Abstract
Background
Although decanoic acid (DA) is thought to act as a muscarinic cholinergic agonist, effect of DA on nociceptive behavioral responses and the excitability of nociceptive neuronal activity under in vivo conditions remain to be determined. The aim of the present study, therefore, was to investigate whether in vivo acute administration of ointment containing DA affects the excitability of nociceptive trigeminal spinal nucleus caudalis (SpVc) neurons associated with hypoalgesia in naïve rats.
Results
After local application of DA, the threshold of escape from mechanical stimulation applied to the shaved orofacial skin was significantly higher than before DA application. Vehicle treatment (without DA) had no significant effect on the escape threshold from mechanical stimulation. Extracellular single unit recordings were made from SpVc wide-dynamic range (WDR) neurons in response to orofacial non-noxious and noxious mechanical stimuli of pentobarbital-anesthetized rats. The mean firing frequency of SpVc WDR neurons in response to noxious, but not non-noxious, mechanical stimuli was inhibited by local application of DA, and the maximum inhibition of discharge frequency of both non-noxious and noxious mechanical stimuli was seen within 1–5 min. The DA-induced short-term inhibitory effects were reversed after approximately 10 min. Pretreatment intravenously with the muscarinic-specific M2 receptor antagonist, methoctramine, abolished the DA-induced suppression of firing frequency of SpVc WDR neurons in response to noxious stimulation. Fluorogold (FG) labeling was identified as the trigeminal ganglion (TG) neurons innervating orofacial skin. FG-labeled small-diameter TG neurons expressed M2 receptor immunoreactivity.
Conclusion
These results suggest that acute DA application induces short-term mechanical hypoalgesia and this effect was mainly due to suppression of the excitability of SpVc WDR neurons via the peripheral M2 receptor signaling pathway in the trigeminal primary afferents. These findings support the idea that DA is a potential therapeutic agent and complementary alternative medicine for the attenuation of trigeminal nociception in the absence of inflammatory/neuropathic conditions.
Keywords: Decanoic acid, trigeminal spinal nucleus, nociception, hypoalgesia, single unit recording, complementary alternative medicine
Introduction
The trigeminal spinal nucleus plays an important role as a relay station for the transmission of orofacial sensory information. This nucleus is functionally subdivided into three nuclei from rostral to caudal: oralis, interpolaris, and caudalis.1 It is well known that the spinal trigeminal nucleus caudalis (SpVc) and the upper cervical (C1–C2) dorsal horn are important relay stations for trigeminal nociceptive inputs from inflammation and tissue injury.1,2 Previous studies reported that inflammation produced by complete Freund’s adjuvant induced hyperexcitability of the SpVc wide-dynamic range (WDR) neurons to mechanical stimuli, with this central sensitization leading to hyperalgesia.2,3 There are also reports that SpVc and C1-C2 WDR neurons contribute to the mechanism of hyperalgesia and/or referred pain associated with dental pain.2,4,5
Recent reports have described the use of complementary and alternative medicines (CAMs), such as herbal medicines and acupuncture, for the treatment of persistent clinical chronic pain,6–8 and the potential effects of diet and dietary supplementation on conditions associated with pain have been the focus of considerable research.9–11 Recently, we have reported that subcutaneous local injection of the dietary constituent and plant polyphenol, resveratrol, into the peripheral receptive field suppresses the excitability of SpVc WDR neurons, possibly by inhibiting sodium channels in the nociceptive nerve terminals of trigeminal ganglion (TG) neurons. Therefore, local injection of resveratrol may act in a manner similar to injection of a local anesthetic agent, providing relief from trigeminal nociceptive pain without side effects.12 As such, resveratrol may be a candidate of CAM.
Another dietary constituent, the fatty acid, decanoic acid (DA), is also known to possess several biological actions, and a previous study using guinea-pig duodenum and jejunum, demonstrated that DA-induced motor activity is reversibly abolished by a muscarinic antagonist, suggesting that DA may act as a muscarinic acetylcholine receptor (mAchR) agonist.13 The mAchR family is involved in a large number of important physiological functions in both the central and peripheral nervous systems, including nociception.14 Pharmacologically, muscarinic receptors can be divided into four subtypes, whereas five different muscarinic receptor genes (M1–M5) have been described.15 The M1-, M3-, and M5-receptor subtypes are coupled to the inositol triphosphate/diacylglycerol pathway resulting in the release of Ca2+ from internal stores and activation of protein kinase C, while M2- and M4-receptor subtype activation results in the inhibition of adenylyl cyclase activity.16 Bernardini et al.17 reported that in electrophysiological experiments using a rat skin-nerve preparation, M2 mAchRs exert an inhibitory or desensitizing influence on the peripheral terminal of C-nociceptors. This desensitization can be explained by the fact that, apart from lowering the intracellular cAMP concentration, M2 mAchRs also affect low-threshold voltage-operated K+ channels which builds up a hyperpolarization force.18 There is evidence that M2 mAchR mRNA is found in the small- and medium-diameter TG neurons,19 and formalin-induced nociceptive behavior is dose-dependently inhibited by M2 mAchR agonists.19 In the trigeminal pain pathway, in vitro electrophysiological preparations have shown that activation of M2 mAchRs inhibits glutamatergic transmission by reducing the Ca2+ influx into primary afferent terminals, which could be a potential target for the treatment of pain from orofacial tissues.20 Taken together, these studies suggest that DA administration induces mechanical hypoalgesia and this effect is mainly due to suppression of the excitability of SpVc WDR neurons via the peripheral M2 mAchR signaling pathway. However, until now no studies have addressed this possibility. Therefore, the aim of the present study was to investigate whether, in the absence of inflammatory/neuropathic conditions, in vivo acute administration of ointment containing DA affects the excitability of nociceptive trigeminal spinal nucleus caudalis (SpVc) neurons associated with hypoalgesia in naïve rats. In addition, we also examined whether M2 mAchR and neurofilament protein-200 (NF-200) marker for myelinated fiber are expressed in the TG neurons by immunohistochemistry.
Methods
This study was approved by the Animal Use and Care Committee of Azabu University and was consistent with the ethical guidelines of the International Association for the Study of Pain.21 Every effort was made to minimize the number of animals used and their suffering.
Local cutaneous application of DA via an ointment
In this study, we made an original ointment for the application of DA (Patent number: P2013-139421A, Japan) to the natural cutaneous tissue. DA (MW = 172.26) is a saturated medium-chain fatty acid with a 10-carbon backbone (C10 H20 O2) and a lipophilic agents (Log P = 4.09). The fur was removed from the cutaneous tissue with depilatory cream. The original ointment containing DA was made in the laboratory and had the following composition: 30% decanoic acid, 15% caffeine, 4% D-mannitol, 31% calcium carbonate, and 20% solvent medium (alcohol, olive oil, and macrogol ointment). DA and D-mannitol and ethanol were purchased from Kanto Chemical, Co, Inc, Japan. Calcium carbonate and caffeine monohydrate were purchased from Wako Pure Chemical Industries, Ltd, Japan. Olive oil was purchased from Nikko Pharmaceutical Company Limited, Japan. Macrogol ointment was purchased from Yoshida Pharmaceutical Company Limited Japan.
Behavioral experiments for measurement of pain threshold
The experiments were performed on adult male Wistar rats (230–310 g body weight, n = 7). One day before the escape threshold experiments, the skin of the rats was carefully shaved on the right side of the buccal region (approximately 100 mm2) using clippers and shaving cream. The mechanical stimulation threshold for escape behavior was measured as described in previous studies.22 In brief, 5 min before DA treatment to the facial skin region, mechanical hypoalgesia was assessed with a set of von Frey hairs (Semmes-Weinstein Monofilaments, North Coast Medical, CA). To evaluate the effect of DA on the rat’s escape threshold, von Frey mechanical stimuli were then applied to the whisker pad in an ascending series of trials, at 1, 3, 5, and 10 min after treatment with DA. Each von Frey stimulation was applied three times in each series of trials. Escape threshold intensity was determined when the rat moved its head away from at least one of the three stimuli. A vehicle control was also tested by using a control ointment (original ointment without DA) with the same protocol.
Electrophysiological experiments for single unit recording of SpVc neurons
Electrophysiological recordings were conducted on 18 adult male Wistar rats (230–310 g body weight). Rats were first anesthetized with pentobarbital sodium (45 mg/kg, i.p.) and maintained, as required, with additional doses of 2–3 mg/kg/h through a cannula into the jugular vein. The level of anesthesia was confirmed by the absence of the corneal reflex and a lack of response to paw pinching. The rectal temperature was maintained at 37 ± 0.5℃ with a homoeothermic blanket during recording. All wound margins were continuously covered with the local anesthetic, 2% lidocaine (Xylocaine), throughout the experiments. The animals were placed in a stereotaxic apparatus and the activity of a single neuron from the SpVc region, according to the coordinates of Paxinos and Watson,23 was recorded extracellularly by means of a glass micropipette filled with 2% pontamine sky blue and 0.5 M sodium acetate. Neuronal activity was amplified (WPI, DAM 80), filtered (0.3-10 KHz), monitored with an oscilloscope (Iwatsu, SS-7672, Tokyo) and recorded for off-line analysis by Power Lab and Chart 5 software (ADI Instruments, UK), as described previously.22
Experimental protocols
Extracellular recordings of SpVc WDR unit activity were made as follows. Mechanical stimulation was used as a stimulus to identify the receptive field quickly and to avoid sensitization of peripheral receptors. Single units that responded to stimulation of the left side of the orofacial skin (whisker pad) with a brush and a set of von Frey hairs were identified. Noxious pinch and mechanical stimulation, which evoked a pain sensation when applied to a human subject, was applied to the orofacial area using von Frey hairs (>15 g: 15, 26, and 60 g) and forceps, as described previously.12,22 After identification of SpVc WDR neurons responding to stimulation of the whisker pad, we determined whether there was spontaneous discharge. The threshold for mechanical stimulation was determined using non-noxious and noxious mechanical stimulation (5 s) with von Frey hairs (1, 2, 4, 6, 10, 15, 26, 60 g) at intervals of 5 s. The mechanical receptive field of neurons was mapped by probing the facial skin with von Frey hairs, and then outlined on a life-sized drawing of a rat on tracing paper. The WDR neuronal discharges induced by mechanical stimulation were quantified by subtracting background activity from evoked activity. Spontaneous discharge frequencies were determined over a period of 2–5 min. If no discharge was recorded, the cell was deemed a silent neuron. The mean firing rate of SpVc WDR neurons evoked by mechanical stimulation was compared before and after drug administration. Post-stimulus histograms (bin = 100 ms) were generated in response to each stimulus. The effects of DA and vehicle treatment on SpVc WDR neuronal activity were evaluated 1, 3, 10, and 20 min after administration because peak effect and recovery were thought to occur during this period. The mean spontaneous and mechanical stimulation-induced discharge rates, as well as the mechanical threshold before and after DA administration, were evaluated in the present study. In this study, the effect of pretreatment with the M2 mAchR-specific antagonist, methoctramine, (1 mM; Sigma-Aldrich, St. Louse, MO) 10 min before DA treatment, on the DA-induced changes in firing of the SpVc WDR neurons responding to mechanical stimulation was also investigated (n = 6).
Identification of recording sites
At the end of recording sessions, rats were deeply anesthetized, and anodal DC currents (30 μA, 5 min) were passed through the recording micropipette. The animals were transcardially perfused with saline and 10% formalin. Frozen coronal sections were cut into 30 µm sections and stained with hematoxylin-eosin. Recording sites were identified from the blue spots, and construction of the electrode tracks was done in combination with micromanipulator readings.
Retrograde labeling of TG neurons innervating facial skin
For immunohistochemical studies, fluorogold (FG, Fluorochrome, Englewood, USA) labeling methods24–26 were used. Adult male Wistar rats (230–250 g body weight, n = 4) were anesthetized with pentobarbital sodium (45 mg/kg, i.p.) before FG solution (0.5% in distilled water, 10 µl) was injected into the shaved facial skin (buccal region) using a Hamilton syringe with a 31-gauge needle.
Immunohistochenmistry for M2 mAchR and NF-200 in the TG neurons
Immunohistochemistry was conducted using the modified method described in our previous studies.24–26 Two days after FG was injected into the facial skin, rats were deeply anaesthetized with pentobarbital sodium (50 mg/kg, i.p.) and transcardially perfused with 50 ml heparinized saline in 0.01 M phosphate buffer saline (PBS), followed by 100 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.3). The trigeminal ganglia were removed and incubated in 4% sucrose (3 × 5 min), 10% sucrose (1 h), and 20% sucrose (2 h) and then in 30% sucrose overnight. Frozen sections were cut at 10 µm with a cryostat (Leica, Nussloch, Germany) and mounted on silane-coated glass slides. Sections were incubated with rabbit anti-M2 mAchR primary antibody (1:200, EMD Millipore, USA) and goat anti-NF200 primary antibody (1:500, EMD Millipore, USA) for 16 h at 4℃, washed, and then incubated in Alexa® 488 Goat anti-rabbit IgG secondary antibody (1:500, Molecular Probes, Eugene, OR) and Alexa®568 goat-anti-mouse IgG secondary antibody (1:1000, Molecular Probes, Eugene, OR), respectively. Labeled cryosections were rinsed consecutively in 0.01 M PBS for 5 min each. The samples were mounted with antifade mounting medium (Molecular Probes). A control experiment was conducted with primary antibody absorption. The fluorescence intensity difference between the staining with primary antibody omitted and the least intense staining was scored as positive. The cell body areas were calculated by assuming that TG neurons were elliptical and their major and minor axes were measured. In this study, we classified TG neurons by measuring the cell diameter as small (<30 µm), medium (30–39 µm), and large (>40 µm).24–26 Every third section was used as the immunohistochemistry and analyzed 20 sections per trigeminal ganglion. At least 100 neurons were measured in each trigeminal ganglion. The data are presented as the percentage of the total (labeled/total populations). Fluorescent images of the stained sections were generated on a Olympus FSX100 all-in one fluorescent microscope (Olympus, Japan). Digital images were analyzed using Olympus Cell Sens microscope imaging software.
Data analysis
Values are expressed as means ± SEM. Statistical analysis was performed using two-way repeated measures analysis of variance, followed by Tukey–Kramer/Dunnett tests (post hoc test) for behavioral and electrophysiological data. P < 0.05 was considered statistically significant.
Results
Effect of DA on the escape threshold from mechanical stimulation
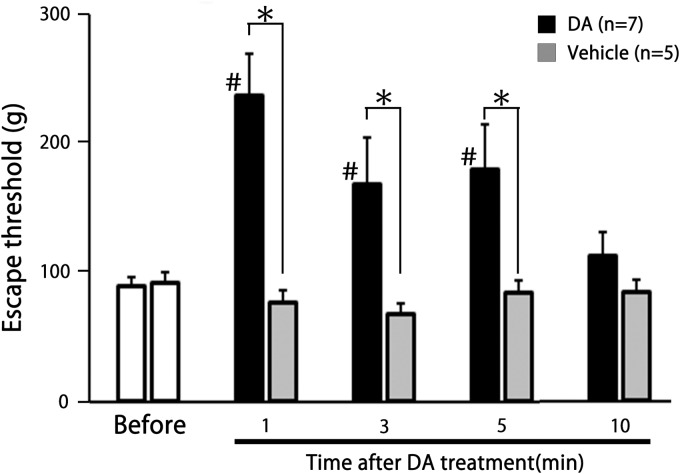
We studied the effect of DA on the escape threshold in seven naïve rats. From 1–5 min after DA treatment of the facial skin, the escape threshold from mechanical stimulation was significantly increased, compared to before DA treatment (88.6 ± 7.4 g vs. 237.1 ± 31.3 g, 168.6 ± 35.7 g and 180.1 ± 33.9 g, for 0 min vs. 1, 3, and 5 min, respectively; F = 17.6, F = 5.4 and F = 5.4, n = 7, P < 0.05) (P < 0.05, n = 7; Figure 1). As shown in Figure 1, the increased escape threshold gradually returned to control levels by 10 min. Vehicle (without DA) treatment had no significant effect on the escape threshold from mechanical stimulation (n = 5). From 1–5 min after DA treatment, escape threshold from mechanical stimulation was significantly increased compared to vehicle control (1, 3, and 5 min, F = 21.3, F = 4.8, and F = 6.9, n = 7; P < 0.05).
Figure 1.
Comparison of the change in escape threshold between decanoic acid (DA) treatment and vehicle treatment in rats. Mechanical stimulation using von Frey hairs was applied to the ipsilateral shaved region of buccal facial skin of naïve rats. Data are mean ± SEM. #P < 0.05; before versus 1, 3, and 5 min after DA treatment (n = 7). *P < 0.05, DA versus vehicle (n = 7 and 5, respectively).
General properties of SpVc WDR neurons responding to mechanical stimulation of orofacial skin
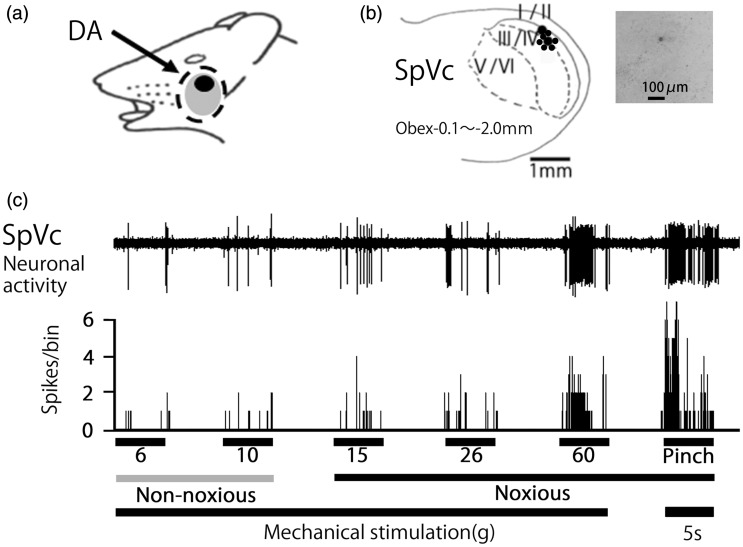
A total of 12 SpVc WDR neurons responding to mechanical stimulation of the whisker pad were tested for a DA effect. The firing of 9 out of 12 of these SpVc WDR neurons responding to mechanical stimulation was inhibited by DA treatment. Inhibition was not observed in one of the units and the remaining two units were inhibited but did not recover to control levels, therefore, 9 SpVc WDR neurons were analyzed for a DA effect in this study. These SpVc neurons responding to non-noxious and noxious mechanical stimulation exhibited a somatic receptive field in the orofacial area (buccal region of the skin) (Figure 2(a)). Recording sites were mainly distributed in the maxillary and mandibular branches (Figure 2(b)). As shown in Figure 2(b), recording sites were found in layers I–III (n = 6, 66%) and IV–V (n = 3, 34%) in the SpVc (obex −1.0 ∼ −2.0 mm). A typical example of histological confirmation of recording site was shown in Figure 2(b) inset. As shown in Figure 2(c), graded mechanical stimulation applied to the most sensitive area of the receptive field showed increased firing frequency of SpVc neurons, proportional to the stimulus intensity. Every neuron recorded belonged to the category of WDR neuron.2,22
Figure 2.
General characteristics of spinal trigeminal nucleus caudalis (SpVc) wide-dynamic range (WDR) neuronal activity in response to mechanical stimulation of orofacial skin. (a) Typical example of receptive field of whisker pad in the facial skin. Shaded area indicates the region applied with decanoic acid (DA). (b) Distribution of SpVc WDR neurons responding to non-noxious and noxious mechanical stimulation of the facial skin (n = 18). Inset: example for histological confirmation of recording site. The number below each drawing indicates the frontal plane in relation to obex. (c) Example of non-noxious and noxious mechanical stimulation-induced firing of SpVc WDR neurons.
Effect of DA on the excitability of SpVc WDR neurons responding to non-noxious and noxious mechanical stimulation
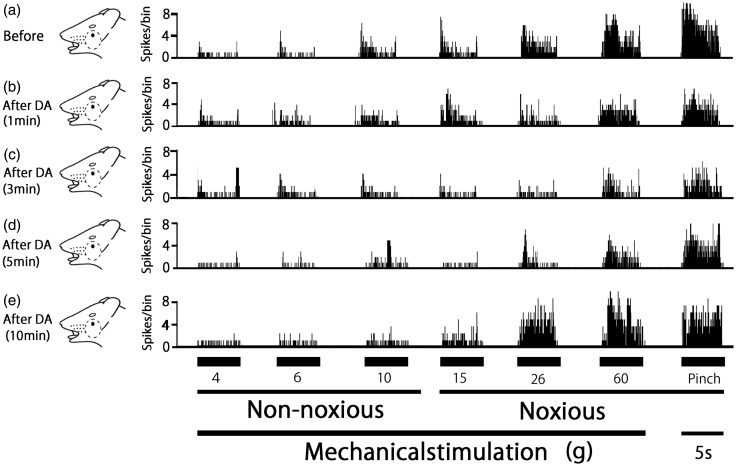
A typical example of the effect of DA treatment on the excitability of SpVc WDR neurons in response to non-noxious mechanical stimulation is shown in Figure 3. One minute after DA treatment, no significant changes were observed in non-noxious (2, 6, and 10 g) mechanical stimulation-evoked SpVc WDR neuronal activity. The time course for SpVc WDR neuronal activity evoked by non-noxious mechanical stimulation is summarized in Figure 4. After DA treatment, there were no significant changes in the mean firing rate of non-noxious mechanical stimulation-evoked SpVc WDR neuronal activity. There were no obvious changes in the size of the receptive field (16.5 ± 0.1 vs. 17.1 ± 0.2 m2, before vs. after DA, respectively) or in the mechanical threshold after DA treatment of the skin.
Figure 3.
Effect of decanoic acid (DA) treatment on spinal trigeminal nucleus caudalis (SpVc) wide-dynamic range (WDR) neuronal activity in response to mechanical stimulation of orofacial skin. (a–c) Typical example of non-noxious (6 and 10 g), noxious (15 and 60 g) mechanical, and noxious pinch stimulation-evoked SpVc WDR neuron activity, before (a) and 1 min (b), 3 min (c), 5 min (d), and 10 min (e) after DA treatment. Receptive field of the whisker pad in the facial skin. Blackened area indicates the location and size of the receptive field.
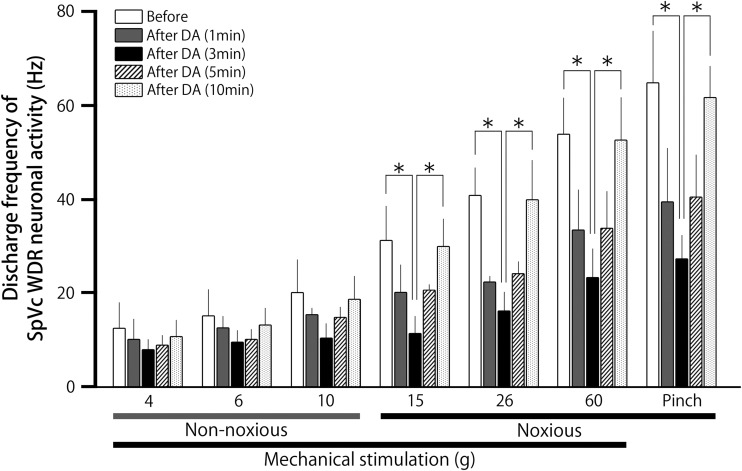
Figure 4.
Time course of the effects of decanoic acid (DA) treatment on the mean firing frequency of spinal trigeminal nucleus caudalis (SpVc) wide-dynamic range (WDR) neurons in response to mechanical stimulation of orofacial skin. #P < 0.05; 6 g versus 10, 15, and 60 g, and pinch stimulation.
Figure 3 also shows typical examples of DA treatment on the excitability of SpVc WDR neurons in response to noxious mechanical and noxious pinch stimulation. Noxious mechanical (15, 26, and 60 g) stimulation-evoked SpVc WDR neuronal activity was obviously inhibited from 1–5 min after DA treatment, but this inhibition disappeared, with activity returning to control levels in approximately 10 min. Similarly, SpVc WDR neuronal activity in response to noxious pinch stimulation was inhibited 1–5 min after DA treatment, with responses returning to control levels within 10 min.
As indicated in Figure 4, the mean firing rates of SpVc WDR neurons evoked by noxious mechanical and pinch stimulation decreased significantly after DA treatment, compared with before DA treatment (15-g stimulus, 31.3 ± 7.2 vs. 11.5 ± 3.6 Hz, F = 7.7, n = 9, P < 0.05; 60-g stimulus, 54.0 ± 7.7 vs. 23.3 ± 6.1 Hz F = 9.9, n = 9, P < 0.05; and pinch stimulus, 65.0 ± 10.9 vs. 27.3 ± 5.1 Hz F = 11.5, n = 9, P < 0.05; before vs. after 3 min DA, respectively). No significant changes were observed in the mean receptive field size after DA administration (14.5 ± 0.1 vs. 16.9 ± 0.3 mm2, before vs. after DA, respectively). There were no changes in the spontaneous firing rate observed after DA treatment. Vehicle treatment had no significant effect on either spontaneous or evoked (non-noxious, noxious mechanical, and pinch stimulation) activity of the SpVc WDR neurons (n = 2; data not shown).
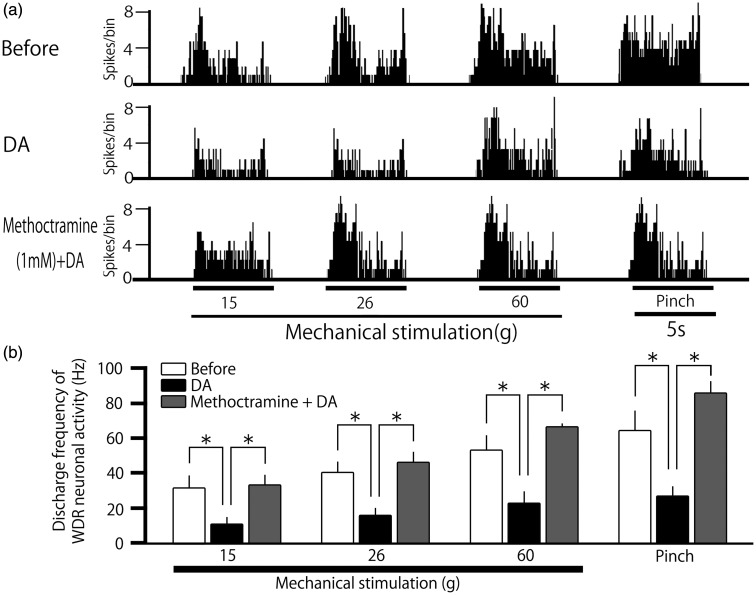
Pretreatment with M2 mAchR antagonist attenuates the DA-induced inhibition of SpVc WDR neurons responding to noxious stimulation
Next, in this study, we tested whether pretreatment with the M2 mAchR antagonist, methoctramine, attenuates the DA-treatment-induced inhibition of SpVc WDR neuronal firing in response to noxious stimulation (n = 6). Figure 5(a) shows a typical example of pretreatment with methoctramine (1 mM, i.v.) on SpVc WDR neuronal activity in response to noxious mechanical stimulation, and summarized results are shown in Figure 5(b). The DA-treatment-induced inhibition of SpVc WDR neuronal firing in response to noxious stimulation was significantly attenuated by pretreatment with methoctramine (15 g, 11.5 ± 3.6 vs. 33.9 ± 5.4 Hz, F = 6.1, n = 6, P < 0.05; 26 g, 16.3 ± 3.9 vs. 47.0 ± 5.1 Hz, F = 10.7, n = 6, P < 0.05; 60 g, 23.3 ± 6.1 vs. 66.9 ± 1.5, F = 9.1, n = 6, P < 0.05; pinch, 27.3 ± 5.1 vs. 86.0 ± 6.5 Hz F = 11.4, n = 6, P < 0.05; for DA vs. DA + methoctramine).
Figure 5.
Pretreatment with an M2 muscarinic acetylcholine receptor (M2 mAchR) antagonist on the decanoic acid (DA)-induced inhibition of spinal trigeminal nucleus caudalis (SpVc) wide-dynamic range (WDR) neurons responding to noxious stimulation. (a) Typical example of the effect of pretreatment with the M2 mAchR antagonist, methoctramine (1 mM, i.v.), on SpVc WDR neuronal activity in response to noxious mechanical stimulation. The DA-treatment-induced inhibition of SpVc WDR neuronal firing in response to noxious stimulation was attenuated by pretreatment with methoctramine. (b) Summarized results showing the effects of pretreatment with methoctramine on the DA-treatment-induced inhibition of SpVc WDR neuronal firing in response to noxious mechanical stimulation. *P < 0.05.
Immunoreactivity of M2 mAchR and NF-200 in the TG neurons innervating facial skin
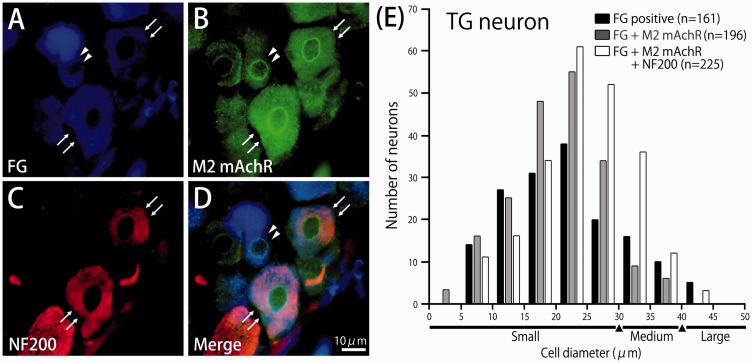
Finally, we examined whether M2 mAchR and neurofilament protein-200 (NF-200) marker for myelinated fiber are expressed in the TG neurons by immunohistochemistry. Two days after FG was injected into the facial skin, TG neurons innervating facial skin were retrogradely labeled (Figure 6(a)). FG-labeled small-diameter TG neurons expressed M2 mAchR immunoreactivity (92%, 181/196) and remaining neurons were medium-diameter TG neurons (8%, 15/196) (Figure 6(b) to (d)). Of the FG-labeled TG neurons, 53% (225/421) also labeled NF-200, myelinated fiber marker (Figure 6(b) to (d)). Figure 6(e) shows a histogram of the cell size spectrum of M2 mAchR- and/or NF-200 positive FG-labeled TG neurons. TG neurons were classified as small diameter (<30 µm), medium diameter (30–40 µm), or large diameter (>40 µm), as described previously.24–26 The FG-labeled TG neurons ranged in size from small to large diameter (9–48 µm; 25.8 ± 7.5 µm, n = 582).The immunoreactivity for M2 mAchR and NF-200 positive FG-labeled TG neurons was not found in large-diameter TG neurons. In the absence of primary antibody, only background staining was evident (data not shown).
Figure 6.
Immunoreactivity of muscarinic acetylcholine M2 receptor (M2 mAchR) and neurofilament protein-200 (NF-200) in the trigeminal ganglion (TG) neurons innervating facial skin. (a) Floresence micrograph showing retrograde FG-labeled TG neurons innervating Facial skin. (b) M2 mAchR immunoreactive TG neurons in the same section. (c) NF-200 immunoreactive TG neurons in the same section. (d) Merged. A typical example of an FG-labeled TG neuron expressing M2 mAchR is indicated by filled triangles. A typical example of FG-labeled TG neurons co-expressing M2 mAchR and NF-200 are indicated by arrows. (e) Histogram showing the cell size spectrum of M2 mAchR and NF-200 positive FG-labeled TG neurons in the trigeminal ganglia.
Discussion
DA treatment-induced short-term and reversible hypoalgesia
Although various pain models have been developed for the trigeminal pain system, we recently used the escape threshold from mechanical stimulation by von Frey hairs to evaluate mechanical hyperalgesia following peripheral inflammation.4,24 Our previous behavioral study showed that inflammation was induced by administration of complete Freund’s adjuvant into the whisker pad24,25 and the threshold of escape from mechanical stimulation applied to the orofacial area in rats with inflammation was significantly lower than in naïve rats. The lowered mechanical threshold in the inflamed rats was returned to control levels by chronic administration of resveratrol.22,24 These results suggest that determination of escape threshold for trigeminal pain can be used to estimate the effect of dietary constituents on pain modulation.22 In this study, we found the following: (1) after local application of DA, the threshold of escape from mechanical stimulation applied to the orofacial area was significantly higher than before DA application; (2) the higher mechanical threshold was observed 3–5 min after DA application and returned to control levels at 10 min; and (3) vehicle treatment had no significant effect on the escape threshold from mechanical stimulation. Taken together, these findings suggest that the dietary constituent, DA, applied to the skin causes a reversible and short-term hypoalgesia.
Mechanism underlying DA treatment-induced hypoalgesia
To further investigate the increased escape threshold from mechanical stimulation induced by local application of DA, we conducted extracellular single unit recordings from SpVc WDR neurons in response to orofacial non-noxious and noxious mechanical stimulation in pentobarbital-anesthetized rats. Orofacial noxious information in the area innervates the trigeminal nerve relay to neurons located in the SpVc and C1–C2 regions and then to higher centers (thalamus and somatosensory cortex). In the SpVc and C1–C2, there are two types of nociceptive neurons, nociceptive-specific (NS) neurons, and WDR neurons, which are classified on their sensitivity to mechanical stimulation applied to the orofacial area, such as the facial skin.27 NS neurons respond only to noxious stimulation (high-threshold mechanical stimulation) of the receptive field, suggesting that NS neurons send encoding stimulus localization to higher centers.27 Since previous studies have demonstrated that WDR neurons in the SpVc region have an important role in the mechanism underlying hyperalgesia and referred pain associated with orofacial pain2,4,5 and also our basic interest was to investigate whether DA treatment affects the neuronal excitability to both non-noxious and noxious stimulation, the focus of the present study was on the effects of DA on SpVc WDR neuronal activity, and we did not examine NS neurons. WDR neurons respond to both noxious (via Aδ- and C-fibers) and non-noxious (via Aβ-fiber) stimulation and have large receptive fields. Graded nociceptive stimulation was applied to the most sensitive area of the receptive field, which exhibited increased firing frequency of SpVc WDR neurons in proportion to stimulus intensity, and many of them also responded to noxious heat stimulation, suggesting that WDR neurons send encoding stimulus intensity to higher centers.28,29
In the present study, we found that the mean firing frequency of SpVc WDR neurons in response to noxious mechanical stimuli was significantly inhibited by local application of DA, and the maximum inhibition of the discharge frequency was seen within 5 min and these inhibitory effects were reversed after approximately 10 min. Bernardini et al.17 demonstrated that M2 mAchRs exert an inhibitory or desensitizing influence on the peripheral terminal of C-nociceptors, possibly by lowering the intracellular cAMP concentration, as well as affecting low-threshold voltage-operated K+ channels which builds up a hyperpolarization force.18 M2 mAchR mRNA is present in the small- and medium-diameter TG neurons,19 while formalin-induced nociceptive behavior is dose-dependently inhibited by M2 mAchR agonists.19 In the present study, we observed that (1) intravenous pretreatment with the muscarinic-specific M2 receptor antagonist, methoctramine, abolished the DA-induced suppression of firing frequency of SpVc WDR neurons in response to noxious stimulation: (2) FG-labeled small diameter TG neurons expressed M2 mAchR immunoreactivity (92%) and remaining neurons were medium-diameter TG neurons (7%). (3) Of the FG-labeled TG neurons, 53% also labeled NF-200 myelinated fiber marker. Taken together, it can be assumed that acute administration of DA intensifies mechanical hypoalgesia and this effect is mainly due to the suppression of excitability of the SpVc WDR neurons via M2 mAchRs expressed in the small- and medium-diameter TG neurons innervating facial skin, inducing desensitization, as described above. As we tested the nociceptive SpVc WDR neurons (secondary), and not the TG (primary) neurons, further studies are needed to clarify whether DA also attenuates the noxious stimulus-induced discharges of the Aδ- and C-TG neurons via the M2 receptor signaling pathway.
Although there is no report for rapid analgesia through peripheral peroxisome proliferator-activated receptor gamma (PPARγ),30 recently it has been reported that PPARγ agonist dose-dependently decreased mechanical hypersensitivity31 and PPARγ is emerging as a new pharmacotherapeutic target for chronic pain.32 Malapaka et al.33 also demonstrated that DA, a 10-carbon fatty acid and a major component of medium chain triglycerideoils, is a direct ligand of PPARγ. Taken together, therefore, it can be assumed that DA inhibits the excitability of nociceptive nerve terminals via PPARγ. Further study needed to elucidate this possibility.
Functional significance for DA treatment-induced hypoalgesia
Recent reports have described the use of CAMs for the treatment of persistent clinical chronic pain,6–8 and the potential effects of diet on conditions associated with pain have been the focus of considerable research.9–11 We have also reported that local administration of the dietary constituent, resveratrol, into the peripheral receptive field suppresses the excitability of SpVc WDR neurons, and may act in a manner similar to injection of a local anesthetic agent, providing relief from trigeminal nociceptive pain without side effects.12
In this study, we made original ointment for the application of DA to the natural cutaneous tissue and we found that in the absence of inflammatory/neuropathic conditions acute administration of the dietary constituent, DA, induced mechanical hypoalgesia, and this inhibitory effect was mainly due to the suppression of excitability of the SpVc WDR neurons via the inhibition of peripheral M2 receptors, possibly via TG neurons. Therefore, it can be speculated that ointment including DA may effectively reduce clinical pain, such as a treatment introduced by injection (i.e., at the time of blood sampling) and post herpatic neuralgia. However, further study need to clarify this possibility. These findings support the idea that DA is a potential therapeutic agent and CAM for treatment related to trigeminal nociception, in the absence of inflammatory/neuropathic conditions, and for the development of a functional analgesic in food.
Conclusion
The present study provides evidence that acute application of ointment containing DA evokes mechanical hypoalgesia and this effect is mainly due to suppression of the excitability of SpVc WDR neurons via the peripheral M2 receptor signaling pathway. These findings support the idea that DA is a potential therapeutic CAM for the treatment of trigeminal nociception, in the absence of inflammatory/neuropathic conditions.
Authors’ contributions
YN, NM, KS, and ST performed the behavioral, electrophysiological, and histological experiments. ST and YS performed immunohistochemical experiments. YA and YS interpreted the data and helped finalize the manuscript. MT participated in the design of the present study and wrote the manuscript. YN and NM contributed equally to this work.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity and their clinical correlates. Crit Rev Oral Biol Med 2000; 11: 57–91. [DOI] [PubMed] [Google Scholar]
- 2.Takeda M, Takahashi M, Mastumoto S. Suppression of neurokinin-1 receptor in trigeminal ganglia attenuates central sensitization following inflammation. J Peripher Nerv Syst 2012; 17: 169–181. [DOI] [PubMed] [Google Scholar]
- 3.Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci 2002; 5(Suppl): 1062–1067. [DOI] [PubMed] [Google Scholar]
- 4.Takeda M, Tanimoto T, Ito M, et al. Role of capsaicin-sensitive afferent inputs from the masseter muscle in the C1 spinal neurons responding to tooth-pulp stimulation in rats. Exp Brain Res 2005; 16: 107–117. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa T, Takeda M, Tanimoto T, et al. Convergence of nociceptive information from temporomandibular joint and tooth-pulp afferents on C1 neurons in the rat. Life Sci 2004; 75: 1465–1478. [DOI] [PubMed] [Google Scholar]
- 6.Rao J, Mihaliak K, Kroenke K, et al. Use of complementary therapies for arthritis among patients of rheumatologists. Ann Intern Med 1999; 131: 409–416. [DOI] [PubMed] [Google Scholar]
- 7.Konvicka JJ, Meyer TA, McDavid AJ, et al. Complementary/alternative medicine use among chronic pain clinic patients. J Perianesth Nurs 2008; 23: 17–23. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg EI, Genao I, Chen I, et al. Complementary and alternative medicine use by primary care patients with chronic pain. Pain Med 2008; 9: 1065–1072. [DOI] [PubMed] [Google Scholar]
- 9.Shir Y, Raja SN, Weissman CS, et al. Consumption of soy diet before nerve injury preempts the development of neuropathic pain in rats. Anesthesiology 2001; 95: 1238–1244. [DOI] [PubMed] [Google Scholar]
- 10.Ernest E. Complementary medicine. Curr Opin Rheumatol 2003; 15: 151–155. [DOI] [PubMed] [Google Scholar]
- 11.Tall JM, Raja SN. Dietary constituents as novel therapeutics for pain. Clin J Pain 2004; 20: 19–26. [DOI] [PubMed] [Google Scholar]
- 12.Shimazu Y, Shibuya E, Takehana S, et al. Local administration of resveratrol inhibits the excitability of nociceptive wide-dynamic range neurons in the rat spinal trigeminal nucleus caudalis. Brain Res Bull 2016; 124: 262–268. [DOI] [PubMed] [Google Scholar]
- 13.Gwynne RM, Thomas EA, Goh SM, et al. Segmentation induced by intraluminal fatty acid in isolated guinea-pig duodenum and jejenum. J Physiol 2004; 556: 557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wess J, Duttaroy A, Gomeza J, et al. Muscarinic receptor subtypes mediating central and peripheral antinociception studied with muscarinic receptor knockout mice: a review. Life Sci 2003; 72: 2047–2054. [DOI] [PubMed] [Google Scholar]
- 15.Caulfield M, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 1998; 50: 279–290. [PubMed] [Google Scholar]
- 16.Felder CC. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB J 1995; 9: 619–625. [PubMed] [Google Scholar]
- 17.Bernardini N, Sauer SK, Haberberger R, et al. Excitatory nicotinic and desensitizing muscarinic (M2) effects on C-nociceptors in isolated rat skin. J Neurosci 2001; 21: 3295–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan ZZ, Williams JT. Muscarine hyperpolarizes a subpopulation of neurons by activating an M2 muscarinic receptor in rat nucleus raphe magnus in vitro. J Neurosci 1994; 14: 1332–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dussor GO, Helesic G, Hargreaves KM, et al. Cholinergic modulation of nociceptive responses in vivo and neuropeptide release in vitro at the level of primary sensory neuron. Pain 2004; 107: 22–32. [DOI] [PubMed] [Google Scholar]
- 20.Jeong SG, Choi IS, Cho JH, et al. Cholinergic modulation of primary afferent glutamatergic transmission in rat medullary dorsal horn neurons. Neuropharmacology 2013; 75: 295–303. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 22.Sekiguchi K, Takehana S, Shibuya E, et al. Resveratrol attenuates inflammation-induced hyperexcitability of trigeminal spinal nucleus caudalis neurons associated with hyperalgesia in rats. Mol Pain 2016; 12: pii: 1744806916643082. doi:10.1177/1744806916643082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 2nd ed New York, NY: Academic Press, 1986. [Google Scholar]
- 24.Takeda M, Takahashi M, Kitagawa J, et al. Brain-derived neurotrophic factor enhances the excitability of small-diameter trigeminal ganglion neurons projecting to the trigeminal nucleus interpolaris/caudalis transition zone following masseter muscle inflammation. Mol Pain 2013; 9: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda M, Tanimoto T, Ikeda M, et al. Temporomandibular joint inflammation potentiates the excitability of trigeminal root ganglion neurons innervating the facial skin in rats. J Neurophysiol 2005; 93: 2723–2738. [DOI] [PubMed] [Google Scholar]
- 26.Takeda M, Tanimoto T, Kadoi J, et al. Enhanced excitability of trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain 2007; 129: 155–166. [DOI] [PubMed] [Google Scholar]
- 27.Sessle BJ. Neural mechanisms and pathways in craniofacial pain. Can J Neurol Sci 1999; 3: S7–S11. [DOI] [PubMed] [Google Scholar]
- 28.Takeda M, Matsumoto S, Sessle BJ, et al. Peripheral and central mechanisms of trigeminal neuropathic pain and inflammatory pain. J Oral Biosci 2011; 53: 318–329. [Google Scholar]
- 29.Iwata K, Imai T, Tsuboi Y, et al. Alteration of medullary dorsal horn neuronal activity following inferior alveolar nerve transection in rats. J Neurophysiol 2001; 86: 2868–2877. [DOI] [PubMed] [Google Scholar]
- 30.Fehrenbacher JC, LoVerme J, Clarke W, et al. Rapid Pain modulation with nuclear receptor ligands. Brain Res Rev 2009; 60: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Churi SB, Abdel-Aleem OS, Tumber KK, et al. Intrathecal rosiglitazone acts at peroxisome proliferator-activated receptor-gamma to rapidly inhibit neuropathic pain in rats. J Pain 2008; 9: 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgenweck J, Griggs RB, Donahue RR, et al. PPARγ activation blocks development and reduces established neuropathic pain in rats. Neuropharmacol 2003; 70: 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malapaka RRV, Khoo S, Zhnag J, et al. Identification and mechanism of 10-carbon fatty acids as modulating ligand of peroxidase ploliferator-activated receptors. J Biol Chem 2012; 287: 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]