Abstract
OBJECTIVE
To investigate whether the use of a belladonna and opium (B&O) rectal suppository administered immediately before ureteroscopy (URS) and stent placement could reduce stent-related discomfort.
METHODS
A randomized, double-blinded, placebo-controlled study was performed from August 2013 to December 2014. Seventy-one subjects were enrolled and randomized to receive a B&O (15 mg/30 mg) or a placebo suppository after induction of general anesthesia immediately before URS and stent placement. Baseline urinary symptoms were assessed using the American Urological Association Symptom Score (AUASS). The Ureteral Stent Symptom Questionnaire and AUASS were completed on postoperative days (POD) 1, 3, and after stent removal. Analgesic use intra-operatively, in the recovery unit, and at home was recorded.
RESULTS
Of the 71 subjects, 65 had treatment for ureteral (41%) and renal (61%) calculi, 4 for renal urothelial carcinoma, and 2 were excluded for no stent placed. By POD3, the B&O group reported a higher mean global quality of life (QOL) score (P = .04), a better mean quality of work score (P = .05), and less pain with urination (P = .03). The B&O group reported an improved AUASS QOL when comparing POD1 with post-stent removal (P = .04). There was no difference in analgesic use among groups (P = .67). There were no episodes of urinary retention. Age was associated with unplanned emergency visits (P <.00) and “high-pain” measure (P = .02)
CONCLUSION
B&O suppository administered preoperatively improved QOL measures and reduced urinary-related pain after URS with stent. Younger age was associated with severe stent pain and unplanned hospital visits.
Ureteral stent placement is one of the most commonly performed urologic procedures and is used in a variety of clinical settings.1 Although the use of ureteral stents is commonplace, it is associated with significant morbidity and cost.2–4 The majority of patients report significant pain, lower urinary tract symptoms, and bother.3 Fourteen percent of patients following endoscopic procedures for nephrolithiasis present to the emergency room, with the most common symptom being pain. Furthermore, acute stent-related symptoms may be misdiagnosed as urinary tract infections, leading to unnecessary antibiotic use.5
The National Institutes of Health called for proposals on how to mitigate stent symptoms in 2015 to address this widely recognized burden. Prior strategies to reduce stent discomfort have focused on pharmacological intervention in addition to manipulating stent characteristics. To date, there is no clear data to prove that altering stent design improves discomfort.4,6–12 Among several medications investigated, only alpha-antagonists have been demonstrated to consistently improve symptoms in randomized trials.13–18 Despite their use, patients still report significant stent-related symptoms and bother, and additional investigation continues.
Belladonna and opium (B&O) rectal suppositories are used for refractory bladder pain and have been shown to reduce postoperative morphine use in patients undergoing radical prostatectomy.19 The pharmacologically active substances in the belladonna extract consist of atropine and scopolamine. Atropine is a potent parasympatholytic and induces smooth-muscle relaxation. Opium is a 20-alkaloid compound that derives the majority of its effect from its morphine content and acts as a narcotic analgesic by increasing the pain threshold. A B&O suppository is locally absorbed and may affect the posterior bladder wall, trigone, and distal ureter. The purpose of this study was to determine whether a B&O suppository administered before manipulation or nociceptive input during ureteroscopy (URS) would reduce postoperative symptoms and improve quality of life (QOL).
METHODS
Study Design
A prospective, single-center, randomized, double-blinded, placebo-controlled study investigating the use of a single B&O suppository (16.2 mg/30 mg) vs placebo suppository given immediately before URS and stent placement was performed at the University of Washington. Placebo suppositories were obtained from a local compounding pharmacy (Key Compounding, Federal Way, WA) composed of MKB fatty acids. This was a registered clinical trial and was approved by the local Institutional Review Board (#44862).
Participants
Individuals who presented to a single provider’s outpatient clinic were screened for recruitment. Eligible participants were ≥18 of age with a planned URS procedure. Exclusion criteria included presence of neurologic disorder (spinal cord injury, multiple sclerosis, spina bifida), allergy to any component of the B&O suppository, surgically altered rectal anatomy, or use of B&O suppository within 1 week of anticipated surgery. Informed consent was obtained before randomization. The institution’s Investigational Drug Service performed block randomization with subject, surgeon, and research team blinded to treatment arm assignment until study conclusion.
Surgical Procedure
Following induction of general anesthesia and before surgical preparation, the circulating nurse administered the study drug (placebo or B&O suppository) without any members of the surgery team present in the operating room. Ureteroscopic laser lithotripsy was performed primarily using a dusting technique with a holmium yttrium-aluminum-garnet laser. The 4 subjects with upper tract urothelial carcinoma were also treated using a holmium laser. A pressurized bag of normal saline irrigation was judiciously used for minimal irrigation. It is common practice to place a 12 French Foley catheter alongside the ureteroscope based on stone volume. After URS was completed, a 6 French Cook Universa Firm Ureteral Stent (polyurethane) was placed and its length was chosen based on surgeon discretion. No subject received a ureteral access sheath or required ureteral balloon dilation. Subjects were prescribed tamsulosin (0.4 mg; #30), docusate (250 mg; #30), and hydrocodone-acetaminophen (5:325 mg; #20). Cystoscopy with stent removal was performed 7–14 days following surgery.
Data Collection
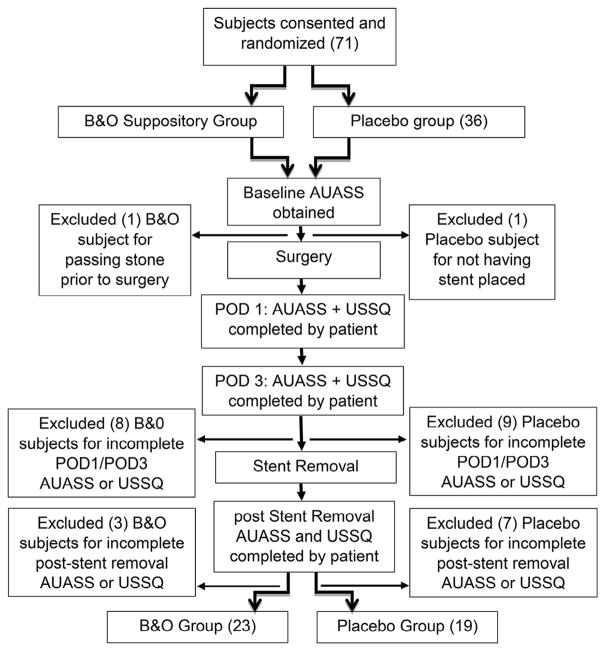
Data were collected by medical record extraction, phone interview, and questionnaires as outlined in Figure 1. Baseline clinical and demographic data included age, gender, medical history, body mass index (BMI), indication for URS, stone size(s) and location(s), and operative details. Baseline urinary symptoms were assessed by the American Urological Association Symptom Score (AUASS) questionnaire. Intraoperative and perioperative medication use was extracted from anesthetic and electronic health records.
Figure 1.
Outline of enrollment and data collection points.
For the follow-up period, the AUASS and the Ureteral Stent Symptom Questionnaire (USSQ)20 were collected on postoperative days (POD) 1, 3, and after stent removal. Stamped addressed envelopes were given to the participants before surgery to return forms. Subjects were telephoned by study staff to maximize the completion rate of forms, and to record any adverse events including side effects and unplanned provider or hospital visits. Subjects were given a medication diary to record narcotic use for POD1–7. Narcotic use was converted to total morphine equivalents.
The USSQ is a validated stent symptom questionnaire that assesses stent symptoms and bother based on 6 different domains. These domains with the possible range of scores include the following: urinary symptoms (11–57), pain (6–80), general health (6–30), work performance (3–15), sexual matters (2–10), and additional problems.
Statistical Analysis
The primary analysis was a comparison of USSQ metrics (pain, urinary bother, general health, work, and sexual satisfaction) between B&O and placebo suppositories. Secondary analyses were performed to compare between the 2 groups’ AUASS, narcotic use, postoperative complications, and unanticipated hospital visits.
The study was powered to detect a minimum of 15% difference in USSQ urinary symptom score.15,18 To detect a difference of this magnitude, with a power of 80% and a significance level of 5%, we calculated that 60 subjects with 30 in each arm were needed. Assuming 15% loss to follow-up, we planned for 70 subjects.
Univariate association of treatment arms with AUASS and USSQ scores at each time point (baseline, POD1, POD3, and post stent) was compared using the Student t test. Paired Student t tests were used to compare change in scores between 2 time points by treatment arm. Multivariate logistic regression was used to estimate the association of outcomes with treatment arm after adjusting for a priori defined variables: age, weight, operative time, and ketorolac use (yes or no). In addition, the postoperative analgesic use was assessed in the multivariate models estimating the treatment effects on the USSQ or AUASS values. Analyses were conducted using STATA software, version 13 (Stata Inc, College Station, TX).
RESULTS
Subject Characteristics
Seventy-one participants were enrolled and randomized from August 2013 to December 2014. Two were excluded because of not having a stent placed (n = 1) and passing the stone immediately before URS (n = 1). Participant characteristics and operative data are listed in Table 1. Of the 69 subjects, 65 were treated for a combination of ureteral (29; 41%) and renal (43; 61%) calculi and 4 were treated for upper tract urothelial cell carcinoma within the renal pelvis. No subjects had chronic indwelling stents. There was no difference in age, gender, BMI, baseline AUASS, operative time, stone burden, and time to stent removal between groups (Table 1).
Table 1.
Subject demographics by treatment group
| Patient Demographics | Placebo (n = 34) | B&O (n = 35) | P Value |
|---|---|---|---|
| Age (y, mean ± STD) | 50 ± 16 | 55 ± 13 | .15 |
| Female (n, %) | 19 (56%) | 17 (49%) | .63 |
| Male (n, %) | 15 (44%) | 18 (51%) | |
| BMI (kg/m2, mean ± STD) | 30 ± 7 | 32 ± 8 .27 | |
| Operative time (min, mean ± STD) | 54 ± 24 | 54 ± 23 | 1.0 |
| Ureteral stone burden (mm, mean ± STD) | 8 ±3 | 7± 2 | .11 |
| Renal stone burden (mm, mean ± STD) | 11 ± 7 | 12 ± 6 | .52 |
| Time to stent removal (d, mean ± STD) | 12 ± 10 | 14 ± 15 | .52 |
B&O, belladonna and opium; BMI, body mass index.
Ureteral Stent Symptom Questionnaire
The USSQ was collected for 75% of the study population at POD1 and POD3 and 57% at post-stent removal (Fig. 1). On POD1, there was no statistically significant difference in the USSQ domains between groups although mean global QOL score was in favor of the B&O group (P = .08). On POD3, the B&O group reported a better mean quality of work score (P = .05) and a higher mean global QOL score (P = .04, Table 2). Following stent removal, the mean global QOL score remained in favor of the B&O group with a trend toward significance (P = .06). The change in the overall pain score domain from POD1 to 3 was more rapid in the B&O as compared with the placebo group (6.9 ± 2.7 vs 3.1 ± 1.7 respectively); however, after adjustment, this difference was not significant (P = .11).
Table 2.
USSQ metrics for placebo vs B&O groups at POD 1, 3, and post-stent removal
| POD 1 | POD 3 | Post-stent | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Placebo | B&O | P Value | Placebo | B&O | P Value | Placebo | B&O | P Value | |
| No. of patients | 26 | 25 | 26 | 25 | 19 | 23 | |||
| Urinary Index Score (mean ± STD) | 32 ± 7.0 | 34 ± 7.0 | .19 | 29 ± 6.0 | 28 ± 8.0 | .3 | 19 ± 5.6 | 18 ± 5.8 | .65 |
| Pain Index Score (mean ± STD) | 26.4 ± 12.7 | 28.7 ± 3.2 | .57 | 23.3 ± 10.9 | 20.9 ± 14.9 | .51 | 7.2 ± 11.7 | 5.4 ± 9.0 | .60 |
| General Health Index | 17 ± 5.0 | 15 ± 5.0 | .24 | 16 ± 4.0 | 14 ± 5.0 | .08 | 11 ± 4.0 | 10 ± 8.0 | .15 |
| Score (mean ± STD) | |||||||||
| Work Performance | 8.0 ± 3.0 | 6.0 ± 3.0 | .25 | 8.5 ± 1.5 | 6.7 ± 2.8 | .05 | 4.8 ± 1.9 | 5.8 ± 2.5 | .4 |
| Score: Quality of work domain (mean ± STD) | |||||||||
| Global quality of life | 5.4 ± 1.2 | 4.4 ± 0.6 | .08 | 5.6 ± 1.3 | 4.5 ± 1.4 | .04 | 5.2 ± 1.2 | 3.9 ± 1.6 | .06 |
| score (mean ± STD) | |||||||||
POD, postoperative day; USSQ, Ureteral Stent Symptom Questionnaire; other abbreviations as in Table 1.
Boldfaced values indicate statistical significance.
When the specific questions within the pain domain were compared at POD3, the placebo group showed higher levels of pain or discomfort when passing urine, with 66.7% reporting “sometimes” to “all of the time” vs only 36.0% in the B&O group reporting this level of discomfort (adjusted P = .03). A non significant trend toward lower levels of pain and discomfort in the B&O group at POD3 for all other specific pain domain questions was observed (data not shown).
AUA Symptom Score
There were no differences between groups in the AUASS metrics at baseline, on POD1, 3, and following stent removal (Table 3). The overall preoperative AUASS QOL domain score was “mixed” (mean score 3.2, standard deviation [SD] 2.0) and increased to “mostly dissatisfied” (mean score 4.0, SD 1.8) at POD1. At stent removal, this measure had decreased to “mostly satisfied” (mean score 1.9, SD 1.6). Comparing the change in QOL metric from baseline with post-stent removal, we found that there was a significant improvement in favor of receiving a B&O suppository (mean change of −2.1, SD 2.1) compared with placebo (mean change of −1.4, SD 2.2) after adjusting for age, BMI, and gender (P = .04).
Table 3.
AUASS and QOL metrics for placebo vs B&O treatment group at baseline, POD 1, 3, and post-stent removal
| Placebo | B&O | P Value | |||
|---|---|---|---|---|---|
|
|
|
||||
| No. of Subjects | Mean ± STD | No. of Subjects | Mean ± STD | ||
| Preoperative baseline | |||||
| Total AUASS | 34 | 10.6 ± 7.5 | 33 | 11.5 ± 5.5 | .61 |
| Quality of life | 3.1 ± 2.2 | 3.3 ± 1.8 | .66 | ||
| POD 1 | |||||
| Total AUASS | 27 | 11.5 ± 7.6 | 26 | 13.8 ± 6.5 | .23 |
| Quality of life | 3.8 ± 2.0 | 4.2 ± 1.5 | .54 | ||
| POD 3 | |||||
| Total AUASS | 27 | 11.2 ± 7.8 | 26 | 14.0 ± 6.9 | .17 |
| Quality of life | 3.9 ± 2.0 | 3.9 ± 1.6 | .89 | ||
| Post-stent removal | |||||
| Total AUASS | 19 | 7.6 ± 7.0 | 21 | 9.8 ± 5.2 | .25 |
| Quality of life | 2.2 ± 2.1 | 1.6 ± 1.1 | .29 | ||
Analgesic Use
There were no significant differences in narcotic administration between groups intraoperatively (P = .26) or in the postanesthesia care unit (P = .34, Table S1). There was also no difference in self-reported cumulative narcotic use between groups on POD1 (P = .39) or on POD3 (P = .674).
A combined dichotomous “high-pain” measure combining extremely high postoperative analgesic use (above 90% percentile) or highest reported levels of pain on the USSQ for POD1 (above 95% percentile) showed no difference between treatment arms, with each group having 9% classified as “high pain.” The mean age of “high-pain” subjects was 45.6 (STD 3.6) years as compared with the mean age of 55.2 (STD 1.9) years for those not experiencing high pain. Younger age was significantly associated with the “high-pain” measure (P = .02) after adjusting for treatment arm.
Adverse Events
There were no study drug-related complications. Specifically, there were no episodes of urinary retention. There were a total of 8 (11%) emergency room (ER) visits: 3 in the B&O group and 5 in the placebo group. There were no hospital admissions or unplanned procedures. A post hoc analysis was performed, and the mean age of subjects who went to the ER was 39.0 (STD 5.1) as compared with 54.5 (STD 1.8) for those who did not. Younger age was significantly associated with visits to the ER (P = .006) after adjusting for treatment arm.
DISCUSSION
B&O rectal suppository use before the manipulation of URS and stent placement results in significantly improved QOL, quality of work, and reduction of pain with urination by day 3 compared with placebo. The B&O group also showed a greater improvement in the AUAQOL metric from pre-to post-stent removal. QOL measures were also in favor of the B&O group POD1 without statistical significance. There were no episodes of urinary retention, and rates of unanticipated provider visits after surgery were similar in both groups. These results support the use of a single preoperative B&O suppository before stent placement with URS.
The immediate preoperative administration was novel in this setting, and our hypothesis centered on the nociceptive theory that the preemptive treatment of pain before manipulation would result in improvement in urinary, pain scores, and QOL. Periureteral anesthetic injections have been tried at the time of stent placement in a small study without clear benefit in pain measures.20 An improvement in various QOL measures was demonstrated in our study. QOL has become an increasingly recognized important outcome and is clearly a significant component to medical care.21 Recurrent stone formers with prior severe stent discomfort may choose therapy based on the expectations of having a stent. The USSQ QOL score assesses the willingness to receive another stent. Subjects who received a B&O overall felt that the stent was more tolerable than did those who received the placebo. This preemptive treatment of stent pain should be kept in mind when conducting further studies.
Prior studies seeking to ameliorate stent discomfort have investigated drug-eluting stents and altering stent composition, length, diameter, and shape to improve symptoms.4,6–12,22,23 Notably, subjects with stents that cross the trigone reported worse urinary symptoms based on AUASS and a nonvalidated questionnaire.11 Whereas no differences in symptoms were seen when comparing a 4.6 with a 6 Fr stent, smaller diameter stents had greater rates of distal migration.9
Pharmacologic interventions have focused on medications that target lower urinary tract symptoms. Anticholinergics have demonstrated mixed results, whereas alpha-antagonists have demonstrated more consistent benefit.24–26 In randomized trials, both alfuzosin and tamsulosin have shown improvement in urinary symptoms and QOL metrics compared with placebo measured by USSQ and AUASS.13–15,17,18 More recently, a randomized study comparing alpha-antagonist alone with alpha-antagonist plus anticholinergic was reported. The study used similar outcome measures and timing to our study by evaluating the AUASS and USSQ within the first few days. They found no difference between groups but did note that symptoms improved with time.16
The development of a validated USSQ has provided a specific tool to evaluate and measure symptoms and has since been used in a number of studies.27 The USSQ explores 6 areas including urinary symptoms, body pain, general health, work performance, sexual matters, and additional problems. Although the questionnaire has certainly been a powerful tool and critical to analyze the impact of ureteral stents on a number of domains, feedback from participants showed high level of burden in completing it. Many subjects interpreted the pain diagram differently from intended. Future study related to the questionnaire may benefit from developing methods to characterize the intensity of symptoms during the first few days, when symptoms are greatest.
Despite these efforts, a challenge for the urology community remains on how to improve the QOL of patients with stents. Approximately 80% of patients are affected by bothersome urinary symptoms and experience stent-related pain that affect daily activities, 32% report sexual dysfunction, and 58% report reduced work capacity resulting in days off work or reduced work efficiency.3 A decrease in overall QOL is noted in 45%–80%. Little is known about the risk factors for severe stent pain. In our study, younger age was a risk factor for pain and adverse outcomes following URS and stent. We observed younger age to be associated with postoperative ER visits, highest reported levels of pain, and consuming the most narcotic pain medication. Identifying populations at highest risk for severe stent pain is useful in counseling patients and choosing therapy including weighing risks and benefits of whether to leave a stent after URS. In addition, it can provide some insight into understanding the mechanisms and etiology of post-stent symptoms. In our study, we noted a 20% improvement in USSQ global QOL, a 22% improvement in the USSQ quality of work score, and an 18% reduction in the USSQ pain score with urination by POD3 compared with placebo.
There were limitations in this study. We underestimated the number of subjects who would not complete the time-sensitive USSQ on POD1 and 3. There were a number of additional domains on the USSQ that trended toward significance in favor of the B&O group, which with higher compliance might have reached statistical significance. All subjects were discharged on an alpha-antagonist, which could have blunted our findings because they have been shown to reduce symptoms. Administration of a single dose B&O suppository may have had a short treatment effect, and whether multiple doses or a higher dose after the procedure would improve outcomes requires further study. Despite the limitations, these results show benefit with no measurable adverse effects of a single B&O suppository to patients at risk for postoperative stent discomfort.
CONCLUSION
A single preoperative B&O suppository improves QOL and lessens urinary pain in patients undergoing URS with stent placement. Younger age has been identified as a risk factor for severe stent-related pain and unplanned hospital visits.
Supplementary Material
APPENDIX. Supplementary Data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.urology.2016.07.035.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Auge BK, Sarvis JA, L’Esperance JO, et al. Practice patterns of ureteral stenting after routine ureteroscopic stone surgery: a survey of practicing urologists. J Endourol. 2007;21:1287–1291. doi: 10.1089/end.2007.0038. [DOI] [PubMed] [Google Scholar]
- 2.Pollard SG, Macfarlane R. Symptoms arising from Double-J ureteral stents. J Urol. 1988;139:37–38. doi: 10.1016/s0022-5347(17)42282-8. [DOI] [PubMed] [Google Scholar]
- 3.Joshi HB, Stainthorpe A, MacDonagh RP, et al. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol. 2003;169:1065–1069. doi: 10.1097/01.ju.0000048980.33855.90. discussion 9. [DOI] [PubMed] [Google Scholar]
- 4.Lange D, Bidnur S, Hoag N, et al. Ureteral stent-associated complications—where we are and where we are going. Nat Rev Urol. 2015;12:17–25. doi: 10.1038/nrurol.2014.340. [DOI] [PubMed] [Google Scholar]
- 5.Scales CD, Jr, Saigal CS, Hanley JM, et al. The impact of unplanned postprocedure visits in the management of patients with urinary stones. Surgery. 2014;155:769–775. doi: 10.1016/j.surg.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lennon GM, Thornhill JA, Sweeney PA, et al. “Firm” versus “soft” double pigtail ureteric stents: a randomised blind comparative trial. Eur Urol. 1995;28:1–5. doi: 10.1159/000475010. [DOI] [PubMed] [Google Scholar]
- 7.Liatsikos EN, Karnabatidis D, Kagadis GC, et al. Application of paclitaxel-eluting metal mesh stents within the pig ureter: an experimental study. Eur Urol. 2007;51:217–223. doi: 10.1016/j.eururo.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 8.Krambeck AE, Walsh RS, Denstedt JD, et al. A novel drug eluting ureteral stent: a prospective, randomized, multicenter clinical trial to evaluate the safety and effectiveness of a ketorolac loaded ureteral stent. J Urol. 2010;183:1037–1042. doi: 10.1016/j.juro.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 9.Erturk E, Sessions A, Joseph JV. Impact of ureteral stent diameter on symptoms and tolerability. J Endourol. 2003;17:59–62. doi: 10.1089/08927790360587342. [DOI] [PubMed] [Google Scholar]
- 10.Joshi HB, Chitale SV, Nagarajan M, et al. A prospective randomized single-blind comparison of ureteral stents composed of firm and soft polymer. J Urol. 2005;174:2303–2306. doi: 10.1097/01.ju.0000181815.63998.5f. [DOI] [PubMed] [Google Scholar]
- 11.Al-Kandari AM, Al-Shaiji TF, Shaaban H, et al. Effects of proximal and distal ends of double-J ureteral stent position on postprocedural symptoms and quality of life: a randomized clinical trial. J Endourol. 2007;21:698–702. doi: 10.1089/end.2007.9949. [DOI] [PubMed] [Google Scholar]
- 12.Miyaoka R, Monga M. Ureteral stent discomfort: etiology and management. Indian J Urol. 2009;25:455–460. doi: 10.4103/0970-1591.57910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damiano R, Autorino R, De Sio M, et al. Effect of tamsulosin in preventing ureteral stent-related morbidity: a prospective study. J Endourol. 2008;22:651–656. doi: 10.1089/end.2007.0257. [DOI] [PubMed] [Google Scholar]
- 14.Beddingfield R, Pedro RN, Hinck B, et al. Alfuzosin to relieve ureteral stent discomfort: a prospective, randomized, placebo controlled study. J Urol. 2009;181:170–176. doi: 10.1016/j.juro.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Deliveliotis C, Chrisofos M, Gougousis E, et al. Is there a role for alpha1-blockers in treating double-J stent-related symptoms? Urology. 2006;67:35–39. doi: 10.1016/j.urology.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 16.Sivalingam S, Streeper NM, Sehgal PD, et al. Does combination therapy with Tamsulosin and Tolterodine improve ureteral stent discomfort compared with Tamsulosin alone? A double-blind, randomized, controlled trial. J Urol. 2016;195:385–390. doi: 10.1016/j.juro.2015.08.104. [DOI] [PubMed] [Google Scholar]
- 17.Dellis AE, Keeley FX, Jr, Manolas V, et al. Role of alpha-blockers in the treatment of stent-related symptoms: a prospective randomized control study. Urology. 2014;83:56–61. doi: 10.1016/j.urology.2013.08.067. [DOI] [PubMed] [Google Scholar]
- 18.Wang CJ, Huang SW, Chang CH. Effects of specific alpha-1A/1D blocker on lower urinary tract symptoms due to double-J stent: a prospectively randomized study. Urol Res. 2009;37:147–152. doi: 10.1007/s00240-009-0182-8. [DOI] [PubMed] [Google Scholar]
- 19.Lukasewycz S, Holman M, Kozlowski P, et al. Does a perioperative belladonna and opium suppository improve postoperative pain following robotic assisted laparoscopic radical prostatectomy? Results of a single institution randomized study. Can J Urol. 2010;17:5377–5382. [PubMed] [Google Scholar]
- 20.Sur RL, Haleblian GE, Cantor DA, et al. Efficacy of intravesical ropivacaine injection on urinary symptoms following ureteral stenting: a randomized, controlled study. J Endourol. 2008;22:473–478. doi: 10.1089/end.2007.9847. [DOI] [PubMed] [Google Scholar]
- 21.Sarkissian C, Noble M, Li J, et al. Patient decision making for asymptomatic renal calculi: balancing benefit and risk. Urology. 2013;81:236–240. doi: 10.1016/j.urology.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 22.Lingeman JE, Preminger GM, Goldfischer ER, et al. Assessing the impact of ureteral stent design on patient comfort. J Urol. 2009;181:2581–2587. doi: 10.1016/j.juro.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn MD, Portis AJ, Kahn SA, et al. Clinical effectiveness of new stent design: randomized single-blind comparison of tail and double-pigtail stents. J Endourol. 2000;14:195–202. doi: 10.1089/end.2000.14.195. [DOI] [PubMed] [Google Scholar]
- 24.Norris RD, Sur RL, Springhart WP, et al. A prospective, randomized, double-blinded placebo-controlled comparison of extended release oxybutynin versus phenazopyridine for the management of postoperative ureteral stent discomfort. Urology. 2008;71:792–795. doi: 10.1016/j.urology.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Park SC, Jung SW, Lee JW, et al. The effects of tolterodine extended release and alfuzosin for the treatment of double-j stent-related symptoms. J Endourol. 2009;23:1913–1917. doi: 10.1089/end.2009.0173. [DOI] [PubMed] [Google Scholar]
- 26.Lee YJ, Huang KH, Yang HJ, et al. Solifenacin improves double-J stent-related symptoms in both genders following uncomplicated ureteroscopic lithotripsy. Urolithiasis. 2013;41:247–252. doi: 10.1007/s00240-013-0554-y. [DOI] [PubMed] [Google Scholar]
- 27.Joshi HB, Newns N, Stainthorpe A, et al. Ureteral stent symptom questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003;169:1060–1064. doi: 10.1097/01.ju.0000049198.53424.1d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.