Abstract
Background
Studies suggest that exaggerated amygdala reactivity is a vulnerability factor for post-traumatic stress disorder (PTSD), however understanding is limited by a paucity of prospective, longitudinal studies. Recent studies in healthy samples indicate that, relative to reactivity, habituation is a more reliable biomarker of individual differences in amygdala function. We investigated reactivity of the amygdala and cortical areas to repeated threat presentations in a prospective study of PTSD.
Methods
Participants were recruited from the emergency department of a large level-I trauma center within 24 hours of trauma. PTSD symptoms were assessed at baseline, and approximately 1, 3, 6, and 12 months post-trauma. Growth curve modeling was used to estimate symptom recovery trajectories. N=31 participated in fMRI around the 1 month assessment, passively viewing fearful and neutral face stimuli. Reactivity (fearful>neutral) and habituation to fearful faces was examined.
Results
Amygdala reactivity, but not habituation, 5–12 weeks post-trauma was positively associated with the PTSD symptom intercept and predicted symptoms at 12 months post-trauma. Habituation in the ventral anterior cingulate cortex (vACC) was positively associated with the slope of PTSD symptoms, such that decreases in vACC activation over repeated presentations of fearful stimuli predicted increasing symptoms.
Conclusions
Findings point to neural signatures of risk for maintaining PTSD symptoms following trauma exposure. Specifically, chronic symptoms were predicted by amygdala hyper-reactivity and poor recovery was predicted by a failure to maintain vACC activation in response to fearful stimuli. The importance of identifying patients at risk following trauma exposure is discussed.
Keywords: trauma, fear, arousal, fMRI, prospective, amygdala
Introduction
Early identification of risk factors that predispose individuals to trauma-related psychopathology, such as post-traumatic stress disorder (PTSD), could help providers prevent or limit symptoms before a disorder develops. Such risk assessment in the peri-traumatic period could benefit a significant proportion of the general population; it has been estimated that 50–60% experience a potentially traumatizing event (1), and 6–8% develop PTSD following trauma exposure (1, 2). Markers of brain function may be particularly powerful biomarkers of risk, as they can provide insight into the mechanisms leading to maladaptive responses to trauma, and potential targets for treatment. Furthermore, understanding the intermediate phenotypes of brain function which underlie PTSD development may lead to novel therapeutic approaches.
Findings from studies of chronic PTSD consistently show an association between symptoms and hyper-reactivity of the amygdala and dorsal aspects of the anterior cingulate cortex (dACC), key brain regions for emotional expression and appraisal (3–7). In addition, PTSD is associated with under-activity and reduced functional connectivity among regions that regulate amygdala function including ventral aspects of the anterior cingulate cortex (vACC)(6, 8–11). This pattern of abnormalities is thought to contribute to hyper-arousal symptoms in PTSD, and impairments in top-down emotion regulation and fear extinction (3, 8, 9, 12). However, the majority of previous research has been conducted cross-sectionally in chronic PTSD, and cannot distinguish between risk factors or acquired features of the disorder.
Recent findings from prospective studies of trauma and PTSD implicate amygdala function as a potential predictor of PTSD. For example, studies of military service members before and after combat deployment showed that amygdala and vACC reactivity increased significantly from pre- to post-combat exposure (13, 14), and that pre-deployment amygdala reactivity to emotionally arousing or risk-related stimuli positively predicted post-deployment PTSD symptoms (14, 15). Perhaps because these studies recruited individuals from highly trained military samples, participants did not show significant levels of PTSD severity post-trauma, and additional studies are needed to determine whether findings generalize to the broader population. A pilot study in a civilian population (N=9) was conducted to assess responses to acute traumas that led to a hospital emergency department visit, and showed that default-mode connectivity between the amygdala and posterior cingulate cortex 6 weeks post-trauma was positively related to PTSD symptoms 12 weeks post-trauma (16). However, amygdala reactivity has not been investigated as a PTSD predictor in a civilian cohort.
Ideal biomarkers of brain function are those that are reliable and minimally influenced by transient day-to-day changes. Test-retest reliability for fMRI measures of reactivity to emotional face stimuli in regions including the amygdala and ACC has been shown to be fair to excellent (17–19). Furthermore, recent studies indicate that amygdala habituation, or the change over time in the response to a repeated stimulus, shows greater test-retest stability within individuals than reactivity (17, 18). Interestingly, individuals with chronic PTSD show an increased initial amygdala response to trauma-related negative stimuli and altered patterns of amygdala habituation relative to controls (20). This heightened initial response, and differences in habituation, may contribute to previous findings of amygdala hyper-reactivity in PTSD. However, no prospective study of PTSD has examined habituation of responses to emotional stimuli.
In the current study, we conducted a prospective longitudinal investigation of PTSD symptoms following acute trauma, measuring brain function using functional magnetic resonance imaging (fMRI) at an early timepoint prior to PTSD diagnosis, approximately 1 month after the index trauma. We investigated neural reactivity and habituation to emotional stimuli as predictors of later PTSD symptom trajectories over the next year. Participants were recruited from an ongoing longitudinal study of biomarkers for PTSD in which individuals who experienced a traumatic event were approached in the emergency department within 24 hours of trauma and assessed for symptoms at 1, 3, 6, and 12 months post-trauma. The fMRI scan took place within 2–3 weeks of the 1-month visit (timeline of visits illustrated in Figure 1b), with M(SD) = 57(14) days following trauma exposure. We hypothesized that heightened reactivity to negative stimuli in the amygdala and dACC, and blunted reactivity in the vACC, would predict later PTSD symptom severity, consistent with the idea that these brain phenotypes reflect vulnerability factors for PTSD. In addition, we assessed the exploratory hypothesis that reduced habituation of the amygdala response to repeated presentations of negative stimuli would predict later PTSD symptoms.
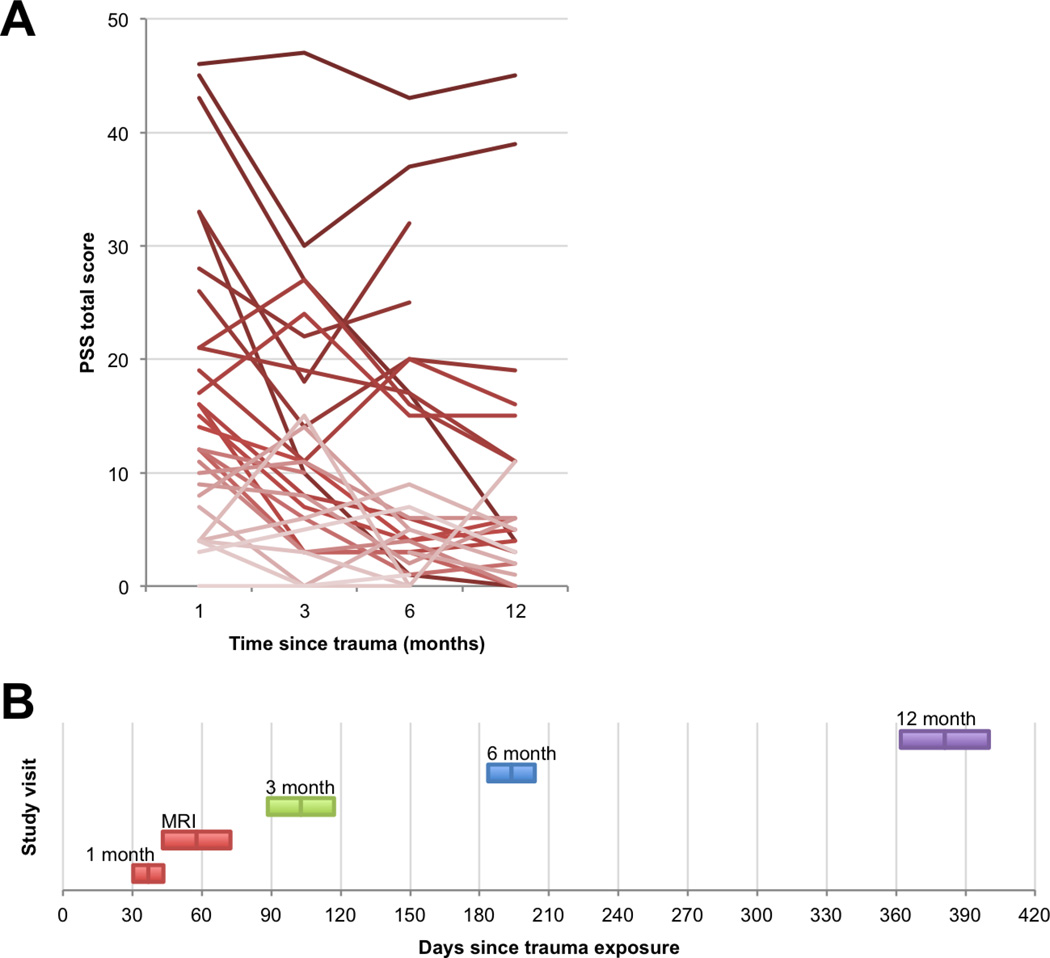
Figure 1.
PTSD symptom trajectories. A: Line graph shows each participant’s PTSD symptom severity scores (PSS total scores), across the four follow-up visits. Line shading (darker to lighter) indicates more severe to less severe symptoms at the 1-month visit. B: Timeline of study visits. Colored bars show M ± SD in the visit delay, relative to study enrollment in the Emergency Department.
Methods and Materials
Participants
38 participants were recruited from a larger study of biomarkers for PTSD. Participants from the parent study who indicated interest in neuroimaging were approached to participate in the neuroimaging study. This add-on study was not designed to be representative of the larger study. Participants were patients in the Emergency Department (ED) of Grady Memorial Hospital in Atlanta, GA, who had experienced a traumatic event within the last 24 hours. Participants were included if they were English-speaking, 18–65 years of age, endorsed a criterion A trauma as defined by the DSM-IV-TR (21), and provided contact information for follow-up visits. Exclusion criteria included previous hospitalization for mental health reasons, current suicidal ideation, attempted suicide in the past 3 months, current intoxication, or altered mental status during the ED visit. After MRI data collection, 7 participants were excluded from further analysis due to anatomical abnormalities such as falx calcification (n=3), head motion > 3mm (n=3), and stimulus presentation malfunction (n=1). Of the final sample of 31, 22 participants were in motor vehicle crashes, 4 were pedestrians who were hit by a vehicle, 3 were motorcycle or bicycle accidents, and 2 were sexual assault victims.
Following the trauma, several participants sought therapy and/or mental health counseling (unrelated to the current study): 6 within the first month, 4 between 1–3 months, 2 between 3–6 months, and 2 between 6–12 months post-trauma. Additional sample characteristics are summarized in Table 1. Table S1 lists prescription medication use and co-morbid diagnoses identified on a Mini International Neuropsychiatric Schedule (MINI)(22) administered during the emergency department visit. Participants provided written informed consent for all parts of the study, and the Institutional Review Boards of Emory University and Grady Memorial Hospital approved the study procedures.
Table 1.
Sample characteristics
| Characteristic | M (SD) or % |
|---|---|
| Age | 31.9 (10.4) |
| Childhood trauma, CTQ | 37.3 (13.0) |
| Gender, Female (%) | 48% |
| Ethnicity, Hispanic (%) | 10% |
| Race (%) | |
| Black | 71 % |
| White | 16% |
| Mixed | 13% |
| Education (%) | |
| Master's degree | 3% |
| Bachelor's degree | 3% |
| Associate's, some college | 55% |
| High School degree | 29% |
| Some high school | 10% |
| Household monthly income (%) | |
| $0 – 249 | 10 % |
| $250 – 499 | 10% |
| $500 – 999 | 13% |
| $1,000 – 1,999 | 20% |
| $2,000 or more | 47% |
| Similar prior trauma? | 42% |
Emergency Department (ED) assessment at the time of trauma exposure
Demographic information and information about the index trauma was gathered using the Standardized Trauma Interview (STI), a 41-item clinician administered interview gathering information on relevant aspects of the trauma at baseline as well as demographic information (23). To assess prior trauma history and baseline PTSD symptoms related to prior trauma, we administered the Posttraumatic Stress Diagnostic Scale (PDS), a 49-item self-report measure (24). Depression symptoms in the two weeks prior to the ED visit were assessed using the Beck Depression Inventory (BDI), a 21-item self-report measure (25). Childhood trauma history was assessed using the Childhood Trauma Questionnaire (CTQ), a 25-item instrument assessing physical, sexual, and emotional abuse, and physical and emotional neglect before age 18, which has shown high reliability and validity relative to external measures of child abuse (26).
Follow-up assessments
PTSD and depression symptoms were assessed 1 month, 3 months, 6 months, and 12 months following the ED visit. PTSD symptom severity in response to the index trauma was measured using the (PSS)(27), a 17-item scale measuring symptom severity assessing DSM-IV-TR criteria for PTSD (21). The PSS items assess the same 17 symptoms assessed by the PDS at the baseline ED visit for prior trauma, using a similar 0–3 scale for frequency. Depression symptoms were measured using the Beck Depression Inventory (BDI), a 21-item scale measuring symptom severity (28).
FMRI study procedure
Participants completed the fMRI session within three weeks [M(SD) = 21(3) days] of the 1 month follow-up assessment [delay relative to index trauma = 57(14) days]. Study procedures followed Stevens et al. (3). Participants passively viewed static fearful and neutral face stimuli, which were presented in blocks of 8 trials, with a total of 30 blocks (15 fearful, 15 neutral) that randomly alternated between the fearful and neutral conditions.
Brain imaging acquisition and analysis
Brain imaging data were acquired on two Siemens 3.0-Tesla Magnetom Trio TIM MRI scanners (Siemens, Malvern, PA) using a 12-channel head coil. N=20 participants were scanned on the first scanner, and N=11 on the second. Functional images were acquired using the Z-SAGA pulse sequence (29) to minimize signal loss due to susceptibility artifacts. Volumes contained 30 axially acquired 4mm thick images with an in-plane resolution of 3.44 × 3.44 mm2 utilizing the parameters: pulse repetition time 3000 ms, TE1/TE2= 30/67ms, FA= 90°. Structural images were acquired using a gradient-echo, T1-weighted pulse sequence (TR=2300ms, TE=2.78ms; 1.2mm × 1.3mm × 1.3mm voxel size).
Preprocessing and statistical analysis was conducted in SPM8 (Wellcome Department of Cognitive Neurology), and details can be found in (30). Briefly, spike and motion artifacts were corrected using ArtRepair software (31). Images were corrected for slice timing, and spatial realignment was applied. Participants with head motion > 3mm over the entire session were excluded from further analyses. Images were normalized with unified segmentation, and smoothed with an 8mm Gaussian kernel.
Blocks of fearful and neutral stimuli were modeled with a boxcar function representing the onset and 8000 ms duration of the block, convolved with a canonical hemodynamic response function. Participant-specific motion parameters were included as covariates. To assess reactivity to threat, contrast images for the fearful versus neutral conditions were entered into group-level random effects analyses. To assess habituation to threat, the first third of the fearful face blocks (5 blocks) were compared to the last third, following analytic strategies used in previous research with healthy samples (18, 32, 33). To investigate whether habituation effects were specific to threat stimuli, we also examined habituation to neutral faces (first third of neutral blocks – last third). Hypothesis-driven regions of interest (ROIs) were constructed in WFUPickAtlas 2.4 software (www.fmri.wfubmc.edu). A bilateral amygdala ROI was defined anatomically using the Automated Anatomical Labeling Toolbox (AAL)(34). To anatomically define the dorsal and ventral aspects of the ACC, we selected primate analogs of rodent prelimbic (PL) and infralimbic (IL) cortex, because the rodent literature provides clear examples of functional differentiation among medial prefrontal cortical areas (e.g., 35, 36). The dACC (PL) was defined using Brodmann Area (BA) 32, and the vACC (IL) was defined using BA 25 (37). To examine regions outside the ROIs, whole-brain analyses were conducted with SPM’s cluster-based False Discovery Rate (FDR) thresholding and an initial threshold of p<.005.
Data analytic strategy
A linear growth curve was estimated in the MPlus (version 7) environment using PSS scores from 1, 3, 6 and 12 months’ post emergency room admission. Two parameters were estimated including the intercept (initial PTSD symptom severity) and slope (change over time in symptoms) using maximum likelihood estimation. The slope and intercept parameters were separately regressed on amygdala, vACC, and dACC reactivity and habituation while controlling for age, gender, exposure to similar previous trauma, and scanner in the overall growth curve model. The intercept and slope values were then saved outside of the MPlus environment for use in additional whole-brain analyses, with age, gender, and scanner as covariates. To improve estimation for saved values, the growth curve was estimated using the larger ED study sample (N=355). However, the only data reported are for the subset (N=31) with neuroimaging data.
Results
Figure 1a and Table 2 show PTSD symptoms related to the index trauma at each of the follow-up visits. Twenty-nine participants of the initial sample of 31 returned for the follow-up assessment at 3 months, 29 at 6 months, and 24 at 12 months post-trauma. The growth curve estimates demonstrated an initial intercept that was significantly greater than 0 (Est=14.92; S.E.=0.67; p<.001) and a significant negative slope (Est=−0.53; S.E.=0.09; p<.001) together indicating significant levels of PTSD symptom severity at 1 month, followed by a significant average decline in symptoms by 12 months post-trauma. However, it is notable that mean symptoms were still moderate at 12 months (M=9.8).
Table 2.
Group summary of symptoms at the post-trauma assessment visits [M(SD)]
| In ED (N=31) |
1 month (N=31) |
3 months (N=29) |
6 months (N=29) |
12 months (N=24) |
|
|---|---|---|---|---|---|
| PTSD symptoms (PSS or PDS) |
4.7(8.2) | 17.2(12.4) | 12.9(10.8) | 10.9(11.7) | 9.8(11.6) |
| Re-experiencing | 0.9(1.7) | 4.7(4.2) | 3.1(3.8) | 2.6(3.4) | 2.0(3.4) |
| Avoidance/Numbing | 1.8(4.0) | 6.2(5.5) | 4.3(4.1) | 3.7(5.0) | 3.3(5.1) |
| Hyper-arousal | 2.0(3.3) | 6.3(4.0) | 5.6(3.9) | 4.6(4.2) | 4.5(4.0) |
| Depression symptoms (BDI) |
9.2(9.2) | 13.5(10.4) | 10.3(8.7) | 9.5(10.2) | 9.0(9.3) |
Reactivity and habituation to fearful face stimuli
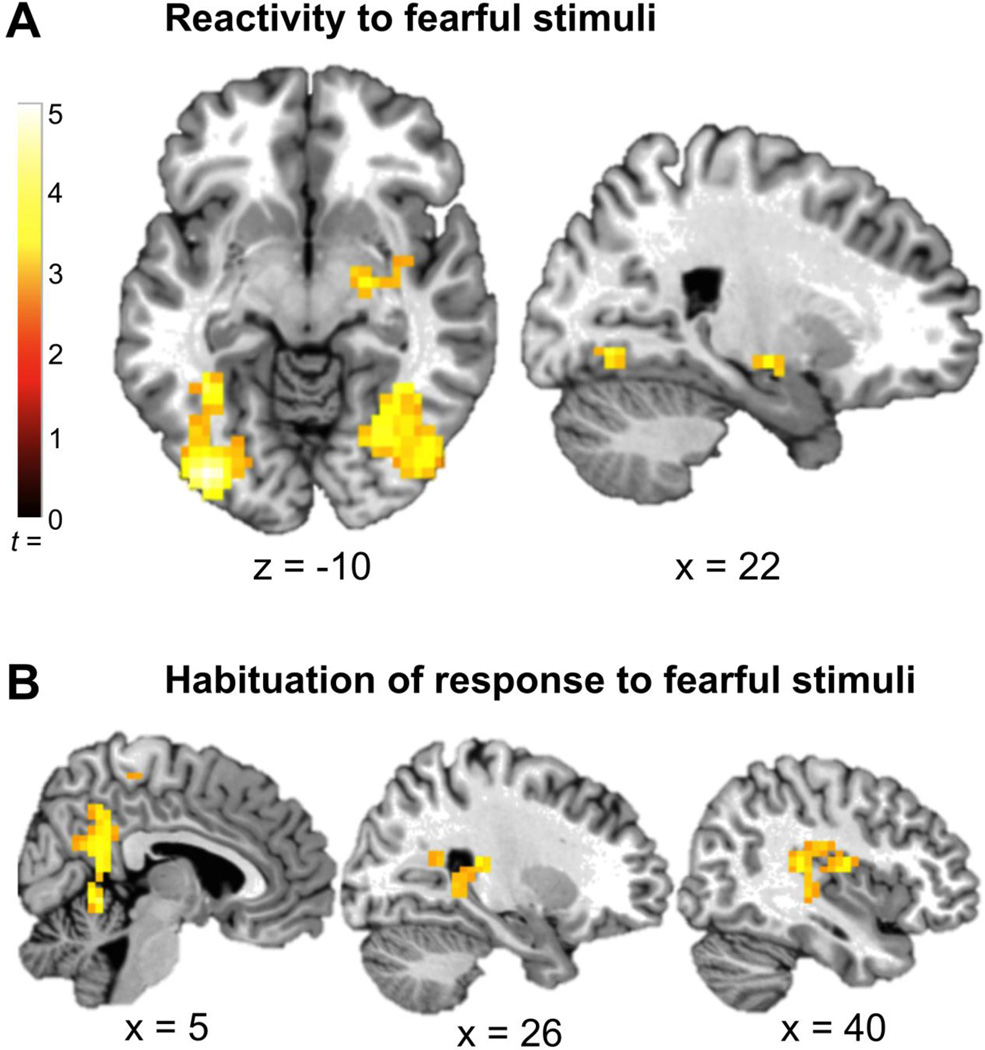
Whole-brain analysis of task-related fMRI activation across the full sample showed significant activation in the right amygdala and bilateral occipital cortex activation for fearful > neutral stimuli, p<.05, corrected (Figure 2a). For the habituation contrast comparing the first 5 > last 5 fearful blocks, there was no significant habituation in any region. Instead there was significant sensitization (increased response from beginning to end of scan) in a large cluster overlapping posterior cingulate cortex, right posterior hippocampus, and right posterior insula, p<.05, corrected (Figure 2b).
Figure 2.
Task-related activation for the full sample. A: The right amygdala (46, 0, −30; Z = 3.48; k = 124) and bilateral occipital cortex (right: 38, −88, 2; Z = 4.33; k = 345; left: =−38, −88, −18; Z = 4.55; k = 218) were significantly activated in the fearful>neutral contrast, p<.05, corrected. B: A cluster overlapping posterior cingulate, right posterior hippocampus, and right posterior insula (2, −56, −6; Z = 3.84; k = 441) showed a pattern of sensitization to the fearful stimuli (first 5 < last 5 fearful blocks), p<.05, corrected.
Associations between fMRI activation and the intercept (initial symptom levels)
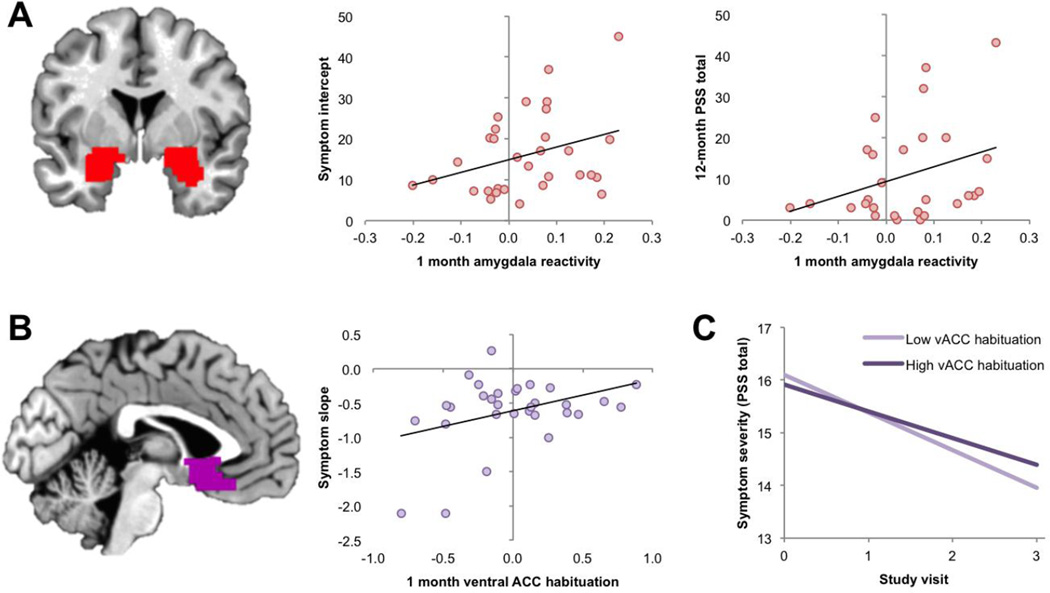
Emotional reactivity in the amygdala ROI was significantly positively associated with the intercept parameter (Est=3.44; S.E.=1.46; p=.02), indicating a positive relationship between amygdala reactivity and symptom levels (Figure 3a). This effect remained significant in follow-up analysis of the N=28 participants without PTSD related to prior trauma (Est=3.43; S.E.=1.50; p<.05). Reactivity in the dACC and vACC ROIs were not associated with the intercept, nor was habituation of responses to fearful or neutral stimuli in any of the ROIs, ps>.20. In whole-brain analyses of the emotional reactivity and habituation contrasts, no region showed a significant correlation with the intercept parameter.
Figure 3.
Measures of fMRI reactivity and habituation that were predictive of later PTSD symptoms. A: Reactivity to fearful faces (fearful > neutral) in the amygdala ROI positively predicted PTSD symptom severity as reflected by the intercept parameter (middle panel) and total symptom severity 12 months post-trauma (right-most panel). Amygdala ROI is overlaid on a representative single-subject brain in MNI space. B: Habituation to fearful faces (first 5 > last 5 fearful blocks) in the ventral ACC ROI positively predicted PTSD symptom slope over the 12 months following trauma, such that more ACC habituation predicted flatter symptom trajectories. ACC ROI is overlaid on a representative single-subject brain in MNI space. C: Slope and intercept of PTSD symptoms over the 4 study visits (0 = 1 month post-trauma, 1 = 3 months, 2 = 6 months, 3 = 12 months), as a function of vACC habituation. For illustrative purposes, the sample was divided into low- and high-habituation groups based on a median split of vACC habituation. Graph shows that higher vACC habituation is associated with a flatter symptom slope over time.
Associations between fMRI activation and symptom trajectories
Emotional reactivity in the amygdala and ACC ROIs was not predictive of the slope (change over time in symptoms; ps>.05). Habituation to fearful stimuli in the vACC ROI demonstrated a positive relationship with the slope parameter (Est=0.94; S.E.=0.40; p=.05; Figure 3b), indicating that greater habituation was associated with a flatter symptom slope (Figure 3c). This effect was stronger in follow-up analysis of the N=28 participants without PTSD related to prior trauma (Est=−2.51; S.E.=0.32; p<.001). Habituation to fearful stimuli in the amygdala and dACC ROIs was not associated with slope, ps>0.10, nor was habituation to neutral stimuli in any of the ROIs, ps>0.30. In whole-brain analyses of the emotional reactivity and habituation contrasts, no region showed a significant correlation with symptom slope.
Contrast estimates for the reactivity and habituation contrasts in the amygdala, vACC, and dACC ROIs were then examined as predictors of 12 month scores as a time-variant covariate nested in the growth curve model. Amygdala reactivity was positively associated with 12 month PSS severity scores (Est=−0.50; S.E.=0.25; p<=0.05) while vACC and dACC reactivity were not (EstvACC= 0.11; S.E.=0.23; p=.63; EstdACC=−.0.23; S.E.=0.30; p=.45). Habituation of the amygdala, vACC, and dACC were not associated with 12 month severity scores (Estamygdala= 2.00; S.E.=1.26; p=.11; EstvACC= 1.49; S.E.=0.97; p=.13; EstdACC=−.24; S.E.=1.33; p=.07).
Discussion
In the current study we examined relationships between emotional brain function and later PTSD symptoms in an acutely traumatized sample. The findings supported the hypothesis that amygdala reactivity would positively predict later symptoms; individuals with a greater amygdala response to fearful faces had greater initial symptom severity and greater severity months post-trauma. This pattern was not related to the amygdala’s habituation to the fearful stimuli, which showed no relationship with current or later PTSD symptoms. In addition, greater vACC habituation to fearful stimuli positively predicted symptom change from 1 to 12 months. Individuals with greater vACC habituation showed a poorer recovery trajectory (flatter slope of recovery) over this time period.
The findings were consistent with previous studies in military samples. A pair of studies by Admon and colleagues found that greater amygdala reactivity before combat deployment predicted greater PTSD symptoms after deployment (14, 15). In addition, Van Wingen and colleagues found that amygdala reactivity increased from pre- to post-deployment, and this was interpreted as an effect of combat stress (13). Extending these findings, we found that those individuals with the highest amygdala reactivity following trauma exposure had the highest overall PTSD symptom levels and were most likely to maintain PTSD symptoms as long as 12 months later. It is possible that stress related to combat or other forms of trauma may increase amygdala reactivity to threatening stimuli, in turn increasing risk for high levels of PTSD symptoms following trauma exposure. An interesting question for future research will be to investigate risk factors prior to or during trauma exposure that explain these important individual differences in amygdala reactivity observed shortly after trauma exposure. This is likely multiply determined by risk factors previously shown to be associated with greater amygdala reactivity and PTSD risk, such as childhood maltreatment (38–40), genomic risk pathways (41–43), and their interaction (44).
Few previous studies have examined patterns of neural habituation as potential contributors to PTSD symptoms. However, theories that posit heightened and inflexible emotional and physiological arousal as a primary contributor to PTSD (45–48) might predict abnormalities in the habituation response to emotionally evocative or threatening stimuli. One previous study showed abnormal patterns of habituation in the amygdala response to trauma-related word stimuli among individuals with PTSD relative to healthy controls (20), but did not examine other brain regions. Here, however, we did not find any relationship between amygdala habituation and current or later PTSD symptoms. Instead, we observed that habituation in vACC, specifically BA 25, positively predicted the slope of PTSD symptom trajectories from 1 to 12 months post-trauma. This indicated that individuals whose vACC response to the fearful stimuli decreased more sharply over the course of the scan showed a slower course of recovery over the year following trauma. There was no association between symptoms and vACC habituation to neutral stimuli, suggesting that the effect was specific to threat stimuli. This was not a finding that we hypothesized, but is interesting given that PTSD is associated with difficulties in regulating arousal (49, 50), and impairments in fear extinction (6, 51), processes that are both mediated by neurons within the vACC (infralimbic cortex in rodents) and their connections with the amygdala (3, 35, 36, 52). Although the current findings regarding ACC habituation are exploratory in nature, it is possible that faster habituation of ACC responses to fearful stimuli may reflect an inability to maintain top-town regulatory control of emotional responses to fearful stimuli, which is predictive of poor recovery trajectories. Additional neuroimaging research specifically probing individual differences in emotion regulation would be helpful in informing the role of vACC function in predicting PTSD recovery outcomes.
The primary limitation of the current study is that it did not capture brain function before trauma onset. However, given the practical difficulty of scanning individuals in the general population both prior to and after trauma, the timepoint of 6–9 weeks following trauma is a reasonable alternative because initial reactions to the traumatic event have subsided, major injuries have healed enough for most individuals to participate in an MRI scan, and the timepoint is before the diagnosis of chronic PTSD at 3 months post-trauma. In addition, this study was conducted in a small pilot sample, and PTSD symptoms were assessed using self-report measures. Additional replication in larger samples with additional varieties of traumas, and replication with clinician-administered interview measures of PTSD, is needed.
To summarize, amygdala hyper-reactivity and vACC habituation to threat predicted later PTSD symptoms in the aftermath of an index trauma. These markers of neural function in the peri-traumatic period suggest that amygdala and ACC function are key targets for early interventions such as psychotherapy or pharmacological treatments administered in the acute aftermath of trauma.
Supplementary Material
Acknowledgments
We would like to acknowledge Debra Houry, M.D., and Abigail Hankin-Wei, M.D. for their generous collaborative efforts on this study. For their tireless work in the emergency department recruiting and assessing participants, we would like to thank Vasiliki Michopoulous, Alex O. Rothbaum, Thomas Crow, Heather Grinstead, Rebecca C. Roffman, Jessica Maples, Lydia Odenat, Loren M. Post, Liza C. Zwiebach, Devika Fiorillo, Kathryn Breazeale, Jessica Morgan, Natasha Mehta, Elicia D. Skelton, Taleesha S. Booker, and Jonathan Zebrowski.
This work was supported by the National Institute of Mental Health (R01 MH094757, R21 MH106902, F32 MH101976, K01 MH102415, and U01 MH110925).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures
The authors declare no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of general psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res. 2013;47:1469–1478. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological psychiatry. 2010;68:433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biological psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 6.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramage AE, Laird AR, Eickhoff SB, Acheson A, Peterson AL, Williamson DE, et al. A coordinate-based meta-analytic model of trauma processing in posttraumatic stress disorder. Human Brain Mapping. 2012 doi: 10.1002/hbm.22155. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, Social Anxiety Disorder, and Specific Phobia. The American journal of psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, et al. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex. 2013;49:1884–1891. doi: 10.1016/j.cortex.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of general psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 11.Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatr Neurosci. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends in cognitive sciences. 2013;17:337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 13.van Wingen GA, Geuze E, Vermetten E, Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Molecular psychiatry. 2011;16:664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, et al. Human vulnerability to stress depends on amygdala's predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences. 2009;106:14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Admon R, Lubin G, Rosenblatt JD, Stern O, Kahn I, Assaf M, et al. Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cerebral Cortex. 2013;23:28–35. doi: 10.1093/cercor/bhr369. [DOI] [PubMed] [Google Scholar]
- 16.Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiat Scand. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- 17.Gee DG, McEwen SC, Forsyth JK, Haut KM, Bearden CE, Addington J, et al. Reliability of an fMRI paradigm for emotional processing in a multisite longitudinal study. Human brain mapping. 2015;36:2558–2579. doi: 10.1002/hbm.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plichta MM, Grimm O, Morgen K, Mier D, Sauer C, Haddad L, et al. Amygdala habituation: a reliable fMRI phenotype. NeuroImage. 2014;103:383–390. doi: 10.1016/j.neuroimage.2014.09.059. [DOI] [PubMed] [Google Scholar]
- 19.Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, et al. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. NeuroImage. 2005;25:1112–1123. doi: 10.1016/j.neuroimage.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biological psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Association AP. Diagnostic and statistical manual-text revision (DSM-IV-TRim, 2000) American Psychiatric Association; 2000. [Google Scholar]
- 22.Pinninti NR, Madison H, Musser E, Rissmiller D. MINI International Neuropsychiatric Schedule: clinical utility and patient acceptance. European Psychiatry. 2003;18:361–364. doi: 10.1016/j.eurpsy.2003.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Foa EB, Rothbaum BO. Treating the trauma of rape: Cognitive-behavioral therapy for PTSD. Guilford Press; 2001. [Google Scholar]
- 24.Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychological assessment. 1997;9:445. [Google Scholar]
- 25.Beck AT, Steer RA, Brown GK. Beck Depression Inventory. 2nd. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 26.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. The American journal of psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 27.Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale-Interview Version and the Clinician-Administered PTSD scale. Journal of traumatic stress. 2000;13:181–191. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical psychology review. 1988;8:77–100. [Google Scholar]
- 29.Heberlein KA, Hu X. Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-shot z-shim: Z-SAGA. Magnetic Resonance in Medicine. 2004;51:212–216. doi: 10.1002/mrm.10680. [DOI] [PubMed] [Google Scholar]
- 30.Kilaru V, Iyer SV, Almli LM, Stevens JS, Lori A, Jovanovic T, et al. Genome-wide gene-based analysis suggests an association between Neuroligin 1 (NLGN1) and posttraumatic stress disorder. Translational psychiatry. doi: 10.1038/tp.2016.69. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazaika P, Whitfield-Gabrieli S, Reiss A. Artifact Repair for fMRI Data from High Motion Clinical Subjects. Human Brain Mapping. 2007 [Google Scholar]
- 32.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 33.Blackford JU, Allen AH, Cowan RL, Avery SN. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nsr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 35.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 36.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN. Circuit-Based Corticostriatal Homologies Between Rat and Primate. Biological psychiatry. 2016 doi: 10.1016/j.biopsych.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Grant MM, Cannistraci C, Hollon SD, Gore J, Shelton R. Childhood trauma history differentiates amygdala response to sad faces within MDD. J Psychiatr Res. 2011;45:886–895. doi: 10.1016/j.jpsychires.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, et al. PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3158–3163. doi: 10.1073/pnas.1318954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 43.Kilaru V, Iyer S, Almli L, Stevens J, Lori A, Jovanovic T, et al. Genome-wide gene-based analysis suggests an association between Neuroligin 1 (NLGN1) and post-traumatic stress disorder. Translational psychiatry. 2016;6:e820. doi: 10.1038/tp.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White MG, Bogdan R, Fisher PM, Munoz KE, Williamson DE, Hariri AR. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes, brain, and behavior. 2012;11:869–878. doi: 10.1111/j.1601-183X.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 46.Norrholm SD, Glover EM, Stevens JS, Fani N, Galatzer-Levy IR, Bradley B, et al. Fear Load: The Psychophysiological Over-expression of Fear as an Intermediate Phenotype Associated with Trauma Reactions. International Journal of Psychophysiology. 2014 doi: 10.1016/j.ijpsycho.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Keane TM, Kolb LC, Kaloupek DG, Orr SP, Blanchard EB, Thomas RG, et al. Utility of psychophysiology measurement in the diagnosis of posttraumatic stress disorder: Results from a department of Veteran's Affairs cooperative study. Journal of consulting and clinical psychology. 1998;66:914. doi: 10.1037//0022-006x.66.6.914. [DOI] [PubMed] [Google Scholar]
- 49.Association AP. Diagnostic and statistical manual of mental disorders, (DSM-5®) Washington, D.C.: American Psychiatric Pub; 2013. [Google Scholar]
- 50.Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biological psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM, et al. Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex. 2015;64:249–259. doi: 10.1016/j.cortex.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.