Abstract
β-catenin is the major effector of the canonical Wnt signaling pathway. Mutations in components of the pathway that stabilize β-catenin result in augmented gene transcription and play a major role in many human cancers. We employed microarrays to identify transcriptional targets of deregulated β-catenin in a human epithelial cell line (293) engineered to produce mutant β-catenin and in ovarian endometrioid adenocarcinomas characterized with respect to mutations affecting the Wnt/β-catenin pathway. Two genes strongly induced in both systems—FGF20 and DKK1—were studied in detail. Elevated levels of FGF20 RNA were also observed in adenomas from mice carrying the ApcMinallele. Both XFGF20 and Xdkk-1 are expressed early in Xenopus embryogenesis under the control of the Wnt signaling pathway. Furthermore, FGF20 and DKK1 appear to be direct targets for β-catenin/TCF transcriptional regulation via LEF/TCF-binding sites. Finally, by using small inhibitory RNAs specific for FGF20, we show that continued expression of FGF20 is necessary for maintenance of the anchorage-independent growth state in RK3E cells transformed by β-catenin, implying that FGF-20 may be a critical element in oncogenesis induced by the Wnt signaling pathway.
Keywords: β-catenin, DKK1, FGF-20, Wnt signaling, Xenopus laevis
Introduction
Wnt proteins are secreted glycoproteins that bind and activate two classes of co-receptors, LDL-related proteins (LRPs) and members of the Frizzled protein family. Signaling initiated by Wnts and their receptors controls a wide variety of cell processes, including cell fate specification, differentiation, migration, and polarity (reviewed in Peifer and Polakis, 2000). β-Catenin is the major effector of the canonical Wnt signaling pathway. In the absence of Wnt, cytosolic β-catenin forms a complex with Axin and adenomatous polyposis coli (APC) proteins, and is rapidly degraded by the ubiquitination–proteosome system. Wnt signaling inactivates the β-catenin destruction complex, so that β-catenin is stabilized, accumulates in the cytoplasm and nucleus, and forms heterodimers with the DNA-binding factors belonging to the LEF/TCF family (reviewed in Huelsken and Behrens, 2002). As a result, significant changes occur in the gene expression program (reviewed in Hecht and Kemler, 2000; Miller et al, 2001). In addition, Wnt signaling can be antagonized extracellularly by secreted factors such as Wnt inhibitory factor-1 (WIF-1), Cerberus, members of the Dickkopf (DKK) family, and soluble Frizzled-related proteins (sFRP) (Kawano and Kypta, 2003).
Genetic alterations that stabilize β-catenin are found in tumors in mice and humans. The most commonly observed genetic alterations involve either the loss of APC or Axin or mutations that affect the amino-terminus of β-catenin, all occurring in a mutually exclusive manner, and the most commonly affected organs are the colon, liver, skin, stomach, ovaries, pancreas, and prostate. For example, loss of APC occurs in 70–80% of human colorectal cancers (reviewed in Bienz and Clevers, 2000) and mutations affecting β-catenin are found in about half of the remaining tumors, implying that stabilization of β-catenin is a major early event in colonic carcinogenesis. Similarly, in ApcMin/+ mice, the loss of the wild-type allele initiates adenoma formation (Moser et al, 1993; Oshima et al, 1995), and expression of an oncogenic form of β-catenin produces adenomas in the mouse intestine (Romagnolo et al, 1999).
Identification of the transcriptional targets of the Wnt/β-catenin signaling pathway is a potentially important means to understand the role of the canonical pathway in oncogenesis and development. A number of candidate target genes have been identified in human cell lines and tumors. (For more information on Wnt pathway targets, consult the Wnt webpage at http://www.stanford.edu/~rnusse/wntwindow.html.)
Our objective in the study reported here has been to identify novel β-catenin target genes that are regulated directly by DNA binding of β-catenin/TCF heterodimers and are potentially relevant to carcinogenesis. To this end, we employed microarray technology to identify genes with significantly altered levels of expression in human epithelial (293) cells expressing mutant (stabilized) β-catenin in which serine 37 has been replaced with alanine; we then compared a list of these genes to a similar list obtained using the same methods to measure the abundance of RNAs in a well-characterized set of primary human ovarian endometrioid adenocarcinomas (OEAs) with and without Wnt pathway defects (Wu et al, 2001). Merging of microarray data from cell culture and OEA tumors revealed at least 17 genes that are regulated in common when similar criteria were applied. Two such genes, FGF20, a putative proto-oncogene (Jeffers et al, 2001), and DKK1, a Wnt pathway antagonist (Glinka et al, 1998), were studied in greater detail in mouse and human tumors, in frog development, in tests of the direct action of β-catenin-TCF heterodimers on gene expression, and in tests for a role of FGF20 during maintenance of the β-catenin-induced transformed state. We present evidence supporting the conclusions that FGF20 and DKK1 are directly regulated by β-catenin during development and tumorigenesis, and that continued expression of FGF20 is required to maintain the anchorage-independent growth state established by Wnt/β-catenin signaling.
Results
Genes regulated by mutant β-catenin in a human epithelial cell line
In an initial effort to identify genes regulated by mutant β-catenin, whether directly or indirectly, we used an efficient virus-based gene delivery system to introduce mutant β-catenin into virtually all cells in a cultured epithelial cell line, the human embryonic kidney cell line 293. This approach obviated a need to select individual clones of β-catenin-expressing cells and thus minimized variations in gene expression that might have been attributed to clonal variation.
293 cells were engineered to produce Tva, the avian leukosis subgroup A virus receptor, allowing efficient infection by the avian retroviral vector RCAS (Fisher et al, 1999). In addition, the β-catenin/TCF reporter construct, pOT, was inserted in the genome of this cell line to monitor β-catenin/TCF activity (Rubinfeld et al, 1993). The resulting cell line, 293Top, was infected with RCAS vectors encoding either GFP, to serve as a control, or HA-tagged β-cateninS37A, a stable mutant protein (Wu et al, 2001). Typically, about 80% of 293 cells were infected with either virus, as judged by anti-HA immunofluorescence or GFP fluorescence (data not shown). In the cells infected with RCAS-β-cateninS37A (293Top-S37A), the pOT reporter was typically induced 100–300-fold compared to cells infected with RCAS-GFP (293Top-GFP; data not shown).
RNA for microarray analysis was isolated 7 days after viral infection from four independently infected cultures, and chromophore-tagged cDNAs were hybridized to human Affymetrix U133A oligonucleotide microarrays to compare the messenger RNA (mRNA) expression profile of 293Top-S37A cells with that of 293Top-GFP cells, as described in greater detail in Materials and Methods. The criteria for identifying genes as up- or downregulated by β-cateninS37A included differential expression of at least two-fold, with a P-value of less than 0.05 using a parametric test.
In all, 62 genes were represented by higher levels of RNA in cells expressing β-cateninS37A than in GFP-expressing cells, and 15 genes were represented by lower RNA levels. Genes previously reported to be regulated by β-catenin-mediated Wnt signaling, such as CCND1 (CyclinD1), ENC1, ABCB1 (MDR1), LEF1, ENPP2 (Autotaxin), MSX1, and MSX2, were among the genes upregulated in the 293 cells expressing β-cateninS37A, as compared to the 293 cells expressing GFP (Supplementary Table 1S). Among the top 20 upregulated genes, we identified several known or suspected proto-oncogenes, such as FGF20 (Jeffers et al, 2001), ETV5 (Ets-5) (Dhulipal, 1997), LMO2 (Rabbitts et al, 1997; Hacein-Bey-Abina et al, 2003), and some genes implicated in Wnt signaling, such as DKK1 (Glinka et al, 1998) and WNT11 (Table I). Reverse transcription followed by semiquantitative polymerase chain reaction-mediated amplification (RT–PCR assay) was used to confirm the findings, with some of the most dramatically upregulated genes by microarray tests (Figure 1A).
Table 1.
List of the twenty most strongly upregulated genes in 293Top cells by mutant β-cateninS37A
| Gene symbol | Gene name | Fold change | P-value | Function |
|---|---|---|---|---|
| FGF20 | Fibroblast growth factor 20 | 17.7 | 0.003 | Signal transduction; cell–cell signaling |
| DKK1 | Dickkopf (Xenopus laevis) homolog 1 | 15.5 | <0.001 | Extracellular Wnt signaling antagonist |
| MEGT1 | Megakaryocyte-enhanced gene transcript 1 | 9.4 | 0.012 | Unknown |
| BIK | BCL2-interacting killer | 9.2 | 0.002 | Apoptotic program; induction of apoptosis |
| EST | GenBank acc. # AK022120 | 9.1 | 0.014 | Unknown |
| ETV5 | Ets variant gene 5 | 6.2 | 0.007 | Transcription factor |
| WNT11 | Wingless-type MMTV integration site, member 11 | 5.3 | 0.006 | Signal transduction; cell–cell signaling; embryogenesis; and morphogenesis |
| SLC2A3 | Solute carrier family 2, member 3 | 5.2 | 0.026 | Glucose transport; carbohydrate metabolism |
| SERPIND1 | Serine proteinase inhibitor, clade D, member 1 | 5 | 0.049 | Plasma glycoprotein; proteinase inhibitor |
| SLC7A8 | Solute carrier family 7, member 8 | 4.9 | 0.007 | Cationic amino-acid transporter |
| ENPP2 | Ectonucleotide pyrophosphatase/phosphodiesterase 2 (autotaxin) | 4.7 | 0.001 | Cell motility; G-protein-linked receptor signaling pathway; transcription factor binding |
| DUSP6 | Dual-specificity phosphatase 6 | 4.6 | 0.024 | Apoptosis; MAPKKK cascade; cell cycle control; inactivation of MAPK |
| LMO2 | LIM domain only 2 (rhombotin-like 1) | 4.2 | <0.001 | Oncogenesis; developmental processes |
| SLC2A14 | Solute carrier family 2, member 14 | 4.0 | 0.001 | Carbohydrate transport |
| ARHGAP26 | GTPase regulator associated with FAK | 3.8 | 0.028 | Neurogenesis; cell growth/maintenance |
| GAD1 | Glutamate decarboxylase 1 (brain, 67 kDa) | 3.6 | 0.003 | Synaptic transmission; glutamate decarboxylation |
| FUT1 | Fucosyltransferase 1 | 3.5 | 0.036 | Carbohydrate metabolism |
| QPTC | Glutaminyl-peptide cyclotransferase (glutaminyl cyclase) | 3.5 | <0.001 | Protein modification |
| RBP1 | Cellular retinol-binding protein 1 | 3.3 | 0.024 | Retinoid binding; vitamin A metabolism |
| ABCB1 |
MDR/TAP member 1 |
3.1 |
0.002 |
Drug resistance; small-molecule transport |
| RNA for microarray analysis was isolated from 293Top cells 7 days after viral infection from four cultures independently infected with either RCAS-β-cateninS37A or RCAS-GFP. The top 20 upregulated genes are shown in descending order with respect to fold increase in RNA levels. P-values were obtained using a parametric test. Known or suspected proto-oncogenes and genes implicated in the Wnt pathway are shown in bold-faced letters. | ||||
Figure 1.
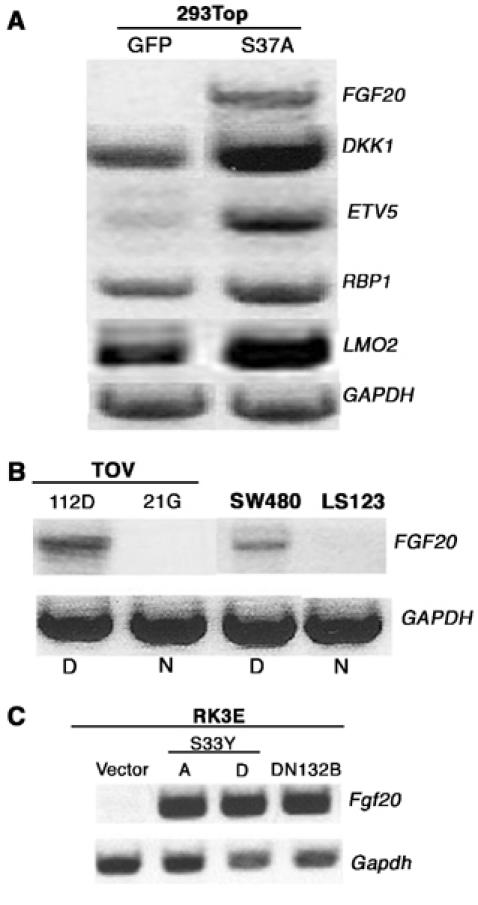
Validation of microarray data by semiquantitative RT–PCR. (A) To verify oligonucleotide microarray results, semiquantitative RT–PCR was used to estimate the amount of RNA from five of the upregulated genes in 293Top cells (Table I). The same total RNA sample was used to prepare probes for microarray hybridization. (B) Expression of FGF20 RNA as assessed by RT–PCR with RNA from OEA cell line TOV112D, ovarian clear cell carcinoma-derived line TOV21G, and colon cancer cell lines SW480 and LS123. The letters D and N refer to β-catenin status (Deregulated and Normal). (C) Measurement of FGF20 RNA from RK3E cells transformed by β-cateninS33Y (clones A and D) and N-terminal-deleted β-cateninΔN132 (ΔN132B) by RT–PCR. GAPDH mRNA was reverse transcribed and amplified to control for the amount of RNA loaded.
FGF20 mRNA is undetectable in 293Top cells that express GFP, although readily observed in cells expressing activated β-catenin. In a survey of normal human tissues with RT–PCR assays, FGF20 RNA was found exclusively in the adult central nervous system, suggesting that expression of FGF20 is tightly controlled in normal development. The gene is, however, expressed in human cancers; for example, FGF20 RNA was detected in five of 15 human colon cancer cell lines (Jeffers et al, 2001). One of those lines, the SW480 line, is known to have deregulated β-catenin due to loss of APC (Munemitsu et al, 1995). We have corroborated this finding with SW480 cells, and we have also found FGF20 RNA in the ovarian endometrioid cell line TOV112D, which contains mutant form of β-catenin (Figure 1B). In contrast, FGF20 RNA is not detectable in TOV21G or LS123, ovarian and colorectal carcinoma lines respectively, harboring wild-type β-catenin (Rutzky et al, 1983; Wu et al, 2001; MN Chamorro, unpublished data, 2004; Figure 1B).
Prior studies have shown that activated mutants of β-catenin promote neoplastic transformation of RK3E cells, a rat epithelial cell line (Kolligs et al, 1999). In agreement with our expression profile of FGF20 in 293Top cells, FGF20 RNA is readily detectable in the β-catenin-transformed RK3E lines by RT–PCR, but not in the parental line (Figure 1C).
Increases in FGF-20 and DKK1 RNA are associated with deregulated β-catenin in human ovarian endometrioid adenocarcinomas
We next extended our findings with 293Top cells by examining the gene expression profiles of our large collection of OEAs. Approximately 40% of these tumors have mutations in CTNNB1 (β-catenin), APC, AXIN1 or AXIN2 (Wu et al, 2001), providing a test of the proposed correlation between Wnt/β-catenin pathway defects and induction of candidate target genes for regulation by β-catenin, as illustrated previously for the β-catenin-regulated genes, ITF-2 and AXIN2 (Kolligs et al, 2002; Leung et al, 2002).
Affymetrix U133A oligo microarrays were again used to profile gene expression in 18 OEAs with an intact β-catenin pathway and 12 OEAs with deregulated β-catenin, in a fashion similar to recently published work using lower density HuGeneFL arrays (Schwartz et al, 2003). By comparing the profiles from tumors with normal versus mutant Wnt/β-catenin pathways, we identified a list of 563 genes differentially regulated by at least 1.75-fold with a P-value of less than 0.05 (Schwartz et al, 2003). To develop a shorter list of genes that are commonly regulated by Wnt/β-catenin signaling in different cell types, we compared our list of regulated genes using modestly different criteria from 293Top cells (two-fold regulation, P<0.05) and OEAs (1.75-fold regulation, P<0.05). By these criteria, at least 17 genes appeared to be regulated in common; 16 of these genes were upregulated and one was downregulated (Table II). It is noteworthy that eight of the 20 most dramatically upregulated genes in 293Top cells are present in the combined list of 16 upregulated genes, including three proto-oncogenes and two genes involved in Wnt signaling (Tables I and II).
Table 2.
List of genes regulated by β-catenin in both 293Top cells and in OEAs
| Gene symbol | Gene name | 293Top fold change | P-values | OEAs fold change | P-values |
|---|---|---|---|---|---|
| FGF-20 | Fibroblast growth factor 20 | 17.70 | 0.003 | 7.39 | <0.001 |
| DKK1 | Dickkopf homolog 1 (Xenopus laevis) | 15.55 | <0.001 | 12.95 | <0.001 |
| ETV5 | Ets variant gene 5 (ets-related molecule) | 6.23 | 0.007 | 1.82 | 0.037 |
| WNT11 | Wingless-type MMTV integration site family, member 11 | 5.32 | 0.006 | 2.20 | 0.011 |
| LMO2 | LIM domain only 2 (rhombotin-like 1) | 4.27 | <0.001 | 4.05 | <0.001 |
| GAD1 | Glutamate decarboxylase 1 | 3.66 | 0.003 | 6.89 | <0.001 |
| QPCT | Glutaminyl-peptide cyclotransferase (glutaminyl cyclase) | 3.57 | <0.001 | 3.90 | <0.001 |
| ABCB1 | ATP-binding cassette, subfamily B (MDR/TAP), member 1 | 3.15 | 0.002 | 1.75 | <0.001 |
| SNK | Serum-inducible kinase | 2.75 | 0.006 | 3.49 | <0.001 |
| IRS1 | Insulin receptor substrate 1 | 2.62 | 0.002 | 2.68 | 0.001 |
| TBX3 | T-box 3 (ulnar mammary syndrome) | 2.49 | <0.001 | 2.17 | 0.024 |
| MSX2 | Msh homeo box homolog 2 (Drosophila) | 2.47 | <0.001 | 6.03 | 0.000 |
| MSX1 | Msh homeo box homolog 1 (Drosophila) | 2.46 | 0.001 | 3.31 | 0.047 |
| DNCI1 | Dynein, intermediate polypeptide 1 | 2.20 | <0.001 | 3.70 | <0.001 |
| CCND1 | Cyclin D1 (PRAD1: parathyroid adenomatosis 1) | 2.05 | <0.001 | 2.90 | 0.001 |
| NMA | Putative transmembrane protein | 2.01 | 0.003 | 7.28 | <0.001 |
| ISYNA1 |
Myo-inositol 1-phosphate synthase A1 |
−2.1 |
<0.001 |
−2.1 |
0.009 |
| Regulated genes are shown in descending order with respect to fold change in upregulation in 293Top cells. | |||||
The two genes that appear to be most highly induced by stabilized β-catenin in both the 293Top cell system and in OEA primary tumors are FGF20 and DKK1 (Table II). Figure 2A demonstrates that both of these genes are expressed at markedly elevated levels in eight of 12 OEAs harboring mutations that deregulate β-catenin, but neither was induced in the 19 tumors lacking such mutations.
Figure 2.
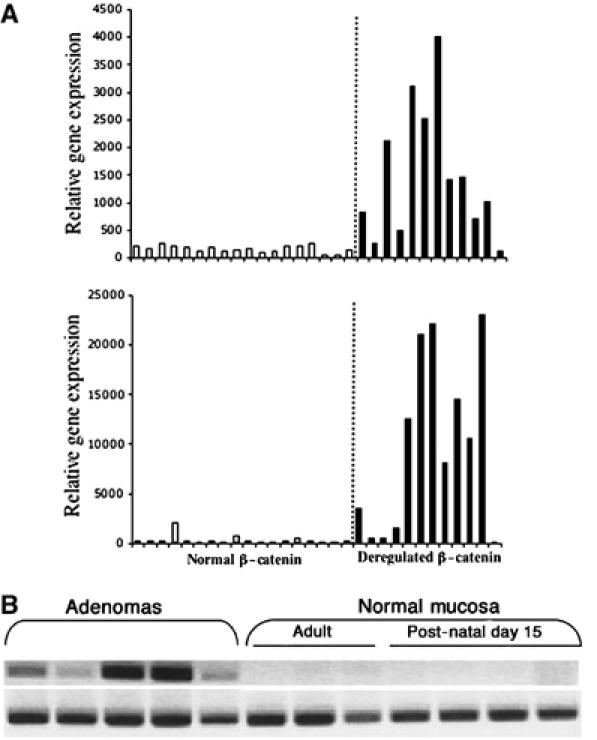
FGF20 and DKK1 gene expression in individual human and mouse tumors. (A) FGF20 (upper panel) and DKK1 (lower panel) gene expression in OEAs. Relative RNA levels were determined by Affymetrix microarray data analysis. White boxes represent the relative gene expression in tumors with an intact Wnt pathway and black boxes represent tumors with a deregulated Wnt pathway. (B) FGF20 RT–PCR products using 200 ng of RNA from adenomas from ApcMin/+ mice and from normal intestinal mucosa samples from 15-day-old and adult ApcMin/+ mice.
FGF-20 is expressed in adenomas from ApcMin/+ mice but not in normal intestinal mucosa
Of the suspected proto-oncogenes on our combined list of candidate targets for regulation by β-catenin, FGF20 displayed the strongest correlation between RNA levels and the status of the Wnt/β-catenin pathway in OEA tumors. We therefore extended our studies of FGF20 expression to other tumor types.
In mice heterozygous for loss-of-function mutations at the Apc locus, such as ApcMin/+ mice and other heterozygous Apc knockout mice, loss of the wild-type allele initiates adenoma formation in the small intestine (Oshima et al, 1995). Using RT–PCR to detect expression of RNA, we found little or no Fgf20 RNA in non-neoplastic intestinal tissues from 15-day-old and adult ApcMin/+ mice, but readily detected Fgf20 RNA in all the adenomas analyzed (Figure 2B).
Stabilization of β-catenin in colonic epithelial cells by loss of APC is an early event in the majority of human colon cancers (Polakis, 2000). In preliminary experiments, we used the RT–PCR assay to measure FGF20 RNA in six primary human colon adenocarcinomas and in three samples of normal colon mucosa. We found FGF20 RNA in half of the tumors, but in none of the normal mucosas (Supplementary Figure IS).
XFGF20 and Xdkk-1 are downstream of the Wnt pathway in Xenopus laevis embryos
Much of our knowledge about the Wnt pathway and its role in vertebrate development comes from studies in Xenopus laevis (reviewed in Harland and Gerhart, 1997; Huelsken and Birchmeier, 2001). We therefore sought to extend the evidence for regulation of some of our candidate genes by the Wnt/β-catenin pathway by testing for transcripts in Xenopus embryos at relevant times in development. Both FGF20 and DKK1 were first identified in X. laevis (Glinka et al, 1998; Koga et al, 1999) and are closely related to their human counterparts (79% amino-acid identity between Xenopus and human FGF-20; 56% identity between Xdkk-1 and human DKK-1). Xdkk-1 is expressed at the beginning of gastrulation (stage 10) in the future anterior endomesoderm, on the dorsal side of the embryo (Glinka et al, 1998). The localization of XFGF20 RNA was previously reported for the neurula stage, but the RNA could be detected from late blastula onwards by RT–PCR (Koga et al, 1999). As in Xenopus early developmental stages are the most accessible experimentally, we confirmed zygotic expression from stage 10 onwards (Figure 3B, left panel), and localized XFGF20 RNA by RT–PCR exclusively to the equatorial region (the marginal zone) of stage 10 embryos, which is fated to produce mesoderm, (Figure 3B, right panel). The pattern of XFGF20 expression is similar to that described for other FGF genes like eFGF and XFGF3 (Isaacs et al, 1994; Schohl and Fagotto, 2003), and for a group of genes expressed in the marginal zone, which require a zygotic Wnt pathway (Figure 3A), such as XmyoD and Xbra (Hoppler et al, 1996; Vonica and Gumbiner, 2002).
Figure 3.
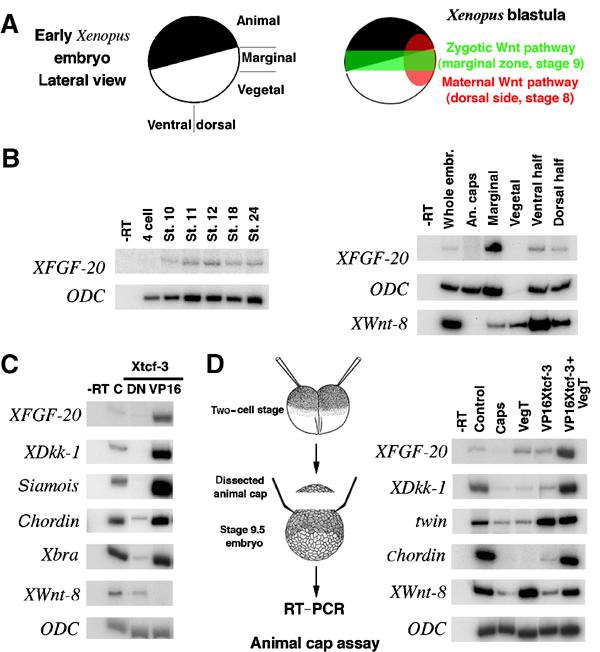
Wnt signaling regulates XFGF20 and Xdkk-1 expression in Xenopus embryos. (A) Schematic description of a pregastrula Xenopus embryo (left panel) and the localizations and timing of the maternal and zygotic Wnt pathways (right panel). (B) XFGF20 is expressed zygotically and localized exclusively in the marginal zone. RT–PCR for XFGF20 in various developmental stages (left panel) and various locations of stage 10 (early gastrula) embryos (right panel). ODC RNA and Xwnt-8 RNA serve as controls for amounts loaded and localization (ODC is normally expressed weakly in vegetal cells). (C) Modulation of the Wnt signaling pathway alters XFGF20 and Xdkk-1 expression. Embryos were injected marginally in each cell at the four-cell stage with 100 pg DN-Xtcf-3 RNA or 20 pg VP16-Xtcf-3 RNA, and collected at stage 9.5 for RT–PCR analysis. XFGF20, Xdkk-1, siamois, chordin, and Xbra all required early activation of the Wnt pathway, while Xwnt-8 is repressed under the same conditions. (D) Synergistic effect of the Wnt pathway and the endomesoderm inducer VegT on ectopic expression of XFGF20 and Xdkk-1 in animal caps. Two-cell stage embryos were injected in the animal pole of each cell with 400 pg VegT RNA or 10 pg VP16 Xtcf-3 RNA, as indicated, and animal caps were cut and collected at stage 9.5 for RT–PCR analysis.
Activating or inhibiting the Wnt pathway had a dramatic effect on FGF20 and DKK1 expression. We injected RNA encoding an activated form of the downstream component of the pathway (VP16Xtcf-3) or a dominant-negative mutant (DNXtcf-3) (Vonica et al, 2000) into the equatorial zone of each blastomere at the four-cell stage (Figure 3C). XFGF20 and Xdkk-1 RNAs were increased by VP16Xtcf-3, and undetectable when Wnt signaling was blocked with DNXtcf-3RNA. Known direct (siamois) (Carnac et al, 1996; Brannon et al, 1997) and indirect (chordin) (Kessler, 1997) target genes of the maternal dorsal Wnt pathway (Figure 3A), as well as the zygotic Wnt target and mesodermal marker Xbra (Smith et al, 1991; Vonica and Gumbiner, 2002), showed a similar response. In contrast, Xwnt-8, a marker of ventral mesoderm (Christian et al, 1991) inhibited by the maternal Wnt pathway, retained expression in embryos injected with DNXtcf-3 RNA, but was absent when VP16Xtcf-3 RNA was injected.
We also asked whether ectopic activation of the Wnt pathway in animal cap explants, which are normally fated to produce only ectoderm, could induce expression of XFGF20 and Xdkk-1 (Figure 3D). On its own, VP16Xtcf-3 can induce both genes only slightly, but induction was significantly enhanced by co-injection of the endomesoderm inducer VegT (Kofron et al, 1999). The indirect target chordin was similarly activated, and the direct Wnt target twin, a homologue of siamois, was strongly activated with VP16Xtcf-3 alone. We conclude that expression of XFGF20 and Xdkk-1 in animal caps is augmented by Wnt signaling. The differences in embryonic expression patterns between the two genes could be a consequence of different requirements for cooperative signaling pathways, responses to different Wnt ligands, or both.
FGF-20 and DKK1 are direct targets of the canonical Wnt signaling pathway
To differentiate between the direct and indirect effects of β-catenin stabilization on the control of FGF20 and DKK1, we took advantage of a dexamethasone-inducible form of VP16 Xtcf-3 (TVGR) (Darken and Wilson, 2001). TVGR RNA was injected alone or in combination with VegT RNA in the animal pole of two cell stage Xenopus embryos (Figure 4). Animal caps were dissected at stage 8.5 and dexamethasone was added in the presence or absence of cycloheximide, an inhibitor of translation. Under these conditions, the transcription of only those genes directly activated by VP16 Xtcf-3 will increase upon addition of the inducer. Cycloheximide treatment decreased the levels of XFGF20 and Xdkk-1 RNA in VegT and TVGR-injected embryos, but addition of dexamethasone significantly raised them. siamois, a direct Tcf target, and, less dramatically, Xbra, showed similar variation. On the contrary, levels of chordin RNA, an indirect Wnt target, did not respond to dexamethasone when translation was inhibited. The low level of siamois induction and the absence of chordin stimulation upon addition of dexamethasone are due to the late timing of Wnt activation, at the limit of competence for activation of dorsal genes (Darken and Wilson, 2001). In conclusion, the injection experiments in frog embryos suggest that expression of XFGF20 and Xdkk-1 is subject to direct regulation by the Wnt/β-catenin signaling pathway.
Figure 4.
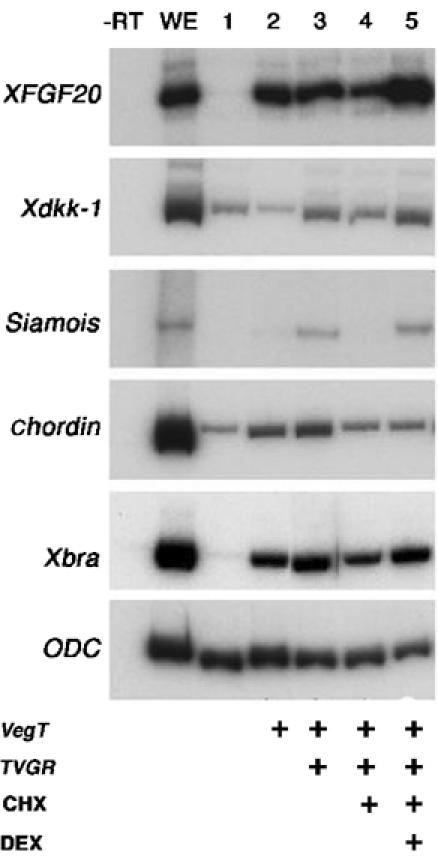
Xenopus XFGF20 and Xdkk-1 are direct targets of the Wnt pathway. Embryos were injected at the two-cell stage in both blastomeres with 400 pg VegT RNA and 20 pg TVGR RNA, and treated with cycloheximide (CHX) and dexamethasone (DEX) as indicated. XFGF20 and Xdkk-1 were induced when DEX was added to CHX-treated caps (compare lanes 4 and 5). Siamois is a control for direct induction by the Wnt pathway, and chordin for indirect induction.
To address the implied direct relationship between the Wnt/β-catenin pathway and the regulation of FGF20 and DKK1 more rigorously, we have performed chromatin immunoprecipitation (ChIP) assays with an anti-β-catenin antibody in the 293Top cells (for ChIP methodology, see Orlando, 2000; Weinmann et al, 2001). Stabilized β-catenin accumulates in the nucleus in a complex with TCF, and the heterodimer influences transcription by binding to TCF recognition sites in DNA (Huelsken and Behrens, 2002). Regulatory domains of direct β-catenin/TCF targets are therefore expected to be enriched in anti-β-catenin immunoprecipitates, as compared to immunoprecipitates obtained with a control antibody. Formaldehyde-fixed chromatin from 293 cells expressing either β-cateninS37A or GFP was subjected to immunoprecipitation with either a rabbit anti-β-catenin antibody or an irrelevant rabbit antibody as a negative control. To determine whether immunoprecipitates were enriched for regulatory DNA sequences from the putative target genes DKK1 and FGF20, we used PCR to amplify the precipitated DNA and determined the amounts of DKK1 and FGF20 regulatory regions as well as DNA from the presumably irrelevant genes GAPDH and LMO7, neither of which shows evidence of regulation by Wnt/β-catenin signaling. As illustrated in Figure 5, the approximately 500 bp regions immediately upstream of the transcript start sites of the DKK1 and FGF20 genes (see Figure 6A for amplified promoter regions) were enriched in the anti-β-catenin immunoprecipitates from cells expressing S37A β-catenin, compared to immunoprecipitates formed with control antibody; no differences were seen with tests of the promoter regions of GAPDH and LMO7. Moreover, no differences in the amounts of these putative regulatory domains were seen with cells expressing GFP. In addition, as positive controls, we showed that β-catenin–DNA complexes from 293Top cells expressing β-cateninS37A are enriched for the promoter regions of the pOT reporter and from the CCND1 (Cyclin D1) gene (Figure 5); the latter has been shown to be a direct target of the β-catenin/TCF complex (Tetsu and McCormick, 1999). Our ChIP data strongly suggest that DKK1 and FGF20, as well as CCND1 and the pOT reporter, are direct targets of β-catenin.
Figure 5.
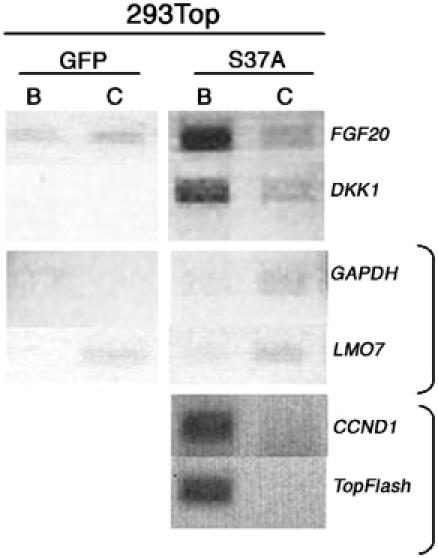
ChIP assay in 293Top cells shows that FGF20 and DKK1 are direct targets of β-catenin. Formaldehyde-fixed chromatin from 293Top cells infected either with RCAS-β-cateninS37A or RCAS-GFP was immunoprecipitated either with anti-β-catenin antibody (B) or with control isogenic antibody (C). The immunoprecipitated DNAs were PCR-amplified using primers mapping to the proximal promoter sequences of FGF20 and DKK1. Amplification of the GAPDH and LMO7 promoters was used as a negative control, and amplification of the TOPFLASH and CCND1 (Cyclin D1) promoters was used as a positive control.
Figure 6.
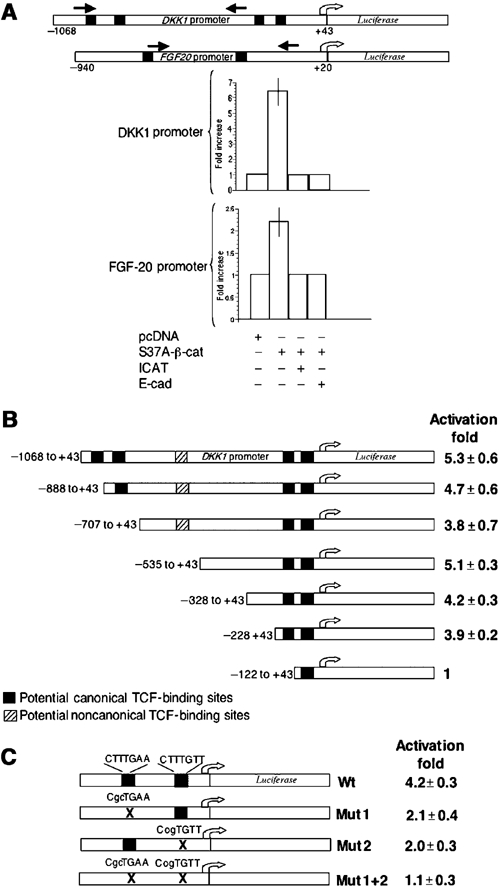
Mapping regions required for response to β-catenin in human DKK1 and FGF20 promoters reporter constructs in 293 cells. (A) Depicted at the top are schematic diagrams of the promoter reporter constructs; black boxes represent potential LEF/TCF binding sites. Black arrows represent location of primers used to amplify immunoprecipitated chromatin in the ChIP assays. The relative activity of the promoter constructs was assessed in 293 cells after transient co-transfections with either empty vector or vector expressing β-cateninS37A in the presence or absence of vectors expressing either ICAT or the C terminus of E-cadherin. To control for transfection efficiency, firefly luciferase activity was normalized to results with Renilla luciferase. (B) DKK1 promoter deletion analysis. 5′ deletions of pDKK1-luciferase were generated using PCR, and their relative activities were measured after transient co-transfections with either empty vector or vector expressing β-cateninS37A, as described in Materials and methods. (C) Mutagenesis analysis of potential LEF/TCF-binding sites in the -–328 to +43 DKK1-luciferase construct. Mutations in the putative TCF-binding sites were generated using QuickChange Site Directed Mutagenesis Kit (Stratagene), and their relative activities assessed as described for panel B.
β-Catenin regulates FGF-20 and DKK1 transcription in 293 cells and Xenopus embryos via TCF-binding sites proximal to the promoter
To further investigate the regulation of DKK1 and FGF20 by β-catenin, genomic fragments containing regions 5′ to the transcriptional start sites were placed upstream of a luciferase reporter gene (DKK1-luciferase and FGF20-luciferase); these constructs were then used to study regulation of luciferase production by mutant β-catenin in 293 cells and in Xenopus embryos.
Inspection of the sequences of the putative regulatory regions revealed four potential TCF-binding sites (CTTTGA/TA/T) in DKK1 and two in FGF20 (Figure 6A). Transient co-transfection of 293 cells with either of the luciferase reporters and a plasmid encoding β-cateninS37A stimulated the DKK1-luciferase reporter about five-fold and the FGF20-luciferase reporter about two-fold. These effects were abolished when ICAT, a β-catenin inhibitor (Tago et al, 2000), or the C-terminus of E-cadherin, which binds to β-catenin, was co-transfected with β-cateninS37A (Figure 6A). The low activity of the FGF20-luciferase reporter suggests that it probably lacks other regulatory sequences for optimal response to mutant β-catenin. Parallel tests with a semi-quantitative RT–PCR assay showed that mutant β-catenin increased the levels of DKK1 RNA from the endogenous gene to the same extent as it increased the luciferase activity from the DKK1 reporter construct (data not shown), suggesting that the DKK1 reporter contains most or all of the sequences necessary for regulation by β-catenin.
A series of 5′ deletions was then used to map the regions of the regulatory domain of the DKK1 promoter required for β-catenin stimulation. No significant changes were observed until the deletions reached the region containing the two TCF-binding sites proximal to the start site (Figure 6B). Site-directed mutations of either of these two sites partially impaired the response β-catenin, while mutations affecting both sites resulted in complete loss of activity (Figure 6C).
Sequence conservation in the regulatory elements of vertebrate promoters is well documented to have occurred during evolution. We therefore tested luciferase reporter constructs containing the human FGF20 and DKK1 promoters in transcription assays in Xenopus embryos (Figure 7). Both wild-type reporters responded strongly to co-injected VP16 Xtcf-3 RNA (Figure 7A and B), while the TCF site-mutated reporters were considerably less sensitive, demonstrating that the Wnt effect is mediated mainly by these sites. In addition, the human FGF20 promoter showed high levels of activity when injected alone in Xenopus embryos, with the greatest activity in the marginal zone, corresponding to the normal pattern of expression of the Xenopus homologue (not shown). This activity was also dependent on intact TCF sites, as the reporter with the mutant TCF sites was significantly less active (Figure 7C). We note that at stage 9 the wild-type FGF20 promoter was more active dorsally, probably due to an earlier onset of transcription there, as has been reported for Xbra (Lerchner et al, 2000; Vonica and Gumbiner, 2002).
Figure 7.
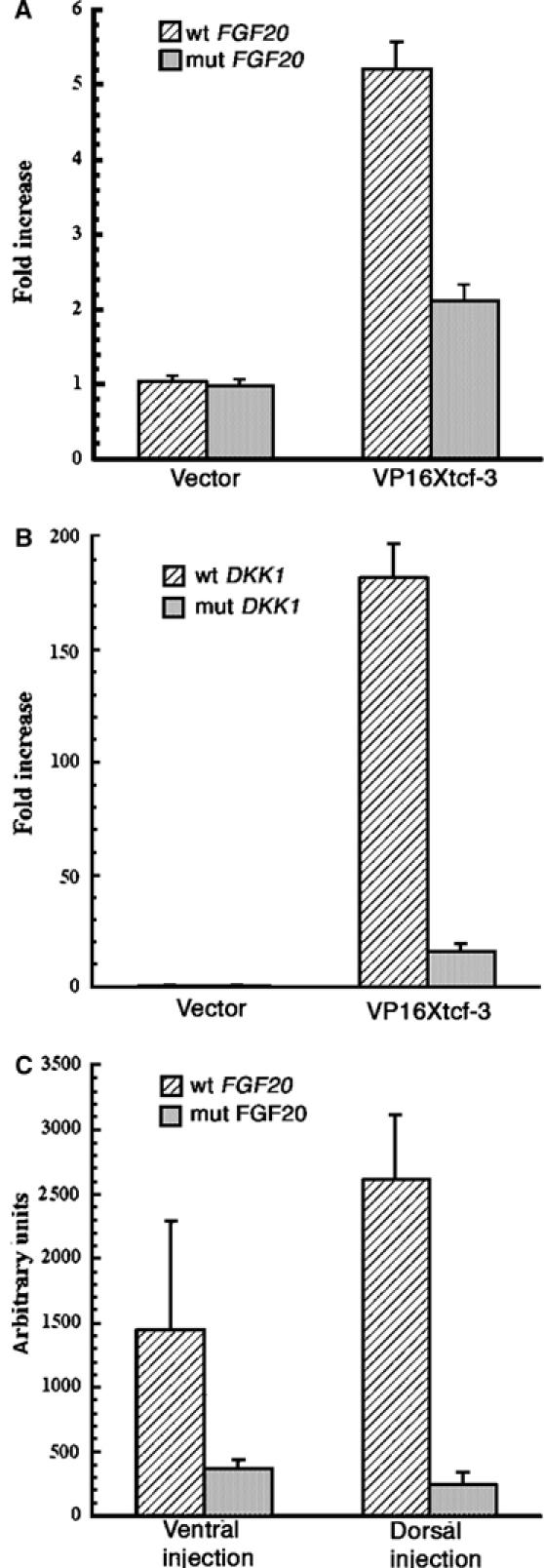
Activation of the human FGF20 and DKK1 promoters by VP16 Xtcf-3 in Xenopus embryos is dependent on intact TCF-binding sites. (A) VP16 Xtcf-3 activates the human FGF20 promoter. Two-cell stage embryos were injected in the animal pole with 20 pg FGF20-luciferase reporter plasmids and 20 pg VP16 Xtcf-3 RNA, and collected at stage 10 for luciferase assays. Mutating the TCF-binding sites reduced activation from 5.2- to 2.1-fold. (B) Activation of the human DKK1 promoter by VP16 Xtcf-3 in Xenopus requires intact TCF-binding sites. In all, 40 pg of wild-type of mutant mouse DKK1-luciferase reporter gene were co-injected with 100 pg VP16 Xtcf-RNA in the animal pole at the two-cell stage and recovered at stage 10. Mutating the TCF-binding sites reduces activation from 180- to 16-fold. (C) Early activation of the human FGF20 promoter by Xenopus endogenous factors is dependent on functional TCF sites. Embryos injected dorsally or ventrally with 40 pg wild-type or mutant reporter DNA were recovered at stage 9 for luciferase assays.
FGF-20 small inhibitory RNA (siRNA) interferes with β-catenin-mediated transformation of RK3E cells
Mutant β-catenin promotes the neoplastic transformation of RK3E cells and renders them capable of anchorage-independent growth, as judged by formation of colonies in soft agar (Kolligs et al, 1999). As shown above, RK3E cells transformed by β-catenin express Fgf20 (Figure 1), and FGF-20 has been reported to induce morphological transformation of mouse 3T3 cells (Jeffers et al, 2001). To evaluate the possibility that production of FGF-20 is required to maintain the transformed phenotype of RK3E cells expressing ectopic β-catenin, we attempted to block the expression of the FGF20 gene in those cells using siRNAs (Brummelkamp et al, 2002).
To this end, we constructed plasmids encoding two siRNA hairpins based on the rat FGF20 gene sequence (siFGF20.A and siFGF20.B); an additional plasmid (siFGF20.C) encodes a mutant form of one of these siRNAs. We initially showed that siFGF20.A and siFGF20.B could reduce the amounts of FGF-20 protein in RK3E cells programmed to express a V5-tagged version of rat FGF-20 (RK3E-FGF20V5; Figure 8A). Subsequently, the two wild-type constructs and the mutant construct, siFGF20.C, were transfected into RK3E cells stably transformed by DNA encoding a mutant β-catenin (clone S33YA; Figure 1). Selection for a co-transfected drug resistance gene generated three polyclonal cultures, RK3E-S33YA-siFGF20.A, RK3E-S33YA-siFGF20.B, and RK3A-S33YA-siFGF20.C, which were used in the subsequent experiments. The cells producing either of the wild-type siRNAs for rat FGF20 contained relatively low levels of FGF20 RNA as compared to the line producing the mutant siRNA, RK3A-S33YA-siFGF20.C, and the starting line, S33YA (Figure 8A and data not shown). When we attempted to grow cells from each of these cultures in soft agar, we found that the cells in which FGF-20 production was inhibited (RK3E-S33YA-siFGF20.A and RK3E-S33YA-siFGF20.B) formed few colonies, whereas the parental cell line and cells expressing the mutant form of FGF20 siRNA formed colonies efficiently in soft agar (Figure 8C). We conclude that continued production of FGF-20 is necessary for maintenance of the anchorage-independent growth capacity of RK3E cells expressing mutant β-catenin.
Figure 8.
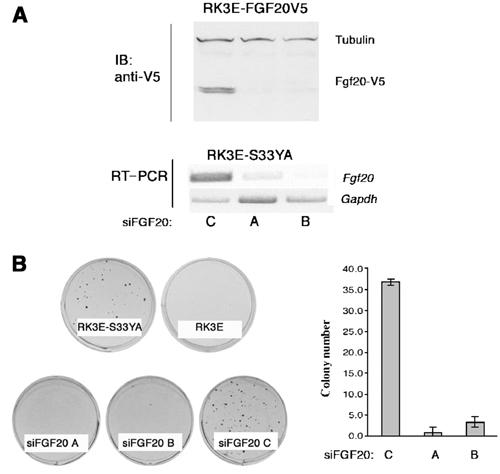
FGF20 siRNA interferes with β-catenin-mediated growth of RK3E cells in soft agar. (A) Effect of FGF20 siRNAs on RK3E cells. Top panel: RK3E cells expressing a v5-tagged version rat FGF20 were transiently transfected with wild-type (lanes A and B) and mutant (lane C) rat FGF20 siRNAs. Lower panel: semiquantitative RT–PCR was carried out with RNAs from RK3E-S33YA cells stably transfected with FGF20 siRNAs. (B) Soft agar assay. 105 cells were plated in 0.4% agarose and incubated for 3 weeks. Cultures were fixed with 1% paraformaldehyde and stained with 0.1% methylene blue and counted. RK3E-S33YA cells form colonies in soft agar as opposed to RK3E cells. Stable RK3E-S33YA cells expressing wild-type FGF20 siRNAs (siFGF20.A and siFGF20.B) formed colonies at a lower efficiency than cells expressing the scrambled FGF20 siRNA. Bar graph at the left represents the number of visible colonies obtained for each cell line from a typical experiment performed in triplicates.
Discussion
The diverse phenotypic changes that occur in different cell types in response to signaling through the Wnt/β-catenin pathway are thought to result principally from altered regulation of transcription rates in response to changes in the nuclear concentration of β-catenin/TCF heterodimers. Consequently, many recent studies have been directed towards the identification of genes that are represented in mRNA pools at higher or lower levels when the Wnt/β-catenin pathway is activated (Conacci-Sorrell et al, 2002; Kielman et al, 2002; van de Wetering et al., 2002; Willert et al, 2002; Schwartz et al, 2003; Shimokawa et al, 2003). Since this pathway governs crucial developmental events and contributes to neoplasia in a variety of cell lineages in many metazoan organisms, these studies of transcriptional control are likely to yield complex results, including the identification of genes that are secondarily regulated by Wnt/β-catenin signaling.
In this study, we have identified and focused on a small number of genes that appear to be strongly induced at the transcriptional level when β-catenin concentrations rise under several biological circumstances: ectopic expression of mutant β-catenin in cultured cell lines; mutations affecting components of the Wnt/β-catenin pathway in cancers arising in intestinal and ovarian cell types; and developmental events involving Wnt/β-catenin signaling in normal Xenopus embryos. Our study was designed to identify novel genes directly regulated by β-catenin that are potentially relevant to cancer development. To achieve this goal, we employed microarray technology to identify genes up- and downregulated, directly or indirectly, in human epithelial (293) cells expressing mutant (stabilized) β-catenin versus control GFP vector. We then compared this list of genes to a similar list obtained by assessing gene expression in a well-characterized set of primary human OEAs with and without Wnt pathway defects. ChIP assays in 293 cells and experiments in frog embryos using an inhibitor of translation were then undertaken to determine whether candidate genes were likely to be direct or indirect tagets of β-catenin/TCF.
We have chosen for special attention two genes that have not previously been reported to be regulated by Wnt/β-catenin signaling, DKK1 and FGF20. These two genes are highly induced by stabilized β-catenin in both the 293Top cell system and in OEA primary tumors (Table II). Both of these genes are expressed at markedly elevated levels in eight of 12 OEAs tumors harboring mutations that deregulate β-catenin, but neither was induced in the 19 tumors lacking such mutations. We do not know why some tumors with deregulated β-catenin do not have increased levels of FGF20 or DKK1 RNA. One possible explanation is that these genes are under the control of other pathways in addition to the Wnt/β-catenin pathway; therefore, it is the integration of signals in the nucleus that dictates the outcome in some cases. Indeed, it has been shown that DKK1 is also regulated by Bmp signaling and the AP-1 family member c-jun (Grotewold and Ruther, 2002). Another explanation would be that the tumors without elevated FGF20 and DKK1 RNAs might have lower levels of β-catenin activity. Very little is known about the regulation of FGF-20, but our data from Xenopus experiments (see below) suggest that it is probably complex. However, in most tumors we studied, activation of the Wnt/β-catenin pathway correlated with augmented expression of these two genes.
We have shown, using ChIP assays, that FGF20 and DKK1 are direct targets of β-catenin in 293Top cells. We have also demonstrated the direct target relationship between the Wnt pathway and expression of XFGF20 and Xdkk-1 in X. laevis using a dexamethasone-inducible form of VP16-Xtcf3 in the presence of dexamethasone and cyclohexamide, and have defined the minimal region necessary for β-catenin regulation of the DKK1 promoter in 293 cells using nested deletions. Mutational analysis of both promoters revealed that they are dependent on LEF/TCF-binding sites in 293Top cells and Xenopus embryos.
Our observations in frog embryos demonstrate that Wnt activation of FGF20 and DKK1 reflects a relation between the Wnt/β-catenin pathway and these targets during normal development; thus, upregulation of these is not an anomaly resulting from highly deregulated β-catenin activity in tumors and in cultured cells. However, it is unlikely that expression of these two genes in intact embryos at the late blastula–early gastrula stages depends on the same Wnt ligand, since patterns of expression for Xdkk-1 and XFGF20 in late blastula and early gastrula Xenopus embryos differ markedly. While Xdkk-1 is present from stage 10 in anterior endomesoderm, forming the leading edge of the dorsally ingressed cells during gastrulation (Glinka et al, 1998), we find XFGF20 to be expressed in the marginal zone, in a territory fated to become mesoderm after gastrulation. The pattern of XFGF20 expression fits a pattern common to an increasing number of genes expressed in the marginal zone. These genes, which have been linked to a zygotic marginal Wnt pathway, include XmyoD (Hoppler et al, 1996; Hamilton et al, 2001), Xpo (Hamilton et al, 2001), Xbra (Vonica and Gumbiner, 2002), and XFGF3 (Schohl and Fagotto, 2003).
The induction of DKK1 by the Wnt/β-catenin pathway is of particular interest because DKK1 encodes a secretory protein that serves as a negative regulator of Wnt binding to the LRP component of the receptor complex (Glinka et al, 1998; Semenov et al, 2001). This implies that signaling events initiated by production of Wnt proteins during normal development might be terminated by production of this inhibitor. In contrast, activation of the Wnt/β-catenin pathway during tumorigenesis by mutations affecting intracellular components of the pathway would be resistant to this auto-regulatory loop. Although upregulation of DKK1 by mutated β-catenin in tumors should not affect β-catenin/TCF activity in cells harboring the oncogenic mutation, it might affect Wnt signaling to stromal cells, as recently suggested by the observation that myeloma cells producing DKK-1 are often associated with lytic bone lesions in patients with multiple myeloma (Tian et al, 2003).
DKK1 and other genes encoding extracellular inhibitors of Wnt signaling are not the only means for auto-inhibition of Wnt signaling. Wnt signaling is also subject to negative feedback regulation in the cytoplasm. For instance, Conductin/Axin2, which binds to β-catenin and induces its degradation, has been shown to be a direct transcriptional target of β-catenin/TCF (Jho et al, 2002; Leung et al, 2002). The ubiquitin receptor ligase, β-TrCP, which is involved in targeting β-catenin to proteosomes, is also activated by Wnt/β-catenin signaling (Spiegelman et al, 2000). In addition, Wingless, the Wnt-1 ortholog in Drosophila, induces the direct expression of naked cuticle, which inhibits Dishevelled and blocks further signaling (Rousset et al, 2001).
FGF20, the other gene induced by Wnt/β-catenin signaling that we have studied intensively in this report, belongs to a large class of genes, the FGFs, encoding secretory proteins with potent mitogenic and angiogenic roles in embryonic development, wound healing, and tumor development (reviewed in Dickson et al, 2000; Powers et al, 2000). Furthermore, activation of expression of FGF3, FGF4, and FGF8 by the mouse mammary tumor virus (MMTV) initiates tumor development in the mammary glands of infected female mice, in a manner similar to activation of Wnt genes. The recently discovered FGF20 gene is expressed during early embryonic development and exclusively in the nervous system in adults (Koga et al, 1999; Jeffers et al, 2001). Ectopic overexpression of FGF20, as for other members of the FGF family, has been shown to transform cells in culture and make them capable of forming tumors in nude mice (Jeffers et al, 2001). Some FGFs in addition to FGF20 also appear to be transcriptional targets for Wnt/β-catenin signaling. FGF4 has recently been reported to be a direct target of β-catenin (Kratochwil et al, 2002), and FGF9 RNA was reported to be present at elevated levels in OEAs with deregulated β-catenin (Schwartz et al, 2003). In Xenopus, expression of XFGF3 was shown to be regulated by the maternal β-catenin activity (Schohl and Fagotto, 2003). Recently, FGF18 was also shown to be a direct target of β-catenin and upregulated in human colon cancers; furthermore, interference with expression of FGF18 suppresses the growth of colon cancer cells in culture (Shimokawa et al, 2003).
Based on the evidence of the involvement of FGFs in tumorigenesis, we have asked whether cell transformation by mutant β-catenin depends on induction of FGF20. Using plasmids encoding inhibitory hairpin RNAs directed against FGF20 RNA, we have found that, when concentrations of FGF20 RNA and protein are reduced in β-catenin transformed rat epithelial cells, the capacity to form anchorage-independent colony is also reduced (Figure 8). The potential relevance of activated FGF20 in β-catenin-induced cancers is further supported by our finding that FGF20 is actively expressed in all five adenomas examined from Min mice, and by our preliminary observations that a subset of human colon cancers and cell lines express FGF20 RNA, whereas normal colon tissues do not (Supplementary data). This is consistent with a large body of evidence that deregulated β-catenin signaling is common in such tumors (Polakis, 2000).
FGF20 and members of the FGF family are not the only presumptive or known proto-oncogenes reported to be induced by Wnt/β-catenin signaling. For example, two well-established proto-oncogenes, c-MYC (He et al, 1998) and CCND1 (CyclinD1) (Shtutman et al, 1999; Tetsu and McCormick, 1999) have been shown to be directly targeted by β-catenin/TCF complexes in colorectal tumor cell lines with APC mutations or activating β-catenin mutations. An oncogenic role for these proto-oncogenes in cancers in which β-catenin is deregulated is possible but not yet proven. On the other hand, genetic experiments with CCND1-deficient mice suggested that Cyclin D1 is not essential for intestinal tumorigenesis in animals carrying the Apcmin allele (Wilding et al, 2002). Analogous experiments with FGF20 null mutants might help to establish the role this gene plays in Wnt/β-catenin-mediated tumorigenesis in the colon, breast and other organs. If FGF20 is commonly induced in various human neoplasms, it might provide a useful marker for detecting cancer through noninvasive methods; in addition, if the gene has a functional role in tumorigenesis, it might also be a useful and accessible therapeutic target.
We concentrated on FGF20 and DKK1 because they were dramatically upregulated in two different settings—a cultured human cell line expressing mutant β-catenin and ovarian tumors with mutations in the Wnt signaling pathway—as observed when we merged lists of Wnt/β-catenin-regulated genes generated by studies being undertaken in our two laboratories. In addition, we chose to study these genes because FGF20 and DKK1 have not been previously reported to be directly regulated by this signaling pathway, and because they are likely to be important targets for transcriptional control in view of their putative roles, respectively, as a proto-oncogene and an inhibitor of Wnt signaling. We note, however, that our merged list of regulated genes contains several other target genes, directly or indirectly regulated by β-catenin, of potential significance with respect to cell signaling and oncogenesis, including LMO2, ETV5 and WNT11. Recently, it has been reported that Wnt-11 promotes transformation of intestinal epithelial cells in culture (Ouko et al, 2004). We are currently investigating, using ChIP assays, whether these genes are directly regulated by β-catenin.
The strategy of focusing on genes that appear to be regulated by Wnt/β-catenin signaling in a multiplicity of settings may have limitations; it is increasingly apparent that transcriptional control reflects the interactions of multiple factors and that the abundance of these factors varies among cell types, thereby diversifying the readout of stereotyped signaling pathways. For instance, levels of c-MYC RNA are increased by the Wnt/β-catenin pathway in colorectal cancer cells, yet no changes were observed in the systems we used here or in immature CD34+ thymocytes stimulated by activation of the Wnt/β-catenin pathway (Staal et al, 2004). However, LMO2, one of the genes most affected by Wnt/β-catenin signaling in our cell and tumor systems, is also highly upregulated in thymocytes (Staal et al, 2004). In this light, it is probable that important regulatory events will occur uniquely in certain cell types; the challenge will be to identify the crucial events by determining the functional consequences of the many changes in gene expression that result from cell signaling.
Materials and methods
Cell lines and tumor samples
To generate 293Top cells, the kidney embryonic epithelial cell line 293 was first transfected with pcDNA6-tva (Fisher et al, 1999), a construct expressing the ALV receptor Tva, using stable calcium phosphate (Stratagene) according to the manufacturer's instructions. After selection with 5 μg/ml blastocidin, one clone was chosen for transfection with the β-catenin luciferase reporter pOT and a plasmid carrying a neomycin-resistant gene. The pOT reporter consists of three LEF/TCF-binding sites and a minimal promoter driving the luciferase gene (Rubinfeld et al, 1993). Cells were then selected in 5 μg/ml blastocidin and 200 μg/ml Geneticin (Life Technology, Inc.) and pooled. The rat kidney epithelial cell lines RK3E, RK3E/S33Y-A, RK3E/S33Y-D, and RK3E/DN132-B were kindly provided by Dr Eric Fearon (University of Michigan) and have been described previously (Kolligs et al, 1999; Schwartz et al, 2003). The ovarian endometrioid tumor sample collection has been described previously in detail (Wu et al, 2001). Quick-frozen human colon tumors and normal colon mucosa were obtained from the MSKCC tumor bank collection. Quick-frozen adenomas and normal small intestinal musosa from Apcmin/+mice were kindly provided by Dr Bert Vogelstein (John Hopkins University).
Viral constructs and infections
The RCAS-GFP virus has been described previously (Holland, 2000). HA-tagged β-cateninS37A cDNA was excised from pMH-S37Aβ-catenin (Zorn et al, 1999) with Asp718 and Apa1, treated with T4 polymerase to form blunt ends, and cloned into the Pme1 site of the ALV retroviral vector RCAS-Y (Dunn et al, 2000). To produce recombinant retroviruses, the retroviral constructs were transfected into DF-1 chicken fibroblasts as described previously (Himly et al, 1998; Schaefer-Klein et al, 1998). To transduce β-cateninS37A and GFP cDNAs into 293Top cells, supernatants from virus producing DF-1 cells were filtered and diluted 1:1 with fresh growth media before infecting cells plated at 10% confluency. This process was repeated 24 h later.
Data analysis
To analyze the expression data, we used two complementary methods. Our first approach was to use the MAS 5.1 (Affymetrix) software to perform a cross-comparison of each experimental sample (293TopS37A) with each of the four reference samples (293TopGFP). We selected 2041 genes that demonstrated at least a two-fold difference in expression in one or more of these comparisons (list 1). As an alternative, we utilized the Genespring 6.0 (Silicon Genetics) software package to identify genes differentially expressed between 293TopS37A and 293TopGFP samples, using a parametric test with filtering (P<0.05) on variances estimated by cross-gene error model (also known as the Rocke-Lorenzato model; Box et al, 1978; Milliken et al, 1984). With this second statistical approach, we identified 976 genes that were differentially regulated (list 2). The intersection of lists 1 and 2 yielded 234 regulated genes (list 3). In all, 77 genes in list 3 demonstrated at least two-fold difference in expression levels. U133A-generated microarray data from the OEAs were processed and analyzed as previously reported for data generated using lower density HuGeneFL arrays (Schwartz et al, 2003). A list of 563 genes with at least 1.75-fold difference in expression in OEAs with deregulated β-catenin relative to wild-type tumors (P<0.05) was obtained.
Supplementary Material
Supplementary data
Supplementary Figure IS
Acknowledgments
We thank E Fearon for providing RK3ES33YA, RK3ES33YD and RK3EDN132B cells, B Vogelstein for providing frozen intestinal mucosa tissues from Apcmin/+ mice, B Gumbiner for providing pCDNA3-ICAT, and F Cong for providing pCDNA-E-cad-C-term. We acknowledge the use of the Genomics Core Lab at the Memorial Sloan Kettering Cancer Center and thank A Viale and H Zhao for advice and discussion regarding microarray analysis, L Schweizer for critical comments on the manuscript, and the members of the Varmus and Cho laboratories for their helpful advice.
References
- Bienz M, Clevers H (2000) Linking colorectal cancer to Wnt signaling. Cell 103: 311–320 [DOI] [PubMed] [Google Scholar]
- Box GEP, Hunter WG, Hunter JS (1978) Statistics for Experimenters. New York: John Wiley and Sons [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D (1997) A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev 11: 2359–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 [DOI] [PubMed] [Google Scholar]
- Carnac G, Kodjabachian L, Gurdon JB, Lemaire P (1996) The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm. Development 122: 3055–3065 [DOI] [PubMed] [Google Scholar]
- Christian JL, McMahon JA, McMahon AP, Moon RT (1991) Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development 111: 1045–1055 [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell ME, Ben-Yedidia T, Shtutman M, Feinstein E, Einat P, Ben-Ze'ev A (2002) Nr-CAM is a target gene of the beta-catenin/LEF-1 pathway in melanoma and colon cancer and its expression enhances motility and confers tumorigenesis. Genes Dev 16: 2058–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darken RS, Wilson PA (2001) Axis induction by wnt signaling: target promoter responsiveness regulates competence. Dev Biol 234: 42–54 [DOI] [PubMed] [Google Scholar]
- Dhulipal PD (1997) Ets oncogene family. Indian J Exp Biol 35: 315–322 [PubMed] [Google Scholar]
- Dickson C, Spencer-Dene B, Dillon C, Fantl V (2000) Tyrosine kinase signalling in breast cancer: fibroblast growth factors and their receptors. Breast Cancer Res 2: 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KJ, Williams BO, Li Y, Pavan WJ (2000) Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development. Proc Natl Acad Sci USA 97: 10050–10055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GH, Orsulic S, Holland E, Hively WP, Li Y, Lewis BC, Williams BO, Varmus HE (1999) Development of a flexible and specific gene delivery system for production of murine tumor models. Oncogene 18: 5253–5260 [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C (1998) Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391: 357–362 [DOI] [PubMed] [Google Scholar]
- Grotewold L, Ruther U (2002) The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. EMBO J 21: 966–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M (2003) LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302: 415–419 [DOI] [PubMed] [Google Scholar]
- Hamilton FS, Wheeler GN, Hoppler S (2001) Difference in XTcf-3 dependency accounts for change in response to beta-catenin-mediated Wnt signalling in Xenopus blastula. Development 128: 2063–2073 [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J (1997) Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol 13: 611–667 [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281: 1509–1512 [DOI] [PubMed] [Google Scholar]
- Hecht A, Kemler R (2000) Curbing the nuclear activities of beta-catenin. Control over Wnt target gene expression. EMBO Rep 1: 24–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himly M, Foster DN, Bottoli I, Iacovoni JS, Vogt PK (1998) The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology 248: 295–304 [DOI] [PubMed] [Google Scholar]
- Holland EC (2000) A mouse model for glioma: biology, pathology, and therapeutic opportunities. Toxicol Pathol 28: 171–177 [DOI] [PubMed] [Google Scholar]
- Hoppler S, Brown JD, Moon RT (1996) Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev 10: 2805–2817 [DOI] [PubMed] [Google Scholar]
- Huelsken J, Behrens J (2002) The Wnt signalling pathway. J Cell Sci 115: 3977–3978 [DOI] [PubMed] [Google Scholar]
- Huelsken J, Birchmeier W (2001) New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev 11: 547–553 [DOI] [PubMed] [Google Scholar]
- Isaacs HV, Pownall ME, Slack JM (1994) eFGF regulates Xbra expression during Xenopus gastrulation. EMBO J 13: 4469–4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers M, Shimkets R, Prayaga S, Boldog F, Yang M, Burgess C, Fernandes E, Rittman B, Shimkets J, LaRochelle WJ, Lichenstein HS (2001) Identification of a novel human fibroblast growth factor and characterization of its role in oncogenesis. Cancer Res 61: 3131–3138 [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F (2002) Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22: 1172–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Kypta R (2003) Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116: 2627–2634 [DOI] [PubMed] [Google Scholar]
- Kessler DS (1997) Siamois is required for formation of Spemann's organizer. Proc Natl Acad Sci USA 94: 13017–13022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielman MF, Rindapaa M, Gaspar C, van Poppel N, Breukel C, van Leeuwen S, Taketo MM, Roberts S, Smits R, Fodde R (2002) Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet 32: 594–605 [DOI] [PubMed] [Google Scholar]
- Kofron M, Demel T, Xanthos J, Lohr J, Sun B, Sive H, Osada S, Wright C, Wylie C, Heasman J (1999) Mesoderm induction in Xenopus is a zygotic event regulated by maternal VegT via TGFbeta growth factors. Development 126: 5759–5770 [DOI] [PubMed] [Google Scholar]
- Koga C, Adati N, Nakata K, Mikoshiba K, Furuhata Y, Sato S, Tei H, Sakaki Y, Kurokawa T, Shiokawa K, Yokoyama KK (1999) Characterization of a novel member of the FGF family, XFGF-20, in Xenopus laevis. Biochem Biophys Res Commun 261: 756–765 [DOI] [PubMed] [Google Scholar]
- Kolligs FT, Hu G, Dang CV, Fearon ER (1999) Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol Cell Biol 19: 5696–5706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolligs FT, Nieman MT, Winer I, Hu G, Van Mater D, Feng Y, Smith IM, Wu R, Zhai Y, Cho KR, Fearon ER (2002) ITF-2, a downstream target of the Wnt/TCF pathway, is activated in human cancers with beta-catenin defects and promotes neoplastic transformation. Cancer Cell 1: 145–155 [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R (2002) FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(−/−) mice. Genes Dev 16: 3173–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerchner W, Latinkic BV, Remacle JE, Huylebroeck D, Smith JC (2000) Region-specific activation of the Xenopus brachyury promoter involves active repression in ectoderm and endoderm: a study using transgenic frog embryos. Development 127: 2729–2739 [DOI] [PubMed] [Google Scholar]
- Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER (2002) Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem 277: 21657–21665 [DOI] [PubMed] [Google Scholar]
- Miller LD, Park KS, Guo QM, Alkharouf NW, Malek RL, Lee NH, Liu ET, Cheng SY (2001) Silencing of Wnt signaling and activation of multiple metabolic pathways in response to thyroid hormone-stimulated cell proliferation. Mol Cell Biol 21: 6626–6639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milliken GA, Johnson DE (1984) Analysis of Messy Data, vol 1: Designed Experiments. Belmont, CA: Wadsworth, Inc. [Google Scholar]
- Moser AR, Mattes EM, Dove WF, Lindstrom MJ, Haag JD, Gould MN (1993) ApcMin, a mutation in the murine Apc gene, predisposes to mammary carcinomas and focal alveolar hyperplasias. Proc Natl Acad Sci USA 90: 8977–8981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P (1995) Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA 92: 3046–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando V (2000) Mapping chromosomal proteins in vivo by formaldehyde-crosslinked-chromatin immunoprecipitation. Trends Biochem Sci 25: 99–104 [DOI] [PubMed] [Google Scholar]
- Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M (1995) Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci USA 92: 4482–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouko L, Ziegler TR, Gu LH, Eisenberg LM, Yang VW (2004) Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J Biol Chem 279: 26707–26715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M, Polakis P (2000) Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 287: 1606–1609 [DOI] [PubMed] [Google Scholar]
- Polakis P (2000) Wnt signaling and cancer. Genes Dev 14: 1837–1851 [PubMed] [Google Scholar]
- Powers CJ, McLeskey SW, Wellstein A (2000) Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer 7: 165–197 [DOI] [PubMed] [Google Scholar]
- Rabbitts TH, Axelson H, Forster A, Grutz G, Lavenir I, Larson R, Osada H, Valge-Archer V, Wadman I, Warren A (1997) Chromosomal translocations and leukaemia: a role for LMO2 in T cell acute leukaemia, in transcription and in erythropoiesis. Leukemia 11 (Suppl 3): 271–272 [PubMed] [Google Scholar]
- Romagnolo B, Berrebi D, Saadi-Keddoucci S, Porteu A, Pichard AL, Peuchmaur M, Vandewalle A, Kahn A, Perret C (1999) Intestinal dysplasia and adenoma in transgenic mice after overexpression of an activated beta-catenin. Cancer Res 59: 3875–3879 [PubMed] [Google Scholar]
- Rousset R, Mack JA, Wharton KA Jr, Axelrod JD, Cadigan KM, Fish MP, Nusse R, Scott MP (2001) Naked cuticle targets dishevelled to antagonize Wnt signal transduction. Genes Dev 15: 658–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P (1993) Association of the APC gene product with beta-catenin. Science 262: 1731–1734 [DOI] [PubMed] [Google Scholar]
- Rutzky LP, Giovanella BC, Tom BH, Kaye CI, Noguchi PD, Kahan BD (1983) Characterization of a human colonic adenocarcinoma cell line, LS123. In vitro 19: 99–107 [DOI] [PubMed] [Google Scholar]
- Schaefer-Klein J, Givol I, Barsov EV, Whitcomb JM, VanBrocklin M, Foster DN, Federspiel MJ, Hughes SH (1998) The EV-O-derived cell line DF-1 supports the efficient replication of avian leukosis-sarcoma viruses and vectors. Virology 248: 305–311 [DOI] [PubMed] [Google Scholar]
- Schohl A, Fagotto F (2003) A role for maternal beta-catenin in early mesoderm induction in Xenopus. EMBO J 22: 3303–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DR, Wu R, Kardia SL, Levin AM, Huang CC, Shedden KA, Kuick R, Misek DE, Hanash SM, Taylor JM, Reed H, Hendrix N, Zhai Y, Fearon ER, Cho KR (2003) Novel candidate targets of beta-catenin/T-cell factor signaling identified by gene expression profiling of ovarian endometrioid adenocarcinomas. Cancer Res 63: 2913–2922 [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X (2001) Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol 11: 951–961 [DOI] [PubMed] [Google Scholar]
- Shimokawa T, Furukawa Y, Sakai M, Li M, Miwa N, Lin YM, Nakamura Y (2003) Involvement of the FGF18 gene in colorectal carcinogenesis, as a novel downstream target of the beta-catenin/T-cell factor complex. Cancer Res 63: 6116–6120 [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A (1999) The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 96: 5522–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG (1991) Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell 67: 79–87 [DOI] [PubMed] [Google Scholar]
- Spiegelman VS, Slaga TJ, Pagano M, Minamoto T, Ronai Z, Fuchs SY (2000) Wnt/beta-catenin signaling induces the expression and activity of betaTrCP ubiquitin ligase receptor. Mol Cell 5: 877–882 [DOI] [PubMed] [Google Scholar]
- Staal FJ, Weerkamp F, Baert MR, van den Burg CM, van Noort M, de Haas EF, van Dongen JJ (2004) Wnt target genes identified by DNA microarrays in immature CD34+ thymocytes regulate proliferation and cell adhesion. J Immunol 172: 1099–1108 [DOI] [PubMed] [Google Scholar]
- Tago K, Nakamura T, Nishita M, Hyodo J, Nagai S, Murata Y, Adachi S, Ohwada S, Morishita Y, Shibuya H, Akiyama T (2000) Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes Dev 14: 1741–1749 [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426 [DOI] [PubMed] [Google Scholar]
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD Jr (2003) The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349: 2483–2494 [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H (2002) The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250 [DOI] [PubMed] [Google Scholar]
- Vonica A, Gumbiner BM (2002) Zygotic Wnt activity is required for Brachyury expression in the early Xenopus laevis embryo. Dev Biol 250: 112–127 [DOI] [PubMed] [Google Scholar]
- Vonica A, Weng W, Gumbiner BM, Venuti JM (2000) TCF is the nuclear effector of the beta-catenin signal that patterns the sea urchin animal–vegetal axis. Dev Biol 217: 230–243 [DOI] [PubMed] [Google Scholar]
- Weinmann AS, Bartley SM, Zhang T, Zhang MQ, Farnham PJ (2001) Use of chromatin immunoprecipitation to clone novel E2F target promoters. Mol Cell Biol 21: 6820–6832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding J, Straub J, Bee J, Churchman M, Bodmer W, Dickson C, Tomlinson I, Ilyas M (2002) Cyclin D1 is not an essential target of beta-catenin signaling during intestinal tumorigenesis, but it may act as a modifier of disease severity in multiple intestinal neoplasia (Min) mice. Cancer Res 62: 4562–4565 [PubMed] [Google Scholar]
- Willert J, Epping M, Pollack JR, Brown PO, Nusse R (2002) A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Zhai Y, Fearon ER, Cho KR (2001) Diverse mechanisms of beta-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res 61: 8247–8255 [PubMed] [Google Scholar]
- Zorn AM, Barish GD, Williams BO, Lavender P, Klymkowsky MW, Varmus HE (1999) Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol Cell 4: 487–498 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary Figure IS


