Abstract
Synthetic peptide vaccines aim to elicit and expand tumor-specific T cells capable of controlling or eradicating the tumor. Despite the high expectations based on preclinical studies, the results of clinical trials using peptide vaccines have been disappointing. Thus, many researchers in the field have considered peptide vaccines as outdated and no longer viable for cancer therapy. However, recent progress in understanding the critical roles of immune adjuvants, modes of vaccine administration and T cell dynamics has lead to a rebirth of this approach and reconsidering the use of peptide vaccines for treating malignant disorders.
Introduction
After the discoveries of Jenner’s cowpox vaccine and Coley’s streptococcus-mediated tumor rejection, the activation of immune system has become an fundamental strategy to prevent or treat a variety of disorders including cancer. While Coley’s immunotherapy relied on antigen-independent immunity, cumulative evidence indicates that tumor-associated antigens (TAAs) can be used as targets in various vaccination strategies aimed at inducing T cell responses that can limit cancer growth [1]. There are several options to induce TAA-specific immune responses: tumor cell/lysate vaccines, recombinant TAA proteins/plasmids, and synthetic peptides encompassing T cell epitopes. Because it is evident that synthetic peptides can elicit antigen-specific T cell responses [2], there were high expectations that peptide vaccines would work as a silver bullet against established malignancies. However, most clinical results using peptide vaccines have not met these expectations and therapeutic efficacy has been disappointing [3]. While most researchers in the field blame the failure of peptide vaccines on the presence of the immunosuppressive tumor environment that inhibits vaccine-induced effector lymphocytes [4], we advocate that the major culprit for lack of success in the majority of clinical studies of cancer vaccines, including peptides, is due to their suboptimal immunogenicity [5]. Realistically, how can anyone expect that a vaccine that generates T cell responses that are barely measurable (e.g., ∼1% of all T cells in blood) will have an effect against an established tumor?
Based on the recent knowledge on peptide vaccine optimization in experimental models [5], it is clear that the selection of peptide formulations, adjuvants and route of administration used in most clinical studies were not the best [6]. Therefore, we believe that there is now an opportunity to generate substantial immune responses and achieve a clinical benefit against established tumors. Although, an HPV- peptide vaccine, using (what we believe) suboptimal adjuvant and route of administration showed complete eradication of pre-malignant lesions [7], it is questionable whether this vaccination strategy would have any therapeutic effect in established disease [8]. In this review, we discuss the recent progress in preclinical models regarding peptide design and formulation, the vaccine delivery method, selection of adjuvants, and the use of combination therapy to improve the efficiency of peptide vaccine, with the hope that these lessons may be taken into the clinic.
Advantages and disadvantages of peptide vaccines compared to other types of cancer immunotherapy
The general principle of an epitope peptide vaccine is to activate and expand TAA-specific T cells. The use of a synthetic peptide has several advantages over other types of antigen. First, GMP-grade peptides containing CD8 or CD4 T cell epitopes are relatively easy to synthesize and are substantially more cost effective compared to recombinant proteins or cell-based vaccines. Because peptide vaccines are a form of active immunotherapy, there is no need for GLP cell-processing centers that are necessary for providing adoptive cell transfer (ACT) therapies, which have shown dramatic clinical responses but with substantial higher costs [9]. Nevertheless, the success of ACT has encouraged the optimization of peptide vaccines by indicating that large numbers of T cells will be required to achieve an anti-tumor effect. Second, peptides derived from either cell surface or intracellular proteins can function as T cell epitopes, which is a distinct advantage to antibody- (e.g., Rituximab, Trastuzumab) or chimeric antigen receptor T cell (CAR-T) -based therapies that can only target cell surface antigens. While tumor antigen loss is a major hurdle for antibody, CAR-T or T cell receptor (TCR) adoptive therapies [10,11], peptide vaccines have more flexibility to confront antigen/epitope loss by the use of multiple-epitopes or switching the peptides to different epitopes, which is technically more challenging in antibody, CAR-T or TCR therapies. Finally, the stimulation of specific TCRs using a single peptide epitope can be quite effective for expanding antigen-specific antitumor T cells, whenever these peptides are administered in the right conditions [12,13]. Because CD8+ T cells (CTLs) that recognize other (irrelevant) antigens can compete for access to antigen-presenting cells (APCs) and cytokines [14], vaccines using proteins, recombinant viruses or tumor cells could be detrimental for mounting effective antitumor T cell responses. Moreover, protein-or long peptide based vaccination may induce antibodies and has the potential risk of inducing anaphylaxis [15], which will be avoided by designing a peptide that do not contain B cell epitopes.
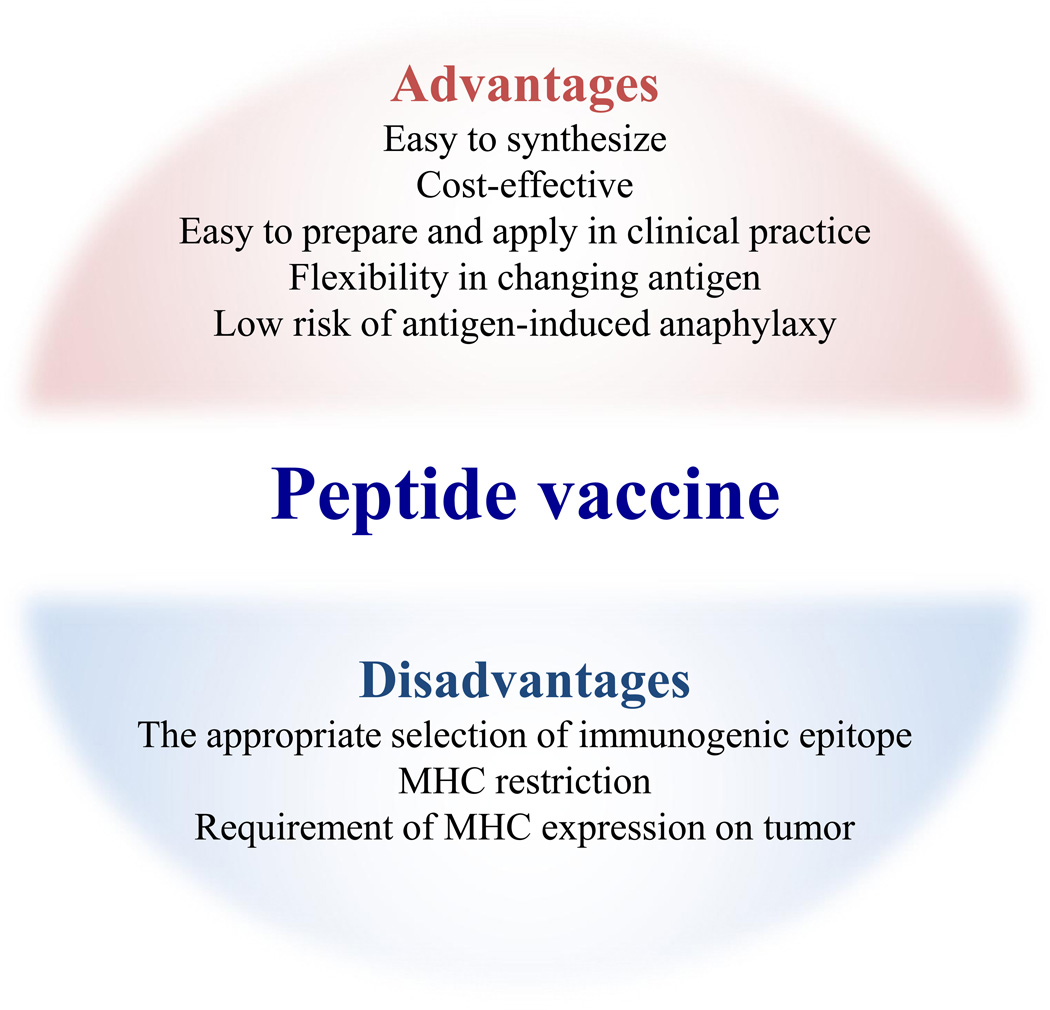
To design effective anti-tumor peptide vaccines, it is important to acknowledge some of the intrinsic disadvantages of this approach (Fig. 1). Because the immunogenicity of a peptide depends on the length and the formulation of peptide [16,17], appropriate selection and in some instances modification of the immunogen is necessary. While MHC-binding algorithm-based predictions have been widely used to select the immunogenic epitope, some algorithms resulted in false-negative (ignorance of effective epitopes) or false-positive results [18,19]. In addition, it will be impossible to produce a global off-the-shelf peptide vaccine due to MHC restriction and vast heterogeneity in MHC alleles in the human population. However, focusing on most prominent MHC alleles and the use of promiscuous peptides that have the ability to bind to more than one MHC allele, would be a partial solution for this problem [20].
Figure 1.
Advantages and disadvantages of peptide vaccine. Peptide vaccine has several advantages over other therapies. Because epitope peptides are easy and considerably cheaper to synthesize, peptide vaccine is highly cost-effective than other immunotherapies such as checkpoint inhibitors and ACT therapy. This therapy can be utilized in any hospitals or clinics because of no requirement of cell processing. The MHC restriction of epitope would be a potential disadvantage by using peptide as an antigen.
Conversely, there are several benefits in genetic-engineered immune therapies over peptide vaccines. Whereas the tumor-recognition activity of CAR construct is confirmed based on the original antibody specificity, predicted epitope peptides require the sometime arduous demonstration that these are able to induce T cells capable of recognizing tumor cells that naturally process and present the same peptide epitope in the context of MHC. On the other hand, CAR-T therapy will be suitable for any patient regardless of the MHC alleles. Although the use of peptide vaccines may not be optimal to treat tumors expressing low levels of MHC, some molecule-targeted therapies such as EGFR and MEK inhibitors increase MHC expression on tumors and could be combined with peptide vaccines [20,21]. Since aberrantly high-affinity TCRs produced by gene-modification can induce severe adverse effects [22], vaccine-induced endogenous T cells would be a more suitable way to treat cancer without toxicities.
Issues related to the selection and delivery of peptide vaccines
Accumulating evidence supports the notion that in addition to CD8 CTLs, CD4+ helper T cells (HTLs) can exhibit direct antitumor effects in addition to their helper function [23]. A clear example was the recent report that ACT of CD4 T cells specific for a mutated antigen induced a dramatic tumor regression in a cholangiocarcinoma patient [24]. Also, the extent of CD4 T cell responses in melanoma patients to peptide vaccines containing a mixture of CD8 and CD4 epitopes correlated with clinical outcome [25]. To elicit antitumor T cells, the initial step is to select an appropriate TAA, which preferentially should be highly immunogenic and absent (or expressed at low levels) in normal tissues. Ideally the TAA should be an essential protein for tumor survival to avoid antigen loss tumor variants. Because cancer testis antigens (CTAs) such as MAGE-A1, -A3 and NY-ESO-1 appear to satisfy these prerequisites, peptides derived from these antigens have been widely used in clinical trials [26,27]. Some TAAs found in cancer stem cells that play critical roles in tumor progression may also be ideal to induce antitumor T cell responses [28]. Recently, the use of neoantigen-derived peptides selected from mutated genes is in vogue for developing therapeutic vaccines to induce antitumor CD8 and CD4 responses [29–32]. Although mutated peptides are promising vaccine targets, technical and economical challenges remain in applying this mode of personalized medicine to the general patient population. Breakthrough in technologies will be necessary to decrease the cost of exome sequencing for identifying relevant immunogenic mutations for each patient’s tumor.
Modification of the amino acid sequence can be a possible strategy to enhance peptide’s immunogenicity. In some instances replacement of amino acids at the anchor residues increases the binding of peptide to MHC followed by the increased chance of TCR stimulation [33]. The length of peptide can also be important to achieve effective CD8 responses. By adding few amino acids to CD8 epitope, the ability and the duration of cross-presentation by APCs was increased [34,35]. It is widely believed that professional APCs such as dendritic cells (DCs) are necessary to present long peptides to naïve T cells to generate productive responses, whereas non-professional APCs (which lack costimulatory molecules) can present short peptides but induce T cell anergy [36]. However, in our experience some short peptides are highly immunogenic when administered systemically (i.v.) in combination with strong adjuvants and costimulatory antibodies [13]. Regardless of peptide length, the amphiphilicity nature of peptide can play a critical role for its immunogenicity [12]. Amphiphilic peptides (long or short) self-assemble into nanostructures that resemble viral particles, which are effectively presented by professional APCs [16]. Our group has shown that the disruption of amphiphilicity inhibited, while peptide sequence modification (elongation) to increase amphiphilicity, dramatically improved CD8 responses [12]. Posttranscriptional modifications (phosphorylation, acetylation) is a sophisticated way to control the function of many proteins, some of which may function as TAAs for inducing antigen-specific T cells [37,38], and constitute promising targets for antitumor peptide vaccines. The combination of CD8 and CD4 epitope would be another strategy to increase the efficacy of peptide vaccine. Although dual activation of tumor-specific CTLs and HTLs induced robust antitumor responses [39], bystander HTLs that were not specific to TAA can augment antitumor CTLs [40] suggesting that non-specific CD4 epitope such as PADRE [41] can be a possible partner with TAA-derived CD8 epitopes.
We believe that the route of peptide vaccine administration is an important factor to regulate the intensity of the immune response. Although the subcutaneous vaccinations (usually with adjuvants such as IFA or alum) are effective to induce antibody responses, we have shown that peptide intravenous vaccinations are far superior to the subcutaneous route suggesting that systemic delivery of peptide delivers antigen to most lymphoid organs and facilitates the recruitment of naïve T cells to the response [12,42]. Moreover, subcutaneous peptide administration in incomplete Freund’s adjuvant (IFA) traps antigen-specific T cells at the injection site, resulting in deletion of T cells via chronic TCR stimulation [43]. Thus, it is not surprising that antitumor responses were barely detected in clinical trials that have used subcutaneous injection of peptide with IFA, which has been the most commonly used approach.
Adjuvant selection and combination therapies
T cells that receive TCR stimulation (signal 1) without appropriate costimulation (signal 2) and cytokines (signal 3) become anergic to the subsequent activation [44,45], the proper stimulation timing and selection of adjuvants is necessary to provides all three signals for a peptide vaccine to be effective in activating and expanding T cells to achieve an antitumor effect. Thus, in addition to DC peptide delivery, proper activation and maturation of these APCs will be critical to accomplish a substantial T cell response. Among all the pattern-recognition receptors (PRRs), members of the Toll-like receptor (TLR) family, known to recognize microorganism products, are known to provide strong activation-maturation signals to APCs [46]. In addition to its activity to induce signal 2 and 3 from APCs, TLR ligands enhance cross-presentation of antigen by recruiting MHC from endosomal recycling compartment [47]. Therefore, the simultaneous injection of TLR ligands with peptides is a practical way to augment the efficacy of vaccine by providing all three signals for the expansion of T cells. Nevertheless, because some TLR ligands may have either immunostimulatory or suppressive activity, it is necessary to select a proper TLR ligand as adjuvant for CTLs or HTLs. For example, TLR3 and TLR9 ligands induced but TLR2 and TLR4 ligands may suppressed CTLs activation using protein-based vaccines [48]. In our hands, poly-IC, which stimulates TLR3, significantly increased CD8+ T cells compared to TLR2 or TLR9 ligand [12]. In addition, poly-IC has the ability to stimulate the intracellular PRR MDA5, an RNA helicase that detects viral dsRNA, resulting in the generation of type-I interferons (IFN-I), which provides signal 3 to the CTLs [49]. Most notably, our results indicate that MDA5 activation by poly-IC is necessary to achieve vast CTL expansions resulting from peptide vaccines [12]. The stimulator of interferon genes (STING) is another intracellular PRR that detects DNA resulting in type 1 interferon production [50]. Recent studies in our laboratory have shown that STING ligands are also potent adjuvants for inducing effective antitumor CTL responses when used in combination with peptide and poly-IC [51]. On the other hand for CD4 responses, our studies reveal that a TLR7 ligand is superior to poly-IC (T. Kumai and E. Celis, manuscript in preparation). Thus, it is important to select a suitable TLR ligand for CTLs and HTLs.
Although the huge CD8 responses generated with peptide and poly-IC can be obtained in the absence of CD4 HTLs [12,52], it is widely accepted that HTLs support CTLs via APCs activation through CD40-CD40L interaction [53]. Taking advantage of this knowledge, the addition of a CD40 agonist (anti-CD40 antibody) to peptide vaccines increased the CD8 response in an HTLs-independent manner [13,52]. In addition to CD40, other costimulatory molecules have the possibility of potentiating peptide vaccines. For example, direct T cell costimulation via 4-1BB and OX-40 together with TCR stimulation leads to robust CD8 and CD4 responses, respectively [54]. ICOS, GITR, and CD27 could be other targetable costimulatory molecules with peptide immunization [55].
Another way to increase T cell responses is through blocking inhibitory pathways that allegedly exist to prevent autoimmunity. Activation of CTLA-4 on activated T cells by CD80 and CD86 on APCs “puts the brakes” on T cell responses and the use of anti-CTLA4 antibodies releases these brakes allowing the progression of T cell response. PD-1 is another negative checkpoint on T cells, which binds to PD-L1 or PD-L2 on tumors and APCs. Substantial evidence demonstrates the antitumor effects of checkpoint inhibitors and these been reviewed elsewhere [56]. The antitumor responses of checkpoint inhibitors are at least partially rely on T cells that respond to mutation-derived epitopes [57]. Accordingly, it is reasonable to combine peptide vaccine with checkpoint inhibitors to enhance the number and function of neoantigen-specific T cells. Because checkpoint inhibitors break immune tolerance, these might also be beneficial for peptide vaccine with non-mutated TAA.
Similar to costimulatory stimulation, addition of Signal 3 using cytokines is an effective method to expand T cells after having received appropriate TCR stimulation (signal 1) and costimulation (signal 2). A problem regarding the provision of signal 3 in vivo is the short half-life of cytokines, requiring the administration of daily high doses, which in most instances leads to severe toxicity. Nevertheless, this drawback can be solved by the use of cytokine-anti-cytokine antibody complexes, which increase the half-life in circulation of the cytokines and in some cases directs its binding to specific receptor on T cells. For example, in mice IL-2 complex with anti-IL-2 antibodies S4B6 or JES6-5H4 preferentially directs IL-2 to the low affinity receptor IL-2Rβ (CD122) expressed on memory CD8 T cells, whereas antibody JES6-1A12 targets IL-2 to the high affinity receptor IL-2Rα (CD25) expressed on recently activated CD8 and CD4 T cells, as well as on CD4 T regulatory cells [58]. Because of these unique characteristics, cytokine-antibody complexes may be a potent venue to enhance the effects of peptide vaccine. Experiments done by our group have shown that peptide vaccination followed by IL-2 complexed with antibody JES6-5H4 resulted in an enhanced expansion of antigen-specific CD8 T cells, capable of overcoming PD1 inhibition leading to tumor eradication in most mice [59].
With the recent knowledge of the dependency on immunity for the effectiveness of conventional antitumor therapies (e.g., radiotherapy), the combination of these classical strategies with active immunotherapy (vaccines) may result in perfect synergy. Recent evidence shows that chemotherapeutic drugs potentiate immune responses by inhibiting immunosuppressive cells or by inducing immunogenic cell death [60,61]. Because targeted radiation augmented TAA-specific CD8 responses by enhancing cross-presentation [62], combining peptide vaccines with beam radiation therapy could also have a synergistic effect.
Conclusions and future perspectives
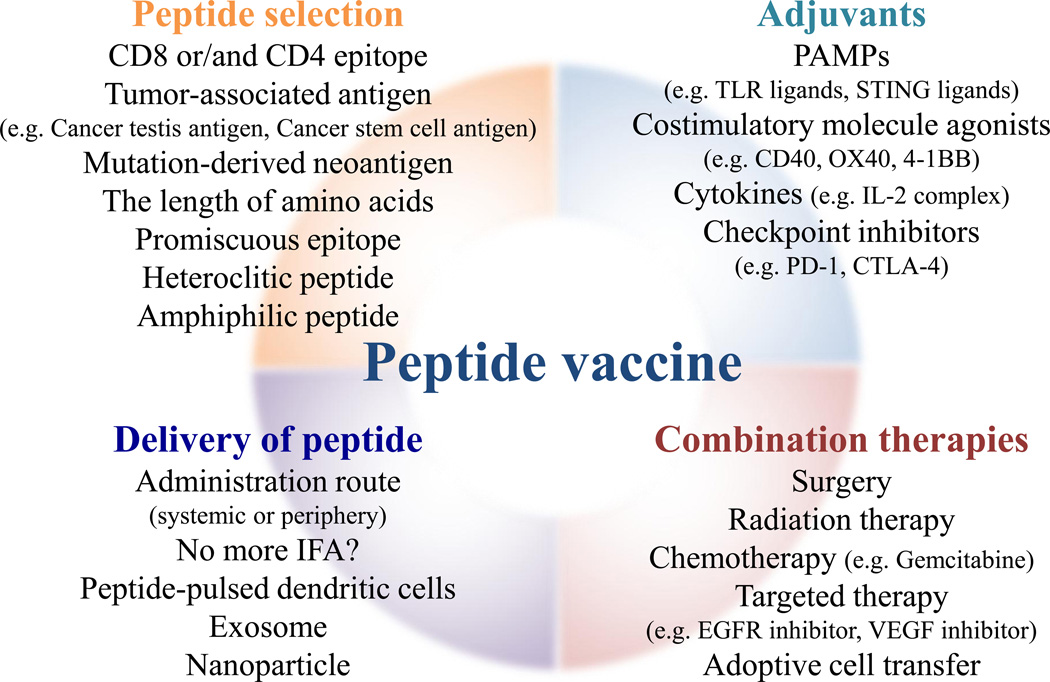
The dramatic positive results observed with some forms of immunotherapy have changed our view on how to treat cancer. Given our comprehensive understanding on how to build up immune responses, there has been a resurgence on the use of peptide vaccines to treat cancer (Fig. 2). To bring the best vaccination strategy to the clinic, we still need to address several issues. While it is favorable to mount a recall response quickly in cancer patients, T cells need to go through expansion and contraction phases to produce memory cells that will be critical for the prevention of tumor recurrences. Thus, elucidation of the appropriate frequency and interval of the vaccine will be necessary. In melanoma patients, PD-1 blockers showed a better survival benefit than CTLA-4 inhibitors [63]. While CTLA-4 blockade improves priming of TAA-specific T cells, PD-1 inhibition unleashes T cell responses that already exist [64,65]. Thus, it would be interesting to explore whether peptide vaccines in cancer patients preferentially prime naïve TAA-specific T cells and/or boosts spontaneously primed TAA-specific T cells. Because immunotherapy induces pseudoprogression of the tumor before regression, the responses to peptide vaccination should be assessed using immune-related response criteria (irRC) instead of RECIST, which has been widely used for chemotherapy [66]. To distinguish responders from non-responders, one must select appropriate biomarkers and realistic immune assays that demonstrate effective T cell responses. In addition, the expression status of specific molecules such as EGFR [67] or the ratio of intratumoral and peritumoral lymphocytes [68] could also be biomarkers candidates for peptide vaccine studies.
Figure 2.
Designing the best protocol for effective peptide vaccine. The main strategies to optimize peptide vaccine are the selection and delivery of peptide, adjuvants, and the combination with other therapies. After selecting an appropriate epitope peptide, peptide needs to deliver through a correct route. Adjuvants are necessary to augment an immunogenicity of the peptide. The combination of other therapies such as radiation and targeted therapy may further increase the efficiency of peptide vaccine.
In summary, it is evident that immunotherapies have become powerful antitumor options and among them, peptide vaccines have a lot to promise. Nevertheless, we are still in the midst of discovering the best approach to translate peptide vaccines into the clinic. We hope that the landscape of peptide vaccines that we have reviewed here may help further understanding of how to improve the efficiency of peptide vaccine that will finally cure cancer patients.
Highlights.
The recent findings in basic immunology revisit the use of peptide vaccine for cancer.
Peptide size, formulation and delivery route are critical for peptide vaccine efficiency.
Neoantigens from mutated genes are strong candidates for peptide vaccines.
The selection of appropriate adjuvants is a prerequisite for obtaining robust T responses.
Peptide vaccines have synergistic effects with checkpoint inhibitors and conventional cancer therapies.
Acknowledgments
This work was supported by a grant from the National Cancer Institute of the National Institutes of Health, R01CA157303, and by start-up funds from Augusta University Georgia Cancer Center and the Georgia Research Alliance (GRA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest
Esteban Celis has filed patent applications based on the use of synthetic peptides and poly-IC combinatorial vaccines. The rights of the patent applications have been transferred to the Moffitt Cancer Center (Tampa, FL). Other authors declare no conflict of interest.
References and recommended reading
* of special interest
** of outstanding interest
- 1.Olsen LR, Campos B, Barnkob MS, Winther O, Brusic V, Andersen MH. Bioinformatics for cancer immunotherapy target discovery. Cancer Immunol Immunother. 2014;63:1235–1249. doi: 10.1007/s00262-014-1627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerlero de Rosbo N, Mendel I, Ben-Nun A. Chronic relapsing experimental autoimmune encephalomyelitis with a delayed onset and an atypical clinical course, induced in PL/J mice by myelin oligodendrocyte glycoprotein (MOG)-derived peptide: preliminary analysis of MOG T cell epitopes. Eur J Immunol. 1995;25:985–993. doi: 10.1002/eji.1830250419. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16:219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 5.Sultan H, Fesenkova VI, Addis D, Fan AE, Kumai T, Wu J, Salazar AM, Celis E. Designing therapeutic cancer vaccines by mimicking viral infections. Cancer Immunol Immunother. 2016 doi: 10.1007/s00262-016-1834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. This study demostrated that a vaccine with HPV-derived long peptides could cure intraepithelial neoplasia
- 8.van Poelgeest MI, Welters MJ, van Esch EM, Stynenbosch LF, Kerpershoek G, van Persijn van Meerten EL, van den Hende M, Lowik MJ, Berends-van der Meer DM, Fathers LM, et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J Transl Med. 2013;11:88. doi: 10.1186/1479-5876-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 10.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leisegang M, Engels B, Schreiber K, Yew PY, Kiyotani K, Idel C, Arina A, Duraiswamy J, Weichselbaum RR, Uckert W, et al. Eradication of large solid tumors by gene therapy with a T cell receptor targeting a single cancer-specific point mutation. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cho HI, Barrios K, Lee YR, Linowski AK, Celis E. BiVax: a peptide/poly-IC subunit vaccine that mimics an acute infection elicits vast and effective anti-tumor CD8 T-cell responses. Cancer Immunol Immunother. 2013;62:787–799. doi: 10.1007/s00262-012-1382-6. This study demostrated that peptide vaccines with poly-IC induced robust CD8 T cell responses via the stimulation of TLR3 and MDA5., and that the amphiphilicity of the peptide and the route of administration were important determinants of the immunogenicity of peptide vaccine
- 13.Cho HI, Celis E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res. 2009;69:9012–9019. doi: 10.1158/0008-5472.CAN-09-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willis RA, Kappler JW, Marrack PC. CD8 T cell competition for dendritic cells in vivo is an early event in activation. Proc Natl Acad Sci U S A. 2006;103:12063–12068. doi: 10.1073/pnas.0605130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quakkelaar ED, Fransen MF, van Maren WW, Vaneman J, Loof NM, van Heiningen SH, Verbeek JS, Ossendorp F, Melief CJ. IgG-mediated anaphylaxis to a synthetic long peptide vaccine containing a B cell epitope can be avoided by slow-release formulation. J Immunol. 2014;192:5813–5820. doi: 10.4049/jimmunol.1302337. This study demostrated that long peptides that contains B cell epitopes induced severe anaphylxis after several doses of vaccine
- 16.Wen Y, Collier JH. Supramolecular peptide vaccines: tuning adaptive immunity. Curr Opin Immunol. 2015;35:73–79. doi: 10.1016/j.coi.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete Freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;179:5033–5040. doi: 10.4049/jimmunol.179.8.5033. [DOI] [PubMed] [Google Scholar]
- 18. Pritchard AL, Burel JG, Neller MA, Hayward NK, Lopez JA, Fatho M, Lennerz V, Wolfel T, Schmidt CW. Exome Sequencing to Predict Neoantigens in Melanoma. Cancer Immunol Res. 2015;3:992–998. doi: 10.1158/2326-6066.CIR-15-0088. This report illustrates a new approach to predict neoantigens in patients by combining whole-exosome sequencing data, mRNA microarrays, and epitope prediction algorithms. However, this approach may lead to false negative results
- 19.Moldovan I, Targoni O, Zhang W, Sundararaman S, Lehmann PV. How frequently are predicted peptides actually recognized by CD8 cells? Cancer Immunol Immunother. 2016 doi: 10.1007/s00262-016-1840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumai T, Matsuda Y, Oikawa K, Aoki N, Kimura S, Harabuchi Y, Celis E, Kobayashi H. EGFR inhibitors augment antitumour helper T-cell responses of HER family-specific immunotherapy. Br J Cancer. 2013;109:2155–2166. doi: 10.1038/bjc.2013.577. This study demonstrated that promiscuous peptides can stimulate T cell responses to a variety of HLA alleles and that EGFR inhibitors upregulate MHC expression on tumors followed by the upregulation of antitumor T cell responses
- 21.Angell TE, Lechner MG, Jang JK, LoPresti JS, Epstein AL. MHC class I loss is a frequent mechanism of immune escape in papillary thyroid cancer that is reversed by interferon and selumetinib treatment in vitro. Clin Cancer Res. 2014;20:6034–6044. doi: 10.1158/1078-0432.CCR-14-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spear TT, Nagato K, Nishimura MI. Strategies to genetically engineer T cells for cancer immunotherapy. Cancer Immunol Immunother. 2016 doi: 10.1007/s00262-016-1842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. Seminal observation that mutation-specific CD4+ T cells can mediate tumor regression in cancer patients
- 25.Slingluff CL, Jr, Lee S, Zhao F, Chianese-Bullock KA, Olson WC, Butterfield LH, Whiteside TL, Leming PD, Kirkwood JM. A randomized phase II trial of multiepitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells for patients with metastatic melanoma (E1602) Clin Cancer Res. 2013;19:4228–4238. doi: 10.1158/1078-0432.CCR-13-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnadas DK, Shusterman S, Bai F, Diller L, Sullivan JE, Cheerva AC, George RE, Lucas KG. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother. 2015;64:1251–1260. doi: 10.1007/s00262-015-1731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rapoport AP, Aqui NA, Stadtmauer EA, Vogl DT, Xu YY, Kalos M, Cai L, Fang HB, Weiss BM, Badros A, et al. Combination immunotherapy after ASCT for multiple myeloma using MAGE-A3/Poly-ICLC immunizations followed by adoptive transfer of vaccine-primed and costimulated autologous T cells. Clin Cancer Res. 2014;20:1355–1365. doi: 10.1158/1078-0432.CCR-13-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishizawa S, Hirohashi Y, Torigoe T, Takahashi A, Tamura Y, Mori T, Kanaseki T, Kamiguchi K, Asanuma H, Morita R, et al. HSP DNAJB8 controls tumor-initiating ability in renal cancer stem-like cells. Cancer Res. 2012;72:2844–2854. doi: 10.1158/0008-5472.CAN-11-3062. [DOI] [PubMed] [Google Scholar]
- 29.Vormehr M, Diken M, Boegel S, Kreiter S, Tureci O, Sahin U. Mutanome directed cancer immunotherapy. Curr Opin Immunol. 2016;39:14–22. doi: 10.1016/j.coi.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 30. Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J, Boegel S, Schrors B, Vascotto F, Castle JC, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–696. doi: 10.1038/nature14426. Interesting finding that the majority of mutation-derived antigens that had an antitumor effect were CD4 T cell epitopes
- 31. Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, Franci C, Cheung TK, Fritsche J, Weinschenk T, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–576. doi: 10.1038/nature14001. Description of a sophisticated method to identify mutation-derived CD8 T cell epitopes by combining mass spectometry and whole-exosome sequencing
- 32.Castle JC, Kreiter S, Diekmann J, Lower M, van de Roemer N, de Graaf J, Selmi A, Diken M, Boegel S, Paret C, et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72:1081–1091. doi: 10.1158/0008-5472.CAN-11-3722. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann S, Mead A, Malinovskis A, Hardwick NR, Guinn BA. Analogue peptides for the immunotherapy of human acute myeloid leukemia. Cancer Immunol Immunother. 2015;64:1357–1367. doi: 10.1007/s00262-015-1762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wei CH, Sherman LA. N-terminal trimer extension of nominal CD8 T cell epitopes is sufficient to promote cross-presentation to cognate CD8 T cells in vivo. J Immunol. 2007;179:8280–8286. doi: 10.4049/jimmunol.179.12.8280. Key observation that the simple extension of peptides can enhance cross-presentation of CD8 epitopes by DCs
- 35.Faure F, Mantegazza A, Sadaka C, Sedlik C, Jotereau F, Amigorena S. Long-lasting cross-presentation of tumor antigen in human DC. Eur J Immunol. 2009;39:380–390. doi: 10.1002/eji.200838669. [DOI] [PubMed] [Google Scholar]
- 36. Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, van der Burg SH, Offringa R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol. 2008;38:1033–1042. doi: 10.1002/eji.200737995. Important finding that long peptides were preferably presented by professional APCs, whereas short peptides were presented by non-professional APCs that lack proper costimulatory moleculess
- 37.Kumai T, Ishibashi K, Oikawa K, Matsuda Y, Aoki N, Kimura S, Hayashi S, Kitada M, Harabuchi Y, Celis E, et al. Induction of tumor-reactive T helper responses by a posttranslational modified epitope from tumor protein p53. Cancer Immunol Immunother. 2014;63:469–478. doi: 10.1007/s00262-014-1533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarling AL, Ficarro SB, White FM, Shabanowitz J, Hunt DF, Engelhard VH. Phosphorylated peptides are naturally processed and presented by major histocompatibility complex class I molecules in vivo. J Exp Med. 2000;192:1755–1762. doi: 10.1084/jem.192.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohtake J, Ohkuri T, Togashi Y, Kitamura H, Okuno K, Nishimura T. Identification of novel helper epitope peptides of Survivin cancer-associated antigen applicable to developing helper/killer-hybrid epitope long peptide cancer vaccine. Immunol Lett. 2014;161:20–30. doi: 10.1016/j.imlet.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Mittal P, St Rose MC, Wang X, Ryan JM, Wasser JS, Vella AT, Adler AJ. Tumor-Unrelated CD4 T Cell Help Augments CD134 plus CD137 Dual Costimulation Tumor Therapy. J Immunol. 2015;195:5816–5826. doi: 10.4049/jimmunol.1502032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim D, Monie A, He L, Tsai YC, Hung CF, Wu TC. Role of IL-2 secreted by PADRE-specific CD4+ T cells in enhancing E7-specific CD8+ T-cell immune responses. Gene Ther. 2008;15:677–687. doi: 10.1038/sj.gt.3303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bridle BW, Nguyen A, Salem O, Zhang L, Koshy S, Clouthier D, Chen L, Pol J, Swift S, Bowdish DM, et al. Privileged Antigen Presentation in Splenic B Cell Follicles Maximizes T Cell Responses in Prime-Boost Vaccination. J Immunol. 2016 doi: 10.4049/jimmunol.1600106. [DOI] [PubMed] [Google Scholar]
- 43. Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–472. doi: 10.1038/nm.3105. Important observation that peptides combined with IFA trap CD8+ and CD4+ T cells in the IFA injection depot, resulting in the apoptosis of T cells by chronic inflammation
- 44. Sckisel GD, Bouchlaka MN, Monjazeb AM, Crittenden M, Curti BD, Wilkins DE, Alderson KA, Sungur CM, Ames E, Mirsoian A, et al. Out-of-Sequence Signal 3 Paralyzes Primary CD4(+) T-Cell-Dependent Immunity. Immunity. 2015;43:240–250. doi: 10.1016/j.immuni.2015.06.023. This study describes that aberrantly high cytokines without appropriate TCR or costimulatory signals induced paralysis in T cells via SOCS3
- 45.Smith TR, Verdeil G, Marquardt K, Sherman LA. Contribution of TCR signaling strength to CD8+ T cell peripheral tolerance mechanisms. J Immunol. 2014;193:3409–3416. doi: 10.4049/jimmunol.1401194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 47. Nair-Gupta P, Baccarini A, Tung N, Seyffer F, Florey O, Huang Y, Banerjee M, Overholtzer M, Roche PA, Tampe R, et al. TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell. 2014;158:506–521. doi: 10.1016/j.cell.2014.04.054. Interesting finding that TLR ligands can augment cross-presentation by recruiting MHC into endosomal recycling compartments of phagosomes
- 48.Mandraju R, Murray S, Forman J, Pasare C. Differential ability of surface and endosomal TLRs to induce CD8 T cell responses in vivo. J Immunol. 2014;192:4303–4315. doi: 10.4049/jimmunol.1302244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Cella M, Gilfillan S, Colonna M. Cutting edge: polyinosinic:polycytidylic acid boosts the generation of memory CD8 T cells through melanoma differentiation-associated protein 5 expressed in stromal cells. J Immunol. 2010;184:2751–2755. doi: 10.4049/jimmunol.0903201. [DOI] [PubMed] [Google Scholar]
- 50.Demaria O, De Gassart A, Coso S, Gestermann N, Di Domizio J, Flatz L, Gaide O, Michielin O, Hwu P, Petrova TV, et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci U S A. 2015;112:15408–15413. doi: 10.1073/pnas.1512832112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, Celis E. STING activator c-di-GMP enhances the anti-tumor effects of peptide vaccines in melanoma-bearing mice. Cancer Immunol Immunother. 2015;64:1057–1066. doi: 10.1007/s00262-015-1713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Assudani D, Cho HI, DeVito N, Bradley N, Celis E. In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells. Cancer Res. 2008;68:9892–9899. doi: 10.1158/0008-5472.CAN-08-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 54.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–5951. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 55.Willoughby JE, Kerr JP, Rogel A, Taraban VY, Buchan SL, Johnson PW, Al-Shamkhani A. Differential impact of CD27 and 4-1BB costimulation on effector and memory CD8 T cell generation following peptide immunization. J Immunol. 2014;193:244–251. doi: 10.4049/jimmunol.1301217. [DOI] [PubMed] [Google Scholar]
- 56.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spangler JB, Tomala J, Luca VC, Jude KM, Dong S, Ring AM, Votavova P, Pepper M, Kovar M, Garcia KC. Antibodies to Interleukin-2 Elicit Selective T Cell Subset Potentiation through Distinct Conformational Mechanisms. Immunity. 2015;42:815–825. doi: 10.1016/j.immuni.2015.04.015. Important discovery on how various anti-IL-2 antibodies can alter the ability of IL-2 to bind to the specific receptors on T cells. In addition to the steric blockade of receptors, antibody also induces the structual change of cytokine
- 59.Cho HI, Reyes-Vargas E, Delgado JC, Celis E. A potent vaccination strategy that circumvents lymphodepletion for effective antitumor adoptive T-cell therapy. Cancer Res. 2012;72:1986–1995. doi: 10.1158/0008-5472.CAN-11-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alizadeh D, Larmonier N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res. 2014;74:2663–2668. doi: 10.1158/0008-5472.CAN-14-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 62.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3:345–355. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 64. Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, Welters MJ, van der Burg S, Kapiteijn E, Michielin O, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6:254ra128. doi: 10.1126/scitranslmed.3008918. This study elucidated that CTLA-4 blockade broadend the diversity of TCRs in the tumor microenvironment suggesting that this therapy primes otherwise ignored TAA-specific T cells
- 65.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol. 2016;34:1510–1517. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz K, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer (NSCLC)., A Retrospective Analysis. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vasaturo A, Halilovic A, Bol KF, Verweij DI, Blokx WA, Punt CJ, Groenen PJ, van Krieken JH, Textor J, de Vries IJ, et al. T-cell Landscape in a Primary Melanoma Predicts the Survival of Patients with Metastatic Disease after Their Treatment with Dendritic Cell Vaccines. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-15-3211. [DOI] [PubMed] [Google Scholar]