Abstract
Objective
To evaluate proinflammatory cytokines and leukocyte subpopulations in cerebrospinal spinal fluid (CSF) and blood of NOMID patients post-treatment; and to compare inflammatory cytokines in CSF and blood in 6 patients treated with two IL-1 blockers, anakinra and canakinumab.
Methods
We immunophenotyped CSF on 17 anakinra-treated pediatric NOMID patients during routine follow-up visits between December 2011 and October 2013 and analyzed CSF cytokine levels in baseline and 3–5 years follow-up samples compared to healthy controls.
Results
Elevated CSF IL-6, IP-10/CXCL10, IL-18 levels, and monocyte and granulocytes counts significantly decreased with anakinra treatment, but did not normalize to control levels, even in patients fulfilling criteria of “clinical remission” (CR). CSF IL-6 and IL-18 levels significantly correlate with measures of blood brain barrier (BBB) function, specifically CSF protein (r=0.75; r=0.81 respectively); and albumin quotient (r=0.79, r=0.68 respectively). Median CSF WBC levels (10.2 vs. 3.7cell/mm3) and CSF IL-6 levels (150.7 vs. 28.5pg/ml) were significantly higher when patients received canakinumab than anakinra despite similar serum cytokine levels.
Conclusions
CSF leukocyte subpopulations and cytokine levels significantly improve with optimized IL-1 blocking treatment but do not normalize. The correlation of CSF IL-6, IP-10/CXCL10 and IL-18 with clinical-laboratory measures of inflammation and BBB function suggests a role as biomarkers in CNS inflammation. The difference in inhibition of CSF biomarkers between two IL-1 blocking agents, anakinra and canakinumab, suggests differences in efficacy in the intrathecal compartment, with anakinra being more effective. Our data indicate that intrathecal immune responses shape CNS inflammation and should be assessed in addition to blood markers.
Clinical trial identifier
(NCT00059748 and NCT00069329)
Keywords: NOMID, CSF biomarkers, Cytokines, Canakinumab, Anakinra
INTRODUCTION
Neonatal-onset multisystem inflammatory disease (NOMID), also referred to as chronic infantile neurologic cutaneous articular (CINCA) syndrome, is the most severe clinical form of the disease spectrum of Cryopyrin-Associated Periodic syndromes (CAPS). CAPS is caused by gain-of-function mutations in the NLRP3 gene that encodes cryopyrin, a protein that is expressed in hematopoietic cells including granulocytes, monocytes, dendritic cells, and non-hematopoietic cells that in the CNS include microglial and reactive astrocytes (1–3). Two-thirds of NOMID patients have de novo germline mutations and about 30% of NOMID patients have somatic mosaicism in NLRP3 (4). Pathogenic NLRP3 mutations lead to constitutive inflammasome activation with secretion of IL-1β and to moderate elevations of IL-18. CAPS patients present with episodic (on the milder end of the disease spectrum) or continuous (on the more severe end of the disease spectrum) systemic inflammation, that includes recurrent fever flares with neutrophilic urticaria, arthralgia, and flares in acute phase reactants. With increasing CAPS severity, CNS and organ inflammation become more prevalent and chronic which leads to organ damage. Cochlear inflammation leads to sensorineural hearing loss; chronic papilledema leads to optic nerve atrophy and peripheral visual field loss; and chronic increased intracranial pressure can lead to brain atrophy and mental retardation. These features are commonly observed in NOMID patients, and are more variable or absent in the milder forms of CAPS (Muckle Wells Syndrome (MWS) and familial cold induced autoinflammatory disease (FCAS) respectively (5; 6).
Treatment with IL-1 blocking agents results in significant clinical and laboratory improvements and has become standard of care. NOMID patients with germline or somatic mosaicism do not differ in disease severity and respond equally to IL-1 blocking treatment (7; 8). Anakinra can penetrate into the CNS and treatment improves CNS inflammation by decreasing CSF pleocytosis, opening pressure and protein levels, papilledema, and inner ear enhancement (6; 8; 9). It currently remains unclear whether NOMID patients with severe CNS inflammation achieve adequate inflammatory control with both short (anakinra) and long-acting IL-1 inhibitors (canakinumab and rilonacept). We have observed that normalization of acute phase reactants in the blood does not preclude persistent, low-grade CNS pleocytosis suggesting ongoing CNS inflammation (10; 11) and dose escalations of the IL-1 blocking agent anakinra, beyond levels needed to control peripheral inflammation are needed to optimally control CNS inflammation (8). In a previous study we observed that IL-6 levels in NOMID CSF in untreated patients were on average six times higher than IL-6 blood levels, suggesting that IL-6 may be produced in the CNS by resident cells, as suggested in CNS inflammation in SLE patients (12) and in neuro-Behçet’s (2). However, neither correlations of IL-6 levels with mononuclear cell subsets in the CSF (6), nor their use as potential biomarker for CNS disease in NOMID have been assessed. This study evaluates 9 cytokines including IL-6 in the CSF compared to serum and correlates cytokine levels with measures of blood brain barrier (BBB) function, and with measures of CNS inflammation, to assess their use as biomarkers for CNS inflammation. We further compare cytokine levels in 6 patients who received anakinra and the long acting IL-1 inhibitor canakinumab in sequence.
PATIENTS AND METHODS
Patients and Controls
Between December 2011 and October 2013 we collected CSF samples for immunophenotyping on 17 consecutive anakinra treated pediatric NOMID patients enrolled in a natural history and treatment study (NCT00069329) who returned for routine follow up. For the cytokine analyses we used archived CSF and plasma or serum samples that were collected, spun and frozen from baseline (prior to patients receiving IL-1 blocking treatments) and follow-up (after starting patients on IL-1 blocking treatment) (NCT00069329). Immunophenotyping of cerebrospinal fluid (CSF) leukocyte subsets was conducted on freshly collected samples (13). Written informed consent was obtained from all patients and/or their legal guardians. One patient was excluded from the analyses of cell populations due to a technical problem with the staining procedure (Supplementary Figures 1A and B). Of the 17 patients, 11 had archived baseline (prior to anakinra treatment) and post-treatment paired CSF and serum samples. For the cytokine analyses we included samples from 4 additional NOMID patients who had matched baseline and post-treatment CSF and serum samples stored, but whose CSF was not immunophenotyped for cell subsets (Supplementary Figure 1A and B). Complete clinical remission (CR) was defined by the following criteria: ESR ≤ 25mm/hr, CRP ≤0.5mg/dL, CSF WBC ≤5 cells/mm3, and protein ≤ 40mg/dL (10). Blood (plasma/serum) and CSF samples from NOMID patients were compared to serum samples from 11 pediatric age matched controls and to 7 CSF samples. Five CSF samples were from healthy adult controls recruited for another study (13) and 2 from pediatric patients with an undifferentiated autoinflammatory who had undergone a diagnostic LP but had no evidence of CNS inflammation by medical history, imaging and CSF markers, (leukocytosis, opening pressure and protein content). The study was conducted in accordance with the Declaration of Helsinki, and the institute responsible Institutional Review Board (IRB) approved the protocol.
Longitudinal cytokine analyses of all available CSF and serum samples were conducted on 21 patients (Supplementary Figure 1B). To assess the relevance of these biomarkers longitudinally, we included six patients who were initially treated with anakinra and were subsequently switched to canakinumab. Three of the 6 patients were ultimately switched back to anakinra.
Assessment of clinical-laboratory CSF parameters
CSF WBC count was assessed in the NIH clinical laboratory from unspun CSF and in Dr. Bielekova’s laboratory from spun and 50-fold concentrated CSF as described (13). Routine CSF protein, glucose levels, calculated CSF/serum albumin quotients and IgG index to assess blood barrier function (14) were obtained from CSF and serum samples where applicable.
Cytokine analysis
CSF and blood cytokines (IL-1β, IL-1ra, IL-6, IL-9, IL-10, IL-12 (p70), IL-12 (p40), IL-17, IP-10/CXCL10, IFN-γ, TNF-α, IL-1 α, IL-18, and IFN-α2) were assessed via Bio-plex multiplex assays (Bio-Rad, Richmond, CA) as previously reported (15). Samples were stored in −80 °C and the majority (except for some baseline blood and CSF samples) had not been previously thawed. Five of the cytokines were either not detectable (IFN-α2, IL-1α) in most of the CSF samples or were not significantly different from controls and did not change with treatment (IL-9, IL-12 (p40)) or were lower than in controls with significant variability and did not change with treatment (IL-17) and were therefore not included in the tables nor further analyzed (Supplementary Table 1A).
Immunophenotyping
We analyzed absolute values of 5 subpopulations of leukocytes which included monocytes, granulocytes, natural killer (NK) cells, CD3, and B cells. Sample preparation and immunophenotyping of leukocytes by flow-cytometry were performed as previously published (13).
Correlation analyses
Correlations of CSF cytokines and blood cytokines with leukocyte subpopulations were mostly assessed post-treatment; only two patients had CSF and blood immunophenotyping preformed prior to initiation of IL-1 blocking therapy. CSF cytokines were also correlated with the following CSF inflammatory parameters, CSF opening pressures, WBC count, total protein, and albumin quotient. Two of the 21 patients had VP shunts and were excluded only from CSF inflammatory parameter correlations; their baseline protein contents were very high consistent with decreased circulation of spinal cord CSF related to the mechanical shunt (16).
Statistics
Statistical analyses and graphing were performed using Graphpad 6 (GraphPad Software, La Jolla CA) and Stata (StataCorp LP, College Station, TX). Differences of non-paired data with parametric or nonparametric distributions were analyzed with Student t-test and Wilcoxon rank-sum test respectively. Paired CSF and blood samples were analyzed with paired Student’s t-test using log 10 transformation of raw values. Correlation studies were assessed with Spearman correlation coefficient. Due to the pilot nature of the study, we provide raw p-values, un-adjusted for multiple comparisons.
RESULTS
Demographic and Clinical Characteristics
The average age at study enrollment was 6.3 ± 5.7 years. Nineteen out of twenty-three patients had germline NLRP3 mutations and 4 had somatic mosaicism (Table 1). Most patients at baseline had been on disease modifying antirheumatic drugs (DMARD) and/or steroids. Those patients who were in complete clinical remission (CR) on anakinra received an average anakinra dose of 4.6 ± 1.4 mg/kg/d and had been on treatment for 2.8 ± 1.7 years, compared to those patients on anakinra who were improved but not in “complete remission” who received an average dose of 3.4 ± 1.4 mg/kg/d and were on anakinra treatment for 1.6 ± 1.3 years. No patient was on steroids or DMARD post-treatment, except for one patient who received methotrexate at the time of the sample collection.
Table 1.
Demographic and baseline characteristics of 23 pediatric NOMID patients
| Demographics | Baseline clinical characteristics | ||
|---|---|---|---|
| Age-years+/− SD | 6.3 ± 5.7 | Growth retardation (< 3rd %) | 17 (71) |
| Age group | CNS damage | ||
| 0–3 years | 11 (46) | Stroke | 2 (8) |
| 4–8 years | 5 (25) | Seizures | 4 (17) |
| 9–12 years | 3 (13) | Papilledema₰ | 21 (100) |
| 13–18 years | 2 (8) | Below average cognitive function (IQ) | 11 (46) |
| ≥18 years | 2 (8) | Extremely low (<70) | 5 (21) |
| Sex | Borderline (70–79) | 2 (8) | |
| Female | 12 (50) | Low average (80–89) | 4 (16) |
| Male | 11 (50) | Abnormalities on brain MRI | |
| Race | Ventriculomegaly | 12 (54) | |
| White | 10 (46) | Arachnoid adhesions | 13 (54) |
| Latino | 8 (33) | Leptomeningeal enhancement τ | 7 (43) |
| Asian | 4 (17) | Dura enhancement τ | 7 (43) |
| Black | 1 (4) | Cochlear enhancement τ | 14 (94) |
| Native American | 0 | VP shunt | 2 (8) |
| NLRP3* mutations | 23 (100) | Inner ear damage (hearing loss) | 19 (83) |
| Mild (>20 to ≤40 dB) | 6 (32) | ||
| Moderate (>40 to ≤70dB) | 7 (37) | ||
| Severe (>70 to ≤95 dB) | 5 (26) | ||
| Profound (>95 dB) | 1 (5) | ||
| Bone damage | |||
| Bone overgrowth | 10 (46) | ||
| Joint contractures | 13 (58) | ||
| Limb length discrepancies | 4 (21) | ||
Except otherwise, values are the number (%) of patients.
recorded in 21 patients
assessed in 15/17 patients, 6/23 patients were on anakinra prior to enrollment
4 patients had germline mosaicism
CSF and blood cytokine levels decrease with IL-1 blocking treatment
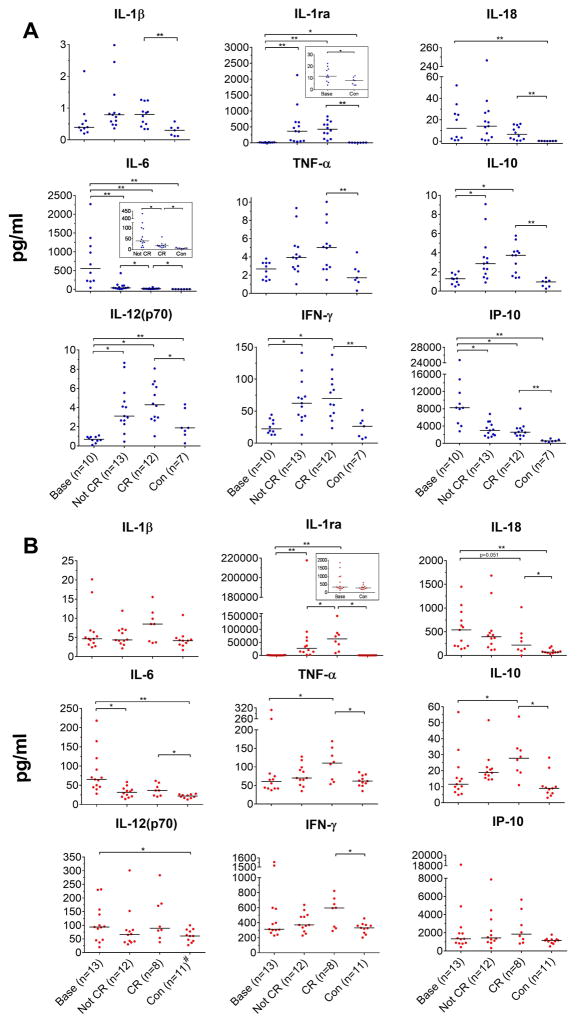
Baseline CSF levels for IL-18 (12.07 vs. 0.29pg/ml), IL-6 (555.31 vs. 5.61pg/ml), and IP-10/CXCL10 (8239.0 vs. 544.19pg/ml) were significantly higher in patients compared to healthy controls, but were not different from healthy controls in the blood for IP-10 (Figure 1A and B). In contrast levels of CSF IL-12(p70) were significantly lower in patients and CSF: TNF, IFNγ and IL-10 levels were not significantly different when compared to healthy controls (Figure 1A). On anakinra treatment, CSF IL-6 and IP-10/CXCL10 levels significantly decreased (to a median of 17.5 pg/ml for IL-6 and 2567.5 pg/ml for IP-10). CSF IL-6 levels were significantly lower in patients in CR compared to those on treatment but not fulfilling CR criteria, but remained increased compared to healthy controls (Figure 1A). CSF IL-18 levels decreased, particularly in those patients with baseline IL-18 values >20pg/ml, but this was not statistically significant. CSF IL-1β, TNF, IL-12(p70), IFNγ, and IL-10 levels increased, albeit by low levels, compared to baseline (significantly for IL-12(p70), IFNγ, and IL-10); and reached statistical significance in patients in CR compared to healthy controls; IL-1β (CR vs HC: 0.8 vs 0.3, in pg/ml, p=0.001); TNF-α (5.02 vs. 1.7pg/ml, p=0.005); IL-10 (3.74 vs. 0.96 pg/ml, p=0.0001); IL-12(p40) (9.6 vs. 1.6 pg/ml, p=0.007); and IFNγ (69.8 vs. 26.3pg/ml, p=0.001).
Figure 1.
CSF and Blood cytokine levels at baseline (base), not in clinical remission (Not CR), and in “complete clinical remission” (CR). A CSF analyses: Statistical analyses were performed of paired CSF samples base versus not in CR (n=5) and base versus CR (n=5), and also of paired samples not in CR versus CR (n=6). Unpaired comparisons between controls (con) (n=7) versus base (n=10) and CR (n=12) were made. B Blood analyses: Statistical analyses were done of paired blood base versus Not in CR (n=5) and base versus CR (n=4) and also of paired samples not in CR versus CR (n=6). Unpaired comparisons between con (n=11) versus base (n=13) and CR (n=8) were made. Paired CSF and blood samples were analyzed with paired Student’s t-test using log 10 transformation of raw values. Unpaired samples were analyzed by unpaired Student’s t-test or Wilcoxon’s rank sum depending on data distribution, *P<0.05, **P<0.005. Blue=CSF and red=blood, control bloods were n=11, #refers to control blood IL-6 which was n=10.
Similar to the CSF, baseline blood IL-18 (median 538.9 vs. 70.4pg/ml, p=0.002) and IL- 6 (65.3 vs. 22.0pg/ml, p= 0.002) levels were significantly higher in untreated NOMID patients than in controls and decreased with treatment, but remained significantly higher relative to healthy controls (Figure 1B). Unlike CSF, baseline IP-10/CXCL10 blood levels were similar to controls. IL-12(p70) levels that were lower in the CSF in NOMID vs. controls were significantly higher in blood in NOMID patients vs. controls. On anakinra, IL-12(p70) levels remained stable while blood TNF-α, IL-10, IFN-γ levels, increased (significant for IL-10 and TNF). Blood IP-10 levels remain unchanged with treatment. As expected, post-anakinra, IL-1Ra blood and CNS levels increased; (as they reflect a sum of both endogenous and recombinant IL-1Ra or anakinra). IL-1β levels, which are only measured at very low levels (6), non-significantly increase.
To compare the amount of cytokine production in the CNS compared to blood, matched CSF and blood samples were analyzed for cytokine CSF/blood ratios in the same assay with appropriate serum and CSF controls. Consistent with our previous report (6) baseline IL-6 levels are higher in the CSF than in blood samples; this was also seen for IP-10 CSF levels. Median CSF/blood ratios were 31 and 11 fold higher than controls, with a median CSF/blood ratio of 5.2 for IL-6, and 8.7 for IP-10/CXCL10 respectively (Supplementary Figure 2).. The CSF/blood ratios for IL-6 and IP-10/CXCL10 progressively dropped with treatment and were lowest at 0.5 and 1.2 respectively when patients were in CR. The median CSF/blood ratios for the other cytokines, including IL-1β and IL-18, are lower in the CSF than blood with levels between 0.05 and 0.2 and did not significantly change on anakinra treatment except the IL-12(p70) and IFN-γ CSF/blood ratios non-significantly increased after treatment, due to a greater increase in the CSF than in blood levels.
CSF monocyte and granulocyte counts significantly decreased but do not fully normalize in remission
NOMID patients have a predominant elevation of innate leukocytes in the CSF (13). Although adaptive immune cells, CD3 and B cells, are mildly increased in the CSF of untreated and anakinra treated patients, only numbers of the innate immune cells (monocytes, granulocytes) were significantly higher in NOMID patients compared to controls (Figure 2). Monocyte and granulocyte numbers decreased post-treatment but monocytes remained on average 3 fold higher in treated NOMID patients in comparison to controls, while neutrophils remained only about 1.5 fold increased (non-significant). B cell counts remained stable and CD3+ T cell counts significantly increased about 3–4 fold with treatment.
Figure 2.
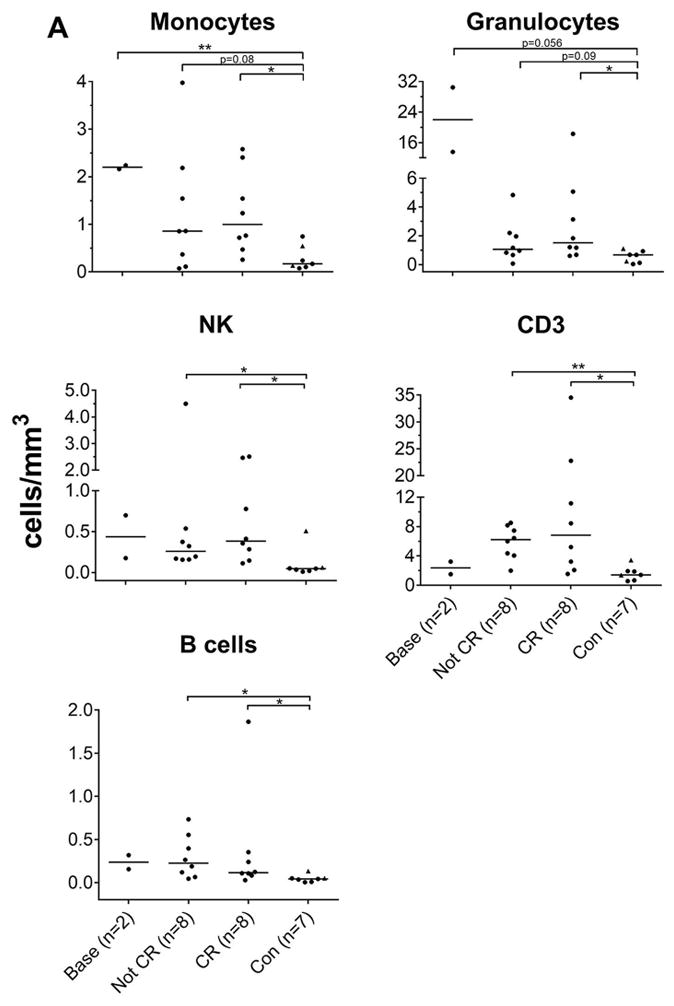
CSF subpopulation leukocyte absolute counts and correlation of CSF IL-6. Statistical analyses was performed between control (con) versus baseline (base), not in clinical remission (not CR), and “complete clinical remission” (CR) using unpaired student’s t-test or Wilcoxon’s rank sum test depending on data distribution, *P<0.05, **P<0.005. Each graph point represents 1 patient’s raw value; medians are depicted as horizontal lines. Triangle points represent pediatric control.
CSF IL-6 and IP-10/CXCL10 levels significantly correlate with monocyte counts when patients are in clinical remission (CR)
To investigate associations between inflammatory cells and cytokines, we correlated CSF cytokines with CSF leukocyte subpopulations. Post-treatment, only CSF IL-6 levels correlated significantly with the number of CSF monocytes (r=0.51, p=0.046). When patients were in remission, the correlation became stronger (r=0.88, p=0.004) and IP-10/CXCL10 levels also correlated significantly with CSF monocytes r=0.81, p=0.015), Supplementary Table 1B and 1C). We found no relationship between IL-18 or the other cytokines measured with subpopulations of CSF leukocytes.
CSF IL-6 and IL-18 correlate with CSF protein and albumin quotient
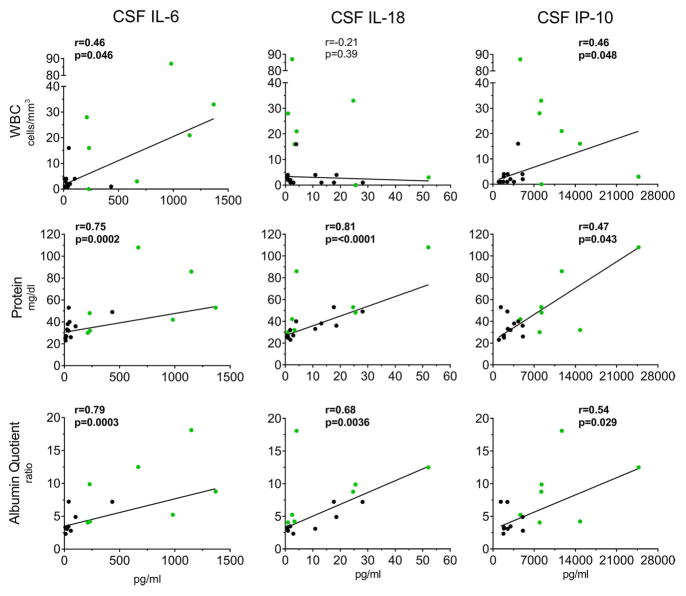
We assessed correlations of IL-18, IL-6 and IP-10 with measures of the BBB function, CSF total protein (TP) and albumin quotient (AQ). BBB function measures correlated strongly with IL-18 (TP: r=0.81, p=<0.0001 and AQ: r=0.68, p=0.0036) and IL-6 (TP: r=0.75, p=0.0002 and AQ: r=0.79, p=0.0003) and moderately with IP-10/CXCL10 levels (TP: r=0.47, p=0.043 and AQ: r=0.54, p=0.029), (Figure 3) but not positively with the other cytokines measured (Supplementary Table 2).
Figure 3.
Correlations between CSF IL-6, IL-18 and IP-10/CXCL10 with CNS inflammatory parameters. Each graph point represents 1 patient’s raw value. For each patient, baseline data (green, n=7) were used and if not available then post-treatment data was included (black, n=12) for a total of n=19 patients. Two patients were excluded because they had VP shunts. Correlation coefficients and p-values were assessed by Spearman’s correlation coefficient. Bolded results in figures indicate statistical significance. Non-linear regression robust straight lines are shown in each graph.
CSF cytokines and CSF WBC counts are higher in the same patients when treated with “optimized” doses of canakinumab compared to “optimized” doses of anakinra
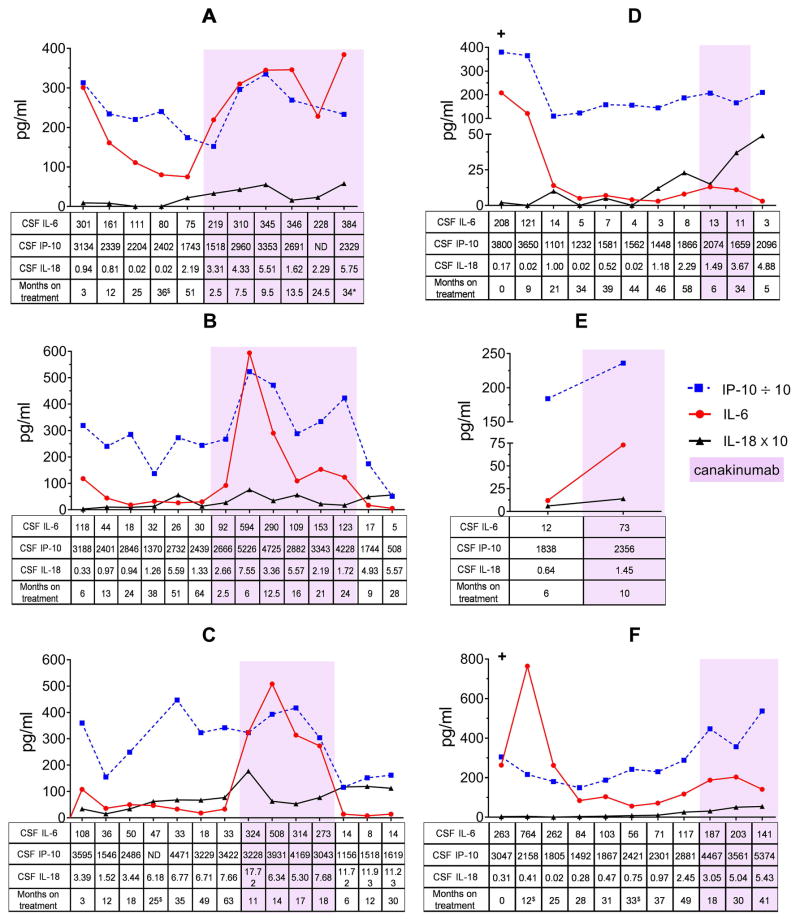
To date there are no comparative studies between anakinra and the long acting monoclonal anit-IL-1β antibody, canakinumab, in CNS inflammation. Since CSF IL-6, IP-10, and IL-18 correlated with clinical measures including monocyte counts and BBB function in anakinra treated NOMID patients, we assessed serial CSF and blood IL-6, IP-10/CXCL10, and IL-18 levels as well as CNS inflammatory markers in 6 pediatric patients who were initially treated with anakinra and were switched to canakinumab, 3 of these patients switched back to anakinra again (Figure 4). We found that CSF IL-6, IP-10/CXCL10, and IL-18 levels and CSF WBC counts were significantly higher when patients were on canakinumab than on anakinra (Table 2) despite only infrequent self-reports of headaches. The CSF cytokine levels and the WBC counts decreased in the 3 patients who switched back to anakinra after canakinumab treatment (Figure 4B–D and Supplementary Table 3B–D), which did not reach statistical significance due to the low number of patients. On both, anakinra and canakinumab treatment, CRP and ESR levels were normal and blood IL-6, IP-10/CXCL10, and IL-18 levels did not significantly differ between treatments (Table 2 and Supplementary Table 3A–F).
Figure 4.
CSF IL-6, IP-10/CXCL10, and IL-18 levels of 6 pediatric NOMID patients who were on anakinra and canakinumab in sequence. All 6 patients were initially on anakinra and were then switched to canakinumab. After canakinumab, 3 patients switched back to anakinra. Depicted on the graphs are values for IP-10/CXCL10 which were divided by 10 and for IL-18 which were multiplied by 10 from their original values shown on the tables. (+) indicates baseline samples and purple highlight indicates results while on canakinumab treatment. ND indicates not done due to no or insufficient CSF sample. $ indicates patients had otitis media or sinusitis at time of procedure, * signifies patient was taking ¼ of prescribed dose
Table 2.
Comparison of CSF and blood cytokine measurements in 6 pediatric NOMID patients on anakinra and subsequent canakinumab treatment
| Measure | anakinra | canakinumab | p-value |
|---|---|---|---|
| CSF IL-6 (pg/ml)+ | |||
| median | 28.5 | 150.7 | 0.004 |
| interquartile range | 14.4–62.7 | 84.3–275.5 | |
| Blood IL-6 (pg/ml)$ # | |||
| median | 3.0 | 2.8 | 0.17 |
| interquartile range | 2.7–5.0 | 2.13–3.0 | |
| CSF IP-10/CXCL10 (pg/ml)+ | |||
| median | 2148.1 | 3119.0 | 0.037 |
| interquartile range | 1904.3–2440.7 | 2458.0–3655.0 | |
| Blood IP-10/CXCL10 (pg/ml)$ # | |||
| median | 906.3 | 1245.1 | 0.82 |
| interquartile range | 878.7–1071.4 | 732.8–1385.7 | |
| CSF IL-18 (pg/ml)+ | |||
| median | 1.3 | 3.4 | 0.028 |
| interquartile range | 0.83–2.3 | 3.2–4.3 | |
| Blood IL-18 (pg/ml)$ # | |||
| median | 179.7 | 145.5 | 0.10 |
| interquartile range | 158.2–332.6 | 131.1–301.6 | |
| CSF WBC (cells/mm3)+‡ | |||
| median | 3.7 | 10.2 | 0.027 |
| interquartile range | 1.6–6.0 | 3.6 –23.9 | |
| CSF protein (mg/dl)+ ¥ | |||
| median | 39.0 | 44.3 | 0.42 |
| interquartile range | 33.7–47.3 | 38.3–46.3 | |
| CSF albumin quotient+ ¥ | |||
| median | 4.4 | 5.2 | 0.89 |
| interquartile range | 3.1–7.0 | 4.0–5.5 | |
Due to small sample size, raw values were transformed into LOGS to be closely normally distributed and analyzed by paired Student t-test. Values reported above are back- transformed medians and 25– 75 quartile ranges.
For each patient CSF measurement, the mean anakinra and mean canakinumab were obtained by taking the average of last 3 anakinra values (prior to canakinumab) and average of the last 3 canakinumab values on steady state. Averages were not calculated for one patient who had one value for both anakinra and canakinumab on steady state, and another patient who had one value on canakinumab on steady state,.
For each patient blood measurement, the mean anakinra and mean canakinumab values were obtained by taking the average of the last 3 anakinra values (prior to canakinumab) and average of the last 2 canakinumab values on steady state. Averages were not calculated for 2 patients who had only one value on canakinumab on steady state.
Blood comparisons were done on 5 patients because one blood sample on canakinumab was not available for one patient
For only CSF WBC due to value of zero, raw values were transformed into ARCSINH.
CSF protein and albumin quotient analyses were performed on 5 patients, CSF protein and albumin quotient were not was not recorded for one patient each on canakinumab on steady state.
DISCUSSION
Increased NLRP3 inflammasome assembly and IL-1β secretion cause the systemic disease manifestations of NOMID. However, the regulation of CNS inflammation, the contribution of CNS resident cells to the development of chronic aseptic meningitis and brain damage and differences of IL-1 blocking treatments on CNS inflammation remain unknown. Our data indicate that IL-6, and IP-10/CXCL10 are highly elevated in the CSF and decrease with treatment but do not normalize in all patients. Furthermore IL-18, IL-6, and IP-10/CXCL10 may be markers for BBB function and difference in treatment responses to the two IL-1 blocking agents anakinra and canakinumab suggest differences in CNS penetration. Collectively, our data suggest a role for serial CSF biomarker measurements in assessing and monitoring “residual” CNS inflammation in NOMID patients on different IL-1 blocking treatments.
Elevated granulocyte counts, which represent over 90% of abnormally elevated cells in the CSF in untreated NOMID patients with aseptic meningitis, virtually normalized with IL-1 blocking treatment. In contrast, CSF monocyte levels decrease but remain significantly elevated even in CR patients. Of the 9 inflammatory cytokines we examined to characterize potential low-grade inflammation, only IL-6 and IP-10/CXCL10 were expressed at higher levels in the CSF compared to corresponding serum samples; both cytokines were significantly lower (albeit not completely normalized) with anakinra compared to canakinumab treatment. Levels were lowest when patients were in CR compared to when patients were on treatment but did not fulfill CR clinical criteria. The presence of higher CSF levels of IL-6 and IP-10 compared to blood levels suggest production of these cytokines in the CNS. Although monocytes in the CSF can produce IL-1, microglia and astrocytes can produce IL-6 and IP-10 in response to IL-1β during active disease and are a likely source for the cytokine production in the CNS in NOMID (17). IP-10/CXCL10 is a known downstream marker of IFN signaling, and NOMID patient blood IP-10/CXCL10 levels are not elevated above control levels. In contrast, IP-10/CXCL10 blood levels are highly elevated in patients with IFN-mediated autoinflammatory diseases (18; 19). While mechanisms leading to elevated IP-10/CXCL10 in the CSF but not the blood in untreated NOMID patients need to be further evaluated, IFN- and STAT1-independent mechanisms of IP-10/CXCL10 upregulation were observed in astrocytes and microglia in the CNS in a murine HIV model (20) and IP-10/CXCL10 production was also observed in brain microvascular endothelial cells that form the BBB in response to exposure to serum from patients with neuromyelitis optica (21). These observations raise the question whether the elevation of CSF IP-10/CXCL10 levels may reflect astrocyte, microglial and/or brain microvascular endothelial cell activation possibly in response to BBB damage in NOMID.
In contrast to IL-6, IP-10/CXCL10 and IL-18 which all decrease with treatment, we observed a small but significant increases in CSF levels of TNFα, IL-12p70, IL-10 and IFNγ with treatment, in the context of significant clinical improvement. The overall CSF levels of these cytokines were much lower in the CSF than in the blood (below 10pg/ml for TNF, IL-12p70 and IL-10, and below 150pg/ml for IFNγ); but the increase in these cytokine levels points to the complexity of interpreting soluble biomarker levels in biological fluids. The measured CSF cytokine levels represent the balance between their “production/secretion” and their “binding/uptake” by target cells and tissues. In that context the small increase in cytokine levels could reflect an altered balance of production and “consumption”; with fewer inflammatory or activated tissue cells being able to “consume/bind” these cytokines.
Upon treatment with anakinra, CSF protein and albumin levels decline (8), suggesting improvement of BBB function. The CSF cytokines IL-6, IP-10/CXCL10, and IL-18 significantly correlate with measures of BBB function. These cytokines, when elevated, have been implied in causing the functional changes of the endothelial cells and astrocytes layer that constitute the BBB and cause or aggravate BBB dysfunction/breakdown (21),(22). While the high levels of IP-10 and IL-6 suggest their production intrathecally, the relatively stable IL-18 CSF/blood ratios before and after anakinra treatment and the lack of correlation of IL-18/WBC suggest that CSF IL-18 may mainly derive from passage across a “leaky” BBB, although low levels of CNS production cannot be ruled out. Interestingly, elevations of IL-1 and IL-18 have been seen in patients with chronic tension headaches (23) and IL-18-mediated microglia/astrocyte interactions have been associated with the development of allodynia and may play a role in patients with persistent headaches (24).
Our study is limited by a small sample size of patients with this rare disease and the fact that we did not correct for the multiple exploratory analyses performed. However the serial, longitudinally collected matched CSF and blood samples (including pre-treatment CSF samples) that were assayed simultaneously, reduced inter-assay variability typically seen with cytokine analyses, and increases the reliability of interpreting our data. The markers we propose need to be validated in further follow-up studies and in other diseases. Multiplex cytokine assays allow for measurement of multiple cytokines simultaneously from a small sample volume, but its utility for the assessment of IL-1β and other low expressing cytokines is limited. IL-1β is highly protein bound and soluble serum, plasma and CSF levels are often below 1 pcg/ml, which is below the assay’s detection range. Although small variations in IL-1β can have large pathological effects as seen in the cryopyrinopathies, IL-1β serum measurements cannot reliably be interpreted thus limiting their use a biomarker. Although IL-6 is not a specific marker for inflammasome activation, it is a known downstream marker of systemic inflammation in CAPS patients (25) and in our study correlated best with clinical markers of CNS inflammation and may be a surrogate for assessing IL-1 activity in CAPS patients.
Our study is the first to compare treatment with two IL-1 blocking agents, anakinra and canakinumab on controlling CNS inflammation in NOMID/CAPS in 6 patients. CSF IL-6, IP-10/CXCL10, and IL-18 levels and CSF WBC counts were significantly higher when patients were in steady state canakinumab compared to anakinra treatment. CRP and ESR in the blood and blood cytokines including IL-6, IP-10/CXCL10 and IL-18 levels were similar during anakinra and canakinumab treatment. However, cytokine levels were lower in the CSF when patients were on anakinra compared to canakinumab treatment, and trended back down when 3 of the 6 patients were switched back to anakinra treatment. Differences in CSF penetration of both drugs may contribute to the differences in low-grade CNS inflammation, which may be particularly relevant in “low inflammatory states” when the BBB is less penetrant. All patients enrolled had chronic CNS inflammation prior to IL-1 blocking treatment. Our CNS data cannot be extrapolated to patients with milder forms of CAPS who have less severe or only intermittent CNS disease.
The long-term clinical implications of chronic low elevation of CSF cytokines including the cytokines IL-1β, IL-6, IL-18, and IP-10 and persistently elevated monocyte counts in NOMID patients are currently not known and complications may take many years to develop. Therefore, correlations with the longer-term development of symptoms and damage need to be studied in the future. Monocytes can confer inflammatory and anti-inflammatory properties (26); they play a role in wound repair and tissue surveillance and their persistence post-treatment may reflect a homeostatic adaptation (27). Similarly, IL-6 has pro- and anti-inflammatory properties; however, chronically elevated CSF IL-6 levels are seen in acute and chronic neurologic conditions (28), and have been correlated with progression of brain atrophy in patients with Neuro-Behcet’s (29). Elevated IL-1β levels have also been associated with Alzheimer’s (30) and with recurrent headaches (23). This study was not designed to investigate the long-term effects of chronic low-grade inflammation but to develop biomarkers that may assist in the assessment and characterization of low-grade inflammation. Our data point to the need for longitudinal assessment of patients’ clinical functions to determine whether the residual inflammation measured is benign, or associated with long-term consequences such as headaches, cognition, and progression of brain atrophy.
In summary, our data indicate that the regulation of inflammation in the CNS compartment cannot be predicted by blood markers of inflammation and that drugs that suppress systemic inflammation may not equally suppress CNS inflammation and suggest a “personalized approach” guided by CSF biomarkers to assess residual inflammation and optimize treatment. Of the markers tested, IL-6, IL-18 and perhaps IP-10/CXCL10 may be used to monitor efficacy of IL-1 blocking therapy in controlling CNS inflammation in NOMID. Differences in blockade of CNS inflammation between anakinra and canakinumab suggest the inclusion of the assessment of CSF inflammation, drug penetration into the CNS and pharmacokinetics of IL-1β inhibitors when designing studies with IL-1β inhibitors in NOMID and other neuroinflammatory diseases.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute of Neurological Disorders and Stroke (NINDS), and by the NIH Clinical Center. The authors would like to thank Usma Hussein for her help in organizing the data. Jackeline Rodriguez-Smith’s research was supported by: The National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors.
Footnotes
COMPETING INTERESTS
RGM received grant support for clinical studies from Regeneron, Novartis, SOBI Inc. and Eli Lilly.
References
- 1.Guarda G, Zenger M, Yazdi AS, Schroder K, Ferrero I, et al. Differential expression of NLRP3 among hematopoietic cells. J Immunol. 2011;186:2529–34. doi: 10.4049/jimmunol.1002720. [DOI] [PubMed] [Google Scholar]
- 2.Hirohata S, Isshi K, Oguchi H, Ohse T, Haraoka H, et al. Cerebrospinal fluid interleukin-6 in progressive Neuro-Behcet’s syndrome. Clinical immunology and immunopathology. 1997;82:12–7. doi: 10.1006/clin.1996.4268. [DOI] [PubMed] [Google Scholar]
- 3.Mankan AK, Dau T, Jenne D, Hornung V. The NLRP3/ASC/Caspase-1 axis regulates IL-1beta processing in neutrophils. Eur J Immunol. 2012;42:710–5. doi: 10.1002/eji.201141921. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka N, Izawa K, Saito MK, Sakuma M, Oshima K, et al. High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: results of an International Multicenter Collaborative Study. Arthritis and rheumatism. 2011;63:3625–32. doi: 10.1002/art.30512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldbach-Mansky R. Current status of understanding the pathogenesis and management of patients with NOMID/CINCA. Curr Rheumatol Rep. 2011;13:123–31. doi: 10.1007/s11926-011-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–92. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka N, Izawa K, Saito MK, Sakuma M, Oshima K, et al. High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: results of an International Multicenter Collaborative Study. Arthritis Rheum. 2011;63:3625–32. doi: 10.1002/art.30512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sibley CH, Plass N, Snow J, Wiggs EA, Brewer CC, et al. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three- and five-year outcomes. Arthritis Rheum. 2012;64:2375–86. doi: 10.1002/art.34409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox E, Jayaprakash N, Pham TH, Rowley A, McCully CL, et al. The serum and cerebrospinal fluid pharmacokinetics of anakinra after intravenous administration to non-human primates. J Neuroimmunol. 2010;223:138–40. doi: 10.1016/j.jneuroim.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibley CH, Plass N, Snow J, Wiggs E, Brewer C, et al. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease (NOMID) treated with anakinra. Arthritis Rheum. 2012 doi: 10.1002/art.34409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sibley CH, Chioato A, Felix S, Colin L, Chakraborty A, et al. A 24-month open-label study of canakinumab in neonatal-onset multisystem inflammatory disease. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirohata S, Miyamoto T. Elevated levels of interleukin-6 in cerebrospinal fluid from patients with systemic lupus erythematosus and central nervous system involvement. Arthritis Rheum. 1990;33:644–9. doi: 10.1002/art.1780330506. [DOI] [PubMed] [Google Scholar]
- 13.Han S, Lin YC, Wu T, Salgado AD, Mexhitaj I, et al. Comprehensive immunophenotyping of cerebrospinal fluid cells in patients with neuroimmunological diseases. J Immunol. 2014;192:2551–63. doi: 10.4049/jimmunol.1302884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci. 2001;184:101–22. doi: 10.1016/s0022-510x(00)00501-3. [DOI] [PubMed] [Google Scholar]
- 15.Houser B. Bio-Rad’s Bio-Plex(R) suspension array system, xMAP technology overview. Archives of physiology and biochemistry. 2012;118:192–6. doi: 10.3109/13813455.2012.705301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox JL, McCullough DC, Green RC. Effect of cerebrospinal fluid shunts on intracranial pressure and on cerebrospinal fluid dynamics. 2. A new technique of pressure measurements: results and concepts. 3. A concept of hydrocephalus. Journal of neurology, neurosurgery, and psychiatry. 1973;36:302–12. doi: 10.1136/jnnp.36.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das S, Mishra MK, Ghosh J, Basu A. Japanese Encephalitis Virus infection induces IL-18 and IL-1beta in microglia and astrocytes: correlation with in vitro cytokine responsiveness of glial cells and subsequent neuronal death. J Neuroimmunol. 2008;195:60–72. doi: 10.1016/j.jneuroim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Ramot Y, Torrelo A, Paller AS, Si N, et al. Mutations in PSMB8 cause CANDLE syndrome with evidence of genetic and phenotypic heterogeneity. Arthritis and rheumatism. 2011 doi: 10.1002/art.33368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371:507–18. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asensio VC, Maier J, Milner R, Boztug K, Kincaid C, et al. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. Journal of virology. 2001;75:7067–77. doi: 10.1128/JVI.75.15.7067-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu F, Nishihara H, Sano Y, Takeshita Y, Takahashi S, et al. Markedly increased IP-10 production by blood-brain barrier in neuromyelitis optica. PLoS One. 2015;10:e0122000. doi: 10.1371/journal.pone.0122000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, et al. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 23.Della Vedova C, Cathcart S, Dohnalek A, Lee V, Hutchinson MR, et al. Peripheral interleukin-1beta levels are elevated in chronic tension-type headache patients. Pain research & management : the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur. 2013;18:301–6. doi: 10.1155/2013/796161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyoshi K, Obata K, Kondo T, Okamura H, Noguchi K. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. J Neurosci. 2008;28:12775–87. doi: 10.1523/JNEUROSCI.3512-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman HM, Rosengren S, Boyle DL, Cho JY, Nayar J, et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364:1779–85. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, et al. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med. 2013;19:713–21. doi: 10.1038/nm.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aubert P, Suarez-Farinas M, Mitsui H, Johnson-Huang LM, Harden JL, et al. Homeostatic tissue responses in skin biopsies from NOMID patients with constitutive overproduction of IL-1beta. PLoS One. 2012;7:e49408. doi: 10.1371/journal.pone.0049408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. International journal of biological sciences. 2012;8:1254–66. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikuchi H, Takayama M, Hirohata S. Quantitative analysis of brainstem atrophy on magnetic resonance imaging in chronic progressive neuro-Behcet’s disease. Journal of the neurological sciences. 2014;337:80–5. doi: 10.1016/j.jns.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Tan MS, Yu JT, Jiang T, Zhu XC, Tan L. The NLRP3 inflammasome in Alzheimer’s disease. Mol Neurobiol. 2013;48:875–82. doi: 10.1007/s12035-013-8475-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.