ABSTRACT
In recent years, long non-coding RNAs (lncRNAs) have attracted the attention of researchers with their involvement in all facets of life. LncRNAs are transcripts of more than 200 nucleotides which lack defined protein coding potential. Although they do not code for proteins, a large number of them are involved in regulating gene expression and translation. The presence of numerous lncRNAs in the human genome has prompted us to investigate the contribution of these molecules to human biology and medicine. In this review, we present the potential role of lncRNAs interlinked to different human diseases and genetic disorders. We also describe their role in cellular differentiation and aging and discuss their potential importance as biomarkers and as therapeutic agents.
KEYWORDS: Biomarkers, epigenetics, gene regulation, human diseases, long non-coding RNA, therapeutic agents
Introduction
The notion that most RNAs act as an intermediate message between DNA and protein has been questioned by the discoveries of new roles for RNAs. It is estimated that > 90 % of the human genome undergoes transcription, however only 2 % of it codes for proteins.1 This results in a large number of RNAs, which do not get translated into proteins. This non-protein coding portion of the genome can be arranged in a variety of categories of non-coding RNAs including transfer RNA (tRNA), ribosomal RNA (rRNA), small nucleolar RNA (snoRNAs), microRNA (miRNAs), small interfering RNA (siRNA), repeat associated siRNA (rasiRNAs) and piwi interacting RNA (piRNAs).2 Although non-coding transcripts such as tRNAs, rRNAs, and spliceosomal RNAs have a wide range of functions and are critical components of cellular machinery, the existence of large pool of non-coding RNAs was initially assumed to be transcriptional noise.3,4 However, mounting evidences have shown that various non-coding RNAs are involved in discrete cellular functions and participate in different regulatory pathways, including chromosomal architecture, in cellular development and differentiation. With the recent advancement in transcriptomic studies, a new class of non-coding RNAs, long non-coding RNAs (lncRNAs) has attracted the focus of scientific community. Many of these lncRNAs have been shown to play specific roles in normal cell functions and diseases.4
In this review, we focus on lncRNAs and discuss their role in pathophysiology of different diseases, and the medical implications of the use of lncRNAs as diagnostic biomarkers or as the basis for novel therapies.
Long non-coding RNA
H19 and Xist are among the first characterized lncRNAs.5,6 Since then, a large number of studies involving DNA tiling arrays,7 next-generation sequencing,8 and transcriptomic studies9-11 have identified thousands of lncRNAs that have been cataloged in various databases such as NONCODE, GENCODE and lncRNAdb.12-14 LncRNAs are generally defined as RNA transcripts with more than 200 nucleotides lacking a clear open reading frame.15 However, some ncRNAs smaller than 200 nucleotides such as BC1 and snaR have been classified in some studies as lncRNAs.14 Additionally, some lncRNAs e.g. lncRNA pncr003:2L in Drosophila are known to code small proteins and peptides.16,17 Since the size definition is purely based on conventional threshold of RNA purification techniques and has no biochemical, structural or functional basis, an alternative definition of lncRNAs has been proposed as ncRNAs that function either as primary or spliced transcripts, independent of existing known classes of small ncRNAs.14 In recent years, there has been dramatic rise in discovery of lncRNAs. To assist in interpreting their function, lncRNAs have been classified based on their length, genomic location of their transcription, association with other functional DNA elements and their subcellular localization.18,19 The simplest classification of lncRNAs is based on their transcription site relative to other genes with different classes described below.
Intergenic lncRNAs and intronic lncRNAs
The transcription sites for intergenic lncRNAs (also called as lincRNAs: long intergenic non-coding RNAs) are located between 2 non-overlapping protein-coding genes. On the other hand, intronic lncRNAs are lncRNAs whose transcripts arise from introns of protein-coding genes.
Sense and antisense lncRNAs
Nearly 70 % of sense transcripts have been reported to have complimentary antisense transcripts.20 Transcription of sense lncRNAs occurs from the same strand of genes that code for protein. These may cover the whole genes or only a fraction of the genes. Antisense lncRNAs (also called as natural antisense transcripts; NAT) are transcribed from the antisense strand overlapping with exonic or intronic region of protein coding genes or cover the entire gene sequence through the introns.
Enhancer and promoter associated lncRNAs
LncRNAs transcribed starting at enhancers are termed enhancer ncRNAs (eRNA). These lncRNAs are involved in forming chromatin loops with promoters that then promotes transcription initiation.21 Transcription of promoter associated lncRNAs (PALR) overlaps the 5′-end of protein coding region comprising the promoter region and first exon or intron.22
Functions of long non-coding RNA
LncRNAs play critical roles in regulation of protein coding genes,23 stem cell pluripotency and differentiation,24 allelic expression,25 cell cycle control,26 apoptosis and senescence.27 LncRNAs can be present in nucleus, cytoplasm and also in mitochondria.28,29 LncRNAs present in different subcellular locations regulate expression of protein coding genes via different mechanisms e.g., controlling chromatin modification, transcription, and translation.
Chromatin modification
In mammalian cells, tissue specific gene expression is controlled by DNA or chromatin modifications. These modifications are performed by a limited number of enzymes (DNA methyltransferases, histone methyltransferases, acetylases, deacetylases etc.) and chromatin-modifying complexes (Polycomb-group and Trithorax-group), many of which lack affinity for particular DNA sequences.30 Various non-coding RNAs including lncRNAs form a network of epigenetic modulators by providing platforms for assembly of these enzymes and chromatin remodeling complexes and guiding them to specific genomic sites (Fig. 1A).31 For example, the HOX transcript antisense RNA (HOTAIR), an lncRNA transcribed from the HOXC locus interacts with polycomb chromatin remodeling complex PRC2 to induce a repressive chromatin state by silencing transcription across 40 kb of the HOXD locus in trans.15 LncRNAs have also been shown to activate transcription by recruiting chromatin-modifying complexes like H3K4 trimethyltransferase MLL1 complex and by activating specific enhancer regions by changing 3-D chromatin conformation.32
Figure 1.
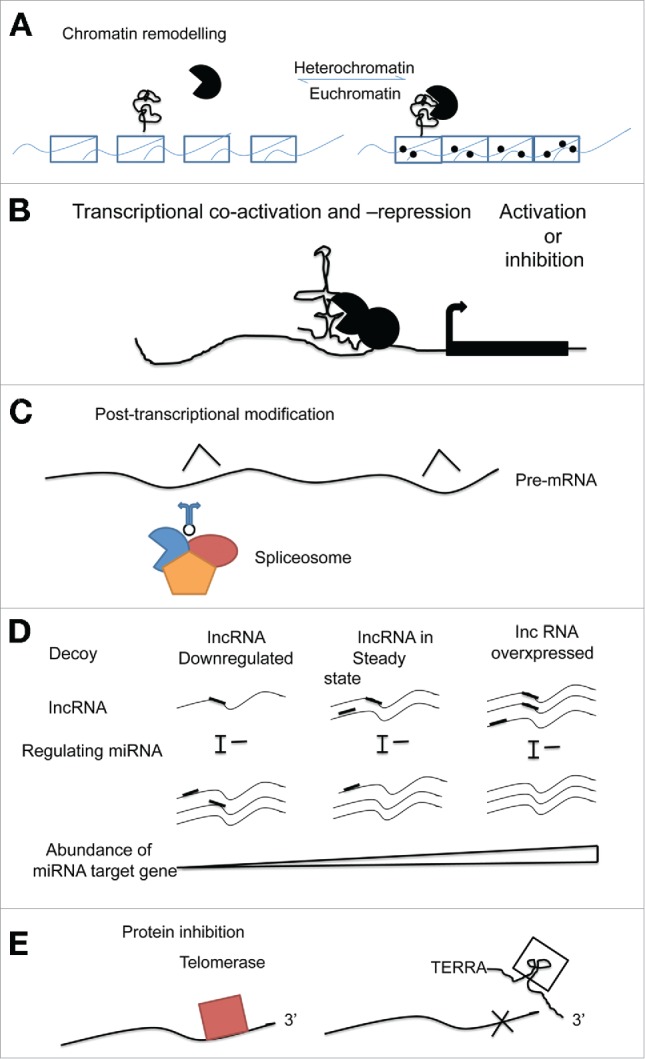
Different mechanisms of functions associated with lncRNAs. They serve in (A) Chromatin modulation, (B) Transcriptional activation and suppression, (C) as post transcriptional machinery, (D) miRNA decoy element and (E) as protein inhibitor (Modified from Cheetham et al. 2013 Br J Cancer, 108:2419-25).
Transcriptional regulation
Different cells experience widespread transcription initiation at enhancers and promoters, however, protein expression takes place in tissue specific manner predicting a major role for lncRNAs associated with these regions (eRNA and PALR) (Fig. 1B).23,33 LncRNAs transcribed from the promoter regions may recruit RNA binding proteins and regulate their function during transcription.34 For example, expression of lncRNAs associated with the cyclin D1 gene promoter is induced by DNA damage signals. Here, lncRNAs act cooperatively to control the activity of RNA binding protein TLS that eventually hinders the histone acetyltransferase activities of CReB binding protein and p300 to silence the expression of cyclin D1.34 The ability of lncRNAs to employ RNA binding proteins to gene promoters greatly increases the transcriptional regulatory machinery.34 LncRNAs also act as co-factors to control activity of transcription factors. In mice, the lncRNA Evf2 transcribed from a conserved distal enhancer controls the binding and action of the transcription factor D1X2 to this enhancer resulting in up-regulation of adjacent protein-coding genes.35
Post-transcriptional regulation
Many genes express antisense lncRNAs, which can overlap with key elements in mRNA to regulate various steps in mRNA processing (Fig. 1C).36 For example, an intron in the 5′ untranslated region of the zinc finger Hox mRNA Zeb2 contains an internal site for ribosome entry, which is required for systematic translation of Zeb2 protein. Antisense lncRNA Zeb2NAT overlaps 5′ splice site of this intron, thus preventing its splicing by spliceosomes leading to accumulation of Zeb2.37
LncRNAs can also inhibit the expression of some specific proteins by forming RNA duplex with the mRNAs.38 Annealing of lncRNA can target protein effecter complexes to the mRNA transcript in a way similar to the targeting done by the RNA-induced silencing complex (RISC) to mRNAs by siRNAs. For example, to achieve X-chromosome inactivation, lncRNAs Xist and Tsix form an RNA duplex which is processed to small RNAs in Dicer dependant manner.38 Some lncRNAs can also act as precursor for miRNAs, e.g. H19/miR-675.39
Medical implications
LncRNAs have versatile contributions to various cellular functions. Mutations or aberrant expressions of lncRNAs may result in cellular dysfunction leading to disease state. Genome-wide association studies (GWAS) have revealed that a large number (88%) of disease associated SNPs reside outside protein coding sequences.40 Of these, 45% SNPs belong to intronic region and 43% SNPs belong to intergenic region of human genome. Many recent studies have implicated lncRNAs in the pathogenesis of various other diseases like Alzheimer's disease, Huntington's disease, psoriasis, diabetes and cardiovascular diseases.41-44 The emerging roles of lncRNAs in diverse disease conditions have paved a new arena to design novel diagnostics and therapeutics. Comparative profiling of lncRNAs isolated from different body fluids such as serum, plasma, urine or sputum can serve as a potential method for early detection of various diseases. For example, identification of lncRNA PCA3 in urine is being used for clinical detection of prostate cancer.45
The current strategies to treat diseases mostly rely on inhibitory drugs. However, to treat certain diseases upregulation of gene expression would be desirable. For example, certain neurological disorders in early stages can be treated with elevated expression of neuro-protective growth factors. In such cases, lncRNAs provide excellent targets for therapeutic agents.46 In the following sections of this review, we will describe what is known about the orchestrating role of lncRNAs in disease pathogenesis, diagnosis and their potential as therapeutic agents and targets.
Development and endocrine glands
LncRNAs play important roles in normal endocrine physiology and development (Table 1) (Fig. 2).47 Several studies have demonstrated the role of lncRNAs in pancreatic β-cell physiology and diabetes.42,48 Transcriptome analysis of human pancreatic β-cells revealed a total of 1128 lncRNAs, some of which showed higher expression on addition of glucose to the β-cell culture. One of these lncRNAs, HI-LNC25 controls the expression of GLIS3 that encodes a transcription factor and regulates expression of insulin and other islet specific transcription factors.42 Comparative analysis of transcriptomes of individuals with and without type-2 diabetes mellitus (T2DM) revealed 493 lncRNAs of which 54 lncRNAs showed expression level correlated to the levels of HbA1c, indicating a direct association with the disease status.48 The regenerative capacity of β-cells in mouse pancreas decreases with age due to up-regulation of the Cdkn2a locus. The lncRNA ANRIL (Antisense Non-coding RNA in the INK4 Locus) is involved in regulation of glucose homeostasis in adult mice by suppressing the expression of Cdkn2a locus that promotes cell division of pancreatic β-cells.49,50 Other lncRNAs that may be involved in T2DM are naPINK1 and KCNQ1OT1 (See Table 1).51,52
Table 1.
LncRNAs expressed in various endocrine tissues. The list represents lncRNAs involved in endocrine function and diseases. Other lncRNAs that are involved in cancer of these tissues are listed in Table 2.
| Organ | lncRNA | Function | Disease association | Ref. |
|---|---|---|---|---|
| Mammary Gland | Pinc Family | Lobuloalveolar differentiation | - | 57 |
| Adipose Tissue | Sra1 | Activates PPARγ to induce adipogenesis | Obesity | 56 |
| lncRAP Family | Adipogenesis | Obesity | 55 | |
| Blnc1 | Thermogenic adipocyte differentiation | - | 122 | |
| PU.1 AS | Blocks translation of PU.1 mRNA and promotes adipocyte differentiation | Obesity | 123 | |
| naPINK1 | Inhibits expression of PINK1 leading to mitochondrial dysfunction | Obesity, Diabetes | 51 | |
| Adrenal Gland | SRA1 | Regulates steroidogenesis | - | 60 |
| Pancreas | ANRIL | Controls expression of Cdkn2a and promotes β-cell proliferation | Diabetes | 49 |
| HI-LNC25 | Regulates level of GLIS3 mRNA | Diabetes | 42 | |
| KCNQ1OT1 | Regulates expression of CDKN1C that controls islet proliferation | Diabetes | 124 | |
| H19 | Regulates expression of IGF2 | Gestational Diabetes | 125 | |
| MEG3 | Unknown | Diabetes | 126 | |
| Pineal Gland | lncSN family | Regulates circadian rhythm | - | 59 |
Figure 2.
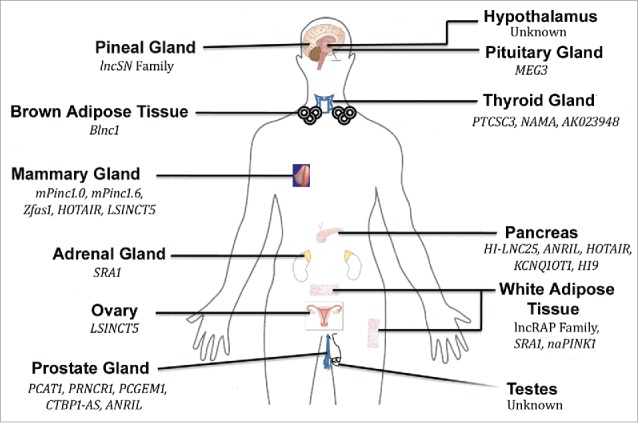
LncRNAs secreted from various endocrine tissues. Tissues are labeled in bold and the involved lncRNAs are also noted. (Modified from Knoll et al. 2015 Nat Rev Endocrinol, 11:151–60)47.
Adipose tissues release a number of chemicals including leptin, which regulates appetite and adiponectin, which helps in regulating various metabolic processes such as glucose metabolism and fatty acid oxidation.53,54 In a recent study, a sum of 175 lncRNAs showed differential expression during adipogenesis. Among them, a group of 10 lncRNAs, named as lncRAP 1–10, were shown to have the capacity to bind the adipogenic transcription factors PPARγ and C/EBPα, and seemed to be required for proper adipocyte differentiation.55 Another lncRNA steroid receptor RNA activator 1 (Sra1) binds and coactivates PPARγ in mice. The mice knock-out for Sra1 locus showed resistance to develop obesity and glucose intolerance with high-fat diet.56
During pregnancy, mammary glands are expanded under the control of hormones estrogen and progesterone. The lncRNA Gb7 or Pinc (Pregnancy induced non-coding RNA) is persistently up-regulated after treatment with estrogen and progesterone in rats. The spliced variants of Pinc, mPinc1.0 and mPinc1.6, show over-expression in the lobulo-alveolar structure of mammary glands during pregnancy, repressed during lactation and again upregulated after involution.57 The interaction of mPinc with PRC2 suggests a role in maintaining repressive chromatin state. In mice, another lncRNA zinc finger antisense 1 (Zfas1) shows down-regulation between pregnancy and lactation and upregulation during lactation and involution. Zfas1 also serves as tumor suppressor and a potent biomarker for breast cancer.58
Pineal gland regulates circadian rhythm by secreting melatonin in accordance with the season and time of day. The tissues from the pineal gland in rat express 112 lncRNAs referred to as lncSNs (lncRNAs, Section on Neuroendocrinology) whose expression oscillates throughout the day. A large fraction (59%) of lncSNs showed higher expression during night.59 In depth analysis of 8 lncSNs showed that their expression was regulated in the suprachiasmatic nuclei of neurons, patterns that were continued in constant darkness indicating their circadian nature.
In adrenal glands, the nuclear receptor NR0B1 coactivates or corepresses another nuclear receptor Steroidogenic factor 1 (SF-1) in a dosage dependent manner.60 The coactivation of SF-1 by NR0B1 is enhanced by non-coding RNA steroid receptor RNA activator (SRA) resulting in activated expression of melanocortin 2 receptor (MC2R) (also known as adreno-corticotropin hormone (ACTH) receptor). The knockdown of SRA1, a variant of SRA in human adrenocortical cells resulted in reduced expression of steroidogenic acute regulatory protein (StAR) and MC2R suggesting a role for the lncRNA SRA in steroidogenesis and adrenal function.60
Besides their roles in maintaining development and physiology of endocrine glands, many lncRNAs are involved in cancer of endocrine glands.47 The lncRNA HOTAIR and long stress induced non-coding RNAs (LSINCTs) show overexpression in breast cancer. The lncRNA MEG3 is a tumor suppressor expressed in pituitary glands. LncRNAs PTCSC3, NAMA and AK023948 show deregulation in thyroid cancers. Two lncRNAs, PCGEM1 and PRNCR1, show over-expression in prostate cancer. PCAT1, a member of a group of 121 lncRNAs identified as prostate cancer associated lncRNA transcripts (PCATs), functions as a transcriptional suppressor in complex with PRC2 leading to inhibition of various tumor suppressor factors such as BRCA2, CENPE and CENPF.47
Cardiovascular disease
Recent studies have shown the influence of lncRNAs in the development of the fetal heart, which involves precise control of gene expression to guide differentiation from pluripotent cells into mesodermal and cardiac cell types. Besides specific proteins, a large number of non-coding RNAs play a part in cellular differentiation. Tissue specific expression of the lncRNAs, Braveheart (Bvht) and Fendrr are associated with early development of the heart in mouse.61,62 These lncRNAs in association with PRC2 regulate expression of genes involved in cardiogenesis. They also control the activity of various transcription factors for mesodermal differentiation.43 Although lncRNAs were initially thought to be non-protein coding sequences, some of them do encode small functional peptides.17 In Drosophila, lncRNA pncr003:2L is translated into 2 polypeptides sarcolamban A and B with 28 and 29 amino acid residues, respectively. These peptides share conserved sequences and structures in different species including humans. These peptides regulate Ca2+ uptake by SERCA2, thereby influencing muscle contraction in heart.16 Mutations in these lncRNAs result in congenital cardiac diseases.
Besides contributing to cardiogenesis, non-coding RNAs are involved in various cardiovascular disorders. A number of GWAS have linked cardiovascular diseases with SNPs in non-coding regions in human genome.63-65 The lncRNA ANRIL located at chromosome 9p21 is associated with a GWAS hotspot for age related diseases such as Alzheimer's disease, coronary disease, type 2 diabetes, endometriosis, glaucoma and cancer.66,67 ANRIL transcription takes place in coronary smooth muscle cells, vascular endothelial cells, and monocyte derived macrophages. The transcript levels of elevated expression of ANRIL are directly correlated to the severity of atherosclerosis.68 Myocardial infarction-associated transcript (MIAT), a 9 kb long lincRNA is expressed in the nuclei of developing neural cells and has also been implicated in retinal cell fate specification. Several variants of MIAT are associated with higher susceptibility to myocardial infarction.69 A SNP (exon 5 11,741 G >A) in MIAT region allows enhanced transcription of this lincRNA. A MIAT variant that has been implicated in splicing regulation shows higher expression in retinal cells in diabetes. Knocking down MIAT led to improvement in retinal microvascular dysfunction caused by diabetes mellitus.70 These results showed that MIAT is involved in pathological angiogenesis and represents a therapeutic target against neovascular diseases.
The involvement of miRNAs in regulation of cardiac specification and differentiation has been well studied. LncRNAs may serve as decoy or competitive endogenous RNAs (ceRNAs) for miRNAs and thus indirectly regulating gene expression (Fig. 1D).71 A recent study showed that a small non-coding RNA miR-489 targets the myeloid differentiation primary response gene 88 (Myd88) to antagonize cardiac hypertrophy.71 The cardiac hypertrophy related factor (CHRF) acts as a ceRNA for miR-489 leading to up-regulation of Myd88 and thereby cardiac hypertrophy.
Although circulatory lncRNAs have been used as a biomarker for cancer; the utility of lncRNAs as biomarkers for cardiovascular diseases is largely unknown. Kumarswamy et al. (2014) found an association between an lncRNA and heart disease.72 They discovered differential regulation of the mitochondrial lncRNA uc022bqs.1 (LIPCAR) at different stages of myocardial infarction in plasma of patients as compared to healthy subjects. With the large number of lncRNAs expressed in human genome, the possibility to identify new biomarkers for the diagnosis of cardiovascular diseases is wide open.
Cell differentiation, apoptosis and cancer
Research in recent years has revealed a major contribution of lncRNAs to almost all phases of life. They are involved in regulating cell cycle, cellular differentiation and cell death. Different environmental changes, stresses, infections, life style changes and aging influence the delicate balance of various biomolecules in the cell leading to perturbed cellular physiology and disease. Cancer is a disease of disturbed cellular division and death. Several lncRNAs have been implicated in the cancer pathophysiology with some of them providing strong targets as biomarkers for diagnosis of diseases and as therapeutic targets to treat the diseases (Table 2).73
Table 2.
LncRNAs associated with carcinogenesis.
| LncRNA | Disease association | Function in Oncogenesis | Functional mechanism | Ref. |
|---|---|---|---|---|
| UCA1 | Bladder cancer | Oncogene/ Biomarker | Promotes cell proliferation and metastasis | 86,127 |
| GAS5 | Breast cancer | Tumor suppressor | inhibits expression of glucocorticoid receptor, induces apoptosis | 128 |
| Zfas1 | Breast cancer | Tumor suppressor | Regulates alveolar development and epithelial cell differentiation | 58 |
| KCNQ1OT1 | Colorectal cancer | Unknown | Imprinting defects | 129 |
| linc-p21 | Colorectal cancer | Tumor suppressor | hnRNP-K mediated gene repression | 80 |
| PTENP1 | Hepatocellular carcinoma | Tumor suppressor | decoy oncomirs targeting PTEN | 130 |
| aHIF | Multiple cancer | Biomarker | Unknown | 131 |
| ANRIL | Multiple cancer | Oncogene | Inhibits the expression of tumor suppressor genes | 132 |
| H19 | Multiple cancer | Tumor suppressor | controls expression of multiple genes involved in growth, proliferation and apoptosis | 133 |
| HOTAIR | Multiple cancer | Oncogene | PRC2 mediated methylation of various genes | 77 |
| HULC | Multiple cancer | Oncogene/ Biomarker | modulates expression of p18 to inhibit apoptosis | 116 |
| MALAT1 | Multiple cancer | Oncogene | controls RNA splicing, promotes cellular mobility | 74,76 |
| MEG3 | Multiple cancer | Tumor suppressor | Inhibits tumor growth | 134 |
| PANDA | Multiple Cancer | Oncogene | Regulates apoptosis | 81 |
| PCA3 | Prostate cancer | Biomarker | Silencing expression of tumor suppressor gene PRUNE2 | 45,135 |
| PCGEM1 | Prostate cancer | Oncogene | promotes cell proliferation | 85 |
| PRNCR1 | Prostate cancer | oncogene | Promotes cancer cell proliferation in association with PCGEM1 | 136 |
| PCAT1 | Prostate Cancer, Hepatocellular carcinoma | Oncogene/ Biomarker | Inhibits expression of tumor suppressor, promotes cell proliferation | 137,138 |
| PTCSC3 | Thyroid Cancer | Tumor suppressor | Decoy miR-574-5p | 139 |
MALAT1 is one of the first lncRNAs to be associated with human lung cancer.74 Since then it has been implicated in the cancer of various other organs including lung, liver, kidney, colon, breast, pancreas, bladder and many more.75 MALAT1, which is involved in control of alternative splicing by modulating the phosphorylation of SR proteins, shows normal expression in various healthy tissues but is up-regulated in cancerous cells. Silencing MALAT1 results in impaired cellular mobility and hence has been linked with cancer metastasis.76 The lncRNA HOTAIR is highly expressed in various cancerous tissues including liver cancer, breast cancer and colon cancer. Overexpression of HOTAIR retargets polycomb repressive complex 2 (PRC2) to new sites in genome including various tumor suppressor genes leading to altered H3K27 methylation, which in turn promotes proliferation and metastasis of cancerous cells (Fig. 3A).77,78 High levels of HOTAIR are associated with relapse for liver carcinoma patients.79 Similar to HOTAIR, lncRNA ANRIL is also associated with several cancer types including acute lymphoblastic leukemia, nasopharyngeal carcinoma, glioma, breast cancer and basal cell carcinoma. ANRIL over-expression represses the expression of the INK4B–ARF–INK4A locus genes containing 3 tumor suppressor genes by recruiting PRC1 and PRC2, resulting in cancer proliferation.66
Figure 3.
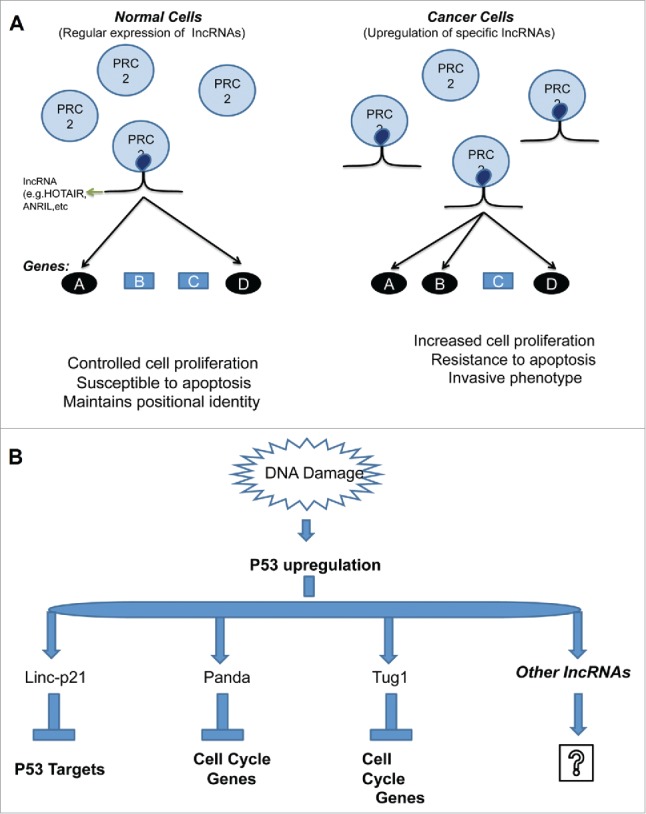
Misexpression of different lncRNAs in cancer that modulate different pathways with diversified mechanisms (A) a group of lncRNAs (e. g. HOTAIR, ANRIL and others) modulate chromatin structure and organization in cis or in trans (PRC2) to alter their expression (B) A group of lncRNAs induced p53 regulated pathway. The lncRNA once activated modulated via different protein partners. (Modified from Niland et al. 2012 Front Genet 3:25)78.
A number of lncRNAs are activated by the tumor suppressor p53 in response to DNA damage (Fig. 3B).78 The tumor suppressor p53 up-regulates lncRNA lincRNA-p21 by binding at its promoter. The lncRNA lincRNA-p21 interacts with heterogeneous nuclear ribonucleoprotein K (hnRNP-K) and represses the expression of multiple genes in the p53 pathway and p53 mediated apoptosis.80 Another p53 induced lncRNA PANDA (P21-associated ncRNA DNA damage activated) down-regulates pro-apoptotic genes by interacting with the transcription factor NF-YA.81 The lncRNA H19 and the H19 derived miR-675 are over-expressed in human colorectal cancer cells, whereas they show normal expression in surrounding tissues.39 LncRNA H19 regulates the expression of several genes within the imprinted gene network, a cluster of genes whose expression depends on the parent contributing them. These genes, including H19 itself and insulin like growth factor 2 (IGF2), are involved in growth, proliferation and apoptosis. The lncRNA H19 forms a ribonucleoprotein complex with methyl-CpG–binding domain protein 1 (MBD1), which interacts with histone lysine methyltransferases to induce methylation of ICRs resulting in repression of genes in this locus.82 Furthermore, downregulation of lncRNA H19 leads to lower levels of p57 and decreases tumor growth.83
Since several lncRNAs are tissue or cell specific and may control the progression of many diseases, they are considered biomarkers for disease diagnosis and as therapeutic targets.84 The prostate cancer associated lncRNA prostate cancer gene 3 (PCA3) is routinely used as a urine test to diagnose prostate cancer risk.45 LncRNA PCGEM1 is expressed in a tissue-specific manner in prostate glands and displays enhanced expression in high risk groups.85 Another cancer related biomarker lncRNA UCA1 (urothelial carcinoma associated 1), which can be detected in urine, shows high sensitivity and specificity for bladder carcinoma.86 Other promising biomarkers for different cancer types include AA174084 found in gastric juice of gastric cancer patients, MALAT1- derived fragment detected in plasma of prostate cancer patients, HULC in plasma of hepatocellular carcinoma patients.87-89 In a successful attempt to treat H19-driven cancer cells, a plasmid preparation BC-819 (DTA-H19) that carries the diphtheria toxin under control of the H19 regulatory sequence was used as an intratumoral injection resulting in reduced tumor size.90 These studies suggest the potential of lncRNAs as biomarkers in cancer diagnosis and targets for treatment.
Immunity and autoimmune diseases
Immunity is the most organized cellular defense of the body against pathogenic agents. It requires correct development, differentiation and activation of immune cells. Th1 helper cells are the major immune cells involved in adaptive immunity against various pathogens. Hundreds of lncRNAs have been identified in CD8+ T cells from human and mouse spleen suggesting their importance in lymphocyte differentiation and activation.91 The lincRNA TMEVPG1 (also named as NeST) in CD8+ T-cells has an important role in controlling Theiler's virus infection. TMEVPG1 along with T cell specific transcription factors T-bet/Stat can up-regulate expression of IFN-γ recruiting H3K4me3 to the ifng gene through interaction with the WDR5 subunit of H3K4 methyltransferase.92 An lncRNA lncDC, which is uniquely expressed in dendritic cells, binds with STAT3 signaling molecule in cytoplasm suggesting a role for lncRNAs in direct control of cellular differentiation and function.93
B-lymphocytes, originating from bone marrow have function in generating antibodies and presenting pathogenic antigens to other immune cells. In the production of antigen receptors, multiple non-coding RNAs have been implicated in regulating variable, diversity and joining [V(D)J] recombination by bringing VH region close to DJH region.94,95 Natural killer cells kill virus infected cells and tumors with proteins like perforin and proteases. Cytotoxic activity of these cells is regulated by many cell surface class I MHC receptors, such as killer cell immunoglobulin-like receptor (KIR). Many KIR genes transcribe antisense lncRNAs that in some cases have been shown to reduce the expression of KIR proteins by overlapping with exon 1 and 2 of genes coding those.96 Macrophages are another class of immune cells that are involved in removal of microbes and other damaged cells from the body by phagocytosis. PTPRJ or CD148, a tyrosine phosphatase with known tumor suppressor activity, is expressed abundantly in macrophages in response to LPS or TLR ligands but down-regulated in response to CSF-1. The lncRNA ptprj-as1 expressed antisense to the ptprj gene and is co-regulated in response to TLR ligands or CSF-1.97 Another non-coding RNA lincRNA-Cox2 or Ptgs2 shows overexpression in dendritic cells after stimulation with TLR4.98 In a recent study, a total of 159 lncRNAs showed induction or suppression in THP1 macrophages treated with Pam3CSK4 in comparison to their expression in non-treated cells.99 In particular, the expression of TNF-α was shown to be regulated by lncRNA linc1992 or THRIL (TNFα and hnRNPL Related Immunoregulatory LincRNA) by interacting with the heterogenous nuclear ribonucleoprotein L (hnRNPL) and binding its promoter. Interestingly, THRIL has been found to be associated with Kawasaki disease, an acute inflammatory disease of childhood.99
Related to the role of non-coding RNAs in regulating the development and activity of different immunological cells, they also have shown their role in various autoimmune diseases (Table 3). Autoimmune thyroid diseases (AITD), Graves' disease and Hashimoto's thyroiditis, are caused by infiltration of the T-cell in the thyroid gland leading to production of anti-thyroid autoantibodies. A SNP Ex9b-SNP10, located in intron 9 of ZFAT gene and promoter region of a non-coding transcript small antisense transcript of ZFAT (SAS-ZFAT), shows association with high risk group of AITD.100 The T-allele of this SNP results in dysregulated B-cell function by up-regulating SAS-ZFAT and down-regulating truncated ZFAT.
Table 3.
LncRNAs involved in immune response.
| LncRNA | Cell involved | Functional mechanism | Associated Disease | Ref. |
|---|---|---|---|---|
| PRINS | Epidermal Cells | Protects cells against stress induced death | Psoriasis | 101 |
| LincRNA-p21 | THP-1 monocytes | Decreased expression induces activity of NFκB | Rheumatoid arthritis | 103 |
| SAS-ZFAT | CD19+ B cells | SNP in SAS-ZFAT promoter region correlates to high risk of autoimmune thyroid disease | Autoimmune thyroid disease | 100 |
| NeST | CD8+ T-cells | Regulates expression of IFNγ by methylation through H3K4 methyltransferase | Microbial infection | 92 |
| GAS5 | T-lymphocytes | Interacts with glucocorticoid receptor and suppresses GR-induced transcriptional activity | Systemic lupus erythematosus | 128 |
| THRIL | THP-1 monocytes | Regulates expression of TNFα via hnRNPL | Kawasaki disease | 99 |
| Lnc-DC | Dendritic cells | Activates transcription factor STAT3 to support cellular differentiation | 93 | |
| Ptprj-as1 | Macrophage | Expressed in response to lipopolysaccharide | 97 |
The hyper-proliferation of keratinocytes in skin of psoriasis patients is induced by infiltrating T-lymphocytes at the dermal-epidermal junction. A non-coding RNA PRINS (Psoriasis susceptibility-related RNA gene Induced by Stress) has been shown to be overexpressed in the psoriatic epidermal cells as compared with healthy skin cells.101 Similarly, another autoimmune disorder rheumatoid arthritis (RA) is the result of joint destruction due to the action of several proteases secreted by T, B, and APC cells in response to an alteration in the synovial microenvironment by proinflammatory cytokines and chemokines. Expression of 85 lincRNAs in CD14+ monocytes from RA patients is regulated by the proinflammatory chemokines TNF-α and IL-6, and showed significant upregulation due to anti-TNFα or anti-IL6 treatment.102 Another non-coding RNA lincRNA-p21 showed reduced expression in RA patients. Treatment of RA patients with methotrexate inhibited the activity of NF-κB by inducing expression of lincRNA-p21 that is thought to regulate gene expression through hnRNP-K mediated repression.80,103 Besides these diseases, lncRNAs have also been associated with various other autoimmune diseases such as systemic lupus erythematosus, juvenile idiopathic arthritis, primary biliary cirrhosis, asthma, celiac disease and inflammatory bowel disease.44,104
Neurological disorders
The onset and progression of many neurological disorders are affected by dysregulation of lncRNAs and genes that they regulate. The best characterized example of neurological diseases controlled by lncRNAs is Alzheimer's disease (AD). Accumulation of extracellular amyloid-β deposits due to increased expression of 2 proteases, β-secretase or β-Site APP-Cleaving Enzyme 1 (BACE1) and γ-secretase, results in AD pathophysiology. An antisense transcript of BACE1 (BACE1-AS), over-expressed in AD patients, forms a RNA duplex with BACE1 mRNA to stabilize it resulting in higher level of BACE1 protein.41 Knocking down BACE1-AS resulted in reduced levels of BACE1-AS and BACE1 lowering the levels of amyloid-β synthesis and aggregation in brain. Thus, BACE1-AS presents a promising therapeutic target to treat AD.105 In contrast to a gradual decrease in the expression in the healthy aging brain, lncRNA BCYRN1/BC200 showed up-regulation in brain of patients with age related AD. The level of translational regulator BCYRN1/BC200 was directly correlated with the severity of the disease.106 BC200 RNA interacts with various RNA-binding proteins that are involved in protein synthesis at postsynaptic sites in neurons.107 Thus, they have role in modulating protein synthesis in dendrites and may contribute to synaptodendritic deterioration in aging brain.
Neurodegenerative disorder spinocerebellar ataxia type 8 (SCA8) that affects muscle and speech coordination is caused by trinucleotide repeats in protein coding gene ataxin 8 (ATXN8), with CAG repeats resulting in poly-Q protein, and in an lncRNA gene ataxin 8 opposite strand (ATXN8OS) with CUG expansion. The lncRNA ATXN8OS interacts with splicing factor MBNL1 in neurons leading to anomalous splicing of GABA-A transporter 4 (GABT4) and loss of GABAergic inhibition in the granular cell layer, which is suggested to contribute to the SCA8 phenotype.108 In another model of SCA8 pathophysiology, the lncRNA ATXN8OS was shown to repress the expression of KLHL1 gene located in close vicinity of ATXN8.109 Reduction in KLHL1 expression decreases neurite outgrowth during development of neurons resulting in brain dysfunction.
Several other lncRNAs have been linked with different cognitive disorders such as schizophrenia, autism spectrum disorders (ASD), and Angelman syndrome. The lncRNA MIAT that interacts with splicing factors QKI and SRSF1 shows down-regulation in brain tissues of schizophrenia patients.110 The loss of MIAT expression was associated with global changes in alternative splicing as observed for DISC1, a gene associated with schizophrenia. Microarray analysis of brain tissues from ASD patient revealed 222 differentially expressed lncRNAs. Most of these lncRNAs were colocalized with protein coding genes associated with brain development.111 Many neurological diseases such as Parkinson's disease, amyolateral sclerosis and AD have defects in mitochondria of the affected neurons, indicating possible role for mitochondrial lncRNAs in neurodegeneration.112
Bio-age
Aging is a biological process during which cells, tissues and organs undergo progressive deterioration leading to loss of function, diseases and death. Different lncRNAs are involved in regulation of cellular activity, proliferation, differentiation, quiescence, senescence, stress response and other functions related to aging by modulating gene expression.27,113 Cellular aging is associated with gradual reduction in the length of telomere, which is controlled by telomerase ribonucleoprotein complex formed by the interaction between protein telomerase reverse transcriptase (TERT) and the lncRNAs TERC (telomerase RNA component) and TERRA (telomeric repeat-containing RNA) that contains telomeric repeats. TERC provide template for synthesis of telomeric repeats to prevent premature aging, whereas TERRA suppresses telomeric elongation by inhibiting TERT activity (Fig. 1E). TERC downregulation or TERRA over-expression are associated with premature aging.27
Several epigenetic factors such as DNA methylation, histone modification and heterochromatin formation are involved in the aging process. Several lncRNAs are involved in age related regulation of these epigenetic alterations. LncRNA X-inactive-specific transcript (XIST), which is involved in X-chromosome silencing in females, is downregulated in aging cells.114 The overexpression of insulin-like growth factor 2 receptor (IGF2R) in senescent cells, as compared with proliferating cells, indicates its role in longevity. The lncRNA Airn controls the expression of Igf2r gene by transcriptional interference with its promoter.115 The other examples of lncRNAs involved in age-related gene methylation include ecCEBP, pRNA, PAPAS, PTENpg1-AS and TARID.
A healthy cell maintains a delicate balance of its protein content. This protein homeostasis is governed by protein biosynthesis, trafficking, and degradation. These processes influence different aspects of cell cycle, proliferation and senescence leading to aging. Various non-coding RNAs play important roles in these processes. Over-expression of lncRNA HULC decreases the expression of tumor suppressor p18 and inhibits apoptosis by autophagy and promotes cell proliferation and metastasis in gastric cancer cells.116,117 The non-coding RNA 7SL interacts with TP53 mRNA and suppresses translation of p53. The RNA-binding protein Hur competitively displaces 7SL to enhance p53 translation, thereby promoting cell cycle arrest and senescence.118 AS Uchl1, an lncRNA with SINEB2 repeats, enhances the expression of ubiquitin carboxyl-terminal hydrolase-1 (UCHL1) inducing senescence.119 The lncRNA-p21 through translation repressors RCK and FMRP suppresses expression of β-catenin and JunB, which are involved in cell proliferation and carcinogenesis.120
During aging various environmental stresses, telomere dysfunction and DNA damage negatively influence the cell cycle progression leading to senescence. Senescence is induced by DNA damage in advancing age that elevates the expression of cell cycle inhibitors, e.g., p53 and p21. The lncRNAs involved in cell cycle regulation and senescence include MALAT1, H19, ANRIL, SRA, HEIH, HULC, UCA1, NcRNACCND1 and others.27 Silencing MALAT1 is associated with enhanced senescence and induced G1/S arrest indicating MALAT1 as a senescence suppressor. The lncRNA HEIH down-regulates the expression of cyclin-dependent kinase inhibitors p16, p21, p27 and p57 assisting tumor cell growth.121 Cell cycle regulator cyclin D1 (CCND1) associated lncRNA NcRNACCND1 is essential for the activity of cyclin-dependent kinases, cdk2 and cdk4 for G1/S transition. Upon exposure to DNA-damaging agents, NcRNACCND1 forms a nucleoprotein complex with protein TLS.34 This complex interacts with CCND1 promoter to inhibit transcription. Thus, lncRNAs are associated with the different aspects of aging and are involved in almost every process in cell cycle, proliferation and senescence.
Conclusion
Extensive research in the field of non-coding RNAs and their roles in maintaining normal physiology as well as in disease pathogenesis suggest that lncRNAs are an important contributor to multiple disease traits. However, the answers of many ncRNAs mediated questions remain uncertain. Although many GWAS have linked them to different diseases, the actual mechanism of disease development is yet to be elucidated. The annotated databases of the lncRNA sequences in human genome are not complete or inaccurate in many cell types. Complete annotation of lncRNAs in specialized cells of different human organs is an important basis required for future work. The potential of lncRNAs as therapeutic targets and as biomarkers for different diseases warrants further investigation to explore their relevance in disease onset and progression. Therefore, we strongly expect that further studies of lncRNAs will reinforce the importance of these novel molecules in human biology and medicine.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Mrs. Hemalatha for helping in formatting and submission of manuscript.
Funding
The work was supported by the CSIR network project of India to U.B. (B.S.C. 0121) and DBT Drosophila project to M.P.B (GAP0362).
References
- 1.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al.. The transcriptional landscape of the mammalian genome. Science 2005; 309:1559-63; PMID:16141072; http://dx.doi.org/ 10.1126/science.1112014 [DOI] [PubMed] [Google Scholar]
- 2.Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: regulators of disease. J Pathol 2010; 220:126-39; PMID:19882673; http://dx.doi.org/ 10.1002/path.2638 [DOI] [PubMed] [Google Scholar]
- 3.Morris KV, Mattick JS. The rise of regulatory RNA. Nat Rev Genet 2014; 15:423-37; PMID:24776770; http://dx.doi.org/ 10.1038/nrg3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Wu Z, Fu X, Han W. lncRNAs: insights into their function and mechanics in underlying disorders. Mutat Res Rev Mutat Res 2014; 762:1-21; PMID:25485593; http://dx.doi.org/ 10.1016/j.mrrev.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 5.Brannan CI, Dees EC, Ingram RS, Tilghman SM. The product of the H19 gene may function as an RNA. Mol Cell Biol 1990; 10:28-36; PMID:1688465; http://dx.doi.org/ 10.1128/MCB.10.1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CJ, Hendrich BD, Rupert JL, Lafreniere RG, Xing Y, Lawrence J, Willard HF. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 1992; 71:527-42; PMID:1423611; http://dx.doi.org/ 10.1016/0092-8674(92)90520-M [DOI] [PubMed] [Google Scholar]
- 7.Kapranov P, Cawley SE, Drenkow J, Bekiranov S, Strausberg RL, Fodor SP, et al.. Large-scale transcriptional activity in chromosomes 21 and 22. Science 2002; 296:916-9; PMID:11988577; http://dx.doi.org/ 10.1126/science.1068597 [DOI] [PubMed] [Google Scholar]
- 8.Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, Rinn JL. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev 2011; 25:1915-27; PMID:21890647; http://dx.doi.org/ 10.1101/gad.17446611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilgner H, Knowles DG, Johnson R, Davis CA, Chakrabortty S, Djebali S, Curado J, Snyder M, Gingeras TR, Guigó R. Deep sequencing of subcellular RNA fractions shows splicing to be predominantly co-transcriptional in the human genome but inefficient for lncRNAs. Genome Res 2012; 22:1616-25; PMID:22955974; http://dx.doi.org/ 10.1101/gr.134445.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, et al.. Landscape of transcription in human cells. Nature 2012; 489:101-8; PMID:22955620; http://dx.doi.org/ 10.1038/nature11233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso CS, Kominsky HD, et al.. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nature biotechnology 2011; 29:742-9; PMID:21804560; http://dx.doi.org/ 10.1038/nbt.1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, et al.. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 2012; 22:1775-89; PMID:22955988; http://dx.doi.org/ 10.1101/gr.132159.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Li H, Fang S, Kang Y, Wu W, Hao Y, Li Z, Bu D, Sun N, Zhang MQ, et al.. NONCODE 2016: an informative and valuable data source of long non-coding RNAs. Nucleic Acids Res 2016; 44:D203-8; PMID:26586799; http://dx.doi.org/ 10.1093/nar/gkv1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amaral PP, Clark MB, Gascoigne DK, Dinger ME, Mattick JS. lncRNAdb: a reference database for long noncoding RNAs. Nucleic Acids Res 2011; 39:D146-51; PMID:21112873; http://dx.doi.org/ 10.1093/nar/gkq1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet 2009; 10:155-9; PMID:19188922; http://dx.doi.org/ 10.1038/nrg2521 [DOI] [PubMed] [Google Scholar]
- 16.Magny EG, Pueyo JI, Pearl FM, Cespedes MA, Niven JE, Bishop SA, Couso JP. Conserved regulation of cardiac calcium uptake by peptides encoded in small open reading frames. Science 2013; 341:1116-20; PMID:23970561; http://dx.doi.org/ 10.1126/science.1238802 [DOI] [PubMed] [Google Scholar]
- 17.Slavoff SA, Mitchell AJ, Schwaid AG, Cabili MN, Ma J, Levin JZ, Karger AD, Budnik BA, Rinn JL, Saghatelian A. Peptidomic discovery of short open reading frame-encoded peptides in human cells. Nat Chem Biol 2013; 9:59-64; PMID:23160002; http://dx.doi.org/ 10.1038/nchembio.1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Laurent G, Wahlestedt C, Kapranov P. The Landscape of long noncoding RNA classification. Trends Genet 2015; 31:239-51; PMID:25869999; http://dx.doi.org/ 10.1016/j.tig.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol 2013; 10:925-33; PMID:23696037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katayama S, Tomaru Y, Kasukawa T, Waki K, Nakanishi M, Nakamura M, Nakamura M, Nishida H, Yap CC, Suzuki M, et al.. Antisense transcription in the mammalian transcriptome. Science 2005; 309:1564-6; PMID:16141073; http://dx.doi.org/ 10.1126/science.1112009 [DOI] [PubMed] [Google Scholar]
- 21.Meseure D, Drak Alsibai K, Nicolas A, Bieche I, Morillon A. Long noncoding RNAs as new architects in cancer epigenetics, prognostic biomarkers, and potential therapeutic targets. Biomed Res Int 2015; 2015:320214; PMID:26448935; http://dx.doi.org/ 10.1155/2015/320214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermüller J, Hofacker IL, et al.. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 2007; 316:1484-8; PMID:17510325; http://dx.doi.org/ 10.1126/science.1138341 [DOI] [PubMed] [Google Scholar]
- 23.Vance KW, Ponting CP. Transcriptional regulatory functions of nuclear long noncoding RNAs. Trends in Genetics 2014; 30:348-55; PMID:24974018; http://dx.doi.org/ 10.1016/j.tig.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinger ME, Amaral PP, Mercer TR, Pang KC, Bruce SJ, Gardiner BB, Askarian-Amiri ME, Ru K, Soldà G, Simons C, et al.. Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome research 2008; 18:1433-45; PMID:18562676; http://dx.doi.org/ 10.1101/gr.078378.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maenner S, Muller M, Becker PB. Roles of long, non-coding RNA in chromosome-wide transcription regulation: lessons from two dosage compensation systems. Biochimie 2012; 94:1490-8; PMID:22239950; http://dx.doi.org/ 10.1016/j.biochi.2011.12.026 [DOI] [PubMed] [Google Scholar]
- 26.Kitagawa M, Kitagawa K, Kotake Y, Niida H, Ohhata T. Cell cycle regulation by long non-coding RNAs. Cell Mol Life Sci 2013; 70:4785-94; PMID:23880895; http://dx.doi.org/ 10.1007/s00018-013-1423-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grammatikakis I, Panda AC, Abdelmohsen K, Gorospe M. Long noncoding RNAs(lncRNAs) and the molecular hallmarks of aging. Aging; (Albany NY: ) 2014; 6:992-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabili MN, Dunagin MC, McClanahan PD, Biaesch A, Padovan-Merhar O, Regev A, Regev A, Rinn JL, Raj A. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol 2015; 16:20; PMID:25630241; http://dx.doi.org/ 10.1186/s13059-015-0586-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rackham O, Shearwood AM, Mercer TR, Davies SM, Mattick JS, Filipovska A. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA 2011; 17:2085-93; PMID:22028365; http://dx.doi.org/ 10.1261/rna.029405.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattick JS, Amaral PP, Dinger ME, Mercer TR, Mehler MF. RNA regulation of epigenetic processes. Bioessays 2009; 31:51-9; PMID:19154003; http://dx.doi.org/ 10.1002/bies.080099 [DOI] [PubMed] [Google Scholar]
- 31.Angrand PO, Vennin C, Le Bourhis X, Adriaenssens E. The role of long non-coding RNAs in genome formatting and expression. Front Genet 2015; 6:165; PMID:25972893; http://dx.doi.org/ 10.3389/fgene.2015.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta RA, et al.. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 2011; 472:120-4; PMID:21423168; http://dx.doi.org/ 10.1038/nature09819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 2007; 130:77-88; PMID:17632057; http://dx.doi.org/ 10.1016/j.cell.2007.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, Tempst P, Rosenfeld MG, Glass CK, Kurokawa R. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature 2008; 454:126-30; PMID:18509338; http://dx.doi.org/ 10.1038/nature06992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng J, Bi C, Clark BS, Mady R, Shah P, Kohtz JD. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes & development 2006; 20:1470-84; PMID:16705037; http://dx.doi.org/ 10.1101/gad.1416106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faghihi MA, Wahlestedt C. Regulatory roles of natural antisense transcripts. Nature reviews Molecular cell biology 2009; 10:637-43; PMID:19638999; http://dx.doi.org/ 10.1038/nrm2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beltran M, Puig I, Peña C, García JM, Álvarez AB, Peña R, Bonilla F, de Herreros AG. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial–mesenchymal transition. Genes & development 2008; 22:756-69; PMID:18347095; http://dx.doi.org/ 10.1101/gad.455708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogawa Y, Sun BK, Lee JT. Intersection of the RNA interference and X-inactivation pathways. Science 2008; 320:1336-41; PMID:18535243; http://dx.doi.org/ 10.1126/science.1157676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsang WP, Ng EK, Ng SS, Jin H, Yu J, Sung JJ, Kwok TT. Oncofetal H19-derived miR-675 regulates tumor suppressor RB in human colorectal cancer. Carcinogenesis 2010; 31:350-8; PMID:19926638; http://dx.doi.org/ 10.1093/carcin/bgp181 [DOI] [PubMed] [Google Scholar]
- 40.Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, Manolio TA. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A 2009; 106:9362-7; PMID:19474294; http://dx.doi.org/ 10.1073/pnas.0903103106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, Finch CE, St Laurent G 3rd, Kenny PJ, Wahlestedt C. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of b-secretase. Nat Med 2008; 14:723-30; PMID:18587408; http://dx.doi.org/ 10.1038/nm1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moran I, Akerman I, van de Bunt M, Xie R, Benazra M, Nammo T, Arnes L, Nakić N, García-Hurtado J, Rodríguez-Seguí S, et al.. Human b cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab 2012; 16:435-48; PMID:23040067; http://dx.doi.org/ 10.1016/j.cmet.2012.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gurha P, Marian AJ. Noncoding RNAs in cardiovascular biology and disease. Circ Res 2013; 113:e115-20; PMID:24311620; http://dx.doi.org/ 10.1161/CIRCRESAHA.113.302988 [DOI] [PubMed] [Google Scholar]
- 44.Sigdel KR, Cheng A, Wang Y, Duan L, Zhang Y. The Emerging Functions of Long Noncoding RNA in Immune Cells: Autoimmune Diseases. J Immunol Res 2015; 2015:848790; PMID:26090502; http://dx.doi.org/ 10.1155/2015/848790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de la Taille A. Progensa PCA3 test for prostate cancer detection. Expert Rev Mol Diagn 2007; 7:491-7; PMID:17892357; http://dx.doi.org/ 10.1586/14737159.7.5.491 [DOI] [PubMed] [Google Scholar]
- 46.Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat Rev Drug Discov 2013; 12:433-46; PMID:23722346; http://dx.doi.org/ 10.1038/nrd4018 [DOI] [PubMed] [Google Scholar]
- 47.Knoll M, Lodish HF, Sun L. Long non-coding RNAs as regulators of the endocrine system. Nat Rev Endocrinol 2015; 11:151-60; PMID:25560704; http://dx.doi.org/ 10.1038/nrendo.2014.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fadista J, Vikman P, Laakso EO, Mollet IG, Esguerra JL, Taneera J, Storm P, Osmark P, Ladenvall C, Prasad RB, et al.. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc Natl Acad Sci U S A 2014; 111:13924-9; PMID:25201977; http://dx.doi.org/ 10.1073/pnas.1402665111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 2006; 443:453-7; PMID:16957737; http://dx.doi.org/ 10.1038/nature05092 [DOI] [PubMed] [Google Scholar]
- 50.Yap KL, Li S, Munoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou MM. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell 2010; 38:662-74; PMID:20541999; http://dx.doi.org/ 10.1016/j.molcel.2010.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheele C, Nielsen AR, Walden TB, Sewell DA, Fischer CP, Brogan RJ, Petrovic N, Larsson O, Tesch PA, Wennmalm K, et al.. Altered regulation of the PINK1 locus: a link between type 2 diabetes and neurodegeneration? FASEB J 2007; 21:3653-65; PMID:17567565; http://dx.doi.org/ 10.1096/fj.07-8520com [DOI] [PubMed] [Google Scholar]
- 52.Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, et al.. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 2010; 42:579-89; PMID:20581827; http://dx.doi.org/ 10.1038/ng.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006; 64:355-65; PMID:16584505; http://dx.doi.org/ 10.1111/j.1365-2265.2006.02474.x [DOI] [PubMed] [Google Scholar]
- 54.Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 2013; 9:191-200; PMID:23671428; http://dx.doi.org/ 10.5114/aoms.2013.33181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun L, Goff LA, Trapnell C, Alexander R, Lo KA, Hacisuleyman E, Sauvageau M, Tazon-Vega B, Kelley DR, Hendrickson DG, et al.. Long noncoding RNAs regulate adipogenesis. Proc Natl Acad Sci U S A 2013; 110:3387-92; PMID:23401553; http://dx.doi.org/ 10.1073/pnas.1222643110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu B, Gerin I, Miao H, Vu-Phan D, Johnson CN, Xu R, Chen XW, Cawthorn WP, MacDougald OA, Koenig RJ. Multiple roles for the non-coding RNA SRA in regulation of adipogenesis and insulin sensitivity. PLoS One 2010; 5:e14199; PMID:21152033; http://dx.doi.org/ 10.1371/journal.pone.0014199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ginger MR, Shore AN, Contreras A, Rijnkels M, Miller J, Gonzalez-Rimbau MF, Rosen JM. A noncoding RNA is a potential marker of cell fate during mammary gland development. Proc Natl Acad Sci U S A 2006; 103:5781-6; PMID:16574773; http://dx.doi.org/ 10.1073/pnas.0600745103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Askarian-Amiri ME, Crawford J, French JD, Smart CE, Smith MA, Clark MB, Ru K, Mercer TR, Thompson ER, Lakhani SR, et al.. SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for breast cancer. RNA 2011; 17:878-91; PMID:21460236; http://dx.doi.org/ 10.1261/rna.2528811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coon SL, Munson PJ, Cherukuri PF, Sugden D, Rath MF, Moller M, Clokie SJ, Fu C, Olanich ME, Rangel Z, et al.. Circadian changes in long noncoding RNAs in the pineal gland. Proc Natl Acad Sci U S A 2012; 109:13319-24; PMID:22864914; http://dx.doi.org/ 10.1073/pnas.1207748109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu B, Yang WH, Gerin I, Hu CD, Hammer GD, Koenig RJ. Dax-1 and steroid receptor RNA activator (SRA) function as transcriptional coactivators for steroidogenic factor 1 in steroidogenesis. Mol Cell Biol 2009; 29:1719-34; PMID:19188450; http://dx.doi.org/ 10.1128/MCB.01010-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grote P, Wittler L, Hendrix D, Koch F, Wahrisch S, Beisaw A, Macura K, Bläss G, Kellis M, Werber M, et al.. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell 2013; 24:206-14; PMID:23369715; http://dx.doi.org/ 10.1016/j.devcel.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, et al.. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 2013; 152:570-83; PMID:23352431; http://dx.doi.org/ 10.1016/j.cell.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ounzain S, Micheletti R, Beckmann T, Schroen B, Alexanian M, Pezzuto I, Crippa S, Nemir M, Sarre A, Johnson R, et al.. Genome-wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart-specific long non-coding RNAs. Eur Heart J 2015; 36:353-68a; PMID:24786300; http://dx.doi.org/ 10.1093/eurheartj/ehu180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang W, Li Y, He F, Wu H. Microarray profiling of long non-coding RNA (lncRNA) associated with hypertrophic cardiomyopathy. BMC Cardiovasc Disord 2015; 15:62; PMID:26141701; http://dx.doi.org/ 10.1186/s12872-015-0056-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li D, Chen G, Yang J, Fan X, Gong Y, Xu G, Cui Q, Geng B. Transcriptome analysis reveals distinct patterns of long noncoding RNAs in heart and plasma of mice with heart failure. PLoS One 2013; 8:e77938; PMID:24205036; http://dx.doi.org/ 10.1371/journal.pone.0077938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pasmant E, Sabbagh A, Vidaud M, Bieche I. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB J 2011; 25:444-8; PMID:20956613; http://dx.doi.org/ 10.1096/fj.10-172452 [DOI] [PubMed] [Google Scholar]
- 67.Congrains A, Kamide K, Katsuya T, Yasuda O, Oguro R, Yamamoto K, Ohishi M, Rakugi H. CVD-associated non-coding RNA, ANRIL, modulates expression of atherogenic pathways in VSMC. Biochem Biophys Res Commun 2012; 419:612-6; PMID:22382030; http://dx.doi.org/ 10.1016/j.bbrc.2012.02.050 [DOI] [PubMed] [Google Scholar]
- 68.Holdt LM, Beutner F, Scholz M, Gielen S, Gabel G, Bergert H, Schuler G, Thiery J, Teupser D. ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler Thromb Vasc Biol 2010; 30:620-7; PMID:20056914; http://dx.doi.org/ 10.1161/ATVBAHA.109.196832 [DOI] [PubMed] [Google Scholar]
- 69.Ishii N, Ozaki K, Sato H, Mizuno H, Saito S, Takahashi A, Miyamoto Y, Ikegawa S, Kamatani N, Hori M, et al.. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet 2006; 51:1087-99; PMID:17066261; http://dx.doi.org/ 10.1007/s10038-006-0070-9 [DOI] [PubMed] [Google Scholar]
- 70.Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li YJ, Tao ZF, Song YC, Chen Q, Jiang Q. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res 2015; 116:1143-56; PMID:25587098; http://dx.doi.org/ 10.1161/CIRCRESAHA.116.305510 [DOI] [PubMed] [Google Scholar]
- 71.Wang K, Liu F, Zhou LY, Long B, Yuan SM, Wang Y, Liu CY, Sun T, Zhang XJ, Li PF. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res 2014; 114:1377-88; PMID:24557880; http://dx.doi.org/ 10.1161/CIRCRESAHA.114.302476 [DOI] [PubMed] [Google Scholar]
- 72.Kumarswamy R, Bauters C, Volkmann I, Maury F, Fetisch J, Holzmann A, Lemesle G, de Groote P, Pinet F, Thum T. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res 2014; 114:1569-75; PMID:24663402; http://dx.doi.org/ 10.1161/CIRCRESAHA.114.303915 [DOI] [PubMed] [Google Scholar]
- 73.Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer 2013; 108:2419-25; PMID:23660942; http://dx.doi.org/ 10.1038/bjc.2013.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et al.. MALAT-1, a novel noncoding RNA, and thymosin b4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 2003; 22:8031-41; PMID:12970751; http://dx.doi.org/ 10.1038/sj.onc.1206928 [DOI] [PubMed] [Google Scholar]
- 75.Wei Y, Niu B. Role of MALAT1 as a Prognostic Factor for Survival in Various Cancers: A Systematic Review of the Literature with Meta-Analysis. Dis Markers 2015; 2015:164635; PMID:26420912; http://dx.doi.org/ 10.1155/2015/164635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt LH, Spieker T, Koschmieder S, Schaffers S, Humberg J, Jungen D, Bulk E, Hascher A, Wittmer D, Marra A, et al.. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol 2011; 6:1984-92; PMID:22088988; http://dx.doi.org/ 10.1097/JTO.0b013e3182307eac [DOI] [PubMed] [Google Scholar]
- 77.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al.. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010; 464:1071-6; PMID:20393566; http://dx.doi.org/ 10.1038/nature08975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Niland CN, Merry CR, Khalil AM. Emerging Roles for Long Non-Coding RNAs in Cancer and Neurological Disorders. Front Genet 2012; 3:25; PMID:22375145; http://dx.doi.org/ 10.3389/fgene.2012.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Z, Zhou L, Wu LM, Lai MC, Xie HY, Zhang F, Zheng SS. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol 2011; 18:1243-50; PMID:21327457; http://dx.doi.org/ 10.1245/s10434-011-1581-y [DOI] [PubMed] [Google Scholar]
- 80.Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, et al.. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010; 142:409-19; PMID:20673990; http://dx.doi.org/ 10.1016/j.cell.2010.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P, et al.. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet 2011; 43:621-9; PMID:21642992; http://dx.doi.org/ 10.1038/ng.848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Monnier P, Martinet C, Pontis J, Stancheva I, Ait-Si-Ali S, Dandolo L. H19 lncRNA controls gene expression of the Imprinted Gene Network by recruiting MBD1. Proc Natl Acad Sci U S A 2013; 110:20693-8; PMID:24297921; http://dx.doi.org/ 10.1073/pnas.1310201110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gabory A, Jammes H, Dandolo L. The H19 locus: role of an imprinted non-coding RNA in growth and development. Bioessays 2010; 32:473-80; PMID:20486133; http://dx.doi.org/ 10.1002/bies.200900170 [DOI] [PubMed] [Google Scholar]
- 84.Yarmishyn AA, Kurochkin IV. Long noncoding RNAs: a potential novel class of cancer biomarkers. Front Genet 2015; 6:145; PMID:25954300; http://dx.doi.org/ 10.3389/fgene.2015.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xue Y, Wang M, Kang M, Wang Q, Wu B, Chu H, Zhong D, Qin C, Yin C, Zhang Z, et al.. Association between lncrna PCGEM1 polymorphisms and prostate cancer risk. Prostate Cancer Prostatic Dis 2013; 16:139-44, S1; PMID:23459097; http://dx.doi.org/ 10.1038/pcan.2013.6 [DOI] [PubMed] [Google Scholar]
- 86.Wang XS, Zhang Z, Wang HC, Cai JL, Xu QW, Li MQ, Chen YC, Qian XP, Lu TJ, Yu LZ, et al.. Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin Cancer Res 2006; 12:4851-8; PMID:16914571; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-0134 [DOI] [PubMed] [Google Scholar]
- 87.Ren S, Wang F, Shen J, Sun Y, Xu W, Lu J, Wei M, Xu C, Wu C, Zhang Z, et al.. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur J Cancer 2013; 49:2949-59; PMID:23726266; http://dx.doi.org/ 10.1016/j.ejca.2013.04.026 [DOI] [PubMed] [Google Scholar]
- 88.Shao Y, Ye M, Jiang X, Sun W, Ding X, Liu Z, Ye G, Zhang X, Xiao B, Guo J. Gastric juice long noncoding RNA used as a tumor marker for screening gastric cancer. Cancer 2014; 120:3320-8; PMID:24986041; http://dx.doi.org/ 10.1002/cncr.28882 [DOI] [PubMed] [Google Scholar]
- 89.Xie H, Ma H, Zhou D. Plasma HULC as a promising novel biomarker for the detection of hepatocellular carcinoma. Biomed Res Int 2013; 2013:136106; PMID:23762823; http://dx.doi.org/ 10.1155/2013/136106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smaldone MC, Davies BJ. BC-819, a plasmid comprising the H19 gene regulatory sequences and diphtheria toxin A, for the potential targeted therapy of cancers. Curr Opin Mol Ther 2010; 12:607-16; PMID:20886393 [PubMed] [Google Scholar]
- 91.Pang KC, Dinger ME, Mercer TR, Malquori L, Grimmond SM, Chen W, Mattick JS. Genome-wide identification of long noncoding RNAs in CD8+ T cells. J Immunol 2009; 182:7738-48; PMID:19494298; http://dx.doi.org/ 10.4049/jimmunol.0900603 [DOI] [PubMed] [Google Scholar]
- 92.Gomez JA, Wapinski OL, Yang YW, Bureau JF, Gopinath S, Monack DM, Chang HY, Brahic M, Kirkegaard K. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus. Cell 2013; 152:743-54; PMID:23415224; http://dx.doi.org/ 10.1016/j.cell.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang P, Xue Y, Han Y, Lin L, Wu C, Xu S, Jiang Z, Xu J, Liu Q, Cao X. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 2014; 344:310-3; PMID:24744378; http://dx.doi.org/ 10.1126/science.1251456 [DOI] [PubMed] [Google Scholar]
- 94.Bolland DJ, Wood AL, Johnston CM, Bunting SF, Morgan G, Chakalova L, Fraser PJ, Corcoran AE. Antisense intergenic transcription in V(D)J recombination. Nat Immunol 2004; 5:630-7; PMID:15107847; http://dx.doi.org/ 10.1038/ni1068 [DOI] [PubMed] [Google Scholar]
- 95.Verma-Gaur J, Torkamani A, Schaffer L, Head SR, Schork NJ, Feeney AJ. Noncoding transcription within the Igh distal V(H) region at PAIR elements affects the 3D structure of the Igh locus in pro-B cells. Proc Natl Acad Sci U S A 2012; 109:17004-9; PMID:23027941; http://dx.doi.org/ 10.1073/pnas.1208398109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wright PW, Huehn A, Cichocki F, Li H, Sharma N, Dang H, Lenvik TR, Woll P, Kaufman D, Miller JS, et al.. Identification of a KIR antisense lncRNA expressed by progenitor cells. Genes Immun 2013; 14:427-33; PMID:23863987; http://dx.doi.org/ 10.1038/gene.2013.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dave RK, Dinger ME, Andrew M, Askarian-Amiri M, Hume DA, Kellie S. Regulated expression of PTPRJ/CD148 and an antisense long noncoding RNA in macrophages by proinflammatory stimuli. PLoS One 2013; 8:e68306; PMID:23840844; http://dx.doi.org/ 10.1371/journal.pone.0068306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al.. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009; 458:223-7; PMID:19182780; http://dx.doi.org/ 10.1038/nature07672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Z, Chao TC, Chang KY, Lin N, Patil VS, Shimizu C, Head SR, Burns JC, Rana TM. The long noncoding RNA THRIL regulates TNFa expression through its interaction with hnRNPL. Proc Natl Acad Sci U S A 2014; 111:1002-7; PMID:24371310; http://dx.doi.org/ 10.1073/pnas.1313768111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shirasawa S, Harada H, Furugaki K, Akamizu T, Ishikawa N, Ito K, Ito K, Tamai H, Kuma K, Kubota S, et al.. SNPs in the promoter of a B cell-specific antisense transcript, SAS-ZFAT, determine susceptibility to autoimmune thyroid disease. Hum Mol Genet 2004; 13:2221-31; PMID:15294872; http://dx.doi.org/ 10.1093/hmg/ddh245 [DOI] [PubMed] [Google Scholar]
- 101.Sonkoly E, Bata-Csorgo Z, Pivarcsi A, Polyanka H, Kenderessy-Szabo A, Molnar G, Szentpali K, Bari L, Megyeri K, Mandi Y, et al.. Identification and characterization of a novel, psoriasis susceptibility-related noncoding RNA gene, PRINS. J Biol Chem 2005; 280:24159-67; PMID:15855153; http://dx.doi.org/ 10.1074/jbc.M501704200 [DOI] [PubMed] [Google Scholar]
- 102.Muller N, Doring F, Klapper M, Neumann K, Schulte DM, Turk K, Schröder JO, Zeuner RA, Freitag-Wolf S, Schreiber S, et al.. Interleukin-6 and tumour necrosis factor-a differentially regulate lincRNA transcripts in cells of the innate immune system in vivo in human subjects with rheumatoid arthritis. Cytokine 2014; 68:65-8; PMID:24721042; http://dx.doi.org/ 10.1016/j.cyto.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 103.Spurlock CF 3rd, Tossberg JT, Matlock BK, Olsen NJ, Aune TM. Methotrexate inhibits NF-kB activity via long intergenic (noncoding) RNA-p21 induction. Arthritis Rheumatol 2014; 66:2947-57; PMID:25077978; http://dx.doi.org/ 10.1002/art.38805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hrdlickova B, Kumar V, Kanduri K, Zhernakova DV, Tripathi S, Karjalainen J, Lund RJ, Li Y, Ullah U, Modderman R, et al.. Expression profiles of long non-coding RNAs located in autoimmune disease-associated regions reveal immune cell-type specificity. Genome Med 2014; 6:88; PMID:25419237; http://dx.doi.org/ 10.1186/s13073-014-0088-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Modarresi F, Faghihi MA, Patel NS, Sahagan BG, Wahlestedt C, Lopez-Toledano MA. Knockdown of BACE1-AS Nonprotein-Coding Transcript Modulates Beta-Amyloid-Related Hippocampal Neurogenesis. Int J Alzheimers Dis 2011; 2011:929042; PMID:21785702; http://dx.doi.org/ 10.4061/2011/929042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mus E, Hof PR, Tiedge H. Dendritic BC200 RNA in aging and in Alzheimer's disease. Proc Natl Acad Sci U S A 2007; 104:10679-84; PMID:17553964; http://dx.doi.org/ 10.1073/pnas.0701532104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin D, Pestova TV, Hellen CU, Tiedge H. Translational control by a small RNA: dendritic BC1 RNA targets the eukaryotic initiation factor 4A helicase mechanism. Mol Cell Biol 2008; 28:3008-19; PMID:18316401; http://dx.doi.org/ 10.1128/MCB.01800-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Daughters RS, Tuttle DL, Gao W, Ikeda Y, Moseley ML, Ebner TJ, Swanson MS, Ranum LP. RNA gain-of-function in spinocerebellar ataxia type 8. PLoS Genet 2009; 5:e1000600; PMID:19680539; http://dx.doi.org/ 10.1371/journal.pgen.1000600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen WL, Lin JW, Huang HJ, Wang SM, Su MT, Lee-Chen GJ, Chen CM, Hsieh-Li HM. et al.. SCA8 mRNA expression suggests an antisense regulation of KLHL1 and correlates to SCA8 pathology. Brain Res 2008; 1233:176-84; PMID:18708037; http://dx.doi.org/ 10.1016/j.brainres.2008.07.096 [DOI] [PubMed] [Google Scholar]
- 110.Barry G, Briggs JA, Vanichkina DP, Poth EM, Beveridge NJ, Ratnu VS, Nayler SP, Nones K, Hu J, Bredy TW, et al.. The long non-coding RNA Gomafu is acutely regulated in response to neuronal activation and involved in schizophrenia-associated alternative splicing. Mol Psychiatry 2014; 19:486-94; PMID:23628989; http://dx.doi.org/ 10.1038/mp.2013.45 [DOI] [PubMed] [Google Scholar]
- 111.Ziats MN, Rennert OM. Aberrant expression of long noncoding RNAs in autistic brain. J Mol Neurosci 2013; 49:589-93; PMID:22949041; http://dx.doi.org/ 10.1007/s12031-012-9880-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Filosto M, Scarpelli M, Cotelli MS, Vielmi V, Todeschini A, Gregorelli V, Tonin P, Tomelleri G, Padovani A. The role of mitochondria in neurodegenerative diseases. J Neurol 2011; 258:1763-74; PMID:21604203; http://dx.doi.org/ 10.1007/s00415-011-6104-z [DOI] [PubMed] [Google Scholar]
- 113.Kim J, Kim KM, Noh JH, Yoon JH, Abdelmohsen K, Gorospe M. Long noncoding RNAs in diseases of aging. Biochim Biophys Acta 2016; 1859:209-21; PMID:26141605; http://dx.doi.org/ 10.1016/j.bbagrm.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abdelmohsen K, Panda A, Kang MJ, Xu J, Selimyan R, Yoon JH, Martindale JL, De S, Wood WH 3rd, Becker KG, et al.. Senescence-associated lncRNAs: senescence-associated long noncoding RNAs. Aging Cell 2013; 12:890-900; PMID:23758631; http://dx.doi.org/ 10.1111/acel.12115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Latos PA, Pauler FM, Koerner MV, Senergin HB, Hudson QJ, Stocsits RR, Allhoff W, Stricker SH, Klement RM, Warczok KE, et al.. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science 2012; 338:1469-72; PMID:23239737; http://dx.doi.org/ 10.1126/science.1228110 [DOI] [PubMed] [Google Scholar]
- 116.Du Y, Kong G, You X, Zhang S, Zhang T, Gao Y, Ye L, Zhang X. Elevation of highly up-regulated in liver cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell proliferation via down-regulating p18. J Biol Chem 2012; 287:26302-11; PMID:22685290; http://dx.doi.org/ 10.1074/jbc.M112.342113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao Y, Guo Q, Chen J, Hu J, Wang S, Sun Y. Role of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: a clinical and in vitro investigation. Oncol Rep 2014; 31:358-64; PMID:24247585; http://dx.doi.org/ 10.3892/or.2013.2850 [DOI] [PubMed] [Google Scholar]
- 118.Abdelmohsen K, Panda AC, Kang MJ, Guo R, Kim J, Grammatikakis I, Yoon JH, Dudekula DB, Noh JH, Yang X, et al.. 7SL RNA represses p53 translation by competing with HuR. Nucleic Acids Res 2014; 42:10099-111; PMID:25123665; http://dx.doi.org/ 10.1093/nar/gku686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carrieri C, Cimatti L, Biagioli M, Beugnet A, Zucchelli S, Fedele S, Pesce E, Ferrer I, Collavin L, Santoro C, et al.. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature 2012; 491:454-7; PMID:23064229; http://dx.doi.org/ 10.1038/nature11508 [DOI] [PubMed] [Google Scholar]
- 120.Yoon JH, Abdelmohsen K, Srikantan S, Yang X, Martindale JL, De S, Huarte M, Zhan M, Becker KG, Gorospe M. LincRNA-p21 suppresses target mRNA translation. Mol Cell 2012; 47:648-55; PMID:22841487; http://dx.doi.org/ 10.1016/j.molcel.2012.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang F, Zhang L, Huo XS, Yuan JH, Xu D, Yuan SX, Zhu N, Zhou WP, Yang GS, Wang YZ, et al.. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology 2011; 54:1679-89; PMID:21769904; http://dx.doi.org/ 10.1002/hep.24563 [DOI] [PubMed] [Google Scholar]
- 122.Zhao XY, Li S, Wang GX, Yu Q, Lin JD. A long noncoding RNA transcriptional regulatory circuit drives thermogenic adipocyte differentiation. Mol Cell 2014; 55:372-82; PMID:25002143; http://dx.doi.org/ 10.1016/j.molcel.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pang WJ, Lin LG, Xiong Y, Wei N, Wang Y, Shen QW, Yang GS. Knockdown of PU.1 AS lncRNA inhibits adipogenesis through enhancing PU.1 mRNA translation. J Cell Biochem 2013; 114:2500-12; PMID:23749759; http://dx.doi.org/ 10.1002/jcb.24595 [DOI] [PubMed] [Google Scholar]
- 124.Travers ME, Mackay DJ, Dekker Nitert M, Morris AP, Lindgren CM, Berry A, Johnson PR, Hanley N, Groop LC, McCarthy MI, et al.. Insights into the molecular mechanism for type 2 diabetes susceptibility at the KCNQ1 locus from temporal changes in imprinting status in human islets. Diabetes 2013; 62:987-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ding GL, Wang FF, Shu J, Tian S, Jiang Y, Zhang D, Wang N, Luo Q, Zhang Y, Jin F, et al.. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes 2012; 61:1133-42; PMID:22447856; http://dx.doi.org/ 10.2337/db11-1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wallace C, Smyth DJ, Maisuria-Armer M, Walker NM, Todd JA, Clayton DG. The imprinted DLK1-MEG3 gene region on chromosome 14q32.2 alters susceptibility to type 1 diabetes. Nat Genet 2010; 42:68-71; PMID:19966805; http://dx.doi.org/ 10.1038/ng.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang F, Li X, Xie X, Zhao L, Chen W. UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Lett 2008; 582:1919-27; PMID:18501714; http://dx.doi.org/ 10.1016/j.febslet.2008.05.012 [DOI] [PubMed] [Google Scholar]
- 128.Lucafo M, De Iudicibus S, Di Silvestre A, Pelin M, Candussio L, Martelossi S, Tommasini A, Piscianz E, Ventura A, Decorti G.et al. Long noncoding RNA GAS5: a novel marker involved in glucocorticoid response. Curr Mol Med 2015; 15:94-9; PMID:25601472; http://dx.doi.org/ 10.2174/1566524015666150114122354 [DOI] [PubMed] [Google Scholar]
- 129.Higashimoto K, Soejima H, Saito T, Okumura K, Mukai T. Imprinting disruption of the CDKN1C/KCNQ1OT1 domain: the molecular mechanisms causing Beckwith-Wiedemann syndrome and cancer. Cytogenet Genome Res 2006; 113:306-12; PMID:16575194; http://dx.doi.org/ 10.1159/000090846 [DOI] [PubMed] [Google Scholar]
- 130.Chen CL, Tseng YW, Wu JC, Chen GY, Lin KC, Hwang SM, Hu YC. Suppression of hepatocellular carcinoma by baculovirus-mediated expression of long non-coding RNA PTENP1 and MicroRNA regulation. Biomaterials 2015; 44:71-81; PMID:25617127; http://dx.doi.org/ 10.1016/j.biomaterials.2014.12.023 [DOI] [PubMed] [Google Scholar]
- 131.Rossignol F, Vache C, Clottes E. Natural antisense transcripts of hypoxia-inducible factor 1alpha are detected in different normal and tumour human tissues. Gene 2002; 299:135-40; PMID:12459261; http://dx.doi.org/ 10.1016/S0378-1119(02)01049-1 [DOI] [PubMed] [Google Scholar]
- 132.Kotake Y, Nakagawa T, Kitagawa K, Suzuki S, Liu N, Kitagawa M, Xiong Y. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene 2011; 30:1956-62; PMID:21151178; http://dx.doi.org/ 10.1038/onc.2010.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Berteaux N, Aptel N, Cathala G, Genton C, Coll J, Daccache A, Spruyt N, Hondermarck H, Dugimont T, Curgy JJ, et al.. A novel H19 antisense RNA overexpressed in breast cancer contributes to paternal IGF2 expression. Mol Cell Biol 2008; 28:6731-45; PMID:18794369; http://dx.doi.org/ 10.1128/MCB.02103-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou Y, Zhang X, Klibanski A. MEG3 noncoding RNA: a tumor suppressor. J Mol Endocrinol 2012; 48:R45-53; PMID:22393162; http://dx.doi.org/ 10.1530/JME-12-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Salameh A, Lee AK, Cardo-Vila M, Nunes DN, Efstathiou E, Staquicini FI, Dobroff AS, Marchi∫ S, Navone NM, Hosoya H, et al.. PRUNE2 is a human prostate cancer suppressor regulated by the intronic long noncoding RNA PCA3. Proc Natl Acad Sci U S A 2015; 112:8403-8; PMID:26080435; http://dx.doi.org/ 10.1073/pnas.1507882112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang L, Lin C, Jin C, Yang JC, Tanasa B, Li W, Merkurjev D, Ohgi KA, Meng D, Zhang J, et al.. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature 2013; 500:598-602; PMID:23945587; http://dx.doi.org/ 10.1038/nature12451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prensner JR, Chen W, Han S, Iyer MK, Cao Q, Kothari V, Evans JR, Knudsen KE, Paulsen MT, Ljungman M, et al.. The long non-coding RNA PCAT-1 promotes prostate cancer cell proliferation through cMyc. Neoplasia 2014; 16:900-8; PMID:25425964; http://dx.doi.org/ 10.1016/j.neo.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yan TH, Yang H, Jiang JH, Lu SW, Peng CX, Que HX, Lu WL, Mao JF. Prognostic significance of long non-coding RNA PCAT-1 expression in human hepatocellular carcinoma. Int J Clin Exp Pathol 2015; 8:4126-31; PMID:26097602 [PMC free article] [PubMed] [Google Scholar]
- 139.Fan M, Li X, Jiang W, Huang Y, Li J, Wang Z. A long non-coding RNA, PTCSC3, as a tumor suppressor and a target of miRNAs in thyroid cancer cells. Exp Ther Med 2013; 5:1143-6; PMID:23599737; http://dx.doi.org/ 10.3892/etm.2013.933 [DOI] [PMC free article] [PubMed] [Google Scholar]


