Abstract
Objective
Symptomatic hand osteoarthritis (SHOA) is a common condition that affects hand strength and function, and causes disability in activities of daily living. Prior studies have estimated lifetime risk for symptomatic knee and hip osteoarthritis to be 45% and 25% respectively. The objective of this study is to estimate overall lifetime risk for SHOA and stratified lifetime risk by potential risk factors.
Methods
We analyzed data for 2,218 adults ≥ 45 years in the Johnston County Osteoarthritis Project, a population-based prospective cohort study in residents of Johnston County, North Carolina. Data were collected in two cycles (1999–2004 and 2005–2010). SHOA was defined as having both self-reported symptoms and radiographic OA in the same hand. Lifetime risk, defined as the proportion of the population who will develop SHOA in at least one hand by age 85, was estimated from models using generalized estimating equations methodology.
Results
Overall, the lifetime risk of SHOA is 39.8% (95% confidence interval (CI): 34.4, 45.3). Nearly one in two women (47.2%; 95% CI: 40.6, 53.9) will develop SHOA by age 85 compared with one in four men (24.6%; 95% CI: 19.5, 30.5). Race-specific estimates are 41.4% (95% CI: 35.5, 47.6) among whites and 29.2% (95% CI: 20.5, 39.7) among blacks. Lifetime risk among individuals with obesity (47.1%, 95% CI: 37.8, 56.7) is 11 percentage point higher than those without obesity (36.1%, 95% CI: 29.7, 42.9).
Conclusion
These findings demonstrate the substantial burden of SHOA overall and in subgroups. Increased use of public health and clinical interventions is needed to address its impact.
Keywords: hand, lifetime risk, osteoarthritis, population-based, prospective cohort, symptoms
The hand is one of the sites most frequently affected by osteoarthritis (OA), characterized by bony enlargements of finger joints and deformities of the hand (1). Many people with hand OA suffer from symptoms of pain or aching, stiffness, loss of mobility, and decreased grip strength, leading to impaired hand function and disability in activities of daily living (2, 3). Contrary to the common belief that it is a disease of older people, hand OA can occur relatively early in life (i.e. middle age), impairing individuals’ capacity to work (4).
Studies of hand OA, particularly epidemiologic assessments of its prevalence and incidence, are sparse. Hand OA with symptoms is associated with the hand functional limitations, disability, and health care utilization (3, 5), hence symptomatic hand OA (SHOA) has both clinical and public health implications. The prevalence estimates for SHOA in the general population of adults among various countries range between 3% and 8% (6–11). Higher prevalences were reported among older adults: prevalence estimates were 13% in men and 26% in women aged > 70 years in the Framingham Study (3), 15% among an Italian community aged ≥65 years (10), and the lowest (5%) among a Chinese population of adults ≥60 years (11). In the Framingham study, the 9-year cumulative incidence (proportion of new cases) of SHOA in adults was 7% (12). The 10-year cumulative incidence of doctor-diagnosed hand OA in a Norwegian adult cohort was 6% (13). The incidence rate of SHOA observed in up to four years among members of the Fallon Community Plan (Massachusetts) was 100/100,000 person years (5).
Lifetime risk is the probability of developing a condition over the course of a lifetime. Lifetime risk has been previously estimated for other chronic conditions, including cancer, heart diseases and diabetes mellitus (14–16). Using data from the Johnston County Osteoarthritis (JoCo OA) project, the lifetime risk of symptomatic knee OA was 45% (17), and 25% for symptomatic hip OA (18). To our knowledge, lifetime risk of SHOA has not been reported. Estimating the lifetime risk of SHOA provides a risk estimate useful for both individuals and those seeking a better understanding of its public health burden. Using longitudinal data from the JoCo OA project, we estimated the overall lifetime risk of SHOA in the population and stratified risk by potential influential factors. It was not our objective to examine the association between risk factors and SHOA, but rather to provide an estimated probability of having SHOA by a certain age, and whether this probability differs by sex, race, education, obesity status, hand injury history, and occupational factors which have been reported to be potential risk factors of OA or may affect the risk factors (19–21).
MATERIALS AND METHODS
Study design and data source
The JoCo OA project is an ongoing population-based prospective cohort study in residents of Johnston County, North Carolina, USA, to monitor the occurrence and natural history of OA. Data collection and evaluation was conducted at baseline during 1991–1997 (n=3,068), and three follow-up cycles (Time 1:1999–2004, Time 2: 2005–2010, and Time 3: 2011–2015). The study protocol used probability sampling to select a representative sample of civilian, noninstitutionalized, black and white men and women aged 45 years and older, who were residents of one of six selected townships, and were physically and mentally capable of completing the study protocol. The protocol included both home interview and clinic visits. Project methods are described in detail elsewhere (22). The study protocol was approved by the Institutional Review Boards of the Centers for Disease Control and Prevention and the University of North Carolina.
Radiographs were obtained during clinic visits by a standard protocol of bilateral postero-anteriorly views of the hands. A single experienced musculoskeletal radiologist read for features of radiographic OA at each of the 30 joints for both hands using standard atlases (23, 24) after the X-ray films were obtained in each data collection cycle. We performed a reliability assessment of the radiographic readings by comparing a sample of those read singly and in series (Appendix), which showed good-to-excellent reliability (kappa coefficient 0.57–0.81 for Time 1, and 0.57–0.84 for Time 2), thus allowing us to use the original (read singly) radiograph readings.
We analyzed data for Time 1 and Time 2 follow-ups in this study because hand symptom and radiographic measurements were collected in these cycles (hand outcomes were not measured at baseline of JoCo OA project). SHOA was defined as having both self-reported symptoms and radiographic OA in the same hand. Participants were considered to have SHOA if they had SHOA in at least one hand. Hand symptoms were ascertained by a “yes” to the question “On most days, do you have pain, aching, or stiffness in your left/right hand?” Hand radiographic OA was defined by meeting the following two criteria (25): (1) a Kellgren-Lawrence grade of ≥2 (i.e. mild to severe radiographic OA) in at least three total joints in each hand excluding the metacarpophalangeal (MCP) joints; and (2) at least one of them being the distal interphalangeal (DIP) joint. The thumb interphalangeal joint was considered as a proximal interphalangeal (PIP) joint, and the carpometacarpal (CMC) joint was included in the count of total number of joints affected as described in criterion (1).
Various definitions of radiographic hand OA have been used in different studies (26). Our definition was based on prior studies on generalized osteoarthritis phenotypes (25, 27, 28), as well as the intent to represent persons commonly seen in clinical settings and OA severe enough that would likely affect quality of life. We believe our definition has both clinical and public health implications, therefore appropriate for lifetime estimation from a population perspective.
We conducted a sensitivity analysis for an alternative definition that includes the MCP joint; the results showed little difference from the analyses excluding the MCP joint, and hence are not shown here. Additional sensitivity analyses using radiographic hand OA as the outcome were also performed for men and women, and persons with and without obesity.
Lifetime risk estimation
Lifetime risk is the cumulative probability, or cumulative incidence of a condition in a cohort – the number of people who develop the disease by a specified age (85 years in this analysis) as a proportion of the total population at risk during the same time period. Lifetime risk estimation of other diseases has typically used life table or modified time-to-event analyses, which require complete registry of the disease or frequent follow-up, respectively. To our knowledge, there are no population-based OA data sources with these characteristics. In addition, there are three methodological challenges to use these conventional methods. First, the time of SHOA onset cannot be precisely determined because OA develops slowly over time. Second, 137 participants in our sample had pre-existing SHOA at their first measurement (prevalent cases) that might thereby be excluded from a time-to-event analysis, meaning we would lose more than one-third of the SHOA cases in the cohort. Third, 70% of the study participants had only one observation at either Time 1 or Time 2 and would be managed through censoring or might be excluded in a typical time-to-event analysis, further reducing the sample size. Because of the considerable attrition between baseline and follow-up commonly seen in cohort studies, life table analysis may result in an overestimation of risk because of the extensive censoring among participants who were absent at follow-up.
We estimated lifetime risk as a model-predicted prevalence of SHOA at age 85 for those who survived to this age, which is equal to the cumulative incidence by age 85 because (1) the joint structural changes defined in radiographic OA are irreversible; (2) SHOA has not been shown to be associated with total mortality (29). We analyzed all individuals with either single or multiple observations using generalized estimating equations (GEE) approach, to account for within-person correlation (i.e., multiple observations per person) and to estimate the population-averaged prediction at a given age. All cohort members, regardless of SHOA status at Time 1, were included in this analysis. The inclusion of both prevalent and incident cases ensures a greater likelihood that persons who have ever had SHOA are included in the estimate.
Study population
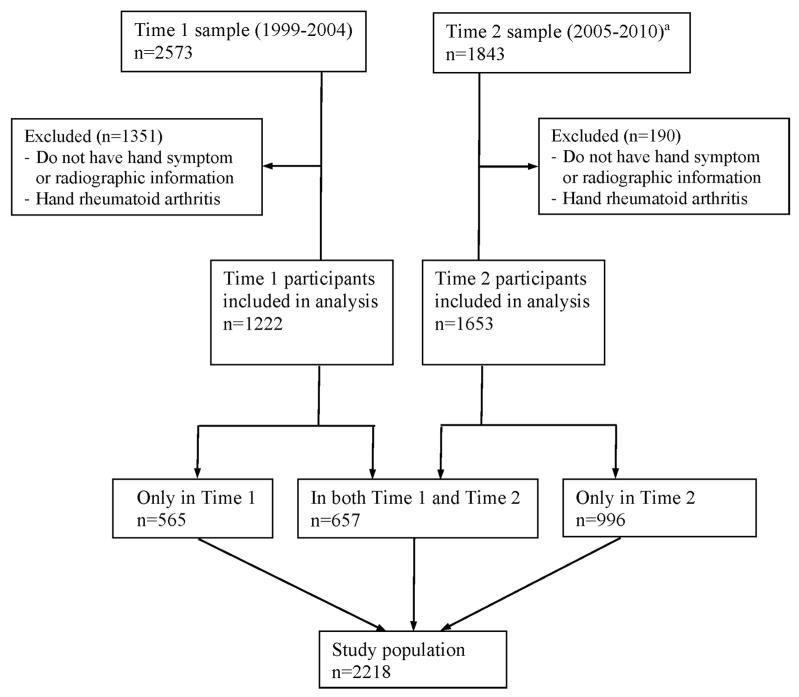
Our study population comprised 2,218 participants with both hand symptom information and radiographs in at least one data collection cycle (Figure 1). Among them, 657 (29.6%) individuals had complete hand outcome information at both Time 1 and Time 2 with a median follow-up time of 6.0 years (range 4.0–8.4 years); the rest of study population had only one observation of hand outcomes at either Time 1 (n=565) or Time 2 (n=996). Participants with radiographic evidence of rheumatoid arthritis (n=11) were excluded from this analysis. All 2,218 eligible participants were included in the data analysis for lifetime risk estimation of SHOA.
Figure 1.
Flowchart for symptomatic hand OA analytic sample: Johnston County Osteoarthritis Project
- Physical/mental unable to participate (n=165)
- Moved away (n=160)
- Deceased (n=358)
- Unknown (n=47)
Statistical analysis
We estimated lifetime risk of SHOA using the GEE methodology. Age served as the independent variable, binary status of SHOA (yes, no) was the outcome variable, and lifetime risk was estimated as the model-predicted probability of developing SHOA at age 85 years using conditional marginal probability. We chose age 85 because it is a reasonable life expectancy for individuals in the US (30). For stratified analysis, we estimated lifetime risk from models which contained the stratification variable (e.g. sex) one at a time and the interaction term of the variable with age. To test the linearity of the age effect, a preliminary analysis was conducted to include the quadratic term age2. We also estimated model-predicted probability of having SHOA for age 45 through 85 years, and plotted cumulative risk curves by age and stratification variables.
To ensure that our estimates were representative of the target population (i.e., the six selected townships in Johnston County, NC), we incorporated sampling weights to account for oversampling of blacks and differential rates of nonresponse at different data collection cycles. The weights were also calibrated to the population counts from the 2000 US Census for those six townships in Johnston County, North Carolina. Additionally, the statistical analyses adjusted for within-cluster correlation from potential correlation of repeated measures for participants (for those with data from both cycles) using an exchangeable working correlation structure, and the within-cluster correlation from the stratified random sampling design at three levels (stratum [townships], the primary [streets] and secondary [households] sampling unit). Taylor series linearization method was used to estimate standard errors of the probabilities and 95% confidence intervals (CI). Adjusted Wald F-statistics were used to test the risk difference between levels of the stratification variables. Statistical testing used α=0.05 level. All analyses were performed using SAS callable SUDAAN software (V.11, RTI International, North Carolina, USA).
Stratification variables
Estimated lifetime risk was stratified for the following six known or potential risk factors for SHOA: sex, race, education, obesity, prior hand injury, and occupational hand activities (19–21). Age and stratification variables were self-reported in an interviewer-administered questionnaire during home interviews. Obesity was defined as body mass index (BMI) ≥30 kg/m2, with participants’ weight and height measured during clinic visits. Prior hand injury for right and left hands was determined by the question, “Has a doctor ever told you that you had broken or fractured any fingers of your hands?” Participants answering ‘yes’ for at least one hand were considered to have prior hand injury. Occupational hand activities were assessed by the question, “For the job that you held longest in your life, how often did you have to use hand-held tools or equipment (pen, keyboard, computer mouse, drill, hairdryer, sander, etc.)?” Participants who answered “Never”, “Seldom” or “Sometimes” were categorized as “less frequent”, and those who answered “Often” or “Always” were categorized as “more frequent”.
RESULTS
Overall, 352 participants had SHOA in at least one hand in the study population. The unweighted prevalence of SHOA was 10.8% at Time 1 and 15.5% at Time 2; and the unweighted prevalence of pain, aching, or stiffness in at least one hand was 46.2% at Time 1 and 36.3% at Time 2.
The average age of the 1,222 study participants at Time 1 was 61.0 years (SD 10.7, range 45–94), with a higher weighted proportion of women than men (60.6% versus 39.4%) and more whites than blacks (71.3% versus 28.7%) (Table 1). About three-fourths of the cohort completed at least high school (75.7%), and nearly half (45.5%) were obese. These characteristics were similar at Time 2 except increased age.
Table 1.
Weighted sociodemographic and clinical characteristics of study population at Time 1 (1999–2004) (n=1222): Johnston County Osteoarthritis Project.
| Unweighted n | Weighted Percentagea (%) | |
|---|---|---|
| Age (years) | ||
| 45–54 | 414 | 29.2 |
| 55–64 | 386 | 31.2 |
| 65–74 | 253 | 22.7 |
| ≥ 75 | 169 | 16.9 |
| Sex | ||
| Women | 812 | 60.6 |
| Men | 410 | 39.4 |
| Race | ||
| White | 769 | 71.3 |
| Black | 453 | 28.7 |
| Education | ||
| Less than high school | 289 | 23.8 |
| High school completed | 617 | 48.0 |
| Vocational school/College and higher | 309 | 27.7 |
| Missing | 7 | 0.5 |
| Obesity (BMI≥30 kg/m2) | ||
| Yes | 603 | 45.5 |
| No | 619 | 54.5 |
| History of hand injury | ||
| Yes | 137 | 11.2 |
| No | 1083 | 88.7 |
| Missing | 2 | 0.1 |
| Occupational hand-held tool use in the longest jobb | ||
| Less frequent | 317 | 25.4 |
| More frequent | 862 | 71.2 |
| Missing | 43 | 3.4 |
Abbreviation: BMI: body mass index
Sampling weights were applied to be representative of the target population
Assessed by the question “For the job that you held longest in your life, how often did you have to use hand-held tools or equipment (pen, keyboard, computer mouse, drill, hairdryer, sander, etc.)?”
Less frequent: Participants answered “Never” or “Seldom” or “Sometimes”
More frequent: Participants answered “Often” or “Always”
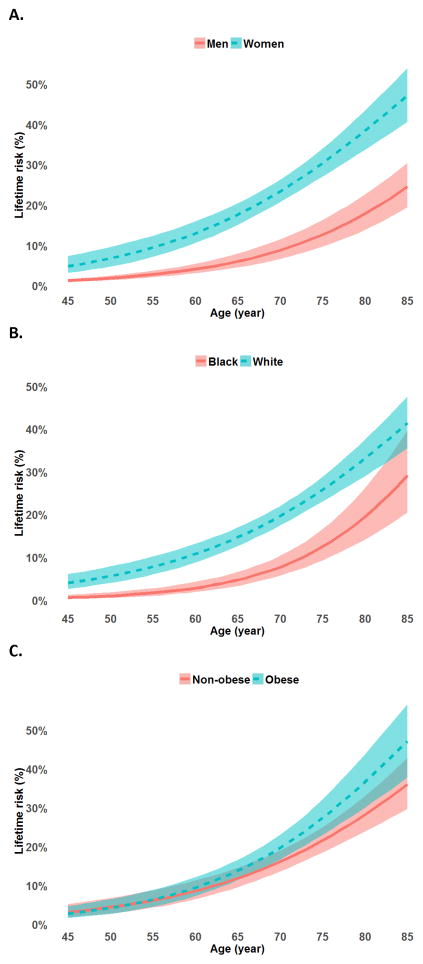
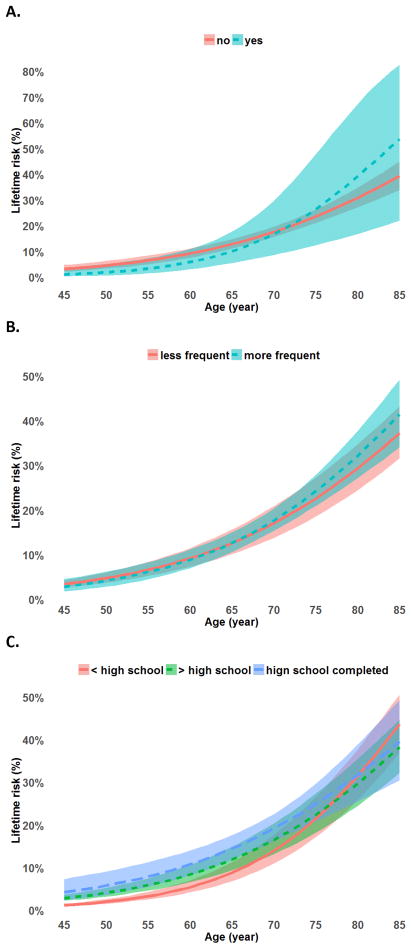
The quadratic term age2 was not statistically significant at α=0.05. First-order continuous age was used in the remaining analyses because its association with lifetime risk provides a simpler (linear) interpretation. The overall lifetime risk of SHOA (i.e. proportion of those who will develop the condition by age 85 years) was 39.8% (95% CI: 34.4, 45.3) (Table 2). Lifetime risk was nearly one in two for women (47.2%; 95% CI: 40.6, 53.9) compared with one in four for men (24.6%; 95% CI: 19.5, 30.5). The cumulative incidence curve of SHOA showed consistently significantly higher risk in women than men at all ages analyzed, and this difference became greater with increasing age (Figure 2a). Lifetime risk of SHOA was 12.2 percentage point higher in whites than blacks (p=0.031), and was 53.7% (95% CI: 22.0, 82.6) in individuals with prior hand injury vs. 39.3% (95% CI: 33.9, 45.0) among those without (p=0.427) (Table 2). The cumulative incidence curve of SHOA indicated a significantly higher risk in whites than blacks throughout middle to older ages (Figure 2b). Lifetime risk of SHOA was 47.1% (95% CI: 37.8, 56.7) among individuals with obesity, and 36.1% (95% CI: 29.7, 42.9) among those without obesity (p=0.063) (Table 2). There was little difference of SHOA cumulative risk for persons before their mid-sixties, but the difference increased with age afterwards (Figure 2c). There was no statistically significant difference in lifetime risk by education or occupational hand-held tool use.
Table 2.
Lifetime risk of symptomatic hand OA (n=2,218), overall and by stratified variables: Johnston County Osteoarthritis Project.
| Percentage | 95% CI | P-valuea | |
|---|---|---|---|
| Overall (at least one hand) | 39.8 | 34.4, 45.3 | n.a. |
|
| |||
| Unilateral | 13.5 | 11.4, 16.1 | n.a. |
|
| |||
| Bilateral | 27.3 | 22.1, 33.2 | n.a. |
|
| |||
| Stratification variablesb | |||
|
| |||
| Sex | |||
| Women | 47.2 | 40.6, 53.9 | <0.0001 |
| Men | 24.6 | 19.5, 30.5 | |
|
| |||
| Race | |||
| White | 41.4 | 35.5, 47.6 | 0.031 |
| Black | 29.2 | 20.5, 39.7 | |
|
| |||
| Education | |||
| Less than high School | 43.7 | 37.0, 50.6 | ≥0.255c |
| High school completed | 39.6 | 30.5, 49.4 | |
| Greater than high School | 38.3 | 32.3, 44.7 | |
|
| |||
| Obesity (BMI≥30 kg/m2) | |||
| Yes | 47.1 | 37.8, 56.7 | 0.063 |
| No | 36.1 | 29.7, 42.9 | |
|
| |||
| Hand injury history | |||
| Yes | 53.7 | 22.0, 82.6 | 0.427 |
| No | 39.3 | 33.9, 45.0 | |
|
| |||
| Occupational hand-held tool use in the longest jobd | |||
| Less frequent | 37.2 | 31.6, 43.2 | 0.392 |
| More frequent | 41.5 | 34.1, 49.2 | |
Abbreviation: BMI, body mass index; CI, confidence intervals
Adjusted Wald F-statistic test P-value (test of the risk difference between levels of the stratification variables)
Estimated from models which contained the stratification variable (e.g. sex) and the interaction term of the variable with age.
The smallest of the three pairwise comparisons
Assessed by the question “For the job that you held longest in your life, how often did you have to use hand-held tools or equipment (pen, keyboard, computer mouse, drill, hairdryer, sander, etc.)?”
Less frequent: Participants answered “Never” or “Seldom” or “Sometimes”
More frequent: Participants answered “Often” or “Always”
Figure 2.
Cumulative risk curves by age for A. Men and women; B. White and Black; C. Obese and non-obese Individuals. The shaded bands represent the 95% confidence intervals for the estimated cumulative risk.
DISCUSSION
Our estimates of lifetime risk from a population-based study of adults suggest that 40% will develop SHOA by age 85 for those live to that age. The lifetime risk of SHOA was slightly lower than that for symptomatic knee OA (45%), but higher than that for symptomatic hip OA (25%) in the same population (17, 18). Similarly, OA prevalences in the general population are usually the highest for the knee, followed by the hand (using definition similar to ours), and lower for the hip (10, 25).
Lifetime risk estimates provides an informative and helpful prediction from the individual’s perspective if they see themselves living to an older age. In addition, it presents the scope of the problem from a population perspective. Given the aging population and increasing life expectancy in the US (31), it is reasonable to expect that more Americans will be affected by this painful and debilitating condition in the years to come.
The lifetime risk was particularly high among women; we estimated that nearly one in two women will develop SHOA by age 85 compared with one in four men. Women are consistently at higher risk of OA than men in epidemiologic studies (19–21), particularly for the hand. In a large prospective cohort study, the adjusted female-to-male rate ratios were 2.5 for hand OA, 1.5 for knee OA, and 1.2 for hip OA; the risk of hand OA risk among women peaked at 60–64 years of age (32). Several hypotheses have been proposed to explain the higher OA risk in women. One probable explanation is a hormonal effect: the higher incidence of OA in women just after menopause suggests that estrogen deficiency may play a role in causing the disease (21). Other hypotheses include differences of pain perception and sensitivity (33), anatomical differences between men and women (34), as well as reporting of pain (3). It has been previously shown in the Framingham study that the prevalence of radiographic hand OA was similar in women and men (94.4% vs. 88.6%), whereas symptomatic hand OA was twice as common in women (26.2% vs. 13.4%) (3). We performed a sensitivity analysis that analyzed radiographic hand OA as the outcome and the lifetime risk at age 85 was 79% (95% CI: 74, 83) for women, and 63% (95% CI: 57, 68) for men. The difference was statistically significant (p<0.01). One possible explanation for the difference between our study and the Framingham study is that their definition was radiographic OA in ≥1 finger joint.
Consistent with our findings, prior studies using data from the JoCo OA project reported that whites were more likely to have SHOA and hand radiographic OA phenotypes as compared with blacks, after adjusting for sex, age and BMI (25, 35). In another population of middle-aged women, the unadjusted prevalence of radiographic OA was found to be similar between black and white women in the DIP, PIP, and CMC joints, but markedly higher in the MCP joints among black females (36). The biological mechanism for these observed differences is unknown and warrant further investigations.
Obesity is an important risk factor for both the incidence and progression of osteoarthritis which is modifiable. The evidence of association is strong between obesity and OA in the load-bearing knee joint, and moderate between obesity and hand OA in different studies (13, 37–42). These studies used multivariate analyses to examine the association between obesity and OA adjusting for other potential confounders (e.g. sex). The goal of our analyses was not to investigate risk factors for SHOA, rather to estimate the scale of the burden and via simple stratification analyses by known risk factors one at a time. Yet it still showed an 11 percentage point difference of SHOA lifetime risk by obesity status. Sensitivity analyses using radiographic hand OA as the outcome estimated the lifetime risk at age 85 to be 78% (95% CI: 72, 84) for people with obesity, and 70% (95% CI: 63, 77) for those without (test-of-difference p-value = 0.076), suggesting the difference of estimated SHOA lifetime risk by obesity may not be fully explained by difference in reporting of pain. Recent studies have suggested that the pathogenesis for hand OA is likely different than that for load-bearing joints (i.e. knee and hip). Unlike knee and hip OA where abnormal biomechanics due to excessive weight plays an essential role, systemic processes, such as aberrant metabolic regulation and inflammation associated with obesity, may be more important than mechanical factors in the pathogenesis and progression of hand OA (43–45).
Joint injury and occupational activities are known modifiable risk factors for OA (19–21). Severe injury to the structure of the knee is strongly linked to the subsequent onset of OA and musculoskeletal symptoms (46, 47). However, similar evidence for the hand is lacking because almost all such injury studies have focused on the knee. The lifetime risk of SHOA was 54% vs. 39% among those with hand injury and those without, respectively, but the small number of participants with hand injury precluded statistically significant findings. Occupational activities that involve repetitive motion, high biomechanical loading, or vibration could initiate or accelerate structural changes in the cartilage, bones, and other joint tissues, and cause injuries (48). Extensive precision pinch or grip seen in occupations such as dentists and textile workers, is associated with increased risk of OA in the DIP joint; whereas forceful and prolonged gripping common in heavy manual work, is associated with increased risk in the MCP joint (48). Our single question on hand-held tool use tried to assess occupational activities that would be relevant to the hand, but did not directly address gripping or pinching, and found no significant association.
The results of our analysis have limitations. First, Johnston County, NC is a lower-income, semi-rural area in the southern US, and our findings may not be generalizable to the US or other populations. However, we expect the lifetime risk of SHOA is likely high in the US given the aging population and the high prevalence of obesity (49), both important risk factors for OA. A comparison of the US and Johnston County populations in 2010 (50) showed 50.8% were women in both populations, approximately one-third were obese (35.7% vs 34.4%), and similar race distributions (white 72.4% vs 74.2%, black 12.6% vs 15.1%). Compared with the US population, the Johnston County population was slightly younger (age≥45 years 39.5% vs 35.6%, age≥65 years 13.0% vs 10.2%), less educated (14.9% vs. 18.8% had not completed high school), and lower income (median annual household income $51,914 vs $49,745). However, Johnston County, NC, had substantially more people living in rural areas (19.3% vs 52%). Second, the hand symptom question was not joint-specific, which may affect specificity of SHOA ascertainment as the joints having radiographic OA and symptoms may not be the same ones. Questions about hand symptoms with more details (e.g. which joints are affected and severity) may help to improve the specificity of SHOA definition. Third, the hand injury question asks participants ever had broken or fractured fingers, which is likely to underestimate less severe hand injuries. Fourth, the assessment of occupational hand tool was limited in two ways: (1) participants self-reported the frequency of hand-held tool use in the longest job held in their lives which may subject to misclassification of exposure due to recall bias; and (2) the occupational hand tool question listed examples ranged from pen, keyboard, and computer mouse to hairdryer, drill, and sander; the latter typically involves vibration and greater force relative to the former. Mixing them together would likely cause non-differential misclassification of occupational exposure, and increase the similarity between the high and low exposure groups, thus result in an underestimate (dilution) of the true difference between stratified lifetime risks. Fifth, although the onset of hand OA occurs most commonly after the age of 40 (21), selecting a study sample with age ≥45 years may miss cases of SHOA in the younger population, which may contribute to a slight underestimation of lifetime risk. Sixth, the method we used did not adjust for potential changes of BMI over time. Seventh, models of stratified lifetime risk estimates only included one factor at a time in addition to age, and residual confounding may exist. Lastly, the limited sample size decreased the precision of the point estimates, and likely prevented detection of statistically significant differences between subgroups.
This study has several strengths. First, our approach provided lifetime risk estimation of SHOA that included all participants with either one or multiple measurements in the analysis, which is otherwise infeasible using conventional methods given the difficulties described earlier. Second, as far as we know the JoCo OA project is the only longitudinal OA cohort in the US that includes oversampling of blacks (33% in the JoCo OA baseline cohort) for analysis of racial differences. Third, the probability sampling design of the JoCo OA project allowed us to make population-based inferences.
Lifetime risk, the probability of developing a condition over a lifetime, is conceptually accessible to the public. It may be easier for the general population understand absolute risk estimates like lifetime risk than relative risk estimates, and motivate healthcare providers/researchers to develop preventive strategies and provide early interventions for hand OA.
SHOA has substantial public health implications considering its high lifetime risk, its effect on functional impairment and disability of the hand, and its association with decreased quality of life. These findings underscore the need for increased use of public health and clinical prevention and intervention measures to address and mitigate the impact of SHOA on individuals and society. There are effective and inexpensive public health interventions (51) as well as other nonpharmacologic and pharmacologic therapies (52) that may help manage OA symptoms, maintain better function, and improve quality of life. Our lifetime risk estimates of SHOA, together with those for the knee and hip (17, 18), further illustrate the significant burden of symptomatic OA.
Supplementary Material
Acknowledgments
Funding sources
Cooperative agreements S043 and S3486 from the Centers for Disease Control and Prevention, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant numbers: 5-P60-AR-30701 and 5-P60-AR49465.
We would like to thank the dedicated staff of the Johnston County Osteoarthritis Project, William Kalsbeek, PhD, for his consultation in sampling design and statistical analysis, and Amy Shi, PhD, for her assistance in hand radiograph reliability assessment.
Appendix Figure.
Cumulative risk curves by age for A. Persons with less than high school, completed high school, and with higher than high school education; B. Persons having less and more frequent occupational hand-held tool use in the longest job; C. Persons with and without prior hand injury. The shaded bands represent the 95% confidence intervals for the estimated cumulative risk.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention
References
- 1.Zhang W, Doherty M, Leeb BF, Alekseeva L, Arden NK, Bijlsma JW, et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009;68(1):8–17. doi: 10.1136/ard.2007.084772. [DOI] [PubMed] [Google Scholar]
- 2.Michon M, Maheu E, Berenbaum F. Assessing health-related quality of life in hand osteoarthritis: a literature review. Ann Rheum Dis. 2011;70(6):921–8. doi: 10.1136/ard.2010.131151. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Niu J, Kelly-Hayes M, Chaisson CE, Aliabadi P, Felson DT. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: The Framingham Study. Am J Epidemiol. 2002;156(11):1021–7. doi: 10.1093/aje/kwf141. [DOI] [PubMed] [Google Scholar]
- 4.Kloppenburg M, Stamm T, Watt I, Kainberger F, Cawston TE, Birrell FN, et al. Research in hand osteoarthritis: time for reappraisal and demand for new strategies. An opinion paper. Ann Rheum Dis. 2007;66(9):1157–61. doi: 10.1136/ard.2007.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis and rheumatism. 1995;38(8):1134–41. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 6.Andrianakos AA, Kontelis LK, Karamitsos DG, Aslanidis SI, Georgountzos AI, Kaziolas GO, et al. Prevalence of symptomatic knee, hand, and hip osteoarthritis in Greece. The ESORDIG study. The Journal of rheumatology. 2006;33(12):2507–13. [PubMed] [Google Scholar]
- 7.Carmona L, Ballina J, Gabriel R, Laffon A, Group ES. The burden of musculoskeletal diseases in the general population of Spain: results from a national survey. Ann Rheum Dis. 2001;60(11):1040–5. doi: 10.1136/ard.60.11.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillon CF, Hirsch R, Rasch EK, Gu Q. Symptomatic hand osteoarthritis in the United States: prevalence and functional impairment estimates from the third U.S. National Health and Nutrition Examination Survey, 1991–1994. Am J Phys Med Rehabil. 2007;86(1):12–21. doi: 10.1097/phm.0b013e31802ba28e. [DOI] [PubMed] [Google Scholar]
- 9.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Prevalence and burden of osteoarthritis: results from a population survey in Norway. The Journal of rheumatology. 2008;35(4):677–84. [PubMed] [Google Scholar]
- 10.Mannoni A, Briganti MP, Di Bari M, Ferrucci L, Costanzo S, Serni U, et al. Epidemiological profile of symptomatic osteoarthritis in older adults: a population based study in Dicomano, Italy. Ann Rheum Dis. 2003;62(6):576–8. doi: 10.1136/ard.62.6.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Xu L, Nevitt MC, Niu J, Goggins JP, Aliabadi P, et al. Lower prevalence of hand osteoarthritis among Chinese subjects in Beijing compared with white subjects in the United States: the Beijing Osteoarthritis Study. Arthritis and rheumatism. 2003;48(4):1034–40. doi: 10.1002/art.10928. [DOI] [PubMed] [Google Scholar]
- 12.Haugen IK, Englund M, Aliabadi P, Niu J, Clancy M, Kvien TK, et al. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2011;70(9):1581–6. doi: 10.1136/ard.2011.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuer EJ, Wun LM, Boring CC, Flanders WD, Timmel MJ, Tong T. The lifetime risk of developing breast cancer. J Natl Cancer Inst. 1993;85(11):892–7. doi: 10.1093/jnci/85.11.892. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353(9147):89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 16.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 17.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis and rheumatism. 2008;59(9):1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy LB, Helmick CG, Schwartz TA, Renner JB, Tudor G, Koch GG, et al. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2010;18(11):1372–9. doi: 10.1016/j.joca.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013;39(1):1–19. doi: 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suri P, Morgenroth DC, Hunter DJ. Epidemiology of osteoarthritis and associated comorbidities. PM R. 2012;4(5 Suppl):S10–9. doi: 10.1016/j.pmrj.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–69. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. The Journal of rheumatology. 2007;34(1):172–80. [PubMed] [Google Scholar]
- 23.Burnett S, Hart DJ, Cooper C, Spector TD. A radiographic atlas of osteoarthritis. London: Springer-Verlag; 1994. [Google Scholar]
- 24.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson AE, Golightly YM, Renner JB, Schwartz TA, Kraus VB, Helmick CG, et al. Brief report: differences in multijoint symptomatic osteoarthritis phenotypes by race and sex: the Johnston County Osteoarthritis Project. Arthritis and rheumatism. 2013;65(2):373–7. doi: 10.1002/art.37775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall M, Dziedzic KS, van der Windt DA, Hay EM. A systematic search and narrative review of radiographic definitions of hand osteoarthritis in population-based studies. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2008;16(2):219–26. doi: 10.1016/j.joca.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Kraus VB, Jordan JM, Doherty M, Wilson AG, Moskowitz R, Hochberg M, et al. The Genetics of Generalized Osteoarthritis (GOGO) study: study design and evaluation of osteoarthritis phenotypes. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2007;15(2):120–7. doi: 10.1016/j.joca.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Nelson AE, Smith MW, Golightly YM, Jordan JM. “Generalized osteoarthritis”: a systematic review. Semin Arthritis Rheum. 2014;43(6):713–20. doi: 10.1016/j.semarthrit.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haugen IK, Ramachandran VS, Misra D, Neogi T, Niu J, Yang T, et al. Hand osteoarthritis in relation to mortality and incidence of cardiovascular disease: data from the Framingham heart study. Ann Rheum Dis. 2015;74(1):74–81. doi: 10.1136/annrheumdis-2013-203789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arias E. United States Life Tables, 2011. Natl Vital Stat Rep. 2015;64(11):1–63. [PubMed] [Google Scholar]
- 31.GKV, VAV Commerce UDo, Administration EaS, Bureau UC, editor. The next four decades: The older population in the United States: 2010 to 2050. 2010. [Google Scholar]
- 32.Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73(9):1659–64. doi: 10.1136/annrheumdis-2013-203355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain. 2012;153(3):602–18. doi: 10.1016/j.pain.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 34.Maleki-Fischbach M, Jordan JM. New developments in osteoarthritis. Sex differences in magnetic resonance imaging-based biomarkers and in those of joint metabolism. Arthritis Res Ther. 2010;12(4):212. doi: 10.1186/ar3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson AE, Renner JB, Schwartz TA, Kraus VB, Helmick CG, Jordan JM. Differences in multijoint radiographic osteoarthritis phenotypes among African Americans and Caucasians: the Johnston County Osteoarthritis project. Arthritis and rheumatism. 2011;63(12):3843–52. doi: 10.1002/art.30610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sowers M, Lachance L, Hochberg M, Jamadar D. Radiographically defined osteoarthritis of the hand and knee in young and middle-aged African American and Caucasian women. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2000;8(2):69–77. doi: 10.1053/joca.1999.0273. [DOI] [PubMed] [Google Scholar]
- 37.Carman WJ, Sowers M, Hawthorne VM, Weissfeld LA. Obesity as a risk factor for osteoarthritis of the hand and wrist: a prospective study. Am J Epidemiol. 1994;139(2):119–29. doi: 10.1093/oxfordjournals.aje.a116974. [DOI] [PubMed] [Google Scholar]
- 38.Oliveria SA, Felson DT, Cirillo PA, Reed JI, Walker AM. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology. 1999;10(2):161–6. [PubMed] [Google Scholar]
- 39.Reyes C, Leyland KM, Peat G, Cooper C, Arden NK, Prieto-Alhambra D. Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: A population-based cohort study. Arthritis Rheumatol. 2016 doi: 10.1002/art.39707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szoeke C, Dennerstein L, Guthrie J, Clark M, Cicuttini F. The relationship between prospectively assessed body weight and physical activity and prevalence of radiological knee osteoarthritis in postmenopausal women. The Journal of rheumatology. 2006;33(9):1835–40. [PubMed] [Google Scholar]
- 41.Visser AW, Ioan-Facsinay A, de Mutsert R, Widya RL, Loef M, de Roos A, et al. Adiposity and hand osteoarthritis: the Netherlands Epidemiology of Obesity study. Arthritis Res Ther. 2014;16(1):R19. doi: 10.1186/ar4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69(4):761–5. doi: 10.1136/ard.2008.106930. [DOI] [PubMed] [Google Scholar]
- 43.Kluzek S, Newton JL, Arden NK. Is osteoarthritis a metabolic disorder? Br Med Bull. 2015;115(1):111–21. doi: 10.1093/bmb/ldv028. [DOI] [PubMed] [Google Scholar]
- 44.Mathiessen A, Slatkowsky-Christensen B, Kvien TK, Hammer HB, Haugen IK. Ultrasound-detected inflammation predicts radiographic progression in hand osteoarthritis after 5 years. Ann Rheum Dis. 2016;75(5):825–30. doi: 10.1136/annrheumdis-2015-207241. [DOI] [PubMed] [Google Scholar]
- 45.Visser AW, de Mutsert R, le Cessie S, den Heijer M, Rosendaal FR, Kloppenburg M, et al. The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: the NEO study. Ann Rheum Dis. 2015;74(10):1842–7. doi: 10.1136/annrheumdis-2013-205012. [DOI] [PubMed] [Google Scholar]
- 46.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2010;18(1):24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Muthuri SG, McWilliams DF, Doherty M, Zhang W. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2011;19(11):1286–93. doi: 10.1016/j.joca.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Felson DT. Do occupation-related physical factors contribute to arthritis? Baillieres Clin Rheumatol. 1994;8(1):63–77. doi: 10.1016/s0950-3579(05)80225-0. [DOI] [PubMed] [Google Scholar]
- 49.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bureau UC. DP-1. Profile of General Population and Housing Characteristics. [Accessed date: 12/23/2016];2010: Johnston County, North Carolina & United States. Available at: https://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml.
- 51.Brady TJ, Jernick SL, Hootman JM, Sniezek JE. Public health interventions for arthritis: expanding the toolbox of evidence-based interventions. J Womens Health (Larchmt) 2009;18(12):1905–17. doi: 10.1089/jwh.2009.1571. [DOI] [PubMed] [Google Scholar]
- 52.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64(4):465–74. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.