Abstract
Purpose
Testicular adrenal rest tumors (TART) are a well-known complication for males with congenital adrenal hyperplasia (CAH), with potential infertility in adulthood. We assessed the prevalence of TART in infant to young adult males presenting to a CAH Comprehensive Care Center.
Materials and Methods
35 males with CAH due to 21-hydroxylase deficiency underwent scrotal ultrasonography (7 males <5 yr, 9 males 5–12 yr, and 19 males >12 yr). There were 32 classical and 3 non-classical CAH. Bone age x-ray (BA) or advanced BA history, glucocorticoid (GC) dose, fludrocortisone (FC) dose, and serum 17-hydroxyprogesterone (17OHP), testosterone, and androstenedione levels within 3 months of the ultrasound were also recorded.
Results
TART were detected (TART+) in 5/35 (14%) patients overall: 1/9 (11%) 5–12 yr and 4/19 (21%) >12 yr. TART were not detected in any patients <5 yr, including one infant in poor hormonal control (youngest TART+ was 6.6 yr). All TART+ had bilateral disease and only one had suspicious physical findings. GC dose and 17OHP did not differ between TART+ and TART-. TART+ were more likely to have advanced BA (100% vs. 42%; p= 0.04) and higher FC dose (p< 0.01). All non-classical males were TART-.
Conclusions
TART were present in young males with classical CAH, but not in infants or toddlers. TART were associated with higher FC requirements and history of advanced BA; however, not all poorly controlled males developed TART. Longitudinal studies are needed to understand individual predisposition to TART, and the age at which to begin screening in CAH.
Keywords: Congenital adrenal hyperplasia, 21-hydroxylase deficiency, testicular adrenal rest tumor
Introduction
Congenital adrenal hyperplasia (CAH) is a potentially life-threatening form of primary adrenal insufficiency characterized by cortisol, aldosterone, and epinephrine deficiencies, as well as androgen excess 1. It is most commonly caused by a mutation in the CYP21A2 gene which encodes the enzyme 21- hydroxylase, an absence or deficiency of which leads to reduced synthesis of cortisol and aldosterone, elevated adrenocorticotropic hormone (ACTH), hyperplasia of the adrenal glands, accumulation of steroid precursors, and excessive production of androgens 2 (Figure 1).
Figure 1.
Steroidogenic pathway in the adrenal gland. A deficiency in the enzyme 21-hydroxylase (shaded) is the most common cause of CAH, accounting for 95% of cases and leading to deficiencies in aldosterone and cortisol (dotted pathways). This results in an accumulation of steroid precursors and excess androgen production (bold).
CAH is typically categorized as classical (severe) or non-classical (mild, late-onset). The classical salt-wasting form (67% of classical individuals) is characterized by a severe reduction in 21-hydroxylase activity, leading to near-complete deficiencies of both cortisol and aldosterone, presentation at or soon after birth (with ambiguous genitalia in females), and risk of early adrenal crisis if untreated 3. The classical simple-virilizing form (33% of classical individuals) is associated with 1–2% of normal 21- hydroxylase activity, and still with risk for adrenal crisis, but to a lesser degree than seen in the salt- wasting form4. Non-classical CAH is characterized by 20–50% of normal 21-hydroxylase activity resulting in a milder phenotype5. Typical presentation occurs later in childhood or adolescence with findings secondary to androgen excess including premature pubarche, growth acceleration, advanced bone age in growing children or hirsutism, acne, delayed menarche/menstrual irregularities and infertility among older patients 6.
Testicular adrenal rest tumors (TART) are a well-known complication in adult males with classical CAH due to 21-hydroxylase deficiency, and can lead to gonadal dysfunction and infertility in adulthood 7. The adrenal rest tissue in the testicle expresses ACTH-specific receptors and is ACTH- responsive, producing steroid hormones, and can itself become hyperplastic 8.TART occur when there is growth of the adrenal rests within the testicular parenchyma. While the resulting tumors are benign, the location of most TART in the rete testis may lead to compressive effects with tubular obstruction and oligo/azospermia 7. In some cases, large TART can compress enough normal tissue to affect both spermatogenesis and testosterone production.
Standard-of-care guidelines suggest that periodic screening should begin in adolescence 9; however, specific recommendations vary and include screening younger children 10,11, with the suggestion that early detection is optimal 7. While TART have been reported in CAH children with a prevalence of 24–33% 10,11, little is known regarding TART in infants and toddlers with CAH. A study of neonatal autopsies of boys with CAH noted the presence of ectopic adrenal tissue in three of seven patients who were < 8 weeks old and in 14 children who were > 14 months 12, but we are not aware of any ultrasonographic studies of testes in infants with CAH. Therefore, we studied the prevalence of TART in boys over a wide age spectrum, from infancy to young adulthood, and the association of TART with specific measures of hormonal control. We hypothesized that TART would be found at a lower prevalence in younger patients and that poor hormonal control would be associated with a greater prevalence of TART.
Materials and Methods
Study Center
We performed a cross-sectional study of males presenting to a CARES Foundation-designated CAH Comprehensive Care Center, which includes a multidisciplinary care team focused on collaborative surgical and medical management of CAH. Inclusion criteria were as follows: males with classical or non-classical CAH due to 21-hydroxylase deficiency confirmed by biochemical and/or genetic testing. Those with CAH due to less common enzyme deficiencies, such as 3β-hydroxysteroid dehydrogenase deficiency or 11β-hydroxylase deficiency, were excluded.
Outcome Measures
Yearly screening ultrasounds are performed as part of clinical care for male patients at our center. Scrotal studies were conducted with a real-time scanner (Toshiba Aplio 500, Toshiba America Medical Systems, Inc., Tustin, CA), using a 14-MHz linear array transducer (Figure 2). Both power and spectral Doppler examinations of the testes were performed. Additional follow-up ultrasounds were performed at 6-month intervals on individuals once TART abnormalities were noted. Patients were recruited from 2013 through 2015. The presence or absence of TART was recorded and diameter measurements (mm) were made. For TART+ patients, previous ultrasounds were reviewed to pinpoint when TART were first detected, in order to identify the index ultrasound for examination of risk factors. Measures of hormonal control were recorded for TART+ patients in relation to the index ultrasound in which TART were first detected.
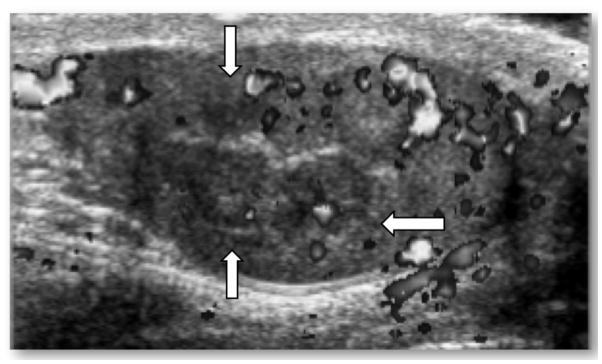
Figure 2.
TART+ doppler ultrasound image of the right testis in a 12 year old male with salt-wasting form of classical CAH, advanced bone age, and very elevated 17-hydroxyprogesterone levels. TART measured 12 mm x 12 mm in diameter (white arrows) and was localized to the testicular hilum (hyperechoic midline strip).
Hormonal control at the time of the ultrasound examination was assessed by serum 17- hydroxyprogesterone (17OHP), androstenedione, and testosterone (all measured by liquid chromatography and tandem mass spectrometry, at Quest Diagnostics Nichols Institute, San Juan Capistrano, CA). Hormone levels were generally measured prior to routine morning CAH medications. 17OHP was categorized as: < 100 (suppressed), 100–1200 (normal), > 1200–5000 (elevated), and > 5000 ng/dL (very elevated). The glucocorticoid (GC) dose was recorded for each patient at the time of the first diagnostic ultrasound. GC dose was expressed as either hydrocortisone (HC) dose (mg/m2/day) or as calculated HC dose equivalents (dexamethasone, 1:80 and prednisone, 1:5). Androstenedione and testosterone were both reported in ng/dL and were further categorized as elevated and not elevated based on age-specific norms.
Longer-term hormonal control was assessed using a history of bone-age advancement. A bone age x-ray of the left hand was taken within 6 months of the time of ultrasound. In cases where the growth plates were already fused, then only a history of advanced bone age at any time was recorded. A bone age ≥2 standard deviation scores above the chronological age was considered to be significantly advanced, likely representing substantial prior exposure to postnatal androgens.
Statistical Analysis
Summary statistics are presented as the proportion or percentage, or mean with standard deviation. Student’s t-test was used for between-group comparisons of continuous data, and Fischer’s exact and Chi-square trend tests were used for between-group comparisons with categorical data and trends between groups with categorical data points. The study was reviewed and approved by the Children’s Hospital Los Angeles institutional review board.
Results
Study Population
We identified 35 male patients with CAH due to 21-hydroxylase deficiency: 27/35 (77%) had the salt-wasting form, 5/35 (14%) had the simple-virilizing form, and 3/35 (9%) had the non-classical form.
Presence of TART in School-Aged and Older Males
TART were found in 5/35 (14%) of patients overall. Seven patients were < 5 years old and none had TART. TART were found in 1/9 (11%) individuals age 5–12 years old and in 4/19 (21%) of patients > 12 years old (Table 1). No TART were detected in males with non-classical CAH.
Table 1.
Testicular adrenal rest tumor prevalence in males with congenital adrenal hyperplasia due to 21-hydroxylase deficiency, and phenotypic subclassification and medication dose in those with and without tumor
| Age (yrs) | ||||
|---|---|---|---|---|
| 0–5 | 5–12 | Greater than 12 | Overall | |
| No. pts | 7 | 9 | 19 | 35 |
| No. TART (%)* | 0 | 1 (11) | 4 (21) | 5 (14) |
| No. CAH type: | ||||
| Salt-wasting | 6 | 17 | 14 | 27 |
| Simple virilizing | 1 | 1 | 3 | 5 |
| Nonclassic | 0 | 1 | 2 | 3 |
| Mean ± SD hydrocortisone dose (mg/m2/day): | ||||
| TART neg | 16.2 ± 4.7 | 15.7 ± 3.1 | 15.4 ± 6.3 | 15.5 ± 5.2 |
| TART pos | – | 19.7 | 16.6 ± 2.2 | 17.2 ± 2.4 |
| Mean ± SD fludrocortisone dose (mg/day): | ||||
| TART neg | 0.08 ± 0.04 | 0.08 ± 0.04 | 0.06 ± 0.05 | 0.07 ± 0.05 |
| TART pos | – | 0.2 | 0.15 ± 0.06 | 0.16 ± 0.05† |
Age trend did not reach statistical significance (p = 0.16).
Significantly higher vs patients without testicular adrenal rest tumor (p <0.01).
Mean TART diameter at initial detection was 6.79 ± 6.14 mm (range 3–20 mm). All TART+ patients had bilateral lesions and none had a palpable lesion. One patient had bilaterally firm testes at the time of diagnosis.
Medication Doses: Glucocorticoid and Fludrocortisone
The GC dose most proximate to the index ultrasound was not statistically different between TART+ (17.2 ± 2.4 mg/m2/day) and TART- patients (15.5 ± 5.2 mg/m2/day, p = 0.49, Table 2). The FC dose was higher in TART+ (0.16 ± 0.05 mg/day) versus TART- patients (0.07 ± 0.05 mg/day, p < 0.01).
Table 2.
Testicular adrenal rest tumors and hormonal control in males with classic congenital adrenal hyperplasia
| Age (yrs) | ||||
|---|---|---|---|---|
| 0–5 | 5–12 | Greater than 12 | Overall | |
| No. pts | 7 | 9 | 19 | 35 |
| Mean ± SD testosterone (ng/dl): | ||||
| TART neg | 17.6 ± 26 | 80.7 ± 205 | 384.6 ± 267 | 232.8 ± 276 |
| TART pos | – | 54 | 326.3 ± 376 | 258.2 ± 336 |
| Mean ± SD androstenedione (ng/dl): | ||||
| TART neg | 33.3 ± 51 | 36.4 ± 32 | 211.3 ± 274 | 124.3 ± 216 |
| TART pos | – | 542 | 142.6 ± 214 | 242.5 ± 265 |
| Mean ± SD 17OHP (ng/dl): | ||||
| TART neg | 5,782 ± 12,410 | 905 ± 1,123 | 4,049 ± 7,425 | 3,778 ± 7,958 |
| TART pos | – | 9,944 | 2,989 ± 3,146 | 4,380 ± 4,135 |
| No. suppressed 17OHP (less than 100 ng/dl): | ||||
| TART neg | 2 | 1 | 2 | 5 |
| TART pos | – | 0 | 1 | 1 |
| No. normal 17OHP (100–1,200 ng/dl): | ||||
| TART neg | 2 | 4 | 6 | 12 |
| TART pos | – | 0 | 1 | 1 |
| No. elevated 17OHP (1,200–5,000 ng/dl): | ||||
| TART neg | 2 | 3 | 4 | 9 |
| TART pos | – | 0 | 0 | 0 |
| No. very elevated 17OHP (greater than 5,000 ng/dl): | ||||
| TART neg | 1 | 0 | 3 | 4 |
| TART pos | – | 1 | 2 | 3 |
| No. advanced bone age (2 SD or greater)/total No.: | ||||
| TART neg | 1/3 | 4/8 | 7/13 | 10/24* |
| TART pos | – | 1/1 | 4/4 | 5/5 |
Other hormonal control measures were statistically similar between groups.
Significantly associated with presence of TART (p = 0.04).
Hormonal Control: CAH Hormone Levels
17OHP levels were elevated in 3/5 (60%) TART+ patients at the time of ultrasound and in 12/30 (40%) TART- patients (Table 2). Overall, TART+ males were not more likely than TART- males to have an elevated 17OHP (p = 0.63). Mean 17OHP levels were also similar between TART+ and TART- males (p = 0.85).
Androstenedione was similar between TART+ and TART- (242 ± 265 vs. 124 ± 216 ng/dL respectively; p = 0.32), as was testosterone (TART+ 258 ± 336 vs. TART- 233 ± 276 ng/dL; p = 0.87). TART+ males were no more likely to have elevated levels of either androstenedione (TART+ 50% vs. TART- 24%; p = 0.30) or testosterone (TART+ 25% vs. TART- 20%; p = 0.99).
Longer-Term Hormonal Control: Advanced Bone Age
All patients with TART had an advanced bone age (≥2 SD of normal), while only 10/24 (42%) TART- patients had an advanced bone age (p = 0.04) (Table 2).
Discussion
The main finding of our study is that males with classical CAH due to 21-hydroxylase deficiency as young as school-age may develop TART; however, these lesions were not detected in infants and toddlers with CAH, by sonographic techniques. Our data support the finding of others that TART prevalence seems to increase with age 10,13, with an overall prevalence of 14% in our cohort. Although some reports of TART and oligospermia in men with non-classical CAH exist 14, they are more commonly associated with classical CAH, and we did not detect TART in any non-classical CAH patients. The presence of TART carries significance for gonadal function and fertility in adulthood 7, with TART potentially leading to infertility by obstruction of the seminiferous tubules and destruction of adjacent testicular tissue. Thus, it is concerning that we and others are finding TART in young children 10,11,13. Although three infants with CAH have previously been reported to be TART+ on autopsy 12, to our knowledge, there are no published reports in which TART have been found by ultrasonography in infants with CAH.
In contrast, the incidence of testicular adrenal rests in newborns without CAH appears low (3.5%) based on examination of autopsy material 15, with a similar incidence to that found in children without CAH who are < 16 years old 16. The predisposition to development of TART could vary among males, due to differing levels of ACTH exposure in utero, for example 15, with TART observed more frequently in male patients with poorly-controlled CAH 7. However, our study demonstrates that the risk factors for development of TART in males with CAH are not yet fully understood. While all males with TART exhibited a significantly advanced bone age, not all had abnormal 17OHP levels around the time of the diagnostic testicular ultrasound. Notably, one infant did not exhibit sonographic evidence for TART on three consecutive scrotal ultrasounds despite consistently poor hormonal control during infancy (17OHP 14,764–33,821 ng/dL). Conversely, there were TART- males who exhibited signs of poor hormonal control, including advanced BA and/or elevated 17OHP. Another study showed no correlation between adrenal hormones and TART overall, although boys with TART were more likely to have a higher androstenedione 11. More CAH patients need to be studied to better assess the relationship between hormonal control and the development of TART over time.
All TART in the current study were found in patients with the salt-wasting (5 of 27) form of classical CAH, and none were found in those with simple-virilizing (0 of 5) or non-classical (0 of 3) forms. In salt-wasting patients on inadequate cortisol replacement, relatively higher levels of ACTH stimulation over long periods of time could provide the necessary environment for the development of TART in susceptible individuals.
There are several limitations to our study. The cross-sectional study design cannot account for hormonal control between visits. It would be ideal to follow patients longitudinally from infancy to adulthood. Ultrasound was used to identify TART; however, histologic verification of these lesions could not be performed. We were also unable to measure Sertoli cell markers (e.g., inhibin B) and gonadotropins to assess gonadal dysfunction. These analytes could be important in any age male with CAH as decreases in functional testicular volume have been found to correspond to lower inhibin B and sperm concentration in adults, with sperm concentration further correlating with both inhibin B and higher FSH levels 17. As well, prepubertal males with CAH have exhibited lower levels of inhibin B and anti-Mullerian hormone correlating with decreased Leydig cell function, especially in those with TART 13.
There is growing evidence that scrotal ultrasounds starting at or near school-age in males with classical CAH due to 21-hydroxylase deficiency could be prudent in the clinical setting. We recommend beginning to screen males with classical CAH at 4–6 years old, the age that we and others have detected TART on scrotal ultrasound. The prevalence of TART increases with age, and thus, initiation of screening during adolescence is a minimum, with more frequent ultrasounds in TART+ males. Variability among individuals with regard to risk factors for developing TART and baseline presence of adrenal rest tissue merits further study in CAH patients.
Conclusions
We conclude that males with classical CAH due to 21-hydroxylase deficiency exhibit a risk for developing TART as young as 6 years old, although very young boys (infants and toddlers) do not appear to exhibit sonographic evidence for TART. Markers of hormonal control were often elevated in our TART+ patients, and advanced bone age was significantly more prevalent in TART+ males. Similarly, higher FC doses were associated with TART, suggesting that more severe salt-wasting occurred in patients with TART. Males with the mildest form of CAH (non-classical) did not exhibit evidence of TART. Since not all patients with markers of poor hormone control had evidence of TART, specific individual risk factors for TART need to be further elucidated.
Acknowledgments
We are very grateful to our patients and their families for their participation. This work was supported by CARES (Congenital Adrenal Hyperplasia Research, Education and Support) Foundation and the Abell Foundation. We thank Shruti Vora, MD, Norma Castaneda, Janet Guerrero, and the administrative assistants in the Children’s Hospital Los Angeles Department of Radiology for their assistance.
Abbreviations
- TART
Testicular Adrenal Rest Tissue
- CAH
Congenital Adrenal Hyperplasia
- 17OHP
17-hydroxyprogesterone
- GC
Glucocorticoid
- HC
Hydrocortisone
- FC
Fludrocortisone
- ACTH
Adrenocorticotropic hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
References
- 1.Kim MS, Ryabets-Lienhard A, Bali B, et al. Decreased adrenomedullary function in infants with classical congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2014;99:E1597–E1601. doi: 10.1210/jc.2014-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speiser PW. Prenatal treatment of congenital adrenal hyperplasia. J Urol. 1999;162:534–536. [PubMed] [Google Scholar]
- 3.Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–36. doi: 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- 4.New MI, Abraham M, Gonzalez B, et al. Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc Natl Acad Sci USA. 2013;110:2611–6. doi: 10.1073/pnas.1300057110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witchel SF, Azziz R. Nonclassic congenital adrenal hyperplasia. Int J Pediatr Endocrinol. 2010;2010:625105. doi: 10.1155/2010/625105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pall M, Azziz R, Beires J, et al. The phenotype of hirsute women: a comparison of polycystic ovary syndrome and 21-hydroxylase-deficient nonclassic adrenal hyperplasia. Fertil Steril. 2010;94:684–9. doi: 10.1016/j.fertnstert.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Claahsen-van der Grinten HL, Hermus AR, Otten BJ. Testicular adrenal rest tumors in congenital adrenal hyperplasia. Int J Ped Endocrinol. 2009;23:209–220. doi: 10.1016/j.beem.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Smeets EE, Span PN, van Herwaarden AE, et al. Molecular characterization of testicular adrenal rest tumors in congenital adrenal hyperplasia: lesions with both adrenocortical and Leydig cell features. J Clin Endocrinol Metab. 2015;3:E524–E530. doi: 10.1210/jc.2014-2036. [DOI] [PubMed] [Google Scholar]
- 9.Speiser PW, Azziz R, Baskin LS, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claahsen-van der Grinten HL, Sweep FC, Blickman JG, et al. Prevalence of testicular adrenal rest tumors in male children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2007;157:339–344. doi: 10.1530/EJE-07-0201. [DOI] [PubMed] [Google Scholar]
- 11.Finkielstain GP, Kim MS, Sinaii N, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97:4429–4438. doi: 10.1210/jc.2012-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanklin DR, Richardson AP, Jr, Rothstein G. Testicular hilar nodules in adrenogenital syndrome. The nature of the nodules Am J Dis Child. 1963;106:243–250. doi: 10.1001/archpedi.1963.02080050245001. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Aguayo A, Rocha A, Rojas N, et al. Testicular adrenal rest tumors and Leydig and Sertoli cell function in boys with classical congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2007;92:4583–4589. doi: 10.1210/jc.2007-0383. [DOI] [PubMed] [Google Scholar]
- 14.Witchel SF. Nonclassic congenital adrenal hyperplasia. Curr Opin Endocrinol Diabetes Obes. 2012;19:151–158. doi: 10.1097/MED.0b013e3283534db2. [DOI] [PubMed] [Google Scholar]
- 15.Bouman A, Hulsbergen-van de Kaa C, Claahsen-van der Grinten HL. Prevalence of testicular adrenal rest tissue in neonates. Horm Res Paediatr. 2011;75:90–93. doi: 10.1159/000316531. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan JG, Gohel M, Kinder RB. Ectopic adrenocortical tissue found at groin exploration in children: incidence in relation to diagnosis, age, and sex. BJU Int. 2005;95:407–410. doi: 10.1111/j.1464-410X.2005.05310.x. [DOI] [PubMed] [Google Scholar]
- 17.Falhammar H, Nystrom HF, Ekstrom U, et al. Fertility, sexuality and testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. 2012;166:441–449. doi: 10.1530/EJE-11-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]