Abstract
Objective
To test the hypothesis that high-fat (HF) diet-induced obesity increases pro-inflammatory cytokine expression, macrophage infiltration and M1 polarization in the infrapatellar fat pad (IFP) prior to knee cartilage degeneration.
Methods
We characterized the effect of HF feeding on knee OA pathology, body adiposity, and glucose intolerance in male C57BL/6J mice and identified a diet duration that induces metabolic dysfunction prior to cartilage degeneration. Magnetic resonance imaging and histomorphology were used to quantify changes in epididymal, subcutaneous, and infrapatellar fat pads and adipocyte sizes. Finally, we utilized targeted gene expression and protein arrays, immunohistochemsitry, and flow cytometry to quantify differences in fat pad inflammatory markers and immune cell populations.
Results
20 weeks of HF diet treatment induced marked obesity, glucose intolerance, and early osteoarthritis (OA), including osteophytes and cartilage tidemark duplication. This duration of HF feeding increased IFP volume. However, it did not increase IFP inflammation, macrophage infiltration, or M1 macrophage polarization as observed in epididymal fat. Furthermore, leptin protein was reduced. This protection from obesity-induced inflammation corresponded with increased IFP fibrosis and the absence of adipocyte hypertrophy.
Conclusion
The IFP does not recapitulate classical abdominal adipose tissue inflammation during the early stages of knee OA in a high-fat diet-induced model of obesity. Consequently, these findings do not support the hypothesis that IFP inflammation is an initiating factor of obesity-induced knee OA. Furthermore, the pro-fibrotic and anti-hypertrophic responses of IFP adipocytes to high-fat feeding suggest that intra-articular adipocytes are subject to distinct spatial-temporal structural and metabolic regulation among fat pads.
Keywords: Obesity, Osteoarthritis, Inflammation, Monocytes/macrophages, Joint Structure
Introduction
Obesity significantly increases the risk of osteoarthritis (OA) (1,2), and clinical and pre-clinical studies support a role for both biomechanical and inflammatory factors in OA pathogenesis (3). OA involves a mosaic pattern of altered systemic and local inflammation (4,5). For example, circulating inflammatory markers can identify symptomatic knee OA at risk for disease progression (6). Locally, cytokines and chemokines are elevated in synovium, cartilage, meniscus, and synovial fluid (7–10). Innate inflammatory mediators drive many facets of obesity-induced metabolic dysfunction, providing a potential link to OA pathogenesis (11–13). Recent studies show that activated synovial macrophages are present in at least some OA joints (14–16), and there is mounting evidence that innate inflammatory networks, such as those involving complement and alarmins, are critical mediators of OA progression and pain (17).
Understanding how metabolic inflammation contributes to OA risk is challenging because obesity triggers systemic and tissue-specific cellular immune responses, particularly within adipose tissue. We previously reported that knee OA develops in proportion to total body fat and the adipokine leptin, but not other cytokines, in high-fat (HF) diet-induced obese mice (18). In humans, leptin is elevated in synovial fluid relative to serum, indicating the potential for local metabolic inflammation (19). In a comparison of patient-matched infrapatellar and subcutaneous fat, the infrapatellar fat pad (IFP) secreted greater levels of adiponectin, adipsin, IL-6, TNF-α, and visfatin per tissue weight (20,21). Clinical imaging studies also support a role for the IFP in OA, linking changes in IFP size or magnetic resonance signal intensity to changes in OA symptoms and structural pathology (22,23). However, most evidence of IFP inflammation is based on samples collected from patients undergoing total joint arthroplasty (24). Therefore, we wanted to understand the effect of obesity on IFP inflammation during the initiation of knee OA.
Macrophages are central mediators of adipose tissue inflammation (25). Resident adipose tissue macrophages suppress inflammation similar to alternatively activated M2 macrophages. Obesity stimulates chemokine-mediated macrophage infiltration and pro-inflammatory (M1) polarization, particularly in abdominal fat. Given the pro-inflammatory phenotype of the IFP in OA joints, we hypothesized that obesity increases knee OA by inducing IFP macrophage infiltration, M1 polarization, and pro-inflammatory cytokine production prior to the development of cartilage degeneration. Previous findings in humans with OA characterized IFP macrophages as primarily M2 regardless of obesity status (26). However, M2-like macrophages are prevalent during wound healing; consequently, the inflammatory characteristics at the end-stage of OA may greatly differ from the onset of disease. Therefore, we evaluated IFP inflammation and macrophage polarization markers prior to the onset of cartilage damage.
We tested our hypothesis using a widely-studied HF diet-induced mouse model of obesity obtained from Jackson Laboratory. We first characterized the temporal effects of HF feeding on knee OA pathology, body adiposity, and glucose tolerance to identify a diet duration that induces metabolic dysfunction prior to cartilage degeneration. We then used magnetic resonance imaging and histomorphology to quantify changes in the size of epididymal, subcutaneous, and infrapatellar fat pads (EFP, SFP, and IFP) and corresponding changes in adipocyte size. Finally, we conducted quantitative gene expression profiling, immunohistochemsitry, and flow cytometry to characterize differences in inflammatory markers and cell types among the different fat pads and in response to a HF diet.
Materials and Methods
Animals
Experiments were conducted in accordance with protocols approved by the AAALAC-accredited Institutional Animal Care and Use Committee at the Oklahoma Medical Research Foundation (OMRF). Male C57BL/6J mice were purchased from the JAX Diet-Induced Obesity (DIO) service (Jackson Laboratory, Bar Harbor, ME), which randomizes mice to diets containing either 10% kcal fat (Control, D12450Bi) or 60% kcal fat (HF, D12492i) beginning at 6 weeks of age (Research Diets, New Brunswick, NJ). DIO animals were purchased at 24 weeks of age and maintained on the same diets in the OMRF vivarium (SPF facility) until experimental endpoints at 26 and 52 weeks of age. Mice were group housed (≤5 animals/cage) in ventilated cages in a temperature-controlled room on 12-h light/dark cycles with ad libitum access to food and water and routine veterinary assessment. Multiple cohorts of animals were purchased from the JAX DIO service to complete all experiments (Supplemental Table 1). Sample sizes for specific experiments are indicated in figure legends.
Body Composition, Fat Pad Volume, and Glucose Analyses
Body fat content excluding the head was measured under isoflurane anesthesia using a dual-energy X-ray absorptiometry system (Lunar PIXImus2; GE LUNAR Corp., Madison, WI) at 24 and 50 weeks of age (27). Infrapatellar, subcutaneous, and peritoneal fat pad volumes were measured in 24 week old control and HF diet animals using water-suppressed magnetic resonance imaging as previously described (28). Intraperitoneal glucose tolerance tests were performed in mice subject to overnight fasting approximately 1 week prior to euthanasia as previously described (29).
Histology and Immunohistochemistry
Animals were euthanized by CO2 asphyxiation, alternating between groups. Intact knees were isolated, processed, and stained with hematoxylin, Fast Green, and Safranin-O for histological grading as described previously (18,30). Modified Mankin OA scoring was conducted by 2 experienced graders under blinded conditions at 4 locations—lateral femur, lateral tibia, medial femur, and medial tibia. Osteophyte severity along the anterior and posterior margins of the medial and lateral tibial plateau was quantified using a 0–3 grading scheme for each region as previously described (31). Epididymal, subcutaneous (femoral-inguinal), and infrapatellar fat pads (EFP, SFP, and IFP, respectively) were harvested following death and stabilized in Zamboni’s Fixative prior to paraffin processing. 6μm sections were collected at 48μm intervals for the EFP and SFP and continuously for the IFP. For adipocyte analyses, two slides per sample were stained with hematoxylin and eosin. Two images per slide were captured using a Nikon E200 microscope and DS-Fi1 digital camera. Adipocytes intersecting a digital grid overlay were manually traced to calculate the average adipocyte area per sample using NIS Elements software (Nikon). For macrophage quantification, three slides per fat pad per animal were prepared for F4/80 staining. Slides underwent antigen retrieval (0.05% Proteinase K), non-specific blocking (5% normal donkey serum in PBS at 0.1% Triton X), and incubation with rat anti-mouse F4/80 monoclonal antibody (1:100, Invitrogen #MF48000) for 1 hour at RT. Slides were washed and stained with Cy™3 secondary antibody (Jackson ImmunoResearch) and Hoechst for visualization and quantitation by fluorescence microscopy using a Nikon Eclipse 80i microscope, X-cite 120Q light source, and DS-Qi1Mc camera. Primary antibody was omitted for negative controls.
Fat pad fibrosis was evaluated by Sirius red staining. Slides were stained in saturated picric acid with 0.1% Sirius Red F3B (VWR), washed in 0.5% acetic acid, and counterstained with 0.5% Harris’s hematoxylin (VWR). Slides were examined under epipolarized UV light with a Nikon E800 microscope equipped with an OMAX 14 MP digital camera and OMAX ToupView acquisition software. Three 10X images per sample were selected for quantification in ImageJ (1.49o) using the colour deconvolution 2 plugin to generate a threshold-based binary image of the epifluorescent signal. Fibrosis was quantified as the area of epifluorescent signal within a central 1400 pixel diameter region of interest free of tissue artifact or vasculature.
RNA and Protein Extraction
Extracted fat pad samples were immediately placed in TRIzol® Reagent (Ambion) on ice and stored at −80°C until homogenization. Although it was not possible to isolate the IFP from the immediate underlying synovium, IFP samples were carefully dissected under a stereomicroscope using McPherson-Vannus scissors and fine forceps to minimize the inclusion of adjacent synovium and other connective joint tissues. To obtain sufficient mRNA, IFPs from both knees were pooled for two animals per analysis sample. EFP and SFP mRNA samples were pooled for the same animal pairs to facilitate comparisons among fat pads. mRNA and protein were isolated following the manufacturer’s protocol, and mRNA was purified using an RNeasy Mini (IFP) and Micro (SFP and EFP) Kit (Qiagen).
Quantitative RT-PCR and Protein Arrays
Gene expression was measured using RT2 Profiler™ PCR Inflammatory Cytokine & Receptors (PAMM-011D) and Extracellular Matrix and Adhesion Molecules (PAMM-013D) Mouse Arrays following manufacturer’s protocol. 200ng mRNA per array was synthesized into cDNA using RT2 First Strand Kit (Qiagen, SABiosciences) following manufacturer’s instructions. Samples were analyzed on a CFX96 thermocycler (Bio-Rad), and gene expression was quantified relative to the geomean of 5 stable reference genes: Gusb, Hprt1, Hsp90ab1, Gapdh, and Actb. Protein samples were diluted in PBS with 1% Triton X-100 and analyzed using the Proteome Profiler™ Mouse Adipokine Array kit (#ARY013, R&D Systems) following manufacturer’s instructions. Membranes were incubated with 250μg protein, and bound antigen was detected by autoradiography using Streptavidin-HRP secondary antibody and ChemiReagent Substrate mix. Densitometry analysis was conducted using a G:BOX imaging system and Gene Tools software (Synegene).
Flow Cytometry
The EFP and IFP tissues were placed in PBS with 5% FBS prior to cell isolation. Stromal vascular fraction cells were obtained by digesting fat at 37°C for 1 hour on an orbital shaker in a buffer containing DMEM/F12 complete medium, 1 mg/mL Type 1A collagenase (Sigma Aldrich), 0.1 mg/mL DNase I, 0.8 mM Zinc Chloride, 1.5% Bovine Serum Albumin, and 25 mM HEPES (Life Technologies). Collagenase activity was neutralized by HBSS medium (#21-022-CV, cellgro Mediatech) and 10% FBS. Mixture was filtered (70μm) and centrifuged at 400g for 10 min at 4°C. Cell filtrate was incubated in 1X red blood cell lysis solution for 1 min at RT followed by DMEM wash and centrifugation. Freshly isolated cells were resuspended in FACS buffer (1X PBS with 5% FBS), counted, and labeled for 30 min on ice with fluorochrome-conjugated monoclonal antibodies (BioLegend) to mouse CD45.2 (104), CD3 (145-2C11), CD19 (6D5), F4/80 (BM8), CD11c (N418), and CD206 (C068C2). Propidium Iodide (PI) was used to determine cell viability (BioLegend). Compensation was done using the BD™ CompBead Anti-Rat and Anti-Hamster Igκ/Negative Control Compensation Particles Set. Experiments were analyzed with a BD LSR II flow cytometer and FlowJo software (Tree Star, Ashland, OR, USA).
Statistical Analyses
Imaging analyses were evaluated in a blinded fashion. Scoring and quantitative analyses were averaged for all images, sections and/or anatomical sites to generate an average value per animal for statistical analyses. Differences in semi-quantitative scores were determined using Mann-Whitney U tests (Prism 6.0f). Gene expression array analyses included a 10% false discovery rate adjustment following the Benjamini-Hochberg procedure as indicated. All other data were analyzed by Student’s t test, one-way ANOVA, or two-way ANOVA followed by Dunnett’s or Holm-Sidak’s multiple comparisons test, respectively (Prism 6.0f). Data are expressed as mean ± SEM unless otherwise stated. n indicates animal numbers per group, and sample sizes are provided in figure legends. P < 0.05 was considered significant.
Results
Time course of high-fat diet-induced knee OA pathogenesis
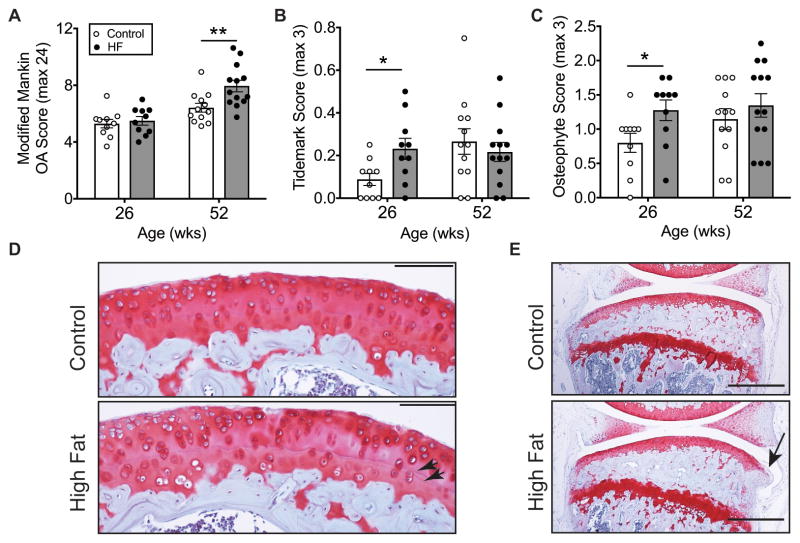
We examined knee OA pathology in mice after 20 and 46 weeks of Control and HF diet feeding. There were no differences in knee OA at 20 weeks based on modified Mankin scoring; whereas, 46 weeks of HF diet increased OA severity (Figure 1A). Between the two time points (i.e., 26 and 52 weeks of age), OA scoring increased 21.2% in Controls and 44.6% in HF diet mice (p=0.0098). Within the modified Mankin sub-component scores, we observed a significant increase in tidemark duplication in HF animals after 20 weeks of diet treatment (Figure 1B, D). HF feeding also accelerated osteophyte formation (Figure 1C, E), indicating the presence of early-stage OA after 20 weeks of HF diet. Therefore, we selected 20 weeks of HF diet treatment to test the hypothesis that obesity induces IFP inflammation prior to the development of overt OA cartilage pathology.
Figure 1. Age-dependent knee OA pathology in high-fat diet-induced obese mice.
Animals were fed either a 10% kcal fat control diet (Control) or a 60% kcal fat high-fat diet (HF) beginning at 6 weeks of age. (A) 46 weeks of a HF diet, but not 20 weeks, increased cartilage OA severity. OA was assessed by the knee cartilage modified Mankin OA score averaged for multiple sections and sites throughout the joint, including the medial and lateral tibia and femur. (B) 20 weeks of a HF diet increased duplication of the tidemark separating the uncalcified and calcified cartilage. After 46 weeks, however, both diet groups were similar. (C) 20 weeks of a HF diet also accelerated the development of tibial osteophytes. However, after 46 weeks, Control and HF diet groups were no longer different. (D) 200X magnification sagittal image of the medial tibia. Arrowheads indicate tidemarks; scale bar = 100μm. (E) 40X magnification sagittal image of the medial compartment of the knee in mice fed a control or HF diet for 20 weeks. Arrow indicates an osteophyte; scale bar = 500μm. Values are mean ± SEM for n=10 per diet (26 wks) and n=12 Control and n=13 HF (52 wks); *p<0.05, **p<0.01.
Distinct infrapatellar adipocyte response to diet-induced obesity and fat pad hypertrophy
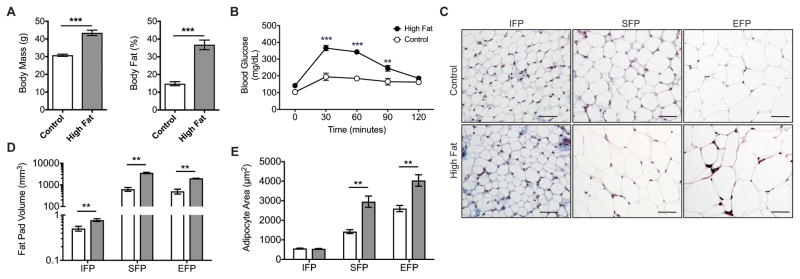
20 weeks of HF feeding increased body mass by 41% (p<0.0001, Figure 2A), and total body fat increased from 14.8% to 36.7% of body mass (p<0.0001, Figure 2A). Fasting blood glucose was not elevated at this time point, but blood glucose remained elevated in HF mice 30–90 minutes following a glucose challenge (Figure 2B). We next compared the effect of HF feeding on the sizes of the EFP, SFP, and IFP depots and their respective adipocytes. In Control diet animals, IFP adipocytes were significantly smaller than adipocytes from the SFP and EFP (Figure 1C). Following 20 weeks of HF feeding, the infrapatellar, subcutaneous, and peritoneal (i.e., epididymal plus mesenteric) adipose tissue volumes increased by 1.54-fold (p=0.005), 5.63-fold (p=0.0004), and 3.98-fold (p=0.0003), respectively (Figure 2D). When adipocyte cross-sectional areas were examined, IFP adipocytes did not become hypertrophic like the SFP and EFP adipocytes (Figure 2C, E). Thus, although the IFP volume increased in response to HF feeding, adipocyte hypertrophy did not contribute to the increase in IFP size.
Figure 2. High-fat diet-induced changes in systemic metabolism and location-specific changes in fat pad and adipocyte size.
Mice were fed a control diet (open bar) or HF diet (closed bar) beginning at 6 weeks of age for 20 weeks. (A) HF diet induced a substantial increase in body mass and body fat (n=8 Control; n=10 HF). (B) Although fasting blood glucose levels were not higher in HF fed animals, they were significantly elevated at 30, 60, and 90 minutes after a glucose challenge, indicating impaired glucose tolerance (n=4 Control, n=5 HF). (C) 200X magnification images of trichrome-stained infrapatellar, subcutaneous, and epididymal fat pad sections (IFP, SFP, and EFP, respectively) by diet group. IFP adipocytes are substantially smaller than SFP or EFP adipocytes for either diet. Bar = 50μm. (D) Water-suppressed magnetic resonance imaging-derived fat pad volumes. Image data were acquired on a 7 T, 30 cm horizontal bore USR Bruker system. A validated automated segmenting procedure (28) and manual segmenting were used for analyses (n=9 per diet for IFP; n=3 per diet for SFP and EFP). (E) HF diet increased adipocyte area in SFP and EFP but not IFP, indicating that adipocyte hypertrophy did not contribute to the increase in IFP size (n=5 per diet per fat pad). **p<0.01, ***p<0.001.
Comparison of high-fat diet-induced changes in infrapatellar versus epididymal fat pad immune cell populations
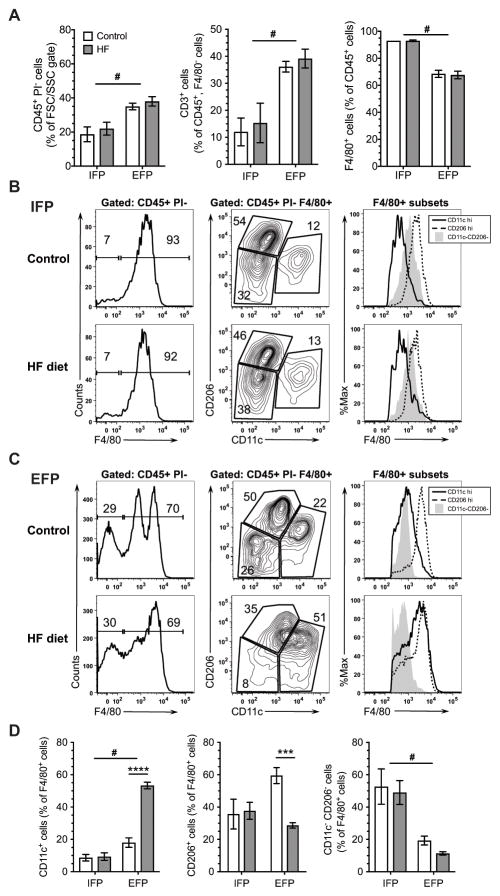
We next evaluated the effect of HF feeding on the immune cell populations in the stromal vascular fraction of the IFP and EFP (Figure 3, Supplemental Figure 1). The EFP contained a greater percentage of CD45+ cells compared to the IFP independent of diet (p<0.0001; Figure 3A). There were also significant differences in T-cell (CD3+) and macrophage (F4/80+) cell populations between EFP and IFP depots (Figure 3A). Notably, the IFP contained a lower percentage of T-cells (p<0.0001) and a higher percentage of macrophages (p<0.0001) compared to the EFP, and these differences were independent of HF diet (Figure 3A). IFP B-cell populations were negligible for either diet.
Figure 3. Effect of a high-fat diet on immune cell populations in the infrapatellar and epididymal fat pads.
Stromal vascular fraction cells were isolated from infrapatellar (IFP) and epididymal (EFP) fat pads after 20 weeks of HF diet. IFP samples were pooled for six animals per experiment, and EFP samples were collected from two of the same six animals. Experiments were repeated three times (N=3 IFP, N=6 EFP). (A) Effect of HF diet on adipose tissue leukocytes (PI-, CD45+) (left), T cells (CD45+, CD3+, F4/80-) (middle), and macrophages (CD45+, F4/80+) (right). (B) Effect of a HF diet on the distribution of F4/80+ adipose tissue leukocytes (PI-, CD45+) in the IFP. Macrophages were further identified within the population of F4/80+ cells based on CD11c and CD206 expression and F4/80 staining intensity among CD11chi, CD206hi, and CD11c−CD206− populations. (C) Effect of a HF diet on the distribution of F4/80+ adipose tissue leukocytes (PI-, CD45+) in the EFP. Unlike the IFP, HF feeding increased the number of CDllc+ cells and shifted the expression of CD11c+ cells from F4/80int to F4/80hi, indicating M1 polarization. (E) Effect of a HF diet on percent of PI-CD45+F4/80+ cells expressing M2 marker CD206 or M1 marker CD11c in the IFP or EFP. #p<0.05 between fat pads; ***p<0.001, ****p<0.0001 between diets.
We then evaluated the effect of a HF diet on CD206+ and CD11c+ adipose tissue macrophages as a function of F4/80 staining (25). In the IFP, the intensity of F4/80 staining and relative proportion of CD206+ and CD11c+ cells did not change with a HF diet (Figure 3B, D). The CD206hi cells were also F4/80hi, as expected for macrophages; whereas, the CD11chi cells had less F4/80 expression, consistent with their potential identity as dendritic cells rather than M1 macrophages (32). The F4/80int CD11c−CD206− cells in the IFP showed a SSC/FSC pattern similar to CD206+ and CD11c+ populations in both the IFP and EFP (data not shown), suggesting that these cells are undifferentiated monocytes rather than neutrophils or eosinophils as recently reported for the EFP (32). In contrast to the IFP, a HF diet significantly altered the intensity of F4/80 staining and relative proportion of CD206+ and CD11c+ cells in the EFP (Figure 3C, D). The proportion of CD11c+ cells and intensity of F4/80 staining in CD11chi cells increased with a HF diet, consistent with an increase in M1-like macrophages. Furthermore, macrophage crown-like structures, another characteristic of adipose tissue inflammation, were increased with a HF diet in the EFP (p=0.019) but were not significantly altered in the SFP, and none were observed in the IFP (data not shown).
Infrapatellar fat pad inflammation is not affected by high-fat diet-induced obesity
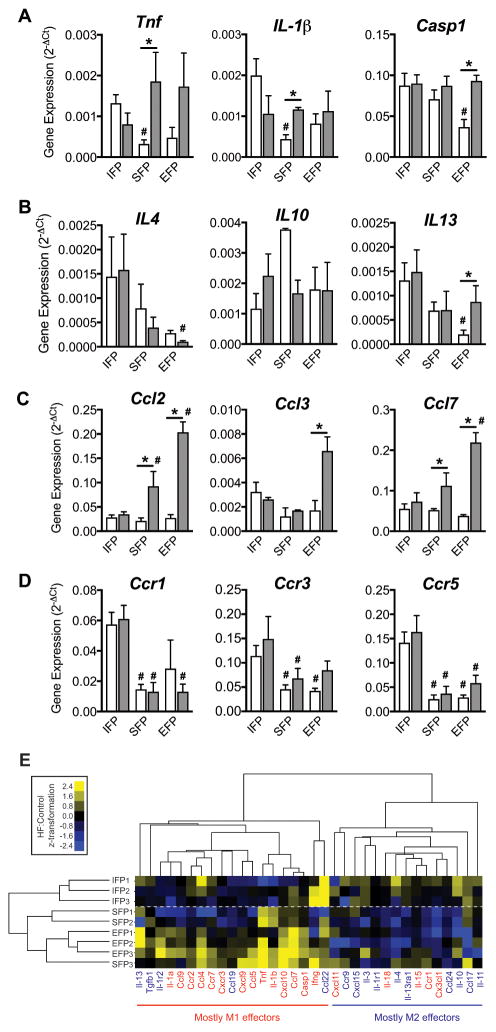
To further characterize fat pad and HF diet-specific effects on adipose tissue inflammation, we compared the expression of pro- and anti-inflammatory genes and chemokine ligand and receptor genes using a targeted gene expression array. In Control diet animals, tumor necrosis factor (Tnf) and Interleukin-1β (Il-1b) were more highly expressed in the IFP compared to the SFP (Figure 4A). The pro-inflammatory mediator, Caspase-1 (Casp1), was also more highly expressed in the IFP compared to the EFP. A HF diet increased the expression of Tnf and Il-1b in the SFP and Casp1 in the EFP, but none were altered in the IFP (Figure 4A). Among key anti-inflammatory genes, Il-4 and Il-13 were more highly expressed in the IFP compared to the EFP, and only Il-13 expression in the EFP increased following a HF diet (Figure 4B). There were no differences in the expression of the macrophage chemokine ligands Ccl2, Ccl3, and Ccl7 in Control diet animals (Figure 4C). However, following a HF diet, Ccl2, Ccl3, and Ccl7 expression increased in the EFP and Ccl2 and Ccl7 expression increased in the SFP, but none occurred in the IFP (Figure 4C). Expression of the chemokine receptors Ccr1, Ccr3, and Ccr5, however, were nearly all elevated in the IFP relative to the other fat pads independent of diet (Figure 4D). Thus, under basal non-obese conditions, the expression of pro-inflammatory genes and chemokine receptor genes was elevated in the IFP compared to subcutaneous and epididymal fat pads, but the IFP was insensitive to HF diet-induced changes.
Figure 4. Effect of a high-fat diet on fat pad specific pro- and anti-inflammatory gene expression.
Gene expression was measured by RT2 Profiler™ PCR Inflammatory Cytokine & Receptors targeted array following 20 weeks of a control or HF diet (open versus closed bar, respectively). Fat pads from both knees were pooled from two animals per gene array. SFP and EFP samples were pooled in parallel from the same animals. Experiments were repeated three times values log-transformed for statistical analyses. (A) Pro-inflammatory, (B) Anti-inflammatory, (C) Chemokine ligand, and (D) Chemokine receptor. Gene expression varied by fat pad type (#p<0.05 versus IFP control) and in response to a high fat diet (*p<0.05 HF versus control within the same fat pad type). (E) HF diet-induced fold-change in gene expression, calculated by the ΔΔCt method, were used to perform an unsupervised cluster analysis of both fat pad type and macrophage polarization effector status. Fat pad samples clustered by anatomical location, and macrophage polarization effector genes clustered primarily by M1 versus M2 effector bias. M1 effector genes tended to be upregulated and M2 effector genes downregulated in EFP and SFP samples.
We then performed an unsupervised cluster analysis of both fat pad type and macrophage polarization effector status on the HF diet-induced fold-change in gene expression (Figure 4E). Although M1 and M2 subtypes can be ambiguous and overlapping, we selected a panel of genes previously associated with these designations to assess general patterns of inflammatory gene expression among the fat pads (33). Depot-specific fat pad samples clustered together based on their HF diet-induced changes in inflammatory gene expression. Notably, the SFP expression patterns more closely followed the EFP than the IFP (Figure 4E). The genes clustered together into two major groups and were significantly associated with the M1 versus M2 gene designations (left and right clusters, respectively, p=0.036; Figure 4A). Consistent with the flow cytometry data, the EFP samples, and to a lesser extent SFP samples, were characterized by an upregulation of M1 effector genes and downregulation of M2 effector genes. The IFP samples, however, did not show a discernable pattern in M1 versus M2 HF diet-induced gene expression. An analysis of adipokine and cytokine protein levels in the IFP also showed minimal effects of a HF diet, with a minor increase in IL-10 (1.19 ± 0.05 HF-induced fold change, P=0.03) and a reduction in leptin (0.61 ± 0.10, P=0.03) (Supplemental Figure 2).
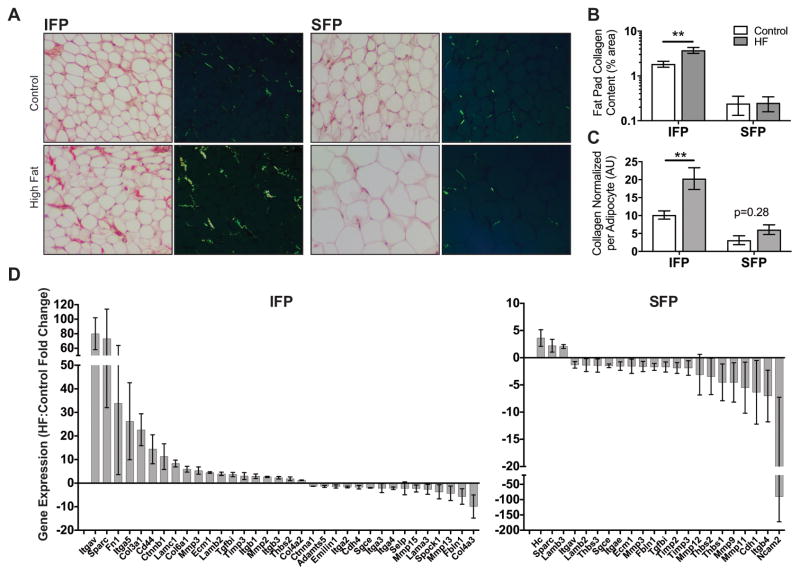
High-fat diet-induced obesity causes a robust increase in infrapatellar fat pad fibrosis
To better understand the factors that differentiate the remodeling response of infrapatellar and subcutaneous fat pads to HF diet-induced obesity, we examined changes in adipose tissue fibrosis and the expression of genetic regulators of extracellular matrix homeostasis. Quantification of Sirius Red staining showed that adipose tissue collagen content was greater in the IFP compared to the SFP in mice fed a Control diet (p=0.003; Figure 5A,B). This difference was magnified following a HF diet where adipose tissue collagen content increased 2.0-fold from 1.84% to 3.74% in the IFP (p=0.001) but did not change in the SFP (0.24% versus 0.25%) (Figure 5B). This same pattern remained even when collagen content was normalized to adipocyte size to account for baseline differences in adipocyte size and HF diet-induced SFP adipocyte hypertrophy (Figure 5C).
Figure 5. HF diet-induced infrapatellar and subcutaneous adipose tissue fibrosis and extracellular matrix gene expression.
(A) Brightfield and epipolarized UV light images of Sirius Red-stained IFP and SFP paraffin sections from mice fed a control or HF diet for 20 weeks. Yellow-green pixels from epipolarized images were quantified to determine adipose tissue collagen content. (B) HF diet significantly increased IFP collagen content but did not alter SFP collagen content. (C) Fat pad collagen content was normalized to the average adipocyte area per fat pad to adjust for anatomical and HF diet-associated differences in adipocyte size. Even with normalization, IFP but not SFP collagen content was elevated by a HF diet. n = 5 per diet. (D) Gene expression was measured by RT2 Profiler™ PCR Extracellular Matrix and Adhesion Molecules targeted array following 20 weeks of a HF diet. Significant differentially expressed genes due to a HF diet are plotted from highest to lowest for the IFP and SFP (p<0.05 based on 95% confidence interval (CI) of fold-change value). Values are mean ± 95% CI; values are also reported in Table S2. Sample sizes same as in Figure 4. **p<0.01.
This pro-fibrotic response in the IFP was accompanied by a greater number of differentially expressed genes involved in extracellular matrix remodeling (Figure 5D; Supplemental Table 2). 34 out of 84 genes were differentially expressed in the IFP in response to a HF diet (19 upregulated and 15 downregulated). The upregulated genes included several that encode extracellular matrix proteins, such as collagens 3, 4, and 6, as well as fibronectin and fibronectin receptors Itga5 and Itgb1. In contrast, 22 genes were differentially expressed in the SFP, with the majority being downregulated (3 upregulated versus 19 downregulated). Only one gene was upregulated in both fat pads in response to a HF diet, secreted protein acidic and rich in cysteine (Sparc), which is a profibrotic glycoprotein that inhibits adipogenesis (34). Three genes were downregulated in the IFP and SFP: fibulin 1 (Fbln1), integrin alpha 4 (Itga4), and sarcoglycan epsilon (Sgce). Several genes were upregulated in the IFP in response to a HF diet that were downregulated in the SFP, including cell adhesion and extracellular matrix binding proteins (Ecm1, Itgav, Lamb2, Tgfbi, and Thbs2) and regulators of matrix proteostasis (Mmp3 and Timp3).
Given these differences in extracellular matrix regulation, we compared differences in gene expression in the IFP relative to the SFP of Control diet mice to gain insight into basal transcriptional differences. We included both inflammatory and extracellular matrix arrays because inflammation can contribute to adipose tissue fibrosis. Out of 168 genes, 10 were upregulated and 16 were downregulated in the IFP (Table 1). A smaller subset retained significance after multiple comparisons correction (4 and 8, respectively). Of these, most were cytokine receptors and chemokines associated with reduced T-cell chemotaxis and increased innate immune responses. These findings suggest that basal differences in adipose tissue inflammatory mediators, rather than the extracellular matrix per se, contribute to the robust pro-fibrotic response in the IFP of obese mice.
Table 1.
IFP Differentially Expressed Genes
| Fold-Regulation (IFP:SFP)
|
||||
|---|---|---|---|---|
| Gene | Mean | 95% CI | p-value | |
|
|
||||
| Up-regulated in the IFP | Ccl17 | 17.4 | [9.54, 25.3] | 0.0122 |
| Ccl19 | 10.9 | [9.64, 12.1] | 0.0008* | |
| Ccr5 | 6.79 | [2.15, 11.4] | 0.0330 | |
| Tnf | 4.54 | [1.46, 7.62] | 0.0385 | |
| Ccr1 | 4.17 | [1.68, 6.67] | 0.0319 | |
| Il13ra1 | 3.45 | [2.82, 4.08] | 0.0035* | |
| Itgam | 3.36 | [3.31, 3.42] | <0.0001* | |
| Thbs3 | 2.06 | [1.28, 2.83] | 0.0279 | |
| Il1r1 | 1.48 | [1.33, 1.62] | 0.0049* | |
| Scye1 | 1.19 | [1.04, 1.34] | 0.0328 | |
|
|
||||
| Down-regulated in the IFP | Adamts2 | −1.26 | [−1.30, −1.23] | <0.0001* |
| Cxcl10 | −1.26 | [−1.87, −0.656] | 0.0039* | |
| Ccl25 | −1.48 | [−2.72, 0.241] | 0.0132 | |
| Cxcl9 | −1.66 | [−1.98, −1.33] | 0.0008* | |
| Mif | −1.66 | [−3.00, −0.335] | 0.0132 | |
| Cxcr3 | −1.72 | [−2.07, −1.38] | 0.0009* | |
| Cdh1 | −1.80 | [−2.95, −0.658] | 0.0089 | |
| Ccl6 | −1.89 | [−3.84, 0.0579] | 0.0237 | |
| Ccl5 | −1.91 | [−2.57, −1.25] | 0.0028* | |
| Col4a2 | −1.94 | [−2.97, −0.914] | 0.0065* | |
| Ccl11 | −2.19 | [−3.05, −1.32] | 0.0040* | |
| Ccr10 | −2.25 | [−3.93, −0.570] | 0.0141 | |
| Thbs1 | −2.41 | [−5.61, 0.787] | 0.0443 | |
| Col4a1 | −2.77 | [−6.28, 0.734] | 0.0436 | |
| Il2rg | −2.85 | [−1.36, −0.811] | 0.0009* | |
| Mmp9 | −10.4 | [−19.1, −1.74] | 0.0298 | |
Values expressed relative to the SFP. Results for control diet only.
Significance retained after controlling for a 10% false discovery rate (exploratory analysis) following the Benjamini-Hochberg procedure.
Discussion
The IFP is considered a paracrine mediator of cartilage catabolism due to the niche it provides for immune cells and its pro-inflammatory phenotype in OA joints (19–21,24). We hypothesized that obesity stimulates IFP inflammation by increasing macrophage infiltration and M1 polarization prior to the development of cartilage degeneration. Although we observed elevated basal IFP inflammation compared to other fat depots, diet-induced obesity did not further increase the number of immune cells in the IFP or induce a pro-inflammatory shift in cytokine expression or macrophage polarization after 20 weeks of HF feeding. Given that this duration of feeding induced early-stage OA changes, such as osteophyte formation and cartilage tidemark duplication, the findings do not support a role for IFP inflammation as a central initiating factor of obesity-induced knee OA. We did not, however, evaluate IFP inflammation after 46 weeks of HF feeding when cartilage degeneration was significantly elevated, raising the possibility that IFP inflammation contributes to OA progression.
Unlike subcutaneous or epididymal adipocytes, IFP adipocytes did not become hypertrophic in response to HF feeding despite an overall increase in fat pad size. This unique response to HF feeding suggests that IFP size increases by hyperplasia (i.e., adipogenesis), which has previously been reported to occur transiently in the epididymal fat of male C57BL/6 mice following high-fat feeding (35). In addition, high-fat feeding also increased IFP fibrosis. Adipose tissue fibrosis is usually associated with adipocyte hypertrophy and obesity-induced metabolic dysfunction (25,36). In this context, M1 adipose tissue macrophages appear to generate pro-inflammatory signals that shift preadipocytes away from an adipogenic lineage and towards a myofibroblast phenotype. The localization of M1 macrophages and dense extracellular matrix deposits in crown-like structures that surround necrotic adipocytes reinforces the pathologic attributes of adipose tissue fibrosis. Therefore, the association of adipose tissue fibrosis with negligible M1 polarization and adipocyte hypertrophy in the IFP of obese mice raises questions about how it occurs and how it impacts joint health. One possibility is that the temporal dynamics of inflammatory activation and resolution are accelerated in the IFP, which would require testing at earlier time points of feeding. Alternatively, IFP fibrosis may occur via physiologic signaling mediators, such as mechanical stress, rather than pathologic pro-inflammatory M1 polarization.
Mechanical signals regulate stem cell fate, adipogenesis, and adipocyte hypertrophy, and the extracellular matrix mediates how cells sense these changes in the environment (37–39). In pre-adipocyte culture models, static tensile strain stimulates adipocyte differentiation; whereas, compressive strain or cyclic tensile strain suppress adipogenesis (reviewed in (40)). Although few studies have examined IFP biomechanical stresses (41), the load bearing aspects of this intra-articular fat pad likely underlie the basal differences in adipocyte size and response to diet-induced obesity. In a previous study, we showed that diet-induced obese mice maintain similar levels of spontaneous locomotor activity despite increased weight gain, consistent with increased joint stresses (18).
Adipocyte size is a key factor regulating adipokine secretion, and our results suggest that obesity-induced IFP fibrosis may limit metabolic inflammation by inhibiting adipocyte hypertrophy. Previous work has shown that subcutaneous adipocytes in the largest size quartile produce more leptin, IL-6, IL-8, CCL2, and G-CSF compared to the smallest quartile, even when normalized to adipocyte surface area (42). We observed few changes in adipokine protein levels in IFPs of HF versus Control mice. One notable exception was leptin, which surprisingly was reduced in HF fed mice. We previously found that IL-1β decreases leptin production in the IFP of rats (43), although in the current study IL-1β gene expression was not increased with a HF diet. Rather, the increased extracellular matrix content may limit leptin production and adipocyte hypertrophy (25,39).
Many suppressors of adipocyte hypertrophy, including collagens (III, IV, and VI) and fibronectin, were transcriptionally upregulated in the IFP following HF feeding. In particular, the matricellular protein SPARC (secreted protein, acidic, and rich in cysteine; also known as osteonectin) negatively regulates adipose tissue expansion by both stimulating fibronectin and suppressing adipogenic transcription factors and genes, including leptin (34). HF feeding induced SPARC expression significantly more in the IFP (73-fold increase) versus the SFP (2.2-fold increase), suggesting that SPARC upregulation is an important mediator of IFP fibrosis with obesity. Consistent with this, we observed that a HF diet increased IFP expression of α5 and β1 integrins, which form a receptor for fibronectin as well as SPARC-mimicking peptides (34). In addition, HF feeding likely stimulated IFP remodeling as shown by differential expression of several matrix metalloproteinases (Mmp2, Mmp3, Mmp13, and Mmp15) and tissue inhibitor of matrix metalloproteinase 3 (Timp3). Furthermore, macrophages are known to regulate extracellular matrix remodeling in adipose tissue (25). We found that the IFP was enriched for M2 macrophages and IL-13 expression, which are associated with fibrosis in other tissues (36).
The clinical significance of the IFP in OA development and progression is an active area of investigation (44,45). Some MRI-based cross-sectional studies have shown positive associations between IFP volume, joint inflammation, and joint pain (46,47); whereas, cross-sectional and longitudinal analyses of other cohorts indicate a protective effect of IFP size on cartilage damage and pain, particularly in women (23,48,49). Age and OA progression also mediate size changes in the IFP (22,43). Our findings show that obesity can increase IFP size in a genetically and environmentally controlled animal model, but increased IFP size is not necessarily associated with increased inflammation. A more detailed understanding of the inflammatory and secretory status of the adipocytes themselves is likely needed to predict the clinical impact of the IFP on OA pathophysiology. Our results parallel recent studies reporting a pro-fibrotic effect of the IFP on synovial fibroblasts (50,51), suggesting that adipocytes may play a central role in modulating joint tissue fibrosis.
Our findings contradict a previous study reporting IFP adipocyte hypertrophy and inflammation in male C57BL/6J mice following HF feeding (52). However, this study utilized a different HF diet composed of Safflower oil and beef tallow versus soybean oil and lard and examined earlier timepoints. The prior study also focused its histological analysis on a single region in the medial compartment, excluding the larger IFP area throughout the inter-condylar notch, which we included in our study. Thus, the prior findings may indicate regional variation in adipocyte responses to obesity. It is also possible that including adjacent synovial tissue could produce an inflammatory phenotype as a recent study reported HF diet-induced increases in synovial macrophages and TNF production (16). Although our study included synovium immediately deep to the IFP, it did not include adjacent peripheral synovium, and we did not observe an increase in TNF expression following HF feeding. This suggests that the synovium, rather than the IFP, is the major source of TNF in the mouse knee. This differs from human data, which show a positive correlation between IFP-derived TNF and body mass index (20). An additional potential limitation of our study is that we only focused on male mice as there is increasing evidence that the specific metabolic links between obesity and OA are sex dependent (53).
In conclusion, the IFP does not recapitulate the classic M1-macrophage mediated inflammation that occurs in abdominal adipose tissue with obesity. This apparent protection from obesity-induced inflammation corresponds with the absence of adipocyte hypertrophy and an increase in adipose tissue fibrosis. These findings suggest that intra-articular adipocytes are subject to distinct spatial-temporal metabolic regulation among fat pads, possibly due to the structural constraints and cyclic biomechanical stresses associated with joints. Additional studies focused on earlier and later time points and the effects of altered joint loading conditions may help clarify the how the IFP contributes to joint health and OA pathogenesis.
Supplementary Material
Acknowledgments
We thank Melinda West, Erin Hutchison, Debra Saunders, and Drs. Yao Fu, Philippe Garteiser, Sabrina Doblas, and Bart Frank for technical assistance and Drs. Mary Beth Humphrey, Yao Fu, and Elise Donovan for advice and intellectual input.
Funding: Supported by the NIH (grants P20-RR-018758 and P20-GM-103441 to Drs. Griffin, Towner, and Lupu, and grant R03-AR-066828 to Dr. Griffin) and the Arthritis Foundation (Arthritis Investigator Award to Dr. Griffin). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Arthritis Foundation.
Footnotes
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Griffin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. TMG.
Acquisition of data. EPB, JH, WPC, SK, RSM, CK, and TMG.
Analysis and interpretation of data. EPB, WPC, SK, RAT, FL, CK, and TMG.
References
- 1.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Annals of the Rheumatic Diseases. 2010;69:761–765. doi: 10.1136/ard.2008.106930. [DOI] [PubMed] [Google Scholar]
- 3.Issa RI, Griffin TM. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiology of Aging & Age-related Diseases. 2012;2 doi: 10.3402/pba.v2i0.17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep. 2013;15:375. doi: 10.1007/s11926-013-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Attur M, Krasnokutsky S, Statnikov A, Samuels J, Li Z, Friese O, et al. Low-grade inflammation in symptomatic knee osteoarthritis: prognostic value of inflammatory plasma lipids and peripheral blood leukocyte biomarkers. Arthritis Rheumatol. 2015;67:2905–2915. doi: 10.1002/art.39279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17:1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rai MF, Patra D, Sandell LJ, Brophy RH. Transcriptome Analysis of Injured Human Meniscus Reveals a Distinct Phenotype of Meniscus Degeneration With Aging. Arthritis Rheum. 2013;65:2090–2101. doi: 10.1002/art.37984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritter SY, Subbaiah R, Bebek G, Crish J, Scanzello CR, Krastins B, et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: comparison with transcriptome analyses of joint tissues. Arthritis Rheum. 2013;65:981–992. doi: 10.1002/art.37823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair A, Gan J, Bush-Joseph C, Verma N, Tetreault MW, Saha K, et al. Synovial chemokine expression and relationship with knee symptoms in patients with meniscal tears. Osteoarthritis Cartilage. 2015;23:1158–1164. doi: 10.1016/j.joca.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courties A, GUALILLO O, Berenbaum F, Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015;23:1955–1965. doi: 10.1016/j.joca.2015.05.016. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=26033164&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 12.Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford) 2015;54:588–600. doi: 10.1093/rheumatology/keu464. [DOI] [PubMed] [Google Scholar]
- 13.Rai MF, Sandell LJ. Inflammatory mediators: tracing links between obesity and osteoarthritis. Crit Rev Eukaryot Gene Expr. 2011;21:131–142. doi: 10.1615/critreveukargeneexpr.v21.i2.30. [DOI] [PubMed] [Google Scholar]
- 14.Daghestani HN, Pieper CF, Kraus VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67:956–965. doi: 10.1002/art.39006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraus VB, McDaniel G, Huebner JL, Stabler TV, Pieper CF, Shipes SW, et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage. 2016 doi: 10.1016/j.joca.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamada D, Maynard R, Schott E, Drinkwater CJ, Ketz JP, Kates SL, et al. Suppressive Effects of Insulin on Tumor Necrosis Factor–Dependent Early Osteoarthritic Changes Associated With Obesity and Type 2 Diabetes Mellitus. Arthritis Rheumatol. 2016;68:1392–1402. doi: 10.1002/art.39561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2014;11:35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin TM, Fermor B, Huebner JL, Kraus VB, Rodriguiz RM, Wetsel WC, et al. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther. 2010;12:R130. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Presle N, Pottie P, Dumond H, Guillaume C, Lapicque F, Pallu S, et al. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthritis Cartilage. 2006;14:690–695. doi: 10.1016/j.joca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H, et al. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Annals of the Rheumatic Diseases. 2011;70:851–857. doi: 10.1136/ard.2010.140046. [DOI] [PubMed] [Google Scholar]
- 21.Distel E, Cadoudal T, Durant S, Poignard A, Chevalier X, Benelli C. The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor. Arthritis Rheum. 2009;60:3374–3377. doi: 10.1002/art.24881. [DOI] [PubMed] [Google Scholar]
- 22.Chuckpaiwong B, Charles HC, Kraus VB, Guilak F, Nunley JA. Age-associated increases in the size of the infrapatellar fat pad in knee osteoarthritis as measured by 3T MRI. J Orthop Res. 2010;28:1149–1154. doi: 10.1002/jor.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan F, Han W, Wang X, Liu Z, Jin X, Antony B, et al. A longitudinal study of the association between infrapatellar fat pad maximal area and changes in knee symptoms and structure in older adults. Ann Rheum Dis. 2015;74:1818–1824. doi: 10.1136/annrheumdis-2013-205108. [DOI] [PubMed] [Google Scholar]
- 24.Ioan-Facsinay A, Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther. 2013;15:225. doi: 10.1186/ar4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Santibanez G, Nien-Kai Lumeng C. Macrophages and the Regulation of Adipose Tissue Remodeling. Annu Rev Nutr. 2014;34:57–76. doi: 10.1146/annurev-nutr-071812-161113. [DOI] [PubMed] [Google Scholar]
- 26.Bastiaansen-Jenniskens YM, Clockaerts S, Feijt C, Zuurmond A-M, Stojanovic-Susulic V, Bridts C, et al. Infrapatellar fat pad of patients with end-stage osteoarthritis inhibits catabolic mediators in cartilage. Ann Rheum Dis. 2012;71:288–294. doi: 10.1136/ard.2011.153858. [DOI] [PubMed] [Google Scholar]
- 27.Johnston SL, Peacock WL, Bell LM, Lonchampt M, Speakman JR. PIXImus DXA with different software needs individual calibration to accurately predict fat mass. Obes Res. 2005;13:1558–1565. doi: 10.1038/oby.2005.191. [DOI] [PubMed] [Google Scholar]
- 28.Garteiser P, Doblas S, Towner RA, Griffin TM. Calibration of a semi-automated segmenting method for quantification of adipose tissue compartments from magnetic resonance images of mice. Metab Clin Exp. 2013;62:1686–1695. doi: 10.1016/j.metabol.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 30.Cai A, Hutchison E, Hudson J, Kawashima Y, Komori N, Singh A, et al. Metabolic enrichment of omega-3 polyunsaturated fatty acids does not reduce the onset of idiopathic knee osteoarthritis in mice. Osteoarthritis Cartilage. 2014;22:1301–1309. doi: 10.1016/j.joca.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraus VB, Huebner JL, DeGroot J, Bendele A. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthritis Cartilage. 2010;18:S35–S52. doi: 10.1016/j.joca.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, et al. Adipose Tissue Dendritic Cells Are Independent Contributors to Obesity-Induced Inflammation and Insulin Resistance. J Immunol. 2016;197:3650–3661. doi: 10.4049/jimmunol.1600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie J, Sage EH. SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J Biol Chem. 2009;284:1279–1290. doi: 10.1074/jbc.M808285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 2015;17:376–385. doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pessin JE, Kwon H. How does high-fat diet induce adipose tissue fibrosis? J Investig Med. 2012;60:1147–1150. doi: 10.231/JIM.0b013e318271fdb9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chun T-H. Peri-adipocyte ECM remodeling in obesity and adipose tissue fibrosis. Adipocyte. 2012;1:89–95. doi: 10.4161/adip.19752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pope BD, Warren CR, Parker KK, Cowan CA. Microenvironmental Control of Adipocyte Fate and Function. Trends in Cell Biology. 2016;26:745–755. doi: 10.1016/j.tcb.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shoham N, Gefen A. Mechanotransduction in adipocytes. J Biomech. 2012;45:1–8. doi: 10.1016/j.jbiomech.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 41.Bohnsack M, Hurschler C, Demirtas T, Rühmann O, Stukenborg-Colsman C, Wirth CJ. Infrapatellar fat pad pressure and volume changes of the anterior compartment during knee motion: possible clinical consequences to the anterior knee pain syndrome. Knee Surg Sports Traumatol Arthrosc. 2005;13:135–141. doi: 10.1007/s00167-004-0561-1. [DOI] [PubMed] [Google Scholar]
- 42.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between Adipocyte Size and Adipokine Expression and Secretion. Journal of Clinical Endocrinology & Metabolism. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 43.Fu Y, Huebner JL, Kraus VB, Griffin TM. Effect of Aging on Adipose Tissue Inflammation in the Knee Joints of F344BN Rats. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2016;71:1131–1140. doi: 10.1093/gerona/glv151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eymard F, Chevalier X. Inflammation of the infrapatellar fat pad. Joint Bone Spine. 2016;83:389–393. doi: 10.1016/j.jbspin.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 45.Roemer FW, Jarraya M, Felson DT, Hayashi D, Crema MD, Loeuille D, et al. Magnetic resonance imaging of Hoffa’s fat pad and relevance for osteoarthritis research: a narrative review. Osteoarthritis Cartilage. 2016;24:383–397. doi: 10.1016/j.joca.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Ballegaard C, Riis RGC, Bliddal H, Christensen R, Henriksen M, Bartels EM, et al. Knee pain and inflammation in the infrapatellar fat pad estimated by conventional and dynamic contrast-enhanced magnetic resonance imaging in obese patients with osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage. 2014;22:933–940. doi: 10.1016/j.joca.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 47.Cowan SM, Hart HF, Warden SJ, Crossley KM. Infrapatellar fat pad volume is greater in individuals with patellofemoral joint osteoarthritis and associated with pain. Rheumatol Int. 2015;35:1439–1442. doi: 10.1007/s00296-015-3250-0. [DOI] [PubMed] [Google Scholar]
- 48.Han W, Cai S, Liu Z, Jin X, Wang X, Antony B, et al. Infrapatellar fat pad in the knee: is local fat good or bad for knee osteoarthritis? Arthritis Res Ther. 2014;16:R145–8. doi: 10.1186/ar4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai J, Xu J, Wang K, Zheng S, He F, Huan S, et al. Association Between Infrapatellar Fat Pad Volume and Knee Structural Changes in Patients with Knee Osteoarthritis. J Rheumatol. 2015;42:1878–1884. doi: 10.3899/jrheum.150175. [DOI] [PubMed] [Google Scholar]
- 50.Eymard F, Pigenet A, Citadelle D, Flouzat-Lachaniette C-H, Poignard A, Benelli C, et al. Induction of an inflammatory and prodegradative phenotype in autologous fibroblast-like synoviocytes by the infrapatellar fat pad from patients with knee osteoarthritis. Arthritis Rheumatol. 2014;66:2165–2174. doi: 10.1002/art.38657. [DOI] [PubMed] [Google Scholar]
- 51.Bastiaansen-Jenniskens YM, Wei W, Feijt C, Waarsing JH, Verhaar JAN, Zuurmond A-M, et al. Stimulation of fibrotic processes by the infrapatellar fat pad in cultured synoviocytes from patients with osteoarthritis: a possible role for prostaglandin f2α. Arthritis Rheum. 2013;65:2070–2080. doi: 10.1002/art.37996. [DOI] [PubMed] [Google Scholar]
- 52.Iwata M, Ochi H, Hara Y, Tagawa M, Koga D, Okawa A, et al. Initial Responses of Articular Tissues in a Murine High-Fat Diet-Induced Osteoarthritis Model: Pivotal Role of the IPFP as a Cytokine Fountain. PLoS ONE. 2013;8:e60706. doi: 10.1371/journal.pone.0060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.June RK, Liu-Bryan R, Long F, Griffin TM. Emerging role of metabolic signaling in synovial joint remodeling and osteoarthritis. J Orthop Res. 2016 doi: 10.1002/jor.23420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.