ABSTRACT
The adult olfactory bulb (OB) continuously receives new interneurons that integrate into the functional neuronal network and that play an important role in odor information processing and olfactory behavior. Adult neuronal progenitors are derived from neural stem cells in the subventricular zone (SVZ) bordering the lateral ventricle. They migrate long distances along the rostral migratory stream (RMS) toward the OB where they differentiate into interneurons, mature, and establish synapses with tufted or mitral cells (MC), the principal neurons in the OB. The plasticity provided by both adult-born and pre-existing early-born neurons depends on the formation and pruning of new synaptic contacts that adapt the functioning of the bulbar network to changing environmental conditions. However, the formation of new synapses occurs over a long time scale (hours-days), whereas some changes in environmental conditions can occur more rapidly, requiring a much faster adjustment of neuronal networks. A new form of structural remodeling of adult-born, but not early-born, neurons was recently brought to light. This plasticity, which is based on the activity-dependent relocation of mature spines of GCs toward the dendrites of active principal cells, may allow a more rapid adjustment of the neuronal network in response to quick and persistent changes in sensory inputs. In this mini-review we discuss the different forms of structural plasticity displayed by adult-born and early-born neurons and the possibility that these different forms of structural remodeling may fulfill distinct roles in odor information processing.
KEYWORDS: activity-dependent plasticity, adult neurogenesis, critical period, olfactory bulb, spine relocation, spine turnover, structural plasticity
Introduction
The OB is probably one of the most plastic regions in the rodent brain. Each and every day, approximately 30,000 to 40,000 new neuronal progenitors reach the OB, and nearly half of them mature and integrate into the bulbar network (Fig. 1).44,52 Some 95% of these neuronal progenitors differentiate into granule cells (GCs) whereas others become periglomerular neurons (PGCs). It has been estimated that 10–15% of GCs and 30% of PGCs are renewed during adulthood (Fig. 1).32,42 Several studies have shown that adult-born cells play a pivotal role in synaptic remodeling and odor information processing.1,6-8,11,30,46,47 OB interneurons form reciprocal dendrodendritic synapses with principal cells that synchronize these glutamatergic relay neurons and lead to fine spatio-temporal tuning of their responses to odors.19,21,50 Both early-born and adult-born interneurons have a high rate of spine turnover that matches the dynamics of postsynaptic sites in MC dendrites.47 This synaptic remodeling constantly sculpts the bulbar network to optimize sensory information processing.47 In addition, a new form of structural plasticity provided by adult-born, but not early-born neurons, that has been recently been brought to light,8 is based on the rapid activity-dependent relocation of mature functional spines of adult-born GCs toward active MC dendrites.8 How do these different forms of structural remodeling “co-operate” to adjust the OB network and are they triggered in response to different sensory inputs?
Figure 1.
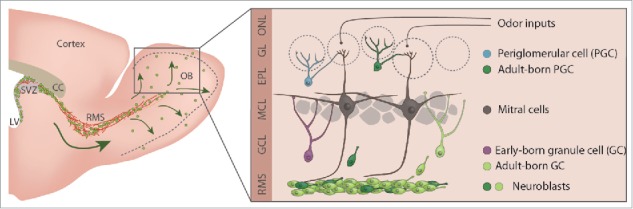
Schematic representation of the mouse forebrain and of olfactory bulb neurogenesis. Schematic drawing of the adult mouse forebrain and illustration showing the OB neuronal circuitry and adult neurogenesis. Early-born and adult-born GCs are shown in different colors.
In this mini-review, following a brief description of the maturational profiles of early-born and adult-born GCs, we discuss the different forms of structural plasticity displayed by these interneurons. We propose that these different types of structural remodeling may provide bulbar networks with distinct temporal levels of adaptability to changing sensory inputs.
Different forms of structural plasticity of GCs in the OB
GCs are anaxonic neurons with basal and apical dendrites.51 Apical dendrites can be divided into proximal and distal domains. The distal domain of apical dendrites forms secondary and tertiary branches in the external plexiform layer (EPL) where they establish dendrodendritic reciprocal synapses with principal neurons.51 Basal and proximal dendrites receive axodendritic synapses formed by the glutamatergic top-down centrifugal projections and collateral axons of MCs51 as well as local OB interneurons.17,45 The synaptic integration of GCs is different during adulthood than during the early postnatal stages. GCs born during embryogenesis and early postnatal life first receive glutamatergic inputs on their proximal and distal dendrites and then later on their basal dendrites.28 In contrast, adult-born GCs receive their first synaptic inputs on their proximal and basal dendrites before forming output dendrodendritic synapses with MCs on their distal dendrites.28,51 This maturational profile allows adult-born GCs to “silently” integrate into the OB network.28 Electrophysiological9 and anatomic14 studies have shown that adult-born GCs receive local GABAergic inputs on their proximal and basal dendrites before any long-ranging glutamatergic inputs.14,43,45 These GABAergic inputs are believed to drive the stages of GC maturation.14,28,43,51 Both early-born and adult-born GCs remain structurally plastic well beyond their synaptic integration period.47 New spines are constantly forming, retracting, and stabilizing on the dendrites of adult-born and early-born GCs. A recent in vivo 2-photon imaging study has shown that approximately 20% of spines are constantly being added to and eliminated from the distal dendrites of GCs47 (Fig. 2). The continuous turnover of GC spines matches the dynamics of gephryn-positive postsynaptic puncta in MCs, and it has been proposed that this persistent synaptic structural plasticity in the adult OB optimizes odor information processing.47
Figure 2.
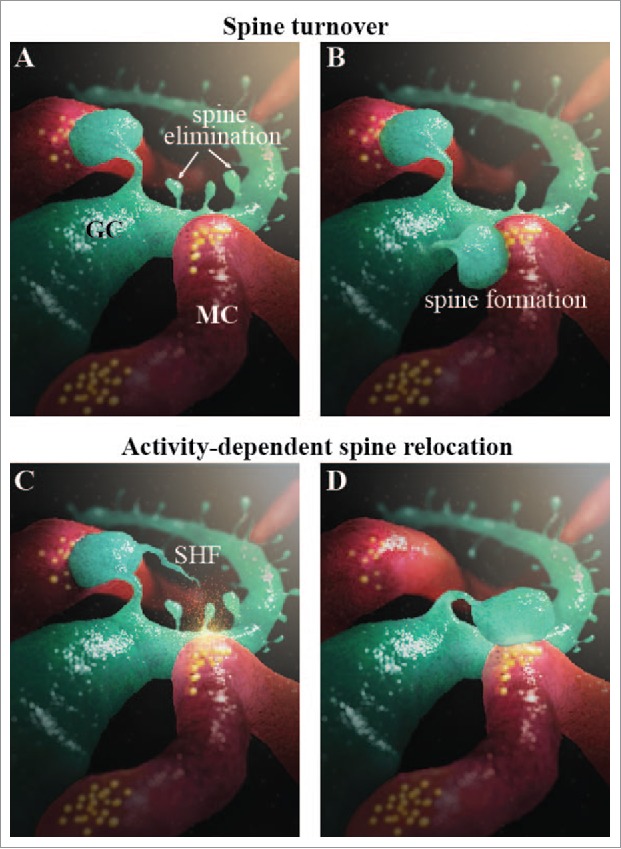
Two forms of structural plasticity in the adult OB. (A-B) Spine turnover represented as the formation and elimination of spines on GC dendrites (green). MC dendrites are shown in red. (C-D) Activity-dependent spine relocation is shown by the relocation of a spine from one MC dendrite to another. The activity-dependent release of glutamate and BDNF is indicated by the yellow cloud.
It should be mentioned, however, that synaptogenesis occurs over a long period of time (from hours to days), whereas changes in environmental conditions may take place very rapidly. It is thus conceivable that a faster structural plasticity should occur in the OB to optimize sensory information processing in a rapidly changing odor environment. We recently uncovered a new form of structural plasticity that is based on the activity-dependent relocation of mature GC spines toward active MC dendrites8 (Fig. 2). Spine relocation occurs within a few minutes8 and thus seems to be perfectly adapted to rapid changes in the odor environment, unlike synaptogenesis, which takes several hours or even days. Spine relocation is preceded by the growth of a thin filopodia-like protrusion from the spine head (spine head filopodia; SHF). SHFs are very motile, and SHF dynamics are controlled by MC-derived glutamate and the level of odor-induced activity.8 The directionality of SHF growth is, however, controlled by MC-derived BDNF. The stimulation of MCs induces the directional growth of SHFs toward an activated principal cell dendrite, which is followed by spine relocation8 (Fig. 2). It is thus possible that SHFs play the role of “microsensors” and actively probe the bulbar microenvironment, promoting the activity-dependent relocation of GC spines from inactive to active MC dendrites. Modeling studies had shown that spine relocation provides the OB with a rapid way to change the set of synchronized MCs and, as such, odor information processing.8 This new form of structural plasticity was observed with mature adult-born, but not early-born GCs, suggesting that adult neurogenesis plays a central role in plasticity and the rapid adaptation to new sensory inputs. While the cellular and molecular bases for such distinct responses are not known, these results are in line with observations showing that adult-born neurons are more responsive to incoming odors than their older counterparts4,37 and that these 2 populations of cells participate differently in the hedonic aspects of odor information processing.41
How do these 2 forms of structural plasticity cooperate to optimize the response of the OB network to a constantly changing odor environment? Since spine relocation and synaptogenesis occur on different time scales, it is likely that these 2 forms of plasticity accommodate rapid and long-lasting changes in the odor environment, respectively. According to this model, spine relocation occurs when there is a rapid and persistent change in the odor environment. The new environmental stimuli may require the synchronization of different sub-populations of MCs, redirecting the relocation of GC spines from inactive to active principal cell dendrites. In contrast, synaptogenesis requires long-lasting changes in sensory inputs. It remains to be shown whether different experimental paradigms based on spontaneous odor exploration or odor learning will induce predominantly one or the other form of structural plasticity or whether these 2 forms of plasticity act simultaneously to adjust bulbar network functioning. Modeling studies suggest that both forms of structural plasticity optimize odor information processing in the OB at different time scales.8,47 However, some sensory information processing, such as the retrieval and/or erasure of previously learned odor information, may require simultaneous rapid and long-lasting changes in the OB network, inducing spine relocation and spine formation/pruning. It is also not known whether relocated spines are preserved in the OB network for weeks or months or whether they are “temporal units” that enable the rapid optimization of the OB network until new GC spines form and replace the relocated spines. Our chronic in vivo imaging results have shown that relocated spines persist for at least 24–48 h following relocation.8 However, it is not known whether these spines can persist for weeks or months in the OB network.
Activity-dependent regulation of different forms of structural plasticity
Not only both types of structural plasticity in the OB optimize odor information processing,8,47 but also the level of sensory input regulates the extent of the structural modifications. Several studies have shown that synaptic integration and the maintenance of GCs are sensory experience dependent.8,30,34,35,44,52 GCs display very high spine dynamics during the critical period of neuron development (14 to 30-day-old cells)29,47 when they are particularly sensitive to the level of odor inputs. Sensory activity during this critical period differentially regulates the synaptogenesis of distinct GC dendritic compartments.46,29,33 Sensory deprivation decreases the spine density of the distal and basal compartments29,46 and increases the spine density of proximal dendrites.29 Interestingly, sensory deprivation after the critical periods of early-born46 and adult-born29 neuron development has no effect on the spine density of distal and basal dendrites, while it increases the spine density of proximal dendrites.29 In addition, although olfactory learning during the critical period of synaptic development increases the PSD95-expressing spine density of proximal and basal dendrites, it increases gephyrin-expressing spine density only in the proximal domain.33 These results suggest that GC synaptogenesis is particularly sensitive to the level of sensory activity during the time window when these neurons are added into the bulbar network. Since different dendritic GC compartments receive distinct inputs,33 these results also suggest that sensory experience-induced structural modifications occur in the input-dependent manner.
Odor-induced activity also regulates spine relocation. First, the stimulation of MCs with a pattern of activity mimicking the responses of these principal cells to odors induces spine relocation toward an activated dendrite.8 Second, while sensory deprivation decreases the number of GC spines,8,29,46 the percentage of spines with SHFs increases.8 Third, sensory deprivation leads to an increase in SHF dynamics and a shorter lifetime.8 As mentioned above, SHFs play an important role in spine relocation, and their directional growth toward activated MC dendrites precedes spine relocation.8 The increased percentage of spines with SHFs suggests that these spines are selectively preserved, at the expense of others, following sensory deprivation. The deletion of BDNF in MCs abolishes the specific preservation of spines with SHFs but does not alter SHF dynamics.8 These observations are compatible with the fact that BDNF is necessary for SHF directionality but not for SHF dynamics.8 Based on these results, we hypothesize that sensory deprivation fosters synaptic competition and that spines with SHFs are selectively preserved because the increase in SHF dynamics leads to a higher probability of spines with SHFs finding an active MC dendrite. This, in turn, allows the formation of functional synapses and thus spine maintenance. This hypothesis is in line with observations that (i) SHFs constantly scrutinize the spine microenvironment for factors released in an activity-dependent manner,8 (ii) sensory deprivation dampens MC activity, thus reducing the availability of these factors, and (iii) BDNF mediates competitive interactions between individual neurons.15 On the other hand, spines without SHFs are less plastic and are eliminated. Further studies are required to elucidate the molecular and cellular mechanisms underlying the specific maintenance of spines with SHFs.
Cellular and molecular mechanisms underlying different forms of structural plasticity
Although the OB undergoes continuous structural modifications, very little is known about the mechanisms underlying spine turnover and relocation. Furthermore, to date, all the studies examining the structural plasticity of GCs have considered them as a homogenous population of neurons. Several GC subtypes have been, however, identified based on immunohistochemical markers. Subpopulations of GCs express calretinin, glycoprotein 5T4, metabotropic glutamate receptor 2 (mGluR2), or Ca2+/calmodulin-dependent protein kinases IIα (CaMKIIα) and IV (CaMKIV).3,27,53 Do all these GC subtypes display the 2 types of structural plasticity, and is the extent of structural modifications similar for different GC subtypes? While these questions have not been explored, it is conceivable that different GC subtypes play a distinct role in OB functioning and odor behavior. 39,48 Their structural plasticity may be also driven differently by distinct odor experiences to adequately optimize OB functioning. Interestingly, although spine relocation is induced by a specific pattern of activity that mimics MCs responses to odors,8 stimulation patterns consisting of the same number of spikes but given in the random order do not induce spine relocation.8 Half of early-born and adult-born GCs express CaMKIIα;53 our unpublished observations), which has been shown to decode patterns of neuronal activity.12 This suggests that the propensity to exhibit spine relocation is determined by specific patterns of neuronal activity and may be displayed by the specific CaMKIIα-expressing GC subtype, for example.
Another aspect that remains largely unexplored is the role of other OB cells in the different forms of structural plasticity. To date, studies on structural plasticity in the OB have largely centered on GCs and MCs, their postsynaptic targets.8,29,30,35,47 The roles of other cells that actively participate in synaptic maintenance and remodeling, such as astrocytes and microglia, remain elusive. Recently, however, the role of microglia in the structural remodeling of OB has begun to emerge.13,20 These cells are sensitive to the level of sensory activity and actively participate in the elimination of adult-born neurons.13 Microglia processes actively survey the OB microenvironment and play a role in synaptic pruning.13 Activated microglia may also migrate toward inhibitory synapses and displace presynaptic inhibitory terminals from cortical neurons,10 a process that may be compatible with the spine relocation observed in the OB.8 Do microglia in the OB play a role in spine relocation? If so, what mechanisms are involved in unwrapping and phagocytosing spines in the case of spine turnover and in promoting activity-dependent spine relocation?
In terms of the molecular factors involved in spine turnover and spine relocation, MC-derived factors such as BDNF and glutamate have been shown to promote these 2 types of structural plasticity. We previously showed that dendritic filopodia formation/retraction on the distal dendrites of immature adult-born neurons is dependent on MC-derived glutamate and the activation of NMDA receptors on GC dendrites.7 This dynamic decreases as adult-born GCs mature and is accompanied by a progressive hyperpolarization of the membrane potential of these cells and an increased Mg2+ block of NMDA receptors.7 Interestingly, after this critical period of GC development, MC-derived glutamate promotes spine maintenance by activating AMPA receptors on GCs,8 which stops the formation of SHFs and reinforces synaptically active spines. These results are in line with observations in other brain regions showing that NMDA receptors trigger the formation of new spines,31,38 whereas AMPA receptors induce synapse stabilization.18 MC-derived BDNF is another factor that is released in an activity-dependent manner.8 BDNF acts via TrkB receptors on GC spines5,8 and induces spine relocation.8 TrkB deletion in adult-born GCs affects dendritic arborization and spine growth.5 These effects have been attributed to the PI3K and phospholipase C-γ (PLCγ) signaling pathways, respectively.5 Further studies will be required to determine which TrkB-induced signaling pathway promotes spine relocation and how the activation of this trophic factor receptor “decodes” the spine formation and/or spine relocation programs.
Although MC activity is a major player in the turnover and relocation of spines, little is known about the intracellular pathways in GCs that mediate these 2 forms of structural plasticity. The actin cytoskeleton is intimately involved in the formation, elimination, stability, motility, and morphology of dendritic spines in other brain regions.22,25,40 Small GTPases such as Rac1, Cdc42, and Rho control actin cytoskeleton polymerization and depolymerization, which leads to spine turnover and changes in spine morphology.23,36 It has recently been shown that the optogenetic photoactivation of Rac1 induces the shrinkage of potentiated spines and the erasure of previously acquired motor learning.23 SHF, in constrast, are enriched in microtubules.26 It would thus be interesting to explore the role of the actin cytosceleton, microtubules, and actin-binding proteins in the various forms of structural plasticity in the OB. Other intracellular signaling pathways, synaptic adhesion molecules, and extracellular matrix metaloproteinases are also likely to emerge as important regulators of different forms of structural plasticity in the OB. It will be important to determine whether they play a general role in structural modifications or exquisitely affect one or the other form of structural plasticity.
Conclusion
Adult-born neurons continuously rewire the bulbar network and make up a population of cells that enable the OB to adapt to an ever-changing odor environment. These structural modifications are manifested at different levels, starting with the addition and elimination of cells and ending with modifications to their synaptic contacts. At least 2 different forms of synaptic modifications, which act on spine turnover and spine relocation, have been described. Other forms of structural modifications such as spine enlargement and spine neck plasticity2,49 may also occur in the OB. Why does the OB need such an elaborate repertoire of structural reorganization, which mechanisms underlie the different forms of structural plasticity, how do all these forms of plasticity cooperate to optimize odor information processing, and how are odor-related memories efficiently stored and recalled in a neuronal network that is undergoing constant structural remodeling? Although these questions remain to be investigated, the high level of structural plasticity in the OB may be related to the fact that it is one of the few regions in the adult brain where the critical period never ends. Other sensory systems also display high levels of activity-dependent structural reorganization during the critical period of their development.16,24 The OB, with its constant supply of new neurons, maintains this high level of plasticity throughout life. It is thus a particularly interesting system for studying the sensory experience-induced reshaping of neuronal connections and their contributions to neural processing.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Mireille Massouh at MassouhBioMedia for the illustrations.
Funding
This work was supported by an operating grant from the National Science and Engineering Research Council of Canada (NSERC) and a grant from the Canadian Institutes of Health Research (CIHR; MOP 105859) to A.S.
References
- [1].Alonso M, Lepousez G, Sebastien W, Bardy C, Gabellec MM, Torquet N, Lledo PM. Activation of adult-born neurons facilitates learning and memory. Nat Neurosci 2012; 15:897-904; PMID:22581183; http://dx.doi.org/ 10.1038/nn.3108 [DOI] [PubMed] [Google Scholar]
- [2].Araya R, Vogels TP, Yuste R. Activity-dependent dendritic spine neck changes are correlated with synaptic strength. Proc Natl Acad Sci U S A 2014; 111:2895-2904; http://dx.doi.org/ 10.1073/pnas.1321869111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Batista-Brito R, Close J, Machold R, Fishell G. The distinct temporal origins of olfactory bulb interneuron subtypes. J Neurosci 2008; 28:3966-3975; PMID:18400896; http://dx.doi.org/ 10.1523/JNEUROSCI.5625-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Belnoue L, Grosjean N, Abrous DN, Koehl M. A critical time window for the recruitment of bulbar newborn neurons by olfactory discrimination learning. J Neurosci 2011; 31:1010-1016; PMID:21248125; http://dx.doi.org/ 10.1523/JNEUROSCI.3941-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bergami M, Vignoli B, Motori E, Pifferi S, Zuccaro E, Menini A, Canossa M. TrkB signaling directs the incorporation of newly generated periglomerular cells in the adult olfactory bulb. J Neurosci 2013; 33:11464-11478; PMID:23843518; http://dx.doi.org/ 10.1523/JNEUROSCI.4812-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Breton-Provencher V, Lemasson M, Peralta MR, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci 2009; 29:15245-15257; PMID:19955377; http://dx.doi.org/ 10.1523/JNEUROSCI.3606-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Breton-Provencher V, Coté D, Saghatelyan A. Activity of the principal cells of the olfactory bulb promotes a structural dynamic on the distal dendrites of immature adult-born granule cells via activation of NMDA receptors. J Neurosci 2014; 34:1748-1759; PMID:24478357; http://dx.doi.org/ 10.1523/JNEUROSCI.3013-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Breton-Provencher V, Bakhshetyan K, Hardy D, Bammann RR, Cavarretta F, Snapyan M, Côté D, Migliore M, Saghatelyan A. Principal cell activity induces spine relocation of adult-born interneurons in the olfactory bulb. Nat Commun 2016; 7:12659; PMID:27578235; http://dx.doi.org/ 10.1038/ncomms12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci 2003; 6:507-518; PMID:12704391 [DOI] [PubMed] [Google Scholar]
- [10].Chen Z, Jalabi W, Hu W, Park H-J, Gale JT, Kidd GJ, Bernatowicz R, Gossman ZC, Chen JT, Dutta R, et al.. Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat Commun 2014; 5:4486; PMID:25047355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cummings DM, Snyder JS, Brewer M, Cameron HA, Belluscio L. Adult neurogenesis is necessary to refine and maintain circuit specificity. J Neurosci 2014; 34:13801-13810; PMID:25297106; http://dx.doi.org/ 10.1523/JNEUROSCI.2463-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 1998; 279:227-230; PMID:9422695; http://dx.doi.org/ 10.1126/science.279.5348.227 [DOI] [PubMed] [Google Scholar]
- [13].Denizet M, Cotter L, Lledo P-M, Lazarini F. Sensory deprivation increases phagocytosis of adult-born neurons by activated microglia in the olfactory bulb. Brain Behav Immun 2016; 60:38-43; PMID:27640898; http://dx.doi.org/ 10.1016/j.bbi.2016.09.015 [DOI] [PubMed] [Google Scholar]
- [14].Deshpande A, Bergami M, Ghanem A, Conzelmann K-K, Lepier A, Götz M, Berninger B. Retrograde monosynaptic tracing reveals the temporal evolution of inputs onto new neurons in the adult dentate gyrus and olfactory bulb. Proc Natl Acad Sci U S A 2013; 110:1152-1161; http://dx.doi.org/ 10.1073/pnas.1218991110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].English CN, Vigers AJ, Jones KR. Genetic evidence that brain-derived neurotrophic factor mediates competitive interactions between individual cortical neurons. Proc Natl Acad Sci U S A 2012; 109:19456-19461; PMID:23129644; http://dx.doi.org/ 10.1073/pnas.1206492109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. Eur J Neurosci 2012; 35:1540-1553; PMID:22607000; http://dx.doi.org/ 10.1111/j.1460-9568.2012.08075.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eyre MD, Antal M, Nusser Z. Distinct deep short-axon cell subtypes of the main olfactory bulb provide novel intrabulbar and extrabulbar GABAergic connections. J Neurosci 2008; 28:8217-8229; PMID:18701684; http://dx.doi.org/ 10.1523/JNEUROSCI.2490-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fischer M, Kaech S, Wagner U, Brinkhaus H, Matus A. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat Neurosci 2000; 3:887-894; PMID:10966619; http://dx.doi.org/ 10.1038/78791 [DOI] [PubMed] [Google Scholar]
- [19].Fukunaga I, Herb JT, Kollo M, Boyden ES, Schaefer AT. Independent control of gamma and theta activity by distinct interneuron networks in the olfactory bulb. Nat Neurosci 2014; 17:1208-1216; PMID:24997762; http://dx.doi.org/ 10.1038/nn.3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grier BD, Belluscio L, Cheetham CEJ. Olfactory sensory activity modulates microglial-neuronal interactions during dopaminergic cell loss in the olfactory bulb. Front Cell Neurosci 2016; 10:178; PMID:27471450; http://dx.doi.org/ 10.3389/fncel.2016.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gschwend O, Abraham NM, Lagier S, Begnaud F, Rodriguez I, Carleton A. Neuronal pattern separation in the olfactory bulb improves odor discrimination learning. Nat Neurosci 2015; 18:1474-1482; PMID:26301325; http://dx.doi.org/ 10.1038/nn.4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci 1998; 18:9835-9844; PMID:9822742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 2015; 525:333-338; PMID:26352471; http://dx.doi.org/ 10.1038/nature15257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res 2005; 147:115-124; PMID:15581701 [DOI] [PubMed] [Google Scholar]
- [25].Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol 2010; 189:619-629; PMID:20457765; http://dx.doi.org/ 10.1083/jcb.201003008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hu X, Viesselmann C, Nam S, Merriam E, Dent EW. Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci 2008; 28:13094-13105; PMID:19052200; http://dx.doi.org/ 10.1523/JNEUROSCI.3074-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Imamura F, Nagao H, Naritsuka H, Murata Y, Taniguchi H, Mori K. A leucine-rich repeat membrane protein, 5T4, is expressed by a subtype of granule cells with dendritic arbors in specific strata of the mouse olfactory bulb. J Comp Neurol 2006; 495:754-768; PMID:16506198; http://dx.doi.org/ 10.1002/cne.20896 [DOI] [PubMed] [Google Scholar]
- [28].Kelsch W, Lin C-W, Lois C. Sequential development of synapses in dendritic domains during adult neurogenesis. Proc Natl Acad Sci U S A 2008; 105:16803-16808; PMID:18922783; http://dx.doi.org/ 10.1073/pnas.0807970105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kelsch W, Lin C-W, Mosley CP, Lois C. A critical period for activity-dependent synaptic development during olfactory bulb adult neurogenesis. J Neurosci 2009; 29:11852-11858; PMID:19776271; http://dx.doi.org/ 10.1523/JNEUROSCI.2406-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kopel H, Schechtman E, Groysman M, Mizrahi A. Enhanced synaptic integration of adult-born neurons in the olfactory bulb of lactating mothers. J Neurosci 2012; 32:7519-7527; PMID:22649230; http://dx.doi.org/ 10.1523/JNEUROSCI.6354-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kwon H-B, Sabatini BL. Glutamate induces de novo growth of functional spines in developing cortex. Nature 2011; 474:100-104; PMID:21552280; http://dx.doi.org/ 10.1038/nature09986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD., et al.. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci 2007; 27:12623-12629; PMID:18003841; http://dx.doi.org/ 10.1523/JNEUROSCI.3812-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lepousez G, Nissant A, Bryant AK, Gheusi G, Greer CA, Lledo PM. Olfactory learning promotes input-specific synaptic plasticity in adult-born neurons. Proc Natl Acad Sci U S A 2014; 111:13984-1389; PMID:25189772; http://dx.doi.org/ 10.1073/pnas.1404991111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Livneh Y, Mizrahi A. Experience-dependent plasticity of mature adult-born neurons. Nat Neurosci 2012; 15:26-28; http://dx.doi.org/ 10.1038/nn.2980 [DOI] [PubMed] [Google Scholar]
- [35].Livneh Y, Feinstein N, Klein M, Mizrahi A. Sensory input enhances synaptogenesis of adult-born neurons. J Neurosci 2009; 29:86-97; PMID:19129387; http://dx.doi.org/ 10.1523/JNEUROSCI.4105-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol 2002; 18:601-635; PMID:12142283; http://dx.doi.org/ 10.1146/annurev.cellbio.18.031802.150501 [DOI] [PubMed] [Google Scholar]
- [37].Magavi SSP, Mitchell BD, Szentirmai O, Carter BS, Macklis JD. Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo. J Neurosci 2005; 25:10729-10739; PMID:16291946; http://dx.doi.org/ 10.1523/JNEUROSCI.2250-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 1999; 283:1923-1927; PMID:10082466; http://dx.doi.org/ 10.1126/science.283.5409.1923 [DOI] [PubMed] [Google Scholar]
- [39].Malvaut S, Saghatelyan A. The role of adult-born neurons in the constantly changing olfactory bulb network. Neural Plast 2015; 2016:e1614329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Matus A, Brinkhaus H, Wagner U. Actin dynamics in dendritic spines: a form of regulated plasticity at excitatory synapses. Hippocampus 2000; 10:555-560; PMID:11075825; http://dx.doi.org/ 10.1002/1098-1063(2000)10:5%3c555::AID-HIPO5%3e3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- [41].Muthusamy N, Zhang X, Johnson CA, Yadav PN, Ghashghaei HT. Developmentally defined forebrain circuits regulate appetitive and aversive olfactory learning. Nat Neurosci 2017; 20:20-23; PMID:27918532; http://dx.doi.org/ 10.1038/nn.4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ninkovic J, Mori T, Götz M. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci 2007; 27:10906-10911; PMID:17913924; http://dx.doi.org/ 10.1523/JNEUROSCI.2572-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Panzanelli P, Bardy C, Nissant A, Pallotto M, Sassoè-Pognetto M, Lledo P-M, Fritschy J-M. Early synapse formation in developing interneurons of the adult olfactory bulb. J Neurosci 2009; 29:15039-15052; PMID:19955355; http://dx.doi.org/ 10.1523/JNEUROSCI.3034-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci 2002; 22:6106-6113; PMID:12122071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pressler RT, Strowbridge BW. Blanes cells mediate persistent feedforward inhibition onto granule cells in the olfactory bulb. Neuron 2006; 49:889-904; PMID:16543136; http://dx.doi.org/ 10.1016/j.neuron.2006.02.019 [DOI] [PubMed] [Google Scholar]
- [46].Saghatelyan A, Roux P, Migliore M, Rochefort C, Desmaisons D, Charneau P, Shepherd GM, Lledo PM. Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron 2005; 46:103-116; PMID:15820697; http://dx.doi.org/ 10.1016/j.neuron.2005.02.016 [DOI] [PubMed] [Google Scholar]
- [47].Sailor KA, Valley MT, Wiechert MT, Riecke H, Sun GJ, Adams W, Dennis JC, Sharafi S, Ming G-L, Song H, et al.. Persistent structural plasticity optimizes sensory information processing in the olfactory bulb. Neuron 2016; 91:384-396; PMID:27373833; http://dx.doi.org/ 10.1016/j.neuron.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Takahashi H, Ogawa Y, Yoshihara S-I, Asahina R, Kinoshita M, Kitano T, Kitsuki M, Tatsumi K, Okuda M, Tatsumi K, et al.. A subtype of olfactory bulb interneurons is required for odor detection and discrimination behaviors. J Neurosci 2016; 36:8210-8227; PMID:27488640; http://dx.doi.org/ 10.1523/JNEUROSCI.2783-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tønnesen J, Katona G, Rózsa B, Nägerl UV. Spine neck plasticity regulates compartmentalization of synapses. Nat Neurosci 2014; 17:678-685; PMID:24657968; http://dx.doi.org/ 10.1038/nn.3682 [DOI] [PubMed] [Google Scholar]
- [50].Urban NN, Sakmann B. Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. J Physiol 2002; 542:355-367; PMID:12122137; http://dx.doi.org/ 10.1113/jphysiol.2001.013491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Whitman MC, Greer CA. Synaptic integration of adult-generated olfactory bulb granule cells: basal axodendritic centrifugal input precedes apical dendrodendritic local circuits. J Neurosci 2007; 27:9951-9961; PMID:17855609; http://dx.doi.org/ 10.1523/JNEUROSCI.1633-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yamaguchi M, Mori K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc Natl Acad Sci U S A 2005; 102:9697-9702; PMID:15976032; http://dx.doi.org/ 10.1073/pnas.0406082102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zou D-J, Greer CA, Firestein S. Expression pattern of alpha CaMKII in the mouse main olfactory bulb. J Comp Neurol 2002; 443:226-236; PMID:11807833; http://dx.doi.org/ 10.1002/cne.10125 [DOI] [PubMed] [Google Scholar]


