Abstract
ASH sensory neurons are required in Caenorhabditis elegans for a wide range of avoidance behaviors in response to chemical repellents, high osmotic solutions and nose touch. The ASH neurons are therefore hypothesized to be polymodal nociceptive neurons. To understand the nature of polymodal sensory response and adaptation at the cellular level, we expressed the calcium indicator protein cameleon in ASH and analyzed intracellular Ca2+ responses following stimulation with chemical repellents, osmotic shock and nose touch. We found that a variety of noxious stimuli evoked strong responses in ASH including quinine, denatonium, detergents, heavy metals, both hyper- and hypo-osmotic shock and nose touch. We observed that repeated chemical stimulation led to a reversible reduction in the magnitude of the sensory response, indicating that adaptation occurs within the ASH sensory neuron. A key component of ASH adaptation is GPC-1, a G-protein γ-subunit expressed specifically in chemosensory neurons. We hypothesize that G-protein γ-subunit heterogeneity provides a mechanism for repellent-specific adaptation, which could facilitate discrimination of a variety of repellents by these polymodal sensory neurons.
Keywords: adaptation, ASH neurons, avoidance, cameleon, gpc-1
Introduction
Detection of aversive sensory stimuli in the environment is an essential feature of animal nervous systems that allows them to avoid noxious chemicals and dangerous conditions. Animals make use of specialized neurons and sensory structures called nociceptors to detect a wide range of aversive and painful stimuli, including toxic chemicals. An unusual feature of nociceptor neurons is that they are often polymodal and can respond to several qualitatively distinct types of sensory stimuli. Bitter taste receptors can also be considered polymodal, as they respond to a variety of chemically diverse toxic compounds. Understanding the mechanisms through which these neurons respond to and discriminate multiple classes of sensory stimuli represents an intriguing problem in sensory biology.
Caenorhabditis elegans contains at least 11 bilaterally symmetric pairs of sensory neurons in the head and two in the tail that function primarily as chemosensors (reviewed in Bargmann and Mori, 1997). ASH, one of these bilateral pairs in the head, has been shown to play a central role in mediating avoidance behaviors in response to chemical repellents as well as several different noxious stimuli. Specifically, ASH is required for behavioral avoidance of water-soluble (e.g. copper, quinine and SDS) and volatile (octanol) chemical repellents, osmotic shock and mechanical stimulation on the tip of the animal's nose (Bargmann et al, 1990; Kaplan and Horvitz, 1993; Troemel et al, 1997; Hart et al, 1999; Sambongi et al, 1999; Hilliard et al, 2002). On this basis, it has been hypothesized that ASH is a polymodal sensory neuron that responds to chemical, osmotic and mechanical stimuli (Kaplan and Horvitz, 1993). However, the nature of the cellular response of ASH to these stimuli has not been examined either in vivo or in vitro. Here, we combine both genetic and physiological methods to examine the nature of the ASH cellular response and how the signaling molecules affect this response.
To monitor the activity of sensory neurons in response to controlled sensory stimuli in living animals, we have used the genetically encoded calcium indicator cameleon (Miyawaki et al, 1997). Cameleon is a fusion of two fluorescent molecules, CFP and YFP, connected by a calmodulin and an M13 domain. Binding to calcium induces a conformational change in the structure of the cameleon protein that leads to an increase in fluorescence resonance energy transfer (FRET) from CFP to YFP. Thus, a transient rise in intracellular calcium leads to an increased ratio between the emission intensity values of YFP and CFP in a cell expressing cameleon. Recent studies have demonstrated that cameleon can be used to detect calcium transients resulting from neuronal or muscle cell activity (Kerr et al, 2000; Suzuki et al, 2003).
In this paper, we present results obtained using cameleon-based calcium imaging to study the cellular responses of C. elegans avoidance neurons to sensory stimuli. We demonstrate that aversive stimuli including chemical repellents and osmotic shock induce Ca2+ transients in ASH neurons, and that these transients correlate temporally with the stimulus onset and are maintained if the stimulus persists. We show also that ASH transients can be induced by nose touch, a mechanical stimulation. Mutations in the TRPV-related osm-9, the L-type voltage gated calcium channel egl-19 and the G-protein α-subunit odr-3 each diminish the transients induced by all repellents. We further show that C. elegans behaviorally adapts to repetitive or persistent application of repellent, and that this loss of behavioral response results in part from sensory adaptation within ASH. Finally, we show that the G-protein γ-subunit GPC-1 facilitates a component of the initial response to quinine and promotes sensory adaptation to copper.
Results
Chemical repellents induce intracellular Ca2+ increase in ASH
To monitor the activity of the ASH sensory neurons upon exposure to soluble repellents and other stimuli, we used the calcium indicator protein cameleon. We first generated transgenic lines in which the calcium-sensitive protein YC2.12 (Nagai et al, 2002) was transcribed under the control of the sra-6 promoter, which directs expression in ASH and two other neurons (ASI and PVQ) (Troemel et al, 1995). Transgenic animals were then glued on hydrated agar pads and placed in a small perfusion chamber under a constant flow of saline buffer. The repellent stimuli were delivered through a glass needle placed in the perfusion chamber near the tip of the animal's head as shown in Figure 1A (see also Materials and methods). The needle, controlled by a miniature-motorized stage, was moved near the head in the ‘on' phase of the recording and far from the head in the ‘off' phase. This system allowed a sharp and rapid stimulus delivery similar to that achieved on the plate using the previously defined drop test assay for repellent sensation (Hilliard et al, 2002).
Figure 1.
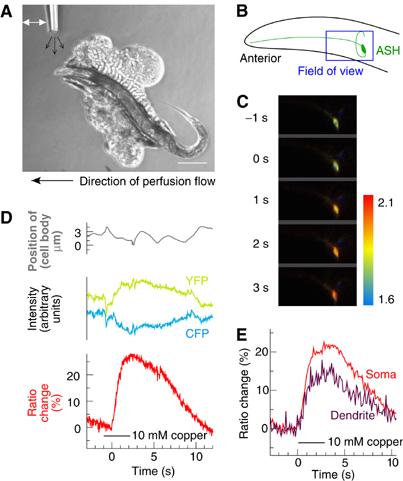
(A) Soluble repellent stimulation conditions. An adult hermaphrodite is glued on a 2% agarose pad placed into a perfusion chamber and bathed in extracellular saline (direction of main flow is indicated with a large black arrow at the bottom of the panel). Soluble repellents are applied via a stream of liquid (small black arrows) from a glass needle. The stimulus is applied and removed by changing the position of the needle (white arrow). Anterior is at left and dorsal is up in this and in all following panels. Scale bar, 200 μm. (B) Diagram of the animal's head with one of the two symmetrical ASH neurons highlighted. From the ASH cell body, the dendrite runs anteriorly until the tip of the head ending with a sensory cilium. On the opposite side of the cell body, the axon makes a turn and enters the nerve ring. The blue box shows the approximate position of the recording field. (C) Calcium transients in ASH visualized with cameleon under control of the sra-6 promoter. Individual frames taken before and during application of 10 mM Cu2+ are shown. Colors indicate YFP/CFP ratio, where high ratio (red) corresponds to high calcium. Color bar indicates ratio scale. Field of view is 70 × 35 μm (corresponding approximately to the blue box in panel B). (D) Calcium imaging in ASH. ASH imaging during a 3 s application of 10 mM Cu2+ (black bar) reveals a change in the YFP/CFP ratio (red line, quantified as % of the baseline level) that can be seen as a reciprocal change in YFP and CFP intensities (yellow and cyan lines). The ratio change does not correspond to motion of the sample (gray line), indicating that it reflects an increase in the calcium transient in ASH. (E) Calcium transients in the ASH cell body (red line) and dendrite (purple line) have similar profiles in response to a 3 s 10 mM Cu2+ stimulus (black bar).
Using this stimulus protocol, we observed reliable increases in the fluorescence ratio of YFP to CFP emission in ASH that correlated temporally with the application of a chemical stimulus (Figure 1B–E). Robust increases in the YFP/CFP ratio signal were observed in the ASH cell body (Figure 1D) as well as in the ASH dendrite (Figure 1E) immediately following the application of a soluble repellent (in this specific case, copper chloride 10 mM). The yellow and cyan intensities showed reciprocal changes in intensity (Figure 1D), as expected for a FRET change caused by an increase in calcium. The movement of the cell did not influence the recording as indicated by the cell body position control trace that does not match the ratio trace. Together, these results demonstrate that it is feasible to monitor calcium transients in ASH with cameleon.
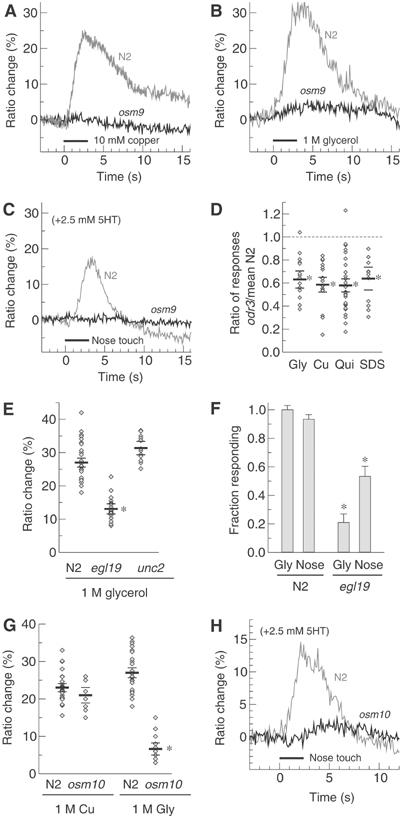
A wide range of aversive stimuli whose avoidance was previously shown to require the ASH neurons induced Ca2+ transients in the ASH cell body (Figure 2A). We observed robust Ca2+ transients while stimulating with the alkaloid quinine, heavy metal ions (copper), detergent (SDS) and hyperosmotic solution (1 M glycerol) (Figure 2A and B). No response was observed to saline buffer alone. In all cases, the neuronal responses were observed at repellent concentrations comparable to the critical concentrations for behavioral avoidance as assayed in the drop test protocol (Hilliard et al, 2002, 2004). Interestingly, we were also able to identify responses to several other aversive stimuli not previously described to require ASH for avoidance. The bitter tastant denatonium evoked strong calcium transients in ASH (Figure 2A), while hypo-osmotic shock (distilled water) produced a modest response (not shown). Stimulation with chemicals that are not aversive, such as NaCl (10 or 50 mM) and lysine (10 or 50 mM), did not evoke calcium responses in ASH (Figure 2A and data not shown). Mutations in unc-13, a gene required for synaptic transmission, did not significantly alter the calcium responses of ASH to repellent stimuli (Figure 2B), indicating that ASH is the primary sensory neuron responsible for detecting these compounds.
Figure 2.

(A) Noxious stimuli trigger calcium transients in ASH. Shown are typical calcium transients in ASH evoked by the noxious stimuli 10 mM Cu2+, 1 M glycerol, 0.1% SDS, 10 mM quinine and 10 mM denatonium; by nose touch with and without 2.5 mM serotonin, in wild type; by nose touch in the presence of 2.5 mM serotonin in unc-13 (synaptic transmission defective) backgrounds; and by the innocuous stimuli of extracellular saline buffer and 50 mM NaCl. Response is plotted as fractional fluorescence ratio change over baseline. Black bars indicate duration of application of each stimulus. (B) Quantification of ASH responses to selected noxious stimuli in N2 and unc-13 backgrounds, and in N2 in the presence of 2.5 mM serotonin. Small diamonds indicate the amplitude of YFP/CFP ratio change during a single application of a repellent (black bar). Horizontal lines indicate mean±s.e.m. The asterisk indicates a significant decrease in Cu2+ response compared to N2 without serotonin (P<0.01). N2 sample sizes (n=number of animals): Cu2+ (n=19); glycerol (n=12); SDS (n=15); quinine (n=11); unc-13 sample sizes: Cu2+ (n=10); glycerol (n=7); 5-HT sample sizes: Cu2+ (n=5); glycerol (n=6).
ASH is also important for responses to nose touch, a reversal behavior induced by a mechanical stimulus on the tip of the nose (Kaplan and Horvitz, 1993; Hart et al, 1999). In the same condition as those used to test chemical repellents, we did not observe calcium responses to nose touch in ASH. Previous studies (Chao et al, 2004) have shown that nose touch escape behavior is significantly more prominent in presence of food, or in the presence of the neuromodulator serotonin (which in C. elegans appears to signal food abundance for many behaviors). When 2.5 mM serotonin was added to the liquid medium, we observed calcium transients in ASH in response to nose touch stimuli (Figure 2A). These transients were also present in unc-13 mutant animals (Figure 2B), indicating that ASH is a primary sensory neuron for nose touch. Exogenous serotonin did not increase ASH responses to chemical repellents or osmotic shock (Figure 2B). Thus, serotonin appeared to specifically potentiate the nose touch modality of the ASH neurons. Even with exogenous serotonin, the typical delay between stimulus application and the calcium response onset seemed to be slightly longer for nose touch than for chemical stimulation. This could be due to the difficulty of giving an optimal nose touch under recording conditions, or the delay might be stimulus-specific. Collectively, these results demonstrate that the ASH neurons are indeed polymodal in their sensory response properties.
We also investigated the effect of repellent stimulus duration on the calcium responses in ASH neurons. In part, these experiments were directed toward understanding an important aspect of the ASH physiology: does ASH primarily sense the presence of repellents, or a change in their concentration? To address this question, we compared the responses to 1, 3 and 10 s stimulations with the soluble repellent copper (Figure 3A). We observed that longer stimulations led to longer lasting responses. Although the slope of the ratio trace decreased as the exposure progressed, indicating a decrease in the rate of calcium influx, the calcium level in the cell appeared elevated throughout even the 10 s exposure. If the stimulus was maintained for 60 s, the ratio signal began to decrease and reached the baseline after approximately 30 s (Figure 3B). These results indicated that sensory adaptation appears to occur in ASH (see also below), although its kinetics are slow enough to maintain a response for a number of seconds after the switch from buffer to repellent. Thus, ASH appeared to respond primarily to the presence of copper rather than to the rate of change in its concentration.
Figure 3.
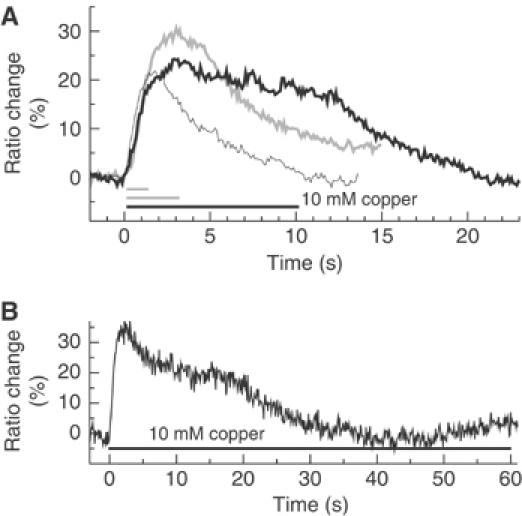
(A) ASH response is maintained throughout persistent stimuli (less than 10 s). Animals were stimulated for 1 s (thin bar), 3 s (gray bar) or 10 s (black bar) with 10 mM Cu2+ and corresponding ratio changes in ASH were recorded (thin line, gray line, black line). (B) ASH adapts to 10 mM Cu2+ within 60 s. Recordings are made as in (A), but stimulation lasted 60 s (black bar).
Calcium transients in ASH require the TRP channel OSM-9 and the L-type calcium channel EGL-19
To determine those molecules necessary for the observed Ca2+ transients in ASH, we studied the ASH Ca2+ responses of different genetic backgrounds containing mutations that affect ASH-dependent behaviors. We first generated cameleon-expressing lines carrying a loss-of-function mutation in osm-9, a TRPV-related cation-selective channel expressed in ASH and localized on the apical cilia of the sensory neuron (Colbert et al, 1997; Tobin et al, 2002). Since osm-9 mutants are defective in a wide range of ASH-mediated avoidance behaviors, the OSM-9 channel has been proposed to mediate the depolarization of ASH following sensory stimulation. Cameleon recordings from ASH in osm-9(ky10) mutant animals indeed showed that Ca2+ responses were eliminated or strongly reduced to all the repellents tested, including copper, hyperosmotic shock and quinine (Figure 4A and B and data not shown). osm-9 mutant animals also showed no response to nose touch stimuli (Figure 4C). These findings support the hypothesis that OSM-9 plays a general role in mediating ASH excitation in response to a wide range of sensory stimuli.
Figure 4.
(A, B) The TRPV-related channel OSM-9 is required for repellent-induced calcium transients. (A) Typical responses of N2 (gray line) and osm-9 (black line) animals to 10 mM Cu2+ and (B) 1 M glycerol are shown. No significant repellent-induced calcium transients have been observed in osm-9 with any repellent. Black bars indicate duration of application of the stimulus. (C) OSM-9 is also required for calcium transients induced by nose touch. (D) Mutation in the G-protein odr-3 reduces response to all repellents. Individual responses are indicated as diamonds. Horizontal bars represent the mean±s.e.m. of the repellent response as a fraction of the mean wild-type response; n⩾6 animals for each condition; reduction is significant in each case (*P<0.001). (E) L-type channels are a major source of observed calcium transients. Individual responses to 1 M glycerol (diamonds) and mean±s.e.m. (horizontal bars) are shown for wild-type, egl-19(ad1006) and unc-2(mu74) strains. A significant reduction is observed for the loss-of-function allele of the L-type channel EGL-19 (P<0.001, asterisk), but not for a putative null of the worm non-L-type channel UNC-2. n⩾7 animals for each condition. (F) Wild-type worms give reliable behavioral responses to 1 M glycerol and nose touch (left bars). egl-19(ad1006) worms give significantly reduced behavioral responses (P<0.01, asterisk), consistent with the reduced calcium transient observed in (E). A minimum of 60 trials were used for each condition, except glycerol on N2 for which 30 trials were used. Error bars indicate expected s.e.m. given observed response rate. (G) osm-10 mutants have a specific deficit for response to high-osmotic shock (1 M glycerol) but not Cu2+. Individual responses (diamonds) and mean±s.e.m. (horizontal lines) are shown. The asterisk indicates a significant difference from wild type (P<0.001). Sample sizes: n=19, wild-type Cu2+; n=5, osm-10 Cu2+; n=12, wild-type gly; n=7, osm-10 gly. (H) Nose touch is diminished in osm-10 mutants. Typical wild-type (gray line) and osm-10 (black line) traces are shown.
Since OSM-9 is reported to localize specifically to sensory cilia, we reasoned that a voltage-gated calcium channel (VGCC) might be required to conduct the depolarization to the cell soma and/or mediate the observed somatic calcium influx. We examined the effects of mutations in egl-19, which encodes the only C. elegans L-type VGCC (Lee et al, 1997), and unc-2, which encodes the only non-L-type, high voltage-activated VGCC (Schafer and Kenyon, 1995), on repellent-evoked calcium transients in ASH. As shown, we observed that the partial loss-of-function mutation egl-19(ad1006), which was shown to cause approximately a 50% reduction in depolarization-induced calcium influx in cultured C. elegans touch neurons (Suzuki et al, 2003), significantly reduced calcium influx in ASH in response to high osmotic strength (Figure 4E). Likewise, we observed a significant deficiency in ASH-mediated escape behaviors in the egl-19 reduction-of-function mutant (Figure 4F). In contrast, a likely null allele of unc-2(mu74) had no measurable effect on calcium influx in ASH (Figure 4E). Thus, the L-type VGCC encoded by egl-19 appears to play an important role in generating the neuronal calcium transients in ASH in response to repellent exposure.
The ASH neurons may express at least nine G-protein α-subunit genes: odr-3, gpa-1, gpa-3, gpa-11, gpa-13, gpa-14, gpa-15, goa-1 and gsa-1 (Zwaal et al, 1997; Roayaie et al, 1998; Jansen et al, 1999). The product of the odr-3 gene has been shown to be important for chemosensory signaling in a number of C. elegans sensory neurons (Zwaal et al, 1997; Roayaie et al, 1998; Jansen et al, 1999; Hilliard et al, 2004); we therefore analyzed the effect of the odr-3(n2150) mutation on calcium responses to repellent stimuli. We found that for all repellent stimuli tested, including copper, SDS, quinine and glycerol, the magnitude of the sensory calcium transient was significantly reduced, with mutant responses ranging from 40 to 60% of those seen in wild type (Table I; Figure 4D). Thus, ODR-3 appeared to be generally important for the cellular response to most if not all repellents sensed by ASH. In contrast, the effects on the ASH responses of another α-subunit, GPA-3, appeared to be more repellent-specific. For example, a deletion allele of gpa-3 significantly reduced the overall ratio change evoked in ASH by stimulation with quinine, but not by stimulation with copper or SDS and only slightly to glycerol (Table I). When we tested gpa-3; odr-3 double mutants, we observed a complete loss of calcium response to all repellents (n⩾5) (data not shown). Together, these results indicate that both ODR-3 and GPA-3 are involved in ASH responses to chemical repellents, with GPA-3 having a more prominent role in quinine response and ODR-3 a more general role for most if not all ASH sensory responses.
Table 1.
Response in ASH to soluble repellents of animals of different genetic backgrounds
| 10 mM Cu2+ | 1 M glycerol | 10 mM quinine | 0.1% SDS | |
|---|---|---|---|---|
| N2 | 23.0±1.1 (19) | 27.0±1.4 (12) | 17.1±1.0 (11) | 21.1±1.3 (15) |
| odr-3(n2150) | 14.5±1.7 (7)** | 15.8±1.9 (7)** | 9.9±1.0 (13)** | 13.4±2.1 (6)* |
| gpa-3(pk35) | 20.2±1.8 (7) | 22.2±2.0 (6)† | 11.5±1.1 (10)** | 21.0±2.0 (6) |
| gpc-1(pk298) | 21.9±1.8 (7) | 32.5±2.0 (6)† | 10.6±1.2 (8)** | 23.2±2.3 (5) |
| Values reported are mean±s.e.m. (sample size) of ratio change amplitude in each population. The asterisks indicate significant difference from wild type: †P<0.05; *P<0.01; **P<0.001. |
Finally, we investigated the Ca2+ response in osm-10 mutant animals. osm-10(n1602) mutants are behaviorally defective in avoidance of high osmotic strength while maintaining mostly unaltered avoidance responses to other repellents and to nose touch (Hart et al, 1999). OSM-10 is a novel intracellular protein expressed in ASH, ASI and in the phasmid neurons (Hart et al, 1999). As predicted, in osm-10 animals, we observed a strong reduction of the Ca2+ transient in response to high osmotic strength and normal Ca2+ levels in response to other chemical repellents (Figure 4G and data not shown). In contrast, we were not able to detect normal Ca2+ transients in response to nose touch (Figure 4H). These results might suggest that FLP and OLQ, two other classes of cells that are necessary for nose touch and do not express osm-10 (Kaplan and Horvitz, 1993; Hart et al, 1999), can account for the behavioral response in osm-10 animals. Alternatively, osm-10 mutants may retain some nose touch response in ASH despite compromised Ca2+ transients, or they may be nose touch defective under imaging conditions, but give more normal responses under more favorable environmental conditions.
ASH sensory neurons reversibly adapt to persistent stimuli
A property of many primary sensory neurons is sensory adaptation, a reduction in response sensitivity after persistent stimulation (repeated or prolonged). To determine whether sensory adaptation occurred in ASH, we first determined the effect of a repeated brief repellent stimulation on sensory adaptation in ASH using the drop test assay. As shown (Figure 5A), the avoidance index (a.i.) measured by the drop test (10 mM Cu2+) underwent a significant decrease (a.i. 0.6 versus 1.0) on the second stimulus presented with a 10 s interstimulus interval (ISI). The avoidance response reached very low values (a.i. 0.1) around the 6th stimulus. To determine whether this behavioral adaptation also reflected changes in the response properties of ASH, immobilized, cameleon-expressing animals were given two stimuli, repeated after a 10 second ISI, with the same repellent. For all repellents tested, the second stimulus gave a response that was significantly smaller (over 20% reduction in amplitude) than the response to the first stimulus (Figure 5B and C and data not shown). Subsequent stimuli caused further reductions in response magnitude that paralleled the reduction in avoidance behavior (Figure 5C). This reduction in response magnitude was reversible after a rest period of 5 min. This reversible adaptation was also observed in unc-13 mutants (Figure 5D), which are defective in synaptic transmission. These findings indicate that ASH undergoes cell-intrinsic, reversible sensory adaptation when repeatedly stimulated, and that these changes in the response properties of ASH underlie part or all of the behavioral plasticity evoked by repeated repellent exposures.
Figure 5.
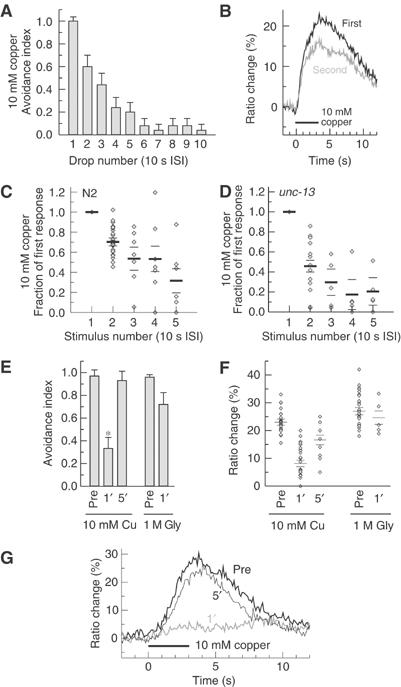
(A) Repeated repellent application causes adaptation of the behavioral avoidance response. The fraction of animals reversing in response to successive drops of 10 mM Cu2+ delivered at 10 s ISI is shown. n=25 animals. (B) Typical ASH traces obtained with successive 10 mM copper stimuli are shown. The second trace (ISI 10 s) was overlaid with the first so that the difference between them is more readily appreciated. Bar indicates the duration of the stimulus. (C) Repeated repellent application causes adaptation of ASH response. Cu2+ (10 mM) was delivered to wild-type animals in 3 s puffs with a 10 s ISI. Plotted is the ratio of the second response to the first for individual trials (diamonds) and mean±s.e.m. (horizontal bars). n=17 animals for the first two stimuli and n=6 for stimuli 3–5. All responses after the first are significantly reduced (P<0.01). (D) Adaptation of ASH response is maintained in the synaptic-transmission-defective unc-13 background. Recording conditions are as in (C). n=8 animals for the first two stimuli and n=4 thereafter. All responses after the first are significantly reduced (P<0.01). (E) Prolonged repellent application causes repellent-specific behavioral adaptation. The fraction of animals reversing in response to a drop of 10 mM Cu2+ before, 1 min after and 5 min after a 1 min exposure to 10 mM Cu2+ is shown. n=24 animals per condition. The fraction of animals reversing in response to a drop of 1 M glycerol before and 1 min after a 1 min exposure to 10 mM Cu2+ is also shown. n>10 animals per condition. The asterisk indicates significant reduction (P<0.001). (F) Prolonged repellent application causes repellent-specific adaptation in ASH response. Copper (left columns): individual calcium transients (diamonds) and means±s.e.m. (horizontal bars) before, 1 and 5 min after a 1 min exposure to 10 mM Cu2+. n=19 before, n=18 1 min after and n=5 5 min after. Glycerol (right columns): individual calcium transients (diamonds) and means±s.e.m. (horizontal bars) before and 1 min after a 1 min exposure to 10 mM Cu2+. n=12 before and n=5 1 min after. Reduction in response to Cu2+ after 1 min is significant (P<0.001) compared to before and 5 min after; 5 min after is also reduced compared to before (P<0.01). (G) Typical calcium transients in response to 10 mM Cu2+ are shown before (thick line), 1 min after (gray line) and 5 min after (thin line) a 1 min exposure to 10 mM Cu2+ (wild-type animals). Bar indicates duration of application of the stimulus.
To further investigate the cellular basis for sensory adaptation in ASH, we used a prolonged stimulus protocol to induce long-lasting adaptation to copper. To assay the effect of prolonged stimulation on behavior, we maintained the animals continuously for 1 min in the presence of 10 mM Cu2+ and assayed for copper avoidance using the drop test after 1 min of recovery period in the absence of repellent. As shown in Figure 5E, the animals pre-exposed to Cu2+ exhibited a significantly reduced avoidance response to this repellent compared to naive animals. However, in the same conditions, animals pre-exposed to Cu2+ showed high sensitivity to glycerol, another repellent detected by ASH (Figure 5E); thus, copper adaptation did not generally inhibit avoidance responses but appeared at least somewhat repellent-specific. Normal sensitivity to copper was restored following a 5 min rest period on repellent-free medium, indicating that the sensory adaptation induced by this procedure was also reversible (Figure 5E).
To assess whether the sensory adaptation to prolonged stimulation reflected changes in the responses of ASH itself, we determined the effect of prolonged copper adaptation on calcium responses in ASH. Immobilized cameleon-expressing animals were given 1 min of continuous repellent stimulation (trace shown in Figure 3B) and then allowed to recover in buffer solution for 1 min or 5 min before applying the testing stimulus (Figure 5F and G). We observed that ASH Ca2+ responses were strongly reduced after 1 min of recovery time, while they were almost completely restored after 5 min of recovery. As in the behavioral assay, adaptation to copper did not markedly impair ASH neuronal responses to a different repellent, glycerol. Thus, the repellent-specific reduction in the avoidance response to copper following a prolonged stimulus also appeared to result, at least in part, from changes in the cellular response properties of ASH itself.
GPC-1 is required for sensory adaptation in ASH
Previous studies (Jansen et al, 2002) indicated that gpc-1, a gene encoding one of two G-protein γ-subunits in the C. elegans genome and expressed specifically in chemosensory neurons, was important for behavioral adaptation to soluble attractants. However, the cellular basis for this defect in adaptation behavior was not determined. Therefore, we investigated the effects of gpc-1 mutations on sensory adaptation in ASH. The initial response of gpc-1 mutant animals to a single application of most repellents was indistinguishable from wild type (Table I). The only exception was quinine; here the gpc-1 mutant showed a partially reduced initial response (ratio change 11 versus 17% in wild type; see Table I). For all other repellents, GPC-1 did not appear to play an important role in the initial sensory response in ASH.
To investigate the effect of gpc-1 on sensory adaptation, we determined the effect of the gpc-1 deletion on behavioral and cellular adaptations to prolonged repellent stimulation. As described previously, we pre-exposed animals on the plate to copper for 1 min, then assayed for response to a copper test stimulus following a 1 min rest period. In this assay, whereas only 35% of wild-type animals exhibited avoidance responses to the copper test stimulus following the treatment, 64% of gpc-1 animals did so (Figure 6A). A similar adaptation defect was seen also at the cellular level: gpc-1 mutant animals showed a significantly larger magnitude of ASH Ca2+ response to the test stimulus (65% of unadapted response) than did wild-type animals subjected to the same treatment (35% of unadapted response) (Figure 6B). We also tested the involvement of GPC-1 in the sensory adaptation induced by repetitive repellent stimuli. As previously shown, wild-type animals showed a significantly reduced behavioral response (60% animals responding) after the second successive Cu2+ stimulus when using an ISI of 10 s. In the same experimental conditions, almost 100% of the gpc-1 animals were still responding (Figure 6C). We extended the repellent stimulation up to 10 successive stimuli and for each experimental point we observed a consistent difference between the wild-type and gpc-1 strain (Figure 6C). At the cellular level, gpc-1 animals showed comparable defects in adaptation: little or no difference was observed between the ASH Ca2+ transients elicited by the first and the second stimuli of copper, glycerol, quinine and SDS (Figure 6D). Together, these results identify a specific role for GPC-1 in ASH sensory adaptation.
Figure 6.
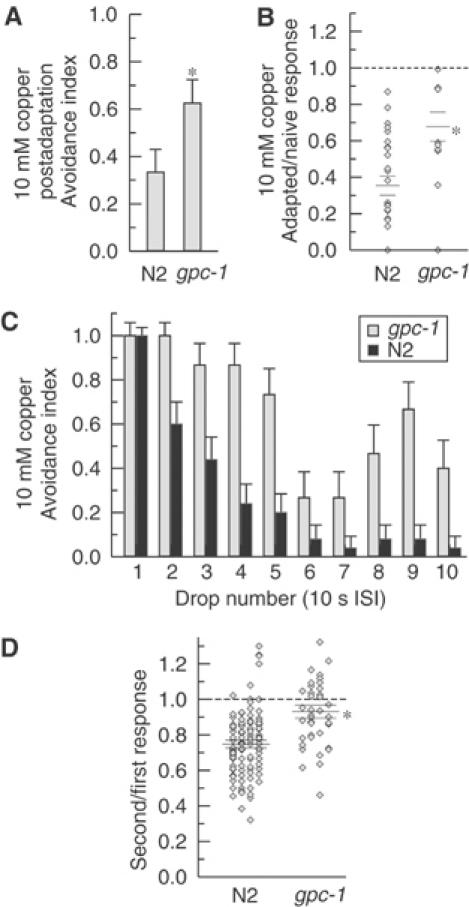
(A) gpc-1 animals are behaviorally defective in adaptation to prolonged repellent stimuli. Wild-type and mutants in the G-protein γ-subunit gpc-1 were tested with a copper test stimulus 1 min after a 1 min exposure to 10 mM Cu2+. gpc-1 animals show significantly less (P<0.01, asterisk) reduction of the avoidance index compared to the wild-type animals. n=24 animals for each condition. (B) gpc-1 animals are defective for ASH adaptation to prolonged stimuli. Wild-type and gpc-1 animals were stimulated before and 1 min after a 1 min exposure to 10 mM Cu2+. gpc-1 animals show significantly less reduction compared with the wild type (P<0.01, asterisk; n=10 gpc-1, n=12 N2). Ratio of second response/first response for individual trials (diamonds) and mean±s.e.m. (horizontal bars) are shown. (C) gpc-1 animals are behaviorally defective for adaptation to repeated stimuli. Wild-type and gpc-1 animals were given 10 successive 10 mM Cu2+ stimuli with an ISI of 10 s. By the second stimulus, gpc-1 animals show a significant difference from wild type (P<0.01, Fisher's exact test). n=15 animals for gpc-1; n=25 for wild type. (D) gpc-1 animals are defective in ASH adaptation to repetitive repellent stimulation. Ca2+ responses in ASH were recorded for two successive stimuli of 3 s with an ISI of 10 s. The ratio of second response to first response for individual trials (diamonds) and mean±s.e.m. (horizontal bars) are shown for the following repellents: 1 M glycerol, 10 mM Cu2+, 10 mM quinine, 0.1% SDS (columns, left to right). gpc-1 showed no significant decrease in response magnitude and was significantly different from wild type (*P<0.001).
Discussion
In this study, we have used in vivo optical imaging to characterize basic response properties of the ASH nociceptor neurons in intact animals. Previous genetic and cell-ablation studies had indicated that these neurons were required for avoidance of a wide range of aversive stimuli, including toxic chemicals, hyperosmotic solutions and nose touch (Bargmann et al, 1990; Kaplan and Horvitz, 1993; Troemel et al, 1997; Hart et al, 1999; Sambongi et al, 1999; Hilliard et al, 2002, 2004). We observed that ASH indeed generated neuronal responses to nearly all previously identified ASH-dependent aversive stimuli, as well as to hypo-osmotic shock and denatonium. Previous observations (Chao et al, 2004) on behavioral responses to nose touch indicated that serotonin (or presence of food) had a potentiating effect on this ASH-mediated avoidance response. Our observation that ASH Ca2+ transients can only be detected in response to nose touch if serotonin is present supports their behavioral observations and reveals that this neurotransmitter is playing a central role directly on the ASH sensory cell. The results presented in this work also indicated that ASH senses the presence of repellent substances more than a change in their concentration. If ASH were designed to sense a repellent gradient, we would have expected the response duration and magnitude to be approximately independent of the duration of the stimulus. Conversely, if ASH neurons sense the presence of repellent more than its change in concentration, then we would expect a prolonged response if the stimulus persists. In fact, we observed that elevated calcium levels were maintained during a prolonged stimulus, although some sensory adaptation did occur. Since most of the repellents sensed by ASH are toxic or harmful to nematode survival, sustained activity inducing longer behavioral avoidance probably provides a selective advantage in the animal's normal environment.
Overall, the response properties of the ASH neurons show interesting parallels with certain mammalian sensory neurons. For example, mammalian nociceptor neurons, like ASH, generate sensory responses to a qualitatively diverse range of stimuli, including chemicals (e.g. capsaicin), heat, acid and cold. Moreover, the OSM-9 channel, which is required for all ASH responses and is likely to be the transduction channel in the ASH sensory cilia, is a close homolog of the TRPV1 (VR-1) capsaicin receptor, which is a transduction channel in mammalian nociceptors (Caterina et al, 1997; Tominaga et al, 1998). It was also shown that another cation channel of the vanilloid subfamily TRPV4 (VR-OAC) with OSM-9 similarity can rescue the osmotic and mechanosensory defects when expressed in ASH in the osm-9 mutant background (Liedtke et al, 2003). Another parallel to ASH are the mammalian bitter taste receptors; like ASH, they respond to a broad range of soluble compounds that all evoke aversive behavioral responses. Moreover, the sensory transduction mechanism in bitter taste neurons occurs through G-protein-mediated activation of a TRP-like cation channel, a mechanism that closely resembles a hypothesized sensory transduction pathway of ASH (Perez et al, 2002).
Thus, the ASH neurons may provide a useful model for understanding the mechanisms by which bitter taste neurons respond and adapt to chemical repellents. We speculate that the restricted number of neurons present in the C. elegans sensory system might have required the consolidation of multiple functions in the same cell. In this view, ASH is indeed polymodal and might have characteristics and abilities belonging to nociceptors, bitter taste receptors and mechanoreceptors.
These studies also provide new insight into the cellular and molecular mechanisms of taste adaptation in C. elegans. Previous studies have demonstrated that nematodes show sensory adaptation to volatile and soluble attractants. We have shown here that C. elegans also adapts to soluble repellents, and that this behavioral adaptation occurs in part through changes in the response properties of the sensory neuron ASH. Furthermore, adaptation to copper did not appreciably affect ASH responses to high osmolarity, indicating that sensory adaptation in these polymodal neurons is at least somewhat repellent-specific. The mechanism for ASH sensory adaptation was shown to involve a specialized G-protein γ-subunit, GPC-1, which also affects quinine sensation but is otherwise unnecessary for acute responses to repellents. gpc-1 mutants were previously shown to exhibit defects in behavioral assays for adaptation to soluble attractants (Jansen et al, 2002). Our results show that GPC-1 is also involved in adaptation to repellents, and demonstrate that the gpc-1 adaptation-defective phenotype results at least in part from effect on the ASH primary sensory neurons. Interestingly, GPC-1 affected not only the persistent adaptation that occurred after prolonged, minute-long repellent exposures, but also the reduction in response magnitude observed in the second of two repeated brief stimuli. Thus, GPC-1 may function relatively early in the process of sensory adaptation in ASH.
How might GPC-1 participate in the process of sensory adaptation? One possibility is that it could facilitate the targeting of a G-protein receptor kinase (GRK) to the membranes of the ASH sensory cilia to inactivate signaling from taste receptors. In mammalian cells, GRK2 has been shown to associate with lipid membranes through interactions with specific Gβγ isoforms that are mediated by its carboxy-terminus (DebBurman et al, 1995; Daaka et al, 1997). This membrane association leads to phosphorylation of the receptor, which in turn recruits arrestin, a protein that prevents coupling of the receptor to its G-protein effector (Morris and Malbon, 1999). Alternatively, Gβγ has been shown in many systems to directly activate second messenger pathways distinct from those activated by their associated α-subunit (Boyer et al, 1989). It is possible that ODR-3, associated with the essential Gγ subunit GPC-2, could activate a second messenger pathway responsible for evoking acute responses to repellents, while GPC-1, perhaps associated with a repellent-specific Gα subunit, could activate a different pathway responsible for adaptation. The availability of both cellular and behavioral assays for these processes in C. elegans should make it possible to identify additional components of the ASH sensory transduction and adaptation pathways and characterize their functions in vivo.
Materials and methods
Strains
Wild-type animals were C. elegans variety Bristol, strain N2. Mutant strains included NL335 gpa-3(pk35) V; CX2205 odr-3(n2150) V; NL792 gpc-1(pk298) X; CX10 osm-9 (ky10) IV; DA1006 egl-19(ad1006) IV; CB55 unc-2(e55) X; NL2338 gpa-3(pk35) V odr-3(n2150) V.
The following lines were obtained in this work:
AQ1444 lin-15(n765) X; ljEx95 [lin-15(+) psra-6∷YC2.12];
AQ1446 odr-3(n2150) V; ljEx95;
AQ1447 gpc-1(pk298) X; ljEx95;
AQ1449 osm-9(ky10) IV; ljEx95;
AQ1451 egl-19(ad1006) IV; ljEx95;
AQ1452 unc-2(mu74) X; ljEx95;
AQ1045 gpa-3(pk35) V; ljEx95;
AQ1182 unc-13(e51) I; ljEx95;
AQ1048 osm-10(n1602) III; ljEx95;
MT3641 osm-10(n1602) III;
MT7929 unc-13(e51) I;
MT8189 lin-15(n765) X.
Worms were grown under uncrowded conditions at 20°C on NGM agar plates seeded with Escherichia coli strain OP50 (Brenner, 1974).
Construction of cameleon expressing plasmids
The psra-6∷YC2.12 expression vector was generated as follows. A 4-kb sra-6 promoter fragment was prepared by PCR on C. elegans genomic DNA with primers GCATGCATCTGTCATGGTCAGTATTT GAGAAG and GCGTCGACGGCAAAATCTGAAATAATAAATATTAAA TTCTGCG followed by NscI and SalI digestion. The cDNA for YC2.12 was kindly provided by Atsushi Miyawaki (RIKEN, Japan) and a SalI site was introduced by PCR. A Fire Lab ‘97 vector kit plasmid L3613 backbone was digested with PstI and EcoRI and the promoter and the cameleon fragments were incorporated by utilizing the compatibility of NscI and PstI ends. The resulting plasmid was injected into lin-15(n765) worms along with lin-15 marker (Huang et al, 1994) to obtain stable extrachromosomal arrays (Mello and Fire, 1995).
Behavioral tests and statistics
See Supplementary data.
Preparation of samples for imaging and delivery of the repellent
A needle-based local perfusion system was used for application of repellents for acute stimulation and adaptation protocols. Individual animals were immobilized on a hydrated 2% agarose pad using cyanoacrylate glue. During the gluing process, the coverslip containing the pad was positioned on ice to reduce animal's movements. The glue was delivered through a drawn glass capillary tube operated by mouth. The tip of the animal's head was always kept free from glue; any animal accidentally glued on the head was discarded. The coverslip with the glued animal was then positioned in the perfusion chamber (RC-26GLP, Warner Instruments) and covered with extracellular saline solution (composition: NaCl 80 mM, KCl 5 mM, D-glucose 20 mM, Hepes 10 mM, MgCl2 5 mM, CaCl2 1 mM; pH adjusted with NaOH to 7.2). Repellents were added to this solution to achieve the final concentration without adjusting the pH, as some repellents are poorly soluble at pH 7.2 (e.g. copper). A variety of lines of evidence, including observation of responses at lower concentration and pH closer to 7.2, and a lack of robust response to solutions of lower pH without repellent, confirm that the effects observed are due primarily to the presence of repellent (data not shown).
The perfusion chamber was positioned under the microscope objective and connected with the constant flow of extracellular saline generated by gravity using 50 ml syringe. The flow was restricted to 1.0 ml/min by inserting a short piece of narrow Teflon tubing in the input line. In these conditions, the animals could typically survive and give responses for over 4 h although in our experiments we did not record from animals that were on the pad for more than 2 h.
The testing substance was delivered using a needle with 50 μm tip diameter made of a drawn glass capillary (1.2 mm OD, 0.69 mm ID borosilicate glass, pulled on a Sutter Instruments P-97 micropipette puller). The needle was connected through a Teflon tube to a 10 ml syringe containing the repellent solution and typically had a flow rate of 20 μl/min. The needle was positioned using a motorized stage (Polytec/PI M-111.1DG microtranslation stage with C-862 Mercury II controller). The position of the needle was perpendicular to the tip of the animal's head at a distance of about 200 μm, in the on phase, as shown in Figure 1A. During recording, in the off phase the needle was moved far from the animal's head, while during the on phase it was moved near so that the repellent flow could reach the sensory cilia. The constant flow of buffer through the perfusion chamber rapidly clears repellents delivered to the animal's head, so the duration of the stimulus is effectively the same as the duration of the on phase. After the recording, the repellent flow was stopped entirely while perfusion was continued to remove any residual repellent from the bath. Each animal was tested with one or more trials with a rest period of at least 5 min between successive trials to allow recovery. To test adaptation to extended exposure to Cu2+, the needle was used to deliver repellent for 60 s, and then used again to deliver a test stimulus after 60 or 300 s.
For the nose touch stimulus, the conditions used were the same as for the chemical repellent stimuli, with the exception that no constant buffer flow was applied in the perfusion chamber. The stimulator used was a needle with 50 μm tip diameter made of a drawn glass capillary (1.2 mm OD, 0.69 mm ID borosilicate glass, pulled on a Sutter Instruments P-97 micropipette puller) with the tip rounded on the flame to allow complete surface contact with the animal's nose. The needle was placed perpendicular to the animal's body at a distance of ∼200 μm from the nose. In the on phase, the blunt glass needle was moved toward the animal so that it could touch the animal's nose, and in the off phase, the needle returned back in its position.
An ASH Ca2+ response was detected following initial exposure to high-intensity light (most notably at 10 × the intensity used in recordings). This response occurred only after the first irradiation, and thereafter did not interfere with calcium responses to sensory stimuli. Consequently, all recordings were conducted following a short pre-exposure to UV light, and stimuli were typically delivered 5–7 s after the beginning of a recording so that any transients induced by excitation light could be distinguished from repellent responses.
Calcium imaging
Optical recordings were performed on a Zeiss Axioskop 2 upright compound microscope fitted with a Hamamatsu Orca ER CCD camera, a Hamamatsu W-View emission image splitter and a Uniblitz Shutter (Vincent Associates). Fluorescence images were acquired and saved using MetaVue 4.6 (Universal Imaging). Samples were typically taken at 10 Hz (100 ms exposure time) with 4 × 4 binning, using a × 63 Zeiss Achroplan water immersion objective. Filter/dichroic pairs were: excitation, 420/40; excitation dichroic 455; CFP emission, 480/30; emission dichroic 505; YFP emission, 535/30 (Chroma).
Image analysis
Image stacks saved with MetaVue were analyzed using a custom program written in Java. Regions of interest around the cell body of the neuron were defined on the YFP channel of the first image by hand; the program then automatically copied the regions to the CFP channel and tracked the neuron through successive frames using a centroid-centering algorithm similar to one previously described (Kerr et al, 2000). The program reports the total intensity recorded in YFP and CFP channels. The ratio was then computed as (YFP intensity)/(CFP intensity)−0.6, where the 0.6 factor corrects for emission crosstalk (CFP bleedthrough into the YFP channel). Motion of the sample was assessed by computing the distance between the current position of the cell body and a reference position (usually the initial position of the cell body). Although this metric does not capture circular motion about the reference position, such motion is rare.
Ratio changes were parameterized using scripts for MATLAB (The Mathworks). The boundaries of a ratio change were determined by multiple criteria, primarily that the average rate of rise over a 0.5 s interval had to be at least 2%/s. Additional criteria were introduced until the results in virtually all cases were equal or superior to results produced by hand. The magnitude of the ratio change was then calculated as (maximum ratio−minimum ratio); the slope was considered to be the slope of the least squares linear fit to the ratio trace in that region.
Supplementary Material
Supplementary material
Acknowledgments
We thank Shawn Lockery and Serge Faumont for their help and suggestion to set up the perfusion system and the delivery of the repellents; Anne Hart and Mike Chao for sharing unpublished results on serotonin and nose touch; Elia di Schiavi and Mara Sapio for advice during the course of this work; Salvatore Arbucci for excellent technical support; Yuedong Wang for advice on biostatistics; and Cori Bargmann, Yun Zhang, Amanda Kahn and Jami Danzker for reading the manuscript. This work was supported by research grants from the Human Frontier Science Program (to WRS and PB) and the NIH (to WRS), an NIH predoctoral training grant (to RK) and an EMBO short-term fellowship (to AJA).
References
- Bargmann CI, Mori I (1997) Chemotaxis and thermotaxis. In C. elegans II, Riddle DL, Bluementhal T, Mayer BJ, Priess JR (eds) pp 717–737. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [PubMed] [Google Scholar]
- Bargmann CI, Thomas JH, Horvitz HR (1990) Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol 55: 529–538 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Waldo GL, Evans T, Northup JK, Downes CP, Harden TK (1989) Modification of AlF-4- and receptor-stimulated phospholipase C activity by G-protein beta gamma subunits. J Biol Chem 264: 13917–13922 [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824 [DOI] [PubMed] [Google Scholar]
- Chao MY, Komatsu H, Fukuto HS, Dionne HM, Hart AC (2004) Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci USA 101: 15512–15517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI (1997) OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci 17: 8259–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daaka Y, Pitcher JA, Richardson M, Stoffel RH, Robishaw JD, Lefkowitz RJ (1997) Receptor and G betagamma isoform-specific interactions with G protein-coupled receptor kinases. Proc Natl Acad Sci USA 94: 2180–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DebBurman SK, Ptasienski J, Boetticher E, Lomasney JW, Benovic JL, Hosey MM (1995) Lipid-mediated regulation of G protein-coupled receptor kinases 2 and 3. J Biol Chem 270: 5742–5747 [DOI] [PubMed] [Google Scholar]
- Hart AC, Kass J, Shapiro JE, Kaplan JM (1999) Distinct signaling pathways mediate touch and osmosensory responses in a polymodal sensory neuron. J Neurosci 19: 1952–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard MA, Bargmann CI, Bazzicalupo P (2002) C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr Biol 12: 730–734 [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Bergamasco C, Arbucci S, Plasterk RHA, Bazzicalupo P (2004) Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. EMBO J 23: 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LS, Tzou P, Sternberg PW (1994) The lin-15 locus encodes two negative regulators of Caenorhabditis elegans vulval development. Mol Biol Cell 5: 395–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G, Thijssen KL, Werner P, van der Horst M, Hazendonk E, Plasterk RH (1999) The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet 21: 414–419 [DOI] [PubMed] [Google Scholar]
- Jansen G, Weinkove D, Plasterk RH (2002) The G-protein gamma subunit GPC-1 of the nematode C. elegans is involved in taste adaptation. EMBO J 21: 986–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JM, Horvitz HR (1993) A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc Natl Acad Sci USA 90: 2227–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr R, Lev-Ram V, Baird G, Vincent P, Tsien RY, Schafer WR (2000) Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron 26: 583–594 [DOI] [PubMed] [Google Scholar]
- Lee RY, Lobel L, Hengartner M, Horvitz HR, Avery L (1997) Mutations in the alpha1 subunit of an L-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J 16: 6066–6076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Tobin D, Bargmann CI, Friedman JM (2003) Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA 100: 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Fire A (1995) DNA transformation. Methods Cell Biol 48: 451–482 [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY (1997) Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388: 882–887 [DOI] [PubMed] [Google Scholar]
- Morris AJ, Malbon CC (1999) Physiological regulation of G protein-linked signaling. Physiol Rev 79: 1373–1430 [DOI] [PubMed] [Google Scholar]
- Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A (2002) A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20: 87–90 [DOI] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF (2002) A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5: 1169–1176 [DOI] [PubMed] [Google Scholar]
- Pinhero JC, Bates DM (2000) Mixed-effects Models in S and S-PLUS. New York, NY: Springer-Verlag [Google Scholar]
- Roayaie K, Crump JG, Sagasti A, Bargmann CI (1998) The G alpha protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron 20: 55–67 [DOI] [PubMed] [Google Scholar]
- Sambongi Y, Nagae T, Liu Y, Yoshimizu T, Takeda K, Wada Y, Futai M (1999) Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans. Neuroreport 10: 753–757 [DOI] [PubMed] [Google Scholar]
- Schafer WR, Kenyon CJ (1995) A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature 375: 73–78 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kerr R, Bianchi L, Frokjaer-Jensen C, Slone D, Xue J, Gerstbrein B, Driscoll M, Schafer WR (2003) In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron 39: 1005–1017 [DOI] [PubMed] [Google Scholar]
- Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann CI (2002) Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35: 307–318 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543 [DOI] [PubMed] [Google Scholar]
- Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI (1995) Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83: 207–218 [DOI] [PubMed] [Google Scholar]
- Troemel ER, Kimmel BE, Bargmann CI (1997) Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell 91: 161–169 [DOI] [PubMed] [Google Scholar]
- Zwaal RR, Mendel JE, Sternberg PW, Plasterk RH (1997) Two neuronal G proteins are involved in chemosensation of the Caenorhabditis elegans Dauer-inducing pheromone. Genetics 145: 715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


