Abstract
We performed a single-institution retrospective analysis of 137 patients with muscle-invasive urothelial carcinoma who underwent neoadjuvant chemotherapy and radical cystectomy to assess the prognostic significance of carcinoma in situ (CIS). The pathologic complete response rates were significantly decreased for patients with CIS identified on transurethral resection of the bladder tumor before treatment. The long-term follow-up data from patients with isolated CIS at cystectomy revealed prolonged progression-free and overall survival.
Background
Carcinoma in situ (CIS) is a poor prognostic finding in urothelial carcinoma. However, its significance in muscle-invasive urothelial carcinoma (MIUC) treated with neoadjuvant chemotherapy (NAC) is uncertain. We assessed the effect of CIS found in pretreatment transurethral resection of bladder tumor (TURBT) biopsies on the pathologic and clinical outcomes.
Materials and Methods
Subjects with MIUC treated with NAC before cystectomy were identified. The pathologic complete response (pCR) rates stratified by TURBT CIS status were compared. The secondary analyses included tumor response, progression-free survival (PFS), overall survival (OS), and an exploratory post hoc analysis of patients with pathologic CIS only (pTisN0) at cystectomy.
Results
A total of 137 patients with MIUC were identified. TURBT CIS was noted in 30.7% of the patients. The absence of TURBT CIS was associated with a significantly increased pCR rate (23.2% vs. 9.5%; odds ratio, 4.08; 95% confidence interval, 1.19–13.98; P = .025). Stage pTisN0 disease was observed in 19.0% of the TURBT CIS patients. TURBT CIS status did not significantly affect the PFS or OS outcomes. Post hoc analysis of the pTisN0 patients revealed prolonged median PFS (104.5 vs. 139.9 months; P = .055) and OS (104.5 vs. 152.3 months; P = .091) outcomes similar to those for the pCR patients.
Conclusion
The absence of CIS on pretreatment TURBT in patients with MIUC undergoing NAC was associated with increased pCR rates, with no observed differences in PFS or OS. Isolated CIS at cystectomy was frequently observed, with lengthy PFS and OS durations similar to those for pCR patients. Further studies aimed at understanding the biology and clinical effect of CIS in MIUC are warranted.
Keywords: CIS, NAC, Pathologic complete response, Transurethral resection of bladder tumor, UC
Introduction
Urothelial carcinoma (UC) of the bladder is a common cancer, with > 76, 000 new cases diagnosed and 16,000 deaths annually in the United States.1 Worldwide, an estimated 450,000 new cases and 165,000 deaths occur annually.2 In muscle-invasive UC (MIUC), radical cystectomy remains a standard treatment approach with significant quality of life effects. Given the substantial morbidity from surgical intervention and metastatic relapse rates approaching 50%, the identification of clinical, pathologic, and molecular factors that affect patient outcomes is critical to the success of personalized patient care.3
When observed, the presence of carcinoma in situ (CIS) has been associated with an aggressive, initially immunotherapy responsive phenotype. CIS has been shown to have a 54% to 83% risk of progression to MIUC if left untreated.4,5 Additionally, extravesical involvement of UC is common with CIS and carries a worse prognosis.6,7 Aggressive intravesical therapy after the initial resection of non–muscle invasive UC (NMIUC) or cystectomy in patients developing relapse after adequate intravesical therapy has typically been recommended.8 Uncertainty regarding the distinct mechanisms responsible for the improved response rates to local immunotherapy in patients with CIS compared with those with high-grade papillary NMIUC (< pT2) underscores the need for improved understanding of the underlying pathobiology of CIS.9,10
In patients with UC, CIS can be present as an isolated finding or simultaneously in association with more invasive tumors within the bladder and upper urinary tract. A study by Shariat et al11 noted significantly increased disease recurrence when CIS was identified on cystectomy specimens performed for NMIBC. Another study by Gupta et al12 revealed increased recurrence rates and decreased cancer-specific survival (CSS) in patients with NMIBC treated with cystectomy if CIS had been identified on precystectomy transurethral resection of bladder tumor (TURBT) samples.12 A meta-analysis by Sylvester et al13 also showed that the presence of concurrent CIS within stage Ta or T1 TURBT specimens correlated with significantly increased risks of disease recurrence and progression. Two large retrospective studies attempted to evaluate whether CIS has prognostic significance specifically in patients with MIUC. Yafi et al14 found that CIS found on TURBT in patients with MIUC led to statistically significant upstaging at cystectomy; however, no significant differences in progression-free survival (PFS), CSS, or overall survival (OS) were observed. Nuhn et al15 evaluated MIUC cystectomy specimens for the presence of CIS and found no associated differences in PFS or CSS.
Level 1 evidence from randomized clinical trials demonstrated significant improvements in OS with the use of neoadjuvant cisplatin-based combination chemotherapy before cystectomy for eligible patients with MIUC.16,17 Furthermore, the neoadjuvant approach represents an attractive setting for drug development, because multiple studies have demonstrated an association between the pathologic response and longer term survival outcomes.17–20 In general, agreement exists that a reduction in stage to a non–muscle-invasive stage (< pT2N0) confers more favorable outcomes. It is less clear whether a pathologic complete response (pCR), defined as no evidence of any tumor of any stage (pT0N0), is necessary for patient benefit compared with simply a reduction to a non–muscle-invasive stage. Because clinical trials now commonly use pCR as a primary endpoint for neoadjuvant MIUC trials, a thorough understanding of the baseline patient factors with an effect on pCR rates is critical. In the only study to analyze the effect of TURBT CIS on the cystectomy pathologic response in MIUC patients who received neoadjuvant chemotherapy (NAC), Parker et al21 reported inferior pCR rates at cystectomy in association with TURBT CIS; however, no effect on survival outcomes was observed. We present the results from our large single-institution retrospective analysis evaluating the pathologic and prognostic significance of pretreatment TURBT CIS in patients with MIUC treated with NAC followed by cystectomy.
Materials and Methods
Study Cohort
The study subjects were identified from the urology and medical oncology department clinical databases of Indiana University Simon Cancer Center (IUSCC). The eligibility criteria included patients of any age with nonmetastatic UC who were documented to have received NAC and whose pathologic reports were available for both prechemotherapy TURBT (or staging biopsy) and post-chemotherapy cystectomy. Patients with clinical lymph node-positive disease found on imaging studies were included as long as NAC and cystectomy had been performed with curative intent. Subjects with components of non–small-cell variant histologic features were allowed; however, subjects with pure nonurothelial histologic features found on TURBT were excluded. Subjects with NMIUC and those with any component of small cell or neuroendocrine features on TURBT were excluded. The institutional review board of the IUSCC approved the present study.
Pathologic Staging and Response Evaluation
All cystectomies were performed at the IUSCC (Indianapolis, IN). All TURBTs and cystectomy pathologic specimens obtained at IUSCC were reviewed by expert genitourinary pathologists. The slides from TURBTs performed at outside institutions were not uniformly reviewed again, and staging was established by a review of the outside pathology reports. Pathologic staging was performed in accordance with the American Joint Committee on Cancer TNM system for UC of the bladder. A pCR was defined by a finding of T0N0 at cystectomy.
Clinical Data Evaluation
The available clinical and demographic information was recorded for all study subjects and included the following: age at TURBT, gender, race, Eastern Cooperative Oncology Group performance status, Karnofsky performance status, baseline laboratory values (creatinine, white blood cell count, hemoglobin, platelet count), TURBT date, clinical stage (including TURBT pathologic findings and other staging diagnostics), presence of variant histologic features on TURBT (types and percentages, if reported), the presence of CIS on TURBT, NAC regimen, cycles of NAC completed, cystectomy date, pathologic stage, date of progression, date of death, and date of the last follow-up visit. The data were tabulated into a clinical database for further statistical analyses (Figure 1). All primary information was gathered from the IUSCC and associated hospital electronic medical record systems.
Figure 1. Schema.
Abbreviations: CIS = carcinoma in situ; MIUC = muscle-invasive urothelial carcinoma; pCR = pathologic complete response; TURBT = transurethral resection of bladder tumor.
Data Verification and Missing Data Methods
The clinical information, including the pathologic reports and stage, was verified by all investigators for accuracy. For the TURBT cases from outside institutions, which were reviewed by IUSCC pathologists, the IUSCC pathology review date was used as the TURBT date if the original TURBT date was not available from the outside institution pathology report. If the original TURBT date was supplied, the original TURBT date was used in all analyses.
Survival Evaluation
PFS was calculated as the duration between the TURBT date and the occurrence of either death from any cause or documented UC recurrence radiographically or clinically. OS was calculated as the duration between the TURBT date and death from any cause. For both PFS and OS analyses, patients lost to follow-up were censored at their last known follow-up date.
Statistical Analysis
The primary outcome of interest in our analysis was the pCR rate. We aimed to test the experimental hypothesis that the finding of CIS in TURBT samples from MIUC patients would be associated with lower pCR rates after NAC. All baseline clinical variables for which data were complete (age at TURBT, gender, race, clinical stage, NAC regimen, presence of variant histologic features, CIS on TURBT) were evaluated by logistic regression analyses for associations with the pCR rate, with the significance level set at P < .05. Continuous variables (ie, age at TURBT) were dichotomized according to the median values. Odds ratios (ORs) and 95% confidence intervals (CIs) were computed for all variables analyzed. The secondary outcomes included comparisons of PFS and OS according to TURBT CIS status. Kaplan-Meier curves were calculated and log-rank analyses used to compare the PFS and OS according to TURBT CIS status and according to pathologic pTis status. χ2 Tests and Student t tests were used to assess the differences in baseline demographic variables between patients with and without CIS on TURBT. χ2 Tests were used in exploratory analyses to identify the baseline factors predictive of pTisN0 status. All analyses were performed using SPSS Statistics, version 22.
Results
Patient Population
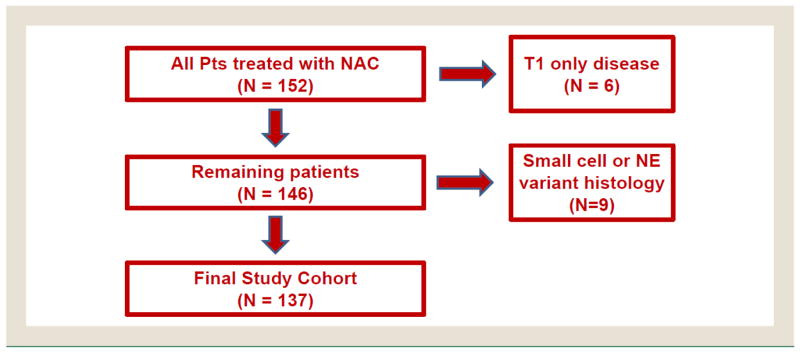
After database review, 152 potential subjects were identified. Of the 152 potential subjects, 6 with stage T1 NMIUC and 9 with small cell carcinoma or neuroendocrine features were excluded, leaving a final study data set of 137 subjects (Figure 2). The study data set was divided into 2 cohorts, with 42 subjects with CIS found on TURBT (30.7%) and 95 subjects without CIS found on TURBT. The patients had documented follow-up data available for an average of 25.4 months (range, 0–264 months).
Figure 2. Analysis Cohort Selection.
Abbreviations: NAC = neoadjuvant chemotherapy; NE = neuroendocrine; Pts = patients.
The patient demographics are listed in Table 1. The study population was a largely white male population, with a mean age of 61.1 years (median, 62.0 years). Most patients had clinical stage T2N0, and 40 patients (29.2%) had clinical lymph node involvement. Cisplatin-based NAC was used in 113 patients (82.5%). The presence of variant histologic features was noted in 46 patients (33.6%). Subjects with CIS found on TURBT were more frequently women, had a greater incidence of clinical stage T2N0 disease, had a lower incidence of clinical lymph node-positive disease, and were more frequently treated with cisplatin NAC. However, none of these differences were statistically significant.
Table 1.
Patient Demographics
| Variable | Full Cohort (n = 137) | CIS TURBT (n = 42) | No CIS TURBT (n = 95) | P Value |
|---|---|---|---|---|
| Age (y) | 61.1 ± 11.0 | 61.5 ± 10.4 | 60.9 ± 11.2 | .94 |
| Creatinine (mg/dL) | 1.2 ± 0.4 | 1.1 ± 0.4 | 1.2 ± 0.4 | .55 |
| WBC count (K/mL) | 7.5 ± 2.4 | 7.4 ± 2.5 | 7.6 ± 2.4 | .74 |
| Hemoglobin (g/dL) | 13.0 ± 2.2 | 13.1 ± 2.3 | 12.9 ± 2.2 | .68 |
| Platelet count (K/mL) | 273.2 ± 99.4 | 278.7 ± 143.4 | 271.4 ± 80.3 | .76 |
| CIS on TURBT | 42 (30.7) | NA | NA | NA |
| Variant histologic features | 46 (33.6) | 16 (38.1) | 30 (31.6) | .46 |
| ECOG PS | .20 | |||
| 0 | 25 (18.2) | 6 (14.3) | 19 (20.0) | |
| 1 | 27 (19.7) | 6 (14.3) | 21 (22.1) | |
| 2 | 5 (3.6) | 3 (7.1) | 2 (2.1) | |
| Unknown | 80 (58.4) | 27 (64.3) | 53 (55.8) | |
| White race | 128 (93.4) | 40 (95.2) | 88 (92.6) | .57 |
| Male gender | 104 (75.9) | 29 (69.0) | 75 (78.9) | .21 |
| Clinical stage | ||||
| T2N0 | 83 (60.6) | 30 (71.4) | 53 (55.8) | .08 |
| T3-T4N0 | 14 (10.2) | 4 (9.5) | 10 (10.5) | .86 |
| Any T, N+ | 40 (29.2) | 8 (19.0) | 32 (33.7) | .08 |
| Cisplatin-containing regimen | 113 (82.5) | 38 (90.5) | 75 (78.9) | .10 |
Data presented as mean ± standard deviation or n (%).
Abbreviations: CIS = carcinoma in situ; ECOG = Eastern Cooperative Oncology Group; PS = performance status; TURBT = transurethral resection of bladder tumor; WBC = white blood cell.
NAC Tumor Response
The pCR rate at cystectomy was lower in patients with CIS found on TURBT (9.5% vs. 23.2%; P = .07; Table 2). Furthermore, a significantly greater frequency of pTisN0M0 stage was observed at cystectomy in patients with CIS found on TURBT (19.0% vs. 3.2%; P < .01). The rates of < pT2N0M0, pN+, and pM1 disease were similar between the 2 groups.
Table 2.
Tumor Response at Cystectomy
| Outcome | CIS TURBT (n = 42) | No CIS TURBT (n = 95) | P Value |
|---|---|---|---|
| Complete response (pCR) | 4 (9.5) | 22 (23.2) | .07 |
| Pathologic TisN0M0 | 8 (19.0) | 3 (3.2) | <.01 |
| Pathologic <T2N0M0 | 18 (42.9) | 35 (36.8) | .51 |
| Pathologic N+M0 | 14 (33.3) | 25 (26.3) | .40 |
| Pathologic M1 | 0 (0.0) | 2 (2.1) | 1.00 |
Abbreviations: CIS = carcinoma in situ; pCR = pathologic complete response; TURBT = transurethral resection of bladder tumor.
Binary and Multinomial Logistic Regression Analyses
Complete data were available for age, TURBT CIS status, variant histologic status, race, gender, pre-NAC clinical stage, and cisplatin NAC status. On binary logistic regression analysis of these variables, age ≤ 62 years (OR, 2.76; 95% CI, 1.06–7.10; P = .035) and nonwhite race (OR, 6.37; 95% CI, 1.58–25.71; P = .009) were statistically associated with increased pCR rates. The absence of CIS on TURBT approached a statistical association with increased pCR rates (OR, 2.86; 95% CI, 0.92–8.91; P = .069). On multinomial logistic regression analysis, the absence of CIS on TURBT (OR, 4.08; 95% CI, 1.19–13.98; P = .025) and nonwhite race (OR, 6.05; 95% CI, 1.17–31.14; P = .031) were significantly associated with increased pCR rates. Both age ≤ 62 years and pre-NAC clinical stage T2N0 showed a trend toward statistical significance. The full results are summarized in Table 3.
Table 3.
Logistic Regression Analysis Results of pCR Rate Stratified by Precystectomy Clinical Variables
| Variable | Patients With pCR/Total | Binary LR Analysis | Multinomial LR Analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Age group | .035 | .059 | |||
| >62 y | 7/64 (10.9) | Ref | Ref | ||
| ≤62 y | 19/73 (26.0) | 2.76 (1.06–7.10) | 2.72 (0.96–7.66) | ||
| CIS found on TURBT | .069 | .025 | |||
| Yes | 4/42 (9.5) | Ref | Ref | ||
| No | 22/95 (23.2) | 2.86 (0.92–8.91) | 4.08 (1.19–13.98) | ||
| Variant histologic features | .559 | .710 | |||
| Yes | 10/46 (21.7) | Ref | Ref | ||
| No | 16/91 (17.6) | 0.77 (0.32–1.86) | 0.83 (0.30–2.25) | ||
| Race | .009 | .031 | |||
| White | 21/128 (16.4) | Ref | Ref | ||
| Nonwhite | 5/9 (55.6) | 6.37 (1.58–25.71) | 6.05 (1.17–31.14) | ||
| Gender | .707 | .724 | |||
| Male | 19/104 (18.3) | Ref | Ref | ||
| Female | 7/33 (21.2) | 1.20 (0.46–3.18) | 0.81 (0.26–2.54) | ||
| Clinical stage | .164 | .058 | |||
| T3-T4N+ found on TURBT | 7/54 (13.0) | Ref | Ref | ||
| T2N0 found on TURBT | 19/83 (22.9) | 1.91 (0.77–4.76) | 2.78 (0.97–7.97) | ||
| Cisplatin-containing regimen | .337 | .229 | |||
| Yes | 24/113 (21.2) | Ref | Ref | ||
| No | 2/24 (8.3) | 0.34 (0.07–1.54) | 0.38 (0.08–1.84) | ||
Data presented as n (%).
Abbreviations: CI = confidence interval; CIS = carcinoma in situ; LR = logistic regression; OR = odds ratio; pCR = pathologic complete response; Ref = reference group; TURBT = transurethral resection of bladder tumor.
Survival Analyses
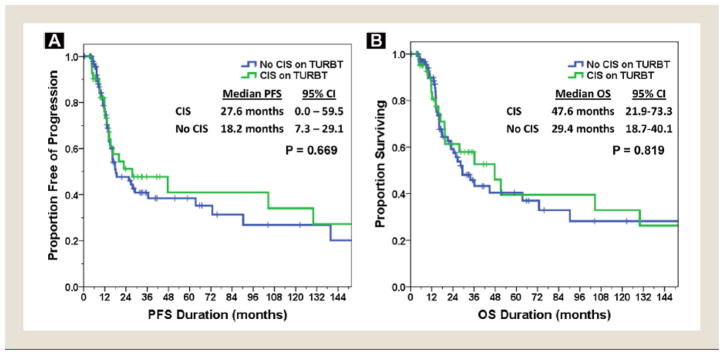
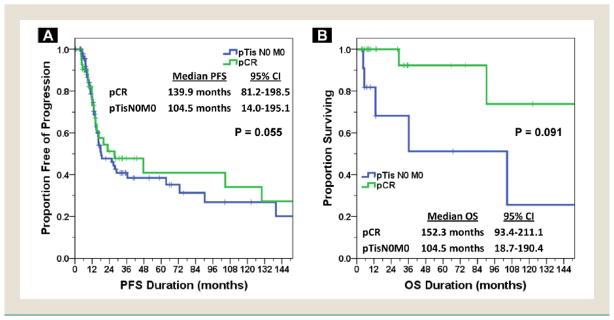
The mean follow-up duration for subjects with and without CIS found on TURBT was 31.0 months (range, 0–204 months) and 22.4 months (range, 0–264 months). The median PFS for the entire cohort was 20.0 months (95% CI, 10.1–29.8 months), with a median OS of 33.8 months (95% CI, 17.3–50.4 months). The median PFS (27.6 vs. 18.2 months) and OS (47.6 vs. 29.4 months) were both increased for patients with CIS present on TURBT; however, neither result was statistically significant on log-rank testing (P = .67 for PFS and P = .82 for OS). Both PFS and OS stratified by TURBT CIS status are depicted in Figure 3. Given the high percentage of patients with pTisN0M0 disease at cystectomy in patients with CIS found on pre-NAC TURBT, an exploratory analysis of PFS and OS between the pCR and pTisN0M0 patients was performed. As depicted in Figure 4, the median PFS was 139.9 and 104.5 months (P = .055) and the median OS was 152.3 and 104.5 months (P = .091) for pCR and pTisN0M0 patients, respectively.
Figure 3. Survival According to Transurethral Resection of Bladder Tumor (TURBT) Carcinoma In Situ (CIS) Status. (A) Progression-free Survival (PFS). (B) Overall Survival (OS).
Abbreviation: CI = confidence interval.
Figure 4. Survival According to Pathologic Complete Response (pCR) Versus pTisN0M0 Status. (A) Progression-free Survival (PFS). (B) Overall Survival (OS).
Abbreviation: CI = confidence interval.
Discussion
MIUC remains a frequent clinical dilemma with significant treatment-associated morbidity, in addition to high relapse rates. The ability to identify patients with MIUC with a greater risk of treatment failure, relapse, and decreased survival could have numerous potential benefits. These theoretically could include designing future studies of more aggressive subsets of patients who might benefit from alternative treatment regimens, evaluating high-risk tumor profiles for a better understanding of the underlying biology of the disease, and detecting potential novel targets for therapy in high-risk patients. Higher primary tumor stage and nodal metastases are known risk factors in MIUC that affect survival and are reflected in the current staging system; however, these can be difficult to accurately assess before definitive therapy, somewhat limiting their usefulness in preoperative treatment decisions.3 Additional factors affecting the risk of relapse in patients with MIUC that are not considered in the current staging system include primary tumor location at the bladder neck or ureterovesical junction (especially with associated hydroureteronephrosis), sarcomatoid, small cell, or micropapillary variant histologic features, and an abnormal immunophenotype for p53, Rb, or vascular endothelial growth factor.18 The presence of CIS on pre-NAC TURBT could be another potential risk-modifying feature in MIUC, because it has been shown to have negative prognostic implications in studies of UC, as discussed previously. Additionally, if found to be a risk-modifying feature of MIUC, pre-NAC TURBT CIS could have clinical utility, because it is an objective finding identifiable by pretreatment pathologic examination. Thus, it could potentially guide further treatment decisions for these patients. Despite this, the presence of pre-NAC TURBT CIS has received limited evaluation in MIUC.
The results of our study revealed significantly decreased pCR rates at cystectomy among patients with pre-NAC CIS in their TURBT samples. This finding is noteworthy, because pCR is a frequently used early endpoint in clinical trial designs in the NAC setting for MIUC. Our results are similar to the results previously reported by Parker et al.21
Interestingly, the survival analysis showed that patients with TURBT CIS had numerically greater median PFS and OS outcomes; however, the differences were not statistically significant. This was unexpected, because we hypothesized that TURBT CIS would not only result in decreased pCR rates but would also result in decreased PFS and OS. A potential explanation for the longer than expected PFS and OS for these patients could be the uncontrolled differences in clinical stage between our cohorts, because more patients in the TURBT CIS cohort had lower stage (T2) disease and more patients in the non–CIS cohort had higher stage (lymph node-positive) disease. In addition, more patients in the TURBT CIS cohort received cisplatin-based treatment, which is the only chemotherapy regimen shown in prospective studies to improve survival. Finally, as hypothesized, differences in survival with residual disease might exist between patients with and without CIS.
In addition to differing pCR rates, subjects with TURBT CIS demonstrated a sixfold increase in the rate of pTisN0M0 at cystectomy. In an exploratory post hoc analysis of PFS and OS among pCR versus pTisN0M0 patients, those achieving a pCR had numerically superior outcomes, although the difference was not statistically significant and PFS and OS were prolonged in both subsets. The number of patients analyzed (pCR, n = 26; pTisN0M0, n = 11) was extremely small. In their previous analysis, Parker et al21 suggested that pTisN0M0 might behave more similarly to a pCR as a potential reason for the lack of a significant survival difference seen in their study. To the best of our knowledge, large previous efforts have not been performed to assess whether a true clinically significant difference exists in the outcomes between patients with pCR versus pTisN0M0 after NAC for MIUC. Given the prolonged median PFS and OS in both groups in our study and the lack of significant survival outcome differences in the study by Parker et al,21 further prospective evaluations of survival outcomes of patients with pCR versus pTisN0M0 are warranted. It is difficult to make a definitive conclusions, given the retrospective nature of our study, small sample size, and post hoc analysis. However, if pTisN0M0 truly results in prolonged survival, this is a critical finding, given that the current standard endpoint in NAC clinical trials is the pCR.
Our analysis demonstrated a markedly increased pCR rate in nonwhite patients with MIUC treated with NAC. It is plausible that different races might have different tumor biology as an explanatory factor. However, caution is warranted in making any definitive conclusions from this finding owing to the extremely small number of nonwhite patients treated in our cohort.
The strengths of our study included the number of NAC MIUC patients evaluated, the use of an objective preoperative variable (ie, the presence vs. absence of TURBT CIS), an objective postoperative endpoint (ie, pCR), cystectomy pathology reports assessed by similarly trained genitourinary pathologists at a single institution, the high overall rate of cisplatin-based regimens received, cystectomies performed by similarly trained urologists at a single institution, and the inclusion only of patients who had received NAC. Despite these strengths, a number of limitations must be acknowledged. Our study was limited to a single institution; thus, the patient outcomes could have been subject to institutional practice patterns, which might vary from the patterns of care in other geographic regions of the United States. Furthermore, the study was a retrospective evaluation spanning > 2 decades. Significant changes in care and management of UC have occurred (eg, an increased use of bacillus Calmette-Guérin in NMIUC, the documented benefit of NAC in MIUC, an increased use of extended lymph node dissection), which might have introduced unaccounted-for confounding variables affecting the clinical outcomes. Although unexpected, the fairly large discrepancy among the clinical stages among our cohorts represents another limitation. However, we attempted to control for this on multivariate analysis. A potential reason for the presence of more clinically limited stage tumors in the TURBT CIS cohort might be related to the knowledge of CIS as a high-risk feature, combined with closer surveillance for NMIUC patients, leading to early recognition of MIUC in these patients. Another limitation was the inherent sampling error and tumor heterogeneity, which can make the identification of TURBT CIS difficult, in addition to the less judicious reporting of CIS in the past. Thus, 3 patients were found to have pTisN0M0 at cystectomy in the non-CIS cohort, likely representing TURBT CIS that was not sampled on the initial biopsy or pathologic assessment. If the finding of CIS is truly prognostic or predictive, a more thorough and standardized evaluation of TURBT specimens and the use of improved techniques of identifying CIS would be advisable. Two such techniques include blue light and narrow band cystoscopy, both of which have been shown to improve the detection of CIS in NMIUC compared with traditional white light cystoscopy.22–25 Finally, our survival analyses were limited by the variable follow-up durations and considerable number of patients censored with an unknown event status.
Conclusion
Our study identified a significant association between the presence of CIS within the pretreatment TURBT sample of MIUC patients treated with NAC and decreased pCR rates at cystectomy. An analysis of survival outcomes, albeit limited by the small retrospective sample sizes, did not show an associated decrease in survival outcomes in association with TURBT CIS histologic features. Furthermore, the finding of pTisN0M0 at cystectomy is common in patients with CIS found on pre-NAC TURBT samples. The clinical consequence of this finding remains undefined, although our data suggest the survival outcomes for these patients might be prolonged. Further delineation of any significant difference between pTisN0M0 and pCR outcomes will likely require prospective validation through large international data registries. Further study of patients with CIS could offer opportunities for improved translational study, which might have important clinical ramifications for both NMIUC and MIUC patients.
Clinical Practice Points.
CIS in isolation or when identified with invasive urothelial cancer has been shown in multiple studies to have poor prognostic implications.
CIS identified in pretreatment biopsy samples of patients treated with NAC results in decreased pCR rates at cystectomy; however, no significant differences in median PFS or OS were found.
Among patients with CIS found on pretreatment biopsies, significant proportions of cystectomy samples revealed CIS only after NAC.
Residual CIS at cystectomy resulted in prolonged median PFS and OS that were not significantly different from the median PFS and OS found with a pCR.
Judicious evaluation and reporting of associated CIS should be performed to further understand the prevalence and prognostic significance of this finding.
Further studies with the aim of understanding the underlying biology of CIS, given its differing clinical behavior, are warranted.
Footnotes
Disclosure
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Sauter G. Bladder cancer. In: Stewart B, Wild C, editors. World Cancer Report 2014. Lyon, France: WHO Press; 2014. [Google Scholar]
- 3.Stenzl A, Cowan NC, De Santis M, et al. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2009;55:815–25. doi: 10.1016/j.eururo.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Lamm D, Herr H, Jakse G, et al. Updated concepts and treatment of carcinoma in situ. Urol Oncol. 1998;4:130–8. doi: 10.1016/s1078-1439(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 5.Skinner DG, Richie JP, Cooper PH, Waisman J, Kaufman JJ. The clinical significance of carcinoma in situ of the bladder and its association with overt carcinoma. J Urol. 1974;112:68–71. doi: 10.1016/s0022-5347(17)59645-7. [DOI] [PubMed] [Google Scholar]
- 6.Chade DC, Shariat SF, Godoy G, et al. Clinical outcomes of primary bladder carcinoma in situ in a contemporary series. J Urol. 2010;184:74–80. doi: 10.1016/j.juro.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solsona E, Iborra I, Ricos JV, Monros JL, Dumont R, Almenar S. Extravesical involvement in patients with bladder carcinoma in situ: biological and therapy implications. J Urol. 1996;155:895–900. doi: 10.1016/s0022-5347(01)66338-9. [DOI] [PubMed] [Google Scholar]
- 8.Babjuk M, Burger M, Zigeuner R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–53. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guérin immunotherapy for recurrent Ta, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group study. J Urol. 2000;163:1124–9. [PubMed] [Google Scholar]
- 10.Sylvester RJ, van der Meijden AP, Witjes JA, Kurth K. Bacillus Calmette-Guérin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol. 2005;174:86–92. doi: 10.1097/01.ju.0000162059.64886.1c. [DOI] [PubMed] [Google Scholar]
- 11.Shariat SF, Palapattu GS, Karakiewicz PI, et al. Concomitant carcinoma in situ is a feature of aggressive disease in patients with organ-confined TCC at radical cystectomy. Eur Urol. 2007;51:152–60. doi: 10.1016/j.eururo.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A, Lotan Y, Bastian PJ, et al. Outcomes of patients with clinical T1 grade 3 urothelial cell bladder carcinoma treated with radical cystectomy. Urology. 2008;71:302–7. doi: 10.1016/j.urology.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 13.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–77. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Yafi FA, Aprikian AG, Chin JL, et al. Impact of concomitant carcinoma in situ on upstaging and outcome following radical cystectomy for bladder cancer. World J Urol. 2014;32:1295–301. doi: 10.1007/s00345-013-1207-z. [DOI] [PubMed] [Google Scholar]
- 15.Nuhn P, Bastian PJ, Novara G, et al. Concomitant carcinoma in situ in cystectomy specimens is not associated with clinical outcomes after surgery. Urol Int. 2011;87:42–8. doi: 10.1159/000325463. [DOI] [PubMed] [Google Scholar]
- 16.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–6. doi: 10.1016/j.eururo.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 18.Millikan R, Siefker-Radtke A, Grossman HB. Neoadjuvant chemotherapy for bladder cancer. Urol Oncol. 2003;21:464–7. doi: 10.1016/s1078-1439(03)00148-0. [DOI] [PubMed] [Google Scholar]
- 19.Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32:1895–901. doi: 10.1200/JCO.2013.53.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonpavde G, Goldman BH, Speights VO, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115:4104–9. doi: 10.1002/cncr.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker WP, Ho PL, Melquist JJ, et al. The effect of concomitant carcinoma in situ on neoadjuvant chemotherapy for urothelial cell carcinoma of the bladder: inferior pathological outcomes but no effect on survival. J Urol. 2015;193:1494–9. doi: 10.1016/j.juro.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Colombo R, Naspro R, Bellinzoni P, et al. Photodynamic diagnosis for follow-up of carcinoma in situ of the bladder. Ther Clin Risk Manag. 2007;3:1003–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Geavlete B, Jecu M, Multescu R, Geavlete P. Narrow-band imaging cystoscopy in non-muscle-invasive bladder cancer: a prospective comparison to the standard approach. Ther Adv Urol. 2012;4:211–7. doi: 10.1177/1756287212454181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidbauer J, Witjes F, Schmeller N, Donat R, Susani M, Marberger M. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol. 2004;171:135–8. doi: 10.1097/01.ju.0000100480.70769.0e. [DOI] [PubMed] [Google Scholar]
- 25.Ye Z, Hu J, Song X, et al. A comparison of NBI and WLI cystoscopy in detecting non-muscle-invasive bladder cancer: a prospective, randomized and multi-center study. Sci Rep. 2015;5:10905. doi: 10.1038/srep10905. [DOI] [PMC free article] [PubMed] [Google Scholar]