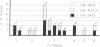Abstract
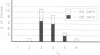
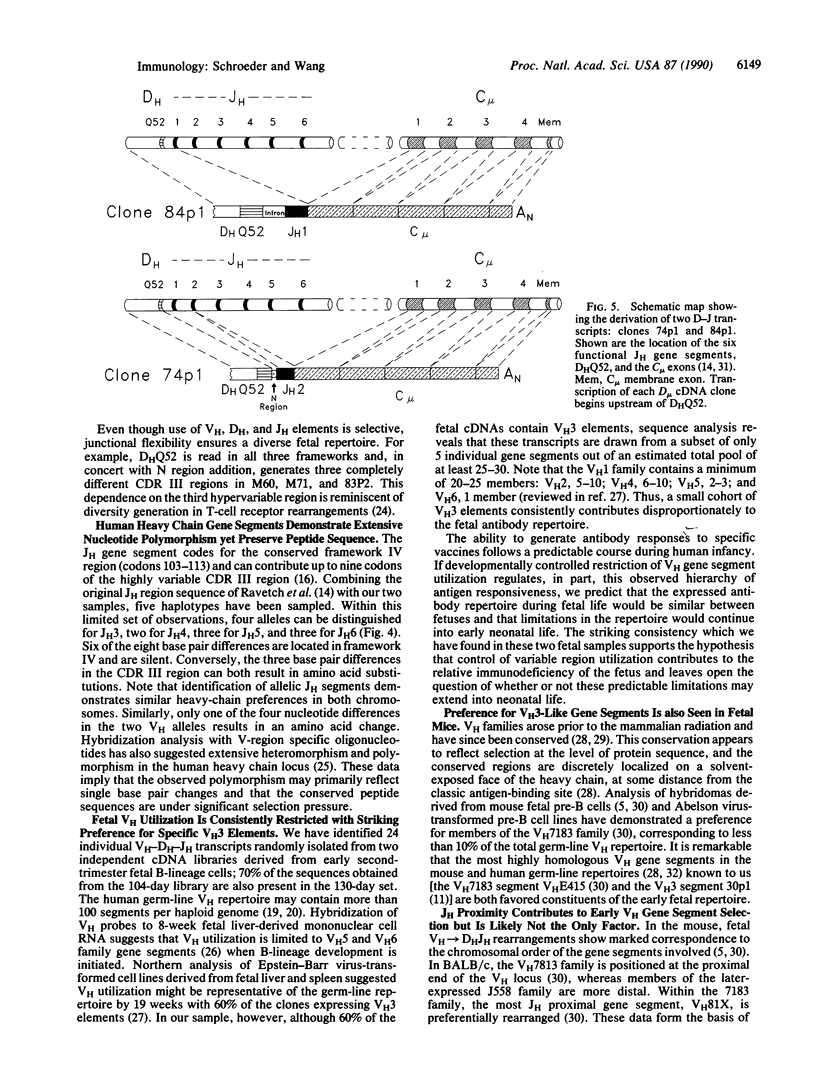
The ability to respond to specific antigens develops in a programmed fashion. Although the antibody repertoire in adults is presumably generated by stochastic combinatorial joining of rearranged heavy variable, diversity, and joining (VH-DH-JH) and light (VL-JL) chains, experimental evidence in the mouse has shown nonrandom utilization of variable gene segments during ontogeny and in response to specific antigens. In this study, we have performed sequence analysis of 104-day human fetal liver-derived, randomly isolated constant region C+ mu transcripts and demonstrate a consistent preference during fetal life for a small subset of three highly conserved VH3 family gene segments. In addition, the data show that this preferential gene segment utilization extends to the DHQ52 and the JH3 and JH4 loci. Sequence analysis of two "sterile" DH-JH transcripts suggests that transcriptional activation of the JH-proximal DHQ52 element may precede initiation of DH-JH rearrangement and influence fetal DH utilization. Sequence comparisons reveal striking nucleotide polymorphism in allelic gene segments which is poorly reflected in the peptide sequence, implying considerable evolutionary selection pressure. Although vertebrate species utilize a variety of strategies to generate their antibody repertoire, preferential utilization of VH3 elements is consistently found during early development. These data support the hypothesis that VH3 gene segments play an essential role in the development of the immune response.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Baumann B., Potash M. J., Köhler G. Consequences of frameshift mutations at the immunoglobulin heavy chain locus of the mouse. EMBO J. 1985 Feb;4(2):351–359. doi: 10.1002/j.1460-2075.1985.tb03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein K. E., Reddy E. P., Alexander C. B., Mage R. G. A cDNA sequence encoding a rabbit heavy chain variable region of the VHa2 allotype showing homologies with human heavy chain sequences. Nature. 1982 Nov 4;300(5887):74–76. doi: 10.1038/300074a0. [DOI] [PubMed] [Google Scholar]
- Bos N. A., Meeuwsen C. G. B cell repertoire in adult antigen-free and conventional neonatal BALB/c mice. I. Preferential utilization of the CH-proximal VH gene family PC7183. Eur J Immunol. 1989 Oct;19(10):1811–1815. doi: 10.1002/eji.1830191008. [DOI] [PubMed] [Google Scholar]
- Buluwela L., Albertson D. G., Sherrington P., Rabbitts P. H., Spurr N., Rabbitts T. H. The use of chromosomal translocations to study human immunoglobulin gene organization: mapping DH segments within 35 kb of the C mu gene and identification of a new DH locus. EMBO J. 1988 Jul;7(7):2003–2010. doi: 10.1002/j.1460-2075.1988.tb03039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buluwela L., Rabbitts T. H. A VH gene is located within 95 Kb of the human immunoglobulin heavy chain constant region genes. Eur J Immunol. 1988 Nov;18(11):1843–1845. doi: 10.1002/eji.1830181130. [DOI] [PubMed] [Google Scholar]
- Christoph T., Krawinkel U. Physical linkage of variable, diversity and joining gene segments in the immunoglobulin heavy chain locus of the mouse. Eur J Immunol. 1989 Aug;19(8):1521–1523. doi: 10.1002/eji.1830190829. [DOI] [PubMed] [Google Scholar]
- Cooper M. D. Pre-B cells; normal and abnormal development. J Clin Immunol. 1981 Apr;1(2):81–89. doi: 10.1007/BF00915383. [DOI] [PubMed] [Google Scholar]
- Cuisinier A. M., Guigou V., Boubli L., Fougereau M., Tonnelle C. Preferential expression of VH5 and VH6 immunoglobulin genes in early human B-cell ontogeny. Scand J Immunol. 1989 Oct;30(4):493–497. doi: 10.1111/j.1365-3083.1989.tb02455.x. [DOI] [PubMed] [Google Scholar]
- Currier S. J., Gallarda J. L., Knight K. L. Partial molecular genetic map of the rabbit VH chromosomal region. J Immunol. 1988 Mar 1;140(5):1651–1659. [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Dersimonian H., Schwartz R. S., Barrett K. J., Stollar B. D. Relationship of human variable region heavy chain germ-line genes to genes encoding anti-DNA autoantibodies. J Immunol. 1987 Oct 1;139(7):2496–2501. [PubMed] [Google Scholar]
- Humphries C. G., Shen A., Kuziel W. A., Capra J. D., Blattner F. R., Tucker P. W. A new human immunoglobulin VH family preferentially rearranged in immature B-cell tumours. Nature. 1988 Feb 4;331(6155):446–449. doi: 10.1038/331446a0. [DOI] [PubMed] [Google Scholar]
- Ichihara Y., Abe M., Yasui H., Matsuoka H., Kurosawa Y. At least five DH genes of human immunoglobulin heavy chains are encoded in 9-kilobase DNA fragments. Eur J Immunol. 1988 Apr;18(4):649–652. doi: 10.1002/eji.1830180426. [DOI] [PubMed] [Google Scholar]
- Jeong H. D., Teale J. M. Comparison of the fetal and adult functional B cell repertoires by analysis of VH gene family expression. J Exp Med. 1988 Aug 1;168(2):589–603. doi: 10.1084/jem.168.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Vakil M., Solvason N. The role of idiotypic interactions and B-cell subsets in development of the B-cell repertoire. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):203–207. doi: 10.1101/sqb.1989.054.01.025. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Perry R. P. Transcriptional and posttranscriptional control of immunoglobulin mRNA production during B lymphocyte development. Nucleic Acids Res. 1986 Jul 11;14(13):5431–5447. doi: 10.1093/nar/14.13.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. E., Wiedemann L. M., Pittet A. C., Strauss S., Nelson K. J., Davis J., Van Ness B., Perry R. P. Nonproductive kappa immunoglobulin genes: recombinational abnormalities and other lesions affecting transcription, RNA processing, turnover, and translation. Mol Cell Biol. 1985 Jul;5(7):1660–1675. doi: 10.1128/mcb.5.7.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawinkel U., Christoph T., Blankenstein T. Organization of the Ig VH locus in mice and humans. Immunol Today. 1989 Oct;10(10):339–344. doi: 10.1016/0167-5699(89)90191-6. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Matsuda F., Kinashi T., Kodaira M., Honjo T. A novel family of variable region genes of the human immunoglobulin heavy chain. J Mol Biol. 1987 Jun 20;195(4):761–768. doi: 10.1016/0022-2836(87)90482-7. [DOI] [PubMed] [Google Scholar]
- Litman G. W., Berger L., Murphy K., Litman R., Hinds K., Erickson B. W. Immunoglobulin VH gene structure and diversity in Heterodontus, a phylogenetically primitive shark. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2082–2086. doi: 10.1073/pnas.82.7.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logtenberg T., Schutte M. E., Inghirami G., Berman J. E., Gmelig-Meyling F. H., Insel R. A., Knowles D. M., Alt F. W. Immunoglobulin VH gene expression in human B cell lines and tumors: biased VH gene expression in chronic lymphocytic leukemia. Int Immunol. 1989;1(4):362–366. doi: 10.1093/intimm/1.4.362. [DOI] [PubMed] [Google Scholar]
- Nickerson K. G., Berman J., Glickman E., Chess L., Alt F. W. Early human IgH gene assembly in Epstein-Barr virus-transformed fetal B cell lines. Preferential utilization of the most JH-proximal D segment (DQ52) and two unusual VH-related rearrangements. J Exp Med. 1989 Apr 1;169(4):1391–1403. doi: 10.1084/jem.169.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J., Wu G. E. The B cell repertoire. FASEB J. 1989 May;3(7):1818–1824. doi: 10.1096/fasebj.3.7.2497040. [DOI] [PubMed] [Google Scholar]
- Parvari R., Avivi A., Lentner F., Ziv E., Tel-Or S., Burstein Y., Schechter I. Chicken immunoglobulin gamma-heavy chains: limited VH gene repertoire, combinatorial diversification by D gene segments and evolution of the heavy chain locus. EMBO J. 1988 Mar;7(3):739–744. doi: 10.1002/j.1460-2075.1988.tb02870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter R. M., Kearney J. F., Chang S. P., Hood L. E. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985 Mar 29;227(4694):1597–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H., Forster A., Milstein C. P. Human immunoglobulin heavy chain genes: evolutionary comparisons of C mu, C delta and C gamma genes and associated switch sequences. Nucleic Acids Res. 1981 Sep 25;9(18):4509–4524. doi: 10.1093/nar/9.18.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Sanz I., Dang H., Takei M., Talal N., Capra J. D. VH sequence of a human anti-Sm autoantibody. Evidence that autoantibodies can be unmutated copies of germline genes. J Immunol. 1989 Feb 1;142(3):883–887. [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Early restriction of the human antibody repertoire. Science. 1987 Nov 6;238(4828):791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Structure and evolution of mammalian VH families. Int Immunol. 1990;2(1):41–50. doi: 10.1093/intimm/2.1.41. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Walter M. A., Hofker M. H., Ebens A., Willems van Dijk K., Liao L. C., Cox D. W., Milner E. C., Perlmutter R. M. Physical linkage of a human immunoglobulin heavy chain variable region gene segment to diversity and joining region elements. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8196–8200. doi: 10.1073/pnas.85.21.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H., Maeda T., Tani Y., Miyake S., Oka Y., Komori T., Ogawa H., Soma T., Minami Y., Sakato N. Selective use of the VHQ52 family in functional VH to DJH rearrangements in a B precursor cell line. J Exp Med. 1987 Aug 1;166(2):607–612. doi: 10.1084/jem.166.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Tutter A., Riblet R. Conservation of an immunoglobulin variable-region gene family indicates a specific, noncoding function. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7460–7464. doi: 10.1073/pnas.86.19.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems van Dijk K., Schroeder H. W., Jr, Perlmutter R. M., Milner E. C. Heterogeneity in the human Ig VH locus. J Immunol. 1989 Apr 1;142(7):2547–2554. [PubMed] [Google Scholar]
- Wu G. E., Paige C. J. VH gene family utilization in colonies derived from B and pre-B cells detected by the RNA colony blot assay. EMBO J. 1986 Dec 20;5(13):3475–3481. doi: 10.1002/j.1460-2075.1986.tb04672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]