of immune hemolytic anemia with gammaglobulin. J Pediatr. 1987;110:817–8. doi: 10.1016/s0022-3476(87)80036-7. (P-V) [DOI] [PubMed] [Google Scholar]
- 59.Oda H, Honda A, Sugita K, et al. High dose intravenous IgG infusion in refractory autoimmune hemolytic anemia (Evans syndrome) J Pediatr. 1985;107:744–6. doi: 10.1016/s0022-3476(85)80405-4. (P-V) [DOI] [PubMed] [Google Scholar]
- 60.Otheo E, Maldonado MS, Muñoz A, Hernández-Jodra M. High-dose intravenous immunoglobulin as single therapy in a child with autoimmune hemolytic anemia. Pediatr Hematol Oncol. 1997;14:487–90. doi: 10.3109/08880019709028781. (P-V) [DOI] [PubMed] [Google Scholar]
- 61.Anderson D, Ali K, Blanchette V, et al. Guidelines on the use of intravenous immunoglobulin for hematologic conditions. Transfus Med Rev. 2007;21:S9–56. doi: 10.1016/j.tmrv.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Dussadee K, Taka O, Thedsawad A, Wanachiwanawin W. Incidence and risk factors of relapses in idiopathic autoimmune hemolytic anemia. J Med Assoc Thai. 2010;93:S165–70. (A-V) [PubMed] [Google Scholar]
- 63.Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transf Med Rev. 2010;24:195–210. doi: 10.1016/j.tmrv.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 64.Lucchini G, Masera N, Foti G, et al. A life threatening paediatric case of acute autoimmune hemolytic anemia (AIHA) successfully cured by plasma exchange and combined immunosuppressive treatment. Transfus Apher Sci. 2009;40:115–8. doi: 10.1016/j.transci.2009.01.020. (P-V) [DOI] [PubMed] [Google Scholar]
- 65.Roy-Burman A, Glader BE. Resolution of severe Donath Landsteiner autoimmune hemolytic anemia temporally associated with institution of plasmapheresis. Crit Care Med. 2002;30:931–4. doi: 10.1097/00003246-200204000-00039. (P-V) [DOI] [PubMed] [Google Scholar]
- 66.McCarthy LJ, Danielson CF, Fernandez C, et al. Intensive plasma-exchange for severe autoimmune hemolytic anemia in a four-month-old infant. J Clin Apher. 1999;14:190–2. doi: 10.1002/(sici)1098-1101(1999)14:4<190::aid-jca8>3.0.co;2-s. (P-V) [DOI] [PubMed] [Google Scholar]
- 67.Berentsen S, Tjønnfjord GE. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012;26:107–15. doi: 10.1016/j.blre.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Lechner K, Jäger U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116:1831–8. doi: 10.1182/blood-2010-03-259325. [DOI] [PubMed] [Google Scholar]
- 69.Nanan R, Scheurlen W, Gerlich M, Huppertz HI. Severe low-titer cold-hemagglutinin disease responsive to steroid pulse therapy. Ann Hematol. 1995;71:101–2. doi: 10.1007/BF01699254. (V-P) [DOI] [PubMed] [Google Scholar]
- 70.Lahav M, Rosenberg I, Wysenbeek AJ. Steroid-responsive idiopathic cold agglutinin disease: a case report. Acta Haematol. 1989;81:166–8. doi: 10.1159/000205552. [DOI] [PubMed] [Google Scholar]
- 71.Meytes D, Adler M, Viraq I, et al. High-dose methylprednisolone in acute immune cold hemolysis. N Engl J Med. 1985;312:318. doi: 10.1056/NEJM198501313120520. [DOI] [PubMed] [Google Scholar]
- 72.Hillen HF, Bakker SJ. Failure of interferon-alpha-2b therapy in chronic cold agglutinin disease. Eur J Haematol. 1994;53:242–3. doi: 10.1111/j.1600-0609.1994.tb00197.x. (A-V) [DOI] [PubMed] [Google Scholar]
- 73.Berentsen S, Tjonnfjord GE, Shammas FV, et al. No response to cladribine in five patients with chronic cold agglutinin disease. Eur J Haematol. 2000;65:88–90. doi: 10.1034/j.1600-0609.2000.9l201.x. (A-V) [DOI] [PubMed] [Google Scholar]
- 74.Berentsen S, Ulvestad E, Gjertsen BT, et al. Rituximab for primary chronic cold agglutinin disease: a prospective study of 37 courses of therapy in 27 patients. Blood. 2004;103:2925–8. doi: 10.1182/blood-2003-10-3597. (P-V) [DOI] [PubMed] [Google Scholar]
- 75.Schollkopf C, Kjeldsen L, Bjerrum OW, et al. Rituximab in chronic cold agglutinin disease: a prospective study of 20 patients. Leuk Lymphoma. 2006;47:253–60. doi: 10.1080/10428190500286481. (P-V) [DOI] [PubMed] [Google Scholar]
- 76.Berentsen S, Randen U, Vagan AM, et al. High response rate and durable remissions following fludarabine and rituximab combination therapy for chronic cold agglutinin disease. Blood. 2010;116:3180–4. doi: 10.1182/blood-2010-06-288647. (P-V) [DOI] [PubMed] [Google Scholar]