Dear Sir,
In 2014, you published the “Principles of treatment and update of recommendations for the management of haemophilia and congenital bleeding disorders in Italy”, produced by the Italian Association of Haemophilia Centre (AICE - Associazione Italiana Centri Emofilia) Directors1. The choice of the type of product for replacement therapy in persons with haemophilia A is a milestone in their care, conditioning the risk of transmission of infectious agents and that of developing factor VIII (FVIII) inhibitors. In particular, AICE recommended choosing recombinant FVIII in previously untreated patients, minimally treated patients and previously treated patients exclusively exposed to recombinant FVIII products. In addition, recombinant products were recommended for human immunodeficiency virus-positive patients with clinical signs of immune deficiency and for previously treated patients exposed to plasma-derived concentrates but not infected by hepatitis C virus or who had cleared the virus spontaneously or following antiviral treatment. Among the latter patients, those being treated with a plasma-derived FVIII product were encouraged to continue its use on the basis of decisions jointly taken with their physicians. Furthermore, previously treated patients with chronic hepatitis C virus infection (HCV-RNA virus positive) or positive for human immunodeficiency virus but without signs of immune deficiency could continue on the plasma-derived or recombinant product they were then using, according to decisions shared with their physicians. On the basis of this AICE scenario, plasma-derived FVIII concentrates were recommended for a minority of persons with haemophilia A.
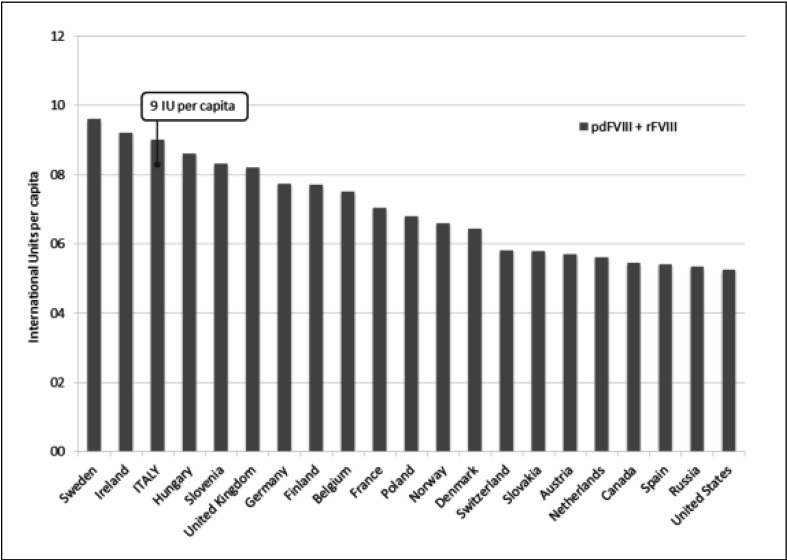
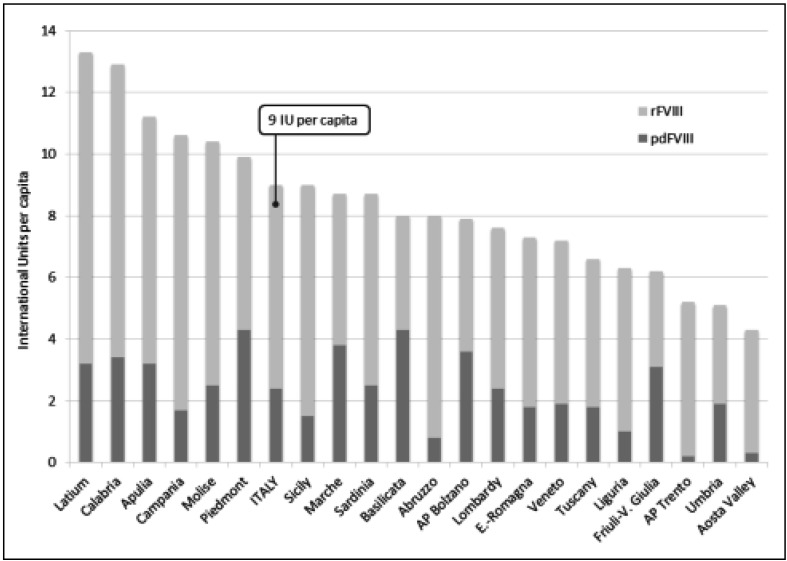
With this preamble, a significant increase in the demand for plasma-derived FVIII was recorded between 2011 and 2014 in Italy2. In 2014, the overall demand for FVIII (recombinant plus plasma-derived) was as high as 547 million IU (9 IU per capita), one of the highest in Europe (Figure 1) even though with a large variability across the Italian regions (range: 4.3–13.3 IU per capita) (Figure 2). The amount of plasma-derived FVIII accounted for about 147 million IU (around 27% of the total demand), with an increase of 58% in comparison with the consumption recorded in 2011.
Figure 1.
Total demands for factor VIII per country in Europe, USA and Canada (2014).
Figure 2.
National and regional total demands for plasma-derived and recombinant factor VIII in Italy (2014).
How can this discrepancy between the increasing trends observed in the national and regional demands for plasma-derived FVIII and the very restrictive current recommendations by AICE be explained? A first reason could be the wider implementation of immune tolerance induction for eradication of FVIII inhibitors in poor-prognosis patients, in whom the use of von Willebrand factor-containing plasma-derived FVIII products is perceived to increase the likelihood of tolerance success. Another explanation could be the implementation of strategies meant to prevent the occurrence of age-related comorbidities and related increased intake of drugs adversely affecting haemostasis such as antiplatelet and anti-inflammatory agents3. This situation may have prompted adult and elderly persons with haemophilia A treated with plasma-derived FVIII to switch from on-demand to prophylactic regimens, in order to control the risk of drug-related bleeding. However, these clinical conditions are unlikely to be sufficient to explain the increased consumption of plasma-derived FVIII observed in Italy. We believe that the perception of these products is indeed changing among Italian patients and physicians, driven by the increased awareness of their safety record documented by the fact that no transmission of blood-borne viral infections occurred in the last 25 years. Moreover, plasma-derived FVIII, in particular the product obtained from contract fractionation of Italian plasma, is offering a valid option in terms of availability, with more economic sustainability for the Italian health care system. Last but not least, the perceived lower immunogenicity of plasma-derived FVIII products may have influenced this treatment approach in previously untreated patients at high risk of inhibitor4. We, therefore, envisage a revision of the AICE recommendations in order to recognise the current role of plasma-derived FVIII concentrates as a valid therapeutic option for the treatment of haemophilia A. This request is strongly supported by the recent results of the Survey of Inhibitors in Plasma Product Exposed Toddlers (SIPPET)5. By means of a randomised design, this multinational investigator-driven study is confirming that plasma-derived FVIII products containing von Willebrand factor are associated with less development of inhibitors than recombinant products of all the currently available generations5.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Rocino A, Coppola A, Franchini M, et al. Principles of treatment and update of recommendations for the management of haemophilia and congenital bleeding disorders in Italy. Blood Transfus. 2014;12:575–98. doi: 10.2450/2014.0223-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Candura F, Lanzoni M, Calizzani G, et al. Rapporti ISTISAN 16/7. Roma: Istituto Superiore di Sanità; 2016. [In Italian.] [Google Scholar]
- 3.Makris M. Prophylaxis in haemophilia should be life-long. Blood Transfus. 2012;10:165–8. doi: 10.2450/2012.0147-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannucci PM, Mancuso ME, Santagostino E. How we choose factor VIII to treat hemophilia. Blood. 2012;119:4108–14. doi: 10.1182/blood-2012-01-394411. [DOI] [PubMed] [Google Scholar]
- 5.Peyvandi F, Mannucci PM, Garagiola I, et al. Source of factor VIII replacement (plasmatic or recombinant) and incidence of inhibitory alloantibodies in previously untreated patients with severe hemophilia A: the multicenter randomized SIPPET study. N Engl J Med. 2016;374:2054–64. [Google Scholar]