Abstract
Introduction
Prior studies have demonstrated an increased risk of cancer-associated scleroderma in patients with RNA polymerase III (POL) autoantibodies and in patients negative for anti-centromere (CENP), anti-topoisomerase-1 (TOPO), and anti-POL antibodies (referred to as CENP/TOPO/POL (CTP)-Negative). In a recent study of 16 CTP-negative scleroderma patients with coincident cancer, we found that 25% had autoantibodies to RNPC3, a member of the minor spliceosome complex. In this investigation, we validated the relationship between anti-RNPC3 antibodies and cancer and examined the associated clinical phenotype in a large sample of scleroderma patients.
Methods
Scleroderma patients with cancer were assayed for CENP, TOPO, POL and RNPC3 autoantibodies. Disease characteristics and the cancer-scleroderma interval were compared across autoantibody groups. The relationship between autoantibody status and cancer-associated scleroderma was assessed by logistic regression.
Results
Of 318 patients with scleroderma and cancer, 70 (22.0%) were positive for anti-POL, 54 (17.0%) for anti-TOPO, and 96 (30.2%) for anti-CENP. Twelve patients (3.8% of overall group or 12.2% of CTP-negatives) were positive for anti-RNPC3. Patients with anti-RNPC3 had a short cancer-scleroderma interval (median 0.9 years). Relative to patients with anti-CENP, patients with anti-RNPC3 (OR 4.3; 95%CI 1.10–16.9; p=0.037) and anti-POL (OR 4.49; 95%CI 1.98–10.2; p<0.001) had a >4-fold increased risk of cancer within 2 years of scleroderma onset. Patients with anti-RNPC3 had severe restrictive lung and gastrointestinal disease, Raynaud’s, and myopathy.
Conclusion
Anti-RNPC3 autoantibodies associate with an increased risk of cancer at scleroderma onset, similar to POL autoantibodies. These data suggest the possibility of cancer-induced autoimmunity in this scleroderma subset.
Introduction
Patients with systemic sclerosis (scleroderma) have an elevated risk of cancer compared to individuals in the general population (1). Recent data have demonstrated that a subset of scleroderma patients has a close temporal relationship between cancer diagnosis and the first clinical signs of scleroderma (2, 3). This clustering is most notable in patients with RNA polymerase III (POL) autoantibodies (2–6), who have a >5 fold increased risk of cancer within 2 years of scleroderma onset (3). Biologic studies strongly suggest paraneoplastic development of autoimmunity and scleroderma in patients with POL autoantibodies. Genetic alterations (somatic mutations and/or loss of heterozygosity) of the POLR3A gene that encodes for POL is also specifically identified in these patients’ cancers, but not cancers from scleroderma patients with other autoantibodies (7). Furthermore, these patients develop mutation-specific T cell immune responses and the development of POL autoantibodies that react with both mutant and wild-type POL proteins (7). In aggregate, these studies suggest a model of cancer-induced autoimmunity in which autoantigen mutation in cancers may trigger the development of anti-tumor immune responses that then result in autoimmunity (8).
In addition to patients with POL autoantibodies, there are other subsets of scleroderma patients who demonstrate a similar clustering of cancer diagnosis with the first clinical signs of scleroderma. This clustering is most notable among older patients developing scleroderma who are positive for antinuclear antibodies (ANA), but negative for the 3 most common scleroderma autoantibodies observed in US cohorts (anti-centromere (CENP), anti-topoisomerase 1 (TOPO), and anti-POL; hereafter referred to as “CENP/TOPO/POL (CTP)-negative”) (2, 3). These individuals likely represent a heterogenous population of scleroderma patients targeting different autoantigens, both known and novel. We recently utilized Phage-Immunoprecipitation Sequencing (PhIP-Seq) and PLATO (Parallel Analysis of in vitro Translated ORFs) (9, 10) to identify unique autoantibodies in CTP-negative scleroderma patients with a clustering of cancer diagnosis and scleroderma onset (11). Specifically, 16 CTP-negative patients with scleroderma, cancer, and a short cancer-scleroderma interval (≤ 5 years) were studied. Four of these 16 patients (25%) had autoantibodies to multiple adjacent peptides within RNPC3 (11), a 65 kDa protein component of the minor spliceosome complex which participates in removal of U12-type introns from pre-mRNA (12, 13). The minor spliceosome complex consists of several small nuclear RNAs and multiple protein components, including SNRNP25, SNRNP35, SNRNP48, PDCD7 and the Sm proteins. RNPC3 has 2 RNA recognition motifs, indicating that it likely contacts one of the small nuclear RNAs of the minor spliceosome. This anti-RNPC3 specificity (also known as anti-U11/U12) has previously been described in scleroderma, with a reported prevalence of 3.2% in the University of Pittsburgh scleroderma cohort (14).
In this investigation, we sought to verify whether anti-RNPC3 antibodies associate with a short cancer-scleroderma interval in a large sample of patients with scleroderma and cancer, as this may be additional evidence supporting a model of cancer-induced autoimmunity. We also compared the prevalence of anti-RNPC3 antibodies in CTP-negative patients with and without cancer to determine whether they are markers of cancer risk overall. Similarly, we examined the clinical phenotype to identify whether any unique clinical characteristics could be a sign of an underlying cancer. Lastly, we assayed anti-RNPC3 antibodies in cancer patients without scleroderma to define whether anti-RNPC3 antibodies are cancer biomarkers more broadly.
Patients and Methods
Study population and associated statistical analyses
Patients with scleroderma and an available serum sample were identified through the IRB-approved Johns Hopkins Scleroderma Center database. All patients have scleroderma defined by 2013 American College of Rheumatology (ACR) classification criteria, 1980 ACR classification criteria, or having at least 3 of 5 CREST (calcinosis, Raynaud’s, esophageal dysmotility, sclerodactyly, telangiectasia) syndrome features (15, 16). Demographic data, symptom onset dates, cutaneous subtype (17), organ-specific severity scores (18, 19), smoking status, and cancer diagnoses (dates, site, histology and therapy) are captured in all patients at the first visit and longitudinally at 6-month intervals for relevant parameters. All clinically obtained pulmonary function tests and echocardiograms are recorded. The date of scleroderma onset was defined by the date of the first scleroderma symptom, either Raynaud’s or non-Raynaud’s. The date of cancer diagnosis was obtained from pathology reports or medical record review when available, and was otherwise defined by patient report. The cancer-scleroderma interval was calculated as the difference between these two dates.
Cancer cohort and autoantibody status
We first examined our entire cohort of scleroderma patients with cancer and an available serum sample (N=325). The closest serum sample to cancer diagnosis was studied for each participant. Autoantibodies against TOPO, POL, and CENP A/B were assayed by enzyme-linked immunosorbent assays using commercially available kits (Inova Diagnostics), and results ≥ 40 units were defined as true positives for our primary analyses. A sensitivity analysis was also performed redefining antibody positivity as ≥20 units. Autoantibodies to RNPC3 were assayed by immunoprecipitation of 35S-methionine labeled protein generated by in vitro transcription and translation from cDNA encoding full length RNPC3 (purchased from Origene Technologies) as described previously (20). Representative data from the immunoprecipitation assay to detect RNPC3 antibodies is shown in Supplemental Figure 1. We restricted our primary analyses to patients who were positive for only 1 scleroderma autoantibody, as previously described (3). Of 325 patients with complete autoantibody data, only 7 patients were excluded from our analyses due to positivity for multiple autoantibodies, largely due to overlap with anti-centromere antibodies. Five of the 7 also had anti-RNPC3 antibodies, only 3 of whom were moderately or strongly positive. Therefore our study population consisted of 318 scleroderma patients with cancer.
Patients were subdivided into 5 autoantibody categories for analysis: anti-POL, anti-TOPO, anti-CENP, anti-RNPC3, and “CENP/TOPO/POL/RNPC3 (CTPR)-negative” (i.e. those who were negative for the 4 tested autoantibodies). Demographics, cancer-scleroderma interval, and scleroderma phenotypic features were compared across autoantibody subgroups. For continuous variables, differences in means were assessed by analysis of variance (ANOVA) unless unequal variances were suggested by Bartlett’s test; in this instance, the Kruskal-Wallis test was applied as a nonparametric test. Dichotomous and categorical variables were compared using the Fisher’s exact test. Characteristics were also compared between anti-RNPC3 positive vs. negative patients using the Student’s t test and Fisher’s exact test where appropriate.
We also performed logistic regression analysis to examine whether anti-RNPC3 and other autoantibodies associate with an increased risk of cancer-associated scleroderma. Cancer-associated scleroderma was defined by a short cancer-scleroderma interval (± 2 years), as previously described (3). The cancer-scleroderma interval was also examined graphically by generating scatterplots of age at scleroderma onset and age at cancer diagnosis for each autoantibody type.
Comparison with CTP-negative scleroderma patients without cancer
Sixty CTP-negative scleroderma patients without cancer were also studied. The prevalence of anti-RNPC3 positivity was compared between CTP-negative patients with cancer and without cancer by the chi-square test. We examined whether the clinical phenotype differed between anti-RNPC3 positive patients with and without cancer using the Wilcoxon-Mann-Whitney test and Fisher’s exact test where appropriate.
Comparison with healthy controls and other disease states
Twenty-five healthy controls, 45 patients with pancreatic cancer, and 35 patients with lupus and cancer were also assayed for anti-RNPC3 as described above.
All statistical analyses were performed using Stata version 13 (StataCorp, College Station, TX). Two sided p-values <0.05 were considered statistically significant. Odds ratios (ORs) with 95% confidence intervals (95% CIs) are provided.
Results
Three hundred eighteen scleroderma patients with cancer were analyzed (Table 1). Seventy patients (22.0%) were positive for anti-POL antibodies, 54 (17.0%) for anti-TOPO, 96 (30.2%) for anti-CENP, and 12 (3.8%) for anti-RNPC3, leaving 86 (27.0%) patients who likely target other specificities (the CTPR-negative group). None of the controls (healthy, pancreatic cancer, or lupus and cancer) had evidence of anti-RNPC3 antibodies.
Table 1.
Characteristics of study population (N=318 patients with SSc and cancer).
| Variable | POL (N=70) | TOPO (N=54) | CENP (N=96) | RNPC3 (N=12) | CTPR-Negative (N=86) | P-Value |
|---|---|---|---|---|---|---|
| Age SSc onset (years), mean (SD) | 52.7 (13.0) | 43.8 (16.3) | 43.8 (14.7) | 50.1 (11.7) | 47.9 (13.7) | 0.0007 |
| Age at cancer diagnosis (years), mean (SD) | 56.3 (10.8) | 52.2 (14.8) | 56.3 (13.4) | 48.6 (13.2) | 56.3 (12.8) | 0.1059 |
| SSc-cancer interval (years), median (IQR) | 1.0 (−1.3, 8.9) | 7.7 (0.3, 14.1) | 11.1 (1.3, 25.8) | 0.9 (−5.0, 2.7) | 7.5 (1.4, 16.7) | 0.0001^ |
| nonRP-cancer interval (years), median (IQR) | 0.8 (−2.0, 5.5), N=69 | 6.0 (−1.4, 13.0), N=52 | 5.3 (−1.5, 13.8), N=95 | −0.1 (−8.5, 0.5) | 3.7 (−1.2, 12.0), N=85 | 0.0021^ |
| RP-cancer interval (years), median (IQR) | 0.8 (−1.7, 8.5), N=68 | 7.7 (0.3, 14.1) | 9.2 (−0.1, 24.6) | 0.9 (−5.0, 2.7) | 8.7 (0.8, 16.4), N=78 | 0.0008^ |
| Disease duration at 1st visit, median (IQR) | 1.6 (1.0, 4.2) | 3.6 (1.5, 12.2) | 13.5 (5.1, 26.2) | 2.2 (1.1, 6.6) | 6.1 (1.0, 12.4) | 0.0001^ |
| Female sex, no. (%) | 52 (74.3) | 43 (79.6) | 86 (89.6) | 12 (100) | 66 (76.7) | 0.024 |
| Race, no. (%) | N=95 | N=85 | 0.001 | |||
| White | 69 (98.6) | 46 (85.2) | 93 (97.9) | 9 (75) | 75 (88.2) | |
| Black | 1 (1.4) | 5 (9.3) | 2 (2.1) | 3 (25) | 8 (9.4) | |
| Other | 0 (0) | 3 (5.6) | 0 (0) | 0 (0) | 2 (2.4) | |
| Smoking, no. (%) | N=95 | 0.912 | ||||
| Never | 33 (47.2) | 27 (50) | 48 (50.5) | 7 (58.3) | 35 (40.7) | |
| Former | 29 (41.4) | 22 (40.7) | 37 (39) | 4 (33.3) | 43 (50) | |
| Current | 8 (11.4) | 5 (9.3) | 10 (10.5) | 1 (8.3) | 8 (9.3) | |
| 2013 ACR classification criteria*, no. (%) | 92 (95.8) | 70 (100) | 54 (100) | 12 (100) | 82 (95.4) | 0.201 |
| Cutaneous subtype, no. (%) | ||||||
| Diffuse | 54 (77.1) | 23 (42.6) | 6 (6.3) | 3 (25) | 29 (33.7) | <0.001 |
| Limited | 16 (22.9) | 31 (57.4) | 90 (93.8) | 9 (75) | 57 (66.3) | |
| Baseline mRSS, median (IQR) | 18 (8, 30), N=69 | 6 (3, 15), N=51 | 2 (2, 4), N=91 | 2 (2, 4) | 4 (2, 13), N=77 | 0.0001^ |
| Baseline Medsger disease severity scores**, no. (%) | ||||||
| Severe RP (pits, ulcers, gangrene) | 9 (12.9) | 25 (46.3) | 32 (33.3) | 7 (58.3) | 21 (25), N=84 | <0.001 |
| Severe GI disease (≥2) | 5 (7.1) | 12 (22.2) | 21 (21.9) | 3 (25) | 26 (30.6), N=85 | 0.005 |
| Severe lung disease (FVC or DLCO <70% pred) | 17 (34), N=50 | 23 (57.5), N=40 | 29 (44.6), N=65 | 8 (88.9), N=9 | 31 (49.2), N=63 | 0.019 |
| Baseline FVC (% predicted), mean (SD) | 84.6 (14.3), N=62 | 73.2 (16.9), N=49 | 90.0 (16.1), N=91 | 66.1 (17.8) | 77.7 (19.5), N=78 | <0.0001 |
| Baseline DLCO (% predicted), mean (SD) | 83.6 (20.2), N=55 | 74.7 (22.4), N=46 | 85.2 (25.1), N=79 | 64.2 (23.4), N=8 | 71.4 (22.1), N=70 | 0.0005 |
| Baseline RVSP (mmHg), median (IQR) | 33.2 (26, 38), N=38 | 31 (27.5, 35.5), N=32 | 35 (28, 43), N=53 | 43 (35, 51), N=6 | 34 (30, 42), N=57 | 0.0206^ |
| FVC ever <70% predicted, no. (%) | 22 (31.4) | 34 (63) | 26 (27.1) | 9 (75) | 36 (41.9) | <0.001 |
| RVSP ever >45 mmHg, no. (%) | 14 (20) | 16 (29.6) | 34 (35.4) | 6 (50) | 33 (38.4) | 0.062 |
| Myopathy ever, no.(%)*** | 10 (14.3) | 5 (9.3) | 6 (6.3) | 4 (33.3) | 15 (17.4) | 0.027 |
| Tendon friction rubs ever, no. (%) | 32 (45.7) | 12 (22.2) | 3 (3.1) | 0 (0) | 8 (9.3) | <0.001 |
| Renal crisis, no. (%) | 9 (12.9) | 1 (1.9) | 1 (1.0) | 1 (8.3) | 6 (7.0) | 0.009 |
| Death | 25 (35.7) | 21 (38.9) | 26 (27.1) | 7 (58.3) | 35 (40.7) | 0.134 |
| Cancer site, no. (%) | NT | |||||
| Female/gynecologic | ||||||
| Breast | 27 (38.6) | 17 (31.5) | 29 (30.2) | 6 (50) | 18 (20.9) | |
| Other gynecologic | 5 (7.1) | 3 (5.6) | 9 (9.4) | 2 (16.7) | 7 (8.1) | |
| Lung | 6 (8.6) | 9 (16.7) | 11 (11.5) | 0 (0) | 6 (7) | |
| Hematologic | 3 (4.3) | 0 (0) | 8 (8.3) | 2 (16.7) | 11 (12.8) | |
| Skin | 8 (11.4) | 12 (22.2) | 24 (25) | 2 (16.7) | 25 (29.1) | |
| Others | 21 (30) | 13 (24.1) | 15 (15.6) | 0 (0) | 19 (22.1) | |
The 4 remaining patients in the anti-CENP group and 3 of the 4 in the CTPR-negative group met at least 3 of 5 CREST criteria; one patient in the CTPR-negative group met 1980 ACR classification criteria.
Severe Raynaud’s defined by a Medsger severity score at baseline ≥2 (pits, ulcers, gangrene); severe lung severity score defined by FVC or DLCO <70% predicted; severe GI severity score defined by requirement of high dose medications for gastroesophageal reflux disease, antibiotics needed for bacterial overgrowth, malabsorption syndrome, episodes of pseudo-obstruction, or requirement of total parenteral nutrition.
Myopathy defined by a history of abnormal muscle enzymes or abnormal findings on electromyography, muscle biopsy or magnetic resonance imaging.
denote analyses performed using the Kruskal-Wallis test; medians and interquartile ranges (IQR) are presented.
Abbreviations: POL=RNA polymerase III; Topo=Topoisomerase 1; Cenp=Centromere; CTPR-Negative=Negative for centromere, topoisomerase 1, RNA polymerase III and RNPC3; SSc=systemic sclerosis; RP=Raynaud’s phenomenon; ACR=American College of Rheumatology; mRSS=modified Rodnan skin score; GI=gastrointestinal; FVC=forced vital capacity; DLCO=diffusing capacity; RVSP=right ventricular systolic pressure; NT=Not tested
RNPC3 autoantibodies associate with a short cancer-scleroderma interval and severe clinical phenotype
The cancer-scleroderma interval was significantly different across the 5 autoantibody subgroups; this finding persisted whether scleroderma onset was defined by Raynaud’s onset (p=0.0008), the first non-Raynaud’s symptom (p=0.0021), or the first symptom (either Raynaud’s or non-Raynaud’s; p=0.0001). Patients with anti-RNPC3 autoantibodies have a short cancer-scleroderma interval (median 0.9 years), similar to that observed for patients with anti-POL antibodies (median 1.0 years). This temporal clustering between cancer and scleroderma for anti-RNPC3 and anti-POL positive patients is illustrated in Figure 1. The red line in each scatterplot represents where the age of cancer diagnosis equals the age at scleroderma onset (i.e., cancer-scleroderma interval=0). Patients with anti-RNPC3 autoantibodies cluster tightly on or around the line of perfect agreement, consistent with cancer-associated scleroderma. Relative to patients with anti-CENP autoantibodies, patients with anti-RNPC3 antibodies (OR 4.3; 95 % CI 1.10, 16.9; p=0.037) and anti-POL antibodies (OR 4.49; 95% CI 1.98, 10.2; p<0.001) have a >4 fold increased risk of cancer within 2 years of scleroderma onset (Table 2). When broadening our reference group to include patients with anti-CENP, anti-TOPO and those who are CTPR-Negative, patients with anti-RNPC3 (OR 3.57; 95% CI 1.01, 12.6; p=0.048) and anti-POL (OR 3.72; 95% 1.99, 6.98; p<0.001) had a >3 fold increased odds of cancer within 2 years of scleroderma onset. These findings persisted in our sensitivity analyses that redefined autoantibody positivity with a lower cutoff of ≥20 units (data not shown).
Figure 1. Relationship between age at cancer diagnosis and age at scleroderma onset.
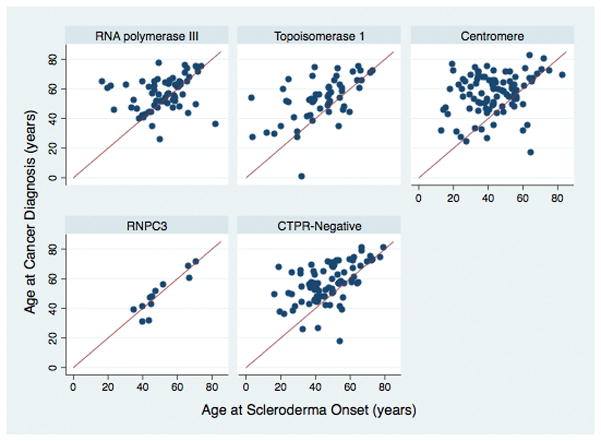
The red line in each graph denotes perfect agreement between age at cancer diagnosis and age at scleroderma onset. CTPR-Negative refers to the group that is negative for centromere, topoisomerase 1, RNA polymerase III and RNPC3 autoantibodies.
Table 2.
Relative odds (95% CI) of cancer-associated scleroderma*
| Autoantibody | Cancer-associated scleroderma |
|---|---|
| Centromere (CENP) | Reference |
| RNA polymerase III (POL) | 4.49 (1.98, 10.2) |
| Topoisomerase 1 (TOPO) | 1.72 (0.65, 4.54) |
| RNPC3 | 4.3 (1.10, 16.9) |
| Remaining (CTPR-Negative) | 1.13 (0.45, 2.87) |
Cancer-associated scleroderma defined as cancer and scleroderma occurring within 2 years of each other (±2 years)
Patients with anti-RNPC3 autoantibodies were 100% female and more likely to be black (25%) (Table 1). There were statistically significant differences in age at scleroderma onset (p=0.0007) and disease duration at first visit (p=0.0001) across autoantibody categories. Patients with anti-RNPC3 and anti-POL antibodies had a mean age of scleroderma onset above 50 years and a shorter time to presentation for clinical evaluation than the other antibody subgroups, likely due to the aggressive phenotype associated with these two autoantibodies (6, 14). While patients with anti-RNPC3 antibodies had less severe cutaneous and articular disease as assessed by subtype, modified Rodnan skin scores, and the presence of tendon friction rubs, they had more severe restrictive lung disease at baseline with lower forced vital capacity and diffusing capacity and higher Medsger lung severity scores. Anti-RNPC3 positive patients also had associated pulmonary hypertension as defined by elevated right ventricular systolic pressure (RVSP) on baseline echocardiography, although it is important to note the small sample size of patients with an estimated RVSP for this analysis. Anti-RNPC3 patients had an associated myopathy (33.3%), severe gastrointestinal disease and severe Raynaud’s phenomenon.
Given our small sample size, pairwise comparisons between the anti-RNPC3 autoantibody group with every other autoantibody group were not performed because of the high likelihood that associations would be observed by chance alone. However, we compared anti-RNPC3-positive and -negative patients and confirmed that anti-RNPC3 positive patients had statistically significant associations with a short cancer-scleroderma interval, severe restrictive lung disease consistent with ILD, a higher baseline RVSP, severe Raynaud’s phenomenon, and history of myopathy (data not shown).
While anti-RNPC3 autoantibodies are not commercially available for clinical use, indirect immunofluorescence patterns on ANA testing may provide insight into CTP-negative patients who could have this specificity. Of the 12 anti-RNPC3 positive patients, 11 had available data on ANA pattern. Nine of the 11 patients (81.8%) had a speckled pattern. As some of the phenotypic features that associate with anti-RNPC3 autoantibodies are similar to that seen in patients with anti-U1RNP, we assessed by ELISA whether anti-U1RNP is also present among these patients. No patient with anti-RNPC3 autoantibodies was moderately to strongly positive for anti-U1RNP.
Patients with anti-RNPC3 antibodies also had a worse prognosis with a shorter time to death compared to patients in the other autoantibody subgroups (Supplemental Figure 2; median survival 9.0 years in anti-RNPC3 vs. >20 years in all other antibody groups; log rank test p<0.0001). While statistical comparisons of all tumor types are not possible with our small sample size, it is noteworthy that most malignancies (66.7%) in the anti-RNPC3 group were female/gynecologic tumors, with 50% being breast cancers (p=0.075 for comparison across antibody groups).
Prevalence of anti-RNPC3 autoantibodies and clinical phenotype of anti-RNPC3 positive patients does not differ by cancer status
Among CTP-negative scleroderma patients, there were no significant differences in the prevalence of anti-RNPC3 antibodies by cancer status (12/98 (12.2%) with cancer were anti-RNPC3 positive, compared to 8/60 (13.3%) of those without cancer; p=0.842).
We examined whether unique phenotypic features could identify the subset of patients with an underlying cancer among anti-RNPC3 positive patients, as clinical differences could aid in risk stratification for cancer screening. Our sample size was limited to 12 anti-RNPC3 positive patients with cancer and 8 anti-RNPC3 positive patients without cancer. There were no statistical differences in age at scleroderma onset, age at cancer diagnosis, disease duration at first visit, gender, race, cutaneous subtype, organ specific severity scores, baseline pulmonary function, myopathy or articular disease (data not shown).
Discussion
This investigation found that scleroderma patients with anti-RNPC3 autoantibodies have a short cancer-scleroderma interval (median 0.9 years), similar to that observed for patients with anti-POL antibodies (median 1.0 years). Relative to patients with anti-CENP autoantibodies, patients with anti-RNPC3 antibodies (OR 4.3; 95 % CI 1.10, 16.9; p=0.037) and anti-POL antibodies (OR 4.49; 95% CI 1.98, 10.2; p<0.001) have a >4 fold increased risk of cancer occuring within 2 years of scleroderma onset. The presence of anti-RNPC3 autoantibodies did not signify an increased risk of cancer overall, but identified a subset of patients who have an increased risk of cancer at the time of the first clinical manifestations of scleroderma. The temporal association between cancer and scleroderma in patients with anti-RNPC3 antibodies is very similar to that observed in patients with anti-POL antibodies, and strongly suggests that additional, serologically-defined subsets of scleroderma patients may have cancer-induced autoimmunity (2, 3, 7, 8). Although the mechanistic relationship between cancer and scleroderma among patients with anti-RNPC3 antibodies is unknown, these data support the idea that cancer might initiate scleroderma-specific immune responses and also provide rationale for targeted malignancy screening at scleroderma onset.
In our study of scleroderma patients with cancer, patients with anti-RNPC3 were more likely to be black and have severe ILD, GI disease, and Raynaud’s phenomenon. Even among a cohort of patients with a history of cancer, patients with anti-RNPC3 had a faster time to death from scleroderma onset than patients without anti-RNPC3, which may be consistent with the aggressive phenotype of these patients. Overall, our findings related to the clinical phenotype and prevalence estimates (3.8% Johns Hopkins, 3.2% Pittsburgh) are similar to those previously described for U11/12 antibodies in the University of Pittsburgh scleroderma cohort (14). Our data suggest that patients with anti-RNPC3 may be more likely to have a myopathy, which has not been described; further study is required to validate this finding in a larger cohort.
Recognizing that the prevalence of anti-RNPC3 antibodies was low in our cohort, we were unable to identify any significant clinical phenotypic differences between anti-RNPC3 positive patients with cancer compared to those without cancer. Anti-RNPC3 autoantibodies were unique to scleroderma and were not found in cancer patients without scleroderma, demonstrating that anti-RNPC3 antibodies are not cancer biomarkers in non-scleroderma populations.
As in patients with POL autoantibodies, these data support the practice of careful cancer screening at the onset of scleroderma in patients with anti-RNPC3 autoantibodies. As we begin to identify more subsets of patients with possible cancer-associated scleroderma, it will be critical to consider evidence based approaches to define the optimal cancer screening algorithm in these patients to maximize cancer dectection while minimizing the risks associated with false positive testing. The approach to cancer screening in scleroderma may vary by autoantibody subtype. In our small sample of anti-RNPC3 positive patients with cancer, 50% of the malignancies were breast cancers, suggesting that mammography is important. The remaining cancers seen in our anti-RNPC3 positive patients were gynecologic, hematologic and skin in origin.
Our study was conducted in the largest cohort of well characterized patients with scleroderma and cancer to date, but it was limited by the small sample size of patients with anti-RNPC3 autoantibodies. While we observed statistical differences, it is important to note that we could not perform pairwise comparisons between each pair of autoantibody groups due to our sample size. In addition, our control group was restricted to scleroderma patients who were CTP-negative without cancer. Lastly, while our data demonstrating a clustering of cancer diagnosis with scleroderma onset suggests a paraneoplastic mechanism of scleroderma onset similar to patients with POL autoantibodies, this investigation did not examine whether genetic or post-translational alterations of RNPC3 are present in the cancer tissue of these patients.
In conclusion, anti-RNPC3 autoantibodies associate with an increased risk of cancer at scleroderma onset, similar to that observed in patients with POL autoantibodies. These data suggest the possibility of cancer-induced autoimmunity in this subset of scleroderma patients, and biologic studies of autoantigen alteration in cancer tissues of anti-RNPC3 patients remains an important priority. Awareness of the association between anti-RNPC3 antibodies and cancer in patients with scleroderma provides an opportunity for early cancer detection and intervention, which may improve overall outcomes.
Supplementary Material
Sera from CTP-negative scleroderma patients with an associated cancer (lanes 2–7), CTP-negative scleroderma patients without an associated cancer (lanes 8 & 9) and healthy control subjects (lanes 10–12) were used to immunoprecipitate 35S-methionine radiolabeled full-length human RNPC3 generated by in vitro transcription and translation. For each set of immunoprecipitations performed, a positive reference immunoprecipitation was included using an anti-FLAG monoclonal antibody (lane 1; the RNPC3 is FLAG-tagged). The scleroderma sera shown in lanes 2–5 and lane 8 have antibodies against RNPC3.
median survival 9.0 years in anti-RNPC3 vs. >20 years in all other antibody groups; log rank test p<0.0001).
Acknowledgments
The authors would like to thank Adrianne Woods and Margaret Sampedro for excellent management of the database and biological samples for this study.
Funding: This work was supported by NIH grants K23 AR061439 (AS) and R01 DE-12354-15A1 (AR & LC-R), the Ira T. Fine Discovery Fund, the Donald B. and Dorothy L. Stabler Foundation, and the Scleroderma Research Foundation. The Johns Hopkins Rheumatic Disease Research Core Center, where the antibody assays were performed, is supported by NIH grant P30 AR-053503.
Footnotes
Conflicts of interest: None
References
- 1.Onishi A, Sugiyama D, Kumagai S, Morinobu A. Cancer incidence in systemic sclerosis: meta-analysis of population-based cohort studies. Arthritis and rheumatism. 2013;65(7):1913–21. doi: 10.1002/art.37969. [DOI] [PubMed] [Google Scholar]
- 2.Shah AA, Rosen A, Hummers L, Wigley F, Casciola-Rosen L. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis and rheumatism. 2010;62(9):2787–95. doi: 10.1002/art.27549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah AA, Hummers LK, Casciola-Rosen L, Visvanathan K, Rosen A, Wigley FM. Examination of autoantibody status and clinical features associated with cancer risk and cancer-associated scleroderma. Arthritis & rheumatology. 2015;67(4):1053–61. doi: 10.1002/art.39022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Airo P, Ceribelli A, Cavazzana I, Taraborelli M, Zingarelli S, Franceschini F. Malignancies in Italian patients with systemic sclerosis positive for anti-RNA polymerase III antibodies. The Journal of rheumatology. 2011;38(7):1329–34. doi: 10.3899/jrheum.101144. [DOI] [PubMed] [Google Scholar]
- 5.Moinzadeh P, Fonseca C, Hellmich M, Shah AA, Chighizola C, Denton CP, et al. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis research & therapy. 2014;16(1):R53. doi: 10.1186/ar4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikpour M, Hissaria P, Byron J, Sahhar J, Micallef M, Paspaliaris W, et al. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a cross-sectional analysis of data from an Australian cohort. Arthritis research & therapy. 2011;13(6):R211. doi: 10.1186/ar3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph CG, Darrah E, Shah AA, Skora AD, Casciola-Rosen LA, Wigley FM, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science. 2014;343(6167):152–7. doi: 10.1126/science.1246886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah AA, Casciola-Rosen L, Rosen A. Review: cancer-induced autoimmunity in the rheumatic diseases. Arthritis & rheumatology. 2015;67(2):317–26. doi: 10.1002/art.38928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larman HB, Zhao Z, Laserson U, Li MZ, Ciccia A, Gakidis MA, et al. Autoantigen discovery with a synthetic human peptidome. Nature biotechnology. 2011;29(6):535–41. doi: 10.1038/nbt.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, Larman HB, Gao G, Somwar R, Zhang Z, Laserson U, et al. Protein interaction discovery using parallel analysis of translated ORFs (PLATO) Nature biotechnology. 2013;31(4):331–4. doi: 10.1038/nbt.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu G, Shah AA, Li MZ, Xu Q, Rosen A, Casciola-Rosen L, et al. Systematic autoantigen analysis identifies a distinct subtype of scleroderma with coincident cancer. Proc Natl Acad Sci U S A. 2016;113(47):E7526–E7534. doi: 10.1073/pnas.1615990113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benecke H, Luhrmann R, Will CL. The U11/U12 snRNP 65K protein acts as a molecular bridge, binding the U12 snRNA and U11-59K protein. EMBO J. 2005;24(17):3057–69. doi: 10.1038/sj.emboj.7600765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netter C, Weber G, Benecke H, Wahl MC. Functional stabilization of an RNA recognition motif by a noncanonical N-terminal expansion. RNA. 2009;15(7):1305–13. doi: 10.1261/rna.1359909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fertig N, Domsic RT, Rodriguez-Reyna T, Kuwana M, Lucas M, Medsger TA, Jr, et al. Anti-U11/U12 RNP antibodies in systemic sclerosis: a new serologic marker associated with pulmonary fibrosis. Arthritis Rheum. 2009;61(7):958–65. doi: 10.1002/art.24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65(11):2737–47. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis and rheumatism. 1980;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 17.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. The Journal of rheumatology. 1988;15(2):202–5. [PubMed] [Google Scholar]
- 18.Clements PJ, Lachenbruch PA, Seibold JR, Zee B, Steen VD, Brennan P, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. The Journal of rheumatology. 1993;20(11):1892–6. [PubMed] [Google Scholar]
- 19.Medsger TA, Jr, Silman AJ, Steen VD, Black CM, Akesson A, Bacon PA, et al. A disease severity scale for systemic sclerosis: development and testing. The Journal of rheumatology. 1999;26(10):2159–67. [PubMed] [Google Scholar]
- 20.Fiorentino DF, Chung LS, Christopher-Stine L, Zaba L, Li S, Mammen AL, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1gamma. Arthritis Rheum. 2013;65(11):2954–62. doi: 10.1002/art.38093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sera from CTP-negative scleroderma patients with an associated cancer (lanes 2–7), CTP-negative scleroderma patients without an associated cancer (lanes 8 & 9) and healthy control subjects (lanes 10–12) were used to immunoprecipitate 35S-methionine radiolabeled full-length human RNPC3 generated by in vitro transcription and translation. For each set of immunoprecipitations performed, a positive reference immunoprecipitation was included using an anti-FLAG monoclonal antibody (lane 1; the RNPC3 is FLAG-tagged). The scleroderma sera shown in lanes 2–5 and lane 8 have antibodies against RNPC3.
median survival 9.0 years in anti-RNPC3 vs. >20 years in all other antibody groups; log rank test p<0.0001).


