Abstract
Virus-specific memory B cells (Bmem) play a crucial role in protecting against variant viruses. The ability to recognize these variant viruses, defined as antibody breadth, is achieved in Bmem populations by two very different pathways, germline-encoded cross-reactivity and affinity-driven, somatic evolution in germinal centers (GCs) for conserved viral epitopes. The latter class of broadly-reactive Bmem cells are not cross-reactive per se, but bind epitopes crucial for viral fitness. Although these conserved epitopes are often weakly immunogenic, the GC reaction is surprisingly permissive for the continued survival/proliferation of B cells that bind with low affinity or react to cryptic epitopes, increasing their chance of memory recruitment. In this review, we discuss the adaptive strategies of B-cell memory to viral antigenic variations.
Introduction
Memory B (Bmem) cells have, for some time, been considered little more than back-ups for long-lived plasma cells that continuously secrete antibody to provide immediate humoral protection to homologous re-infection [1]. Bmem cells, however, express BCR repertoires that are more broadly-reactive to viral variants than do populations of long-lived plasma cells, and thereby potentiate broader protection than that afforded by circulating antibody [2–4]. Germinal centers (GCs) provide a microenvironment for an affinity-driven selection of memory precursors based on competition for antigen and T-cell help [5], which acts, on average, to increase the affinity and specificity of “winner” B-cell clones but also narrows clonal diversity by removing “loser” clones [6,7].
The observation of an early, GC-independent pathway for entry to the memory cell pools lead to a model proposing that the recruitment of low-affinity/broad specificity (polyreactive) B cells into a Bmem compartment serves to increase the breadth of humoral protection [8–10]. On the other hand, the extensive accumulation of V(D)J mutations in later, broadly neutralizing Abs (bNAbs) illustrates the contribution of the GC pathways to protective breadth [11,12]. Here, we discuss recent observations on the nature of clonal selection in GCs elicited by complex viral antigens and propose a comprehensive model that accounts for the development of the early and later forms of broadly-reactive Bmem cells, with a specific focus on immune responses to influenza virus and HIV-1.
Viral strategies for evading Bmem cell responses
Viral envelope proteins (e.g., influenza hemmagglutinin and HIV-1 Env) are the primary targets of efficacious, neutralizing Abs [13–15], but the rapidity with which mutations in these structural proteins accrue enables viral escape from protective Ab [13,15]. Influenza viruses acquire these escape mutations seasonally, within infected populations, while HIV-1 mutates in chronically infected individuals to escape autologous immunity. This escape mechanism for maintaining viral fitness in the face of the immune response appears to have resulted in the evolution of viral envelope proteins comprising variable regions with lesser impact on viral function and conserved regions necessary for transmission and replication [16]. It is epitopes within these conserved, functional regions that are potential targets for broadly-neutralizing Abs (bNAbs). Often, viruses employ a number of strategies to obscure the conserved domains from Ab surveillance, with the functional result that the conserved domains are weakly immunogenic or immunologically subdominant. In consequence, this adaptation restricts the efficacy of standard immunization protocols to elicit high-affinity Ab to conserved but cryptic domains [17].
Another viral strategy for evading immune surveillance that has become increasingly apparent is structural mimicry of host antigens by epitopes within conserved envelope domains. This mimicry serves to mitigate the generation of bNAbs through the effects of immune tolerance [17,18]. Haynes et al. first noted auto- and polyreactivity among bNAbs to HIV-1 [19] and later studies showing impaired B-cell development in knock-in mice expressing HIV-1 bNAb BCR transgenes have demonstrated the efficacy of host mimicry [20]. Although the generality of host mimicry as a mechanism to escape immune control remains to be established, the frequent polyreactivity of influenza bNAbs suggest that neutralizing epitopes that elicit auto- or polyreactive antibody may also act to increase the viral fitness of influenza [21].
Counteraction by germline-encoded cross-reactivity of Bmem cells
After immunization with haptenated protein, Bmem cells quickly develop in primary responses, in a week or less, within or outside GCs [8–10]. These early differentiative pathways to humoral memory are relatively less dependent on extensive GC responses, and promote the recruitment of low-affinity/polyreactive B cells, most bearing IgM BCRs, into the memory pool. On exposure to related, variant viruses, these early, cross-reactive Bmem cells are postulated to serve as the evolutionary templates for mutation and selection in secondary GCs where they generate high-affinity BCR repertoires to the novel epitopes expressed on variant viruses[9,10,22–24]. The early recruitment of memory repertoires is also supported by the idea that memory precursors are not selected solely on the basis of BCR affinity but by other criteria [25], the details of which are discussed later.
This early type of antibody breadth is the product of virus-specific BCR that, nonetheless, are characterized by binding sites capable of interactions with disparate antigen ligands. In consequence, early Bmem cells react with low affinity to structural variants of epitopes that arise in mutant viruses. This model is supported by the findings of McGargill’s laboratory showing that rapamycin treatment enhances the breadth of protective Ab responses to influenza viruses in correlation with attenuation of GC responses [26]. This intriguing correlation sets the stage for additional studies to provide a fuller mechanistic explanation of a seemingly counter-intuitive relationship: less selection equals broader protection.
Protection against influenza virus is also mediated by low affinity/polyreactive IgM Abs from B-1 B cells [27], likely reflecting low-affinity interaction between influenza virus and host receptors [28,29]. Whereas the polyreactive IgM Abs provide rapid, albeit low levels of protection, vastly more effective responses to secondary infections are provided by high-affinity, isotype-switched antibodies, a point illustrated by the significantly reduced humoral protection of AID-deficient mice in comparison to normal controls [30].
Cross-reactive Bmem cells from the early memory pathways are important, both to provide immediate humoral protection and subsequently to supply evolutionary templates on which later GC mutation and selection – in both primary and secondary responses - act. Essentially, the early Bmem pathways illustrate the benefits and limitations of diversity in the primary BCR repertoire. These early BCR are selected for rapid clonal expansion by their frequency and avidity for antigen [31], but are limited by their modest potency in the absence of somatic evolutionary refinement [30].
GC pathways for fine-tuning Bmem specificity to conserved, functional epitopese
If the early pathways to Bmem cell populations act to provide the templates through a limited selection, Ab fitness, as represented by increasing affinity and specificity to viral conserved domains, is achieved through V(D)J mutation and selection in the later, GC pathways to memory. Indeed, HIV bNAbs are recognized for their extraordinary frequencies of somatic mutations [11]. Influenza bNAbs that target the conserved stem domains are also significantly more mutated than neutralizing Abs to less well conserved HA regions [12]. Although the frequencies and kinetics of V(D)J somatic mutation differ between HIV-1 and influenza bNAbs, both bNAb types are similar in that they expand the breadth of specificity through the accumulation of V(D)J mutations [12,32,33]. In the case of HIV-1, longitudinal analyses have successfully traced bNAb lineages over periods of years and demonstrated the evolution of Ab neutralization breadth during episodes of V(D)J diversification and selection by serial virus escape mutants [32,33]. Intriguingly, during the latent phase when bNAbs emerge, lymph node T cells including follicular helper T (TFH) cells serve as viral reservoirs and continuously release viruses that express mutations that can facilitate escape from humoral immunity [34]. These findings establish a paradigm of virus and B-cell co-evolution that proceeds side-by-side during chronic HIV-1 infection. Significantly, these periodic, co-evolutionary responses focus BCR selection and affinity maturation on those virus epitopes that remain stable, i.e., on conserved HIV-1 epitopes that cannot readily be altered by mutational change.
Virus and BCR co-evolution also contributes to the generation of broadly reactive Bmem cells that recognize invariant regions of influenza virus escape mutants [35]. In contrast to HIV-1 chronicity, influenza virus replications are transient, nonetheless signs of persistent viral replication in the lungs of infected mice can be detected for at least 1 month following pulmonary infection [35,36]. This extended period of viral replication and antigen exposure is reflected in the delayed contraction of local GCs ectopically formed in lungs at sites of viral replication [35]. Importantly, comparing the frequencies of strain-specific and broadly-reactive GC B cells during this persistent GC phase, broadly-reactive B cells were found to be significantly enriched in lung GCs relative to splenic GCs in infected mice [35]. The role for ectopic GCs in generating broadly reactive Bmem populations nicely fits with previous observations in mice and humans that respiratory influenza infections increase significantly the breadth of serum IgG responses compared to homologous vaccination by other parenteral routes [37,38].
Permissive selection for maintaining GC clonal diversity
How GCs select BCR repertoires over time is fundamental to understand affinity maturation and specificity breadth achieved in bNAb responses. A major obstacle to the selective expansion of high affinity, broadly-reactive B cells specific for the conserved neutralizing regions of viruses, is the weak immunogenicity or immunological subdominance of the associated viral epitopes [17]. As we discussed earlier, conserved neutralization domains that are crucial to virus survival, replication, and/or transmission are often concealed from immune responses and this concealment is likely responsible for the poor immunogenicity of these epitopes [17].
Recent advances in determining BCR affinity and clonal diversity in GCs elicited by complex, polyepitopic antigens has revealed that BCR selection in GCs is permissive for a diverse spectrum of affinity and clonality. In contrast to responses to genetically restricted hapten responses, B cells activated by complex antigens establish GCs that not only begin with substantial clonal diversity but may actually grow more clonally diverse with time, as B cell clones that were rare initially become numerous [31,39]. A recent and surprising finding by Kuraoka et al.[31] was that half of all GC B cells elicited by complex protein antigens do not bind the native form of the eliciting antigen, even though the non-binding B cells acquire V(D)J mutations and expand clonally at rates and extents similar to that of antigen-binding GC B cells [31]. The proliferation and V(D)J mutations that accrue to GC B cells responding to this unknown agent, called “dark antigen” by Kuraoka [31], most likely represent responses to neoepitopes generated by physiological processes that modify antigen in vivo. Even highly stable antigen immunogens carefully adjuvanted to preserve structural stability elicit robust “dark antigen” responses (M. Kuraoka and G. Kelsoe, unpublished). We think it likely that humoral responses to dark antigen neoepitopes are common, but overlooked by standard assay methods utilizing native antigen as an assay ligand.
These recent observations of interclonal GC selection make some useful points. First, even in the presence of significant affinity maturation, unrecognized modes of GC selection allow B cells that are initially rare and/or bear low affinity BCR to remain viable and continue within GCs for much longer periods than previously thought [31]. This permissive environment provides an opportunity for less fit, lower affinity and/or polyreactive B cells to enter memory B-cell pools where they may be useful templates for later evolutionary refinement. Second, the observation of GC B-cell responses to dark antigen suggests that complex protein antigens are subjected to structural modifications that may change the balance of epitopic antigenicity in vivo ([31]; M. Kuraoka and G. Kelsoe, unpublished).
Of note, viral envelop proteins undergo substantial conformational and/or structural modifications during viral replication [13,15]. If these modifications promote the exposure, generation, and recognition of epitopes that are normally cryptic, in some instances including conserved epitopes, to GC B cells, viral replication per se may limit the structural constraints that normally minimize the activation and selection of B cells capable of generating bNAbs that recognize stable and conserved virus epitopes. Moreover, the infection-associated, immunological milieu may also attenuate any immunological tolerance that mitigates antigen-driven selection of GC B cells expressing auto- or polyreactive BCR, as B-cell intrinsic signaling through IFN-γ, the cytokine upregulated after virus infection, are suggested to regulate GC B cell tolerance to autoantigens [40,41]. Together, it is tempting to speculate that these viral and immunological factors account for the enhanced elicitation of broadly-reactive antibodies after infection [37,38] and elevated GC selection for broad reactivity at the sites of virus replication [35].
How broadly-reactive B cells are recruited and maintained in the memory pool?
After bNAb B cells are selected, they are often recruited into the memory compartment rather than the long-lived plasmacyte compartment [2–4]. Shinnakasu et al. [42] have shown the strength of T-cell helper activity provided by TFH cells is among the key determinants for fate decision into the memory compartment; weak signals instruct GC B cells into the memory pools by increasing expression of the Bach2 transcription factor (reviewed in page xx – xx). Broadly-reactive B cells may be guided into the memory compartment by a similar mechanism, as the subdominant nature of the conserved domains lowers the accessibility of BCR ([21,43], Y. Adachi and Y. Takahashi, unpublished), thereby limiting the amount of antigens presented to TFH cells.
The maintenance of broadly-reactive Bmem cells is crucial to sustain the capacity for broad protection to variant viruses. BCR polyreactivity has negative effects on the maintenance of IgG+ memory B cells, and may reduce the life span of broadly-reactive memory B cells [44]. Memory B cells are also maintained in the peripheral tissues where Bmem cells with unique phenotypes localize as a tissue-resident memory compartment [35,45,46]. Tissue residency shortens the time for Ab production on secondary infection and substantially improves protective efficacy [47]. Intriguingly, broadly-reactive Bmem cells are enriched in tissue-resident memory pools, where they may potentiate broad protection at infection sites [35]. Where and how tissue-resident Bmem cells are maintained remains important questions to be addressed.
Concluding remarks
We discussed multiple pathways for memory B cell development, and have highlighted a possible functional partition between the early GC-independent and late GC-dependent pathways. We propose that permissive GC selection based on conformationally modified antigens may be the basis for selecting BCR repertoires targeting conserved viral epitopes, the sites of vulnerability. Whereas antibody secreted by long-lived plasma cells is exactly directed towards past infections and antigen exposures, these non-dividing cells are eventually lost in the absence of additional recruitment by homologous challenge. Bmem cells, on the other hand, can persist for extended periods through their capacity for self-renewal even when they carry BCR that are cross-reactive for variant viruses. In this way, the breadth of Bmem cells is a key feature of long-lasting memory for future virus infection that have altered their antigenic profiles through mutation. We now know Bmem cells are not simply a back-up for long-lived plasma cells but a cell compartment that helps to anticipate virus cells should evolution. A deeper understanding of the biology of broadly-reactive Bmem be an important goal for both basic and translational immunology.
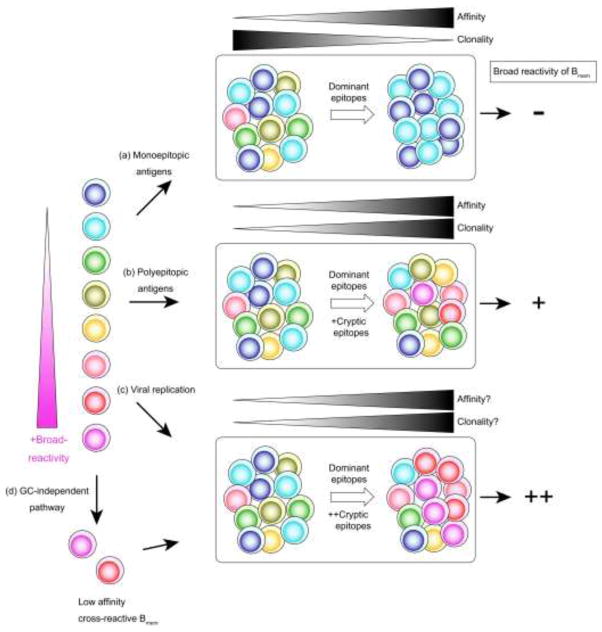
Figure. Takahashi and Kelsoe.
Proposed model for GC selection and broad-reactivity of Bmem cells after three types of antigen priming. (a) Monoepitopic antigens recruit B cells with better accessibility to antigens into GCs where antigens and TFH cells select somatic variants with high affinity/specificity, resulting in increased affinity and reduced clonality. (b) Polyepitopic antigens elicit GCs where conformational modification of selecting antigens increases the survival and proliferation of B cells that bind to cryptic/conserved epitopes. (c) Viral replication induces substantial conformational modification of antigens that exposes the cryptic/conserved epitopes and promotes the selection of broadly-reactive B cells. (d) GC-independent pathway elicits low-affinity/specificity Bmem cells which save germline-encoded cross-reactivity for the later GC responses.
Highlights.
Viral conserved domains are often concealed from the humoral responses.
Memory B cells counteract with viral mutations by germline-encoded cross-reactivity.
GC reactions fine-tune the specificity of memory B cells toward the conserved domains.
Permissive GC selection allows the fine-tuning of memory specificity.
Broadly-reactive B cells may be recruited into the memory pool with an attenuated T-cell help.
Acknowledgments
This work was supported in part by Emerging/Re-emerging Infectious Diseases Project from Japan Agency for Medical Research and Development, AMED and JSPS KAKENHI Grant Number 16K15296 (to Y.T.), and by NIH awards AI100645, HHSN272201000053C, and AI117892 (to G.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109:9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purtha WE, Tedder TF, Johnson S, Bhattacharya D, Diamond MS. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J Exp Med. 2011;208:2599–2606. doi: 10.1084/jem.20110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu Y, Zhang Z, Sheehan J, Avnir Y, Ridenour C, Sachnik T, Sun J, Hossain MJ, Chen LM, Zhu Q, et al. A broadly neutralizing anti-influenza antibody reveals ongoing capacity of haemagglutinin-specific memory B cells to evolve. Nat Commun. 2016;7:12780. doi: 10.1038/ncomms12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 6.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 7.Jacob J, Przylepa J, Miller C, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J Exp Med. 1993;178:1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med. 2012;209:597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaji T, Ishige A, Hikida M, Taka J, Hijikata A, Kubo M, Nagashima T, Takahashi Y, Kurosaki T, Okada M, et al. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J Exp Med. 2012;209:2079–2097. doi: 10.1084/jem.20120127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ. A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity. 2016;44:116–130. doi: 10.1016/j.immuni.2015.12.004. This paper clearly confirms the early memory B cell development before the GC reaction starts without using GC-deficient mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 12.Pappas L, Foglierini M, Piccoli L, Kallewaard NL, Turrini F, Silacci C, Fernandez-Rodriguez B, Agatic G, Giacchetto-Sasselli I, Pellicciotta G, et al. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature. 2014;516:418–422. doi: 10.1038/nature13764. [DOI] [PubMed] [Google Scholar]
- 13.Ward AB, Wilson IA. Insights into the trimeric HIV-1 envelope glycoprotein structure. Trends Biochem Sci. 2015;40:101–107. doi: 10.1016/j.tibs.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 15.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 16.Corti D, Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu Rev Immunol. 2013;31:705–742. doi: 10.1146/annurev-immunol-032712-095916. [DOI] [PubMed] [Google Scholar]
- 17*.Kelsoe G, Verkoczy L, Haynes BF. Immune System Regulation in the Induction of Broadly Neutralizing HIV-1 Antibodies. Vaccines (Basel) 2014;2:1–14. doi: 10.3390/vaccines2010001. Nicely introducing the conept of host mimicry as a important to mitigate humoral immune responses to HIV-1 and proposing several vaccine strategies to overcome the evolutionary adaptation of HIV-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, Yang G, Wiehe K, Nicely NI, Vandergrift NA, Rountree W, Bonsignori M, Alam SM, Gao J, Haynes BF, et al. Polyreactivity and autoreactivity among HIV-1 antibodies. J Virol. 2015;89:784–798. doi: 10.1128/JVI.02378-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 20.Verkoczy L, Diaz M, Holl TM, Ouyang YB, Bouton-Verville H, Alam SM, Liao HX, Kelsoe G, Haynes BF. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc Natl Acad Sci U S A. 2010;107:181–186. doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Andrews SF, Huang Y, Kaur K, Popova LI, Ho IY, Pauli NT, Henry Dunand CJ, Taylor WM, Lim S, Huang M, et al. Immune history profoundly affects broadly protective B cell responses to influenza. Sci Transl Med. 2015;7:316ra192. doi: 10.1126/scitranslmed.aad0522. This paper desribes several important findings including a poor accesibility and polyreactivity of Abs to influenza conserved domains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaji T, Furukawa K, Ishige A, Toyokura I, Nomura M, Okada M, Takahashi Y, Shimoda M, Takemori T. Both mutated and unmutated memory B cells accumulate mutations in the course of the secondary response and develop a new antibody repertoire optimally adapted to the secondary stimulus. Int Immunol. 2013;25:683–695. doi: 10.1093/intimm/dxt030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuccarino-Catania GV, Sadanand S, Weisel FJ, Tomayko MM, Meng H, Kleinstein SH, Good-Jacobson KL, Shlomchik MJ. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat Immunol. 2014;15:631–637. doi: 10.1038/ni.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McHeyzer-Williams LJ, Milpied PJ, Okitsu SL, McHeyzer-Williams MG. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nat Immunol. 2015;16:296–305. doi: 10.1038/ni.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarlinton DM, Smith KG. Dissecting affinity maturation: a model explaining selection of antibody-forming cells and memory B cells in the germinal centre. Immunol Today. 2000;21:436–441. doi: 10.1016/s0167-5699(00)01687-x. [DOI] [PubMed] [Google Scholar]
- 26*.Keating R, Hertz T, Wehenkel M, Harris TL, Edwards BA, McClaren JL, Brown SA, Surman S, Wilson ZS, Bradley P, et al. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat Immunol. 2013;14:1266–1276. doi: 10.1038/ni.2741. This paper describes very surprising findings that immune suppressive drug enhances the breadth of humoral responses to influenza viruses, in contrast to our expectation. The data is supportive for the concept that germline-encoded cross-reactivity may provide the broad protection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauter NK, Bednarski MD, Wurzburg BA, Hanson JE, Whitesides GM, Skehel JJ, Wiley DC. Hemagglutinins from two influenza virus variants bind to sialic acid derivatives with millimolar dissociation constants: a 500-MHz proton nuclear magnetic resonance study. Biochemistry. 1989;28:8388–8396. doi: 10.1021/bi00447a018. [DOI] [PubMed] [Google Scholar]
- 29.Xiong X, Coombs PJ, Martin SR, Liu J, Xiao H, McCauley JW, Locher K, Walker PA, Collins PJ, Kawaoka Y, et al. Receptor binding by a ferret-transmissible H5 avian influenza virus. Nature. 2013;497:392–396. doi: 10.1038/nature12144. [DOI] [PubMed] [Google Scholar]
- 30.Harada Y, Muramatsu M, Shibata T, Honjo T, Kuroda K. Unmutated immunoglobulin M can protect mice from death by influenza virus infection. J Exp Med. 2003;197:1779–1785. doi: 10.1084/jem.20021457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Kuraoka M, Schmidt AG, Nojima T, Feng F, Watanabe A, Kitamura D, Harrison SC, Kepler TB, Kelsoe G. Complex Antigens Drive Permissive Clonal Selection in Germinal Centers. Immunity. 2016;44:542–552. doi: 10.1016/j.immuni.2016.02.010. This paper finds that GC selection for polyepitopic antigens is permissive for a wide range of BCR affinity and diversity of clonality. Surprisingly, the sustantial fractions of GC B cells do not detectably bind immunogen, indicating the structural modification of presented antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonsignori M, Zhou T, Sheng Z, Chen L, Gao F, Joyce MG, Ozorowski G, Chuang GY, Schramm CA, Wiehe K, et al. Maturation Pathway from Germline to Broad HIV-1 Neutralizer of a CD4-Mimic Antibody. Cell. 2016;165:449–463. doi: 10.1016/j.cell.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boritz EA, Darko S, Swaszek L, Wolf G, Wells D, Wu X, Henry AR, Laboune F, Hu J, Ambrozak D, et al. Multiple Origins of Virus Persistence during Natural Control of HIV Infection. Cell. 2016;166:1004–1015. doi: 10.1016/j.cell.2016.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Adachi Y, Onodera T, Yamada Y, Daio R, Tsuiji M, Inoue T, Kobayashi K, Kurosaki T, Ato M, Takahashi Y. Distinct germinal center selection at local sites shapes memory B cell response to viral escape. J Exp Med. 2015;212:1709–1723. doi: 10.1084/jem.20142284. This paper finds GC responses in the lungs after influenza virus infeciton as the sites for B cells selection for the conserved domains, serving as the source of broadly-reactive memory B cells in the same tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim TS, Hufford MM, Sun J, Fu YX, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med. 2010;207:1161–1172. doi: 10.1084/jem.20092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, et al. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One. 2011;6:e25797. doi: 10.1371/journal.pone.0025797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, Garcia-Sastre A, Palese P, Treanor JJ, et al. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol. 2013;87:4728–4737. doi: 10.1128/JVI.03509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Tas JM, Mesin L, Pasqual G, Targ S, Jacobsen JT, Mano YM, Chen CS, Weill JC, Reynaud CA, Browne EP, et al. Visualizing antibody affinity maturation in germinal centers. Science. 2016;351:1048–1054. doi: 10.1126/science.aad3439. This paper uses very elegant technologies to successfully follow the clonal diversity of individual GCs. Similar to the reference 31, the authors find the most GCs retain substantial clonal diversity, but at the same time, they find a limited clonality in a subset of GCs referred as “clonal bursts”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domeier PP, Chodisetti SB, Soni C, Schell SL, Elias MJ, Wong EB, Cooper TK, Kitamura D, Rahman ZS. IFN-gamma receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity. J Exp Med. 2016;213:715–732. doi: 10.1084/jem.20151722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson SW, Jacobs HM, Arkatkar T, Dam EM, Scharping NE, Kolhatkar NS, Hou B, Buckner JH, Rawlings DJ. B cell IFN-gamma receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J Exp Med. 2016;213:733–750. doi: 10.1084/jem.20151724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42**.Shinnakasu R, Inoue T, Kometani K, Moriyama S, Adachi Y, Nakayama M, Takahashi Y, Fukuyama H, Okada T, Kurosaki T. Regulated selection of germinal-center cells into the memory B cell compartment. Nat Immunol. 2016;17:861–869. doi: 10.1038/ni.3460. The authors elegantly show low-affinity GC B cells are recruited into the memory pool by high expression of Bach2. In addition, the author show the inverse correlation of Bach2 exression with the strength of T-cell help, proposing an instuctive model for memory B cell recruitment by weak T-cell help. [DOI] [PubMed] [Google Scholar]
- 43.Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 44.Gitlin AD, von Boehmer L, Gazumyan A, Shulman Z, Oliveira TY, Nussenzweig MC. Independent Roles of Switching and Hypermutation in the Development and Persistence of B Lymphocyte Memory. Immunity. 2016;44:769–781. doi: 10.1016/j.immuni.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joo HM, He Y, Sangster MY. Broad dispersion and lung localization of virus-specific memory B cells induced by influenza pneumonia. Proc Natl Acad Sci U S A. 2008;105:3485–3490. doi: 10.1073/pnas.0800003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen M, Hong MJ, Sun H, Wang L, Shi X, Gilbert BE, Corry DB, Kheradmand F, Wang J. Essential role for autophagy in the maintenance of immunological memory against influenza infection. Nat Med. 2014;20:503–510. doi: 10.1038/nm.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Onodera T, Takahashi Y, Yokoi Y, Ato M, Kodama Y, Hachimura S, Kurosaki T, Kobayashi K. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc Natl Acad Sci U S A. 2012;109:2485–2490. doi: 10.1073/pnas.1115369109. [DOI] [PMC free article] [PubMed] [Google Scholar]