Abstract
Background
To limit the potential short and long-term morbidity of lymphadenectomy, sentinel lymph node (SLN) biopsy has been proposed for endometrial cancer. The principle of SLN biopsy relies on removal of a small number of lymph nodes that are the first drainage basins from a tumor and thus the most likely to harbor tumor cells. While the procedure may reduce morbidity, efficacy data is limited and little is known about how commonly the procedure is performed.
Objective
We examined the patterns and predictors of use of SLN biopsy and outcomes of the procedure in women with endometrial cancer who underwent hysterectomy.
Methods
We used the Perspective database to identify women with uterine cancer who underwent hysterectomy from 2011–2015. Billing and charge codes were used to classify women as having undergone lymphadenectomy, SLN biopsy, or no nodal assessment. Multivariable models were used to examine clinical, demographic, and hospital characteristics with use of SLN biopsy. Length of stay and cost were compared among the different methods of nodal assessment.
Results
Among 28,362 patients, 9327 (32.9%) did not undergo nodal assessment, 17,669 (62.3%) underwent lymphadenectomy, and 1366 (4.8%) underwent SLN biopsy. SLN biopsy was performed in 1.3% (95% CI, 1.0–1.6%) of abdominal hysterectomies, 3.4% (95% CI, 2.7–4.1%) of laparoscopic hysterectomies, and 7.5% (95% CI, 7.0–8.0%) of robotic-assisted hysterectomies. In a multivariable model, more recent year of surgery was associated with performance of SLN biopsy. Compared to abdominal hysterectomy, those undergoing laparoscopic (aRR=2.45; 95% CI, 1.89–3.18) and robotic-assisted (aRR=2.69; 95% CI, 2.19–3.30) hysterectomy were more likely to undergo SLN biopsy. Among women who underwent minimally invasive hysterectomy, length of stay and cost were lower for SLN biopsy compared to lymphadenectomy.
Conclusion
The use of SLN biopsy for endometrial cancer increased from 2011–2015. The increased use was most notable in women who underwent a robotic-assisted hysterectomy.
Introduction
Although lymphadenectomy is commonly performed as part of the surgical treatment of endometrial cancer, the role of the procedure remains controversial.1,2 Node dissection provides important prognostic information, may help guide adjuvant treatment, and has been suggested in some studies to have a therapeutic effect.1–4 More recently, however, randomized trials have failed to show a survival benefit for lymphadenectomy.5,6 Importantly, these studies also demonstrated that lymphadenectomy was associated with an increased risk of complications and called into question the value of the procedure.5,6
To limit the potential short and long-term morbidity of lymphadenectomy, sentinel lymph node (SLN) biopsy has been proposed for endometrial cancer.7–10 The principle of SLN biopsy relies on removal of a small number of lymph nodes that are the first drainage basins from a tumor and thus the most likely to harbor tumor cells.11,12 SLN biopsy has been extensively validated for a number of other solid tumors and is now in the standard of care in breast cancer, vulvar cancer, and melanoma.13–15
Despite the potential benefits of SLN biopsy for endometrial cancer, the appropriate role of the procedure is uncertain. While some studies have examined the performance of SLN biopsy compared to lymphadenectomy, whether SLN biopsy can be used in lieu of lymphadenectomy and in which patients is unknown.11 Further, as randomized trials have been unable to demonstrate a survival benefit even with full lymphadenectomy, some have questioned why SLN biopsy would be utilized at all for apparent early-stage endometrial cancer.5,6 Given the limited data and uncertainty surrounding SLN biopsy for endometrial cancer, we examined the patterns and predictors of use of SLN biopsy in women with newly diagnosed endometrial cancer undergoing surgery.
Methods
Data Source
We analyzed the Perspective database (Premier, Inc., Charlotte, North Carolina), an all-payer database that has been utilized to examine treatment patterns and quality of care of hospitalized patients in the United States.16 Perspective captures data from over 500 hospitals and includes demographic characteristics, medical diagnoses and procedures as well as data on medication and device utilization. This study was deemed exempt by the Columbia University Institutional Review Board.
Patients and Procedures
Women with a diagnosis of uterine cancer (ICD-9 179, 182.x) who underwent hysterectomy from January 2011 to March 2015 were analyzed. Patients were stratified based on the route of hysterectomy into the following groups: abdominal hysterectomy (ICD-9 68.3, 68.39, 68.4, 68.49, 68.6, 68.69, 68.9), laparoscopic hysterectomy (ICD-9 68.31, 68.41, 68.51, 68.61, 68.71) and robotically assisted hysterectomy (any hysterectomy code in combination with ICD-9 17.4x). To limit the number of women with carcinomatosis, patients who underwent concurrent extended abdominal surgery (small or large bowel resection, splenectomy, diaphragm resection, liver resection, and bladder resection) were excluded.
The cohort was stratified based on performance of lymphadenectomy. Performance of lymphadenectomy was based on the identification of either an ICD-9 code for lymph node evaluation (ICD-9 40.1, 40.11, 40.2, 40.29, 40.3, 40.5, 40.50, 40.52, 40.53, 40.59) or a CPT that included nodal evaluation (CPT 58200, 58210, 58548, 38500, 38562, 38564, 38571, 38570, 38572, 38589, 38770, 38780). Sentinel lymph node biopsy was identified based on the presence of a CPT code for radiopharmaceutical mapping (38792, 38900, 78195, 78800, 78801) or of a hospital charge code for a substance used for sentinel lymph node biopsy (technetium-99, isosulfan blue, patent blue, sulphan blue, or indocyanine green). We also identified patients with a charge code for methylene blue, which could have been used for either SLN biopsy or evaluation of the genitourinary tract. The primary analysis of SLN biopsy did not include patients who had a charge code for methylene blue. A sensitivity analysis was conducted in which patients who had a charge code for methylene blue were considered to have undergone SLN biopsy. Patients with codes for both a lymphadenectomy and SLN biopsy were categorized as having undergone SLN biopsy. Women who did not have codes for either lymphadenectomy or SLN biopsy were classified as not having undergone nodal evaluation.
Covariates and Outcomes
Demographic characteristics included age at the time of hysterectomy (<40, 40–49, 50–59, 60–69, ≥70), year of the surgical procedure (2011–2015), marital status (married, single, other/unknown), race (white, black, other/unknown) and insurance coverage (commercial, Medicare, Medicaid, uninsured, unknown). The Elixhauser comorbidity index was used to perform risk adjustment for medical comorbidities. Patients were classified as having 0, 1, or ≥2 comorbidities.17
Hospital characteristics examined included hospital location (urban vs rural), whether the hospital was a teaching hospital (non-teaching vs teaching), hospital bed size (<400, 400–600, >600 beds), and region of the country in which the hospital was located (Northeastern, Midwest, South, West). Procedure volume was estimated as annualized hospital volume and calculated as the total number of hysterectomies performed by a given hospital divided by the number of quarters in which that hospital performed at least one operation and multiplied by 4. Annualized hospital volume was included as a continuous variable in the regression models.
Length of stay and hospital cost were analyzed. Length of stay was estimated as the number of hospital days from the time the hysterectomy was performed until discharge from the hospital.18 Cost was captured from hospital-level costs that are reported by facilities. Perspective captures cost data through a log of all services, treatments, and items that are billed to a patient during the hospital stay.16 Within the database, approximately three-quarters of hospitals report direct cost based on procedural accounting, while the remaining 25% of hospitals estimate cost based on Medicare cost-to-charge ratios.19,20 The total hospital cost for the index admission in which the hysterectomy was performed was captured for each patient. The recorded cost was adjusted for inflation using the Consumer Price Index and reported in 2015 U.S. dollars.21 To limit the influence of extreme costs, costs estimates were winsorized to the 3rd and 97th percentiles.
Statistical Analysis
Frequency distributions between categorical variables were compared using χ2 tests. The trends in use of SLN biopsy over time, stratified by mode of hysterectomy are reported using Cochran-Armitage trend tests. Cost data and length of stay are reported as medians with interquartile ranges, and compared using the Kruskal-Wallis non-parametric tests.
The association between the clinical, demographic, and hospital characteristics and performance of SLN biopsy were examined using multivariate random-intercept Poisson regression models. These models include all of the reported covariates as well as a hospital-specific intercept to account for clustering at the hospital-level. A sensitivity analysis was performed in which the cohort was limited to only women who underwent a minimally invasive hysterectomy (laparoscopic or robotic-assisted). A second sensitivity analysis was undertaken and included only patients who underwent surgery in 2014–2015. Results are reported as adjusted risk ratios (aRR) and 95% confidence intervals.
Quantile (median) regression models were developed to estimate the association between SLN biopsy and cost after adjusting for other clinical and hospital characteristics.22 This methodology directly estimates the adjusted median costs for each covariate, while 95% confidence intervals were derived based on bootstrap resampling methods.23 Results are reported as differences in the median from the referent group and 95% confidence intervals. All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, North Carolina). All statistical tests were two-sided.
Results
A total of 28,362 patients treated at 463 hospitals were identified. The cohort included 9480 (33.4%) women who underwent abdominal hysterectomy, 3915 (13.8%) who underwent laparoscopic hysterectomy, and 14,967 (52.8%) who had a robotically assisted procedure (Table 1). Overall, 9327 (32.9%) patients did not undergo lymph node assessment, 17,669 (62.3%) underwent lymphadenectomy, and 1366 (4.8%) underwent SLN biopsy. SLN biopsy was performed in 1.3% (95% CI, 1.0–1.6%) of abdominal hysterectomies, 3.4% (95% CI, 2.7–4.1%) of laparoscopic hysterectomies, and 7.5% (95% CI, 7.0–8.0%) of robotic-assisted hysterectomies.
Table 1.
Demographic and clinical characteristics by lymph node assessment in women with endometrial cancer.
| Sentinel lymph node biopsy | Lymph node dissection | No nodal assessment | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| All | 1,366 | (4.8) | 17,669 | (62.3) | 9,327 | (32.9) | |
| Age (years) | <0.001 | ||||||
| <40 | 42 | (5.0) | 432 | (51.8) | 360 | (43.2) | |
| 40–49 | 85 | (3.6) | 1,286 | (54.1) | 1,008 | (42.4) | |
| 50–59 | 352 | (4.6) | 4,617 | (60.7) | 2,634 | (34.6) | |
| 60–69 | 491 | (4.9) | 6,552 | (64.8) | 3,073 | (30.4) | |
| ≥70 | 396 | (5.3) | 4,782 | (64.4) | 2,252 | (30.3) | |
| Year | <0.001 | ||||||
| 2011 | 100 | (1.6) | 4,251 | (66.0) | 2,091 | (32.5) | |
| 2012 | 160 | (2.3) | 4,484 | (63.1) | 2,464 | (34.7) | |
| 2013 | 446 | (6.2) | 4,412 | (61.3) | 2,345 | (32.6) | |
| 2014 | 525 | (8.3) | 3,750 | (59.3) | 2,054 | (32.5) | |
| 2015 | 135 | (10.6) | 772 | (60.3) | 373 | (29.1) | |
| Marital status | <0.001 | ||||||
| Married | 572 | (4.4) | 8,093 | (62.6) | 4,268 | (33.0) | |
| Single | 446 | (3.9) | 7,213 | (62.5) | 3,887 | (33.7) | |
| Other/unknown | 348 | (9.0) | 2,363 | (60.9) | 1,172 | (30.2) | |
| Race | <0.001 | ||||||
| White | 969 | (4.8) | 12,308 | (61.1) | 6,865 | (34.1) | |
| Black | 71 | (2.5) | 1,893 | (66.0) | 906 | (31.6) | |
| Other/unknown | 326 | (6.1) | 3,468 | (64.8) | 1,556 | (29.1) | |
| Insurance status | <0.001 | ||||||
| Commercial | 669 | (5.2) | 7,846 | (61.4) | 4,260 | (33.4) | |
| Medicare | 578 | (4.8) | 7,690 | (63.7) | 3,801 | (31.5) | |
| Medicaid | 72 | (4.3) | 974 | (58.6) | 616 | (37.1) | |
| Uninsured | 22 | (2.1) | 665 | (61.9) | 388 | (36.1) | |
| Unknown | 25 | (3.2) | 494 | (63.3) | 262 | (33.6) | |
| Hospital location | <0.001 | ||||||
| Urban | 1,360 | (5.0) | 16,892 | (62.3) | 8,847 | (32.7) | |
| Rural | 6 | (0.5) | 777 | (61.5) | 480 | (38.0) | |
| Hospital teaching status | <0.001 | ||||||
| Teaching | 958 | (5.7) | 10,678 | (63.5) | 5,175 | (30.8) | |
| Non-teaching | 408 | (3.5) | 6,991 | (60.5) | 4,152 | (35.9) | |
| Hospital bed size | <0.001 | ||||||
| <400 | 614 | (5.4) | 6,723 | (58.8) | 4,106 | (35.9) | |
| 400–600 | 292 | (3.8) | 4,861 | (62.9) | 2,573 | (33.3) | |
| >600 | 460 | (5.0) | 6,085 | (66.2) | 2,648 | (28.8) | |
| Hospital region | <0.001 | ||||||
| Northeastern | 102 | (1.9) | 3,384 | (62.9) | 1,893 | (35.2) | |
| Midwest | 241 | (5.4) | 2,685 | (59.7) | 1,573 | (35.0) | |
| South | 601 | (4.4) | 8,861 | (64.5) | 4,285 | (31.2) | |
| West | 422 | (8.9) | 2,739 | (57.8) | 1,576 | (33.3) | |
| Comorbidity (Elixhauser index) | <0.001 | ||||||
| 0 | 216 | (8.8) | 1,463 | (59.4) | 786 | (31.9) | |
| 1 | 261 | (4.9) | 3,436 | (64.2) | 1,653 | (30.9) | |
| ≥2 | 889 | (4.3) | 12,770 | (62.2) | 6,888 | (33.5) | |
| Route of Hysterectomy | <0.001 | ||||||
| Abdominal | 119 | (1.3) | 5,960 | (62.9) | 3,401 | (35.9) | |
| Laparoscopic | 132 | (3.4) | 2,014 | (51.4) | 1,769 | (45.2) | |
| Robotic | 1,115 | (7.5) | 9,695 | (64.8) | 4,157 | (27.8) | |
| Annualized hospital volume median (IQR) | 125 | (44–211) | 60 | (36–89) | 49 | (21–76) | <0.001 |
IQR: interquartile range, P values calculated using χ2 tests.
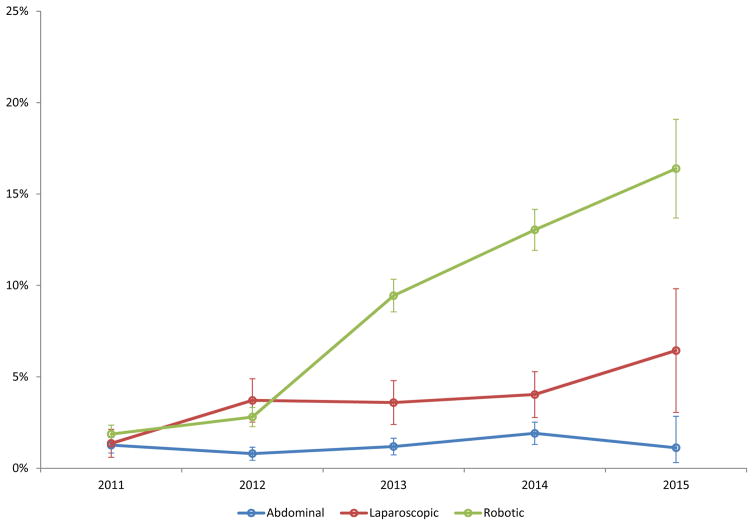
Among the women who underwent SLN biopsy, 850 (62.2%) had the procedure performed using indocyanine green, 523 (38.3%) using isosulfan blue and 133 (9.7%) with technetium-99. Use of indocyanine green (P<0.0001) and isosulfan blue (P<0.0001) both increased over time while technetium-99 use (P=0.19) remained stable (Table 2). The use of SLN biopsy for women who underwent abdominal hysterectomy was 1.3% in 2011 and then rose to 1.9% in 2014 and declined slightly to 1.1% in 2015 (P=0.09) (Figure 1). For laparoscopic hysterectomy, the rate of SLN biopsy rose from 1.4% in 2011 to 6.4% in 2015 (P<0.001). For robotic hysterectomy, the rate of SLN biopsy rose year after year from 1.9% in 2011 to 16.4% in 2015 (P<0.001).
Table 2.
Use of sentinel node techniques over time.
| 2011 | 2012 | 2013 | 2014 | 2015 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| N | % | N | % | N | % | N | % | N | % | ||
| SLN biopsy | 100 | 1.6 | 160 | 2.3 | 446 | 6.2 | 525 | 8.3 | 135 | 10.6 | <0.001 |
| Indocyanine green | 44 | 0.7 | 102 | 1.4 | 320 | 4.4 | 292 | 4.6 | 92 | 7.2 | <0.001 |
| Isosulfan blue | 14 | 0.2 | 20 | 0.3 | 180 | 2.5 | 247 | 3.9 | 62 | 4.8 | <0.001 |
| Technetium-99 | 43 | 0.7 | 26 | 0.4 | 28 | 0.4 | 30 | 0.5 | 6 | 0.5 | 0.19 |
P-values were from Cochran-Armitage trend tests. Techniques are not mutually exclusive.
Figure 1.
Use of sentinel lymph node biopsy in women with endometrial cancer stratified by route of hysterectomy (P-values from Cochran-Armitage trend test: abdominal 0.09; laparoscopic <0.001; robotic <0.001). Error bars represent 95% confidence intervals.
In a multivariate model, more recent year of surgery was associated with performance of SLN biopsy. Compared to women operated on in 2011, the adjusted risk ratio for SLN biopsy for those treated in 2014 was 5.06 (95% CI, 4.06–6.30) and in 2015 was 6.26 (95% CI, 4.80–8.16) (Table 3). Similarly, compared to abdominal hysterectomy, those undergoing laparoscopic (aRR=2.45; 95% CI, 1.89–3.18) and robotic-assisted (aRR=2.69; 95% CI, 2.19–3.30) hysterectomy were more likely to undergo SLN biopsy. Patients with 2 or more comorbidities were also more likely to undergo SLN biopsy. There was no association between hospital characteristics and use of SLN biopsy.
Table 3.
Multivariable models of predictors of sentinel lymph node biopsy.
| Entire Cohort aRR | Minimally invasive hysterectomy aRR | 2014–2015 aRR | |
|---|---|---|---|
| Age | |||
| <40 | Referent | Referent | Referent |
| 40–49 | 0.68 (0.47–0.99)* | 0.64 (0.43–0.93)* | 0.79 (0.47–1.31) |
| 50–59 | 0.90 (0.65–1.24) | 0.84 (0.60–1.16) | 0.85 (0.54–1.34) |
| 60–69 | 1.01 (0.73–1.40) | 0.92 (0.66–1.28) | 0.87 (0.55–1.37) |
| ≥70 | 1.04 (0.73–1.47) | 0.91 (0.64–1.30) | 0.91 (0.56–1.49) |
| Year | |||
| 2011 | Referent | Referent | – |
| 2012 | 1.46 (1.14–1.88)* | 1.89 (1.41–2.53)* | – |
| 2013 | 3.41 (2.73–4.26)* | 4.50 (3.46–5.85)* | – |
| 2014 | 5.06 (4.06–6.30)* | 6.66 (5.13–8.64)* | Referent |
| 2015 | 6.26 (4.80–8.16)* | 8.43 (6.24–11.40)* | 1.17 (0.96–1.42) |
| Marital status | |||
| Married | Referent | Referent | Referent |
| Single | 0.95 (0.83–1.08) | 0.96 (0.84–1.10) | 1.02 (0.85–1.21) |
| Other/unknown | 1.24 (0.95–1.63) | 1.22 (0.91–1.64) | 1.22 (0.79–1.89) |
| Race | |||
| White | Referent | Referent | Referent |
| Black | 0.92 (0.71–1.18) | 0.85 (0.64–1.13) | 0.98 (0.69–1.39) |
| Other/unknown | 0.95 (0.80–1.12) | 0.91 (0.76–1.08) | 1.07 (0.84–1.35) |
| Insurance status | |||
| Commercial | Referent | Referent | Referent |
| Medicare | 0.89 (0.76–1.04) | 0.90 (0.77–1.06) | 0.91 (0.72–1.13) |
| Medicaid | 0.89 (0.69–1.14) | 0.90 (0.69–1.18) | 0.84 (0.59–1.21) |
| Uninsured | 0.72 (0.47–1.10) | 0.65 (0.39–1.08) | 0.61 (0.30–1.26) |
| Unknown | 1.19 (0.79–1.79) | 1.21 (0.78–1.86) | 1.05 (0.53–2.08) |
| Hospital location | |||
| Urban | Referent | Referent | – |
| Rural | 0.72 (0.23–2.26) | 0.61 (0.09–3.99) | – |
| Hospital teaching status | |||
| Teaching | Referent | Referent | Referent |
| Non-teaching | 0.81 (0.42–1.59) | 0.75 (0.30–1.87) | 0.53 (0.21–1.35) |
| Hospital bed size | |||
| <400 | Referent | Referent | Referent |
| 400–600 | 0.61 (0.30–1.23) | 0.65 (0.26–1.63) | 0.41 (0.15–1.13) |
| >600 | 0.49 (0.22–1.11) | 0.28 (0.09–0.89)* | 0.36 (0.12–1.08) |
| Hospital region | |||
| Northeastern | Referent | Referent | Referent |
| Midwest | 0.83 (0.35–2.00) | 1.11 (0.35–3.57) | 1.04 (0.31–3.52) |
| South | 0.80 (0.37–1.73) | 0.81 (0.28–2.37) | 0.63 (0.21–1.88) |
| West | 0.92 (0.36–2.39) | 1.15 (0.32–4.05) | 2.07 (0.55–7.83) |
| Comorbidity (Elixhauser) | |||
| 0 | Referent | Referent | Referent |
| 1 | 1.08 (0.89–1.30) | 1.12 (0.93–1.36) | 1.06 (0.82–1.36) |
| ≥2 | 1.19 (1.01–1.41)* | 1.22 (1.03–1.44)* | 1.11 (0.89–1.38) |
| Hysterectomy | |||
| Abdominal | Referent | – | Referent |
| Laparoscopic | 2.45 (1.89–3.18)* | Referent | 2.17 (1.40–3.36)* |
| Robotic | 2.69 (2.19–3.30)* | 1.06 (0.87–1.28) | 3.39 (2.41–4.79)* |
| Volume (increase in 1 unit) | 1.003 (0.995–1.012) | 1.009 (0.998–1.021) | 1.008 (0.997–1.020) |
P-value <0.05
aRR: adjusted risk ratio
Mixed-effect models were fitted accounting for hospital-level clustering using random-intercept log-Poisson regression models. The covariates included age, year, marital status, race, insurance status, hospital location, teaching status, bed size, region, comorbidity, route of hysterectomy and annualized hospital volume. Hospital location was not adjusted for in the model limiting to 2014–2015 because of convergence issue.
In a model limited to women who underwent minimally invasive hysterectomy, year of surgery and increased comorbidity remained associated with SLN biopsy (Table 2). In this model, women treated at larger hospitals were less likely to undergo SLN biopsy. In an analysis limited to 2014 and 2015, mode of hysterectomy was the only factor associated with SLN biopsy; those women who underwent either a laparoscopic or robotic-assisted hysterectomy were more likely to undergo SLN biopsy.
Among women who underwent a laparoscopic or robotic-assisted hysterectomy, the median length of stay was 0 days (IQR, 0-1) in women who underwent no nodal assessment, 1 day (IQR, 0-1) in those who had a lymphadenectomy, and 0 days (IQR, 0-1) in women who underwent SLN biopsy (Table 4). The median hospital cost for women who underwent a minimally invasive hysterectomy without nodal assessment was $8877 (IQR, $6974–11,352). In comparison, the median cost of minimally invasive hysterectomy with lymphadenectomy was $10,259 (IQR, $7807–13,034) and $9550 (IQR, $7278–13,124) for SLN biopsy. In an adjusted model, compared to no nodal assessment, lymphadnectomy was associated with $1418 (95% CI, $1300–1536) in increased median costs while SLN biopsy was associated with a $950 (95% CI, $697–1204) increase in median cost.
Table 4.
Length of stay and cost associated with lymph node assessment in women with endometrial cancer who underwent robotic-assisted or laparoscopic hysterectomy.
| Sentinel lymph node biopsy | Lymph node dissection | No nodal assessment | |||||
|---|---|---|---|---|---|---|---|
| Median | (IQR) | Median | (IQR) | Median | (IQR) | P-value | |
| Length of stay | 0 | (0–1) | 1 | (0–1) | 0 | (0–1) | <0.001 |
| Total cost (adjusted) | |||||||
| Univariable | $9550 | ($7278–13,124) | $10,259 | ($7807–13,034) | $8877 | ($6974–11,352) | <0.001 |
| Multivariable (difference of median and 95% CI) | $950 | ($697–1204) | $1418 | ($1300–1536) | Referent | ||
Adjusted cost: Cost was adjusted for inflation using the Consumer Price Index to 2015 US dollars and winsorized to 3rd and 97th percentiles.
P-value was from Kruskal-Wallis test. IQR: interquartile range.
Multivariable median regression model were fit using interior point algorithm with resampling method (1000 repeats) to compute confidence interval, adjusted for age, year, marital status, race, insurance status, hospital location, teaching status, bed size, region, comorbidity, route of hysterectomy, annualized hospital volume, any complication and lymph node assessment.
In a sensitivity analysis, a total of 1123 women had a charge code for methylene blue (Supplemental Table 1). If methylene blue was used for SLN biopsy in all of these women, the total rate of SLN biopsy increased to 8.5% within the cohort. In this analysis the predictors of SLN biopsy were similar to those seen in the analysis without methylene blue (Supplemental Table 2). Likewise, trends for length of stay and cost were similar (Supplemental Table 3).
Comment
We noted that, despite the unclear role of sentinel lymph node biopsy in endometrial cancer, the use of the procedure increased rapidly from 2011 to 2015. The increased use of the procedure was most notable in women who underwent a robotic-assisted hysterectomy. Compared to lymphadenectomy, sentinel lymph node biopsy was associated with lower hospital costs even after adjusting for the route of surgery.
To date, much of the data examining SLN biopsy for endometrial cancer has focused on the identification of a SLN and on comparisons between different mapping techniques.12 A recent systematic review that included 17 studies reported that the SLN detection rates in endometrial cancer ranged from 60–100%.12 Reports that included more than 100 patients had detection rates of >80%.12 Studies that have directly examined the ability of SLN biopsy to identify nodal metastases have noted mixed findings.7–10 The multicenter, prospective SENTI-ENDO study, which included 133 patients, reported a sensitivity of 84% and negative predictive value of 97% for the detection of metastatic disease.7 A single institution study that included 498 women noted a false negative rate for the detection of positive nodes of 15%.8
A number of technical factors influence the accuracy of SLN biopsy for endometrial cancer.8,11 Given that endometrial tumors are not readily visualized prior to hysterectomy, the optimal injection site for SLN mapping remains uncertain.11,12 While cervical injection is most commonly used, some studies have also used hysteroscopic localization or injection of the uterine fundus.11,12 While many studies have used a combination of blue dye and technetium-99, the optimal dye or combination is uncertain.7,11,12 Recent studies have suggested that indocyanine green may be superior to blue dyes.24,25 Lastly, pathologic assessment is a critical component of optimizing the detection of metastatic disease.11,26 In addition to routine hematoxylin and eosin staining, ultrastaging with immunohistochemistry to detect micrometastases and isolated tumors cells is often performed.26,27 However, the prognostic significance of these findings on clinical outcomes remains uncertain.27
We noted that the route of hysterectomy was an important factor in use of SLN biopsy. In particular, those women who underwent a robotically assisted hysterectomy were more likely to have a SLN procedure. Currently available robotic technology is often equipped with near infrared fluorescence imaging to allow performance of SLN biopsy with indocyanine green.24,28 Given that use of SLN biopsy increased much more rapidly in women undergoing robotic-assisted hysterectomy, the easy access of this technology with the robotic platform may be one factor driving the diffusion of SLN biopsy. Prior work has suggested that surgeons are often influenced to use technological advances when they are readily available even in the absence of data.29,30
Compared to lymphadenectomy, SLN biopsy was associated with lower costs. Among women who underwent minimally invasive hysterectomy, the adjusted cost of SLN biopsy was approximately $700 lower than for lymphadenectomy. The lower cost for SLN biopsy is likely multifactorial; the currently available agents used for SLN mapping are relatively inexpensive and the time to perform a sentinel node biopsy is likely substantially less than a full lymphadenectomy. Prior studies of breast cancer have also reported that the costs of SLN biopsy are lower than axillary dissection both in the short and long-term.31
While our study benefits from the inclusion of a large cohort of women, we recognize several important limitations. First, coding for SLN biopsy relies on a number of procedural and charge codes. Although this methodology has been used in other analyses, a small number of women who underwent SLN biopsy may not have been captured.16,32 Second, we cannot accurately distinguish those women who underwent SLN biopsy followed by lymphadenectomy and those women who only underwent sentinel node assessment. A priori the goal of our study was to examine patterns of use of SLN biopsy and not specifically examine performance characteristics. Third, our analysis was limited to acute, in-hospital outcomes. Many of the potential benefits of SLN biopsy, such as a reduced rate of lymphedema, will require long-term follow-up. Similarly, we were unable to examine the impact of SLN biopsy on survival which clearly warrants further evaluation. Lastly, our dataset lacks a number of important demographic factors such as body mass index as well as tumor characteristics such as stage and histology as well as patient preferences that likely influenced the use of SLN biopsy.
While SLN biopsy may be safe, patient selection criteria for the procedure remain controversial. Many have advocated SLN biopsy for low-risk, early stage tumors. However, among these women the risk of nodal disease is low and nodal assessment has not been shown to improve outcomes, calling into question the utility of SLN biopsy in this population.5,6 Alternatively, women with higher grade tumors with myometrial invasion are at greater risk for nodal metastases. For these patients, however, there is greater concern that nodal metastases may be missed given the appreciable false negative rates of SLN biopsy in some series. Currently, the National Comprehensive Cancer Network Guidelines suggest that SLN biopsy may be considered in select patients with endometrial cancer.33 Moving forward, randomized studies as well as prospective observational studies would be of great value to help define the role of SLN biopsy for endometrial cancer.
Supplementary Material
Acknowledgments
Funding: Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01CA134964) are recipients of grants from the National Cancer Institute.
Footnotes
Disclosure: The authors report no conflicts of interest or disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wright JD, Huang Y, Burke WM, et al. Influence of Lymphadenectomy on Survival for Early-Stage Endometrial Cancer. Obstet Gynecol. 2016;127:109–18. doi: 10.1097/AOG.0000000000001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMeekin DS. Designing the next lymphadenectomy trial: what should we learn for our prior experiences. Gynecol Oncol. 2012;126:1–2. doi: 10.1016/j.ygyno.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Cragun JM, Havrilesky LJ, Calingaert B, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005;23:3668–75. doi: 10.1200/JCO.2005.04.144. [DOI] [PubMed] [Google Scholar]
- 4.Sharma C, Deutsch I, Lewin SN, et al. Lymphadenectomy influences the utilization of adjuvant radiation treatment for endometrial cancer. Am J Obstet Gynecol. 2011;205:562.e1–9. doi: 10.1016/j.ajog.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–16. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 6.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–36. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballester M, Dubernard G, Lecuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO) Lancet Oncol. 2011;12:469–76. doi: 10.1016/S1470-2045(11)70070-5. [DOI] [PubMed] [Google Scholar]
- 8.Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;125:531–5. doi: 10.1016/j.ygyno.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 9.How J, Gotlieb WH, Press JZ, et al. Comparing indocyanine green, technetium, and blue dye for sentinel lymph node mapping in endometrial cancer. Gynecol Oncol. 2015;137:436–42. doi: 10.1016/j.ygyno.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Naoura I, Canlorbe G, Bendifallah S, Ballester M, Darai E. Relevance of sentinel lymph node procedure for patients with high-risk endometrial cancer. Gynecol Oncol. 2015;136:60–4. doi: 10.1016/j.ygyno.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Smith B, Backes F. The role of sentinel lymph nodes in endometrial and cervical cancer. J Surg Oncol. 2015;112:753–60. doi: 10.1002/jso.24022. [DOI] [PubMed] [Google Scholar]
- 12.Cormier B, Rozenholc AT, Gotlieb W, Plante M, Giede C Communities of Practice Group of Society of Gynecologic Oncology of C. Sentinel lymph node procedure in endometrial cancer: A systematic review and proposal for standardization of future research. Gynecol Oncol. 2015;138:478–85. doi: 10.1016/j.ygyno.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 13.Rao R, Euhus D, Mayo HG, Balch C. Axillary node interventions in breast cancer: a systematic review. JAMA. 2013;310:1385–94. doi: 10.1001/jama.2013.277804. [DOI] [PubMed] [Google Scholar]
- 14.Kyrgidis A, Tzellos T, Mocellin S, et al. Sentinel lymph node biopsy followed by lymph node dissection for localised primary cutaneous melanoma. Cochrane Database Syst Rev. 2015;5:CD010307. doi: 10.1002/14651858.CD010307.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrie TA, Patel A, Martin-Hirsch PP, et al. Sentinel node assessment for diagnosis of groin lymph node involvement in vulval cancer. Cochrane Database Syst Rev. 2014;6:CD010409. doi: 10.1002/14651858.CD010409.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. Jama. 2013;309:689–98. doi: 10.1001/jama.2013.186. [DOI] [PubMed] [Google Scholar]
- 17.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–33. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 18.Schiavone MB, Herzog TJ, Ananth CV, et al. Feasibility and economic impact of same-day discharge for women who undergo laparoscopic hysterectomy. Am J Obstet Gynecol. 2012;207:382.e1–9. doi: 10.1016/j.ajog.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. Jama. 2010;303:2359–67. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 20.Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Jama. 2010;303:2035–42. doi: 10.1001/jama.2010.672. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Labor Bureau of Labor. [Accessed September 1, 2011, 2011];Statistics Consumer Price Index. 2011 at ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt.
- 22.Koenker R. Quantile Regression. New York: Canbridge University Press; 2005. [Google Scholar]
- 23.He X, Hu F. Markov chain marginal bootstrap. J Am Statist Assoc. 2002;97:783–95. [Google Scholar]
- 24.Holloway RW, Bravo RA, Rakowski JA, et al. Detection of sentinel lymph nodes in patients with endometrial cancer undergoing robotic-assisted staging: a comparison of colorimetric and fluorescence imaging. Gynecol Oncol. 2012;126:25–9. doi: 10.1016/j.ygyno.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Sinno AK, Fader AN, Roche KL, Giuntoli RL, 2nd, Tanner EJ. A comparison of colorimetric versus fluorometric sentinel lymph node mapping during robotic surgery for endometrial cancer. Gynecol Oncol. 2014;134:281–6. doi: 10.1016/j.ygyno.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Kim CH, Soslow RA, Park KJ, et al. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging. Int J Gynecol Cancer. 2013;23:964–70. doi: 10.1097/IGC.0b013e3182954da8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St Clair CM, Eriksson AG, Ducie JA, et al. Low-Volume Lymph Node Metastasis Discovered During Sentinel Lymph Node Mapping for Endometrial Carcinoma. Ann Surg Oncol. 2016;23:1653–9. doi: 10.1245/s10434-015-5040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi EC, Ivanova A, Boggess JF. Robotically assisted fluorescence-guided lymph node mapping with ICG for gynecologic malignancies: a feasibility study. Gynecol Oncol. 2012;124:78–82. doi: 10.1016/j.ygyno.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Barbash GI, Glied SA. New technology and health care costs--the case of robot-assisted surgery. N Engl J Med. 2010;363:701–4. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 30.Barkun JS, Aronson JK, Feldman LS, et al. Evaluation and stages of surgical innovations. Lancet. 2009;374:1089–96. doi: 10.1016/S0140-6736(09)61083-7. [DOI] [PubMed] [Google Scholar]
- 31.Classe JM, Baffert S, Sigal-Zafrani B, et al. Cost comparison of axillary sentinel lymph node detection and axillary lymphadenectomy in early breast cancer. A national study based on a prospective multi-institutional series of 985 patients 'on behalf of the Group of Surgeons from the French Unicancer Federation'. Ann Oncol. 2012;23:1170–7. doi: 10.1093/annonc/mdr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright JD, Tergas AI, Burke WM, et al. Uterine Pathology in Women Undergoing Minimally Invasive Hysterectomy Using Morcellation. Jama. 2014 doi: 10.1001/jama.2014.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) [Accessed January 21, 2017];Uterine Neoplasms. Version I.2017. at https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.