Abstract
Background
About 15% to 25% of people with diabetes will develop a foot ulcer. These wounds are often resistant to healing; therefore, people with diabetes experience lower limb amputation at about 20 times the rate of people without diabetes. If an ulcer does not heal with standard wound care, other therapeutic interventions are offered, one of which is hyperbaric oxygen therapy (HBOT). However, the effectiveness of this therapy is not clearly known. The objectives of this health technology assessment were to assess the safety, clinical effectiveness, and cost-effectiveness of standard wound care plus HBOT versus standard wound care alone for the treatment of diabetic foot ulcers. We also investigated the preferences and perspectives of people with diabetic foot ulcers through lived experience.
Methods
We performed a review of the clinical and economic literature for the effectiveness and cost-effectiveness of hyperbaric oxygen therapy, as well as the budget impact of HBOT from the perspective of the Ministry of Health and Long-Term Care. We assessed the quality of the body of clinical evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. To better understand the preferences, perspectives, and values of patients with diabetic foot ulcers and their experience with HBOT, we conducted interviews and administered an online survey.
Results
Seven randomized controlled trials and one nonrandomized controlled trial met the inclusion criteria. Comparing standard wound care plus HBOT with standard wound care alone, we found mixed results for major amputation rates (GRADE quality of evidence: low), a significant difference in favour of standard wound care plus HBOT on ulcers healed (GRADE quality of evidence: low), and no difference in terms of adverse events (GRADE quality of evidence: moderate).
There is a large degree of uncertainty associated with the evaluation of the cost-effectiveness of standard wound care plus HBOT. However, results appear to suggest that this treatment results in lower costs and better outcomes than standard wound care alone. Funding HBOT will result in a budget impact of $4 million per year in immediate treatment costs for the Ontario Ministry of Health and Long-Term Care. This cost decreases to $0.5 million per year when downstream costs are considered.
There is a substantial daily burden of care and emotional weight associated with living with diabetic foot ulcers, both of which are compounded by concern regarding possible amputation. Patients feel that HBOT is an effective treatment and reported that they were satisfied with how their ulcers healed and that this improved their quality of life.
Conclusions
The evidence makes it difficult to draw any definitive conclusions on the clinical and cost effectiveness of standard wound care plus HBOT versus standard wound care alone for the treatment of diabetic foot ulcers.
BACKGROUND
Health Condition
Diabetes is a metabolic disease in which the body has difficulty producing insulin. This leads to high blood glucose levels, which can damage organs, blood vessels, and nerves. Diabetes affects 10.2% of the Ontario population.1 In Canada, the incidence of new cases of diabetes has held steady at around 0.6%; however, cumulatively, this leads to an increase in overall population rates, and these rates are expected to continue to rise.2
For several reasons, people with diabetes are especially susceptible to lower limb and foot wounds that do not heal.3 People with diabetes may experience nerve damage, which can lead to weakness and numbness in the foot in addition to pain.3 This numbness can predispose patients to foot injuries, either through trauma or by continuing to walk on a severe blister or callus without feeling any pain.4 Additionally, diabetes can cause the skin to become very dry and prone to cracking, increasing the risk for infection.4 People with diabetes may also experience peripheral artery disease, which can cause a hardening and obstruction of blood vessels in the lower leg and foot. This condition results in poor circulation, which can make fighting infection more difficult and make patients more susceptible to ulcers.4 Other factors that may contribute to complications with wound healing include poor tissue perfusion (spread of oxygen in the body), bacterial infection, malnutrition, and poor control of blood glucose levels.5
It is estimated that about 15% to 25% of people with diabetes develop a foot ulcer in their lifetime.6,7 These wounds are often resistant to treatment and difficult to heal; therefore, people with diabetes experience lower limb amputation at about 20 times the rate of people without diabetes.8
Patients with diabetic foot ulcers are treated with standard wound care (detailed below). It may be that many patients end up being referred to hyperbaric oxygen therapy (HBOT) clinics when healing is not achieved with standard wound care alone.
Clinical Need and Target Population
Standard wound care for patients with diabetic foot ulcers consists of four phases:
-
1.
Assessing the wound, including examining the size and looking for signs of infection and ischemia, most often using the Wagner Ulcer Classification System,9 which categorizes ulcers based on wound depth and the presence of infection (Table A1)
-
2.
Planning the best course of treatment, based on goals mutually agreed upon by the health care provider and patient
-
3.
Implementing the plan, including mitigating risk factors (e.g., managing blood glucose levels), debriding the wound, providing infection control, and redistributing pressure applied to the foot ulcer by using offloading devices
-
4.
Evaluating of the progress of wound healing on an ongoing basis, making adjustments to the treatment plan as necessary10
To optimize local wound care, it is suggested to follow the TIME framework: tissue debridement, inflammation and infection control, moisture balance (optimal dressing selection), and epithelial edge advancement.11
Biologic agents include growth factors (e.g., becaplermin gel), which promote wound bed vascularization, and pharmacotherapeutics (e.g., rusalatide acetate, tetrachlorodecaoxide), which encourage the formation of granulation tissue (new connective tissue and blood vessels).10 However, clinical experts consulted for this review stated that these agents are not currently available in Ontario.
Negative pressure wound therapy involves delivering 100% oxygen at subatmospheric pressure to the wound using a dressing.10 This technology has been reported to be an effective treatment option for the management of diabetic foot ulcers.12
In HBOT, 100% oxygen is administered to the patient at an increased atmospheric pressure; this is the technology being evaluated in this health technology assessment.
Although many guidelines discuss the use of HBOT, these same guidelines acknowledge that there is insufficient evidence to support the routine use of HBOT in the management of diabetic foot ulcers.10,13–15
Technology
In HBOT, a patient enters a hyperbaric chamber that fits either a single or multiple individuals and is exposed to 100% oxygen while the atmospheric pressure is increased.16 There are many different ways of administering HBOT. In some centres, patients receives treatment five times a week for 90 minutes per session. The total number of sessions varies based on the response of the wound to treatment. Patients may receive 40 or more sessions in total.
Wound healing occurs through various phases of regeneration, and oxygen is an integral part of this process, as it is needed for tissue growth.17 While the exact mechanism by which HBOT works is unknown, it is theorized that HBOT improves the oxygen concentration in a person's blood, thereby increasing the amount of oxygen reaching the areas that need to heal.17 It is theorized that HBOT may be able to support these mechanisms by increasing the amount of oxygen in the blood and directing it to the regions where it is needed most.
Overall, there are few risks associated with HBOT. The most common risk is ear discomfort requiring equalization of the middle ear pressure, but no long-term side effects have been reported.18 The more serious side effects of HBOT include rupture of the ear membranes and sinus trauma.18 Additionally, there is a very small risk of oxygen poisoning upon being exposed to 100% oxygen at increased atmospheric pressure; this can lead to mild or moderate symptoms of blurred vision, nausea, and dizziness, and, rarely, more severe symptoms, such as seizures or difficulty breathing because of chest tightness.18,19
Equity
The rate of diabetes is higher among patients who are older and is disproportionately represented in lower-income, South Asian, Asian, African, Hispanic, and Aboriginal populations.2,8
Currently, the majority of the available HBOT units are concentrated in the southern and southeastern regions of Ontario.20 As such, they can possibly accommodate the majority of the Ontario population; however, it could be a challenge for those in northern and northwestern regions to access a clinic. Additionally, current practice consists of daily HBOT sessions for four to six weeks.21 It may be difficult for some patients to find the time, arrange the transportation, and cover out-of-pocket expenses to make this commitment.
Context
Hyperbaric oxygen therapy is currently publicly funded by the Ministry of Health and Long-Term Care for 14 indications, including the “enhancement of healing in selected problem wounds.”22 There are an estimated 47 HBOT units in Canada, of which 12 are located in Ontario.20
Given the uncertainty of the available evidence, the present review was undertaken in response to a request in January 2016 from the Ontario Health Technology Advisory Committee to evaluate primary studies examining the effect of HBOT on diabetic foot ulcers.
Research Questions
What are the safety and effectiveness of standard wound care plus HBOT compared with standard wound care alone for the treatment of diabetic foot ulcers?
What is the cost-effectiveness of standard wound care plus HBOT compared with standard wound care alone for the treatment of diabetic foot ulcers?
What is the budget impact of funding HBOT for the treatment of diabetic foot ulcers?
What are the values and preferences of patients with diabetic foot ulcers and their family members in terms of wound care treatments, including HBOT?
CLINICAL EVIDENCE REVIEW
Objectives
The objectives of this study were to assess the safety and effectiveness of standard wound care plus HBOT compared with standard wound care alone for the treatment of diabetic foot ulcers.
Methods
Research questions are developed by Health Quality Ontario in consultation with experts, end users, and/or applicants in the topic area.
Sources
We performed a literature search on February 16, 2016, using no date limits, in Ovid MEDLINE, Ovid MEDLINE In-Process, Ovid Embase, EBSCO Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database.
Search strategies were developed by medical librarians using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.23 Database auto-alerts were created in MEDLINE, Embase, and CINAHL and monitored for the duration of the HTA review. See Appendix 1 for full details, including all search terms.
Literature Screening
A single reviewer reviewed the abstracts, and, for those studies meeting the eligibility criteria, we obtained full-text articles. We also examined reference lists for any additional relevant studies not identified through the search.
Inclusion Criteria
English-language full-text publications
Systematic reviews, randomized controlled trials, and cohort studies
Studies comparing standard wound care (including vascular and wound assessment, offloading, infection control, debridement, and dressing changes) plus HBOT with standard wound care alone
Studies involving 20 to 60 administered HBOT treatment sessions (parameters selected based on current practice in Ontario)
Studies involving HBOT treatment administered for 90 to 120 minutes at 2.0 to 3.0 atmospheres absolute (ATA) (parameters selected based on current practice in Ontario)
Studies including one month or more of follow-up
Studies in which the population of interest included patients with type 1 or type 2 diabetes presenting with diabetic foot ulcers that had not healed after four weeks despite receiving standard wound care
See Appendix 2 for further information on the included studies.
Exclusion Criteria
Animal and in vitro studies
Editorials, case reports, and commentaries
Studies in which the control group was treated with a therapeutic intervention other than standard wound care (e.g., negative pressure wound therapy)
Studies with five or fewer participants per group
Studies comparing wound healing using topical 100% oxygen to standard wound therapy
Outcomes of Interest
Primary outcomes of interest were as follows:
Major amputations
Ulcers healed
Adverse events
All-cause mortality
Secondary outcomes of interest were as follows:
Minor amputations
Ulcer size reduction
Time to heal
Quality of life
Data Extraction
We extracted relevant data on study characteristics, risk-of-bias items, and PICOT (population, intervention, comparison, outcome, time) using a standardized data form. The form collected information about the following:
Sources (i.e., citation information, contact details, study type)
Methods (i.e., study design, study duration and years, participant allocation, allocation sequence concealment, blinding, reporting of missing data, reporting of outcomes, and whether the study compared two or more groups)
Baseline characteristics of the patients included in the studies, including those based on the PROGRESS-Plus categories (place of residence, race/ethnicity, occupation, gender, religion, education, socioeconomic status, and social capital)24
Outcomes (i.e., outcomes measured, number of participants for each outcome, number of participants missing for each outcome, outcome definition and source of information, unit of measurement, upper and lower limits [for scales], and time points at which the outcomes were assessed)
We contacted authors of the studies when required to clarify information provided in the publications.
Statistical Analysis
We conducted analyses to compare intervention groups (patients receiving standard wound care plus HBOT) with control groups (patients receiving standard wound care alone) using Review Manager, version 5.3.25 Statistical significance was assumed when P < .05. Pooled results were expressed as the mean difference for continuous data and odds ratio or risk difference for categorical data using a fixed-effects model where there was considered to be low between-study heterogeneity based on interventions and populations described or an I2 less than or equal to 30%.26 Where fixed-effects models were considered inappropriate, a random-effects model was applied; where data pooling was considered inappropriate, data were summarized narratively.
Where outcomes could be meta-analyzed and where appropriate (based on clinical and statistical heterogeneity), we performed subgroup and sensitivity analyses for the following considerations:
Number of HBOT treatments
Use of a sham treatment
Transcutaneous oximetry measures
Length of follow-up
Wagner grade
Quality of Evidence
The quality of the body of evidence for each outcome was examined according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria.27 The overall quality was determined to be high, moderate, low, or very low using a step-wise, structural methodology.
See Appendix 3 for details of the GRADE analysis.
Expert Consultation
Expert consultation on the appropriate use of HBOT was sought. Experts consulted included physicians and nurses with specialties in the areas of HBOT and wound care. The role of the expert advisors was to provide guidance on the standard Ontario practice for wound care and on HBOT for the treatment of diabetic foot ulcers. They provided advice during the development of the clinical evidence review plan and supported the contextualization of the evidence and results, as available. However, the statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted experts.
Results
Literature Search
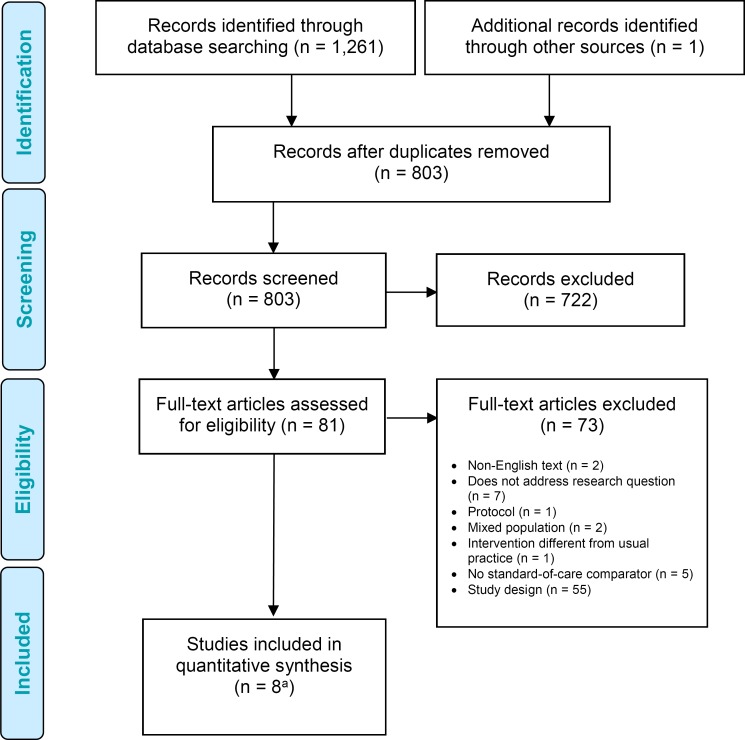
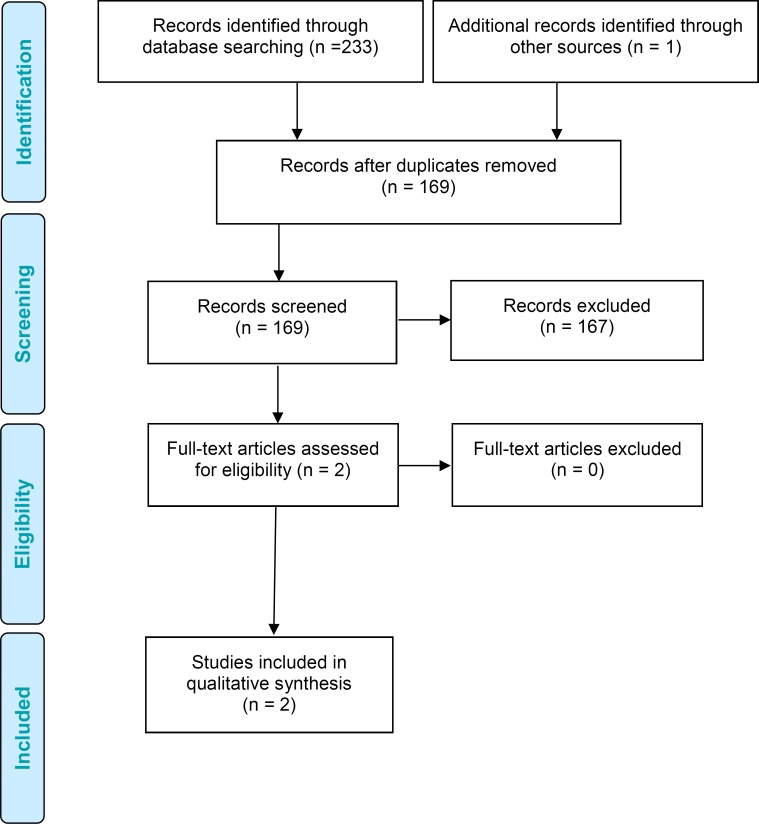
The database search yielded 1,261 citations published. After removing duplicates, we reviewed titles and abstracts to identify potentially relevant articles. We obtained the full texts of these articles for further assessment. Six randomized controlled trials28–33 (two articles reported on the same population) and one nonrandomized controlled trial34 met the inclusion criteria. We hand-searched the reference lists of the included studies, along with health technology assessment websites and other sources, to identify additional relevant studies. One citation (a randomized controlled trial35) was added, for a total of eight.
Our search identified a few systematic reviews on this topic. A summary of the reviews is available in Appendix 4 (Table A8). There was a range of inclusion criteria across the various systematic reviews identified. Some reviews limited study designs, and others examined various therapies for healing diabetic foot ulcers. Some systematic reviews conducted pooled analyses, whereas others determined that pooling was inappropriate because of heterogeneity of the interventions, populations, and/or outcome measurements. Available published pooled analyses related to the primary outcomes of interest (major amputations and ulcer healing) in the population of interest (patients with diabetes) are also summarized in Appendix 4. We used A Measurement Tool to Assess Systematic Reviews (AMSTAR) to assess the methodological quality of systematic reviews (see Appendix 3).36 Given the different approaches of the various systematic reviews and the uncertainty of the evidence on clinical effectiveness, we conducted our own systematic review of primary studies.
Figure 1 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 1: PRISMA Flow Diagram.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
aEight articles, two articles with the same population but one outcome reported in a separate article.
Source: Adapted from Moher et al.37
Characteristics of Included Populations
We included six randomized controlled trials (eight articles, including two articles with the same population) and one nonrandomized controlled trial in the clinical evidence review. All studies compared the effectiveness of standard wound care plus HBOT (“HBOT group”) with standard wound care alone (“standard care group”) for the treatment of patients with diabetic foot ulcers. The studies took place in Canada,35 France,31 Italy,30 Sweden,32–34 Turkey,29 and the United Kingdom.28 The studies varied in many ways. The overall characteristics of the included studies are summarized in Appendix 2 (Table A2). The baseline characteristics of the populations are captured below in Tables 1 and 2. Additional baseline characteristics can be found in Appendix 2 (Tables A3 and A4).
Table 1:
Baseline Characteristics of HBOT Groups in Included Studies
| Faglia et al, 199630 | Kalani et al, 200234,a | Abidia et al, 200328 | Kessler et al, 200331 | Duzgun et al, 200829 | Londahl et al, 2010,32 201133 | Fedorko et al, 201635 | |
|---|---|---|---|---|---|---|---|
| Age ± SD (years) | 61.7 ± 10.4 | 54 ± 14 | 72 ± 12.6 | 60.2 ± 9.7 | 58.1 ± 11.03 | 69 (37–95)b | 61 ± 12 |
| Male (%) | 77 | 71 | 75 | 71 | 74 | 78 | 63 |
| Type of diabetes (%) | Not measured | ID: 65 NID: 35 | Not measured | 1: 14 2: 86 | Not measured | 1: 24 2: 76 | 1: 12 2: 88 |
| Insulin therapy (%) | 60 | 65 | 50 | 92.8 | 82 | 90 | Not measured |
| Average diabetes duration ± SD (years) | 16 ± 10 | 28 ± 12 | 13 ± 9.9 | 18.2 ± 13.2 | 16.9 ± 6.24 | 20 (1–63)c | 19.1 ± 11.5 |
| Wagner grade (%) | 2: 11.5 | Not measured | 2: 100 | Not measured | 2: 6 | 2: 24 | 2: 46.9 |
| 3: 25.7 | 3: 38 | 3: 51 | 3: 44.9 | ||||
| 4: 62.8 | 4: 50 | 4: 24 | 4: 8.2 | ||||
| Ulcer surface area ± SD (cm2) | Not measured | 10.77 ± 15.28 | 1.06 (0.12–8.23)d | 2.31 ± 2.18 | Not measured | 3.1 (0.6–55)d | 3.8 ± 4.8 (0.0–19.6)c |
| Average ulcer duration (range) | Not measured | Not measured | 6 months (2–18 months) | Not measured | Not measured | 9 months (3–44 months) | 235 ± 227 days (28–1,080 days) |
| TcPO2 of foot dorsum ± SD (mm Hg) | 23.25 ± 10.6 | Basale: 22 ± 12 During O2 inhalation: 198 ± 135 | 46 ± 15 | 45.6 ± 18.1 | Not measured | Not measured | Not measured |
Abbreviations: ID, insulin dependent; NID, not insulin dependent; O2, oxygen; SD, standard deviation; TcPO2, transcutaneous oxygen pressure.
Kalani et al was a nonrandomized controlled trial.
Age given with range, not ±SD.
Average diabetes duration given with range, not ± SD.
Ulcer surface area given with range.
Measured basal TcPO2 and TcPO2 during O2 inhalation.
Table 2:
Baseline Characteristics of Standard Care Groups in Included Studies
| Faglia et al, 199630 | Kalani et al, 200234,a | Abidia et al, 200328 | Kessler et al, 200331 | Duzgun et al, 200829 | Londahl et al, 201032 | Fedorko et al, 201635 | |
|---|---|---|---|---|---|---|---|
| Age ±SD (years) | 65.6 ± 9.1 | 65 ± 11 | 70 ± 6.6 | 67.6 ± 10.5 | 63.3 ± 9.15 | 68 (28–86)b | 62 ± 12 |
| Male (%) | 64 | 86 | 50 | 69 | 54 | 84 | 70 |
| Type of diabetes (%) | Not measured | ID: 43 NID: 57 | Not measured | 1: 15 2: 85 | Not measured | 1: 42 2: 58 | 1: 2 2: 98 |
| Insulin therapy (%) | 66.7 | 43 | 63 | 92.3 | 90 | 91 | Not measured |
| Average diabetes duration ± SD (years) | 19 ± 9 | 26 ± 17 | 10 ± 6.3 | 22.1 ± 13.1 | 15.88 ± 5.56 | 23 (3–54)c | 12.4 ± 10.0 |
| Wagner grade (%) | 2: 15.2 | Not measured | 1: 12.5 | Not measured | 2: 12 | 2: 27 | 2: 42.6 |
| 3: 24.2 | 2: 87.5 | 3: 36 | 3: 62 | 3: 53.7 | |||
| 4: 60.6 | 4: 40 | 4: 11 | 4: 3.7 | ||||
| Ulcer surface area ± SD (cm2) | Not measured | 4.49 ± 9.24 | 0.78 (0.18–8.66)d | 2.82 ± 2.43 | Not measured | 2.8 (0.6–39)d | 3.6 ± 5.7 (0.1–26.9)d |
| Average ulcer duration (range) | Not measured | Not measured | 9 months (3–60 months) | Not measured | Not measured | 10 months (3–39 months) | 336 ± 528 days (28–3,650 days) |
| TcPO2 of foot dorsum ± SD (mm Hg) | 21.29 ± 10.7 | Basale: 25 ± 10 During O2 inhalation: 185 ± 88 | 43 ± 19 | 45.2 ± 24.2 | Not measured | Not measured | Not measured |
Abbreviations: ID, insulin dependent; NID, not insulin dependent; O2, oxygen; SD, standard deviation; TcPO2, transcutaneous oxygen pressure.
Kalani et al34 was a nonrandomized controlled trial.
Age given with range, not ±SD.
Average diabetes duration given with range, not ± SD.
Ulcer surface area given with range.
Measured basal TcPO2 and TcPO2 during O2 inhalation.
The average age of participants across studies ranged from 54 to 72 years in the HBOT group and from 62 to 70 years in the standard care group. No information was given on the PROGRESS-Plus categories except for gender (information regarding place of residence, race/ethnicity, occupation, religion, education, socioeconomic status, and social capital was not provided). Males made up 54% to 86% of the samples across treatment groups and studies. In the studies that reported type of diabetes and Wagner grade, 58% to 98% of patients had type 2 diabetes, and ulcers ranged from Wagner grades 2 to 4 across arms and studies, with the majority of patients having a grade 3 ulcer (25.7–51% in the HBOT group and 24.2–62% in the standard care group).
All study sample sizes were small (ranging from 8 to 50 participants in each arm), and interventions varied in terms of the number of daily sessions (one or two), number of HBOT treatments (20–60), and method of treatment (within a hyperbaric chamber that fits either a single [monoplace] or multiple [multiplace] patients). The result was a heterogeneous body of evidence.
While all studies stated that the control groups received standard wound care, insufficient information was provided on the elements composing this care. Three studies met all requirements for standard wound care (vascular and wound assessment, offloading, infection control, debridement, and dressing changes).28,30,32 Four studies partially met the requirements for standard wound care.29,31,34,35 One study29 did not distinguish between ulcers attributed to ischemia versus peripheral ischemia and/or prolonged pressure that went undetected because of neuropathy. Another two studies stated only that offloading was done and did not provide details on the offloading process.31,34 In three studies, patients in control groups also received a sham treatment (hyperbaric air).28,32,33,35 Two studies included patients who were admitted to hospital with care being continued as outpatients.30,31
The follow-up times of the studies varied from one month to three years, with some studies not stating the exact follow-up time.
Methodological Quality of the Included Studies
The complete results of the clinical evidence quality assessment for included studies are presented in Appendix 3. A total of seven studies were applicable to the research question. The methodological quality of these studies was assessed, and large heterogeneity was found to exist across studies. Risk-of-bias limitations consisted of unclear allocation concealment, unclear blinding, and a lack of intention-to-treat analyses in almost all included studies. Where possible, we summarized subgroup analyses narratively, and we summarized each outcome narratively.
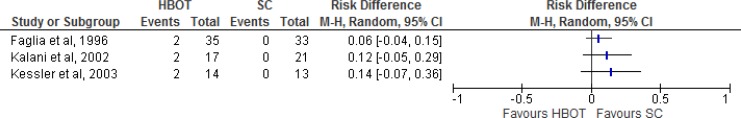
Results for Major Amputations
Major amputation rates were measured in four of the seven studies (three randomized controlled trials and one nonrandomized controlled trial; Table 3, Figure 2). Faglia et al30 defined a major amputation as one occurring below or above the knee; Kalani et al34 defined it as below the knee; Abidia et al28 did not define major amputation; and Londahl et al32 defined it as above the ankle. Comparing HBOT with standard care, there were varying results for major amputation rates. The quality of evidence was very low for major amputations according to the GRADE criteria.
Table 3:
GRADE Evidence Profile for Comparison of HBOT and Standard Care on Major Amputation
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| 3 (RCTs) | No serious limitations | Serious limitations (−1)a | No serious limitations | Serious limitations (−1)b | Undetected | No other considerations | ⊕⊕ Low |
| 1 (NRCT)34 | No serious limitations | No serious limitationsa | No serious limitations | Serious limitations (−1)b | Undetected | No other considerations | ⊕ Very Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HBOT, hyperbaric oxygen therapy; NRCT, nonrandomized controlled trial; RCT, randomized controlled trial.
I2 was about 80%, but no clear explanation was provided. (Kalani et al34 did not provide baseline characteristics for multiple factors [e.g., prior amputations, previous vascular issues]; however, the three other RCTs provided information on comorbidities, and there were no significant differences in baseline characteristics between arms. Abidia et al28 included three patients (one in HBOT, two in sham) with chronic obstructive pulmonary disease who should not have been included as this condition is a contraindication for HBOT). Also, Londahl et al32 had more strict exclusion criteria than the other studies.
Lack of power to determine a difference in amputation rates between the HBOT and standard care groups. Fedorko et al was not included because their definition of major amputation was not consistent with that of other studies.
Figure 2: Risk Difference for HBOT Versus Standard Care on Major Amputations.
Abbreviations: CI, confidence interval; HBOT, hyperbaric oxygen therapy; M-H, Mantel-Haenszel; SC, standard care.
Sources: Faglia et al,30Kalani et al,34Abidia et al,28and Londahl et al.32
At an unspecified follow-up date, Faglia et al30 reported three major amputations (8.6%) in the HBOT group and 11 (33.3%) in the standard care group (P = .016). At the three-year follow-up, Kalani et al34 reported two major amputations (12%) in the HBOT group and seven (33%) in the standard care group (no P value given). At an unspecified follow-up date (presumably the one-year follow-up), Abidia et al28 found that one patient (12.5%) in the HBOT group and one (12.5%) in the standard care group had undergone a major amputation. At the one-year follow-up, Londahl et al32 found that three patients (7.8%) in the HBOT group and one (2.7%) in the standard care group had had amputations. In the HBOT group, two major amputation were performed within two months after inclusion, and one was performed 191 days after inclusion. The one amputation in the standard care group was performed 98 days after inclusion. Estimates from the four studies are given below.
Faglia et al30 reported major amputation rates by Wagner grade. Of the four patients with a Wagner grade 3 ulcer in the HBOT group, one had a major amputation, and of 22 patients with a Wagner 4 grade ulcer, two had an amputation. No patients with a Wagner grade 2 ulcer had a major amputation (zero of four patients). In the standard care group, 11 of 20 patients with a Wagner grade 4 ulcer had a major amputation. No patients with a Wagner 2 grade ulcer (zero of five patients) or a Wagner 3 grade ulcer (zero of eight patients) had a major amputation. There was a significant difference between the HBOT and standard care groups in major amputation rate for patients with Wagner grade 4 ulcers (P = .002). Other studies reporting major amputation rates did not provide a breakdown by Wagner grade.
Fedorko et al35 reported “freedom from having or meeting the criteria for amputation” as their primary outcome. This definition of major amputation differed from other studies. The criteria for amputation used by Fedorko et al35 were (1) lack of significant progress in wound healing over the follow-up period, indicating an ongoing risk of severe systemic infection related to the wound; (2) persistent deep infection involving bone and tendons (antibiotics required, hospitalization required, and/or pathogen involved); (3) inability to bear weight on the affected limb; and (4) pain causing significant disability. The criteria used for determining whether a participant should undergo amputation were determined through digital photographs, were not validated, and were not aligned with guidelines used by vascular surgeons. Primary amputation is necessary only for the unsalvageable diabetic foot. Indications include wet gangrene (infection plus ischemia), life-threatening sepsis, extensive muscle necrosis, revascularization being technically impossible, and the patient being bed-ridden or having a functionally useless limb.38
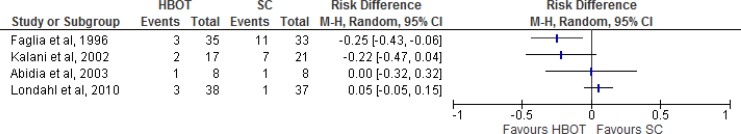
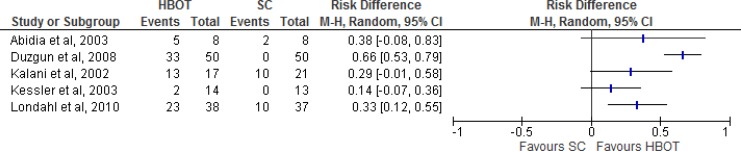
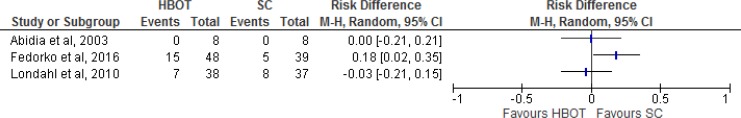
Results for Ulcers Healed
The outcome of ulcers healed was measured in five of the seven studies (four randomized controlled trials and one nonrandomized controlled trial; Table 4, Figure 3). The evidence shows a statistically significant difference in favour of HBOT versus standard care (Figure 4). However, according to the GRADE assessment, the quality of evidence is low. Kalani et al34 and Kessler et al31 did not define ulcer healing. Abidia et al28 defined complete healing as complete epithelialization of the ulcer. Duzgun et al29 defined ulcer healing as total closure of the wound without the need for surgical intervention in the operating room (i.e., complete cure achieved with bedside debridement). In Londahl et al,32 an ulcer was considered healed when it was completely covered by epithelial regeneration and remained so until the next visit in the study. Wagner grade 4 ulcers were considered healed when the gangrene had separated and the ulcer below was completely covered by epithelial regeneration.
Table 4:
GRADE Evidence Profile for Comparison of HBOT and Standard Care on Ulcers Healed
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| 4 (RCTs) | Serious limitations (−1)a | No serious limitationsb | Serious limitations (−1)c,d | No serious limitationse | Undetected | No other considerations | ⊕⊕ Low |
| 1 (NRCT)34 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕ Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HBOT, hyperbaric oxygen therapy; NRCT, nonrandomized controlled trial; RCT, randomized controlled trial.
Unclear allocation concealment, unclear blinding, and a lack of intention-to-treat analyses.
Including Duzgun et al29 makes the I2 value jump from 0% to 80%.
The interventions varied across studies in terms of how many sessions were given (20–60), how many sessions occurred daily (1 vs. 2), and whether treatment was given in a monoplace or multiplace hyperbaric chamber.
Standard care was not delivered to the control groups in the same way across studies, and several standard care treatment protocols did not meet standard wound care guidelines.
The overall result may be inflated as one study (Duzgun et al29) reported no ulcers healed in the standard care group, which may not be accurate.
Figure 3: Risk Difference for HBOT Versus Standard Care on Ulcers Healed.
Abbreviations: CI, confidence interval; HBOT, hyperbaric oxygen therapy; M-H, Mantel-Haenszel; SC, standard care.
Sources: Abidia et al,28Duzgun et al,29Kalani et al,34Kessler et al,31and Londahl et al.32
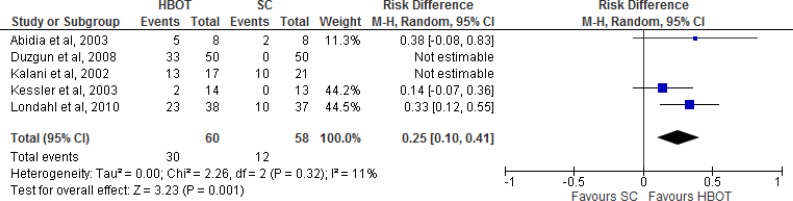
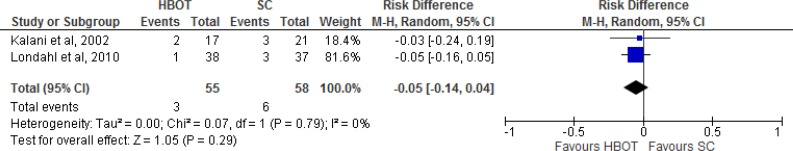
Figure 4: Summary of Risk Difference for HBOT Versus Standard Care on Ulcers Healed, Excluding Duzgun et al and Kalani et ala.
Abbreviations: CI, confidence interval; HBOT, hyperbaric oxygen therapy; M-H, Mantel-Haenszel; SC, standard care.
aStudies included were randomized controlled trials with a standard outcome definition for ulcers healed.
Sources: Abidia et al,28 Duzgun et al,29 Kalani et al,34 Kessler et al,31 and Londahl et al.32
At a three-year follow-up, Kalani et al34 found that 13 of 17 patients (76.5%) in the HBOT group and 10 of 21 patients (47.6%) in the standard care group had achieved complete ulcer healing.
At a four-week follow-up, Kessler et al31 reported complete ulcer healing in two patients in the HBOT group and in no patients in the standard care group.
Abidia et al28 measured complete ulcer healing at three time points. At six weeks, five of eight patients in the HBOT group and one of eight patient in the standard care group had achieved complete healing. At six months, the five patients from the HBOT group continued to demonstrate complete ulcer healing, and two patients in the standard care group had achieved complete ulcer healing. At one year, five patients in the HBOT group continued to demonstrate complete healing, whereas no patients in the standard care group had achieved complete healing. We therefore assumed that the two patients in the standard care group who had achieved complete healing experienced a recurrence of the same ulcer between the six-month and one-year time points. However, since this was not confirmed, we included these two patients in our assessment of risk difference for HBOT versus standard care on ulcers healed (Figure 4).
At an unspecified follow-up date, Duzgun et al29 found that 33 patients (66%) had experienced ulcer healing without surgery in the HBOT group versus no patients (0%) in the standard care group. However, this result does not mean that no patients in the standard care group achieved ulcer healing during the study. Duzgun et al29 also measured wound healing or coverage requiring debridement of the wound in an operating room, an amputation, or the use of a flap or skin graft. Eight patients (16%) in the HBOT group and 50 (100%) in the standard care group achieved wound closure through operative debridement of the wound, an amputation, or the use of a flap or skin graft.
At a one-year follow-up, Londahl et al32 found that 23 of 38 patients (52%) in the HBOT group and 10 of 37 patients (27%) in the standard care group experienced complete ulcer healing (P = .009).
Figure 4 shows the summary estimate for ulcers healed, excluding the studies by Duzgun et al29 and Kalani et al.34 Duzgun et al29 was excluded because its outcomes were not defined in the same way as in the other included studies. Kalani et al34 was excluded because it was a nonrandomized controlled trial. For the outcome of ulcers healed comparing HBOT with standard care, we found a risk difference of 0.25 (95% confidence interval [CI] 0.10–0.41), which is statistically significant (P = .001). It should be noted that there were variations in the interventions, comparators, and follow-up times of the three studies included in the meta-analysis.
Results for Adverse Events
Six of the seven studies (five randomized controlled trials and one nonrandomized controlled trial) measured adverse events (Table 5, Figures 5 and 6). The evidence showed that common adverse events occurred in both the HBOT groups and in the standard care groups that included sham treatments (hyperbaric air). The quality of evidence was moderate according to the GRADE criteria.
Table 5:
GRADE Evidence Profile for Comparison of HBOT and Standard Care on Adverse Events
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| 6 (RCTs) | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕ Moderate |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HBOT, hyperbaric oxygen therapy; RCT, randomized controlled trial.
Unclear allocation concealment, unclear blinding, and a lack of intention-to-treat analyses.
Figure 5: Risk Difference for HBOT Versus Standard Care (Without Sham) on Adverse Events.
Abbreviations: CI, confidence interval; HBOT, hyperbaric oxygen therapy; M-H, Mantel-Haenszel; SC, standard care.
Sources: Faglia et al,30 Kalani et al,34 and Kessler et al.31
Figure 6: Risk Difference for HBOT Versus Standard Care (With Sham) on Adverse Events.
Abbreviations: CI, confidence interval; HBOT, hyperbaric oxygen therapy; M-H, Mantel-Haenszel; SC, standard care.
Sources: Abidia et al,28 Fedorko et al,35 and Londahl et al.32
Table 6 presents the reported adverse events that occurred across the six studies. Adverse events occurred in the control groups of studies that administered a sham treatment to patients, with one exception.28,32,35 In the study by Abidia et al,28 no adverse events were reported in either the HBOT or standard care group.
Table 6:
Adverse Events Occurring in Included Studies
| Author, Year | HBOT (n) | Control (n) | Types of Adverse Event |
|---|---|---|---|
| Without sham treatment | |||
| Faglia et al, 199630 | 2/35 | 0/33 | Barotraumatic otitis |
| Kalani et al, 200234 | 2/17 | 0/21 | Cataracts Pain in ears |
| Kessler et al, 200331 | 2/14 | 0/13 | Barotraumatic otitis |
| With sham treatment | |||
| Abidia et al, 200328 | 0/8 | 0/8 | N/A |
| Londahl et al, 201032 | 7/38 | 8/37 |
HBOT
Control
|
| Fedorko et al, 201635 | 15/48 | 5/39 |
HBOT
Control
|
Abbreviations: HBOT, hyperbaric oxygen therapy; N/A, not available.
Results for All-Cause Mortality
Only two of the seven studies measured all-cause mortality (one randomized controlled trial and one nonrandomized controlled trial; Table 7, Figure 7). Overall, three patients from the HBOT groups and six patients from the standard care groups died, but this difference was not significant. The quality of evidence was moderate according to the GRADE criteria.
Table 7:
GRADE Evidence Profile for Comparison of HBOT and Standard Care on All-Cause Mortality
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)a | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| 1 (NRCT)34 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)a | Undetected | No other considerations | ⊕ Very Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HBOT, hyperbaric oxygen therapy; NRCT, nonrandomized controlled trial; RCT, randomized controlled trial.
Wider confidence intervals that, if aligned with true estimate, can either increase or decrease risk of mortality.
Figure 7: Risk Difference With Summary Estimate for HBOT Versus Standard Care on All-Cause Mortality.
Abbreviations: CI, confidence interval; HBOT, hyperbaric oxygen therapy; M-H, Mantel-Haenszel; SC, standard care.
Kalani et al34 reported two deaths in the HBOT group. One patient died of multiorgan failure and the other of progressive heart failure. In the standard care group, three patients died: two of cerebral infarction (stroke) and one of progressive heart failure after an acute myocardial infarction (heart attack). Londahl et al32 reported one patient death in the HBOT group, caused by multiple organ failure 20 days after study inclusion. Three patients in the standard care group died: two of myocardial infarction, one at 162 days and one at 218 days; and one of sepsis from an infected foot ulcer at 144 days.
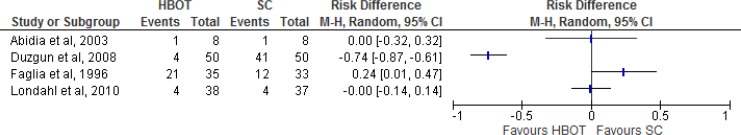
Results for Minor Amputations
Of the seven studies, four randomized controlled trials reported the minor amputation rate (Table 8, Figure 8). Faglia et al30 defined a minor amputation as the removal of a toe or forefoot. Abidia et al28 and Londahl et al32 did not define minor amputation. Duzgun et al29 defined a minor amputation as one occurring distal to (forward of) the metatarsophalangeal joint (the joint between the toes and the foot) or proximal to (close to) the metatarsophalangeal joint. The quality of evidence was very low according to the GRADE criteria.
Table 8:
GRADE Evidence Profile for Comparison of HBOT and Standard Care on Minor Amputations
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| 4 (RCTs) | Serious limitations (−1)a | Serious limitations (−1)b | Serious limitations (−1)c,d | No serious limitations | Undetected | No other considerations | ⊕ Very Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HBOT, hyperbaric oxygen therapy; RCT, randomized controlled trial.
Unclear allocation concealment, unclear blinding, and a lack of intention-to-treat analyses.
No trend in estimates; some studies had more minor amputations in the HBOT group, and others had more minor amputations in the standard care group.
The interventions varied across studies in terms of how many sessions were given (35–60), how many sessions occurred daily (1 vs. 2), and whether treatment was given in a monoplace or multiplace hyperbaric chamber.
Standard care was not delivered to the control groups in the same way across studies, and several standard care treatment protocols did not meet standard wound care guidelines. Fedorko et al was not included because their definition of minor amputation was not consistent with that of other studies.
Figure 8: Risk Difference for HBOT Versus Standard Care on Minor Amputations.
Abbreviations: CI, confidence interval; HBOT, hyperbaric oxygen therapy; M-H, Mantel-Haenszel; SC, standard care.
Sources: Londahl et al,32 Fedorko et al,35 Faglia et al,30 Duzgun et al,29 and Abidia et al.28
Faglia et al30 stated that, by an unspecified time point, five forefeet and 16 toes had been amputated in the HBOT group, and four forefeet and eight toes had been amputated in the standard care group. By an unspecified follow-up date (presumably the one-year follow-up), Abidia et al28 reported one minor amputation in the HBOT group versus none in the standard care group. By an unspecified time point, Duzgun et al29 reported four minor amputations in the HBOT group and 41 in the standard care group. At the one-year follow-up, Londahl et al32 reported four minor amputations in each of the HBOT and standard care groups.
Fedorko et al35 measured minor amputations using the same criteria as for major amputations, which precluded their inclusion in the GRADE rating for minor amputations. However, only one minor amputation actually occurred in the standard care group within the study period.
Results for Ulcer Size Reduction
Of the seven studies, four randomized controlled trials measured wound size reduction, each in a slightly different way (Table 9). The evidence showed no difference in ulcer size reduction between HBOT and standard care at different time points. The quality of evidence was low according to the GRADE criteria.
Table 9:
GRADE Evidence Profile for Comparison of HBOT and Standard Care on Ulcer Size Reduction
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| 4 (RCTs) | No serious limitations | No serious limitations | Serious limitations (−1)a,b | Serious limitations (−1)c | Undetected | No other considerations | ⊕⊕ Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HBOT, hyperbaric oxygen therapy; RCT, randomized controlled trial.
The interventions varied across studies in terms of how many sessions were given (35–60), how many sessions occurred daily (one vs. two), and whether treatment was given in a monoplace or multiplace hyperbaric chamber.
Standard care was not delivered to the control groups in the same way across studies, and several standard care treatment protocols did not meet standard wound care guidelines.
Abidia et al28 had only eight patients in each study arm. Ulcer size reduction was reported as a percentage, with a range from −206% to 100%. A negative number indicates that an ulcer had grown in size. The wide range is likely a result of the small sample size.
Abidia et al28 measured the reduction in wound ulcer surface area by tracing the ulcers onto a transparent sheet and then converting the tracings into digital images. Surface area was calculated using a special software program (SigmaScan, Systat Software, Inc., Chicago, IL). Ulcer assessment also included ulcer depth measurement and looking for clinical signs of infection.
Kessler et al31 measured the baseline wound ulcer surface area in square centimetres using a computer program and calculated the percentage of wound ulcer surface area reduction between baseline and day 15, between days 15 and 30, and between baseline and day 30.
Fedorko et al35 measured wound width manually and took computerized measurements of wound surface area and perimeter from high-resolution calibrated digital photos.
Abidia et al28 also measured the average reduction in ulcer size at two time points. At 6 weeks, ulcer size reduction was 100% (range 34–100%) for patients in the HBOT group and 52% (range −29–100%) for those in the standard care group (P = .027). However, at 6 months, there was no significant difference in ulcer size reduction between the HBOT and standard care groups (100% HBOT, range −206–100%, vs. 95% standard care, range 0–100%).
Kessler et al31 measured average reduction in ulcer size at three time points. At day 14, average ulcer size reduction for patients in the HBOT group was 41.8% ± 25.5% and for those in the standard care group was 21.7% ± 16.9% (P = .0037). From days 14 to 28, average ulcer size reduction for patients in the HBOT group was 48.1% ± 30.3% and for those in the standard care group was 41.7% ± 27.3% (not significant). From 0 to 4 weeks, average ulcer size reduction was 61.9% ± 23.3% in the HBOT group and 55.1% ± 21.5% in the standard care group (not significant).
Fedorko et al35 measured reduction of the ulcer surface area. At 12 weeks, there was an average reduction in ulcer surface area of 1.9 cm2 in the HBOT group and 1.8 cm2 in the standard care group. The adjusted mean difference between groups was 0.037 cm2 (P = .949).
Results for Time to Heal
Two of the seven studies (one randomized controlled trial and one nonrandomized controlled trial) measured time to heal (Table 10). The evidence showed no definitive difference in healing time for ulcers treated by HBOT versus standard care. The quality of evidence was low according to the GRADE criteria. Kalani et al34 measured mean healing time. Londahl et al32 presented healing rates and percentage of ulcers healed in a graph at 0, 1, 2, 3, 6, 9, and 12 months.
Table 10:
GRADE Evidence Profile for Comparison of HBOT and Standard Care on Time to Heal
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | Very serious limitations (−2)a | Undetected | No other considerations | ⊕⊕ Low |
| 1 (NRCT)34 | No serious limitations | No serious limitations | No serious limitations | Very serious limitations (−2)a | Undetected | No other considerations | ⊕ Very Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HBOT, hyperbaric oxygen therapy; NRCT, nonrandomized controlled trial; RCT, randomized controlled trial.
The range of time to heal was quite wide. Median time would have been a more appropriate measure for time to heal.
Kalani et al34 found that, on average, the time to heal in the HBOT group was 15 ± 7 months (range 3–30 months) and in the standard care group was 15 ± 4 months (range 8–18 months).
Londahl et al32 showed that, at 3 and 6 months, there was no difference in ulcers healed between the HBOT and standard care groups. However, at 9 and 12 months, there was a significant difference in ulcers healed between the HBOT and standard care groups (P < .01). At 9 months, about 57% of ulcers were healed in the HBOT group compared with about 19% in the standard care group. At 12 months, about 61% of ulcers were healed in the HBOT group compared with about 27% in the standard care group.
Results for Quality of Life
Of the seven studies, two randomized controlled trials measured quality of life (Table 11) and showed varying results for HBOT versus standard care. The quality of this evidence was moderate according to the GRADE criteria. In a 2011 publication, Londahl et al33 reported on quality of life for the sample from their 2010 study.32 Londahl et al33 and Abidia et al28 measured quality of life using the Short-Form 36-item health survey (SF-36). The SF-36 is a self-reporting questionnaire that measures physical and mental function across eight domains: physical functioning, bodily pain, general health perception, vitality, social functioning, role limitations owing to physical health, role limitations owing to emotional health, and mental health. Abidia et al28 also measured anxiety and depression using the Hospital Anxiety and Depression Scale.
Table 11:
GRADE Evidence Profile for Comparison of HBOT and Standard Care on Quality of Life
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| 2 (RCTs) | No serious limitations | No serious limitations | No serious limitations | No serious limitationsa | Undetected | No other considerations | ⊕⊕⊕ Moderate |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HBOT, hyperbaric oxygen therapy; RCT, randomized controlled trial.
Quality of life was determined based on two small sample sizes.
On the Hospital Anxiety and Depression Scale, Abidia et al28 found significant improvements from baseline in the depression scores for both the HBOT and standard care groups (P = .011 and P = .023, respectively). For the standard care group, there was also a significant improvement in the anxiety domain (P = .042). On the SF-36 scale, the HBOT group showed a significant improvement in the general health and vitality domains (P = .012 for each) but no improvement in any domains for the standard care group. Lastly, no significant difference was found in quality of life between the two groups overall.
Londahl et al33 found that, for the HBOT group, there was a significant difference between the pretreatment and post-treatment SF-36 mental summary scores and in two of the eight SF-36 domains (no P values given). No significant differences were found for any SF-36 domains between pretreatment and post-treatment for the standard care group.
Variation Across Included Studies
Heterogeneity exists in this body of evidence. The variations and limitations among studies are captured in Table 12.
Table 12:
Variations and Limitations Across Included Studies
| Author, Year | Limitations and Variations |
|---|---|
| Faglia et al, 199630 |
|
| Kalani et al, 200234 |
|
| Abidia et al, 200328 |
|
| Kessler et al, 200331 |
|
| Duzgun et al, 200829 |
|
| Londahl et al, 201032 |
|
| Fedorko et al, 201635 |
|
Abbreviations: ATA, atmospheres absolute; HBOT, hyperbaric oxygen therapy; ID, insulin dependent; NID, not insulin dependent.
Variation across the studies included in this review was most notable in terms of intervention, control, setting, and timing. The interventions varied in terms of the number of daily sessions (one vs. two), number of HBOT treatments (20–60), and whether the treatment occurred in a monoplace or multiplace hyperbaric chamber. Only three studies met guideline requirements for standard wound care (vascular and wound assessment, offloading, infection control, debridement, and dressing changes).28,30,32 However, details of the standard wound care protocol employed were not provided in two of these studies.28,32
Experts consulted for this review stated the importance of proper offloading for these patients. Six studies28,30–32,34,35 reported providing offloading devices to patients in both HBOT and standard care groups but did not provide a description of the devices provided, and only one study stated explicitly that patients had to be properly offloaded for a month without signs of healing before being considered for HBOT.31
Two studies involved inpatients30,31; the others were conducted in an outpatient setting. All studies varied in follow-up time, which ranged from one month to three years.
Discussion
Results Compared With Other Studies
Mirroring what the included studies tell us about HBOT compared with standard care for the treatment of diabetic foot ulcers, multiple systematic reviews report varying results.39–41 Some reviews combine results, whereas others do not. Some systematic reviews state that there is a lack of evidence in favour of HBOT over standard care, and that which exists is generally of poor quality, but others report favourable results for HBOT over standard care (see Appendix 4). In this health technology assessment, the some outcomes of the included studies were not meta-analyzed where there was substantial clinical heterogeneity across studies.
Ongoing Studies
There is a multicentre randomized controlled trial (DAMOCLES) underway in the Netherlands examining the effectiveness and cost-effectiveness of HBOT for diabetic foot ulcers.42 According to the protocol, the authors plan on recruiting 275 patients from approximately 35 hospitals, making this the largest trial ever addressing HBOT for the treatment of diabetic foot ulcers. As of December 2016, the trial website (www.amc.nl/web/CRU/Damocles/Algemeen-en-nieuws.htm) indicates that enrollment for the trial is complete and that the researchers were expecting to begin analyzing the initial results in November 2016. The results of this study may improve our understanding of the effectiveness of HBOT compared with standard care for the treatment of diabetic foot ulcers.
Patient Selection
More research should not only examine the effectiveness of HBOT for the treatment of diabetic foot ulcers, but also the appropriate patient selection criteria for this technology. The only patient selection criterion for HBOT is an ulcer of Wagner grade 3 or 4. Another criterion may be transcutaneous oximetry (TcPO2) as measured while breathing regular air or 100% oxygen. Kalani et al34 found that the only significant difference between patients who underwent amputation and those whose ulcers healed was the TcPO2 measurement during 100% oxygen inhalation (142 ± 65 mm Hg vs. 234 ± 110 mm Hg, P = .03). Measuring TcPO2 at the wound site allows for the identification of patients whose ulcers are in the “Goldilocks zone,” where hypoxia is present but not severe enough that surgery would be the only option to improve blood flow; such wounds may benefit from the presence of additional oxygen.43–45 Further, a retrospective analysis found that TcPO2 measured while patients breathed regular air under a pressure of 200 mm Hg within a hyperbaric chamber provided the best single predictor of HBOT responsiveness.43 However, it is important to note that transcutaneous oximetry is not publicly funded in Ontario. Faglia et al30 performed a multivariate analysis of major amputation on all considered variables and found that negative prognostic factors were a low ankle-brachial index value (P = .013) and a high Wagner grade (P = .022). Lastly, understanding how comorbid conditions affect healing is important; for example, conditions such as renal failure negatively correlate with healing using HBOT. Experts consulted for this review commented on the urgent need for more research to clarify the role of HBOT in the management of diabetic foot ulcers and to identify those patients most likely to respond to HBOT.
It is important to acknowledge the variation in wound care across the studies reviewed. The experts consulted for this review emphasized that proper offloading is very important for patients with diabetic foot ulcers and that quality research is needed to achieve standardized and measurably adequate foot protection as a prerequisite to further study of other adjunct therapies, including HBOT.
Conclusions
We are uncertain about the effects of HBOT on diabetic foot ulcers because the GRADE assessment of almost all outcomes in the studies reviewed was low or very low. Our conclusions on the effectiveness of standard wound care plus HBOT compared with standard wound care alone for the treatment of diabetic foot ulcers for each outcome are as follows:
Results were inconsistent for major amputations (GRADE quality of evidence: low)
There was a significant improvement in ulcers healed in patients who received HBOT versus those who received standard care (GRADE quality of evidence: low)
The most common adverse events associated with HBOT were short-term, such as barotraumatic otitis, the inability to equalize middle ear pressure, and worsening of cataracts (GRADE quality of evidence: moderate)
There was no difference in all-cause mortality between patients treated with HBOT and those treated with standard care (GRADE quality of evidence: moderate)
There was no difference in ulcer size reduction between patients treated with HBOT and those treated with standard care (GRADE quality of evidence: low)
Results were inconsistent for minor amputations (GRADE quality of evidence: very low)
Results were inconsistent for average time to heal (GRADE quality of evidence: low)
Results were inconsistent for quality of life (GRADE quality of evidence: moderate)
The evidence makes it difficult to draw any definitive conclusions on the effectiveness of standard wound care plus HBOT versus standard wound care alone for the treatment of diabetic foot ulcers.
ECONOMIC EVIDENCE REVIEW
Objective
The objective of this study was to review the literature on the cost-effectiveness of standard wound care plus HBOT compared with standard wound care alone for the treatment of diabetic foot ulcers.
Methods
Sources
We performed an economic literature search on February 17, 2016, using Ovid MEDLINE, Ovid MEDLINE In-Process, Ovid Embase, EBSCO Cumulative Index to Nursing & Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database, for studies published from inception to February 17, 2016. Database auto-alerts were created in MEDLINE, Embase, and CINAHL and monitored for the duration of the HTA review. We also extracted economic evaluation reports developed by health technology assessment (HTA) agencies by searching the websites of HTA agencies such as Canadian Agency for Drugs and Technologies in Health (CADTH), Institute of Health Economics, Institut national d'excellence en santé et en services sociaux (INESSS), McGill University Health Centre Health Technology Assessment Unit, and Cost-Effectiveness Analysis (CEA) Registry. Finally, we reviewed the reference lists of the included economic literature for any additional relevant studies not identified through the systematic search.
We based our search terms on those used in the clinical evidence review of this report and applied economic filters to the search results. The full search strategy can be found in Appendix 1. The final search strategy was peer-reviewed using the PRESS Checklist.23 See Appendix 1 for full details, including all search terms.
Literature Screening
Study eligibility criteria for the literature search are listed below. A single reviewer reviewed titles and abstracts, and, for those studies meeting the inclusion/exclusion criteria, we obtained full-text articles.
Inclusion Criteria
English-language full-text publications
Studies from inception to February 17, 2016
Studies in patients with diabetic foot ulcers
Studies comparing HBOT with standard wound care for the treatment of diabetic foot ulcers
Exclusion Criteria
Studies in which the comparator did not include standard wound care
Costing studies
Outcomes of Interest
Full economic evaluations: cost–utility analyses, cost-effectiveness analyses, cost–benefit analyses
Data Extraction
We extracted relevant data on the following:
Study design and perspective
Population and comparator
Interventions
Outcomes (i.e., health outcomes, costs, and cost-effectiveness)
We contacted authors of the studies to provide unpublished data where required.
Methodological Appraisal
We determined the usefulness of each identified study for decision-making by applying a modified methodological checklist for economic evaluations that was originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom. The original checklist is used to inform development of clinical guidelines by NICE.46 We modified the wording of the questions to remove references to guidelines and to make it Ontario specific. The original NICE checklist has two sections: an applicability section and a methodological quality section. Only the first section was used for our review. From this checklist, studies were deemed directly applicable, partially applicable, or not applicable to the research question. A summary of the number of studies judged to be directly applicable, partially applicable, or not applicable to the research question is presented.
Results
Literature Search
The database search yielded 233 citations published from inception to February 17, 2016. After duplicates were removed, there were 169 articles. We excluded a total of 166 articles based on information in the title and abstract. We then obtained the full texts of two potentially relevant articles for further assessment.47,48 Figure 9 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 9: PRISMA Flow Diagram.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Source: Adapted from Moher et al.37
Two economic evaluations met the inclusion criteria. We hand-searched the reference lists of the included studies and health technology assessment websites to identify other relevant studies, and one additional citation was reviewed in full text but was excluded as it was a repeat of one of the included economic evaluations.
Study Applicability
One Canadian study48 was deemed fully applicable after a review using the modified methodological checklist. We considered the study by Guo et al47 to be partially applicable, as the authors used a United States private payer perspective. The results of the modified methodological checklist for economic evaluations applied to the included articles are presented in Appendix 5. A summary of the two studies reviewed is presented in Table 13.
Table 13:
Results of Economic Literature Review—Summary
| Author, Year, Location | Study Design and Perspective | Population/Comparator | Interventions | Results | ||
|---|---|---|---|---|---|---|
| Health Outcomes | Costs | Cost-Effectiveness | ||||
| Guo et al, 2003, United States47 | Cost–utility analysis Decision model 12-year model United States societal perspective | 60-year-old patients with severe diabetic foot ulcers (Wagner grade 3 or higher) | HBOT Standard care | 0.61 QALY gained per person for HBOT | Incremental cost of $1,371 per person for HBOT | $2,255/QALY |
| Chuck et al, 2008, Canada48 | Cost–utility analysis Decision model 12-year model Canadian provincial ministry of health | 65-year-old patients with diabetic foot ulcers | HBOT Standard care | HBOT = 3.64 QALYs Standard care = 3.01 QALYs | HBOT = $40,695 Standard care = $49,786 | HBOT dominates standard care |
Abbreviations: HBOT, hyperbaric oxygen therapy; QALY, quality-adjusted life year.
Methodological Quality of the Included Studies
Guo et al47 developed a decision model incorporating healed ulcers, minor amputations, major amputations, and death states. The authors extended the model over a 12-year time frame because they estimated that a 60-year-old would have a 20-year life expectancy and that people with diabetes are expected to have a shorter life expectancy by about 8 years compared with those without diabetes. Incremental costs were calculated by subtracting the cost of HBOT from the cost of minor and major amputations averted. Additional downstream costs were excluded. Costs for standard care were also excluded from the model, as patients in both arms received standard care, and the costs thus cancelled when calculating the incremental cost. The authors assumed that mortality rates remained constant throughout the duration of the model and that there would be no ulcer recurrence. For sensitivity analyses, the minimum and maximum bounds of HBOT efficacy found in the literature were included in the model. The authors calculated one-way sensitivity analyses of each parameter to determine the variables that had the most impact.
The model developed by Chuck et al48 was based on that created by Guo et al.47 This study included an unhealed ulcer state not found in the model by Guo et al. The model by Chuck et al also had a 12-year time frame, matching the life expectancy of a 65-year-old person with diabetes residing in Alberta. The mortality rate increases each year in this model. Patients were assumed to remain in their first-year health state for the remainder of their life expectancy. For example, a patient who was unhealed after the first year was considered to remain unhealed. Similarly, it was considered that patients who were healed by the end of the first year would not experience another foot ulcer. Two sensitivity analyses were conducted. In the first analysis, the probability of healing with HBOT was decreased by 10%. For the second analysis, the cost of HBOT was increased until there was no longer an incremental cost savings.
Discussion
The results of published economic evaluations of HBOT for the treatment of diabetic foot ulcers suggest that HBOT may be cost-effective compared with standard care. Guo et al observed an incremental cost of $2,255 per quality-adjusted life year (QALY), which is well below the commonly used threshold of $50,000 per QALY.47 The study by Chuck et al presented even more favourable results for HBOT, observing overall lower costs and better QALYs compared with standard care.48 Both studies present HBOT as good value for money as a result of the major and minor amputations averted and improved QALYs owing to the higher ulcer healing rates observed with HBOT versus standard care.
Although the study by Chuck et al is Canadian specific, it was conducted eight years ago. Since then, two additional randomized controlled trials have been published that provide additional evidence on the efficacy of HBOT for the treatment of diabetic foot ulcers.32,49 We decided that an updated primary economic evaluation was warranted given the new data available.
Conclusions
Standard wound care plus HBOT appears to be cost-effective compared with standard wound care alone for the treatment of diabetic foot ulcers.
PRIMARY ECONOMIC EVALUATION
The published economic evaluations identified in the literature review addressed the use of HBOT for the treatment of diabetic foot ulcers. The last publication is now eight years old. Since then, more clinical research evaluating this treatment has been published.32,49 To account for these new data, we conducted a primary economic evaluation that considers all clinical evidence available.
Objective
The objective of this study was to assess the cost-effectiveness of standard wound care plus HBOT compared with standard wound care alone for the treatment of diabetic foot ulcers within the context of the Ontario Ministry of Health and Long-Term Care.
Methods
The information presented in this report follows the reporting standards set out by the Consolidated Health Economic Evaluation Reporting Standards Statement.50
Type of Analysis
A cost–utility analysis was conducted, as utility data related to diabetic foot ulcers are available.
Target Population
The study population consisted of men and women aged 68 years and older presenting with diabetic foot ulcers and without a history of lower limb amputation. The age 68 years was selected as it has been identified as the average age of patients in Ontario presenting with diabetic foot ulcers.51
Perspective
We conducted this analysis from the perspective of the Ontario Ministry of Health and Long-Term Care.
Interventions
We evaluated standard wound care plus HBOT compared with standard wound care alone for the treatment of diabetic foot ulcers. For the purposes of our evaluation, standard wound care consisted of patient education by a health care provider, offloading, debridement, infection control, moisture balance, and frequent re-evaluation.52
Discounting and Time Horizon
We applied an annual discount rate of 5% to both costs and QALYs. We used a lifelong time horizon in all analyses. We used a cut-off age of 85 years, as this is the reported life expectancy for women with diabetes in Canada. (Life expectancy for men with diabetes in Canada is 80 years).53
Model Structure
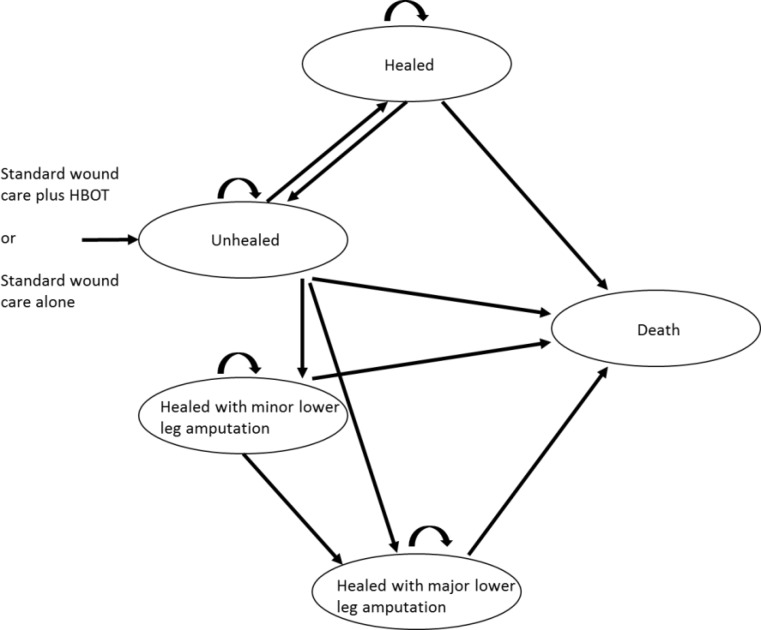
We developed a state transition model adapted from the model constructed by Chuck et al.48 Our model consisted of five health states that consider the clinical outcomes of healing, amputation, and death (Figure 10). These five states are as follows.
Figure 10: Model Structure.
Healed
This state defines patients whose foot ulcers have completely healed through initial treatment (via standard care plus HBOT or standard care alone) or subsequent standard care but excludes healing as a result of amputation. An ulcer is considered healed when sufficient epithelial regeneration has occurred to completely cover the surface area of the wound.32 Patients in this state are considered to be at risk of a subsequent foot ulcer or death throughout the duration of the model.
Unhealed
This state defines patients whose foot ulcers remain unhealed despite initial treatment and who continue to receive standard care. Patients in this state are considered to be at risk for lower limb amputation or death.
Minor Lower Leg Amputation: Healed
In this state, patients have undergone an amputation at or below the ankle. For our analysis, such patients were considered to have completely healed and not at risk of a subsequent ulcer or further minor or major amputation.
Major Lower Leg Amputation: Healed
In this state, patients have undergone an amputation above the ankle. For our analysis, such patients were considered to have completely healed and not at risk of a subsequent ulcer or further minor or major amputation.
Death
In each health state, there is a probability that a patient will transition to the death state. In our model, the death state represents all-cause mortality.
Clinical Outcome and Utility Parameters
Clinical outcomes and utility parameters were extracted from various sources. The details of each model parameter are explained below. Where data were not available for a specific year, transition probabilities were estimated through linear extrapolation of the data available.
Clinical Outcomes
Healed State Mortality
Patients in the healed state were assumed to have the same mortality risk as people with diabetes without foot ulcers. The base case additional risk of mortality experienced by people with diabetes was based on a study by Lind et al.54 The rate ratio extracted from the study was for an age cohort of 65 to 74 years. The rate ratio was multiplied by the age- and sex-adjusted mortality rates reported in life tables published by Statistics Canada.55 The annual probability of mortality was calculated as 2.4% in the first year, reaching 11.9% by the end of the model duration. The transition probability for each year is presented in Appendix 6.
Unhealed State Mortality
The base case additional mortality risk for patients with foot ulcers compared with people with diabetes without ulcers was extracted from a meta-analysis conducted by Brownrigg et al.56 Based on these data, the annual mortality risk for patients with diabetic foot ulcers was observed to be 4.6% in the first year, increasing to 22.6% by the end of the model. Details of this analysis can be found in Appendix 6.
Major and Minor Lower Leg Amputation State Mortality
The base case mortality rates associated with major and minor lower leg amputation were based on results from Fortington et al.57 In this study, mortality rates were reported at one and five years post-amputation. Details on the annual transition probabilities can be found in Appendix 6. Table 14 provides the major and minor lower leg amputation parameter inputs for our economic evaluation.
Table 14:
Major and Minor Lower Leg Amputation Mortality Parameter Inputs for Economic Evaluation
Foot Ulcer Recurrence
The base case probability of a patient in the healed state transitioning back to the unhealed state because of ulcer recurrence was based on three-year ulcer-free survival data from Pound et al.58 In this study, annual ulcer-free survival was extracted and converted into the probability of ulcer recurrence. It was assumed that there was no additional ulcer recurrence after three years. The transition probability was calculated to be 0.37 in the first year, 0.11 in the second year, and 0.20 in the third year.
Lower Leg Amputation
The base case probability of transitioning from the unhealed state to a major or minor lower leg amputation state treated by HBOT versus standard care was calculated based on data reported by Londahl et al for the first year.32 In the base case, it was assumed that major and minor amputation rates were identical for both treatment arms. Transition probabilities to amputation states were based on data reported by Hopkins et al.51 Since there are currently no published data on amputation rates after three years, a conservative assumption of no additional amputations occurring after three years was used for our model. Extracting data from Hopkins et al,51 we considered femur, knee, tibia, and fibula amputations to be major amputations. Ankle, tarsal (hindfoot and midfoot), metatarsal (forefoot), phalanx (forefoot), and toe amputations were considered minor amputations. Tables 15 and 16 provide the major and minor lower leg amputation parameter inputs for our economic evaluation.
Table 15:
Major Lower Leg Amputation Parameter Inputs for Economic Evaluation
Table 16:
Minor Lower Leg Amputation Parameter Inputs for Economic Evaluation
| Model Parameter | Annual Probability | Reference | |
|---|---|---|---|
| HBOT | Standard Care | ||
| Minor lower leg amputation, first year | 0.08 | 0.09 | Londahl et al32 |
| Minor lower leg amputation, after first year | 0.04–0.07 | 0.04–0.07 | Hopkins et al51 |
Abbreviation: HBOT, hyperbaric oxygen therapy.
Ulcer Healing
The base case ulcer healing rates for patients receiving either HBOT or standard care were based on the one-year follow-up results of Londahl et al.32 In the analysis, the probability of ulcer healing was 52% for HBOT and 29% for standard care. The three-year healing rates observed in the standard care arm of the study by Kalani et al (48%) were assumed to be an accurate reflection of long-term healing rates for our study population.34 Using these data, the annual probability of healing in the second and third years was calculated as 14%. (See Appendix 6 for detailed calculations). We assumed that both HBOT and standard care resulted in the same healing rate after one year and that the probability of healing was 14% annually for the remainder of the model.
Utilities
We used the utility values reported by Ragnarson Tennvall et al in our base case analysis (Table 17).59 This study cohort consisted of patients treated for diabetic foot ulcers. Results were stratified based on the patient's current status (i.e., current foot ulcer with no previous amputation, healed foot ulcer with no previous amputation, minor amputation, or major amputation).
Table 17:
Utilities Used in the Economic Model59
| Health State | Utility, Base Case |
|---|---|
| Unhealed | 0.44 |
| Healed | 0.60 |
| Minor lower leg amputation | 0.61 |
| Major lower leg amputation | 0.31 |
Cost Parameters
Hyperbaric Oxygen Therapy Costs
Costs for HBOT treatment consist of the physician cost for the initial assessment to determine eligibility, the physician cost at each HBOT session, and the overhead facility cost per HBOT session. The list of initial assessment billing codes is presented in Appendix 6 (Table A19). Three different sets of physician billing codes can be used for HBOT depending on where the physician is physically located in relation to the HBOT unit during treatment. For the base case analysis, we assumed that the physician is located inside the HBOT unit but not within the hyperbaric chamber with the patient or patients. This assumption is based on information provided by clinical experts (written communication, April 11, 2016; April 25, 2016).
We derived overhead facility costs from information provided by a clinical expert (written communication, April 11, 2016). The calculations are presented in Appendix 6 (Table A20). The overhead facility costs were assumed to be the same for both monoplace and multiplace chamber facilities. According to expert opinion, multiplace chamber facilities have higher initial capital costs than monoplace chamber facilities but may provide greater versatility in patient management. The overhead facility costs for multiplace chamber facilities are likely to differ from those of monoplace chamber facilities, but not markedly so (written communication, June 10, 2016).
According to expert opinion, the mean total number of HBOT sessions used to treat diabetic foot ulcers is between 40 and 45, with each session lasting 90 minutes (written communication, April 11, 2016; April 25, 2016). For the base case analysis, we assumed that all patients receive 45 sessions. The HBOT treatment costs per session used in our model are presented in Table 18.
Table 18:
Per-Session HBOT Treatment Costs Used in the Economic Model
| Variable | Unit Cost, $ | Quantity | Total Cost, $ | Reference |
|---|---|---|---|---|
| Initial assessment physician cost | 166.05 | 1 | 166.05 | Ontario Schedule of Benefits for Physician Services,60 expert opinion |
| Overhead cost per session (base case) | 100 | 1 | 100.00 | Expert opinion |
| Physician HBOT cost per session (base case) | 71.85 for first ¼ hour; 35.90 for each subsequent ¼ hour | 5 for each subsequent ¼ hour | 251.35 | Ontario schedule of benefits for physician services,60 expert opinion |
| Physician pre- and post-session assessment (base case) | 31.45 | 2 | 62.90 | Ontario schedule of benefits for physician services,60 expert opinion |
Abbreviation: HBOT, hyperbaric oxygen therapy.
Healed Health State Costs
Patients whose foot ulcers heal will continue to incur diabetes-related health care costs. We extracted diabetes-related health care costs from a costing study by Rosella et al.61 In this study, the authors calculated the mean annual cost for a cohort of Ontarians with diabetes. The costs were inflated to 2015 dollars, using inflation numbers reported by Statistics Canada.62 The costs included in our model are presented in Table 19.
Table 19:
Costs for Patients Whose Foot Ulcers Healed Used in the Economic Model
| Variable | Mean Cost per Year, $ (SD) | Reference |
|---|---|---|
| Woman with diabetes aged | 18,103.70a | Rosella et al, 201661 |
| 65–74 years | (87,989.60) | |
| Man with diabetes aged | 87,989.60 | Rosella et al, 201661 |
| 65–74 years | (106,861.00) | |
| Sex-adjusted person with diabetes aged 65–74 years | 20,729.23 |
Abbreviation: SD, standard deviation.
Sample calculation: $17,403 × 126.6 (2015 consumer price index inflation) ÷ 121.7 (2012 consumer price index inflation) = $18,103.70.
Unhealed Health State Costs
The ongoing costs for patients whose foot ulcers remain unhealed were calculated by extracting the cost ratio of mean expenditures for the treatment of foot ulcers in patients with diabetes over 65 years of age reported by Ramsey et al.63 The study reported only first- and second-year costs; we used the mean cost for the first two years as the annual cost in the unhealed state in our model. The costs included in the model are presented in Table 20.
Table 20:
Costs for Patients Whose Foot Ulcers Remain Unhealed Used in the Economic Model
| Variable | Mean Healed Ulcer Cost per Year, $ | Mean Cost Ratio (SD) | Mean Unhealed Ulcer Cost per Year, $ | Reference |
|---|---|---|---|---|
| First year | 20,729.23 | 2.67 (0.68) | 55,347a | Ramsey et al63 |
| Second year | 87,989.60 | 1.56 (0.45) | 32,338 | Ramsey et al63 |
| Annual cost, unhealed state | 43,842 |
Abbreviation: SD, standard deviation.
Sample calculation: $20,729.23 × 2.67 = $55,347.
Lower Leg Amputation State Costs
The total costs of surgical lower leg amputations were calculated from administrative data collected in Ontario using IntelliHealth Ontario databases. All codes used to identify this cohort are presented in Appendix 6 (Table A21). All records of lower leg amputations entered into inpatient hospital databases in 2014 were collected. We calculated the cost of an inpatient stay by multiplying the reported resource intensity weight by the cost per hospital stay reported by the Canadian Institute for Health Information.64 The resource intensity weight is a standardized measure that estimates the level of health care resource utilization of a patient compared with the average hospitalization for a patient with the same disease profile. The hospital-related surgical costs included in the model are presented in Table 21. We calculated the physician costs for performing amputations based on the average cost for a major amputation and the average cost for a minor amputation reported in the Ontario Schedule of Benefits for Physician Services.60 The mean physician cost for a major lower leg amputation is $483.61 and for a minor lower leg amputation is $318.20. Details of the fee codes for these procedures are presented in Appendix 6 (Table A22).
Table 21:
Hospital-Related Surgical Costs Associated With Lower Leg Amputations Used in the Economic Model
| Variable | Mean Resource Intensity Weight (SD) | Cost per Typical Hospital Stay, $ | Mean Cost per Hospital Stay, $ (SD) | Reference |
|---|---|---|---|---|
| Major lower leg amputation | 5.41 (8.24) | 5,283 | 28,598a (43,545) | CIHI64 |
| Minor lower leg amputation | 3.17 (2.80) | 5,283 | 16,759 (14,783) | CIHI64 |
Abbreviations: CIHI, Canadian Institute for Health Information; SD, standard deviation.
Sample calculation: 5.41 × $5,283 = $28,598.
For patients who underwent a lower leg amputation, the health care costs incurred in the years after the surgical procedure were calculated from the ratio of the costs for the amputation versus the healed study cohort observed in a study by Apelqvist et al.65 In this study, data for the first and second year following major and minor amputations were collected. In our model, we assumed that follow-up costs were the same for every year after the first year. The calculated cost ratios and resulting costs per year are presented in Table 22.
Table 22:
Annual Costs Following Surgical Amputation of the Lower Leg
| Variable | Annual Cost, Patient With Healed Ulcer, $ | Cost Ratio Compared to Healed Ulcer | Annual Cost, Patient With Lower Leg Amputation, $ | Reference |
|---|---|---|---|---|
| Major lower leg amputation, first year | 20,729 | 4.54 | 94,110a | Apelqvist et al65 |
| Major lower leg amputation, after first year | 20,729 | 2.77 | 57,420 | Apelqvist et al65 |
| Minor lower leg amputation, first year | 20,729 | 2.22 | 46,018 | Apelqvist et al65 |
| Minor lower leg amputation, after first year | 20,729 | 2.23 | 46,226 | Apelqvist et al65 |
Sample calculation: $20,729 × 4.54 = $94,110.
Analysis
We calculated the base case of this analysis by using the input parameters presented above. We assessed variability and uncertainty in the model through one-way and probabilistic sensitivity analyses. To determine the impact of simultaneously varying numerous variables within the assigned distributions, we conducted a probabilistic sensitivity analysis by running simulations of the model. Several of the model parameters reported uncertainty by providing values for 95% confidence intervals or standard deviations. We used this information to develop distributions of the data for probabilistic sensitivity analyses. The values where uncertainty was presented and the type of distribution used are presented in Table 23.
Table 23:
Model Variable Distributions
| Variable | Distribution | Reference |
|---|---|---|
| Annual cost of diabetes care | Gamma | Rosella et al61 |
| Cost of major lower leg amputation | Gamma | Administrative dataa |
| Cost of minor lower leg amputation | Gamma | Administrative dataa |
| Annual cost following lower leg amputation | Gamma | Administrative dataa |
| Diabetes mortality ratio | Normal | Lind et al54 |
| Diabetic foot ulcer mortality ratio | Normal | Brownrigg et al56 |
| Probability of ulcer healing in first year | Beta | Londahl et al32 |
| Probability of major amputation | Beta | Londahl et al,32 Hopkins et al51 |
| Probability of minor amputation | Beta | Longdahl et al,32 Hopkins et al51 |
Data provided by IntelliHealth Ontario.
We also conducted one-way sensitivity analyses by varying specific model variables and examining the impact on the results. The variables and ranges are presented in Table 24.
Table 24:
Variables Varied in One-Way Sensitivity Analyses
| Variable | Value | Reference |
|---|---|---|
| Healed state to death state (sensitivity analysis 1) | 0.03–0.20 | Iversen et al66 |
| Healed state to death state (sensitivity analysis 2) | 0.083 | Chuck et al48 |
| Unhealed state to death state (sensitivity analysis 1) | 0.09–0.98 | Morbach et al67 |
| Unhealed state to death state (sensitivity analysis 2) | 0.083 | Chuck et al48 |
| Major lower leg amputation state to death state (sensitivity analysis 1) | 0.28–0.48 | Jones et al68 |
| Major lower leg amputation state to death state (sensitivity analysis 2) | 0.133 in first year 0.083 in other years | Chuck et al48 |
| Minor lower leg amputation state to death state (sensitivity analysis 2) | 0.083 | Chuck et al48 |
| Healed state to unhealed state (sensitivity analysis) | 0.07–0.35 | Waaijman et al69 |
| Major lower leg amputation (sensitivity analysis 1) | HBOT: 0.01–0.09 SC: 0.01–0.33 | Faglia et al30 (1st year) Hopkins et al51 (2nd and 3rd years) |
| Major lower leg amputation (sensitivity analysis 2) | HBOT: 0.01–0.11 SC: 0.001–0.01 | Abidia et al28 (1st year) Hopkins et al51 (2nd and 3rd years) |
| Major lower leg amputation (sensitivity analysis 3) | HBOT: 0.01–0.08 SC: 0.01–0.10 | Londahl et al32 Hopkins et al51 (1st to 3rd years) |
| Major lower leg amputation (sensitivity analysis 4) | HBOT: 0.01–0.02 SC: 0.02–0.08 | Morbach et al67 |
| Minor lower leg amputation (sensitivity analysis 1) | HBOT: 0.02–0.60 SC: 0.02–0.36 | Faglia et al30 (1st year) Hopkins et al51 (2nd and 3rd years) |
| Minor lower leg amputation (sensitivity analysis 2) | HBOT: 0.02–0.11 SC: 0–0.02 | Abidia et al28 (1st year) Hopkins et al51 (2nd and 3rd years) |
| Minor lower leg amputation (sensitivity analysis 3) | HBOT: 0.02–0.08 SC: 0.02–0.10 | Londahl et al32 Hopkins et al51 (1st to 3rd years) |
| Diabetic foot ulcer healing, with 3-year data (sensitivity analysis 1) | HBOT: 0.25–0.52 SC: 0.13–0.29 | Londahl et al32 Kalani et al34 |
| Diabetic foot ulcer healing, first year (sensitivity analysis 2) | HBOT: 0.63 SC: 0 | Abidia et al28 |
| Utility values | 0.84–0.68 | Redekop et al70 |
| Utility values for standard care | 0.76 | O'Reilly et al71 |
| Per-session physician cost for HBOT (physician in chamber with patient) | $356.20 | Ontario Schedule of Benefits for Physician Services60 |
| Per-session physician cost for HBOT (physician in the HBOT facility and available if necessary) | $35.75 | Ontario Schedule of Benefits for Physician Services60 |
| Number of HBOT sessions | 40 | Expert opinion |
| Physician cost billed as minor assessment | $273.05 | Ontario schedule of benefits for physician services,60 expert opinion |
Abbreviations: HBOT, hyperbaric oxygen therapy; SC, standard care.
Main Assumptions
The major assumptions for this model are as follows:
Patients receiving a lower leg amputation will not have a foot ulcer recurrence on that limb
Patients receiving a minor lower leg amputation will not require a major lower leg amputation
The annual health care costs for a patient with an unhealed foot ulcer in the second year will be the same in subsequent years
The annual health care costs for a patient with a major or minor lower leg amputation in the third year will be the same in subsequent years
The amputation and healing rates for the treatment (HBOT) and comparator (standard care) arms will be the same after the first year
Most of the outcome parameters in our model were based on clinical trials that used multiplace hyperbaric chambers for HBOT treatment; we assumed that results from these studies would be generalizable to HBOT treatment using monoplace hyperbaric chambers
Generalizability
The findings of this economic analysis cannot be generalized to all patients with diabetic foot ulcers. They may, however, be used to guide decision-making about the specific patient populations addressed in the trials investigated by Health Quality Ontario.
Expert Consultation
In April and May 2016, expert consultation was solicited on the use of HBOT for the treatment of diabetic foot ulcers. Members of the consultation included clinical scientists who have conducted research in the topic area and physicians practising hyperbaric medicine. The role of the expert advisors was to contextualize the evidence, provide research guidelines, and provide advice on the treatment of diabetic foot ulcers using HBOT. However, the statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted experts.
Results
Base Case Analysis
The results from the base case analysis are presented in Table 25. In the base case, HBOT was associated with lower total costs than standard care. Treatment with HBOT also resulted in improved overall QALYs compared with standard care. Thus, HBOT dominated standard care in our base case analysis.
Table 25:
Base Case Analysis Results
| Strategy | Average Total Costs, $ | Incremental Cost,a $ | Average Total Effects | Incremental Effectb | ICERc |
|---|---|---|---|---|---|
| HBOT | 232,800 | −3,700 | 3.73 | 0.02 | HBOT dominates |
| Standard care | 236,500 | 3.71 |
Abbreviation: HBOT, hyperbaric oxygen therapy; ICER, incremental cost-effectiveness ratio.
Incremental cost = average costs (HBOT) - average costs (standard care).
Incremental effect = average effects (HBOT) - average effects (standard care).
ICER = incremental costs ÷ incremental effects.
Sensitivity Analysis
The incremental cost and incremental QALYs calculated for each simulation of the probabilistic sensitivity analysis are illustrated in Figure 11. The cost-effectiveness acceptability curve is presented in Figure 12. The results from the cost-effectiveness acceptability curves present a 45% probability of HBOT being cost-effective compared with standard care at a cost-effectiveness threshold of $0, a 50% probability at a $50,000 threshold, and a 57% probability at a $100,000 threshold.
Figure 11: Incremental Cost and Incremental QALY Results From the Probabilistic Sensitivity Analysis.
Abbreviation: QALY, quality-adjusted life year.
Figure 12: Cost-Effectiveness Acceptability Curve.
Abbreviation: HBOT, hyperbaric oxygen therapy.
The results of the one-way sensitivity analysis are presented in Table 26. The incremental cost ranged from -$48,600 to $8,600 for HBOT. The incremental QALY ranged from 0.04 in favour of standard care to 1.04 in favour of HBOT.
Table 26:
One-Way Sensitivity Analysis Results
| Scenario | Incremental Cost,a $ | Incremental Effect, QALYsb | Result |
|---|---|---|---|
| Healed state to death state (sensitivity analysis 1, Iversen et al66) | −4,790 | −0.01 | HBOT has lower cost but worse outcomes |
| Unhealed state to death state (sensitivity analysis 1, Morbach et al67) | 3,045 | 0.12 | HBOT has higher cost but better outcomes |
| Major lower leg amputation state to death state (sensitivity analysis 1, Jones et al68) | −4,868 | 0.01 | HBOT dominates |
| Mortality rates used in model by Chuck et al48 (sensitivity analysis 1, Chuck et al48) | −7,848 | −0.10 | HBOT has lower cost but worse outcomes |
| Healed state to unhealed state (sensitivity analysis, Waaijman et al69) | 2,400 | 0.20 | HBOT has higher cost but better outcomes |
| Major and minor lower leg amputation (sensitivity analysis 1, Faglia et al,30 Hopkins et al51) | 8,642 | 1.04 | HBOT dominates |
| Major and minor lower leg amputation (sensitivity analysis 2, Abidia et al,28 Hopkins et al51) | −13,595 | −0.04 | HBOT has lower cost but worse outcomes |
| Major and minor lower leg amputation (sensitivity analysis 3, Hopkins et al51 for standard care only) | −11,300 | 0.16 | HBOT dominates |
| Major lower leg amputation (sensitivity analysis 4, Morbach et al67) | −8,312 | 0.09 | HBOT dominates |
| Diabetic foot ulcer healing, 3-year data (sensitivity analysis 1, Londahl et al,32 Kalani et al34) | −9,319 | 0.06 | HBOT dominates |
| Diabetic foot ulcer healing, first year (sensitivity analysis 2, Abidia et al28) | −48,563 | 0.16 | HBOT dominates |
| Utility values (Redekop et al70) | −3,703 | −0.01 | HBOT has lower cost but worse outcomes |
| Utility values for standard care (O'Reilly et al71) | −3,703 | 0.13 | HBOT dominates |
| Per-session physician cost for HBOT (physician in chamber with patient) | −1,905 | 0.02 | HBOT dominates |
| Per-session physician cost for HBOT (physician in the HBOT facility and available if necessary) | −15,638 | 0.02 | HBOT dominates |
| Number of HBOT sessions | −5,675 | 0.02 | HBOT dominates |
| Physician cost billed as minor assessment | −5,469 | 0.02 | HBOT dominates |
Abbreviations: HBOT, hyperbaric oxygen therapy; QALY, quality-adjusted life year.
Incremental cost = average costs of HBOT – average costs of standard care.
Incremental effect = average effects of HBOT – average effects of standard care.
Limitations
There are several limitations to our analysis. First, almost all studies that evaluated healing and amputation rates associated with HBOT used a multiplace hyperbaric chamber, whereas HBOT facilities in Ontario use monoplace chambers. For our model, we assumed that the outcomes would be the same for both monoplace and multiplace chambers; however, the accuracy of this assumption is unknown. Second, there are few clinical trial data beyond one year. It is therefore unknown what the long-term benefits of HBOT may be. One expert mentioned that HBOT may result in reduced long-term recurrence rates because of the angiogenesis (development of new blood vessels) associated with HBOT (oral communication, April 27, 2016), but there is a need for clinical studies to explore this hypothesis. Third, our model assumed that a patient who undergoes a major or minor amputation will not undergo a subsequent amputation. In reality, patients who undergo lower leg amputations are at risk for a subsequent amputation on the same leg or the other leg. Health states for a second minor or major amputation were not included based on a lack of costing and utility data to inform these health states. Although our cost estimates for amputations incorporated the cost of subsequent amputations, our utility values for amputation states were based on first amputations. This means that the total QALYs for patients in the amputation states in our model are likely an overestimation. The impact of this on the overall results is unknown since HBOT was found to be associated with a slightly higher major amputation rate and a lower minor amputation rate compared with standard care in the first year.
Discussion
Given the uncertainty in the clinical outcomes and costs associated with HBOT, the results of our cost-effectiveness analysis were associated with a large degree of uncertainty. As observed in our probabilistic sensitivity analysis, results varied, sometimes showing that treatment with HBOT results in lower costs and better QALYs than standard care, other times showing that treatment with HBOT results in higher costs and worse QALYs than standard care. Compared with standard care, treatment with HBOT has a slightly lower probability of being cost-effective at lower willingness-to-pay thresholds but a slightly higher probability of being cost-effective at higher willingness-to-pay thresholds. However, in our base case evaluation of the cost-effectiveness of HBOT compared with standard care for the treatment of diabetic foot ulcers, we found HBOT to be less costly and to result in better QALYs.
In an economic evaluation of HBOT conducted by Chuck et al,48 the authors observed a total 12-year cost of $40,695 and 3.64 QALYs for HBOT compared to $49,786 and 3.01 QALYs for standard care.48 The incremental cost savings with HBOT reported in this analysis were about $9,000. The reported incremental cost was higher than that observed in our analysis. The difference is likely a result of the different data sources used for costing the healed state. In both studies, the cost of care for a person with diabetes was used as the cost of the healed health state. The cost of the healed state used in the study by Chuck et al was based on the cost of care for a person with diabetes in Saskatchewan in 1997.48 Our study used data based on a diabetes cohort in Ontario from 2004 to 2012.54 The higher costs found in our analysis may be a result of the rising cost of health care over time and/or the difference in the cost of health care between Saskatchewan and Ontario.
The incremental QALY benefit for HBOT was observed to be 0.63 in the study by Chuck et al48; this is much higher than that observed in our study. The difference in incremental QALYs is a result of the differences in clinical outcomes inputted into the model. In the study by Chuck et al,48 results from the clinical outcomes showed improved healing rates, a higher probability of minor lower leg amputation, and a lower probability of major lower leg amputations for HBOT compared with standard care. In the base case, the clinical outcomes included in our model showed improved healing rates but a lower probability of minor lower leg amputation and a higher probability of major lower leg amputation. The results of Chuck et al were based on outcome differences in the first year and the assumption of there being no change in health state (with the exception of mortality) after the first year.48
The clinical outcomes used in our model were based on the aggregated results of the clinical literature review. In a study by Guo et al, HBOT was observed to have a cost-per-QALY of $2,100.47 In this analysis, costs consisted of initial treatment and major and minor amputations experienced in the first year. Guo et al did not consider downstream costs.47 As a result, the cost of HBOT was observed to be higher than that of standard care. Our study included the downstream costs associated with HBOT and standard care; by including these in our analysis, we found a cost savings associated with HBOT. The sensitivity analyses of Chuck et al and Guo et al were limited and thus not comparable to our analysis.47,48
In our one-way sensitivity analyses, most results were consistent with the base case. Analyses that were contrary to the results of the base case show how uncertain the base case results are. The results of the one-way sensitivity analyses revealed the sensitivity of the results to clinical outcomes. This was reiterated in the probabilistic sensitivity analysis results showing incremental costs and incremental effects of simulation both in favour of and against HBOT versus standard care. The resulting probability that HBOT is cost-effective is close to equal to the probability that standard care is cost-effective.
Our study has several strengths. First, our analysis incorporated diabetic foot ulcer recurrence in the healed state. This prevented the benefits of ulcer healing from being overestimated. Second, the clinical outcomes in our model were based on an extensive review of the clinical literature. Third, many of the model inputs, such as physician costs, mean number of sessions per person, and overhead costs, were confirmed by clinical experts. Fourth, our analysis is the only one to date that has included diabetic foot ulcer recurrence. Including this possibility allowed for a more accurate portrayal of the disease course of diabetic foot ulcers, thus producing more accurate results.
Conclusions
Following our analysis, we are uncertain of the value for money of standard wound care plus HBOT versus standard wound care alone for the treatment of diabetic foot ulcers. Treatment with standard wound care plus HBOT may be less costly and result in better QALYs compared with standard wound care alone for the treatment of diabetic foot ulcers. The results of our analysis suggest that additional clinical studies evaluating rates of death, ulcer healing, and lower extremity amputation in patients with diabetic foot ulcers who undergo treatment with standard wound care plus HBOT may help reduce this uncertainty.
BUDGET IMPACT ANALYSIS
We conducted a budget impact analysis from the perspective of the Ontario Ministry of Health and Long-Term Care to determine the estimated cost burden for intervention over the next five years. All costs are reported in 2016 Canadian dollars.
Objective
The objective of this study was to determine the budget impact of funding HBOT for the treatment of diabetic foot ulcers.
Methods
Target Population
The current incidence of diabetic foot ulcers in the Ontario population is 36.9 per 100,000 persons.51 Given that the total population of Ontario is estimated to be 13.8 million people,72 the total number of new cases of diabetic foot ulcers in Ontario is expected to be 5,100 per year (13.8 million × 0.00037). The proportion of patients with diabetic foot ulcers eligible for HBOT is about 30%.73 In total, it is estimated that the total number of new cases each year eligible for HBOT is about 1,500 (5,100 × 0.3).
One estimate of the incidence of diabetic foot ulcers has been reported by Hopkins et al.51 Using this information and data on the proportion of patients with diabetic foot ulcers eligible for HBOT (30%), we were able to calculate the total number of patients in Ontario who would be eligible for HBOT in a given year and incorporate this information into a scenario analysis. Several other estimates of the incidence of diabetes have been reported in the literature.74,75 We incorporated this information into the calculation of the total number of patients eligible for HBOT and analyzed each result in a separate scenario analysis. One estimate of the incidence of diabetes in Ontario was reported by the Public Health Agency of Canada for fiscal year 2008/09 (0.6%).74 Ontario incidence rates of diabetes stratified by local health integration network have also been reported by the Institute for Clinical Evaluative Sciences.75 The upper (1.2%) and lower (0.8%) results observed in this report were included as separate analyses. The total number of patients with diabetic foot ulcers was calculated by multiplying the total number of Ontarians with diabetes by the proportion with diabetic foot ulcers (6%).76 Using the total number of patients with diabetic foot ulcers, we calculated the total number of patients eligible for HBOT based on the different estimates of the incidence of diabetes. The total number of patients eligible for HBOT is presented in Table 27. We assumed that the number of patients eligible for HBOT would remain steady throughout the five-year analysis period. This assumption was based on a report observing stable incidence rates over the last decade.77
Table 27:
Total Number of Patients Eligible for HBOT per Year for Base Case and Scenario Analyses
| Variable | Diabetes Incidence Rate | Total Number of Ontarians with Diabetes | Total Number of Patients with Diabetic Foot Ulcers | Total Number of Patients Eligible for HBOT | Reference |
|---|---|---|---|---|---|
| Scenario 1 | Not applicable | Not applicable | 5,100a | 1,500b | Hopkins et al51 |
| Scenario 2 | 0.006 | 82,753c | 4,965d | 1,489e | PHAC74 |
| Scenario 3 (diabetes incidence rate from ICES, upper value) | 0.012 | 107,578 | 6,455 | 1,936 | Booth et al75 |
| Scenario 3 (diabetes incidence rate ICES, lower value) | 0.008 | 168,264 | 10,095 | 3,029 | Booth et al75 |
Abbreviations: HBOT, hyperbaric oxygen therapy; ICES, Institute for Clinical Evaluative Sciences; PHAC, Public Health Agency of Canada.
Sample calculation: 13,792,100 (population of Ontario) × 0.00037 = 5,100.
Sample calculation: 5,100 × 0.3 = 1,500.
Sample calculation: 13,792,100 (population of Ontario) × 0.006 = 82,753.
Sample calculation: 82,753 × 0.06 = 4,965.
Sample calculation: 4,965 × 0.3 = 1,489.
According to experts, there are about 20 monotherapy chambers, two dual-place chambers, and three multiplace chambers in Ontario that adhere to guidelines on the implementation of HBOT as articulated by Health Canada and the Undersea & Hyperbaric Medical Society (written communication, May 2, 2016; May 13, 2016; June 10, 2016). According to expert consultation, the number of new patients with diabetic foot ulcers treated with HBOT in a monoplace chamber per year ranges from four to nine per chamber (written communication May 2, 2016; May 4, 2016). Assuming an average turnover of six new patients with diabetic foot ulcers per chamber per year at all centres in the province, we estimate that approximately 120 patients are treated in monoplace chambers in Ontario each year. Assuming that a dual-place chamber has the capacity to treat 1.5 times the number of patients and that a multiplace chamber can treat four times as many patients compared to a monoplace chamber, we estimate that an additional 81 patients are treated with HBOT per year (9 in dual-place chambers and 72 in multiplace chambers). In total, we estimate that about 200 patients with diabetic foot ulcers receive HBOT treatment in Ontario each year. In the base case analysis, we assumed that this number would not change in subsequent years as it represents maximum chamber volume based on current capacity. In separate scenario analyses, we analyzed the budget impact if there were to be a progressive increase in capacity with the goal of having enough chambers to treat all new cases in five years for all scenarios included in our analysis. The number of patients receiving HBOT per year was estimated using linear extrapolation (Table 28).
Table 28:
Total Number of Patients with Diabetic Foot Ulcers Receiving HBOT per Year for Base Case and Scenario Analyses
| Variable | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Base case assuming no increase in capacity | 200 | 200 | 200 | 200 | 200 |
| Scenario analysis 1 assuming an increase in capacity (incidence rate from Hopkins et al51) | 465a | 731 | 996 | 1,261 | 1,527 |
| Scenario analysis 2 assuming increase in capacity (incidence rate from PHAC74) | 458 | 716 | 974 | 1,232 | 1,489 |
| Scenario analysis 3 assuming increase in capacity (incidence rate from ICES [Booth et al75], upper value) | 547 | 895 | 1,242 | 1,589 | 1,936 |
| Scenario analysis 4 assuming increase in capacity (incidence rate from ICES [Booth et al75], lower value) | 766 | 1,331 | 1,897 | 2,463 | 3,029 |
Abbreviations: HBOT, hyperbaric oxygen therapy; PHAC, Public Health Agency of Canada.
Sample calculation: (1,527 [estimated total number of patients with diabetic foot ulcers eligible for HBOT] – 200 [current number of patients treated]) ÷ 5 [years] + 200 [number of patients treated last year] = 465.
Resource and Costs
For the base case of the budget impact analysis, we conducted two analyses. The first examined the upfront direct costs associated with HBOT calculated in the primary economic evaluation. This analysis included the physician costs for initial assessment, costs of providing HBOT, costs of pre- and post-session assessments, and overhead costs per HBOT session (per-patient cost of $4,980, assuming 45 HBOT sessions per patient; see Table 18 for calculations). The second analysis examined total costs over five years for patients receiving HBOT during this period. This analysis examined the annual incremental cost of HBOT and included all downstream costs. Both analyses used the results from the primary economic evaluation. The annual HBOT costs per person per year are presented in Table 29. For the scenario analyses, we did not include the initial capital costs of transforming current facilities or building new facilities to increase capacity.
Table 29:
Total HBOT Costs per Person per Year
| Variable | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Upfront direct cost of HBOT | $18,800 | 0 | 0 | 0 | 0 |
| All incremental costs for HBOT | $16,800 | −$3,923 | −$4,876 | −$3,481 | −$2,393 |
Abbreviation: HBOT, hyperbaric oxygen therapy.
Analysis
To obtain the total cost of providing HBOT for patients with diabetic foot ulcers, we multiplied the annual cost of HBOT by the total number of patients eligible for HBOT each year.
To calculate the total upfront direct cost of HBOT, the upfront direct cost of HBOT per patient was multiplied by the total number of patients eligible for HBOT each year for five years.
To calculate the total cost of HBOT, the annual total cost per patient, extracted from the primary economic evaluation, was multiplied by the total number of patients eligible for HBOT each year and calculated over time.
Expert Consultation
In April and May 2016, expert consultation was solicited on the use of HBOT for the treatment of diabetic foot ulcers. Members of the consultation included clinical scientists who have conducted research in the topic area and physicians practising hyperbaric medicine. The role of the expert advisors was to contextualize the evidence on the treatment of diabetic foot ulcers using HBOT. However, the statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted experts.
Results
Base Case
The total upfront treatment cost of HBOT for the treatment of diabetic foot ulcers given current capacity is estimated to be $3.7 million per year ($18,800 [upfront cost of HBOT]) × 200 [total number of patients treated per year given current capacity]). The total cost of providing this treatment, including the downstream costs, over a five-year time frame is estimated to be $3.3 million in the first year and $0.5 million by the fifth year. Table 30 provides the budget impact results for each year up to five years. The detailed calculation of the budget impact can be found in Appendix 6 (Table A24).
Table 30:
Budget Impact Analysis: Base Case Results for Publicly Funding HBOT for the Treatment of Diabetic Foot Ulcers Given Current Capacity
| Total Budget Impact, $ million | ||||
|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
| 3.3 | 2.6 | 1.7 | 1.0 | 0.5 |
Sensitivity Analysis
Assuming that there is a gradual expansion of HBOT facilities in Ontario to meet the needs of all eligible patients within five years, the total budget impact is estimated to be between $13 million and $27 million per year. Table 31 provides the budget impact results for each year up to five years. Detailed calculations of the budget impact sensitivity analyses can be found in Appendix 6 (Tables A25 to A32).
Table 31:
Scenario Analyses: Total Budget Impact per Year for HBOT
| Total Budget Impact, $ million | |||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Scenario analysis 1 | 7.8 | 10.5 | 11.8 | 12.5 | 12.8 |
| Scenario analysis 2 | 7.7 | 10.3 | 11.5 | 12.2 | 12.5 |
| Scenario analysis 3 | 9.2 | 13.0 | 15.0 | 16.1 | 16.6 |
| Scenario analysis 4 | 12.8 | 19.5 | 23.3 | 25.5 | 26.7 |
Discussion
The annual upfront cost of treating patients with diabetic foot ulcers with HBOT given Ontario's current hyperbaric chamber capacity is $3.7 million. When all downstream costs are considered, there is an annual cost of $0.5 million by the fifth year of HBOT funding. If there is an expansion of infrastructure to meet the needs of all patients with diabetic foot ulcers eligible for HBOT in Ontario within five years, the cost per year may be between $13 million and $27 million by the fifth year, excluding the capital cost of building and expanding facilities. The wide range in results observed across scenario analyses highlights the uncertainty found in the results of our budget impact analysis.
One budget impact analysis has been conducted from a Canadian public health care payer perspective.48 In this analysis, the total upfront treatment cost of HBOT was multiplied by the number of estimated patients eligible for HBOT. The analysis considered different rates of increasing HBOT capacity and provided budget impact estimates for if all eligible HBOT patients were to be treated by one year, two years, and four years. The budget impact of funding HBOT including all downstream costs was not considered in the analysis by Chuck et al.48
Our evaluation has several strengths. The estimate of the current number of patients treated with HBOT in Ontario is based on information from several clinical experts. Also, most of the inputs for calculating the total number of patients eligible for HBOT are based on Ontario sources. The cost inputs in the budget impact analysis are based on the results of our primary economic evaluation. Further, our economic evaluation estimated both the upfront and downstream costs of HBOT from an Ontario health care perspective.
Our analysis also has several limitations. First, our scenario analyses did not include the capital costs of expanding existing or creating new HBOT facilities to meet the demand of all eligible patients. Second, our estimates of the number of patients with diabetic foot ulcers eligible for HBOT did not consider barriers to treatment. A certain proportion of patients eligible for HBOT may refuse treatment for a number of reasons or may not be able to reach or arrange transportation to a treatment centre. These factors would lower the total upfront treatment cost of HBOT per year, but also reduce the total cost savings of HBOT per year when downstream costs are considered. Third, our analyses included the overhead costs of treating patients with diabetic foot ulcers at HBOT facilities with monoplace hyperbaric chambers. There may be differences in overhead costs for facilities with dual-place and multiplace chambers compared with monoplace hyperbaric chambers.
Conclusions
The budget impact of funding HBOT for the treatment of diabetic foot ulcers with current capacity is close to $4 million per year in upfront treatment costs, but this cost is reduced to $0.5 million per year by the end of five years if downstream costs are considered. If there is an expansion of capacity with the goal of treating all patients with diabetic foot ulcers eligible for HBOT, the potential cost could be between $13 million and $27 million per year by the fifth year depending on the estimate of diabetic foot ulcer incidence used. These calculations do not include the infrastructure cost of creating more capacity.
PUBLIC AND PATIENT ENGAGEMENT
Background
Public and patient engagement explores preferences, perspectives, and values through the lived experience of a person with a health condition, including the impact the condition and its treatment has on the patient, the patient's family or other caregivers, and the patient's personal environment. Public and patient engagement intends to increase awareness and build appreciation for the needs, priorities, and preferences of the person at the centre of a treatment program. The insights gained through public and patient engagement provide an in-depth picture of lived experience through an intimate look at the preferences, perspectives and values that underpin the experience.
Lived experience is a unique source of evidence about the personal impact of a health condition and how that condition is managed, including what it is like to navigate the health care system with that condition and how technologies may or may not make a difference in people's lives. Information shared from lived experience can also identify gaps or limitations in published research (e.g., outcome measures that do not reflect what is important to those with lived experience).78–80 Additionally, lived experience can also provide information or perspectives on the ethical and social value implications of technologies and treatments. When the needs, priorities, preferences, and values of those with lived experience are not adequately explored in the published literature, Health Quality Ontario makes an effort to reach out to and directly speak with people in Ontario who live with the health condition being investigated, including those who may have experience with the intervention in question.
The impact of diabetes, in particular diabetic foot ulcers, on patients and families was perceived at the outset of this project to have a substantial bearing on quality of life. To better understand the true impact on quality of life, we spoke directly with patients with diabetes who have experienced foot ulcers and who may or may not have had experience with HBOT for the treatment of diabetic foot ulcers. Understanding and appreciating these patients' day-to-day functioning and experience of treatments for diabetic foot ulcers, including HBOT, help to contextualize the potential value of HBOT from a lived experience perspective.
Methods
Engagement Plan
Engagement as a concept captures a range of efforts used to involve the public and patients in various domains and stages of health technology assessment decision-making.81 Rowe and Frewer have outlined three types of engagement: communication, consultation, and participation.82 Communication constitutes a one-way transfer of information from the sponsor to the patient, whereas participation involves the sponsor and patient collaborating through realtime dialogue. Consultation refers to the sponsor seeking out and soliciting information (e.g., experiential input) from the public, patients, and caregivers affected by the technology or intervention in question.83
For this study, our engagement plan was consultation. Within this typology, the engagement design focused on an interview methodology to examine the lived experience of patients with diabetes who have diabetic foot ulcers, including those having undergone treatment with HBOT. The qualitative interview was selected as an appropriate methodology because it allowed Health Quality Ontario staff to deeply explore the meaning of central themes in the lived experience of the participants. The main task of interviewing is to understand the meaning of what participants say.84 Interviews are particularly useful for getting the story behind a participant's experiences, which was the objective of our study. The sensitive nature of exploring quality-of-life issues is another factor supporting the use of interviews for this project.
For participants who did not wish to speak directly to Health Quality Ontario staff via interview, we made an anonymous online survey available that allowed participants to share their lived experience (Appendix 9).
Participant Recruitment
The recruitment strategy for this project consisted of an approach called purposive sampling to actively recruit participants with direct lived experience of diabetic foot ulcers.85–88 Staff of Health Quality Ontario's Patient, Caregiver, and Public Engagement group reached out to patients with diabetes through a variety of partner organizations, including the Ontario Centres for Complex Diabetes Care, wound care advocacy and support groups, diabetes associations, and hyperbaric treatment centres across the province, and through word of mouth with interview participants who contacted other appropriate participants after being interviewed.
Inclusion Criteria
A broad range of participants was sought, including those having experienced diabetic foot ulcers and treatment with HBOT. Patients of various ages were sought, as we assumed that various stage-of-life commitments (e.g., school, work, family) are likely to impact the choices patients with diabetic foot ulcers face in terms of treatment options and outcomes sought. We also wanted a broad geographic representation, as we assumed that access to HBOT facilities varies from region to region across the province.
Exclusion Criteria
No exclusion criteria were defined.
Participants
Patient, Caregiver, and Public Engagement staff at Health Quality Ontario engaged with 21 patients with diabetic foot ulcers from across the province, as well as family members of these patients, through interviews; a further one patient completed the online survey. Of the patients, 13 had been treated with HBOT at centres in the Greater Toronto Area and Ottawa. All participants, whether having undergone treatment with HBOT or not, were familiar with various treatment options for diabetic foot ulcers, including a number of offloading devices. Patients reported ages ranging from early 20s to early 70s.
Approach
Interview
At the beginning of each interview, the Patient, Caregiver, and Public Engagement interviewer explained the purpose of the health technology assessment process (including the role and mandate of Health Quality Ontario and the Ontario Health Technology Advisory Committee), the risks associated with participation, and how the participant's personal health information would be protected. These issues were explained to participants verbally and through a letter of information. Written consent was then obtained from participants prior to commencing the interview. See Appendix 7 for the letter of information and consent form used. Interviews were recorded and transcribed.
Interview questions focused on the impact of diabetic foot ulcers on the patient's and family's quality of life, experiences with other health interventions designed to manage diabetic foot ulcers, the patient's direct experience with HBOT, and any perceived benefits or limitations of HBOT. See Appendix 8 for the interview guide.
The interview was semistructured, consisting of a series of open-ended questions. Interviews lasted for about 20 to 35 minutes, with questions derived from a list developed by the Health Technology Assessment International (HTAi) Interest Group on Patient and Citizen Involvement in HTA (PCIG) to elicit lived experience specific to the impact of a health technology or intervention on lived experience and quality of life.89
Survey
Patient, Caregiver, and Public Engagement interviewers verbally explained to participants the purpose of the health technology assessment process (including the role and mandate of Health Quality Ontario and the Ontario Health Technology Advisory Committee), the risks associated with participation, and how the participants' personal health information would be protected. These issues were also explained to participants through a letter of information), which included a link to the online survey. Once at the survey website, participants were required to give consent prior to beginning the survey. Survey results were collated.
Survey questions focused on the impact of diabetic foot ulcers on patients' and family members' quality of life, experiences with other health interventions designed to manage diabetic foot ulcers, patients' direct experiences with HBOT, and any perceived benefits or limitations of HBOT. See Appendix 9 for the survey questions.
Data Extraction and Analysis
Patient, Caregiver, and Public Engagement staff selected a modified version of a grounded theory methodology to analyze transcripts of participant interviews and survey responses, because this method captures elements of lived experience and allows for themes to emerge and be compared among participants. The inductive nature of grounded theory follows an iterative process of eliciting, documenting, and analyzing responses while simultaneously collecting and analyzing data using a constant comparative approach.90,91 Through this approach, staff coded transcripts and responses and compared themes using the qualitative data analysis software program NVivo (QSR International, Doncaster, Victoria, Australia). NVivo enables the identification and interpretation of patterns in the interview data about the meaning and implications of the lived condition from the patients' perspective and of what is important in their daily lived experience of diabetic foot ulcers and HBOT.
Results
Physical and Emotional Experience of Living With Diabetic Foot Ulcers
Because of the spectrum of potential diabetes symptoms and wound severity, patients with diabetic foot ulcers reported varying degrees of impact from the burden of their disease. Participants consistently reported mobility challenges, and many mentioned a decreased ability to leave the home or engage in activities outside the home in which they had participated before developing foot ulcers. Participants also reported decreased walking, visiting with friends, cancelled vacations, and an inability to drive. For those who were employed at the time foot ulcers developed, challenges arose with regard to performing work duties, and leaves of absence or modified work duties were often required. Both patients and family members reported being impacted by this change in the patient's ability to work:
Up until it really got bad, I wasn't doing much too differently ‘cause I didn't know. After that, I was being told to stay off my feet as much as possible, and at that time I was working, so I had to take the time off to just stay off the feet.
Especially now you can't walk, you're off work, you've got a family to raise and children, and now you can't work, and, oh, gee, you're spiralling down into the abyss pretty quick.
Following an initial experience with the complicated nature of diabetic foot ulcers and treatments, patients reported an increased awareness and vigilance when it came to the status of their foot health. On several occasions, patients reported monitoring small cuts or bruises closely and carefully maintaining the skin of the foot. Patients also reported an increased number of appointments with physicians, chiropodists, and wound care clinics, as well as a tendency to get off their feet when symptoms of swelling, redness, or calluses arose:
In addition, I perform daily wound care, plus I wear custom orthotics, and I'm committed to daily applications of moisturizers.
However, inherent in this increased vigilance is the emotional burden of constant stress and the fear of developing a foot ulcer. This emotional burden was expressed by both patients and family members. Patients reported clearly understanding that a foot ulcer could eventually have dire consequences, such as amputation:
…At home, her life shrank to her house, essentially, and to her bed. She was spending a lot of time in bed sleeping, and she was overwhelmed in trying to deal with all of this and deal with the inevitable fear of still this potential amputation looming over her head…
I live in constant fear that the “other shoe will drop” and either the ulcer will return or occur elsewhere.
So having these things is incredibly terrifying for people. Most diabetics will not admit that they have a problem until it becomes self-evident to the family around them and they're forced into care.
Such an emotional burden often necessitated the support of family members, and this was reported by patients. Those with family supports spoke of gratitude for their aid and acknowledged the difficulties that they would have faced if not for their family, both in terms of physical and emotional well-being. Because of the physical limitations imposed by diabetic foot ulcers, family members often help patients get to and from treatment centres and serve as advocates when evaluating treatment options:
Without my family, without my close friends, I don't know where I would have been. I don't think I would have been in my home; I wouldn't have been able to manage on my own those early months.
Patient Experience of Various Diabetic Foot Ulcer Treatments
Patients reported familiarity with a wide variety of treatment options for diabetic foot ulcers, including dressings, bandages, silver nitrate, packing, and offloading devices, including total contact casts, air casts, removable cast walkers, orthopedic shoes, ankle foot orthoses, Charcot restraint orthotic walker (CROW) boots, felt padding, wheelchairs, crutches, canes, and walkers. Patients reported encountering these treatment options in the community at hospitals, wound care clinics, and chiropody clinics, and in the home through nursing visits arranged through community care access centres.
Preventing amputation was reported as top of mind when patients discussed the potential benefits of various offloading devices and wound care treatments. A majority of patients interviewed had undergone amputation, including single-toe, multiple-toe, foot, and below-the-knee amputations. The physical and emotional impacts of amputation were made clear by the patients interviewed:
Got my confidence back, and, you know, I felt pretty low, I must admit. Not nice to…you've had a member of your body attached to you for 66 years and, all of sudden, it's gone. It was a pretty traumatic experience to go through.
With this mindset, patients reported that the main goal of any therapy was the successful healing of diabetic foot ulcers. Patients reported a high tolerance for devices and treatments described as inconvenient, burdensome, or uncomfortable as long as they successfully treated the wound. Patients stated that treatments for diabetic foot ulcers could take a long time and that healing was often slow, frustrating, and inconsistent. Patients also reported frequent setbacks; for example, high out-of-pocket costs associated with travel or lack of time sometimes caused patients to halt their course of treatment early, causing newly healed wounds to reopen. For this reason, patients consistently reported a willingness to try alternative treatment methods if recommended by their health care provider or if alternative treatments showed an increased rate of healing:
Well, it was a little bit cumbersome and heavy and hot, but I knew the downside if it didn't get healed up; I was kind of [would] probably face a further amputation.
A few years ago, we tried the air cast; it didn't work. We tried orthopedic shoes; they didn't work. We tried different types of shoes; [they] didn't work. We even tried sort of like a cap, sort of like a brace that keeps the foot straight that comes down the back of the calf and under the foot. And these were all specifically made to my foot and my leg and didn't work. I would have problems, then the wound would open up, then, boom, I'd be back in the cast again.
This home care and the ulcer had been going on for two and a half years approximately. And this was a last-ditch effort for me, so I was going to do whatever had to be done to get this over with.
Patient Knowledge of HBOT
Most patients reported having heard about HBOT through chance circumstances; for example, through a neighbour, friend, or health care provider who happened to mention it, at which point patients frequently undertook their own research on the subject. Others reported being prompted by imminent amputation to search for any alternative to heal their wounds. Patients stated that when they told their health care providers that they wanted to try HBOT, the response was mixed; some health care providers were supportive and willing to provide a referral, whereas others did not recommend it. Several patients expressed frustration at the lack of information on HBOT available in the health care community and lamented that HBOT was not presented as a viable treatment option for their diabetic foot ulcers:
They never mentioned it. And the doctor over at the hospital never mentioned it. My personal physician never mentioned it.
It wasn't till I really pushed my personal physician that he gave me a referral. And then I got everything going.
Patients reported having mixed expectations regarding the effectiveness of HBOT. The misgivings of health care providers were reported as one of the factors influencing patient expectations. However, patients also reported feeling frustrated and discouraged after their ulcers failed to heal following other treatments, such as offloading devices:
I was a little leery. I thought, “Oh, what now?” I mean, after two years, is anything going to work? But I went with an open mind.
Given previous failed treatment attempts, patients repeatedly mentioned the fear of amputation as motivation to seek out HBOT and to continue HBOT treatment for as long as needed. Many patients felt that HBOT was a last resort to prevent amputation:
[The surgeon's] option was amputation, and I was saying, “You know what? That's not the option that we want. We want to explore every other possibility we can to see if we can get this wound to heal first before we use amputation as a last resort.”
I'd rather try the hyperbaric, and if it didn't work, then fine; at least I've exhausted all my opportunities. But when they don't even tell you that's an option, that just does not make sense.
And when you have a choice between having an amputation…and all the implications that would involve, having wheelchairs, prosthetics, and stuff like that…[and trying HBOT], I thought the 240-kilometre drive was well worth the trip [to reach an HBOT facility].
HBOT Treatment
Patients reported very high satisfaction with HBOT treatment. It was seen as simple and relaxing. Patients described the clinics and staff at HBOT health care facilities as efficient, caring, and effective. Patients appreciated that comprehensive standard wound care was integrated into their HBOT treatment and said that they felt sure that the combination of standard wound care and HBOT increased the healing of their diabetic foot ulcers:
It was the easiest thing in the world because there are individual chambers that they just put you in [and] close you up, and you watch TV for a couple of hours or a movie or something. It was a break. I used to have a doze.
Access to HBOT was reported as a moderate barrier. Patients stated that HBOT treatment was fairly time-consuming, requiring a number of hours each day for several weeks or months. Patients reported experiencing emotional stress regarding the daily commitment required to receive HBOT treatment, but they also reported feeling thankful they had the financial means and time available to complete their treatment regimens. While the patients we interviewed all completed their courses of HBOT treatment, whether ultimately successful or not, many spoke of other patients they knew who had had to stop their treatment sessions early because of an unsustainable cost burden of time or travel. Patients expressed frustration at the out-of-pocket costs involved in HBOT treatment:
She said she's burned through her retirement fund. Like, why should you go through your retirement fund to save a limb? You shouldn't have to choose between going bankrupt or losing a limb, I think.
However, most patients compared those costs to the anticipated costs of an amputation and reported strong opinions that any out-of-pocket expenses were worthwhile:
So when I think about if you're looking at cost, what would it cost to amputate a person's foot and then with the aftercare? What would that cost compared to what it cost him or the system to do the, say, 30 treatments, or whatever it was, at the clinic? What wins in terms of saving money?
Perceptions of the Effectiveness of HBOT
Patients reported very high satisfaction with HBOT and with its perceived ability to heal their diabetic foot ulcers:
Basically, it gave me back the life that I'm used to living. I mean, if I hadn't have done that and they'd cut the leg off, I don't know where I'd be right now.
Many felt HBOT was the only reason they had avoided amputation. Several patients had undergone previous amputation and regretted that they had not learned of HBOT in time to potentially prevent those amputations:
But, you know, that's a good question that you're asking me because I thought to myself, “By god, what if I'd gone to hyperbaric?” I could have still had my left leg, you know. But I never thought about it, and doctors didn't tell me about it at the time.
Discussion
From speaking directly with patients with diabetic foot ulcers, it is clear that they and their families face difficult challenges. All patients mentioned the physical toll of living with diabetic foot ulcers, with a reduced ability to participate in social and physical activities mentioned most often. Patients also spoke of the emotional burden of the constant vigilance associated with the care of their wounds, as well as that associated with the fear of a new wound developing.
Patients stated that pursuing a variety of treatments was necessary to avoid the frightening possibility of an amputation or to prevent further amputations. The goal of all treatments was the same: to reduce the size of a foot ulcer, to have the ulcer heal over, and to prevent the ulcer's reoccurrence. Patients who sought out HBOT reported difficulty in achieving wound healing by other means. Many were facing imminent amputation and saw HBOT as a final option.
Hyperbaric oxygen therapy was presented by health care providers as an effective means of treating diabetic foot ulcers by increasing oxygen and blood flow to the damaged areas. However, patients reported great frustration at the lack of information about HBOT available in the broader health care community. Patients reported frequently having to take it upon themselves to research HBOT and advocate for this treatment with their health care providers.
The treatment of HBOT itself was described as simple and pleasant, though time-consuming. Patients were required to attend daily sessions for at least one hour at a time, and treatment courses could last for months. The patients we interviewed all completed full courses of treatment; however, many spoke of other patients they had met who could not commit the time or bear the cost burden of attending the number of sessions required to complete a full course of treatment. Avoiding amputation was a key motivating factor for those patients who completed their courses of treatment. Patients also believed that the integrated care they received— standard wound care services were provided in conjunction with HBOT at the HBOT clinics patients attended—contributed to the successful treatment of their wounds.
Patients perceived that treatment with HBOT improved the healing of their diabetic foot ulcers and described being very satisfied with HBOT and how their wounds healed with HBOT. Patients stated that the duration of HBOT treatment varied according to the severity of their wounds, with more severe wounds requiring a longer course of treatment. Several patients reported immediately seeking out HBOT when a diabetic foot ulcer recurred, rather than first seeking out standard care, because they felt previous treatments with HBOT had been effective. Patients reported being able to return to their previous lifestyle and daily physical activities upon complete wound healing, although careful monitoring and caution were often necessary.
Conclusions
For patients with diabetic foot ulcers, there is a substantial daily burden of care and emotional weight associated with the condition. The worrisome possibility of amputation leads patients to carefully monitor and care for their feet and to seek out effective means of treatment. Patients felt that the information available in the health care community on HBOT as a treatment option for diabetic foot ulcers was difficult to obtain. However, when such information was provided by the patients' own health care providers, the patients felt better informed. Patients who received HBOT for the treatment of diabetic foot ulcers felt HBOT was a highly effective treatment, reporting satisfaction with wound healing and an improvement in their quality of life. Although patients felt that receiving HBOT was a simple process, they also felt it required a substantial time commitment, and they incurred associated costs. For the patients we interviewed, the perceived effectiveness of HBOT outweighed the costs of travel, time commitment, and other out-of-pocket costs required to complete treatment.
Acknowledgments
The medical editors were Kara Stahl, Susan Harrison, and Elizabeth Jean Betsch. Others involved in the development and production of this report were Arshia Ali, Stacey Brener, Kellee Kaulback, Ana Laing, Claude Soulodre, Mark Weir, Andrée Mitchell, Anil Thota, Vivian Ng, Laura Williams, Nancy Sikich, and Irfan Dhalla.
We are grateful to several clinical experts for their insight, feedback, and contextualization of the evidence.
ABBREVIATIONS
- AMSTAR
A Measurement Tool to Assess Systematic Reviews
- CI
Confidence interval
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- HBOT
Hyperbaric oxygen therapy
- QALY
Quality-adjusted life-year
- RCT
Randomized controlled trial
- SD
Standard deviation
- SF-36
Short-Form 36-item health survey
GLOSSARY
- Adverse event
Any unexpected problem that happens during treatment, regardless of the cause or severity.
- Barotraumatic otitis
The discomfort produced by an imbalance of air pressure in the ear canal. Most commonly caused by rapid change in altitude, as during takeoff or landing on an aircraft.
- Base case
The scenario with the lowest possible value for which the assertion holds true.
- Cost–utility analysis
A type of analysis that estimates the value for money of an intervention by weighing the cost of the intervention against the improvements in length of life and quality of life. The result is expressed as a dollar amount per quality-adjusted life year (QALY).
- Discount rate
A tool in determining the present value of some future cost or benefit. The discount rate takes into account the time value of money (a sum of money is worth more today than it will be tomorrow), and also factors in any uncertainty over whether the expected benefit will materialize at all.
- Incremental cost
The extra cost associated with using one test or treatment instead of another.
- Incremental cost-effectiveness ratio (ICER)
Determines “a unit of benefit” for an intervention by dividing the incremental cost by the effectiveness. The incremental cost is the difference between the cost of the treatment under study and an alternative treatment. The effectiveness is usually measured as additional years of life or as quality-adjusted life years (QALYs).
- Ischemia
A condition where a part of the body does not receive enough blood, causing tissue damage.
- Meta-analysis
A technique to determine the current state of research into a specific defined topic of study by combining the results of all studies on that topic.
- Offloading
A technique that encourages healing of wounds or injuries by reducing pressure on the affected areas.
- Quality-adjusted life year (QALY)
A measurement that takes into account both the number of years gained by a patient from a procedure and the quality of those extra years (e.g., ability to function, freedom from pain). One QALY is expressed as a number between 0 (no benefit) and 1 (perfect health). The QALY is commonly used as an outcome measure in cost–utility analyses.
- Randomized controlled trial
A type of study in which subjects are assigned randomly into different groups, with one group receiving the intervention or treatment under study and the other group(s) receiving a different intervention or treatment (or a placebo or no treatment) in order to determine the effectiveness of one approach over another.
- Risk difference
The difference in the risk of a particular outcome between the technique under study and some other comparison technique.
- Sensitivity analysis
Every evaluation contains some degree of uncertainty. Study results can vary depending on the values taken by key parameters. Sensitivity analysis is a method that allows estimates for each parameter to be varied to show the impact on study results. There are various types of sensitivity analyses. Examples include deterministic, probabilistic, and scenario.
- Sepsis
The presence of bacteria or their toxins in the blood or tissues.
- Systematic review
A type of research that assesses all studies evaluating a specific treatment compared with another treatment.
APPENDICES
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <January 2016>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to February 12, 2016>, EBM Reviews - Database of Abstracts of Reviews of Effects <1st Quarter 2016>, EBM Reviews - Health Technology Assessment <1st Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2016 Week 07>, All Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
| 1 | Diabetic Foot/ (17388) |
| 2 | Foot Ulcer/ (5795) |
| 3 | ((diabet* adj4 (foot or feet or ulcer*)) or DFU or lower leg wound* or ((unheal* wound* or ulcer*) adj2 (foot or feet))).tw. (22693) |
| 4 | or/1–3 (29803) |
| 5 | exp Skin Ulcer/ (94496) |
| 6 | Foot Diseases/ (18637) |
| 7 | exp Leg Injuries/ (189428) |
| 8 | Wound Healing/ (170038) |
| 9 | (skin ulcer* or varicose ulcer* or leg ulcer* or ((lower extremit* or lower leg*) adj ulcer*) or ((foot or feet) adj disease*) or (leg adj2 (injur* or wound*)) or (wound* adj2 heal*)).tw. (127732) |
| 10 | or/5–9 (496866) |
| 11 | exp Diabetes Mellitus/ (1041997) |
| 12 | exp Diabetes Complications/ (798819) |
| 13 | (diabet* or MODY or IDDM or NIDDM).tw. (1158317) |
| 14 | or/11–13 (1362645) |
| 15 | 10 and 14 (35830) |
| 16 | 4 or 15 (43719) |
| 17 | Hyperbaric Oxygenation/ (23748) |
| 18 | ((hyperbar* adj2 (oxygen* or O2 or chamber or chambers or session or sessions or therap* or treatment*)) or HBOT or HBO or HBO2 or oxygen chamber*).tw. (22670) |
| 19 | or/17–18 (31048) |
| 20 | 16 and 19 (1114) |
| 21 | 20 use pmoz,cctr,coch,dare,clhta,cleed (480) |
| 22 | limit 21 to english language [Limit not valid in CDSR,DARE; records were retained] (425) |
| 23 | diabetic foot/ (17388) |
| 24 | foot ulcer/ (5795) |
| 25 | ((diabet* adj4 (foot or feet or ulcer*)) or DFU or lower leg wound* or ((unheal* wound* or ulcer*) adj2 (foot or feet))).tw. (22693) |
| 26 | or/23–25 (29803) |
| 27 | exp skin ulcer/ (94496) |
| 28 | foot disease/ (21170) |
| 29 | exp leg injury/ (189198) |
| 30 | wound healing/ (170038) |
| 31 | ulcer healing/ (6490) |
| 32 | (skin ulcer* or varicose ulcer* or leg ulcer* or ((lower extremit* or lower leg*) adj ulcer*) or ((foot or feet) adj disease*) or (leg adj2 (injur* or wound*)) or (wound* adj2 heal*)).tw. (127732) |
| 33 | or/27–32 (502663) |
| 34 | exp diabetes mellitus/ (1041997) |
| 35 | (diabet* or MODY or IDDM or NIDDM).tw. (1158317) |
| 36 | or/34–35 (1362645) |
| 37 | 33 and 36 (36072) |
| 38 | 26 or 37 (43905) |
| 39 | hyperbaric oxygen/ (14305) |
| 40 | ((hyperbar* adj2 (oxygen* or O2 or chamber or chambers or session or sessions or therap* or treatment*)) or HBOT or HBO or HBO2 or oxygen chamber*).tw. (22670) |
| 41 | or/39–40 (28054) |
| 42 | 38 and 41 (1104) |
| 43 | 42 use emez (677) |
| 44 | limit 43 to english language [Limit not valid in CDSR,DARE; records were retained] (603) |
| 45 | 22 or 44 (1028) |
| 46 | 45 use pmoz (348) |
| 47 | 45 use emez (603) |
| 48 | 45 use cctr (32) |
| 49 | 45 use coch (18) |
| 50 | 45 use dare (14) |
| 51 | 45 use clhta (9) |
| 52 | 45 use cleed (4) |
| 53 | remove duplicates from 45 (706) |
CINAHL
| # | Query | Results |
|---|---|---|
| S1 | (MH “Diabetic Foot”) | 5,997 |
| S2 | (MH “Foot Ulcer”) | 983 |
| S3 | ((diabet* N4 (foot or feet or ulcer*)) or DFU or lower leg wound* or ((unheal* wound* or ulcer*) N2 (foot or feet))) | 7,770 |
| S4 | S1 OR S2 OR S3 | 7,770 |
| S5 | (MH “Skin Ulcer+”) | 21,939 |
| S6 | (MH “Foot Diseases”) | 1,761 |
| S7 | (MH “Leg Injuries+”) | 27,491 |
| S8 | (MH “Wound Healing”) | 14,938 |
| S9 | (skin ulcer* or varicose ulcer* or leg ulcer* or ((lower extremit* or lower leg*) N1 ulcer*) or ((foot or feet) N1 disease*) or (leg N2 (injur* or wound*)) or (wound* N2 heal*)) | 25,343 |
| S10 | S5 OR S6 OR S7 OR S8 OR S9 | 65,113 |
| S11 | (MH “Diabetes Mellitus+”) | 107,501 |
| S12 | (diabet* or MODY or IDDM or NIDDM) | 139,150 |
| S13 | S11 OR S12 | 139,719 |
| S14 | S10 AND S13 | 7,856 |
| S15 | S4 OR S14 | 9,033 |
| S16 | (MH “Hyperbaric Oxygenation”) | 1,688 |
| S17 | ((hyperbar* N2 (oxygen* or O2 or chamber or chambers or session or sessions or therap* or treatment*)) or HBOT or HBO or HBO2 or oxygen chamber*) | 2,007 |
| S18 | S16 OR S17 | 2,007 |
| S19 | S15 AND S18 | 238 |
| S20 | S15 AND S18 Limiters - English Language | 233 |
Economic Evidence Search
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <January 2016>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to February 12, 2016>, EBM Reviews - Database of Abstracts of Reviews of Effects <1st Quarter 2016>, EBM Reviews - Health Technology Assessment <1st Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2016 Week 07>, All Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
| 1 | Diabetic Foot/ (17390) |
| 2 | Foot Ulcer/ (5795) |
| 3 | ((diabet* adj4 (foot or feet or ulcer*)) or DFU or lower leg wound* or ((unheal* wound* or ulcer*) adj2 (foot or feet))).tw. (22702) |
| 4 | or/1–3 (29812) |
| 5 | exp Skin Ulcer/ (94504) |
| 6 | Foot Diseases/ (18638) |
| 7 | exp Leg Injuries/ (189439) |
| 8 | Wound Healing/ (170056) |
| 9 | (skin ulcer* or varicose ulcer* or leg ulcer* or ((lower extremit* or lower leg*) adj ulcer*) or ((foot or feet) adj disease*) or (leg adj2 (injur* or wound*)) or (wound* adj2 heal*)).tw. (127793) |
| 10 | or/5–9 (496949) |
| 11 | exp Diabetes Mellitus/ (1042086) |
| 12 | exp Diabetes Complications/ (798833) |
| 13 | (diabet* or MODY or IDDM or NIDDM).tw. (1158716) |
| 14 | or/11–13 (1363052) |
| 15 | 10 and 14 (35837) |
| 16 | 4 or 15 (43732) |
| 17 | Hyperbaric Oxygenation/ (23750) |
| 18 | ((hyperbar* adj2 (oxygen* or O2 or chamber or chambers or session or sessions or therap* or treatment*)) or HBOT or HBO or HBO2 or oxygen chamber*).tw. (22676) |
| 19 | or/17–18 (31054) |
| 20 | 16 and 19 (1115) |
| 21 | economics/ (247553) |
| 22 | economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (716010) |
| 23 | economics.fs. (370751) |
| 24 | (econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw. (659184) |
| 25 | exp “costs and cost analysis”/ (496990) |
| 26 | cost*.ti. (226179) |
| 27 | cost effective*.tw. (238611) |
| 28 | (cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (149246) |
| 29 | models, economic/ (128895) |
| 30 | markov chains/ or monte carlo method/ (115414) |
| 31 | (decision adj1 (tree* or analy* or model*)).tw. (32250) |
| 32 | (markov or markow or monte carlo).tw. (95290) |
| 33 | quality-adjusted life years/ (25501) |
| 34 | (QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (47762) |
| 35 | ((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw. (92764) |
| 36 | or/21–35 (2202819) |
| 37 | 20 and 36 (215) |
| 38 | limit 37 to english language [Limit not valid in CDSR, DARE; records were retained] (197) |
| 39 | 38 use pmoz,cctr,coch,dare,clhta (78) |
| 40 | 20 use cleed (4) |
| 41 | or/39–40 (82) |
| 42 | diabetic foot/ (17390) |
| 43 | foot ulcer/ (5795) |
| 44 | ((diabet* adj4 (foot or feet or ulcer*)) or DFU or lower leg wound* or ((unheal* wound* or ulcer*) adj2 (foot or feet))).tw. (22702) |
| 45 | or/42–44 (29812) |
| 46 | exp skin ulcer/ (94504) |
| 47 | foot disease/ (21171) |
| 48 | exp leg injury/ (189209) |
| 49 | wound healing/ (170056) |
| 50 | ulcer healing/ (6490) |
| 51 | (skin ulcer* or varicose ulcer* or leg ulcer* or ((lower extremit* or lower leg*) adj ulcer*) or ((foot or feet) adj disease*) or (leg adj2 (injur* or wound*)) or (wound* adj2 heal*)).tw. (127793) |
| 52 | or/46–51 (502746) |
| 53 | exp diabetes mellitus/ (1042086) |
| 54 | (diabet* or MODY or IDDM or NIDDM).tw. (1158716) |
| 55 | or/53–54 (1363052) |
| 56 | 52 and 55 (36079) |
| 57 | 45 or 56 (43918) |
| 58 | hyperbaric oxygen/ (14305) |
| 59 | ((hyperbar* adj2 (oxygen* or O2 or chamber or chambers or session or sessions or therap* or treatment*)) or HBOT or HBO or HBO2 or oxygen chamber*).tw. (22676) |
| 60 | or/58–59 (28060) |
| 61 | 57 and 60 (1105) |
| 62 | Economics/ (247553) |
| 63 | Health Economics/ or exp Pharmacoeconomics/ (211238) |
| 64 | Economic Aspect/ or exp Economic Evaluation/ (384435) |
| 65 | (econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw. (659184) |
| 66 | exp “Cost”/ (496990) |
| 67 | cost*.ti. (226179) |
| 68 | cost effective*.tw. (238611) |
| 69 | (cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (149246) |
| 70 | Monte Carlo Method/ (48693) |
| 71 | (decision adj1 (tree* or analy* or model*)).tw. (32250) |
| 72 | (markov or markow or monte carlo).tw. (95290) |
| 73 | Quality-Adjusted Life Years/ (25501) |
| 74 | (QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (47762) |
| 75 | ((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw. (92764) |
| 76 | or/62–75 (1808440) |
| 77 | 61 and 76 (208) |
| 78 | limit 77 to english language [Limit not valid in CDSR,DARE; records were retained] (190) |
| 79 | 78 use emez (114) |
| 80 | 41 or 79 (196) |
| 81 | 80 use pmoz (45) |
| 82 | 80 use emez (114) |
| 83 | 80 use cctr (3) |
| 84 | 80 use coch (17) |
| 85 | 80 use dare (6) |
| 86 | 80 use clhta (7) |
| 87 | 80 use cleed (4) |
| 88 | remove duplicates from 80 (155) |
CINAHL
| # | Query | Results |
|---|---|---|
| S1 | (MH “Diabetic Foot”) | 5,997 |
| S2 | (MH “Foot Ulcer”) | 983 |
| S3 | ((diabet* N4 (foot or feet or ulcer*)) or DFU or lower leg wound* or ((unheal* wound* or ulcer*) N2 (foot or feet))) | 7,770 |
| S4 | S1 OR S2 OR S3 | 7,770 |
| S5 | (MH “Skin Ulcer+”) | 21,939 |
| S6 | (MH “Foot Diseases”) | 1,761 |
| S7 | (MH “Leg Injuries+”) | 27,491 |
| S8 | (MH “Wound Healing”) | 14,938 |
| S9 | (skin ulcer* or varicose ulcer* or leg ulcer* or ((lower extremit* or lower leg*) N1 ulcer*) or ((foot or feet) N1 disease*) or (leg N2 (injur* or wound*)) or (wound* N2 heal*)) | 25,343 |
| S10 | S5 OR S6 OR S7 OR S8 OR S9 | 65,113 |
| S11 | (MH “Diabetes Mellitus+”) | 107,501 |
| S12 | (diabet* or MODY or IDDM or NIDDM) | 139,150 |
| S13 | S11 OR S12 | 139,719 |
| S14 | S10 AND S13 | 7,856 |
| S15 | S4 OR S14 | 9,033 |
| S16 | (MH “Hyperbaric Oxygenation”) | 1,688 |
| S17 | ((hyperbar* N2 (oxygen* or O2 or chamber or chambers or session or sessions or therap* or treatment*)) or HBOT or HBO or HBO2 or oxygen chamber*) | 2,007 |
| S18 | S16 OR S17 | 2,007 |
| S19 | S15 AND S18 | 238 |
| S20 | (MH “Economics”) | 10,467 |
| S21 | (MH “Economic Aspects of Illness”) | 6,258 |
| S22 | (MH “Economic Value of Life”) | 503 |
| S23 | MH “Economics, Dental” | 100 |
| S24 | MH “Economics, Pharmaceutical” | 1,734 |
| S25 | MW “ec” | 135,301 |
| S26 | (econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*) | 197,455 |
| S27 | (MH “Costs and Cost Analysis+”) | 80,439 |
| S28 | TI cost* | 35,036 |
| S29 | (cost effective*) | 22,845 |
| S30 | AB (cost* N2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)) | 14,607 |
| S31 | (decision N1 (tree* or analy* or model*)) | 4,221 |
| S32 | (markov or markow or monte carlo) | 2,169 |
| S33 | (MH “Quality-Adjusted Life Years”) | 2,431 |
| S34 | (QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs) | 4,252 |
| S35 | ((adjusted N1 (quality or life)) or (willing* N2 pay) or sensitivity analys?s) | 8,768 |
| S36 | S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 | 258,437 |
| S37 | S19 AND S36 | 37 |
| S38 | S19 AND S36 Limiters - English Language | 37 |
Appendix 2: Additional Information on Included Studies
Table A1:
Wagner Grade Classification
| Grade | Description |
|---|---|
| 1 | Superficial diabetic ulcer |
| 2 | Deep ulcer
|
| 3 | Deep ulcer with abscess or osteomyelitis |
| 4 | Gangrene to portion of forefoot |
| 5 | Extensive gangrene of foot |
Source: Wagner F.9
Table A2:
Characteristics of Included Studies
| Author, Year | Study Design and Sample Size | Inclusion Criteria | Exclusion Criteria | Intervention | Control | Follow-Up |
|---|---|---|---|---|---|---|
| Studies with 2 HBOT sessions daily | ||||||
| Kessler et al, 200331 | RCT HBOT: 14 Control: 13 | Pts who had type 1 or 2 diabetes; Wagner grades 1–3; ulcer for > 3 mo despite glycemic stabilization, absence of local infection, and satisfactory offloading measures; TcPO2 > 30 mm Hg at dorsum of foot; or nonproliferating retinopathy | No exclusion criteria specified | Pts of both groups were hospitalized for 2 wk, then followed as outpatients for 2 wk. Pts in HBOT group underwent two 90-minute daily sessions of 100% O2 breathing in a multiplace hyperbaric chamber pressurized at 2.5 ATA. This regimen lasted 5 d/wk for 2 consecutive wk. HBOT sessions included period of compression in air for 15 min, followed by three 30-min breathing periods, separated by 5-min intervals of air breathing, and then decompression period of 15 min | Providing pts with orthopedic device to remove mechanical stress and pressure at site of ulcer during walking; optimization of metabolic control required SC insulin administration (2 or 3 injections during the day or 1 bedtime treatment); in cases of chronic infection, pts were given antibiotics according to microbiological test results | Outcomes were measured at baseline, day 15, and day 30 (4-wk follow-up) |
| Studies with 2 HBOT sessions daily | ||||||
| Duzgun et al, 200829 | RCT HBOT: 50 Control: 50 | Pts who had type 1 or 2 diabetes; no restriction on Wagner grade; ulcer for 4 wk despite receiving wound care; no restriction on diagnosis of osteomyelitis made on basis of bone biopsy at time of surgical intervention | Contraindications for HBOT (untreated pneumothorax; chronic obstructive pulmonary disease; history of otic surgery; upper respiratory tract infection; fever; history of idiopathic convulsion; hypoglycemia; current corticosteroid, amphetamine, catecholamine, or thyroid hormone use) | Standard treatment was supplemented by HBOT treatments administered at maximum pressure of 2.0 ATA, in monoplace hyperbaric chamber employing a volume of 10 m3 at 2–3 ATA for 90 min. Treatment was administered as 2 sessions/d, followed by 1 session on following day, alternating throughout course of therapy, which typically extended for 20–30 d | Standard treatment included daily wound care, including dressing changes and local debridement at bedside, as well as amputation if indicated. Infection controls were carried out by clinical follow-up and by performing culture antibiograms of surgically obtained specimens to determine appropriate antibiotic therapy | Overall mean duration of follow-up was 92 ± 12 wk |
| Studies with 1 HBOT session daily | ||||||
| Faglia et al, 199630 | RCT HBOT: 35 Control: 33 | Pts who had type 1 or 2 diabetes; Wagner grades 2–4; ulcer for 30 d despite receiving wound care; all pts were examined for various diabetes-related conditionsa | No exclusion criteria were specified | Pts breathed 100% O2 in multiplace hyperbaric chamber, pressurized with air, wearing a soft helmet. Pressure was 2.5 ATA in first phase and 2.4–2.2 ATA in second phase. Scheme consisted of daily session (90 min for each session) in first phase and weekly (5/7) session in reparative phase | Vascular assessmentb with aggressive and radical debridement was performed by consultant surgeon. After surgical curettage, wound was cleaned with uncoloured topical antimicrobial agents and wadded with occlusive dressing. Dressing, with debridement if necessary, was carried out not less than twice daily when necrosis or exudate was present, daily when ulcer was clean, and every 2 d during granulation period. During hospitalization, all pts were provided with orthopedic devices to remove mechanical stress and pressure at ulcer site, while maintaining ambulation. Orthotic consisted of alkaform insole molded in plastic cast and extra-deep special shoe with rigid sole allowing insertion of bandaged foot | Not specified, but could be as long as 176 d (according to measurement of outcome) |
| Studies with 1 HBOT session daily | ||||||
| Kalani et al, 200234 | NRCTc HBOT: 17 Control: 21 | Pts who had type 1 or 2 diabetes; ulcer for 2 mo; TcPO2 < 40 mm Hg that increased to ≥ 100 mm Hg or at least 3 times the baseline value during inhalation of 100% O2 | No exclusion criteria specified (pts had to refrain from smoking and coffee 2 h before assessment) | Daily treatment sessions were given at pressure of 250 kPa, equivalent to 15 m H2O, in acrylic monoplace chamber pressurized with 100% O2, allowing pts to breathe without masks or hoods. Pass-throughs allowed continued intravenous therapy and monitoring when needed. Length and frequency of treatment sessions were 90 min and 5 times weekly, respectivelyd | All pts were treated with non–weight-bearing protective shoes, orthotics, improvement of metabolic control, blood pressure, and nutrition. Regular control of offloading was performed | 3 y |
| Studies with 1 HBOT session daily | ||||||
| Abidia et al, 200328 | RCT HBOT: 8 Control: 8 | Pts who had type 1 or 2 diabetes; ulcer for 6 wk despite optimum medical management for more than 6 wk since presenting; no occlusive arterial disease; acceptable metabolic control of diabetes | Pts for whom vascular surgery, angioplasty, or thrombolysis was planned | Given in multiplace chamber via hood at pressure of 2.4 ATA for 90 min daily, 5 d/wk, totaling 30 sessions. Decompression time was extended to 20 min to avoid giving O2 supplement during decompression to control group without compromising their safety | Sham treatment of hyperbaric air. All pts regularly attended specialized multidisciplinary clinic comprising diabetic physician, vascular surgeon, chiropodist, and specialist nurse. All pts underwent diagnostic angiography as part of their diagnostic assessment. Vascular intervention was by clinician choice. Occlusive arterial disease was confirmed by ABI < 0.8 (or great toe brachial pressure index < 0.9 if calf vessels were incompressible). Wound care was standardized for all pts and included offloading, aggressive debridement, and dressing to ensure that moist wound environment was maintained. Antibiotic therapy was given if there were clinical signs of infection | Pts were assessed at baseline, after 15 treatments, after 30 treatments, and 6 wk later. Two more follow-up visits were performed at 6 mo and 1 y |
| Studies with 1 HBOT session daily | ||||||
| Londahl et al, 201032 | RCT HBOT: 49 Control: 45 | Pts who had type 1 or 2 diabetes; ulcer below ankle for > 3 mo despite being treated at diabetic foot clinic for < 2 mo; pts with adequate distal perfusion; nonreconstructible peripheral vascular | Contraindications for HBOT (severe chronic obstructive pulmonary disease, malignancy, or untreated thyrotoxicosis), current drug or alcohol misuse, vascular surgery in lower limbs within 2 mo, participation in another study, or suspected poor compliance | Multiplace hyperbaric chamber 5 d/wk for 8 wk (40 treatment sessions). Treatment period could be extended to 10 wk, but number of treatments was not allowed to exceed 40. HBOT session included period of compression in air for 5 min, followed by treatment period at 2.5 ATA for 85 min, and then decompression period of 5 min | Pts from both groups could be treated in same session, as study gases were administered by masks, and air or 100% O2 entered chamber through separate double-blinded pipes. Study treatment was given as adjunct to regular treatment at multidisciplinary diabetes foot clinic, which included treatment of infection, revascularization,e debridement, offloading, and metabolic control according to high international standards | Outcomes were measured at 3-mo intervals until 1 y |
| Studies with 1 HBOT session daily | ||||||
| Fedorko et al, 201635 | RCT HBOT: 49 Control: 54 | Pts who had type 1 or 2 diabetes; Wagner grades 2–4; ulcer for 4 wk despite receiving wound care | Any conditions precluding safe treatment in a hyperbaric chamber; impending urgent amputation owing to ongoing or exacerbated infection; exposed calcaneus bone with no prospect of weight-bearing potential even if defect were to heal; and pts with major large-vessel, peripheral arterial disease who were possible candidates for revascularization by open surgery or endovascular procedure (as assessed by vascular surgeon) or those who had undergone such a procedure within past 3 mo | Participants entered monoplace hyperbaric chamber 5 d/wk for 6 wk (30 sessions). HBOT consisted of breathing 100% O2 for 90 min at 244 kPa of pressure, with 5-min intervals of breathing air for every 30 min of O2 | Sham sessions consisted of breathing air at ∼125 kPa of pressure (equivalent to breathing 27% O2 by face mask) on same schedule as intervention. Weekly clinical assessments for 12 wk included comprehensive wound care. Care was provided by multidisciplinary team led by wound care physician at study site and included infection control, debridement, prescriptions for offloading devices, and advanced wound care dressings | 12 wk |
Abbreviations: ABI, ankle-brachial index; ATA, atmospheres absolute; BPG, bypass graft; H2O, water; HBOT, hyperbaric oxygen therapy; NRCT, non–randomized controlled trial; O2, oxygen; PTA, percutaneous transluminal angioplasty; pt, patient; RCT, randomized controlled trial; SC, subcutaneous; TcPO2, transcutaneous oxygen pressure.
Diabetic retinopathy (fundus oculi assessed by ophthalmologist); albumin excretion rate (mg/24 h, average of three 24-h collections, measured with nephelometer); renal impairment (creatinine > 133 pmol/L, measured via the Jaffe method; arterial hypertension (systolic blood pressure > 160 mm Hg or diastolic blood pressure > 95 mm Hg or antihypertensive therapy); coronary artery disease (resting electrocardiogram and B-mode echocardiography); obesity (body mass index, measured in kg/m2; > 24 for women, > 25 for men); dyslipidemia (total cholesterol > 6.20 mmol/L, measured with colorimeter [Boehringer Mannheim, Mannheim, Germany]; or high-density lipoprotein cholesterol < 0.90 mmol/L for men and < 1.16 mmol/L for women, measured via polyethylene glycol 6000; or triglycerides > 2.25 mmol/L, measured with colorimeter; or hypolipidemic therapy).
In subjects with ABI < 0.0 or TcPO2 < 5 mm Hg, a therapy with prostacyclin was established, and arteriography by intra-arterial digital subtraction technique was performed if there were no contraindications (creatinine > 221 mcmol/L or paraproteinemia). In these subjects, the opportunity and possibility of carrying out PTA or BPG was assessed. Focal stenosis involving > 50% of vessel lumen was considered an indication for PTA. Stenosis completely occluding the lumen or occluding more than 10 cm contraindicated PTA. When PTA was impossible, angiogram was evaluated by vascular surgeons for BPG. Bypasses were performed when angiography showed patent vessel in continuity with foot.
Stopped randomization after 14 patients (7 in each arm).
First four patients underwent 40 sessions of HBOT; remaining 13 patients underwent 60 sessions.
All patients were assessed by vascular surgeon at time of inclusion, and only patients with adequate distal perfusion or nonreconstructable peripheral vascular disease were included in study.
Table A3:
Additional Baseline Characteristics of HBOT Groups in Included Studies
| Faglia et al, 199630 | Kalani et al, 200234 | Abidia et al, 200328 | Kessler et al, 200331 | Duzgun et al, 200829 | Londahl et al, 201032 | Fedorko et al, 201635 | |
|---|---|---|---|---|---|---|---|
| Length and width of ulcer ± SD (range) | Not measured | Not measured | Not measured | Not measured | Not measured | Not measured | Length: 2.5 ± 1.8 cm (0.5–8.9 cm) Width: 1.5 ± 1.0 cm (0.5–4.5 cm) |
| Ulcer depth in mm ± SD (range) | Not measured | Not measured | 2.3 (0.5–4) | Not measured | Not measured | Not measured | 0.6 ± 1.3 (0.0–9.0) |
| Ulcer location (%) | Not measured | Not measured | Not measured | Not measured | Not measured | Toe: 35 Plantar forefoot: 27 Heel: 16 Dorsal foot: 2 Lateral and medial malleoli (either side of the ankle): 6 | Not measured |
| Toe blood pressure ± SD (mm Hg) | 0.65 ± 0.28a | 48 ± 18 | 0.47 ± 0.24b | Not measured | Not measured | ≤ 60: 57% ≤ 35: 33% | Not measured |
| Previous vascular surgery (%) | Not measured | Not measured | Not measured | Not measured | Not measured | 57 | 12.2 |
| Smoking habits (%) | Smoking habits not defined (assumed current smoker) Current: 31.4 | Current: 23.5 | Current: 12.5 | Not measured | Currentc: 40 | Current: 22 Previous: 41 Pack-yearsd: 26 (range 1–47) | Current: 49 |
| Comorbidities (%) | Previous major amputation: 0 Previous minor amputation: 0 Previous ulcer: 25.7 Background retinopathy: 34.2 Proliferative retinopathy: 37.1 Microalbuminuria: 34.3 Proteinuria: 22.8 Renal impairment: 11.4 Hypertension: 54.2 Hyperlipidemia: 31.4 CAD: 40 Prior stroke: 8.6 Infection: 91.4 Infection recovery: 74.2 Polymicrobial infection: 57 Bone lysis: 31.4 Osteopenia: 42.8 Mönckeberg sclerosis: 60 Peripheral angiography: 88.5 | Not measured | Chronic obstructive pulmonary disease: 12.5 Cardiac failure: 25 Previous angioplasty: 0 Previous bypass surgery: 25 Previous major amputation: 0 Previous minor amputation: 12.5 Previous ulcers: 37.5 | CAD: 14.2 Renal impairment: 35.7 Carotid arteriopathy: 7.1 | Hypertension: 64 | Previous myocardial infarction: 25 Previous stroke: 16 Congestive heart failure: 35 Atrial fibrillation: 25 Renal transplant: 4 Nephropathy: 90 Dialysis: 6 Hypertension: 61.1 Hyperlipidemia: 88 Previous major amputation: 14 Previous minor amputation: 32 Charcot foot: 4 | Hypertension: 65.3 Hyperlipidemia: 67.3 Previous stroke: 8.2 Previous amputation: 6 |
| Mobility (%) | Not measured | Not measured | Not measured | Not measured | Not measured | Walking with support: 38 Walking without support: 43 Wheelchair: 18 | Not measured |
Abbreviations: CAD, coronary artery disease; HBOT, hyperbaric oxygen therapy; SD, standard deviation.
Measured by ankle-brachial index.
Measured by the great toe–brachial pressure index.
“Current smokers” in this study were defined as active smokers or those who had quit within two months of presentation.
Nonsmokers excluded.
Table A4:
Additional Baseline Characteristics of Standard Care Groups in Included Studies
| Faglia et al, 199630 | Kalani et al, 200234 | Abidia et al, 200328 | Kessler et al, 200331 | Duzgun et al, 200829 | Londahl et al, 201032 | Fedorko et al, 201635 | |
|---|---|---|---|---|---|---|---|
| Length and width of ulcer ± SD (range) | Not measured | Not measured | Not measured | Not measured | Not measured | Not measured | Length: 2.5 ± 1.9 cm (0.5–10.5 cm) Width: 1.7 ± 1.2 cm (0.3–5.0 cm) |
| Ulcer depth in mm ± SD (range) | Not measured | Not measured | 1.6 (0.5–4) | Not measured | Not measured | Not measured | 0.6 ± 0.7 (0.0–4.5) |
| Ulcer location (%) | Not measured | Not measured | Not measured | Not measured | Not measured | Toe: 47 Plantar forefoot: 24 Heel: 7 Dorsal foot: 0 Lateral and medial malleoli (either side of the ankle): 7 | Not measured |
| Toe blood pressure ± SD (mm Hg) | 0.64 ± 0.25a (unit not provided) | 54 ± 31 | 0.44 ± 0.3b (unit not provided) | Not measured | Not measured | ≤ 60: 57 ≤ 35: 29 | Not measured |
| Previous vascular surgery (%) | Not measured | Not measured | Not measured | Not measured | Not measured | 49 | 13 |
| Smoking habits (%) | Smoking habits not defined (assumed current smoker) Current: 36.4 | Current: 28.5 | Current: 25 | Not measured | Currentc: 72 | Current: 29 Previous: 38 Pack-yearsd: 25 (range 4–73) | Current: 55.6 |
| Comorbidities (%) | Previous major amputation: 0 Previous minor amputation: 30.3 Previous ulcer: 36.4 Background retinopathy: 39.4 Proliferative retinopathy: 27.3 Microalbuminuria: 27.3 Proteinuria: 21.2 Renal impairment: 27.3 Hypertension: 51.6 Hyperlipidemia: 24.2 CAD: 45.4 Prior stroke: 12.1 Infection: 84.8 Infection recovery: 51.6 Polymicrobial infection: 51.6 Bone lysis: 27.3 Osteopenia: 63.6 Mönckeberg sclerosis: 60.6 Peripheral angiography: 78.8 | Not measured | Chronic obstructive pulmonary disease: 25 Cardiac failure: 25 Previous angioplasty: 12.5 Previous bypass surgery: 37.5 Previous major amputation: 0 Previous minor amputation: 25 Previous ulcers: 50 | CAD: 30.8 Renal impairment: 46.1 Carotid arteriopathy: 7.6 | Hypertension: 56 | Previous myocardial infarction: 33 Previous stroke: 16 Congestive heart failure: 27 Atrial fibrillation: 33 Renal transplant: 2 Nephropathy: 80 Dialysis: 7 Hypertension: 73 Hyperlipidemia: 87 Previous major amputation: 7 Previous minor amputation: 47 Charcot foot: 9 | Hypertension: 75.9 Hyperlipidemia: 72.2 Previous stroke: 7.4 Previous amputation: 7 |
| Mobility (%) | Not measured | Not measured | Not measured | Not measured | Not measured | Walking with support: 31 Walking without support: 44 Wheelchair: 24 | Not measured |
Abbreviations: CAD, coronary artery disease; HBOT, hyperbaric oxygen therapy; SD, standard deviation.
Measured by ankle-brachial blood pressure.
Measured by the great toe–brachial pressure index.
“Current smokers” in this study were defined as active smokers or those who had quit within 2 months of presentation.
Nonsmokers excluded.
Appendix 3: Clinical Evidence Quality Assessment
Table A5:
AMSTAR Scores of Included Systematic Reviews
| Author, Year | AMSTAR Scorea | (1) Provided Study Design | (2) Duplicate Study Selection | (3) Broad Literature Search | (4) Considered Status of Publication | (5) Listed Excluded Studies | (6) Provided Characteristics of Studies | (7) Assessed Scientific Quality | (8) Considered Quality in Report | (9) Methods to Combine Appropriate | (10) Assessed Publication Bias | (11) Stated Conflict of Interest |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bishop and Mudge, 201492 | 3 | X | ✓ | X | X | ✓ | X | X | X | X | X | |
| De Laet et al, 200893 | 9 | ✓ | X | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓b | ✓ |
| Game et al, 201539 | 9 | ✓ | ✓ | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ | X | ✓ |
| Goldman, 200940 | 9 | ✓ | X | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓c | ✓ |
| Hailey et al, 200794 | 7 | ✓ | ✓ | ✓ | ✓ | X | ✓ | X | X | ✓ | X | ✓ |
| Hinchliffe et al, 200895 | 9 | ✓ | X | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓c | ✓ |
| Huang et al, 201515 | 10 | ✓ | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓ | ✓c | ✓ |
| Kranke et al, 201541 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Lui et al, 201396 | 9 | ✓ | ✓ | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| MAS, 200597,d | 9 | ✓ | X | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓c | ✓ |
| Navarro and Bornstein, 201220 | 6 | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | X | X | X | ✓ |
| O'Reilly et al, 201371 | 9 | ✓ | ✓ | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ | X | ✓ |
| Ritchie et al, 200898 | 9 | ✓ | ✓ | ✓ | ✓ | X | ✓ | ✓ | ✓ | X | ✓ | ✓ |
| Roeckl-Wiedmann et al, 200599 | 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| Stoekenbroek et al, 2014100 | 9 | ✓ | ✓ | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ | X | ✓ |
Abbreviation: AMSTAR, A Measurement Tool to Assess Systematic Reviews.
Maximum possible score is 11. Details of AMSTAR score are described in Shea et al.36
Publication bias mentioned as a possibility; see section 3.5, p. 38 of systematic review.
Publication bias presumed to have been assessed as it is a criterion of GRADE, the tool used to assess quality.
Publication by Health Quality Ontario, formerly Medical Advisory Secretariat.
Our first consideration was study design; we started with the assumption that randomized controlled trials are high quality, whereas observational studies are low quality. We then took into account five additional factors—risk of bias, inconsistency, indirectness, imprecision, and publication bias. Limitations in these areas resulted in downgrading the quality of evidence. Finally, we considered three main factors that may raise the quality of evidence: the large magnitude of effect, the dose-response gradient, and any residual confounding factors.101 For more detailed information, please refer to the latest series of GRADE articles.101
As stated by the GRADE Working Group, the final quality score can be interpreted using the following definitions:
| High | We are very confident that the true prognosis (probability of future events) lies close to that of the estimate |
| Moderate | We are moderately confident that the true prognosis (probability of future events) is likely to be close to the estimate, but there is a possibility that it is substantially different |
| Low | Our confidence in the estimate is limited: the true prognosis (probability of future events) may be substantially different from the estimate |
| Very Low | We have very little confidence in the estimate: the true prognosis (probability of future events) is likely to be substantially different from the estimate |
Table A6:
GRADE Evidence Profile for Comparison of HBOT and Standard Care for the Treatment of Diabetic Foot Ulcers
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Major Amputations | |||||||
| 3 (RCTs) | No serious limitations | Serious limitations (−1)a | No serious limitations | Serious limitations (−1)b | Undetected | No other considerations | ⊕⊕ Low |
| 1 (NRCT) | No serious limitations | No serious limitationsa | No serious limitations | Serious limitations (−1)b | Undetected | No other considerations | ⊕ Very Low |
| Ulcers Healed | |||||||
| 4 (RCTs) | Serious limitations (−1)c | No serious limitationsd | Serious limitations (−1)e,f | No serious limitationsg | Undetected | No other considerations | ⊕⊕ Low |
| 1 (NRCT) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕ Low |
| Adverse Events | |||||||
| 6 (RCTs) | Serious limitations (−1)c | No serious limitations | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Mortality | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)h | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| 1 (NRCT) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)h | Undetected | No other considerations | ⊕ Very Low |
| Minor Amputations | |||||||
| 4 (RCTs) | Serious limitations (−1)c | Serious limitations (−1)i | Serious limitations (−1)e,f | No serious limitations | Undetected | No other considerations | ⊕ Very Low |
| Ulcer Size Reduction | |||||||
| 3 (RCTs) | No serious limitations | No serious limitations | Serious limitations (−1)e,f | Serious limitations (−1)j | Undetected | No other considerations | ⊕⊕ Low |
| Time to Heal | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | Very serious limitations (−2)k | Undetected | No other considerations | ⊕⊕ Low |
| 1 (NRCT) | No serious limitations | No serious limitations | No serious limitations | Very serious limitations (−2)k | Undetected | No other considerations | ⊕ Very Low |
| 2 (RCTs) | No serious limitations | No serious limitations | No serious limitations | No serious limitationsl | Undetected | No other considerations | ⊕⊕⊕ Moderate |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HBOT, hyperbaric oxygen therapy; NRCT, nonrandomized controlled trial; RCT, randomized controlled trial.
I2 was about 80%, but no clear explanation for this result was provided. (Kalani et al34 did not provide baseline characteristics for multiple factors [e.g., prior amputations, previous vascular issues]; however, the three other RCTs provided information on comorbidities, and there were no significant differences in baseline characteristics between arms in these three studies. Abidia et al28 included three patients (one in HBOT, two in sham) with chronic obstructive pulmonary disease; these patients should not have been included in the study as chronic obstructive pulmonary disease is a contraindication for HBOT). Also, Londahl et al32 had more strict exclusion criteria than the other studies.
Lack of power to determine a difference in amputation rates between the HBOT and standard care groups.
Unclear allocation concealment, unclear blinding, and a lack of intention-to-treat analyses.
Including Duzgun et al makes the I2 value jump from 0% to 80%.
The interventions varied across studies in terms of how many sessions were given (20–60), how many sessions occurred daily (1 vs. 2), and whether treatment was given in a monoplace or multiplace hyperbaric chamber.
Standard care was not delivered to the control groups in the same way across studies, and several standard care treatment protocols did not meet standard wound care guidelines.
The overall result may be inflated as one study (Duzgun et al29) reported no ulcers healed in the standard care group, which may not be accurate.
Wider confidence intervals, which, if aligned with true estimate, may either increase or decrease risk of mortality.
No trend in estimates; some studies reported more minor amputations in the HBOT group, whereas others reported more minor amputations in the standard care group.
Abidia et al28 had only eight patients in each study arm. Ulcer size reduction was reported as a percentage, with a range from −206% to 100%. A negative number indicates that an ulcer had grown in size. The wide range is likely a result of the small sample size.
The range of time to heal was quite wide. Median time would have been a more appropriate measure for time to heal.
Quality of life was determined based on two small sample sizes.
Table A7:
Risk of Bias Among Randomized Controlled Trials for the Comparison of HBOT and Standard Care for the Treatment of Diabetic Foot Ulcers
| Author, Year | Allocation Concealment | Blinding | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| Faglia et al, 199630 | Limitationsa | Limitationsb | No limitations | No limitations | No limitations |
| Kalani et al, 200234 | Limitationsa | Limitationsb | No limitationsc | No limitations | Limitationsd |
| Abidia et al, 200328 | No limitations | No limitations | Limitationse | No limitations | Limitationsf |
| Kessler et al, 200331 | Limitationsa | No limitations | Limitationse | No limitations | No limitations |
| Duzgun et al, 200829 | Limitationsa | Limitationsb | No limitationse | No limitations | No limitations |
| Londahl et al, 2010,32 201133 | No limitations | No limitations | No limitations | No limitations | Limitationsg |
| Fedorko et al, 201635 | No limitations | No limitations | Limitationsh | Limitationsi | No limitations |
Abbreviation: HBOT, hyperbaric oxygen therapy.
Authors do not state whether patients were aware of the treatment group to which they were allocated.
Authors did not specify if patients, physicians, or data analysts were blinded to treatment group.
No dropouts reported.
Stopped randomization after 14 patients (seven in each arm).
No intention-to-treat analysis.
Included three patients (one in HBOT and two in control arm) who had chronic obstructive pulmonary disease, a contraindication to HBOT.
Included patients with chronic heart failure (35% in HBOT arm and 27% in control arm), which can be a contraindication for HBOT if too severe (severity of disease in patients not specified).
Used digital photos to assess unvalidated criteria for major and minor amputation.
Authors planned to report on Short-Form 36 (SF-36) domain scores, EuroQoL 5D (EQ-5D) summary scores, and Diabetic Foot Ulcer–Short-Form (DFS-SF) scores but did not include these in publication.
Appendix 4: Additional Material for Clinical Evidence Assessment
Table A8:
Summary of Systematic Reviews of HBOT for the Treatment of Diabetic Foot Ulcers
| Author, Year | Search Dates | No. of Studiesa | Conclusion | Results of Pooled Analyses (95% CI) | AMSTAR | |
|---|---|---|---|---|---|---|
| Major Amputation (Range) | Complete Wound Healing | |||||
| Bishop and Mudge, 201492 | Not specified | 17 | Overall wound healing improved and major amputations were reduced across included studies, but more research is needed | NR | NR | 3 |
| De Laet, 200871 | Not specified | 4 | Evidence is currently insufficient to expand reimbursement in Belgium for HBOT, regardless of indication. Decision-makers should consider linking expanding reimbursement with a proper randomized trial to better study effectiveness and cost-effectiveness | 3 studies: RR 0.31 (0.13–0.71) | NR | 9 |
| Game et al, 201539 | 2010–June 2014 | 11 | Evidence is minimal and generally of poor quality. Little evidence supports use of newer adjunct therapies, except for negative pressure wound therapy for postoperative wounds | NR | NR | 9 |
| Goldman, 200940 | To 2008 | 10 | Strong level of evidence shows that HBOT reduces amputation rate in patients with diabetic foot ulcers, as it promotes full wound healing. Cost-effectiveness should be considered for leg ulcers, as less costly alternatives may be available | 7 studies: OR 0.242 (0.137–0.428) | 6 studies: OR 9.992 (3.972–25.132) | 9 |
| Hailey et al, 200794 | Not specified | 9 | HBOT as adjunct therapy for diabetic foot ulcers compared with standard care alone was considered both effective and cost-effective. Guidelines should be developed to identify patients who would benefit most | NR | NR | 7 |
| Hinchliffe, 200895 | To 2006 | 6 | Of the 9 interventions examined (including wound preparation using antiseptics, bioengineered skin and grafts, and stem cell therapies), there was some evidence suggesting possible benefit from using hydrogels as de-sloughing agents, negative pressure wound therapy, resection of plantar ulcers, and systemic HBOT | NR | NR | 9 |
| Huang et al, 201515 | To April 2015 | 9 | UHMS recommends that HBOT be offered during acute postoperative management as adjunct to standard care. HBOT should not be used for diabetic foot ulcers of Wagner grade 2 or lower, Wagner grade 3 or higher that do not show substantial improvement with 30 d of standard care, or Wagner grade 3 or higher that have had surgical intervention (e.g., debridement, amputation, incision and drainage of deep space abscesses) | 5 studies: Peto OR 0.23 (0.12–0.43) | 3 studies; outcome is unhealed wound: RR 0.48 (0.30–0.77) | 10 |
| Kranke et al, 201541 | To February 2015 | 10 | HBOT as adjunct to standard care resulted in significant improvement in healing diabetic foot ulcers in short term (6 wk), but benefits were not sustained in long term (1 y) | 5 studies: RR 0.36 (0.11–1.18) | 6 wk 5 studies: RR 2.35 (1.19–4.62) 1y 3 studies: RR 9.53 (0.44–207.76) Peto OR 7.58 (4.33–13.29) | 11 |
| Liu et al, 201396 | To April 2012 | 13 | Healing improved and major amputations were reduced among patients who received HBOT vs. those who did not | 11 studies: RR 0.29 (0.19–0.44) | 10 studies: RR 2.33 (1.51–3.60) | 9 |
| MAS, 200597,b | 2003–2004c | 4 | HBOT might prevent major amputations; however, limitations in body of evidence resulted in decision not to claim benefit for wound healing in general or for prevention of minor amputations | NR | NR | 9 |
| Navarro and Bornstein, 201220 | 2005– 2010 | 10 | HBOT can be clinically effective as adjunct therapy for diabetic foot ulcers. However, cost benefits are contingent on appropriate and timely patient referral. Results for other indications are mixed. Overall literature on appropriate use of HBOT is scarce | NR | NR | 6 |
| O'Reilly et al, 201371 | To November 2012 | 12 | Use of HBOT had no significant impact on amputation rates among RCTs examined. However, limited evidence from available RCTs meant overall benefits and harms could not be conclusively determined | 4 studies: RR 0.40 (0.07–2.23)d | 4 studies; outcome is unhealed wound: RR 0.54 (0.26–1.13)c | 9 |
| Ritchie et al, 200898 | To July 2007 | 13 | Poor quality of evidence made conclusions unreliable. HBOT might reduce risk of major amputation, but benefit may result simply from having a good TcPO2 | NR | NR | 9 |
| Roeckl-Wiedmann et al, 200599 | To 2003 | 5 | Some evidence suggests HBOT reduces risk of major amputation among patients with diabetes, but type of ulcer (i.e., venous, arterial, pressure) not reported | 3 studies: RR 0.31 (0.13–0.71) | 2 weeks 2 studies: RR 4.78 (0.94–24.24) | 10 |
| Stoekenbroek et al, 2014100 | To August 2013 | 7 | Some evidence suggests HBOT improves healing of diabetic leg ulcers in patients with concomitant ischemia. However, evidence from large, high-quality studies is needed before adding HBOT to routine patient care | NR | NR | 9 |
Abbreviations: AMSTAR, A Measurement Tool to Assess Systematic Reviews; CI, confidence interval; HBOT, hyperbaric oxygen therapy; MAS, Medical Advisory Secretariat (now Health Quality Ontario); NR, not reported; OR, odds ratio; RCT, randomized controlled trial; RR, relative risk; TCPO2, transcutaneous oxygen pressure; UHMS, Undersea and Hyperbaric Medical Society.
Only studies included in various reviews as part of their evaluation of diabetic foot ulcer healing are listed.
Publication by Health Quality Ontario, formerly Medical Advisory Secretariat.
This review was an update of a Cochrane Review that ran a search from 2000 to 2003; therefore, the search for this review was only from 2003 to 2004.
Results are based on pooled estimates of RCTs. Pooled estimates from observational studies had significant results in favour of HBOT for all three outcomes.
Appendix 5: Modified Methodological Checklist for Economic Evaluations
This checklist was modified from the quality appraisal checklist used by the National Institute for Health and Care Excellence to determine whether an economic evaluation provides useful decision-making information to public health advisory committees. The checklist does not assess the quality of the study or the quality of the reporting.
| Study title: Cost-effectiveness of adjunctive hyperbaric oxygen in the treatment of diabetic ulcers | ||
| First author/year: Guo S/2003 | ||
| Checklist completed by: Brian Chan | ||
| APPLICABILITY (relevance to question under review) | ||
| Item | Yes/Partly/No/Unclear/NA | Comments |
| Is the study population similar to the question? | Yes | |
| Are the interventions similar to the question? | Yes | |
| Is the health care system in which the study was conducted similar to the current Ontario context? | No | |
| Was/were the perspective(s) clearly stated, and what were they? | Yes, United States societal | |
| Are estimates of treatment effect from the best available source? | Yes | |
| Are all future costs and outcomes discounted? | Yes | 3% |
| Is the value of health effects expressed in terms of quality-adjusted life years? | Yes | |
| Are costs and outcomes from other sectors fully and appropriately measured and valued? | No | |
| Overall Judgment (directly applicable/partially applicable/not applicable): Partially applicable | ||
| Comments Regarding Judgement: From a US private payer perspective. | ||
Source: National Institute for Health and Care Excellence. The guidelines manual: appendices B-I, appendix G: Methodology checklist: economic evaluations [Internet]. London (UK): The Institute; 2012 [cited 2014 Oct 6]. Available from: http://publications.nice.org.uk/the-guidelines-manual-appendices-bi-pmg6b/appendix-g-methodology-checklist-economic-evaluations
| Study title: Cost-effectiveness and budget impact of adjunctive hyperbaric oxygen therapy for diabetic foot ulcers | ||
| First author/year: Chuck AW/2008 | ||
| Checklist completed by: Brian Chan | ||
| APPLICABILITY (relevance to question under review) | ||
| Item | Yes/Partly/No/Unclear/NA | Comments |
| Is the study population similar to the question? | Yes | |
| Are the interventions similar to the question? | Yes | |
| Is the health care system in which the study was conducted similar to the current Ontario context? | Yes | |
| Was/were the perspective(s) clearly stated and what were they? | Yes, Ministry of Health | |
| Are estimates of treatment effect from the best available source? | Yes | |
| Are all future costs and outcomes discounted? | No | |
| Is the value of health effects expressed in terms of quality-adjusted life years? | Yes | |
| Are costs and outcomes from other sectors fully and appropriately measured and valued? | No | |
| Overall Judgment (directly applicable/partially applicable/not applicable): Directly applicable | ||
| Comments Regarding Judgement: | ||
Source: National Institute for Health and Care Excellence. The guidelines manual: appendices B-I, Appendix G: Methodology checklist: economic evaluations [Internet]. London (UK): The Institute; 2012 [cited 2014 Oct 6]. Available from: http://publications.nice.org.uk/the-guidelines-manual-appendices-bi-pmg6b/appendix-g-methodology-checklist-economic-evaluations
Appendix 6: Detailed Calculations for Economic Model Inputs
Table A9:
Base Case Calculation of Mortality for People With Diabetes54
| Year | Mortality Risk in General Population | Sex-Adjusted Transition Probability From Healed State to Death State | Mortality Risk for People With Diabetes | |
|---|---|---|---|---|
| Male | Female | |||
| 1 | 0.0168 | 0.0105 | 0.0146 | 0.0244 |
| 2 | 0.0185 | 0.0116 | 0.0161 | 0.0269 |
| 3 | 0.0204 | 0.0128 | 0.0177 | 0.0296 |
| 4 | 0.0225 | 0.0142 | 0.0196 | 0.0327 |
| 5 | 0.0248 | 0.0157 | 0.0216 | 0.0360 |
| 6 | 0.0273 | 0.0174 | 0.0238 | 0.0397 |
| 7 | 0.0300 | 0.0193 | 0.0263 | 0.0439 |
| 8 | 0.0331 | 0.0215 | 0.0290 | 0.0484 |
| 9 | 0.0365 | 0.0238 | 0.0320 | 0.0535 |
| 10 | 0.0402 | 0.0265 | 0.0354 | 0.0590 |
| 11 | 0.0443 | 0.0295 | 0.0391 | 0.0652 |
| 12 | 0.0488 | 0.0328 | 0.0432 | 0.0721 |
| 13 | 0.0538 | 0.0365 | 0.0477 | 0.0797 |
| 14 | 0.0594 | 0.0407 | 0.0528 | 0.0882 |
| 15 | 0.0654 | 0.0455 | 0.0584 | 0.0975 |
| 16 | 0.0722 | 0.0507 | 0.0646 | 0.1079 |
| 17 | 0.0796 | 0.0567 | 0.0715 | 0.1194 |
Table A10:
Sensitivity Analysis Calculation of Mortality for People With Diabetes66
| Year | Sex-Adjusted Transition Probability From Healed State to Death State | Mortality Risk for People With Diabetes |
|---|---|---|
| 1 | 0.0146 | 0.0334 |
| 2 | 0.0161 | 0.0368 |
| 3 | 0.0177 | 0.0406 |
| 4 | 0.0196 | 0.0448 |
| 5 | 0.0216 | 0.0494 |
| 6 | 0.0238 | 0.0545 |
| 7 | 0.0263 | 0.0601 |
| 8 | 0.0290 | 0.0664 |
| 9 | 0.0320 | 0.0733 |
| 10 | 0.0354 | 0.0810 |
| 11 | 0.0391 | 0.0894 |
| 12 | 0.0432 | 0.0989 |
| 13 | 0.0477 | 0.1093 |
| 14 | 0.0528 | 0.1209 |
| 15 | 0.0584 | 0.1337 |
| 16 | 0.0646 | 0.1479 |
| 17 | 0.0715 | 0.1637 |
Table A11:
Base Case Calculation of Sex-Adjusted Mortality for Patients With Diabetic Foot Ulcers56
| Year | Transition Probability From Healed State to Death State | Transition Probability From Unhealed State to Death State |
|---|---|---|
| 1 | 0.0244 | 0.0461 |
| 2 | 0.0269 | 0.0508 |
| 3 | 0.0296 | 0.0560 |
| 4 | 0.0327 | 0.0617 |
| 5 | 0.0360 | 0.0681 |
| 6 | 0.0397 | 0.0751 |
| 7 | 0.0439 | 0.0829 |
| 8 | 0.0484 | 0.0915 |
| 9 | 0.0535 | 0.1011 |
| 10 | 0.0590 | 0.1116 |
| 11 | 0.0652 | 0.1233 |
| 12 | 0.0721 | 0.1363 |
| 13 | 0.0797 | 0.1507 |
| 14 | 0.0882 | 0.1666 |
| 15 | 0.0975 | 0.1843 |
| 16 | 0.1079 | 0.2039 |
| 17 | 0.1194 | 0.2257 |
Table A12:
Sensitivity Analysis Extrapolation of Mortality for Patients With Diabetic Foot Ulcers67
| Year | Transition Probability From Unhealed State to Death State |
|---|---|
| 1 | 0.154 |
| 2 | 0.105 |
| 3 | 0.117 |
| 4 | 0.095 |
| 5 | 0.105 |
| 6 | 0.091 |
| 7 | 0.100 |
| 8 | 0.111 |
| 9 | 0.125 |
| 10 | 0.143 |
| 11 | 0.166 |
| 12 | 0.199 |
| 13 | 0.249 |
| 14 | 0.332 |
| 15 | 0.496 |
| 16 | 0.984 |
| 17 | 0.984 |
Table A13:
Base Case and Sensitivity Analysis Extrapolation of Mortality for Patients Who Have Undergone Major or Minor Amputation
| Year | Transition Probability From Major Lower Leg Amputation State to Death State (Base Case)57 | Transition Probability From Minor Lower Leg Amputation State to Death State (Base Case)57 | Transition Probability From Major Lower Leg Amputation State to Death State (Sensitivity Analysis)68 |
|---|---|---|---|
| 1 | 0.640 | 0.300 | 0.483 |
| 2 | 0.111 | 0.146 | 0.219 |
| 3 | 0.125 | 0.172 | 0.280 |
| 4 | 0.143 | 0.207 | 0.388 |
| 5 | 0.167 | 0.261 | 0.635 |
| 6 | 0.200 | 0.353 | 0.040 |
| 7 | 0.250 | 0.547 | 0.044 |
| 8 | 0.333 | 0.048 | 0.048 |
| 9 | 0.500 | 0.053 | 0.053 |
| 10 | 0.059 | 0.059 | 0.059 |
| 11 | 0.065 | 0.065 | 0.065 |
| 12 | 0.072 | 0.072 | 0.072 |
| 13 | 0.080 | 0.080 | 0.080 |
| 14 | 0.088 | 0.088 | 0.088 |
| 15 | 0.098 | 0.098 | 0.098 |
| 16 | 0.108 | 0.108 | 0.108 |
| 17 | 0.119 | 0.119 | 0.119 |
Table A14:
Calculation of Diabetic Foot Ulcer Healing
| Calculation | Result | |
|---|---|---|
| Probability of ulcer healing from year 1 to year 3 | [0.48 (cumulative 3-year healing) −0.29 (cumulative 1-year healing)]/(1 - 0.29) | 0.27 |
| Rate of ulcer healing | -log(1 - 0.27)/2 | 0.16 |
| Probability of ulcer healing in year 2 and year 3 | 1 - exp((−0.16)*1) | 0.14 |
Table A15:
Base Case and Sensitivity Analysis Extrapolation of Diabetic Foot Ulcer Recurrence for Patients Whose Ulcers Have Healed
| Year | Transition Probability From Healed State to Unhealed State (Base Case)58 | Transition Probability From Healed State to Unhealed State (Sensitivity Analysis)69 |
|---|---|---|
| 1 | 0.37a | 0.35b |
| 2 | 0.11 | 0.07 |
| 3 | 0.20 | 0 |
| 4 | 0 | 0 |
| 5 | 0 | 0 |
| 6 | 0 | 0 |
| 7 | 0 | 0 |
| 8 | 0 | 0 |
| 9 | 0 | 0 |
| 10 | 0 | 0 |
| 11 | 0 | 0 |
| 12 | 0 | 0 |
| 13 | 0 | 0 |
| 14 | 0 | 0 |
| 15 | 0 | 0 |
| 16 | 0 | 0 |
| 17 | 0 | 0 |
Sample calculation: Study reports 63% remain ulcer free at 1 year; in other words, recurrence expected in 37% at 1 year.
Sample calculation: Study reports 56% remain ulcer free at 2 years; in other words, recurrence expected in 44% 2 years, cumulatively. The incremental recurrence probability is calculated as the difference between the 2-year and 1-year cumulative recurrence rates divided by the percentage of remaining cohort: (0.44 - 0.37)/(1 - 0.37).
Table A16:
Base Case and Sensitivity Analysis: Clinical Outcomes and Extrapolation of Major Lower Leg Amputation Risk for Patients with Diabetic Foot Ulcers
| Year | HBOT/SC Transition Probability From Unhealed State to Major Lower Leg Amputation State (Base Case)32,51 | HBOT/SC Transition Probability From Unhealed State to Major Lower Leg Amputation State (Sensitivity Analysis 1)30,51 | HBOT/SC Transition Probability From Unhealed State to Major Lower Leg Amputation State (Sensitivity Analysis 2)28,51 | HBOT/SC Transition Probability From Unhealed State to Major Lower Leg Amputation State (Sensitivity Analysis 2)32,51 | HBOT/SC Transition Probability From Unhealed State to Major Lower Leg Amputation State (Sensitivity Analysis 2)32,67 |
|---|---|---|---|---|---|
| 1 | 0.06/0.02 | 0.09/0.33 | 0.11/0.11 | 0.06/0.10 | 0.06/0.09 |
| 2 | 0.08/0.08 | 0.08/0.08 | 0.08/0.08 | 0.08/0.08 | 0.08/0.02 |
| 3 | 0.05/0.05 | 0.05/0.05 | 0.05/0.05 | 0.05/0.05 | 0.05/0.02 |
| 4 | 0 | 0 | 0 | 0 | 0.01/0.02 |
| 5 | 0 | 0 | 0 | 0 | 0/0.02 |
| 6 | 0 | 0 | 0 | 0 | 0/0.02 |
| 7 | 0 | 0 | 0 | 0 | 0/0.02 |
| 8 | 0 | 0 | 0 | 0 | 0/0.02 |
| 9 | 0 | 0 | 0 | 0 | 0/0.02 |
| 10 | 0 | 0 | 0 | 0 | 0/0.02 |
| 11 | 0 | 0 | 0 | 0 | 0/0.02 |
| 12 | 0 | 0 | 0 | 0 | 0/0.02 |
| 13 | 0 | 0 | 0 | 0 | 0/0.02 |
| 14 | 0 | 0 | 0 | 0 | 0/0.02 |
| 15 | 0 | 0 | 0 | 0 | 0/0.02 |
| 16 | 0 | 0 | 0 | 0 | 0/0.02 |
| 17 | 0 | 0 | 0 | 0 | 0/0.02 |
Abbreviations: HBOT, hyperbaric oxygen therapy; SC, standard care.
Table A17:
Base Case and Sensitivity Analysis Extrapolation of Minor Lower Leg Amputation Risk for Patients With Diabetic Foot Ulcers
| Year | HBOT/SC Transition Probability From Unhealed State to Minor Lower Leg Amputation State (Base Case)32,51 | HBOT/SC Transition Probability From Unhealed State to Minor Lower Leg Amputation State (Sensitivity Analysis 1)30,51 | HBOT/SC Transition Probability From Unhealed State to Minor Lower Leg Amputation State (Sensitivity Analysis 2)28,51 | HBOT/SC Transition Probability From Unhealed State to Minor Lower Leg Amputation State (Sensitivity Analysis 2)32,51 |
|---|---|---|---|---|
| 1 | 0.08/0.09 | 0.60/0.36 | 0.11/0 | 0.08/0.10 |
| 2 | 0.07/0.07 | 0.07/0.07 | 0.07/0.07 | 0.07/0.07 |
| 3 | 0.04/0.04 | 0.04/0.04 | 0.04/0.04 | 0.04/0.04 |
| 4 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 |
| 11 | 0 | 0 | 0 | 0 |
| 12 | 0 | 0 | 0 | 0 |
| 13 | 0 | 0 | 0 | 0 |
| 14 | 0 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 |
| 17 | 0 | 0 | 0 | 0 |
Abbreviations: HBOT, hyperbaric oxygen therapy; SC, standard care.
Table A18:
Base Case and Sensitivity Analysis Extrapolation of Ulcer Healing for Patients With Diabetic Foot Ulcers
| Year | HBOT/SC Transition Probability From Unhealed State to Healed State (Base Case)32,34 | HBOT/SC Transition Probability From Unhealed State to Healed State (Sensitivity Analysis 1)32,34 | HBOT/SC Transition Probability From Unhealed State to Healed State (Sensitivity Analysis 2)28,34 |
|---|---|---|---|
| 1 | 0.52/0.29 | 0.52/0.29 | 0.63/0 |
| 2 | 0.14/0.14 | 0.29/0.14 | 0.14/0.14 |
| 3 | 0.14/0.14 | 0.29/0.14 | 0.14/0.14 |
| 4 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
| 5 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
| 6 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
| 7 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
| 8 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
| 9 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
| 10 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
| 11 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
| 12 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
| 13 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
| 14 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
| 15 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
| 16 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
| 17 | 0.14/0.14 | 0.14/0.14 | 0.14/0.14 |
Abbreviations: HBOT, hyperbaric oxygen therapy; SC, standard care.
Table A19:
Physician Billing Codes Included for HBOT60
| Physician Billing Code | Cost, $ |
|---|---|
| Initial assessment | |
| A005 Consultation fee | 77.20 |
| G489 Venipuncture (adolescent or adult) | 3.54 |
| G310 Electrocardiogram – twelve-lead technical component | 6.60 |
| G313 Electrocardiogram – twelve-lead professional component | 4.45 |
| J304 Flow volume loop, volume vs flow study technical component | 18.55 |
| J304 Flow volume loop, volume vs flow study professional component | 10.75 |
| J327 Repeat flow volume loop after bronchodilator technical component | 2.81 |
| J327 Repeat flow volume loop after bronchodilator professional component | 6.45 |
| G440 Puretone threshold audiometry with or without bone conduction technical component | 10.30 |
| G525 Puretone threshold audiometry with or without bone conduction professional component | 5.85 |
| G120 Impedance plethysmography professional component | 7.00 |
| G121 Impedance plethysmography technical component | 12.55 |
| Physician assessment pre- and post-HBOT session | |
| A014 Partial assessment (base case) | 31.45 |
| A001 Minor assessment (sensitivity analysis) | 21.70 |
| Physician billing for HBOT session | |
| G804 Physician in hyperbaric unit but not in chamber with patient, per session per patient – first ¼ hour (base case) | 71.85 |
| G805 Physician in hyperbaric unit but not in chamber with patient, per session per patient – after first ¼ hour (per ¼ hour) (base case) | 35.90 |
| G807 Physician not in constant attendance – not in hyperbaric unit, supervision (sensitivity analysis) | 35.75 |
Table A20:
Calculation of Overhead Costs per HBOT Session for the Treatment of Diabetic Foot Ulcers
| Variable | Cost, $ |
|---|---|
| Total overhead cost per year | 650,000 |
| Fraction of overhead costs per year for treatment of diabetic foot ulcers (assuming 60% of all patients have diabetic foot ulcers) | 390,000 |
| Overhead cost per patient for treatment of diabetic foot ulcers (about 90 patients per year) | 4,300 |
| Overhead cost per patient for treatment of diabetic foot ulcers (40–45 sessions per person) | 100 |
Table A21:
Surgical Procedure and Diagnosis Codes Used to Identify Lower Leg Amputation Cohort102
| Variable | Codes |
|---|---|
| Major lower leg amputation procedure | 1.VC.93 (amputation of femur) |
| 1.VG.93 (amputation, knee joint) | |
| 1.VQ.93 (amputation, tibia and fibula) | |
| Minor lower leg amputation procedure | 1.WA.93 (amputation, ankle joint) |
| 1.WE.93 (amputation, tarsal bones and intertarsal joints) | |
| 1.WI.93 (amputation, first metatarsal bone and first metatarsophalangeal joint) | |
| 1.WJ.93 (amputation, tarsometatarsal joints, other metatarsal bones and other metatarsophalangeal joints [forefoot]) | |
| Diabetic foot ulcer diagnosis | E10.* (type 1 diabetes mellitus) |
| E11.* (type 2 diabetes mellitus) |
Table A22:
Physician Billing Codes for Lower Leg Amputation60
| Variable | Cost, $ | ||
|---|---|---|---|
| Primary Surgeon | Assistant Surgeon | Anaesthesiologist | |
| Major lower leg amputation procedure | |||
| R624 – tibia/fibula | 306.30 | 72.24a | 105.07b |
| R625 – knee | 305.25 | 72.24 | 105.07 |
| R626 – femur | 306.30 | 72.24 | 105.07 |
| Minor lower leg amputation procedure | |||
| R620 – Metatarsal/phalanx disarticulation | 155.90 | 72.24 | 90.06c |
| R621 – Ray (single) | 217.15 | 72.24 | 90.06 |
| R623 – Symes | 285.80 | 72.24 | 105.07 |
| R622 – Transmetatarsal/transtarsal | 235.75 | 72.24 | 105.07 |
| R619 – Terminal Symes | 144.80 | 72.24 | 90.06 |
Sample calculation: $12.04 × 6.
Sample calculation: $15.01 × 7.
Sample calculation: $15.01 × 6.
Table A23:
Number of Patients per Year Eligible and Expected to Be Treated With HBOT Given No Increase in HBOT Capacity (Base Case)
| Cohort | Number of Patients | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 | 200 | 191 | 190 | 189 | 188 |
| Year 2 | 200 | 191 | 190 | 189 | |
| Year 3 | 200 | 191 | 190 | ||
| Year 4 | 200 | 191 | |||
| Year 5 | 200 | ||||
| Total | 200 | 391 | 581 | 770 | 958 |
Abbreviation: HBOT, hyperbaric oxygen therapy.
Table A24:
Total Budget Impact per Year for HBOT Given No Increase in HBOT Capacity (Base Case)
| Cohort | Total Budget Impact, $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 | 0.7 | −0.75 | −0.9 | −0.7 | −0.5 |
| Year 2 | 0.72 | −0.8 | −0.9 | −0.7 | |
| Year 3 | 0.7 | −0.8 | −0.9 | ||
| Year 4 | 0.7 | −0.8 | |||
| Year 5 | 0.7 | ||||
| Total | 0.7 | −0.03 | −1.0 | −1.6 | −2.1 |
Table A25:
Number of Patients per Year Eligible and Expected to Be Treated for HBOT Given an Increase in HBOT Capacity (Scenario Analysis 1)
| Cohort | Number of Patients | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 | 465 | 444 | 442 | 439 | 437 |
| Year 2 | 731 | 697 | 694 | 690 | |
| Year 3 | 996 | 950 | 946 | ||
| Year 4 | 1,261 | 1,203 | |||
| Year 5 | 1,527 | ||||
| Total | 465 | 1,175 | 2,135 | 3,344 | 4,806 |
Table A26:
Number of Patients per Year Eligible and Expected to Be Treated for HBOT Given an Increase in HBOT Capacity (Scenario Analysis 2)
| Cohort | Number of Patients | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 | 458 | 437 | 435 | 432 | 430 |
| Year 2 | 716 | 683 | 679 | 676 | |
| Year 3 | 974 | 929 | 924 | ||
| Year 4 | 1,232 | 1,175 | |||
| Year 5 | 1,490 | ||||
| Total | 458 | 1,153 | 2,092 | 3,272 | 4,695 |
Table A27:
Number of Patients per Year Eligible and Expected to Be Treated for HBOT Given an Increase in HBOT Capacity (Scenario Analysis 3)
| Cohort | Number of Patients | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 | 547 | 522 | 520 | 517 | 514 |
| Year 2 | 895 | 853 | 849 | 844 | |
| Year 3 | 1,242 | 1,185 | 1,179 | ||
| Year 4 | 1,589 | 1,516 | |||
| Year 5 | 1,936 | ||||
| Total | 547 | 1,417 | 2,615 | 4,140 | 5,989 |
Table A28:
Number of Patients per Year Eligible and Expected to Be Treated for HBOT Given an Increase in HBOT Capacity (Scenario Analysis 4)
| Cohort | Number of Patients | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 | 766 | 730 | 727 | 723 | 718 |
| Year 2 | 1,331 | 1,270 | 1,264 | 1,257 | |
| Year 3 | 1,897 | 1,810 | 1,801 | ||
| Year 4 | 2,463 | 2,350 | |||
| Year 5 | 3,029 | ||||
| Total | 766 | 2,061 | 3,894 | 6,260 | 9,155 |
Table A29:
Total Budget Impact per Year for HBOT Given an Increase in HBOT Capacity (Scenario Analysis 1)
| Cohort | Total Budget Impact, $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 | 7.8 | −1.7 | −2.2 | −1.5 | −1.0 |
| Year 2 | 12 | −2.7 | −3.4 | −2.4 | |
| Year 3 | 16.7 | −3.7 | −4.6 | ||
| Year 4 | 21.2 | −4.7 | |||
| Year 5 | 25.6 | ||||
| Total | 7.8 | 10.5 | 11.8a | 12.5 | 12.8 |
Results may differ because of rounding.
Table A30:
Total Budget Impact per Year for HBOT Given an Increase in HBOT Capacity (Scenario Analysis 2)
| Cohort | Total Budget Impact, $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 | 7.7 | −1.7 | −2.1 | −1.5 | −1.0 |
| Year 2 | 12.0 | −2.7 | −3.3 | −2.4 | |
| Year 3 | 16.3 | −3.6 | −4.5 | ||
| Year 4 | 20.7 | −4.6 | |||
| Year 5 | 25.0 | ||||
| Total | 7.7 | 10.2 | 11.5 | 12.2 | 12.5 |
Table A31:
Total Budget Impact per Year for HBOT Given an Increase in HBOT Capacity (Scenario Analysis 3)
| Cohort | Total Budget Impact, $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 | 9.2 | −2.0 | −2.5 | −1.8 | −1.2 |
| Year 2 | 15.0 | −3.3 | −4.1 | −2.9 | |
| Year 3 | 20.8 | −4.6 | −5.7 | ||
| Year 4 | 26.7 | −5.9 | |||
| Year 5 | 7.0 | ||||
| Total | 9.2 | 13.0 | 15.0 | 16.0 | 32.5 |
Table A32:
Total Budget Impact per Year for HBOT Given an Increase in HBOT Capacity (Scenario Analysis 4)
| Cohort | Total Budget Impact, $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 | 12.8 | −2.9 | −3.5 | −2.5 | −1.7 |
| Year 2 | 22.3 | −5.0 | −6.2 | −4.4 | |
| Year 3 | 31.8 | −7.1 | −8.8 | ||
| Year 4 | 41.3 | −9.2 | |||
| Year 5 | 50.8 | ||||
| Total | 2.8 | 1.9 | −1.7 | −6.9 | −13.1 |
Appendix 7: Letter of Information
SUMMARY
Health Quality Ontario (HQO) is reviewing two treatments for diabetic leg wounds: total contact casting and hyperbaric oxygen therapy to better understand whether these treatment options should be funded by the health care system. As part of this review we would like to hear from patients who suffer from diabetic leg wounds to better understand their and their family's lived experience of the condition, expectations and experiences with accessing and receiving existing treatments, and their experience (if any) with TCC and HBOT.
Currently undergoing total contact casting or hyperbaric oxygen therapy is not necessary to be involved in this review. We are interested in hearing from patients with diabetic leg wounds and their families who may or may not be considering either of these treatments.
WHAT DO YOU NEED FROM ME?
-
✓
Willingness to share your story
-
✓
Indicate your preference for sharing your story:
-
∘
Phone interview
-
∘
In-person interview (for individuals in Toronto)
-
∘
-
✓
Consent to have your story used and recorded by HQO
WHAT YOUR PARTICIPATION INVOLVES
If you agree to participate, you will be asked questions about your lived experience with diabetic leg wounds, existing therapies, and potentially total contact casting or hyperbaric oxygen therapy, if you have undergone these therapies or may be considering them.
Participation is voluntary. You may refuse to participate, refuse to answer any questions or withdraw before your story is captured. Withdrawal will in no way affect care you receive.
HOW WILL MY INPUT BE USED?
Health Quality Ontario will be summarizing the input we receive from patients and families and combining this with clinical and economic evidence reviews about total contact casting and hyperbaric oxygen therapy. This combined evidence review is then used to support the Ontario Health Technology Advisory Committee in coming to a recommendation about public funding.
CONFIDENTIALITY
All information collected from you will be kept confidential, and privacy will be protected except as required by law. The results of this review will be published; however, no identifying information will be released or published. Any records containing information from your interview will be stored securely.
RISKS TO PARTICIPATION
There are no known physical risks to participating. Some participants may experience discomfort or anxiety after speaking about their lived experience. If this is the case, please contact any staff.
HOW CAN I SHARE MY STORY WITH HEALTH QUALITY ONTARIO?
If you are interested in speaking over the phone or in person, please contact David Wells (contact information below).
If you wish to share your story anonymously, you can also fill out an online survey here: http://hqontario.fluidsurveys.com/s/Wound_TCC_HBOT/
HEALTH QUALITY ONTARIO STAFF
David Wells
Program Analyst, Patient, Family and Public Engagement
Tel: (416) 323-6868 Email: David.Wells@hqontario.ca
Consent and Release Form
This form is to be read and completed in accordance with the following instructions before it can be signed.
-
1.
I,_____, allow Health Quality Ontario (Ontario Health Quality Council) to use to inform the development of an evidence-based review:
Check off all appropriate boxes:
-
a)
_____ a recording of my voice
-
b)
_____ a quotation or summary of my opinion that I expressed during an interview
-
c)
_____ name & contact information
-
a)
-
2.
Please read the following paragraphs before affixing your signature under section 3.
-
a)
Personal information collected pursuant to, and on this form, will be used for purposes described on this form and for no other purpose. Health Quality Ontario (Ontario Health Quality Council) acknowledges that you have provided this personal information freely and voluntarily. If you have any questions about this collection of this personal information, contact:
Director, Communications
Tel: (416) 323-6868, ext. 223
-
b)
By signing this form as indicated below, you agree to hereby release and forever discharge the Health Quality Ontario (Ontario Health Quality Council), its officers, employees, agents and representatives from any and all claims, demands, expenses, actions, causes of action and for any and all liability howsoever caused, arising out of, or in any way related to the collection, use and disclosure of information, recordings and images authorized to be collected pursuant to, or on this form.
-
c)
By signing this form as indicated below, you agree to forever waive any and all rights that you may have to the use of information and recordings that are authorized to be collected pursuant to, or on this form; and you acknowledge that all information, recordings and images shall hereafter remain the exclusive property of the Health Quality Ontario (Ontario Health Quality Council).
-
a)
-
3.
Signature is to be affixed in the appropriate space provided below.
I have read this form after it was completed, I understand and agree to be bound by its contents, and I am eighteen (18) years of age or over.
Signature:_____
Print name:_____
Date:_____
Appendix 8: Patient Interview
Introduction
History of diabetes and wounds
Lived Experience
What is the day-to-day routine, quality of life?
What is the impact on partner/spouse?
How much self-care is involved?
Mobility?
Therapies
What therapies have been used to treat leg/foot wounds? Successes/failures?
Is accessibility to therapies an issue (are you able to take advantage of all potential therapies?)
What are the expectations for these therapies long term?
Are the any side effects or risks with the therapies that you have experienced?
Was it difficult to weigh potential benefits and risks when deciding on which therapies to go with?
Are the any other therapies you are considering? How do you go about making a decision?
HBOT
What have you heard of HBOT?
Do you feel you have enough information to understand risk/benefits and make a decision?
What are you expectations of the therapy?
What was the therapy like? What were the challenges to getting it done?
What was you understanding of the therapies needed afterwards? Is this different than expected?
Are there unexpected consequences from the therapy?
Appendix 9: Survey—Diabetic Wound Care Therapies
Purpose
This survey is designed to study the lived experience of diabetic patients who have wounds to the legs and/or feet. The survey will ask you about treatment options and the values that help guide you to make choices surrounding treatment options.
Confidentiality
All details shared through this survey will be kept confidential, and privacy will be protected except as required by law. The results of this review will be published; however, no identifying information will be released or published. Any records containing information from your interview will be stored securely. Participation is voluntary. You may refuse to participate, refuse to answer any questions or withdraw at any time. Withdrawal will in no way affect care you receive.
Consent
I hereby allow Health Quality Ontario to use this survey to inform the development of a health technology assessment of diabetic wound care therapies.
-
◯
Yes
-
◯
No
Where do you currently live in Ontario?
-
◯
Northern Ontario
-
◯
Eastern Ontario
-
◯
Greater Toronto Area (GTA)
-
◯
Central Ontario (excluding GTA)
-
◯
Southwest Ontario
Are you currently living with diabetes?
-
◯
Yes
-
◯
No
At any time, have you suffered from a wound that requires medical care (for example, bandages, dressings, a walking boot, casting, etc.)?
-
◯
Yes, to my legs only
-
◯
Yes, to my feet only
-
◯
Yes, to both my legs AND feet
-
◯
No
Leg Wounds: Lived Experience
Please describe your day-to-day routine of care for your leg wounds.

What was/is the impact of your leg wounds on your mobility?

What was/is the impact of your leg wounds on your family or caregiver?

Is there any other part of your daily lived experience with diabetic leg wounds that you would like to share with us?

Leg Wounds: Therapies
Do you suffer from one of the following medical conditions: emphysema, heart failure, peripheral arterial disease, severe claustrophobia, severe obstructive pulmonary disease, or cancer?
-
◯
Yes
-
◯
No
Have you ever been offered hyperbaric oxygen therapy (HBOT) as a treatment for your wounds?
-
◯
Yes
-
◯
No
Foot and Leg Wounds: Therapies
Have you undergone hyperbaric oxygen therapy to treat your wounds?
-
◯
Yes
-
◯
No
What were your expectations or goals prior to this therapy (consider duration, outcomes, need to repeat, etc.)?

Please describe the therapy from your experience in terms of the procedure itself.

Please describe what your life has been like since undergoing the procedure.

Were there any unexpected consequences or side effects of your HBOT treatment?

Please describe other therapies you are using to treat your leg wounds (for example, dressings, bandages, etc.)
How did you choose the current therapies you are using to treat your leg wounds?
-
◯
Doctor recommended it
-
◯
Wound care specialist recommended it
-
◯
It was easiest
-
◯
It was cheapest
-
◯
It seemed to work best
-
◯
Other (please describe)_____
What did you expect from these therapies (for example, how long did you think the therapies would last, how well did you expect them to work, etc.)?

Have you ever had to choose between types of treatment for your leg wounds due to cost/access/ease of use, etc.?
-
◯
Yes
-
◯
No
If you responded yes to the question above, please describe the situation and result.

Do you currently use a wound care clinic to help treat your wounds?
-
◯
Yes
-
◯
No
Why not?
-
◯
Distance to wound care clinic is too great
-
◯
Costs too much
-
◯
I don't think I need it
-
◯
It hasn't helped in the past
-
◯
Other; please specify:_____
Please add any final comments or anything else you wish to share about your experience below.

Author contributions
This report was developed by a multidisciplinary team from Health Quality Ontario. The clinical epidemiologist was Anna Lambrinos, the health economist was Brian Chan, the patient engagement specialist was David Wells, and the medical librarian was Corinne Holubowich.
KEY MESSAGES
People with diabetes are at risk of developing foot ulcers. When a foot ulcer does not heal despite standard wound care, patients may try additional treatments such as hyperbaric oxygen therapy. In hyperbaric oxygen therapy, a patient enters a chamber that fits a single or multiple individuals and is exposed to 100% oxygen while the atmospheric pressure is increased. To receive this treatment, patients visit a clinic five times a week for four to six weeks and sit in a hyperbaric chamber for about 90 minutes at each visit.
In this health technology assessment, we compared the safety and effectiveness of standard wound care plus hyperbaric oxygen therapy versus standard wound care alone for patients with diabetic foot ulcers. We also looked at how much hyperbaric oxygen therapy costs and whether it is cost-effective (good value for money) and spoke with patients to learn about their experiences with hyperbaric oxygen therapy.
We found that hyperbaric oxygen therapy and standard wound care appear to be given in many different ways. Therefore, the findings of the studies we reviewed differed on how effective hyperbaric oxygen therapy was in terms of the rate of major amputations undergone by patients receiving standard wound care plus hyperbaric oxygen therapy versus standard wound care alone. However, our analysis showed that standard wound care plus hyperbaric oxygen therapy results in an improvement in ulcer healing compared with standard wound care alone. The studies we reviewed found that standard wound care plus hyperbaric oxygen is as safe as standard wound care alone. The evidence makes it difficult to draw any definitive conclusions on the effectiveness of standard wound care plus hyperbaric oxygen therapy versus standard wound care alone for the treatment of diabetic foot ulcers. Our economic analysis found that there is a large degree of uncertainty regarding whether standard wound care plus hyperbaric oxygen therapy is cost-effective versus standard wound care alone. The budget impact of funding hyperbaric oxygen therapy for the treatment of diabetic foot ulcers with current capacity is close to $4 million per year. Patients with whom we spoke who had undergone hyperbaric oxygen therapy for the treatment of diabetic foot ulcers reported that they felt it was a highly effective treatment, they were satisfied with their wound healing, and they felt an improvement in their quality of life. Patients also said that although the process of receiving hyperbaric oxygen therapy was simple, it required a substantial time commitment and has associated costs.
Contributor Information
Health Quality Ontario:
Anna Lambrinos, Brian Chan, David Wells, and Corinne Holubowich
About Health Quality Ontario
Health Quality Ontario is the provincial advisor on the quality of health care. We are motivated by a single-minded purpose: Better health for all Ontarians.
Who We Are
We are a scientifically rigorous group with diverse areas of expertise. We strive for complete objectivity, and look at things from a vantage point that allows us to see the forest and the trees. We work in partnership with health care providers and organizations across the system, and engage with patients themselves, to help initiate substantial and sustainable change to the province's complex health system.
What We Do
We define the meaning of quality as it pertains to health care, and provide strategic advice so all the parts of the system can improve. We also analyze virtually all aspects of Ontario's health care. This includes looking at the overall health of Ontarians, how well different areas of the system are working together, and most importantly, patient experience. We then produce comprehensive, objective reports based on data, facts and the voice of patients, caregivers and those who work each day in the health system. As well, we make recommendations on how to improve care using the best evidence. Finally, we support large scale quality improvements by working with our partners to facilitate ways for health care providers to learn from each other and share innovative approaches.
Why It Matters
We recognize that, as a system, we have much to be proud of, but also that it often falls short of being the best it can be. Plus certain vulnerable segments of the population are not receiving acceptable levels of attention. Our intent at Health Quality Ontario is to continuously improve the quality of health care in this province regardless of who you are or where you live. We are driven by the desire to make the system better, and by the inarguable fact that better has no limit.
References
- (1).Canadian Diabetes Association. Diabetes charter backgrounder - Ontario [Internet]. Toronto (ON): The Association; 2015 [cited 2015 Nov 23]. Available from: http://www.diabetes.ca/getmedia/5941b1c2-8b03-45bf-8db0-e18b969d9aa6/diabetes-charter-backgrounder-ontario-english.pdf.aspx
- (2).Hux JE, Tang M. Patterns of prevalence and incidence of diabetes. In: Hux JE, Booth GL, Slaughter PM, Laupacis A, editors. Diabetes in Ontario: an ICES practice atlas. Toronto (ON): Institute for Clinical Evaluative Sciences; 2003. p. 1.1–1.18. [Google Scholar]
- (3).Canadian Diabetes Association. A step towards good health [Internet]. Toronto (ON): The Association; 2015 [cited 2015 June]. Available from: http://www.diabetes.ca/diabetes-and-you/healthy-living-resources/foot-care/a-step-towards-good-health
- (4).American Diabetes Association. Foot complications [Internet]. Alexandria (VA): The Association; c1995–2016 [updated 2015 May 26; cited 2015 Nov 24]. Available from: http://www.diabetes.org/living-with-diabetes/complications/foot-complications/
- (5).Ontario Wound Care Interest Group. Fewer wounds, faster healing: framework for an Ontario would care strategy [Internet]. Toronto (ON): OntWIG Executive Group; 2012 [cited 2015 Nov 25]. Available from: http://ontwig.rnao.ca/ontwig-framework-document
- (6).Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affect Disord. 2015; 177 : 7–21. [DOI] [PubMed] [Google Scholar]
- (7).Ndip A, Ebah L, Mbako A. Neuropathic diabetic foot ulcers - evidence-to-practice. Int J Gen Med. 2012; 5: 129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Public Health Agency of Canada. Diabetes in Canada: facts and figures from a public health perspective. Ottawa (ON): The Agency; 2011. [Google Scholar]
- (9).Wagner F. The dysvascular foot: a system of diagnosis and treatment. Foot Ankle. 1981; 2: 64–122. [DOI] [PubMed] [Google Scholar]
- (10).Registered Nurses' Association of Ontario. Assessment and management of foot ulcers for people with diabetes. 2nd ed. Toronto (ON): The Association; 2013. [Google Scholar]
- (11).Liu RT, Kleiman EM, Nestor BA, Cheek SM. The hopelessness theory of depression: A quarter-century in review. Clinical Psychology: Science and Practice. 2015; 22 (4): 345–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Medical Advisory Secretariat. Negative pressure wound therapy: an evidence update. Ont Health Technol Assess Ser. 2010; 10 (22): 1–28. [PMC free article] [PubMed] [Google Scholar]
- (13).National Institute for Health and Care Excellence. Diabetic foot problems: prevention and management. London: The Institute; 2015. [PubMed] [Google Scholar]
- (14).Bowering K, Embil JM. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada: foot care. Can J Diabetes. 2013; 37(suppl 1): S135–49. [Google Scholar]
- (15).Huang ET, Mansouri J, Murad MH, Joseph WS, Strauss MB, Tettelbach W, et al. A clinical practice guideline for the use of hyperbaric oxygen therapy in the treatment of diabetic foot ulcers. Undersea Hyperb Med. 2015; 42 (3): 205–47. [PubMed] [Google Scholar]
- (16).Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes. 2015; 6 (1): 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Thackham JA, McElwain DL, Long RJ. The use of hyperbaric oxygen therapy to treat chronic wounds: a review. Wound Repair Regen. 2008; 16 (3): 321–30. [DOI] [PubMed] [Google Scholar]
- (18).Plafki C, Peters P, Almeling M, Welslau W, Busch R. Complications and side effects of hyperbaric oxygen therapy. Aviat Space Environ Med. 2000; 71 (2): 119–24. [PubMed] [Google Scholar]
- (19).Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996; 334 (25): 1642–8. [DOI] [PubMed] [Google Scholar]
- (20).Navarro P, Bornstein S. Hyperbaric oxygen therapy for difficult wound healing in Newfoundland & Labrador [Internet]. St. John's (NL): Newfoundland & Labrador Centre for Applied Health Research, Memorial University; 2012. [cited 2015 Dec 1]. Available from: http://www.nlcahr.mun.ca/CHRSP/HBOT_FULL_REPORT.pdf [Google Scholar]
- (21).Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011; 127 Suppl 1: 131S–41S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Ministry of Health and Long-Term Care. Diagnostic and therapeutic procedures [Internet]. Toronto (ON): The Ministry; 2015. [cited 2016 Jul 7]. Available from: http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/j_diagth.pdf [Google Scholar]
- (23).McGowan J, Sampson M, Salzwedel D, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016; 75: 40–6. [DOI] [PubMed] [Google Scholar]
- (24).O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014; 67 (1): 56–64. [DOI] [PubMed] [Google Scholar]
- (25).Cuijpers P, Cristea IA, Karyotaki E, Reijnders M, Huibers MJH. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta-analytic update of the evidence. World Psychiatry. 2016; 15 (3): 245–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Higgins JPT, Altman DG, editors, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions, version 5.1.0 [updated March 2011]. London: The Cochrane Collaboration; 2011. Available from: www.cochrane-handbook.org. [Google Scholar]
- (27).Parikh SV, Quilty LC, Ravitz P, Rosenbluth M, Pavlova B, Grigoriadis S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 2. Psychological treatments. Canadian Journal of Psychiatry. 2016; 61 (9): 524–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Abidia A, Laden G, Kuhan G, Johnson BF, Wilkinson AR, Renwick PM, et al. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomised-controlled trial. Eur J Vasc Endovasc Surg. 2003; 25 (6): 513–8. [DOI] [PubMed] [Google Scholar]
- (29).Duzgun AP, Satir HZ, Ozozan O, Saylam B, Kulah B, Coskun F. Effect of hyperbaric oxygen therapy on healing of diabetic foot ulcers. J Foot Ankle Surg. 2008; 47 (6): 515–9. [DOI] [PubMed] [Google Scholar]
- (30).Faglia E, Favales F, Aldeghi A, Calia P, Quarantiello A, Oriani G, et al. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. A randomized study. Diabetes Care. 1996; 19 (12): 1338–43. [DOI] [PubMed] [Google Scholar]
- (31).Kessler L, Bilbault P, Ortega F, Grasso C, Passemard R, Stephan D, et al. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: a prospective randomized study. Diabetes Care. 2003; 26 (8): 2378–82. [DOI] [PubMed] [Google Scholar]
- (32).Londahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care. 2010; 33 (5): 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Londahl M, Landin-Olsson M, Katzman P. Hyperbaric oxygen therapy improves health-related quality of life in patients with diabetes and chronic foot ulcer. Diabet Med. 2011; 28 (2): 186–90. [DOI] [PubMed] [Google Scholar]
- (34).Kalani M, Jorneskog G, Naderi N, Lind F, Brismar K. Hyperbaric oxygen (HBO) therapy in treatment of diabetic foot ulcers. Long-term follow-up. J Diabetes Complications. 2002; 16 (2): 153–8. [DOI] [PubMed] [Google Scholar]
- (35).Fedorko L, Bowen JM, Jones W, Oreopoulos G, Goeree R, Hopkins RB, et al. Hyperbaric oxygen therapy does not reduce indications for amputation in patients with diabetes with nonhealing ulcers of the lower limb: a prospective, double-blind, randomized controlled clinical trial. Diabetes Care. 2016; 39 (3): 392–9. [DOI] [PubMed] [Google Scholar]
- (36).Cox RC, Olatunji BO. A systematic review of sleep disturbance in anxiety and related disorders. J Anxiety Disord. 2016; 37: 104–29. [DOI] [PubMed] [Google Scholar]
- (37).MacQueen GM, Frey BN, Ismail Z, Jaworska N, Steiner M, Lieshout RJV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 6. Special populations: Youth, women, and the elderly. Canadian Journal of Psychiatry. 2016; 61 (9): 588–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Weledji EP, Fokam P. Treatment of the diabetic foot - to amputate or not? BMC Surg. 2014; 14: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Game FL, Apelqvist J, Attinger C, Hartemann A, Hinchliffe RJ, Londahl M, et al. Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diabetes Metab Res Rev. 2015; 32(Suppl 1): 154–68. [DOI] [PubMed] [Google Scholar]
- (40).Goldman RJ. Hyperbaric oxygen therapy for wound healing and limb salvage: a systematic review. PM R. 2009; 1 (5): 471–89. [DOI] [PubMed] [Google Scholar]
- (41).Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2015; 6: CD004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Stoekenbroek RM, Santema TB, Koelemay MJ, van Hulst RA, Legemate DA, Reekers JA, et al. Is additional hyperbaric oxygen therapy cost-effective for treating ischemic diabetic ulcers? Study protocol for the Dutch DAMOCLES multicenter randomized clinical trial? J Diabetes. 2015; 7 (1): 125–32. [DOI] [PubMed] [Google Scholar]
- (43).Fife CE, Buyukcakir C, Otto GH, Sheffield PJ, Warriner RA, Love TL, et al. The predictive value of transcutaneous oxygen tension measurement in diabetic lower extremity ulcers treated with hyperbaric oxygen therapy: a retrospective analysis of 1,144 patients. Wound Repair Regen. 2002; 10 (4): 198–207. [DOI] [PubMed] [Google Scholar]
- (44).Strauss MB, Bryant BJ, Hart GB. Transcutaneous oxygen measurements under hyperbaric oxygen conditions as a predictor for healing of problem wounds. Foot Ankle Int. 2002; 23 (10): 933–7. [DOI] [PubMed] [Google Scholar]
- (45).Londahl M, Katzman P, Hammarlund C, Nilsson A, Landin-Olsson M. Relationship between ulcer healing after hyperbaric oxygen therapy and transcutaneous oximetry, toe blood pressure and ankle-brachial index in patients with diabetes and chronic foot ulcers. Diabetologia. 2011; 54 (1): 65–8. [DOI] [PubMed] [Google Scholar]
- (46).National Institute for Health and Care Excellence. Process and methods guides. The guidelines manual: appendix G: methodology checklist [Internet]. London (UK): The Institute; 2012. [cited 2015 May 27]. Available from: http://publications.nice.org.uk/the-guidelines-manual-appendices-bi-pmg6b/appendix-g-methodology-checklist-economic-evaluations [Google Scholar]
- (47).Guo S, Counte MA, Gillespie KN, Schmitz H. Cost-effectiveness of adjunctive hyperbaric oxygen in the treatment of diabetic ulcers. Int J Technol Assess Health Care. 2003; 19 (4): 731–7. [DOI] [PubMed] [Google Scholar]
- (48).Chuck AW, Hailey D, Jacobs P, Perry DC. Cost-effectiveness and budget impact of adjunctive hyperbaric oxygen therapy for diabetic foot ulcers. Int J Technol Assess Health Care. 2008; 24 (2): 178–83. [DOI] [PubMed] [Google Scholar]
- (49).Ma L, Li P, Shi Z, Hou T, Chen X, Du J. A prospective, randomized, controlled study of hyperbaric oxygen therapy: effects on healing and oxidative stress of ulcer tissue in patients with a diabetic foot ulcer. Ostomy Wound Manage. 2013; 59 (3): 18–24. [PubMed] [Google Scholar]
- (50).Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. ISPOR health economic evaluation publication guidelines—CHEERS Good Reporting Practices Task Force. Value Health. 2013. Mar-Apr; 16 (2): 231–50. [DOI] [PubMed] [Google Scholar]
- (51).Hopkins RB, Burke N, Harlock J, Jegathisawaran J, Goeree R. Economic burden of illness associated with diabetic foot ulcers in Canada. BMC Health Serv Res. 2015; 15: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Woo KY, Botros M, Kuhnke J, Evans R, Alavi A. Best practices for the management of foot ulcers in people with diabetes. Adv Skin Wound Care. 2013; 26 (11): 512–24; quiz 225–6. [DOI] [PubMed] [Google Scholar]
- (53).Loukine L, Waters C, Choi BC, Ellison J. Impact of diabetes mellitus on life expectancy and health-adjusted life expectancy in Canada. Popul Health Metr. 2012; 10 (1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Lind M, Garcia-Rodriguez LA, Booth GL, Cea-Soriano L, Shah BR, Ekeroth G, et al. Mortality trends in patients with and without diabetes in Ontario, Canada and the UK from 1996 to 2009: a population-based study. Diabetologia. 2013; 56 (12): 2601–8. [DOI] [PubMed] [Google Scholar]
- (55).Statistics Canada. Life tables, Canada, provinces and territories, 2009 to 2011. Ottawa (ON): Statistics Canada; 2015. [Google Scholar]
- (56).Brownrigg JR, Davey J, Holt PJ, Davis WA, Thompson MM, Ray KK, et al. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: a meta-analysis. Diabetologia. 2012; 55 (11): 2906–12. [DOI] [PubMed] [Google Scholar]
- (57).Fortington LV, Geertzen JH, van Netten JJ, Postema K, Rommers GM, Dijkstra PU. Short and long term mortality rates after a lower limb amputation. Eur J Vasc Endovasc Surg. 2013; 46 (1): 124–31. [DOI] [PubMed] [Google Scholar]
- (58).Pound N, Chipchase S, Treece K, Game F, Jeffcoate W. Ulcer-free survival following management of foot ulcers in diabetes. Diabet Med. 2005; 22 (10): 1306–9. [DOI] [PubMed] [Google Scholar]
- (59).Ragnarson Tennvall G, Apelqvist J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J Diabetes Complications. 2000; 14 (5): 235–41. [DOI] [PubMed] [Google Scholar]
- (60).Ontario Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act [Internet]. Toronto (ON): The Ministry; 2015. [cited 2015 Jul 28]. Available from: http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/sob_master11062015.pdf [Google Scholar]
- (61).Rosella LC, Lebenbaum M, Fitzpatrick T, O'Reilly D, Wang J, Booth GL, et al. Impact of diabetes on healthcare costs in a population-based cohort: a cost analysis. Diabet Med. 2016; 33 (3): 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Statistics Canada. Consumer price index, historical summary [Internet]. Ottawa (ON): Statistics Canada; 2016. [cited 2016 May 13]. Available from: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/econ46a-eng.htm [Google Scholar]
- (63).Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999; 22 (3): 382–7. [DOI] [PubMed] [Google Scholar]
- (64).Canadian Institute for Health Information. Cost of a standard hospital stay [Internet]. Ottawa (ON): The Institute; 2016. [cited 2016 May 13]. Available from: http://yourhealthsystem.cihi.ca/hsp/inbrief?lang=en#!/indicators/015/cost-of-a-standard-hospital-stay-cshs/;mapC1;mapLevel2;/ [Google Scholar]
- (65).Apelqvist J, Ragnarson-Tennvall G, Larsson J, Persson U. Long-term costs for foot ulcers in diabetic patients in a multidisciplinary setting. Foot Ankle Int. 1995; 16 (7): 388–94. [DOI] [PubMed] [Google Scholar]
- (66).Iversen MM, Tell GS, Riise T, Hanestad BR, Ostbye T, Graue M, et al. History of foot ulcer increases mortality among individuals with diabetes: ten-year follow-up of the Nord-Trondelag Health Study, Norway. Diabetes Care. 2009; 32 (12): 2193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Morbach S, Furchert H, Groblinghoff U, Hoffmeier H, Kersten K, Klauke GT, et al. Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care. 2012; 35 (10): 2021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Jones WS, Patel MR, Dai D, Vemulapalli S, Subherwal S, Stafford J, et al. High mortality risks after major lower extremity amputation in Medicare patients with peripheral artery disease. Am Heart J. 2013; 165 (5) 809–15, 815.e1. [DOI] [PubMed] [Google Scholar]
- (69).Waaijman R, de Haart M, Arts ML, Wever D, Verlouw AJ, Nollet F, et al. Risk factors for plantar foot ulcer recurrence in neuropathic diabetic patients. Diabetes Care. 2014; 37 (6): 1697–705. [DOI] [PubMed] [Google Scholar]
- (70).Redekop WK, Stolk EA, Kok E, Lovas K, Kalo Z, Busschbach JJ. Diabetic foot ulcers and amputations: estimates of health utility for use in cost-effectiveness analyses of new treatments. Diabetes Metab. 2004; 30 (6): 549–56. [DOI] [PubMed] [Google Scholar]
- (71).O'Reilly D, Pasricha A, Campbell K, Burke N, Assasi N, Bowen JM, et al. Hyperbaric oxygen therapy for diabetic ulcers: systematic review and meta-analysis. Int J Technol Assess Health Care. 2013; 29 (3): 269–81. [DOI] [PubMed] [Google Scholar]
- (72).Statistics Canada. Population by year, by province and territory [Internet] Ottawa (ON): Statistics Canada; 2015. [cited 2016 May 16]. Available from: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo02a-eng.htm [Google Scholar]
- (73).Agence d'evaluation des technologies et des modes d'intervention en sante. Hyberbaric oxygen therapy in Quebec (AETMIS 2000-3 RE) Montreal (QC): The Agency; 2001. [Google Scholar]
- (74).Public Health Agency of Canada. Diabetes in Canada: Facts and figures from a public health perspective: Ottawa, ON: Public Health Agency of Canada; 2011. [Google Scholar]
- (75).Booth GL, Polsky JY, Gozdyra P, Cauch-Dudek K, Kiran T, Shah BR, et al. Regional measures of diabetes burden in Ontario. Toronto (ON): Institute for Clinical Evaluative Sciences; 2012. [Google Scholar]
- (76).Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers and amputation. Wound Repair Regen. 2005; 13 (3): 230–6. [DOI] [PubMed] [Google Scholar]
- (77).Abraham TM, Pencina KM, Pencina MJ, Fox CS. Trends in diabetes incidence: the Framingham Heart Study. Diabetes Care. 2015; 38 (3): 482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011; 4 (1): 1–10. [DOI] [PubMed] [Google Scholar]
- (79).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012; 5 (3): 199–211. [DOI] [PubMed] [Google Scholar]
- (80).Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee: Toronto (ON): Queen's Printer for Ontario; 2015. [Google Scholar]
- (81).Tjornhoj-Thomsen T, Hansen HP. Knowledge in health technology assessment: who, what, how? Int J Technol Assess Health Care. 2011; 27 (4): 324–9. [DOI] [PubMed] [Google Scholar]
- (82).Rowe G, Frewer LJ. A typology of public engagement mechanisms. Sci Technol Hum Values. 2005; 30 (2): 251–90. [Google Scholar]
- (83).Brundisini F, Giacomini M, DeJean D, Vanstone M, Winsor S, Smith A. Chronic disease patients' experiences with accessing health care in rural and remote areas: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser. 2013; 13 (15): 1–33. [PMC free article] [PubMed] [Google Scholar]
- (84).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage Publications; 1996. [Google Scholar]
- (85).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage Publications; 1999. p. 33–45. [Google Scholar]
- (86).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods. Thousand Oaks (CA): Sage Publications; 1994. p. 23–41. [Google Scholar]
- (87).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage Publications; 2002. [Google Scholar]
- (88).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage Publications; 1998. [Google Scholar]
- (89).Health Technology Assessment International Interest Group on Patient and Citizen Involvement in HTA. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2015 Aug 5]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (90).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990; 13 (1): 3–21. [Google Scholar]
- (91).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage Publications; 1994. p. 273–85. [Google Scholar]
- (92).Bishop AJ, Mudge E. Diabetic foot ulcers treated with hyperbaric oxygen therapy: a review of the literature. Int Wound J. 2014; 11 (1): 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Fonagy P, Cottrell D, Phillips J, Bevington D, Glaser D, Allison E. What works for whom? A critical review of treatments for children and adolescents., 2nd ed. New York, NY: Guilford Press; US; 2015. [Google Scholar]
- (94).Hailey D, Jacobs P, Perry DC, Chuck AW, Morrison A, Boudreau R. Hyperbaric oxygen therapy for diabetic foot ulcer. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2007. [Google Scholar]
- (95).Hinchliffe RJ, Valk GD, Apelqvist J, Armstrong DG, Bakker K, Game FL, et al. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2008; 24 (Suppl 1): S119–44. [DOI] [PubMed] [Google Scholar]
- (96).Liu R, Li L, Yang M, Boden G, Yang G. Systematic review of the effectiveness of hyperbaric oxygenation therapy in the management of chronic diabetic foot ulcers. Mayo Clin Proc. 2013; 88 (2): 166–75. [DOI] [PubMed] [Google Scholar]
- (97).Medical Advisory Secretariat. Hyperbaric oxygen therapy for non-healing ulcers in diabetes mellitus: an evidence-based analysis. Ont Health Technol Assess Ser. 2005; 5 (11): 1–28. [PMC free article] [PubMed] [Google Scholar]
- (98).Ritchie K, Bakker S, Craig J, Macpherson K, Mandava L, McIntosh H, et al. The clinical and cost effectiveness of hyperbaric oxygen therapy. Edinburgh (Scotland): NHS Quality Improvement Scotland; 2008. [Google Scholar]
- (99).Roeckl-Wiedmann I, Bennett M, Kranke P. Systematic review of hyperbaric oxygen in the management of chronic wounds. Br J Surg. 2005; 92 (1): 24–32. [DOI] [PubMed] [Google Scholar]
- (100).Stoekenbroek RM, Santema TB, Legemate DA, Ubbink DT, van den Brink A, Koelemay MJ. Hyperbaric oxygen for the treatment of diabetic foot ulcers: a systematic review. Eur J Vasc Endovasc Surg. 2014; 47 (6): 647–55. [DOI] [PubMed] [Google Scholar]
- (101).Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011; 64 (4): 380–2. [DOI] [PubMed] [Google Scholar]
- (102).Canadian Institute for Health Information. Canadian classification of health interventions, volume 3 [Internet]. Ottawa (ON): The Institute; 2010. Available from: https://www.cihi.ca/en/cci_volume_three_2015_en.pdf [Google Scholar]