Abstract
Background
Docetaxel is one of the primary drugs used for treating castration resistant prostate cancer (CRPC). Unfortunately, over time patients invariably develop resistance to docetaxel therapy and their disease will continue to progress. The mechanisms by which resistance develops are still incompletely understood. This study seeks to determine the involvement of miRNAs, specifically miR-181a, in docetaxel resistance in CRPC.
Methods
Real-time PCR was used to measure miR-181a expression in parental and docetaxel resistant C4-2B and DU145 cells (TaxR and DU145-DTXR). miR-181a expression was modulated in parental or docetaxel resistant cells by transfecting them with miR-181a mimics or antisense, respectively. Following transfection, cell number was determined after 48 h with or without docetaxel. Cross resistance to cabazitaxel induced by miR-181a was also determined. Western blots were used to determine ABCB1 protein expression and rhodamine assays used to assess activity. Phospho-p53 expression was assessed by western blot and apoptosis was measured by ELISA in C4-2B TaxR and PC3 cells with inhibited or overexpressed miR-181a expression with or without docetaxel.
Results
miR-181a is significantly overexpressed in TaxR and DU145-DTXR cells compared to parental cells. Overexpression of miR-181a in parental cells confers docetaxel and cabazitaxel resistance and knockdown of miR-181a in TaxR cells re-sensitizes them to treatment with both docetaxel and cabazitaxel. miR-181a was not observed to impact ABCB1 expression or activity, a protein which was previously demonstrated to be highly involved in docetaxel resistance. Knockdown of miR-181a in TaxR cells induced phospho-p53 expression. Furthermore, miR-181a knockdown alone induced apoptosis in TaxR cells which could be further enhanced by the addition of DTX.
Conclusions
Overexpression of mir-181a in prostate cancer cells contributes to their resistance to docetaxel and cabazitaxel and inhibition of mir-181a expression can restore treatment response. This is due, in part, to modulation of p53 phosphorylation and apoptosis.
Keywords: prostate cancer, miR-181a, docetaxel, resistance
Introduction
In the United States, prostate cancer is the second leading cause of cancer related deaths and the most commonly diagnosed cancer in men with an estimated 220,800 new cases yearly (1,2). Androgen deprivation therapy (ADT), the initial line of treatment for this disease, reduces circulating androgen levels to reduce tumor growth. While androgen deprivation therapy is initially effective at reducing prostate cancer growth, after 2–3 years of treatment, most patients will progress to castration resistant prostate cancer (CRPC) which is defined as progression of prostate cancer in the presence of castrate levels of circulating testosterone (3,4). CRPC is often heralded by hyper-activated AR signaling leading to the transcription of downstream target genes and tumor growth despite insignificant levels of androgen present in the patient.
The most commonly prescribed first-line therapy for CRPC is docetaxel. Docetaxel functions by binding free tubulin in cells and causing the formation of stable microtubules which prevents depolymerization resulting in inhibition of mitosis and induction of apoptosis (5–7). Like with many drugs, over time cancer cells develop resistance to docetaxel and prostate tumor growth will again proceed regardless of the presence of the drug.
Docetaxel resistance has been well studied and a number of contributing mechanisms have been identified. Several of these are related to increased activation of pathways involved in cell survival. (8–13). Similarly, increased expression of inflammatory molecules such as interleukin (IL) 6, IL-8, chemokine ligand 2 (CCL2), transforming growth factor-β1 (TGF-β1) and macrophage inhibitory cytokine-1 (MIC-1) have been tied to promoting docetaxel resistance (14–18). Conversely, reduced activity or expression of wild type p53 is also linked to insensitivity to docetaxel (19). Others have determined that docetaxel resistant prostate cancer cells have increased expression and/or activity of multi-drug resistance proteins such as ABCB1 which excretes the docetaxel from the cells before it can elicit its therapeutic effect (12,20).
Another broad class of molecules that are linked to drug resistance in cancer is miRNAs. miRNAs are non-coding RNAs ranging from 18–25 nucleotides in length that modulate a variety of biological processes, including cancer progression. Studies in other cancer types have identified a number of miRNAs associated with docetaxel resistance. In non-small cell lung cancer, increased expression of miR-27b was found to promote docetaxel resistance through inhibition of EGFR expression (21). Zhang et al. found that miR-3646, miR-3658, miR-4438, miR-1246, and miR-574-3p are all upregulated in docetaxel resistant breast cancer cells and inhibition of miR-3646 was demonstrated to improve treatment response (22). In prostate cancer, studies investigating the role of miRNAs in docetaxel resistance have found that overexpression of miR-2, miR-200c and miR-205 and reduced expression of miR-143 promotes resistance and disease progression (23–25).
Another miRNA commonly linked to drug resistance in cancer is miR-181a. Studies in breast and cervical cancer have demonstrated that miR-181a promotes resistance to doxorubicin and cisplatin respectively (26,27). The aim of the presented study is to characterize the role of miR-181a in docetaxel resistant prostate cancer and to create a more complete picture of the miRNAs involved in prostate cancer docetaxel resistance. Data from this study provide insight into docetaxel resistance and the role of miRNAs in prostate cancer progression.
Materials and Methods
Cell culture
C4-2B cells were provided and authenticated by Dr. Leland Chung (Cedars-Sinai Medical Center, Los Angeles, CA). Cells were cultured in RPMI-1640 containing 10% complete FBS with 100 U/mL penicillin and 0.1 mg/mL streptomycin and maintained at 37°C in a humidified incubator with 5% CO2. TaxR cells were created by culturing parental C4-2B cells in gradually increasing concentrations of docetaxel and thereafter maintained in 5 nmol/L docetaxel-containing media (20). Parental C4-2B cells were passaged alongside the docetaxel treated cells as an appropriate control. DU145 parental and docetaxel resistant DU145 (DU145-DTXR) cells were also created in the same manner (20). Docetaxel (CAS#114977-28-5) was purchased from TSZ CHEM.
rtPCR
Total RNA was isolated from cell lines using either Trypsin (for gene analysis) or with the mirVana miRNA Isolation Kit for miRNA analysis (Ambion.) MiRNA expression for miR-181a was confirmed by rtPCR using the NCode miRNA qRT-PCR Kit (ThermoFisher) following the manufacturer’s instructions. Forward primer sequence for miR-181a was 5′-AACATTCAACGCTGTCGGTGAGT. The reverse, universal primer sequence was supplied with the kit.
Cell Growth Assay
C4-2B and TaxR cells were seeded at 50,000 cells per well in 12-well plates. Mimics or inhibitors and the appropriate controls for miRNA-181a (Life Technologies) were transfected into C4-2B (mimics), TaxR cells (inhibitors) or PC3 (mimics and inhibitors) at a final concentration of 30 nM using Lipofectamine 2000. The following day, the media was changed and cells were treated with 0.5, 1 or 5 nM docetaxel or cabazitaxel as indicated or DMSO as vehicle control. After 48 hours of treatment, cell number was determined using a Beckman Coulter particle counter. Twenty microliters of sample were diluted in 10 mL Isotone II diluent (Beckman Coulter) and each sample was counted three times. Two wells per treatment per experiment were used and three replicate experiments were conducted.
Western Blot
Whole cell, nuclear, and cytosolic protein extracts were resolved on SDS– PAGE and proteins were transferred to nitrocellulose membranes. After blocking for 1 hour at room temperature in 10% non-fat dry milk in PBS/0.1% Tween-20, membranes were incubated overnight at 4°C with the indicated primary antibodies at a 1:1000 dilution. (ABCB1; Santa Cruz Biotechnologies, phospho-p53 (ser15); Cell Signaling Technology.) Following secondary antibody incubation (1:5000), proteins were visualized with an enhanced chemiluminescence detection system (Millipore, Billerica, MA). Tubulin (1:2500) was used as the loading control.
Rhodamine Assay
C4-2B and TaxR cells were seeded in 12-well plates at a density of 50,000 cells per well and then transfected with miR-181a mimic or inhibitor respectively as described above. The day after transfection, a control group of TaxR cells was treated with elacridar as a positive control for increasing cellular rhodamine retention. Forty-eight hours after transfection, cells were incubated with 1 μmol/L rhodamine 123 for 4 hours. The cells were then washed four times with PBS and fluorescence pictures were taken. Treatments were conducted in triplicate and the experiment was repeated two times.
Cell Death ELISA
CWR22Rv1, C4-2B MDVR and LNCaP-BicR cells were seeded on 12-well plates (50,000 cells/well). One day after transfecting miR-181 mimic or antisense constructs into TaxR or PC3 cells as indicated, the cells were treated with 5 nM docetaxel or vehicle control for 48 hours. Mono- and oligonucleosomes in the cytoplasmic fraction were measured by the Cell Death Detection ELISA kit (Roche, Cat. NO. 11544675001) as described previously (28). Briefly, cells were collected and homogenized in 400 μL of incubation buffer. Wells of a 96-well plate were coated with anti-histone antibodies and incubated with the lysates, horseradish peroxidase-conjugated anti-DNA antibodies, and the substrate. Absorbance was measured at 405 nm.
Statistical Analysis
All data are presented as means +/− standard error of the mean (SEM). Significance is defined as p ≤ 0.05 as determined by one-way ANOVA in JMP.
Results
Docetaxel Resistant Cells Have Increased Expression of miR-181a
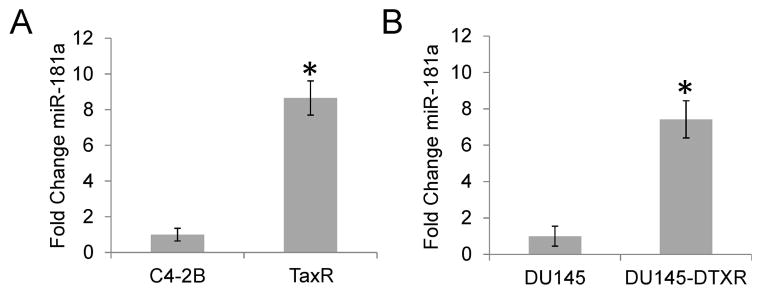
We have previously generated several docetaxel resistant prostate cancer cell sublines including TaxR from C4-2B and DU145-DTXR from DU145 cells (20). We have identified that ABCB1 overexpression and activation confers resistance to both TaxR and DU145-DTXR cells. Although knock down of ABCB1 expression or inhibition of ABCB1 activity using small molecule inhibitors, such as elacridar, can resensitize these resistant cells to docetaxel treatment, the response is only partial, suggesting additional mechanisms are associated with docetaxel resistance. Studies investigating the role of miRNAs in docetaxel resistance have found that altered expression of several miRNAs, including miR-2, miR-200c and miR-205, promotes resistance and disease progression (23–25). In addition, studies have shown that overexpression of miRNA181 contributes to cell survival in the presence of doxorubicin and cisplatin in breast and cervical cancer (26,27). In order to determine if miR-181a is increased in docetaxel resistant cells compared to parental cell lines, rtPCR for miR-181a was performed on parental and docetaxel resistant cell lines. As seen in Fig. 1A–B, miR-181a expression is significantly increased in both TaxR (8.6 fold) and DU145-DTXR (7.4 fold) cells compared to parental C4-2B and DU145 cells respectively.
Figure 1.
miR-181a is increased in docetaxel resistant cells. A. rt-PCR was performed on RNA isolated from parental C4-2B and TaxR cells for miR-181a. B. rt-PCR data for RNA isolated from DU145 and DU145-DTXR cells. Results are presented as mean fold change ± SEM of three replicate experiments conducted in duplicate. * denotes p≤0.05
miR-181a Overexpression Induces Docetaxel Resistance
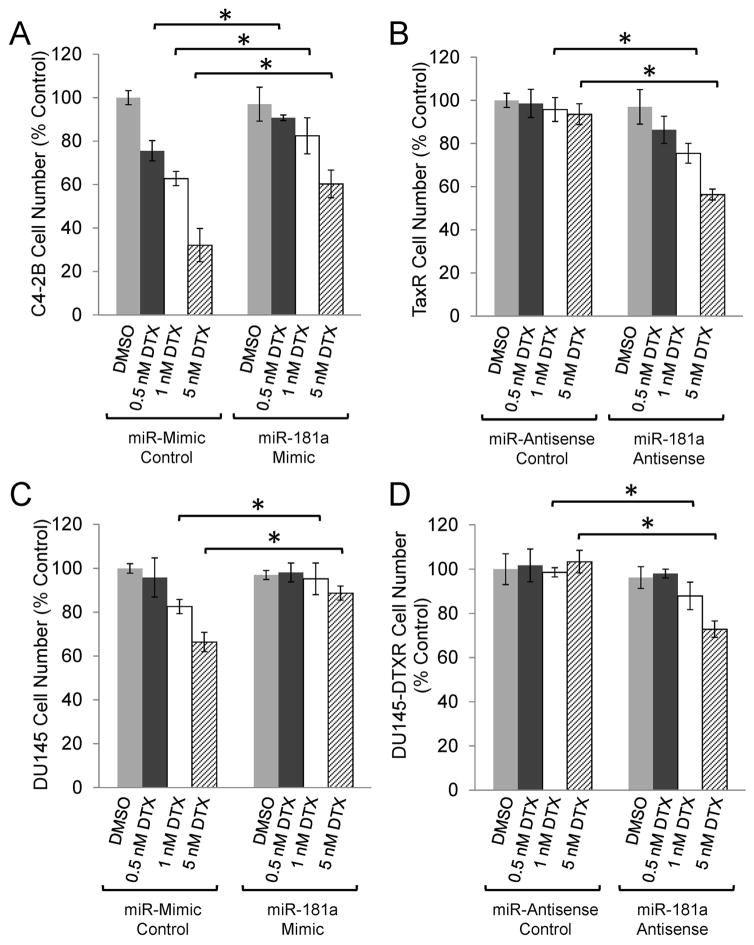
To test whether the increased expression of miR-181a has functional implications in prostate cancer cells, miR-181a was overexpressed in parental cells using miRNA mimics and knocked down in docetaxel resistant cells with miRNA inhibitors and cell number was determined following 48 hours of treatment with docetaxel or vehicle control. Overexpression of miRNA-181a significantly improved cell survival in the presence of increasing doses of docetaxel compared to the miR-mimic control in parental C4-2B cells (Fig. 2A). Conversely, knockdown of miR-181a resensitized docetaxel resistant TaxR cells to docetaxel treatment; at the highest docetaxel concentration (5 nM) no change in cell number was observed in control transfected cells, however there was a 44% reduction in cell number in TaxR cells in response to 5 nM docetaxel when transfected with the miR-181a antisense (Fig. 2B). Likewise, parental DU145 cells had increased cell survival following transfection with miR-181a mimics in response to increased concentrations of docetaxel (Fig. 2C) and inhibition of miR-181a resensitized docetaxel resistant DU145-DTXR cells to treatment (Fig. 2D). Together, these data demonstrate that miR-181a promotes docetaxel resistance in prostate cancer and that targeting this miRNA can re-sensitize cells to docetaxel therapy.
Figure 2.
miR-181a expression promotes docetaxel resistance. A. Parental C4-2B cells were transfected with miR-181a mimics or control and then treated with the indicated dose of docetaxel (DTX) for 48 hours. B. TaxR cells were transfected with miR-181a antisense or control and then treated with the indicated dose of docetaxel for 48 hours. C. Parental DU145 cells were transfected with miR-181a mimics or control and then treated with the indicated dose of docetaxel for 48 hours. B. DU145-DTXR cells were transfected with miR-181a antisense or control and then treated with the indicated dose of docetaxel for 48 hours. Cell number was determined by Coulter Counter. Data are presented as percent control ± SEM of three replicate experiments conducted in duplicate. * denotes p≤0.05 between the indicated groups.
Knockdown of miR-181a Resensitizes TaxR cells to Cabazitaxel treatment
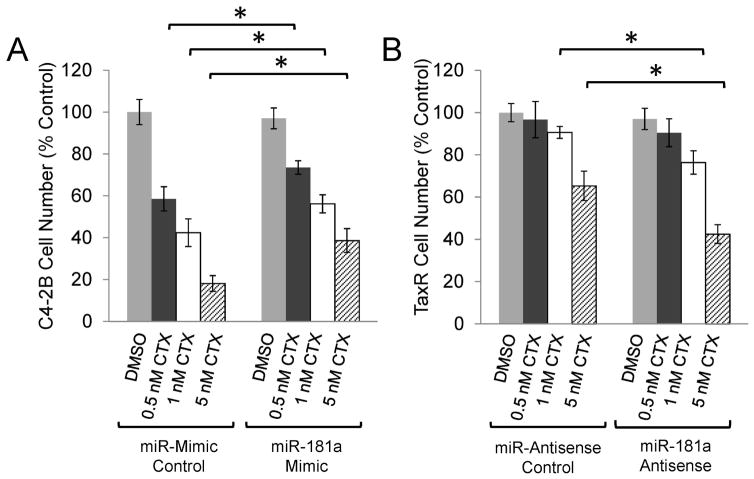
While cabazitaxel is known to be beneficial when administered post-docetaxel therapy, the survival benefit is modest, suggesting that there may be cross resistance between these two taxanes (29). To determine if miR181a is associated with cabazitaxel resistance, cell growth assays were conducted in parental and TaxR cells transfected with miR-181a mimics or antisense respectively and subjected to cabazitaxel treatment as indicated. miR-181a overexpression in C4-2B cells induces resistance to cabazitaxel as indicated by the increase in cell survival compared to cells transfected with the control constructs; miR-mimic control transfected cells attained 58%, 42% or 18% of the cell number from the DMSO treated cells in response to 0.5, 1, or 5 nM cabazitaxel respectively. When transfected with miR-181a mimics, cell number increased to 74%, 56% and 39% of control in response to the same doses of cabazitaxel (Fig. 3A). TaxR cells displayed cross resistance to cabazitaxel and knockdown of miR-181a in these cells successfully re-sensitized them to treatment with 1 or 5 nM cabazitaxel as seen by a reduction from 90% to 76% of control or from 65% to 43% of control respectively when miR-181a was inhibited (Fig. 3B). These data suggest that similar resistance pathways occur between docetaxel and cabazitaxel and that inhibition of miR-181a could be beneficial for both.
Figure 3.
miR-181a knockdown resensitizes TaxR cells to cabazitaxel. A. Parental C4-2B cells were transfected with miR-181a mimics or control and then treated with the indicated dose of cabazitaxel (CTX) for 48 hours. B. TaxR cells were transfected with miR-181a antisense or control and then treated with the indicated dose of cabazitaxel for 48 hours. Cell number was determined by Coulter Counter. Data are presented as percent control ± SEM of three replicate experiments conducted in duplicate. * denotes p≤0.05 between the indicated groups.
miR-181a Does Not Alter ABCB1 Expression or Activity
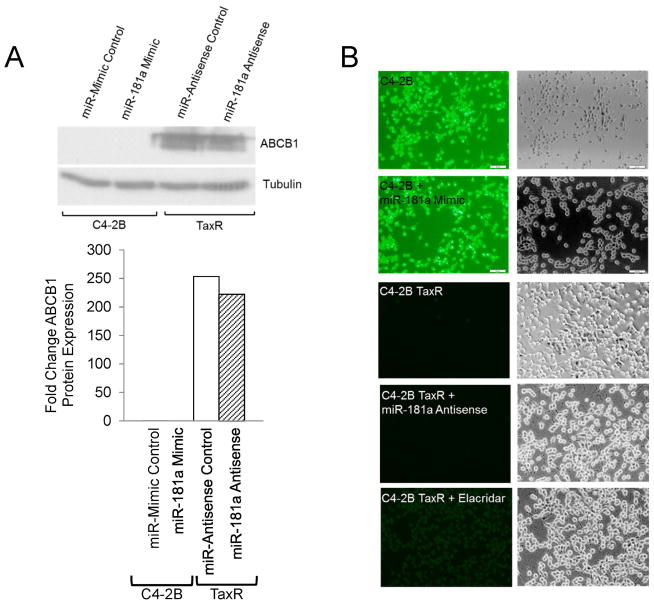
Next we wanted to determine the mechanisms behind miR-181a induced docetaxel resistance. Previous data demonstrate that ATP Binding Cassette Subfamily B Member 1 (ABCB1) is significantly overexpressed in docetaxel resistant cells (20). Furthermore, targeting ABCB1 expression or activity was observed to improve resistant cell response to docetaxel (20,30). Therefore, we decided to measure ABCB1 expression and efflux activity in response to miR-181a over and under expression in C4-2B parental and TaxR cells respectively. As seen in Fig. 4A, modulating miR-181a expression had no effect on ABCB1 expression as determined by Western blot. Rhodamine assays were used to measure ABCB1 efflux activity. Overexpression of miR-181a was incapable of inducing rhodamine efflux in C4-2B cells and miR-181a knockdown in TaxR cells did not inhibit efflux (Fig. 4B). These data suggest that modulation of ABCB1 expression or activity is not a mechanism of action attributed to miR-181a-mediated resistance to docetaxel in prostate cancer.
Figure 4.
ABCB1 is not involved in miR-181 induced docetaxel resistance. A. Cell lysates from parental and TaxR cells transfected with miR-181 mimic or antisense respectively, along with the appropriate control, were subjected to Western blot for ABCB1. B. A rhodamine assay was used to determine ABCB1 activity in parental and TaxR cells transfected with miR-181 mimic or antisense respectively, along with the appropriate controls. Data are representative of two replicate experiments conducted in triplicate.
miR-181a Inhibition Induces p53 Phosphorylation and Apoptosis
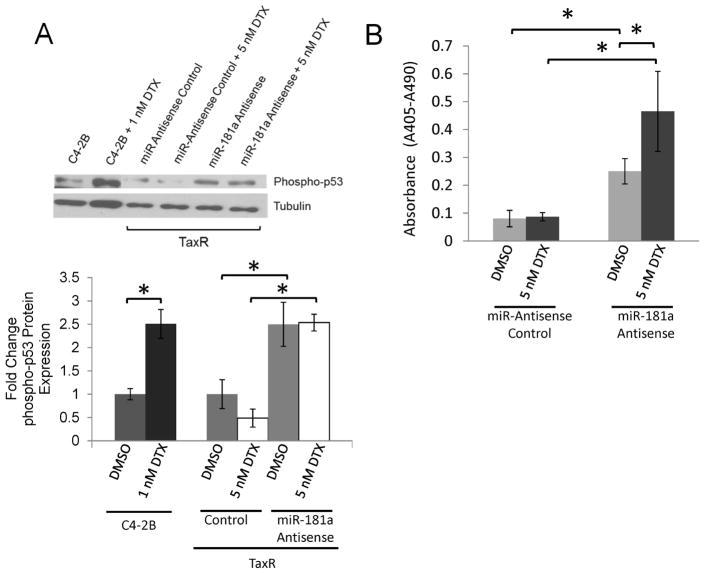
Another potential mechanism associated with docetaxel resistance is loss of p53 phosphorylation in response to docetaxel. Knowing that ABCB1 was not involved in miR-181a mediated docetaxel resistance, we next investigated whether miR-18a could influence p53 and apoptosis. As seen in Fig. 5A, docetaxel treatment induced a 2.5 fold increase in phospho-p53 expression in parental C4-2B cells but not in TaxR cells, consistent with the previous report (19). Knockdown of miR-181a significantly increased p53 phosphorylation by 2.5 fold in both the presence and absence of docetaxel in TaxR cells. Supplementarily, knockdown of miR-181a in TaxR cells induced apoptosis which was further enhanced by co-treatment with docetaxel (Fig. 5B).
Figure 5.
miR-181a promotes p53 phosphorylation and apoptosis. A. Cell lysates from TaxR cells transfected with anit-miR-181a or control and treated ± 5 nM docetaxel (DTX) were subjected to Western blot for phosph-p53. Parental C4-2B and C4-2B cells treated with 5 nM docetaxel were used as control for phosph-p53 induction. B. Cell death ELISA from TaxR cells transfected with anit-miR-181a or control and treated ± 5 nM docetaxel. Results are presented as means ±SEM of three replicate experiments conducted in duplicate. * denotes p≤0.05 between indicated groups.
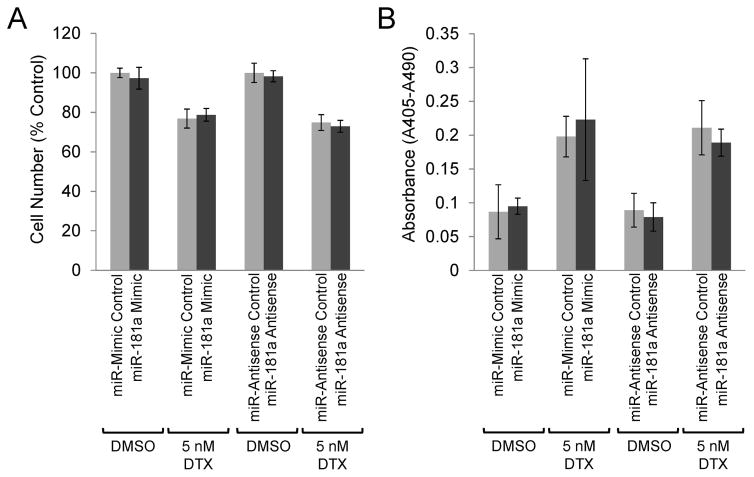
Apoptosis was also assessed in p53-null PC3 prostate cancer cells in the presence or absence of docetaxel with either over- or under-expressed miR181a. As seen in Fig. 6A–B, neither transfection with miR-181 mimics or miR-181 antisense altered the response of PC3 cells to docetaxel in regards to cell number (Fig 6A) or induction of apoptosis (Fig 6B). These data, together with the data above, suggest that miR-181a functions, at least in part, by modulating p53 activity and inducing apoptosis to alter docetaxel sensitivity.
Figure 6.
Functional p53 is required for miR-181a mediated docetaxel resistance. p53-null PC3 cells were transfected with miR-181 mimics or antisense, along with the appropriate controls, and treated with 5 nM docetaxel (DTX). After 48 hours treatment, cell number (A) was determined by Coulter counter and apoptosis (B) was determined by cell death ELISA. Results are presented as means ±SEM of three replicate experiments conducted in duplicate.
Discussion
In the presented study, we utilized docetaxel resistant prostate cancer cell lines to investigate the role of miR-181a in docetaxel resistance. We found that miR-181a was significantly upregulated in two separate docetaxel resistant cell lines and that inhibition of its expression improved the response to docetaxel. Furthermore, overexpression of miR-181a in parental cell lines induced resistance.
miR-181a is a relatively well studied miRNA that has a wide range of known functions. The highest expression levels of miR-181a in a healthy setting are in the thymus and it is highly involved in T-cell function and sensitivity (31,32). Furthermore, miR-181a is known to be critical for organ development and differentiation, particularly through its modulation of zinc-finger proteins (33–35). In regards to cancer, miR-181a dysregulation is observed in several tumor types. Interestingly, both up- and down-regulation of miR-181a is associated with tumor formation as well as with drug resistance. Downregulation of miR-181a has been observed in squamous lung cell carcinoma, oral squamous cell carcinoma, glioblastomas, and non-small-cell lung cancer (36–39). Conversely, breast cancer cells and hepatocellular carcinoma cells have increased expression of miR-181a (40,41). In hepatocellular carcinoma cells, increased expression of miR-181a induces resistance to sorafenib, a multi-kinase inhibitor, by suppressing RASSF1 (42). Additionally, miR-181a overexpression was also found to promote paclitaxel resistance in ovarian cancer cells (43).
Not only did miR-181a promote docetaxel resistance, but we found that its overexpression also induced resistance to cabazitaxel in sensitive cell lines and knockdown of miR-181a in docetaxel resistant cells (which are cross resistant to cabazitaxel) re-sensitized them to cabazitaxel treatment as well. Cabazitaxel is currently approved only for the treatment of patients who have previously undergone docetaxel treatment (29,44). The fact that cells that are resistant to docetaxel also display resistance to cabazitaxel is concerning for this patient population and makes identifying mechanisms of cross resistance important. The fact that inhibition of miR-181a improved both response to docetaxel and cabazitaxel suggests that overexpression of this miRNA is likely one of these mechanisms of cross resistance.
Several genes and molecular pathways have been identified that contribute to docetaxel resistance. Overexpression of Notch and Hedgehog signaling his linked to docetaxel resistance in DU145 and CWR22v1 cells and down regulation of CDH1 and IFIH1 has been identified in docetaxel resistant PC3 and DU145 cells and confirmed in tumors from docetaxel resistant prostate cancer patients (17,45). Studies have previously determined that ABCB1 expression is greatly increased in docetaxel resistant cells and that targeting ABCB1 can improve treatment response (20,30). miR-181a has been demonstrated in other cancer types to impact expression of ABCG2, another member of the ATP Binding Cassette family of transporters similar to ABCB1 (46). Therefore, we sought to determine whether miR-181a could alter ABCB1 expression or activity as part of its mechanism of action for inducing docetaxel resistance in prostate cancer cells. However, we found no change in either ABCB1 protein levels or drug efflux activity, suggesting that increased retention of docetaxel is not the mechanism by which miR-181a induces resistance in our model.
Other studies have found that in docetaxel resistant prostate cancer cells, treatment with docetaxel fails to initiate phosphorylation of p53 whereas in sensitive cells, docetaxel treatment causes a robust phosphorylation of p53 (19). miR-181a has been demonstrated to impact apoptosis and p53 signaling in previous studies in other tissue types. Therefore, we next sought to determine whether miR-181a altering p53 signaling could be involved in the observed docetaxel resistance in our model. We found that inhibition of miR-181a alone was capable of inducing p53 phosphorylation in both the presence and absence of docetaxel. When apoptosis was measured, we observed that while miR181a inhibition on its own induced apoptosis, addition of docetaxel to the miR-181a inhibited cells increased apoptosis further still. The fact that there is increased apoptosis in response to docetaxel but equal expression of phospo-p53 in the miR181a knockdown cells suggests that inhibition of miR-181a is priming the p53 signaling pathway to respond to treatment with docetaxel.
miRNA-181a and p53 have a convoluted relationship. Not only can miR-181a impact p53 signaling, but there is a p53-response element located upstream of miR-181a creating a feedback loop (47). Interestingly, studies indicate that increased p53 may not directly correlate to increased miR-181a expression; parallel sequencing of miRNAs following induction of p53 expression found that the sequence corresponding to the complementary arms of miR-181a’s hairpin was more highly expressed than the mature strand, which suggests p53 may actually have a negative effect on mature miR-181a (48). Ongoing studies in our laboratory are seeking to determine a more precise role for miR-181a and p53 signaling in docetaxel resistant prostate cancer.
In summary, the presented study demonstrates that miR-181a is significantly upregulated in docetaxel resistant prostate cancer cell lines. Inhibition of miR-181a resensitizes resistant cell lines to docetaxel and overexpression of miR-181a promotes resistance to docetaxel therapy in resistant and sensitive cells respectively. Furthermore, we determined that modulation of p53 phosphorylation is a likely mediator of miR-181a mediated docetaxel resistance. Data from this study are an important step forward towards understanding the mechanisms behind docetaxel resistance and will lead to improved treatment strategies for prostate cancer patients.
Acknowledgments
This work was supported in part by grants NIH/NCI CA168601, CA179970, DOD PC150229, and US Department of Veterans Affairs, ORD VA Merits I01BX0002653.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Cookson MS, Roth BJ, Dahm P, Engstrom C, Freedland SJ, Hussain M, Lin DW, Lowrance WT, Murad MH, Oh WK, Penson DF, Kibel AS. Castration-resistant prostate cancer: AUA Guideline. The Journal of urology. 2013;190(2):429–438. doi: 10.1016/j.juro.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Saad F, Hotte SJ. Guidelines for the management of castrate-resistant prostate cancer. Canadian Urological Association journal = Journal de l’Association des urologues du Canada. 2010;4(6):380–384. doi: 10.5489/cuaj.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagher R, Li N, Abraham S, Rahman A, Sridhara R, Pazdur R. Approval summary: Docetaxel in combination with prednisone for the treatment of androgen-independent hormone-refractory prostate cancer. Clin Cancer Res. 2004;10(24):8147–8151. doi: 10.1158/1078-0432.CCR-04-1402. [DOI] [PubMed] [Google Scholar]
- 6.McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785(2):96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Shelanski ML, Gaskin F, Cantor CR. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gan L, Wang J, Xu H, Yang X. Resistance to docetaxel-induced apoptosis in prostate cancer cells by p38/p53/p21 signaling. The Prostate. 2011;71(11):1158–1166. doi: 10.1002/pros.21331. [DOI] [PubMed] [Google Scholar]
- 9.Domingo-Domenech J, Oliva C, Rovira A, Codony-Servat J, Bosch M, Filella X, Montagut C, Tapia M, Campas C, Dang L, Rolfe M, Ross JS, Gascon P, Albanell J, Mellado B. Interleukin 6, a nuclear factor-kappaB target, predicts resistance to docetaxel in hormone-independent prostate cancer and nuclear factor-kappaB inhibition by PS-1145 enhances docetaxel antitumor activity. Clin Cancer Res. 2006;12(18):5578–5586. doi: 10.1158/1078-0432.CCR-05-2767. [DOI] [PubMed] [Google Scholar]
- 10.Patterson SG, Wei S, Chen X, Sallman DA, Gilvary DL, Zhong B, Pow-Sang J, Yeatman T, Djeu JY. Novel role of Stat1 in the development of docetaxel resistance in prostate tumor cells. Oncogene. 2006;25(45):6113–6122. doi: 10.1038/sj.onc.1209632. [DOI] [PubMed] [Google Scholar]
- 11.Zemskova M, Sahakian E, Bashkirova S, Lilly M. The PIM1 kinase is a critical component of a survival pathway activated by docetaxel and promotes survival of docetaxel-treated prostate cancer cells. J Biol Chem. 2008;283(30):20635–20644. doi: 10.1074/jbc.M709479200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Neill AJ, Prencipe M, Dowling C, Fan Y, Mulrane L, Gallagher WM, O’Connor D, O’Connor R, Devery A, Corcoran C, Rani S, O’Driscoll L, Fitzpatrick JM, Watson RW. Characterisation and manipulation of docetaxel resistant prostate cancer cell lines. Mol Cancer. 2011;10:126. doi: 10.1186/1476-4598-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Codony-Servat J, Marin-Aguilera M, Visa L, Garcia-Albeniz X, Pineda E, Fernandez PL, Filella X, Gascon P, Mellado B. Nuclear factor-kappa B and interleukin-6 related docetaxel resistance in castration-resistant prostate cancer. The Prostate. 2013;73(5):512–521. doi: 10.1002/pros.22591. [DOI] [PubMed] [Google Scholar]
- 14.Singh RK, Lokeshwar BL. Depletion of intrinsic expression of Interleukin-8 in prostate cancer cells causes cell cycle arrest, spontaneous apoptosis and increases the efficacy of chemotherapeutic drugs. Mol Cancer. 2009;8:57. doi: 10.1186/1476-4598-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian DZ, Rademacher BL, Pittsenbarger J, Huang CY, Myrthue A, Higano CS, Garzotto M, Nelson PS, Beer TM. CCL2 is induced by chemotherapy and protects prostate cancer cells from docetaxel-induced cytotoxicity. The Prostate. 2010;70(4):433–442. doi: 10.1002/pros.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiota M, Kashiwagi E, Yokomizo A, Takeuchi A, Dejima T, Song Y, Tatsugami K, Inokuchi J, Uchiumi T, Naito S. Interaction between docetaxel resistance and castration resistance in prostate cancer: implications of Twist1, YB-1, and androgen receptor. The Prostate. 2013;73(12):1336–1344. doi: 10.1002/pros.22681. [DOI] [PubMed] [Google Scholar]
- 17.Marin-Aguilera M, Codony-Servat J, Kalko SG, Fernandez PL, Bermudo R, Buxo E, Ribal MJ, Gascon P, Mellado B. Identification of docetaxel resistance genes in castration-resistant prostate cancer. Mol Cancer Ther. 2012;11(2):329–339. doi: 10.1158/1535-7163.MCT-11-0289. [DOI] [PubMed] [Google Scholar]
- 18.Mimeault M, Johansson SL, Batra SK. Marked improvement of cytotoxic effects induced by docetaxel on highly metastatic and androgen-independent prostate cancer cells by downregulating macrophage inhibitory cytokine-1. Br J Cancer. 2013;108(5):1079–1091. doi: 10.1038/bjc.2012.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C, Zhu Y, Lou W, Nadiminty N, Chen X, Zhou Q, Shi XB, deVere White RW, Gao AC. Functional p53 determines docetaxel sensitivity in prostate cancer cells. The Prostate. 2013;73(4):418–427. doi: 10.1002/pros.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Y, Liu C, Nadiminty N, Lou W, Tummala R, Evans CP, Gao AC. Inhibition of ABCB1 expression overcomes acquired docetaxel resistance in prostate cancer. Mol Cancer Ther. 2013;12(9):1829–1836. doi: 10.1158/1535-7163.MCT-13-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Wang Q, Zhou XM, Zhu JP, Li T, Huang M. MicroRNA-27b reverses docetaxel resistance of non-small cell lung carcinoma cells via targeting epithelial growth factor receptor. Molecular medicine reports. 2016 doi: 10.3892/mmr.2016.5332. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Zhong S, Xu Y, Yu D, Ma T, Chen L, Zhao Y, Chen X, Yang S, Wu Y, Tang J, Zhao J. MicroRNA-3646 Contributes to Docetaxel Resistance in Human Breast Cancer Cells by GSK-3beta/beta-Catenin Signaling Pathway. PLoS One. 2016;11(4):e0153194. doi: 10.1371/journal.pone.0153194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi GH, Ye DW, Yao XD, Zhang SL, Dai B, Zhang HL, Shen YJ, Zhu Y, Zhu YP, Xiao WJ, Ma CG. Involvement of microRNA-21 in mediating chemo-resistance to docetaxel in androgen-independent prostate cancer PC3 cells. Acta Pharmacol Sin. 2010;31(7):867–873. doi: 10.1038/aps.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z, Li P, Zhang W, Wu H, Feng N, Wang Z, Hua L, Wang X. miR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol Cell Biochem. 2011;350(1–2):207–213. doi: 10.1007/s11010-010-0700-6. [DOI] [PubMed] [Google Scholar]
- 25.Puhr M, Hoefer J, Schafer G, Erb HH, Oh SJ, Klocker H, Heidegger I, Neuwirt H, Culig Z. Epithelial-to-mesenchymal transition leads to docetaxel resistance in prostate cancer and is mediated by reduced expression of miR-200c and miR-205. Am J Pathol. 2012;181(6):2188–2201. doi: 10.1016/j.ajpath.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Niu J, Xue A, Chi Y, Xue J, Wang W, Zhao Z, Fan M, Yang CH, Shao ZM, Pfeffer LM, Wu J, Wu ZH. Induction of miRNA-181a by genotoxic treatments promotes chemotherapeutic resistance and metastasis in breast cancer. Oncogene. 2016;35(10):1302–1313. doi: 10.1038/onc.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Ke G, Han D, Liang S, Yang G, Wu X. MicroRNA-181a enhances the chemoresistance of human cervical squamous cell carcinoma to cisplatin by targeting PRKCD. Exp Cell Res. 2014;320(1):12–20. doi: 10.1016/j.yexcr.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Lou W, Armstrong C, Zhu Y, Evans CP, Gao AC. Niclosamide suppresses cell migration and invasion in enzalutamide resistant prostate cancer cells via Stat3-AR axis inhibition. The Prostate. 2015;75(13):1341–1353. doi: 10.1002/pros.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO Investigators T. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Liu C, Armstrong C, Lou W, Sandher A, Gao AC. Anti-androgens inhibit ABCB1 efflux and ATPase activity and reverse docetaxel resistance in advanced prostate cancer. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303(5654):83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 32.Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol. 2009;10(11):1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li YG, Zhang PP, Jiao KL, Zou YZ. Knockdown of microRNA-181 by lentivirus mediated siRNA expression vector decreases the arrhythmogenic effect of skeletal myoblast transplantation in rat with myocardial infarction. Microvascular research. 2009;78(3):393–404. doi: 10.1016/j.mvr.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Saunders LR, Sharma AD, Tawney J, Nakagawa M, Okita K, Yamanaka S, Willenbring H, Verdin E. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY) 2010;2(7):415–431. doi: 10.18632/aging.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang S, Wu S, Ding J, Lin J, Wei L, Gu J, He X. MicroRNA-181a modulates gene expression of zinc finger family members by directly targeting their coding regions. Nucleic Acids Res. 2010;38(20):7211–7218. doi: 10.1093/nar/gkq564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao W, Shen H, Liu L, Xu J, Xu J, Shu Y. MiR-21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J Cancer Res Clin Oncol. 2011;137(4):557–566. doi: 10.1007/s00432-010-0918-4. [DOI] [PubMed] [Google Scholar]
- 37.Shin KH, Bae SD, Hong HS, Kim RH, Kang MK, Park NH. miR-181a shows tumor suppressive effect against oral squamous cell carcinoma cells by downregulating K-ras. Biochem Biophys Res Commun. 2011;404(4):896–902. doi: 10.1016/j.bbrc.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 38.Ciafre SA, Galardi S, Mangiola A, Ferracin M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM, Farace MG. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334(4):1351–1358. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 39.Gao W, Yu Y, Cao H, Shen H, Li X, Pan S, Shu Y. Deregulated expression of miR-21, miR-143 and miR-181a in non small cell lung cancer is related to clinicopathologic characteristics or patient prognosis. Biomed Pharmacother. 2010;64(6):399–408. doi: 10.1016/j.biopha.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 40.Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, Deng C, Wauthier E, Reid LM, Ye QH, Qin LX, Yang W, Wang HY, Tang ZY, Croce CM, Wang XW. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50(2):472–480. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, Jacob S, Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008;283(44):29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azumi J, Tsubota T, Sakabe T, Shiota G. miR-181a induces sorafenib resistance of hepatocellular carcinoma cells through downregulation of RASSF1 expression. Cancer Sci. 2016;107(9):1256–1262. doi: 10.1111/cas.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Xu QH, Dong YH, Li GX, Yang L, Wang LW, Li HY. MiR-181a upregulation is associated with epithelial-to-mesenchymal transition (EMT) and multidrug resistance (MDR) of ovarian cancer cells. European review for medical and pharmacological sciences. 2016;20(10):2004–2010. [PubMed] [Google Scholar]
- 44.Paller CJ, Antonarakis ES. Cabazitaxel: a novel second-line treatment for metastatic castration-resistant prostate cancer. Drug design, development and therapy. 2011;5:117–124. doi: 10.2147/DDDT.S13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, Bonal DM, Charytonowicz E, Gladoun N, de la Iglesia-Vicente J, Petrylak DP, Benson MC, Silva JM, Cordon-Cardo C. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012;22(3):373–388. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiao X, Zhao L, Ma M, Bai X, He M, Yan Y, Wang Y, Chen Q, Zhao X, Zhou M, Cui Z, Zheng Z, Wang E, Wei M. MiR-181a enhances drug sensitivity in mitoxantone-resistant breast cancer cells by targeting breast cancer resistance protein (BCRP/ABCG2) Breast Cancer Res Treat. 2013;139(3):717–730. doi: 10.1007/s10549-013-2607-x. [DOI] [PubMed] [Google Scholar]
- 47.Boominathan L. The tumor suppressors p53, p63, and p73 are regulators of microRNA processing complex. PLoS One. 2010;5(5):e10615. doi: 10.1371/journal.pone.0010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A, Meister G, Hermeking H. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6(13):1586–1593. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]