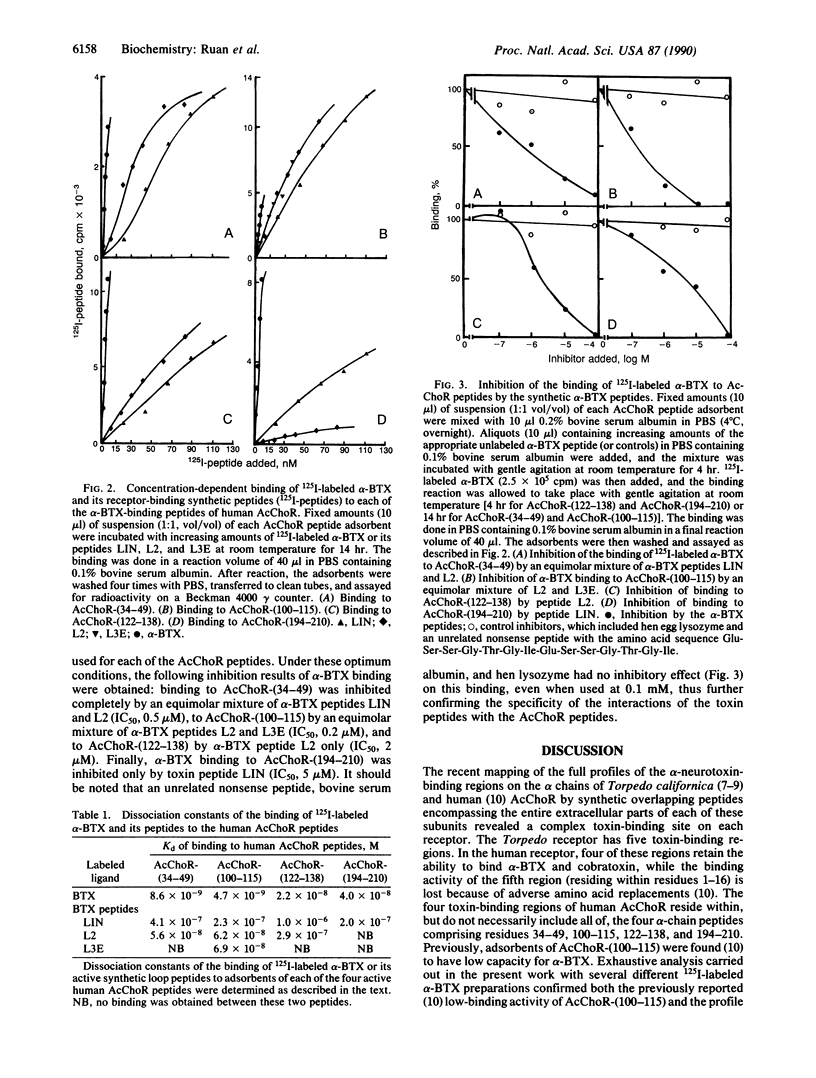
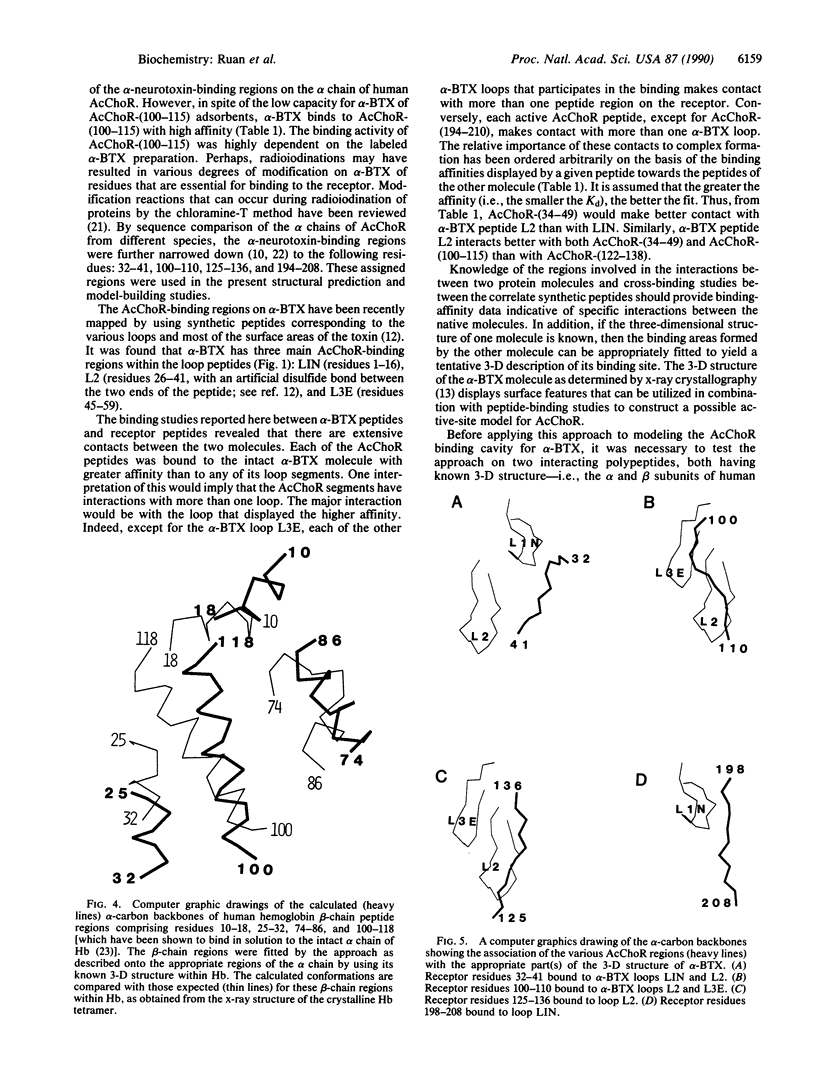
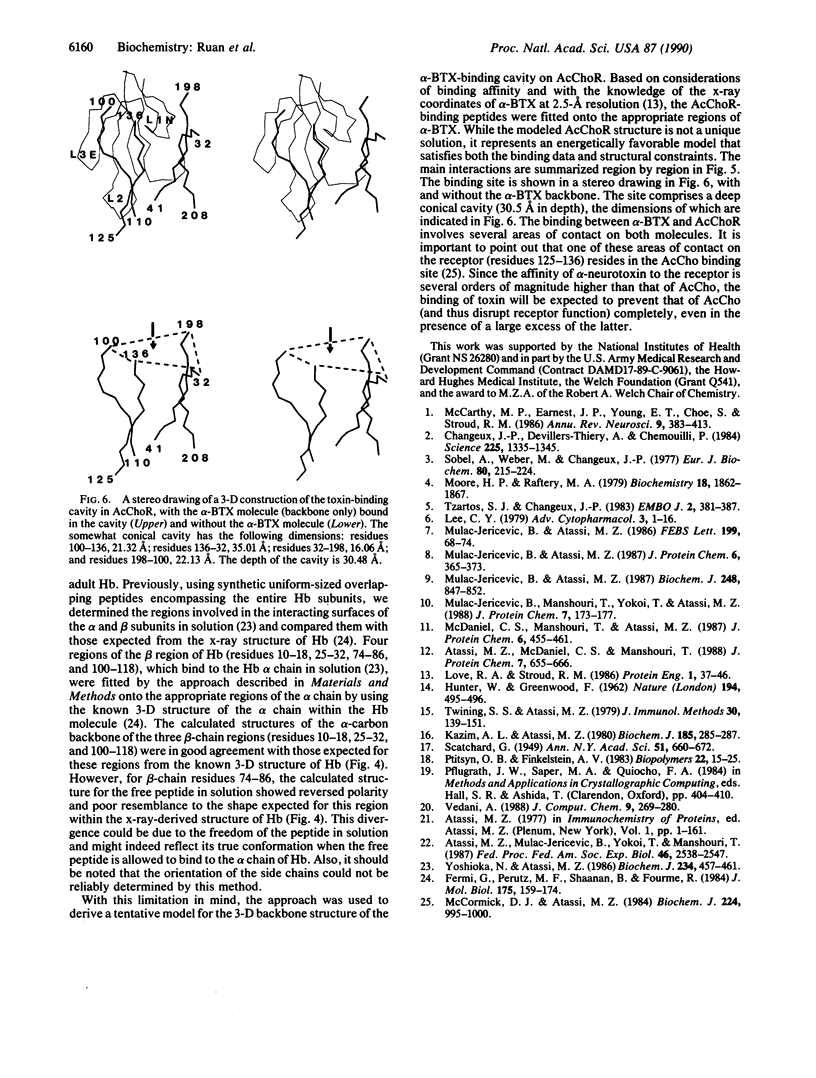
Abstract
In previous studies from this laboratory, the binding regions of alpha-neurotoxins on human and Torpedo acetylcholine (AcCho) receptors (AcChoRs) and the binding regions for the receptor on the toxin were characterized with synthetic peptides of the respective molecules. In the present work, peptides representing the active regions of one molecule are each allowed to bind to each of the active-region peptides of the other molecule. Thus, the interaction of three alpha-bungarotoxin (alpha-BTX) synthetic loop peptides with four synthetic peptides representing the toxin-binding regions on human AcChoR permitted the determination of the region-region interactions between alpha-BTX and the human receptor. Based on the known three-dimensional structure of the toxin, the active peptides of the receptor were then assembled to their appropriate toxin-contact regions by computer model building and energy minimization. This allowed the three-dimensional construction of the toxin-binding cavity on human AcChoR. The cavity appears to be conical, 30.5 A in depth, involving several receptor regions that make contact with the alpha-BTX loop regions. One AcChoR region (within residues 125-136) involved in the binding to alpha-BTX also resides in a known AcCho-binding site, thus demonstrating in three dimensions a critical site involved in both AcCho activation and alpha-BTX blocking. The validity of this approach was first established for three of four peptides corresponding to regions on the beta chain of human hemoglobin involved in binding to the alpha chain. Thus, studying the interaction between peptides representing the binding regions of two protein molecules may provide an approach in molecular recognition by which the binding site on one protein can be described if the three-dimensional structure of the other protein is known.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z., McDaniel C. S., Manshouri T. Mapping by synthetic peptides of the binding sites for acetylcholine receptor on alpha-bungarotoxin. J Protein Chem. 1988 Oct;7(5):655–666. doi: 10.1007/BF01024881. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Mulac-Jericevic B., Yokoi T., Manshouri T. Localization of the functional sites on the alpha chain of acetylcholine receptor. Fed Proc. 1987 Jun;46(8):2538–2547. [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Fermi G., Perutz M. F., Shaanan B., Fourme R. The crystal structure of human deoxyhaemoglobin at 1.74 A resolution. J Mol Biol. 1984 May 15;175(2):159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. Haemoglobin binding with haptoglobin. Unequivocal demonstration that the beta-chains of human haemoglobin bind to haptoglobin. Biochem J. 1980 Jan 1;185(1):285–287. doi: 10.1042/bj1850285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y. Recent advances in chemistry and pharmacology of snake toxins. Adv Cytopharmacol. 1979;3:1–16. [PubMed] [Google Scholar]
- Love R. A., Stroud R. M. The crystal structure of alpha-bungarotoxin at 2.5 A resolution: relation to solution structure and binding to acetylcholine receptor. Protein Eng. 1986 Oct-Nov;1(1):37–46. doi: 10.1093/protein/1.1.37. [DOI] [PubMed] [Google Scholar]
- McCarthy M. P., Earnest J. P., Young E. F., Choe S., Stroud R. M. The molecular neurobiology of the acetylcholine receptor. Annu Rev Neurosci. 1986;9:383–413. doi: 10.1146/annurev.ne.09.030186.002123. [DOI] [PubMed] [Google Scholar]
- McCormick D. J., Atassi M. Z. Localization and synthesis of the acetylcholine-binding site in the alpha-chain of the Torpedo californica acetylcholine receptor. Biochem J. 1984 Dec 15;224(3):995–1000. doi: 10.1042/bj2240995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. P., Raftery M. A. Studies of reversible and irreversible interactions of an alkylating agonist with Torpedo californica acetylcholine receptor in membrane-bound and purified states. Biochemistry. 1979 May 15;18(10):1862–1867. doi: 10.1021/bi00577a003. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B., Atassi M. Z. Profile of the alpha-bungarotoxin-binding regions on the extracellular part of the alpha-chain of Torpedo californica acetylcholine receptor. Biochem J. 1987 Dec 15;248(3):847–852. doi: 10.1042/bj2480847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B., Atassi M. Z. Segment alpha 182-198 of Torpedo californica acetylcholine receptor contains second toxin-binding region and binds anti-receptor antibodies. FEBS Lett. 1986 Apr 7;199(1):68–74. doi: 10.1016/0014-5793(86)81225-x. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B., Manshouri T., Yokoi T., Atassi M. Z. The regions of alpha-neurotoxin binding on the extracellular part of the alpha-subunit of human acetylcholine receptor. J Protein Chem. 1988 Apr;7(2):173–177. doi: 10.1007/BF01025247. [DOI] [PubMed] [Google Scholar]
- Ptitsyn O. B., Finkelstein A. V. Theory of protein secondary structure and algorithm of its prediction. Biopolymers. 1983 Jan;22(1):15–25. doi: 10.1002/bip.360220105. [DOI] [PubMed] [Google Scholar]
- Sobel A., Weber M., Changeux J. P. Large-scale purification of the acetylcholine-receptor protein in its membrane-bound and detergent-extracted forms from Torpedo marmorata electric organ. Eur J Biochem. 1977 Oct 17;80(1):215–224. doi: 10.1111/j.1432-1033.1977.tb11874.x. [DOI] [PubMed] [Google Scholar]
- Twining S. S., Atassi M. Z. Use of immunoadsorbents for the study of antibody binding to sperm whale myoglobin and its synthetic antigenic sites. J Immunol Methods. 1979;30(2):139–151. doi: 10.1016/0022-1759(79)90088-7. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J., Changeux J. P. High affinity binding of alpha-bungarotoxin to the purified alpha-subunit and to its 27,000-dalton proteolytic peptide from Torpedo marmorata acetylcholine receptor. Requirement for sodium dodecyl sulfate. EMBO J. 1983;2(3):381–387. doi: 10.1002/j.1460-2075.1983.tb01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka N., Atassi M. Z. Subunit interacting surfaces of human haemoglobin. Localization of the alpha-subunit-beta-subunit interacting surfaces on the beta-chain by a comprehensive synthetic strategy. Biochem J. 1986 Mar 1;234(2):457–461. doi: 10.1042/bj2340457. [DOI] [PMC free article] [PubMed] [Google Scholar]


