Abstract
Introduction
Smokers are often told to use nicotine lozenge when craving or withdrawal symptoms occur. This may be too late to prevent lapses. This study assessed if nicotine lozenge use prior to a common smoking trigger can minimize trigger induced increases in craving and withdrawal symptoms.
Methods
Eighty-four smokers completed two laboratory sessions in random order. At one session, nicotine lozenge was given immediately after a stressor (to approximate current recommended use – i.e., after craving and withdrawal symptoms occur); at the other session subjects were randomized to receive nicotine lozenge at time points ranging from immediately to 30 minutes prior to the stressor. Withdrawal symptoms and urge to smoke were measured using the Minnesota Nicotine Withdrawal Scale and the Questionnaire of Smoking Urges (QSU).
Results
Relative to receiving lozenge after the stressor, a smaller increase in pre-stressor to post-stressor withdrawal symptom scores occurred when lozenge was used immediately prior (p=0.03) and 10 minutes prior (p=0.044) to the stressor. Results were similar for factors 1 and 2 of the QSU when lozenge was used immediately prior to the stressor (p<0.03) and for factor 1 of the QSU when lozenge was used 10 minutes prior to the stressor (p=0.028). Absolute levels of withdrawal symptoms and urge to smoke severity were also lower when lozenge was given prior versus after a stressor.
Conclusions
Administering the nicotine lozenge prior to a smoking trigger can decrease trigger induced craving and withdrawal symptoms. Future studies are needed to determine if such use would increase cessation rates.
Keywords: Smoking, Withdrawal symptoms, Craving, Nicotine Lozenge
1. Introduction
The morbidity and mortality associated with smoking is well characterized, resulting in approximately 480,000 deaths annually in the United States (USDHHS 2014). New treatments for tobacco dependence are rarely introduced, with only two non-nicotine pharmacological aids currently available. The low success rates and the infrequent introduction of new therapies necessitate that the efficacy of currently available therapies be maximized.
Use of a single nicotine replacement therapy (NRT) typically doubles cessation rates relative to placebo with additional benefit observed when combination NRT (i.e., combining two dosage forms) is used (Fiore et al. 2008). In combination therapy, nicotine gum or lozenge is typically used as needed in combination with scheduled use of the nicotine patch (Fiore et al. 2008; Puska 1995; Steinberg et al. 2009). As monotherapy, nicotine gum or lozenges are frequently used on an as needed basis following an initial period of scheduled use (Fiore et al. 2008; Kozlowski et al. 2007; McNeill et al. 2001; USPHS 2000). Therefore, current counseling methods frequently advise smokers to use these products when they need them (i.e., when symptoms of craving or tobacco withdrawal are present); (Fant et al. 1999; Kozlowski et al. 2007; Sweeney et al. 2001). Although one study found that half of smokers who lapse after an acute craving episode do so within 11 minutes of the onset of craving (Ferguson and Shiffman 2009; Shiffman et al. 1996), substantial concentrations of nicotine are not attained until approximately 10 to 15 minutes after starting use of these products (Benowitz et al. 1988; Kotlyar et al. 2007). These data suggest that waiting to use nicotine lozenge or gum until craving or withdrawal symptoms are present may not result in nicotine delivery quickly enough to avert a smoking lapse.
The purpose of this study was to provide initial information assessing if using nicotine lozenge prior to exposure to a smoking trigger results in smaller trigger induced increases in withdrawal symptoms and urge to smoke than using this product after these symptoms have already occurred (i.e., after smoking trigger exposure). Exposure to a stressful task was chosen as the smoking trigger for this study since similar tasks when presented in a laboratory setting have been shown to decrease the ability to resist smoking, increase craving and withdrawal symptom severity, and increase smoking topography measures (e.g., puff volume) (Kotlyar et al. 2011; McKee et al. 2011; Pomerleau and Pomerleau 1987). In a naturalistic setting, smokers attempting to quit frequently report that stressful events lead to smoking relapse (Cummings et al. 1985). Therefore stress is likely to be an important trigger in smoking lapses. Our hypothesis was that exposure to a smoking trigger (i.e., a stress task) would result in larger increases in withdrawal symptoms and urge to smoke when nicotine lozenge is used immediately after the stressor than if lozenge was used prior to stress exposure.
2. Methods
2.1 Design
In this randomized, cross-over study subjects participated in two laboratory sessions at which withdrawal symptoms and urge to smoke were assessed prior to and following exposure to a common smoking trigger (i.e., a stressful situation). Each subject participated in one session at which a 4 mg nicotine lozenge (mint flavor) was given immediately after the stressor (Figure 1: Condition A) in order to approximate current recommended use of lozenge when used on an as needed basis – i.e., after craving and withdrawal symptoms begin. This served as the control condition. Each subject participated in another session at which they were randomized to one of four experimental groups: receive a 4 mg nicotine lozenge either immediately prior (condition B), 10 minutes prior (condition C), 20 minutes prior (condition D), or 30 minutes prior to the stressor (condition E) (Figure 1). The time-frames of administration span the range from when the impending trigger is presumably more predictable but nicotine concentrations during the trigger would be relatively low (i.e. Condition B) to when predicting the trigger would be more difficult but nicotine concentrations during the trigger would be highest (i.e., Condition E).
Figure 1.
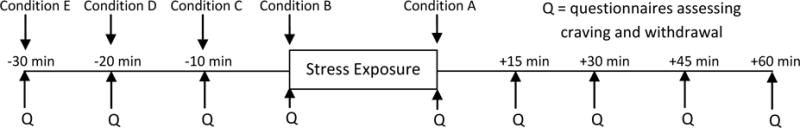
Outline of each laboratory assessment with subjects assigned to condition A at one laboratory session and one of the other conditions at the other laboratory session. The order of laboratory assessments (i.e., condition A vs. other condition) was assigned randomly.
The two laboratory sessions were separated by at least 3 days and the order of laboratory sessions (i.e., control vs. experimental) was assigned randomly. In a two-step randomization process, subjects were first randomized to experimental condition (i.e., conditions B – E) and then to the order in which they receive their assigned experimental condition or the control condition (i.e., condition A). The nicotine lozenge was selected among the four non-patch dosage forms since it is available without a prescription (and therefore more commonly used) and is well tolerated.
2.2 Subjects
Volunteers were recruited through flyers and newspaper advertisements. Initial eligibility was assessed via a phone interview and confirmed at a screening visit at which written informed consent was obtained. This study was approved by the University of Minnesota Institutional Review Board. Study visits occurred at the University of Minnesota between December 2011 and January 2014.
Eligible participants were between the ages of 18 and 65, smoked at least 8 cigarettes daily and identified stress as a smoking trigger. Individuals were excluded who reported a current unstable medical or psychiatric condition (for example, necessitating medication changes within the past 3 months), substance abuse (other than nicotine) within six months of the study, use of medication that could interfere with study outcomes (e.g., psychoactive medications), use of smoking cessation medications in the past month, a history of severe motion sickness (due to the virtual reality (VR) technology utilized to present the stressor), and women who were pregnant or breastfeeding. Medical history was based on subject self-report. A urine pregnancy test confirmed that women were not pregnant at enrollment.
Smokers not identifying stress as a smoking trigger would be unlikely to react to the stressor thereby precluding any possible medication effect. We asked smokers to list 3 smoking triggers relevant to them and only enrolled those who listed stress (or a specific stressful situation) as a trigger.
As the stress task was presented in a VR environment, subjects were immersed in the environment at the screening visit to allow for adaptation so that responses seen at subsequent visits were due to the stressor rather than the novelty of the environment.
2.3 Laboratory sessions
Subjects abstained from smoking overnight prior to each morning laboratory session (confirmed via an exhaled carbon monoxide of ≤ 8 ppm). Upon arrival, subjects relaxed in a quiet room for 90 minutes after which questionnaires assessing nicotine withdrawal and urge to smoke were completed. Those randomly assigned to Condition E for that laboratory session then used one nicotine lozenge. All subjects again completed questionnaires at 20 minutes, 10 minutes, and immediately prior to the stressor. Those assigned to Condition D received a lozenge after completing the 20 minute pre-stressor questionnaires, those assigned to Condition C after completing the 10 minute pre-stressor questionnaires and those assigned to Condition B after completing the pre-stressor questionnaires. All subjects were therefore exposed to the stressor 2 hours after beginning the session (Figure 1). To confirm that the speech task was sufficiently stressful to induce a physiological response, blood pressure and heart rate were measured at 1 to 3 minute intervals starting after the initial 90 minute relaxation period and continuing through the remainder of the laboratory session.
The stressor was presented via an immersive VR environment (software and hardware from Virtually Better, Decatur, Georgia) previously demonstrated to induce a physiological stress response (Kotlyar et al. 2008). The VR visor was placed after the last set of pre-stress questionnaires was completed. Subjects were then presented with a somewhat stressful standardized scenario and asked to spend three minutes preparing and three-minutes delivering a speech addressing how they would handle the scenario. Speeches were prepared while in a waiting room within the VR environment and delivered while seated with a virtual audience around a conference table. The speech task was based on the Trier Social Stress Test (al’Absi et al. 2003; Back et al. 2008; Kirschbaum et al. 1993). After the stress task, the visor was removed and questionnaires were completed every 15 minutes over the next hour. Those assigned to condition A (i.e., the control condition) used the nicotine lozenge immediately after completing the initial post-stressor questionnaires.
2.4 Outcome Measures
The Minnesota Nicotine Withdrawal Scale (MNWS) was used to measure withdrawal symptom severity with scores calculated by adding the 7 withdrawal symptom related items (Hughes and Hatsukami 1998; Hughes 1992; Hughes and Hatsukami 1986). To assess craving, the brief ten item version of the Questionnaire of Smoking Urges (QSU) was used (Cox et al. 2001). A score for each of two factors was calculated with factor 1 reflecting an intention and desire to smoke and factor 2 reflecting anticipation of relief from negative affect (Toll et al. 2006).
2.5 Statistical analysis
Baseline demographics were summarized using mean and standard deviation for continuous variables and frequency and percent for categorical items. Comparisons among groups were performed using one-way ANOVA and chi-square tests for the continuous and categorical demographic variables, respectively. The study’s primary outcome was pre- to post-stress change in MNWS withdrawal scores (calculated as the post-stressor score minus the pre-stressor score). Secondary outcomes were pre- to post- stress change in QSU factor 1 and 2 scores and post-stress symptom severity of withdrawal symptom, QSU factor 1 and QSU factor 2 scores calculated as post-stressor score minus baseline (i.e., −30 minutes) score to adjust for baseline. Each outcome was analyzed according to the cross-over design using a mixed model approach that included fixed effects for experimental vs. control condition (treatment or control) and first or second lab session (order of treatment) plus a random effect due to each subject receiving both an experimental and control condition within a pre-assigned sequence. The model-based results for the difference between controls and experimental conditions are reported as least squared (LS) means and 95% confidence intervals. P-values were considered significant at the 0.05 level. Effect sizes (LS mean/standard error) were calculated for withdrawal scores. The analysis was run separately for each of the four experimental conditions and again with the experimental condition added as another fixed covariate to compare the four experimental conditions. An adjusted alpha level of <0.01 was considered significant for the comparisons among the four conditions. Nicotine dependence severity, as assessed at the screening visit using the Fagerstrom Test for Nicotine Dependence (FTND) score (Heatherton et al. 1991,) was considered as a potential covariate in this last model for each outcome but was not found to be significant (all p-values>0.05) and was not included in the final analysis. There were no systematic order effects among the outcomes of interest. The order of lab sessions was only significant for one measure in one experimental condition (i.e., withdrawal symptom scores for experimental group D). Therefore, the mean values in the text and figures combined the data from the two subgroups with the same experimental condition. Physiological response to the stressor was assessed by subtracting the average of the measurements observed during the 30 minute relaxation period immediately preceding the speech task from the average of the measures obtained during the stress task. This change was evaluated using a two-sided, one sample t-test after averaging the results from the two lab sessions. Statistical analysis was performed using SAS 9.3 (SAS Institute Inc., Cary NC).
3. Results
Ninety-eight subjects completed the first laboratory session and 84 completed both sessions (figure 2). No significant differences in baseline demographic characteristics were found among groups (all p-values>0.1) (Table 1). Systolic blood pressure (BP) and heart rate (HR) measurements were available for both laboratory sessions for 74 subjects and diastolic BP for 73 subjects. The mean (SD) increase from the relaxation period to the period during speech delivery was 11.0 (8.0) mmHg for systolic BP, 7.6 (5.7) mmHg for diastolic BP and 11.1 (7.3) beats per minute for HR (all p-values <0.001). These increases are similar to those reported in other studies using a speech stressor in smokers (Kotlyar et al. 2006; Wardle et al. 2011) suggesting that this task presented within the virtual environment was effective at inducing a stressful state.
Figure 2.
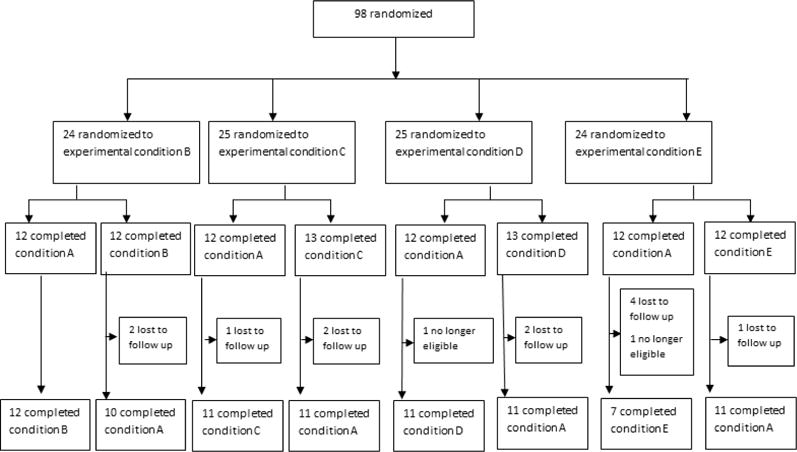
Flow of subjects through the study
Table 1.
Baseline demographics for those assigned to each condition
| Immediately before stressor (n=22) |
10 Minutes Before Stressor (n=22) |
20 Minutes Before Stressor (n=22) |
30 Minutes Before Stressor (n=18) |
p-value* | |
|---|---|---|---|---|---|
| (n=12 receiving control condition at 1st lab) | (n=11 receiving control condition at 1st lab) | (n=11 receiving control condition at 1st lab) | (n=7 receiving control condition at 1st lab) | ||
|
| |||||
| Age: mean (SD) | 37.2 (10.6) | 39.7 (10.9) | 38.1 (16.2) | 37.9 (14.1) | 0.93 |
|
| |||||
| Female subjects: n (%) | 8 (36.4%) | 10 (45.5%) | 14 (63.6%) | 9 (50.0%) | 0.33 |
|
| |||||
| Race: n (%) | |||||
| Caucasian | |||||
| African | 14 (63.6%) | 11 (50.0%) | 16 (72.7%) | 14 (77.8%) | |
| American | 7 (31.8%) | 8 (36.4%) | 4 (18.2%) | 1 (5.6%) | |
| American | 0 (0.0%) | 1 (4.6%) | 1 (4.6%) | 1 (5.6%) | 0.25† |
| Indian | 0 (0.0%) | 1 (4.6%) | 0 (0.0%) | 0 (0.0%) | |
| Asian | 1 (4.6%) | 0 (0.0%) | 1 (4.6%) | 1 (5.6%) | |
| More than one race | 0 (0.0%) | 1 (4.6%) | 0 (0.0%) | 1 (5.6%) | |
| Other | |||||
|
| |||||
| Number of cigarettes per day: mean (SD) | 14.4 (4.4) | 18.5 (8.8) | 14.7 (6.3) | 17.3 (7.1) | 0.14 |
|
| |||||
| Fagerstrom Test for Nicotine Dependence: mean (SD) | 4.5 (2.2) | 5.7 (2.2) | 4.3 (1.8) | 4.7 (2.2) | 0.14 |
|
| |||||
| BMI: mean (SD) | 25.6 (5.1) | 27.7 (7.3) | 25.5 (5.8) | 26.5 (6.5) | 0.61 |
The p-values are based on the ANOVA or the Chi-square tests
The p-value reflects the comparison of Caucasian, African American and other
3.1 Effect of nicotine lozenge timing on stress induced changes in withdrawal symptom and craving intensity
Among subjects who completed both laboratory sessions, magnitude of change from immediately pre-stressor to post-stressor (i.e., from time −0 to time +0 in Figure 3) in withdrawal symptom scores was smaller in Condition B (p=0.03; effect size=2.35) and Condition C (p=0.044; effect size=2.15) than in Condition A (i.e., the control condition). No significant difference from the control condition was seen in Condition D (effect size=0.30) or Condition E (effect size=0.16) (Figure 3). For factor 1 of the QSU, magnitude of change from pre-stress to post-stress was smaller in Conditions B and C than in Condition A (p values < 0.03) with no significant differences between Conditions D or E and Condition A (Table 2). For factor 2 of the QSU, a smaller increase was only seen in Condition B than in the control condition (p=0.025) (table 2).
Figure 3.
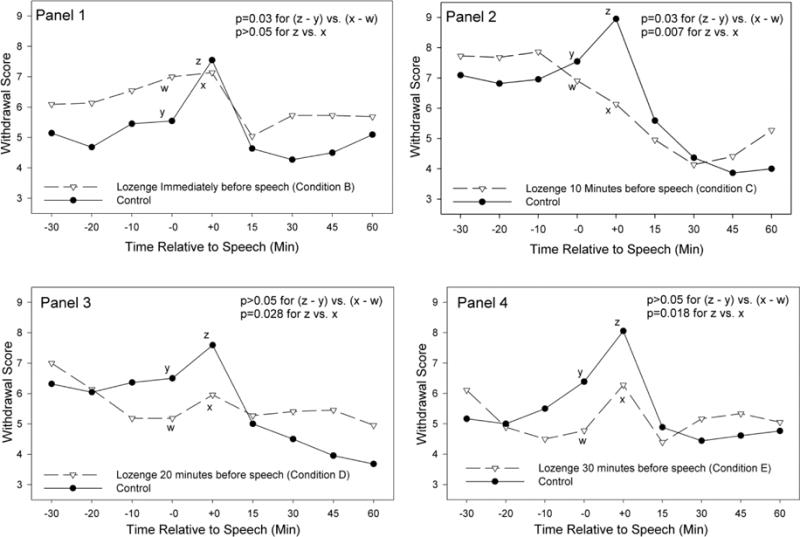
Withdrawal symptom severity when subjects received nicotine lozenge after stress exposure (i.e., control) and when subjects received nicotine lozenge immediately before (panel 1), 10 minutes (panel 2), 20 minutes (panel 3), and 30 minutes (panel 4) before speech. The speech task occurred between time −0 and +0 with assessments prior to the stressor indicated as (−) time and those after as (+) time. p value for (z – y vs. x – w) indicates significance of difference in stress response magnitude between experimental and control condition. p value for z vs. x indicates significance of difference in post stress withdrawal symptoms severity (where the value at time −30 was subtracted from the value at time +0 to adjust for baseline) between the experimental and control condition.
Table 2.
Mean (SD) of symptom score at baseline (i.e., 30 minutes prior to stressor) on factors 1 and 2 of the Questionnaire of Smoking Urges (QSU) and Least Squared Mean (95% CI) of difference in response between control and active conditions. Stress response was calculated as change in symptom severity (post-stress minus pre-stress). Stress response difference between control and active was calculated by subtracting the stress response for the experimental condition from stress response for its control condition. Post stress severity difference was calculated by subtracting post-stress severity score in experimental condition (controlled for baseline) from severity score in its control condition (controlled for baseline). Asterisk (*) indicates difference in score between experimental and control at p<0.05.
| Condition B | Condition C | Condition D | Condition E | ||||||
|---|---|---|---|---|---|---|---|---|---|
| QSU Factor 1 | Experimental | Control | Experimental | Control | Experimental | Control | Experimental | Control | |
| Baseline | 28.7 (7.8) | 26.8 (7.4) | 31.8 (2.9) | 30.3 (5.7) | 28.1 (8.1) | 28.9 (6.7) | 29.1 (6.9) | 30.4 (4.5) | |
| Stress response difference (control vs. active) | 5.17 (2.43, 7.91)* | 2.45 (0.29, 4.61)* | −1.05 (−3.60, 1.51) | −1.34 (−4.09, 1.40) | |||||
| Post stress severity difference (control vs. active) | 4.03 (1.49, 6.56)* | 4.82 (2.23, 7.42)* | 6.27 (2.97, 9.57)* | 6.61 (1.98, 11.24)* | |||||
| QSU Factor 2 | Experimental | Control | Experimental | Control | Experimental | Control | Experimental | Control | |
| Baseline | 10.5 (4.4) | 9.7 (4.5) | 13.5 (4.0) | 13.0 (3.2) | 9.5 (3.8) | 9.6 (4.1) | 10.3 (3.9) | 9.8 (4.0) | |
| Stress response difference (control vs. active) | 2.39 (0.34, 4.43)* | 0.68 (−1.00, 2.36) | 0.00 (−1.69, 1.69) | −0.23 (−1.11, 0.65) | |||||
| Post stress severity difference (control vs. active) | 2.43 (0.28, 4.58)* | 1.93 (0.11, 3.75)* | 2.59 (0.48, 4.70)* | 3.66 (1.60, 5.72)* | |||||
Comparing among the experimental conditions, a smaller stressor response in factor 1 of the QSU was observed in Condition B than in either Condition D or E (both p<0.01) and in Condition C compared to Condition E (p<0.01) (Table 2). No other significant differences among experimental groups were seen at the p<0.01 significance level.
3.2 Effect of nicotine lozenge timing on withdrawal symptom and craving severity experienced immediately after a stressor
Among subjects who completed both laboratory sessions, post-stressor severity in withdrawal symptom scores was lower in Condition C, (p=0.007; effect size 3.04), Condition D (p=0.028; effect size 2.37) and Condition E (p=0.018; effect size=2.63) compared to Condition A (i.e., the control condition) (Figure 3). No significant difference was found between Condition B and Condition A (effect size=1.64) (Figure 3). For both factor 1 and factor 2 of the QSU, symptom severity was lower in each of the experimental conditions than in the control condition (all p values <0.01 for QSU factor 1; all p values <0.04 for QSU factor 2) (Table 2).
Comparing the experimental conditions, post-stress symptom severity in the factor 1 score of the QSU was lower in Condition D than in Condition B (p<0.01). No other significant differences among experimental groups were seen at the p<0.01 significance level.
4. Discussion
This study demonstrated that the timing of nicotine lozenge administration, relative to the occurrence of a smoking trigger, has substantial effects on the extent to which withdrawal symptoms and craving are experienced. Specifically, significant differences were seen in both overall symptom severity and in the increase in symptom severity occurring in response to a smoking trigger based on the timing of nicotine lozenge administration. These results suggest that timing of nicotine lozenge use can be optimized to reduce the intensity of withdrawal symptom and craving severity observed after exposure to a smoking trigger and to reduce the extent to which craving and withdrawal symptom severity increase in response to a smoking trigger.
Previous studies assessing medicinal nicotine effects on trigger induced symptoms have primarily examined either the nicotine patch or nicotine gum given after (rather than before) exposure to a smoking trigger. The studies with nicotine patches suggest that although it is effective at reducing the background (i.e., baseline) level of craving, it has limited effect in blunting the increase in cravings that occurs when smokers are presented with smoking triggers (Havermans et al. 2003; Morissette et al. 2005; Rohsenow et al. 2007; Tiffany et al. 2000; Waters et al. 2004). Studies of more rapid acting nicotine dosage forms have also assessed the use of these products after cue exposure. Such studies have found that more rapid nicotine release formulations (i.e., rapid-release gum, film) result in more rapid declines in craving than standard formulations and that active nicotine gum results in more rapid craving declines than placebo gum (Du et al. 2014; Niaura et al. 2005; Shiffman et al. 2003). However, these studies do not address whether craving can be prevented by taking products prior to smoking trigger exposure.
Our findings that using nicotine lozenge prior to a stressor reduces overall levels of withdrawal and craving severity is consistent with multiple studies finding that medicinal nicotine use reduces withdrawal and craving symptom intensity (West and Shiffman 2001). Lower post-stress levels of craving severity observed when taking the lozenge further in advance of the stressor (i.e., 20 minutes vs. immediately prior to) is consistent with the pharmacokinetics of nicotine lozenge since nicotine concentrations increase for approximately 30 minutes after starting use (Benowitz et al. 1988; Kotlyar et al. 2007). Interestingly, the effect on stress response was observed when smokers took the lozenge either immediately prior to or 10 minutes prior to the stressor while no effect was seen when the lozenge was taken further in advance of the stressor. This is consistent with a study in which the use of nicotine lozenge 30 minutes prior to cue exposure did not affect cue induced craving increases (Schlagintweit et al. 2014). The reasons for this result are not clear. The average time from when subjects took the lozenge (in Condition B) to when they began completing the post-stressor questionnaire was approximately 9 minutes which is when the most rapid increases in nicotine concentrations would be expected (Benowitz et al. 1988; Kotlyar et al. 2007). Rapidly increasing nicotine concentrations may therefore be necessary for smaller stress induced craving and withdrawal symptom scores to occur. It is also possible that taking the nicotine lozenge in close proximity to the stressor provided smokers with a coping mechanism to be able to better respond to the stressor, consistent with literature demonstrating that the expectation of receiving nicotine decreases craving severity (Schlagintweit et al. 2014). The lack of a placebo condition in this initial study precludes the ability to determine the mechanism (i.e., pharmacologic vs behavioral) by which stress induced craving and withdrawal symptom increases were attenuated.
Regardless of the mechanism, the data suggest that providing a lozenge prior to but in relatively close temporal proximity to a stressor decreases the extent to which craving and withdrawal symptoms increase as a result of stress exposure. Since smoking triggers likely become increasingly predictable as their occurrence becomes more imminent, these results suggest that many smokers may be able to use nicotine lozenges in a manner that would decrease craving and withdrawal symptom response to stressors. Future studies addressing the mechanism by which this occurs will allow for the identification of additional interventions (either behavioral or pharmacologic) to further decrease trigger induced craving and withdrawal symptoms.
Additional limitations of the current study are that the stress induced increases in symptom severity were relatively small, that this study only tested one smoking trigger, and that it is not known if an attenuation of craving and withdrawal symptoms in a laboratory setting would lead to increased smoking cessation rates in a naturalistic setting. The small increases in symptom severity may have been due to a ceiling effect observed in these symptoms since subjects were asked to abstain from smoking overnight prior to each laboratory session. For example, the pre-stress QSU factor 1 score in the control condition (i.e., Condition A) was approximately 30 out of a maximum score of 35. It is not clear how the magnitude of these increases compare to the magnitude of stress induced increases that would occur during a smoking cessation attempt. Although baseline (i.e., pre-stress) craving and withdrawal symptom severity in the initial days of a cessation attempt are likely to be high (similar to what occurred in this study), baseline symptom severity would likely decrease over time. Further research is needed to determine if the effects of nicotine lozenge on stress induced craving and withdrawal symptom severity differ depending on how long the smoker has been abstinent It is noteworthy that despite the relatively small stress induced changes in craving that occurred in the control condition, changes were significantly smaller when subjects took the nicotine lozenge in close proximity prior to the stressor. These data suggest that advising smokers to use nicotine lozenge prior to anticipated smoking trigger exposure may have benefits in decreasing craving and withdrawal symptom severity. Further research is needed to determine if such an approach is applicable across multiple smoking triggers and most importantly if it would result in increased smoking cessation success. This is particularly true as there is debate regarding the relative importance of cue induced changes in craving as opposed to absolute levels of craving on predicting successful cessation (Ferguson and Shiffman 2009; Perkins 2012; Sayette and Tiffany 2013). The results of this study are promising in that both absolute levels of craving and cue induced cravings were decreased by altering the timing of nicotine lozenge administration.
In summary, this study found that using nicotine lozenge prior to (rather than after) exposure to a stressor resulted in smaller stress induced increases in withdrawal symptom and urge to smoke severity as well as lower overall post-stressor severity of these symptoms. These data suggest that it may be possible to increase the efficacy of rapid acting forms of medicinal nicotine (such as the nicotine lozenge or nicotine gum) by altering the instructions provided to smokers about how to most effectively use these products. However, a clinical study is needed to determine if this is indeed the case.
Highlights.
Nicotine lozenge is often recommended to be used on an as needed basis which for many smokers means when symptoms of craving and withdrawal are present
Using nicotine lozenge after craving and withdrawal symptoms occur may be too late to prevent relapse
Using nicotine lozenge prior to (rather than after) exposure to a smoking trigger resulted in smaller trigger induced increases in withdrawal symptom and urge to smoke severity as well as lower overall post-stressor severity of these symptoms
Acknowledgments
Funding Sources:
This work was supported by the National Institutes of Health (grant #’s R21DA029689, UL1TR000114). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
This study was designed by MK, JV, CL and DKH. The research was performed by MK, JV, AMM and EA. Data was analyzed by BL and MK. The manuscript was written by MK and BL with all authors reviewing and contributing to the final version of manuscript
Conflict of Interest:
Dr. Kotlyar has received grant funding through the Global Research Award for Nicotine Dependence (GRAND) program funded by Pfizer.
References
- al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74:401–10. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- Back SE, Waldrop AE, Saladin ME, Yeatts SD, Simpson A, McRae AL, Upadhyaya HP, Contini Sisson R, Spratt EG, Allen J, Kreek MJ, Brady KT. Effects of gender and cigarette smoking on reactivity to psychological and pharmacological stress provocation. Psychoneuroendocrinology. 2008;33:560–8. doi: 10.1016/j.psyneuen.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Sheiner L, Jacob P., 3rd Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44:23–8. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Cummings KM, Jaen CR, Giovino G. Circumstances surrounding relapse in a group of recent exsmokers. Prev Med. 1985;14:195–202. doi: 10.1016/0091-7435(85)90035-0. [DOI] [PubMed] [Google Scholar]
- Du D, Nides M, Borders J, Selmani A, Waverczak W. Comparison of nicotine oral soluble film and nicotine lozenge on efficacy in relief of smoking cue-provoked acute craving after a single dose of treatment in low dependence smokers. Psychopharmacology (Berl) 2014;231:4383–91. doi: 10.1007/s00213-014-3586-2. [DOI] [PubMed] [Google Scholar]
- Fant RV, Owen LL, Henningfield JE. Nicotine replacement therapy. Prim Care. 1999;26:633–52. doi: 10.1016/s0095-4543(05)70121-4. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36:235–43. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz L, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Orleans PDMCT. Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Clinical Practice Guidelines. U.S. Department of Health and Human Services, Public Health Service; Rockville, MD: 2008. Treating tobacco use and dependence: 2008 Update. [Google Scholar]
- Havermans RC, Debaere S, Smulders FT, Wiers RW, Jansen AT. Effect of cue exposure, urge to smoke, and nicotine deprivation on cognitive performance in smokers. Psychol Addict Behav. 2003;17:336–9. doi: 10.1037/0893-164X.17.4.336. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tob Control. 1998;7:92–3. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Tobacco withdrawal in self-quitters. J Consult Clin Psychol. 1992;60:689–97. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ’Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kotlyar M, Brauer LH, al’absi M, Adson DE, Robiner W, Thuras P, Harris J, Finocchi ME, Bronars CA, Candell S, Hatsukami DK. Effect of bupropion on physiological measures of stress in smokers during nicotine withdrawal. Pharmacol Biochem Behav. 2006;83:370–9. doi: 10.1016/j.pbb.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Kotlyar M, Donahue C, Thuras P, Kushner MG, O’Gorman N, Smith EA, Adson DE. Physiological response to a speech stressor presented in a virtual reality environment. Psychophysiology. 2008;45:1034–7. doi: 10.1111/j.1469-8986.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- Kotlyar M, Drone D, Thuras P, Hatsukami DK, Brauer L, Adson DE, al’Absi M. Effect of stress and bupropion on craving, withdrawal symptoms, and mood in smokers. Nicotine Tob Res. 2011;13:492–7. doi: 10.1093/ntr/ntr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyar M, Mendoza-Baumgart MI, Li ZZ, Pentel PR, Barnett BC, Feuer RM, Smith EA, Hatsukami DK. Nicotine pharmacokinetics and subjective effects of three potential reduced exposure products, moist snuff and nicotine lozenge. Tob Control. 2007;16:138–42. doi: 10.1136/tc.2006.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, Giovino GA, Edwards B, Difranza J, Foulds J, Hurt R, Niaura R, Sachs DP, Selby P, Dollar KM, Bowen D, Cummings KM, Counts M, Fox B, Sweanor D, Ahern F. Advice on using over-the-counter nicotine replacement therapy-patch, gum, or lozenge-to quit smoking. Addict Behav. 2007;32:2140–50. doi: 10.1016/j.addbeh.2007.01.030. [DOI] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A, Foulds J, Bates C. Regulation of nicotine replacement therapies (NRT): a critique of current practice. Addiction. 2001;96:1757–68. doi: 10.1080/09652140120089508. [DOI] [PubMed] [Google Scholar]
- Morissette SB, Palfai TP, Gulliver SB, Spiegel DA, Barlow DH. Effects of transdermal nicotine during imaginal exposure to anxiety and smoking cues in college smokers. Psychol Addict Behav. 2005;19:192–8. doi: 10.1037/0893-164X.19.2.192. [DOI] [PubMed] [Google Scholar]
- Niaura R, Sayette M, Shiffman S, Glover ED, Nides M, Shelanski M, Shadel W, Koslo R, Robbins B, Sorrentino J. Comparative efficacy of rapid-release nicotine gum versus nicotine polacrilex gum in relieving smoking cue-provoked craving. Addiction. 2005;100:1720–30. doi: 10.1111/j.1360-0443.2005.01218.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Subjective reactivity to smoking cues as a predictor of quitting success. Nicotine Tob Res. 2012;14:383–7. doi: 10.1093/ntr/ntr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF. The effects of a psychological stressor on cigarette smoking and subsequent behavioral and physiological responses. Psychophysiology. 1987;24:278–85. doi: 10.1111/j.1469-8986.1987.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Puska KH, Vartiainen E, Urjanheimo EL, Gustavsson G, Westin A. Combined use of nicotine patch and gum compared with gum alone in smoking cessation: a clinical trial in North Karelia. Tobacco Control. 1995;4:231–5. [Google Scholar]
- Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, MacKinnon SV, Sirota AD, Kaplan GB. High-dose transdermal nicotine and naltrexone: effects on nicotine withdrawal, urges, smoking, and effects of smoking. Exp Clin Psychopharmacol. 2007;15:81–92. doi: 10.1037/1064-1297.15.1.81. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Tiffany ST. Peak provoked craving: an alternative to smoking cue-reactivity. Addiction. 2013;108:1019–25. doi: 10.1111/j.1360-0443.2012.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagintweit HE, Good KP, Barrett SP. The impact of nicotine lozenges and stimulus expectancies on cigarette craving. J Psychopharmacol. 2014;28:773–779. doi: 10.1177/0269881113519508. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–79. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Shadel WG, Niaura R, Khayrallah MA, Jorenby DE, Ryan CF, Ferguson CL. Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacology. 2003;166:343–50. doi: 10.1007/s00213-002-1338-1. [DOI] [PubMed] [Google Scholar]
- Steinberg MB, Greenhaus S, Schmelzer AC, Bover MT, Foulds J, Hoover DR, Carson JL. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447–54. doi: 10.7326/0003-4819-150-7-200904070-00004. [DOI] [PubMed] [Google Scholar]
- Sweeney CT, Fant RV, Fagerstrom KO, McGovern JF, Henningfield JE. Combination nicotine replacement therapy for smoking cessation: rationale, efficacy and tolerability. CNS Drugs. 2001;15:453–67. doi: 10.2165/00023210-200115060-00004. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J Consult Clin Psychol. 2000;68:233–40. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Toll BA, Katulak NA, McKee SA. Investigating the factor structure of the Questionnaire on Smoking Urges-Brief (QSU-Brief) Addict Behav. 2006;31:1231–9. doi: 10.1016/j.addbeh.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS. The health consequences of smoking - 50 years of progress: A report of the Surgeon General. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- USPHS. Reducing tobacco use: a report of the Surgeon General. Dept. of Health and Human Services, Dept. of Health and Human Services; 2000. [Google Scholar]
- Wardle MC, Munafo MR, de Wit H. Effect of social stress during acute nicotine abstinence. Psychopharmacology (Berl) 2011;218:39–48. doi: 10.1007/s00213-010-2150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J Consult Clin Psychol. 2004;72:1136–43. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- West R, Shiffman S. Effect of oral nicotine dosing forms on cigarette withdrawal symptoms and craving: a systematic review. Psychopharmacology. 2001;155:115–22. doi: 10.1007/s002130100712. [DOI] [PubMed] [Google Scholar]


