Abstract
Purpose:
One-year monitoring of patients receiving intraperitoneal (IP) 212Pb-TCMC-trastuzumab to provide long-term safety and outcome data. A secondary objective was to study 7 tumor markers for correlation with outcome.
Methods:
Eighteen patients with relapsed intra-abdominal human epidermal growth factor receptor-2 expressing peritoneal metastases were treated with a single IP infusion of 212Pb-TCMC-trastuzumab, delivered <4 h after 4 mg/kg IV trastuzumab. Seven tumor markers were studied for correlation with outcome.
Results:
Six dose levels (7.4, 9.6, 12.6, 16.3, 21.1, 27.4 MBq/m2) were well tolerated with early possibly agent-related adverse events being mild, transient, and not dose dependent. These included asymptomatic, abnormal laboratory values. No late renal, liver, cardiac, or other toxicity was noted up to 1 year. There were no clinical signs or symptoms of an immune response to 212Pb-TCMC-trastuzumab, and assays to detect an immune response to this conjugate were negative for all tested. Tumor marker studies in ovarian cancer patients showed a trend of decreasing Cancer antigen 72-4 (CA 72-4) aka tumor-associated glycoprotein 72 (TAG-72) and tumor growth with increasing administered radioactivity. Other tumor markers, including carbohydrate antigen (CA125), human epididymis protein 4 (HE-4), serum amyloid A (SAA), mesothelin, interleukin-6 (IL-6), and carcinoembryonic antigen (CEA) did not correlate with imaging outcome.
Conclusions:
IP 212Pb-TCMC-trastuzumab up to 27 MBq/m2 seems safe for patients with peritoneal carcinomatosis who have failed standard therapies. Serum TAG-72 levels better correlated to imaging changes in ovarian cancer patients than the more common tumor marker, CA125.
Key Words: Pb-212-radioimmunotherapy, cancer, ovarian, tumor marker, intraperitoneal
Low toxicity intraperitoneal (IP) treatment continues to be an unmet need for disease that spreads through the cavity such as ovarian and pancreatic cancer. IP chemotherapy has improved survival of ovarian cancer patients but carries risk of life-threatening toxicity, and has not become the standard at most institutions.1 Radiopharmaceuticals have greater potential than external beam radiation due to dose-limiting tolerance of normal organs. β-emitting radiopharmaceuticals have shown modest impact but have also been used at dose-limiting toxicity levels.2–5 Targeted α-emitter radiopharmaceuticals, as implemented in this report, have the potential advantages of improved efficacy with less toxicity than β-emitters.
For targeted radionuclide therapy, the high ionization density of α-particles is attractive but their development/implementation has been challenging compared with the more widely available β-emitters.6,7 With the large helium particle emitted, α-decay results in significantly higher energy delivery (linear energy transfer) than β-decay, which results in higher cell-killing effectiveness. Human cell culture studies showed the relative biological effectiveness (RBE) greater for α-particles than that for β-radiation or kilovoltage photons8; this has been confirmed in other preclinical as well as early clinical trials but the RBE range has been variable from ∼1 to 20.9 The clinical experience where 213Bi-HuM195 and 90Y-HuM195 therapy could be directly compared in leukemic patients suggested that the RBE of α-emitter therapy will vary with cell type, geometry, and endpoints utilized.10 Another advantage of α-particles over β-radiation is the limited range of only a few cell diameters. This spares normal tissues but does limit optimal use to selected clinical applications. Appropriate clinical settings for use of high potency α-particles with short half-lives are those where the targeting is very specific and rapid or other conditions, such as into a resection cavity or tumor mass, where there is limited exposure to normal tissues. Because of many hurdles, implementation of systemic administration using antibody targeted α conjugates has been limited to a few studies, mainly in patients with leukemia, lymphoma, and metastatic melanoma.7,11–13 Limited experience with nonsystemic administration has included intralesional melanoma sites, intracavity or intralesional for brain tumors, and intraperitoneal infusion.14–18 Whereas reports of others show more extensive pharmacokinetics and dosimetry of another α-emitter conjugate administered to the peritoneal cavity (211At-Mx35 F(Ab’)2), our following report is the first therapeutic IP administration where safety was the primary objective posttherapy.16,18,19 Targeted α-conjugate therapy has thus far been well tolerated but initial dose levels have been modest to minimize risks to patients undergoing investigational treatment. This first-in-human clinical trial of IP 212Pb-TCMC2-(4-isothiocyanobenzyl)-1,4,7,10-teraaza-1,4,7,10-tetra-(2-carbamonyl methyl)-cyclododecane-trastuzumab was initiated after extensive murine and nonhuman primate investigations provided biodistribution, safety, and antitumor efficacy data.20–24 In this phase I study, a single IP infusion of 212Pb-TCMC-trastuzumab was escalated over 6 dose levels with toxicity monitoring to confirm the safety of this agent.
This trial, like many other investigations, studied serum tumor markers as indicators of therapeutic efficacy that could be easily and quickly monitored. This is particularly relevant given the limitations inherent in image-based quantification of peritoneal metastatic disease. Seven tumor markers were studied for their correlation to clinical outcome 6 weeks posttherapy. These included carcinoembryonic antigen (CEA), which is used for monitoring patients with gastrointestinal cancer and a minority of patients with other malignancies.25 Carbohydrate antigen (CA125) was monitored in the ovarian cancer patients as this has historically been the standard marker for monitoring of disease response to treatment.26–29 Human epididymis protein 4 (HE-4), serum amyloid A (SAA), mesothelin, interleukin-6 (IL-6), and tumor-associated glycoprotein (TAG-72), were also chosen for study based on prior reports of tumor association.30–36
METHODS
Details of the trial design and agent preparation have been previously reported.17 Briefly, this trial provided a single IP 212Pb-TCMC-trastuzumab infusion <4 h after 4 mg/kg IV trastuzumab in patients with human epidermal growth factor receptor-2 (HER-2) expressing malignancy that had failed standard therapies. Modifications were made after patient 10 to allow patients with HER-2 of 1+ in ≥10% of cells. Modification was also made to discontinue the saturated solution of potassium iodide as imaging showed no thyroid localization and no abnormal laboratory values had been observed in >1 year for the initial 3 patients. The diuretic regimen was also shortened as there was no evidence of renal localization or toxicity and patients had difficulty with side effects such that most were noncompliant with the entire prescribed regimen. Monitoring over the duration of 1 year included clinical findings, laboratory values, cardiac studies, immunologic assays, serum tumor marker levels, and computed tomographic scans. Patients had clinical and/or laboratory posttherapy evaluations 7 times in the first 6 weeks. If there was no toxicity, scheduled monitoring was extended to 6-, and then to 12-week intervals. Cardiac monitoring used electrocardiogram and echocardiograms. Enzyme linked immunosorbent assay testing of 6-week serum samples was performed to determine if there was any evidence of an immune response to TCMC-trastuzumab. A standard anti-drug antibody assay was developed. Briefly, the anti-Her-2 antibody was both the coat and a biotinylated primary detection antibody. Patient serum or polyclonal antibodies raised against TCMC-trastuzumab was the analyte. In the presence of bivalent anti TCMC-trastuzumab antibodies, the biotinylated trastuzumab antibody becomes linked to the trastuzumab coat. HRP-streptavidin is then added to develop a signal. Toxicity was defined using Common Terminology Criteria for Adverse Events (version 4.03 National Cancer Institute). Imaging interpretation and lesion measurements from baseline (<4 w pretreatment) and 6-week interval posttreatment CT scans were performed as an independent review by Imaging Endpoints (Scottsdale, AZ). Lesion measurements were compared by pretreatment and posttreatment volumes as a modification from RECIST criteria described in the original study design that uses tumor diameter products.37 Levels of standard tumor markers CA125 and CEA were obtained at institution laboratories; commercially available kits were used for other markers HE-4 (Fujirebio Diagnostics Inc., in vitro diagnostics), Cancer antigen (CA 72-4) (TAG-72) (DRG International, Research Use Only), SAA (Life Technologies, Ruo), Mesothelin (Aviscera Bioscience Inc., Ruo), IL-6 (Abcam, Ruo). Serum for tumor markers was obtained pretreatment, at 6 weeks, and at additional timepoints for CEA and CA125 in most patients. Statistical analysis used least squares linear regression to fit the data.
RESULTS
Toxicity
Eighteen patients (age 46 to 83) treated at 6 dose levels (7.4, 9.6, 12.6, 16.3, 21.1, 27.4 MBq/m2) were monitored for at least 1 year or until death. Seventeen patients were treated at the University of Alabama at Birmingham, and 1 colon cancer patient was treated at the University of California at San Diego. Sixteen patients were females with ovarian cancer and 2 males had colon cancer. Treatment was well tolerated. Mild acute adverse effects were associated with the investigational agent as previously presented for the initial 16 patients.18 Other than 2 patients who had transient abdominal pain associated with agent plus saline administration, possibly related adverse events were mainly grade 1, transient, asymptomatic laboratory abnormalities that were not dose related.
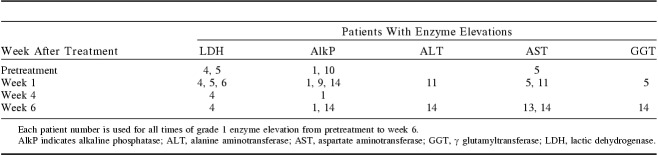
The patients are numbered 1 to 18 by the order of treatment. Monitoring of the enzymes lactic dehydrogenase (LDH), alkaline phosphatase (AlkP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ glutamyltransferase (GGT) showed no elevation among patients 2, 3, 7, 8, 12, 15, 16, 17, and 18. Table 1 shows the time and frequency of patients who developed grade 1 liver function test elevations. As shown in Table 1, 4 patients had elevations before treatment and 6 others experienced elevation to grade 1 level after treatment. These minor fluctuations did not appear dose related; all except patient 14 were below dose level 5 and none occurred at the highest dose level.
TABLE 1.
Summary of Enzyme Abnormalities by Date
Whereas hematologic toxicity has been dose-limiting in prior IP radionuclide conjugate studies, the mean platelet counts, total white blood cell counts, and neutrophil counts remained normal after a mean equivalent dose to marrow of 0.002 to 0.14 cGy/MBq. Only 2 patients had transient decreased counts to grade 1 (platelets 142,000/μL, WBC 3500/μL without neutropenia). Anemia was not tracked for toxicity as 6 patients were anemic pretreatment with hemoglobin of 9.8 to 11.2 g/dL. The percent change at 6 weeks for these patients was from a loss of 3.8% to a gain of 16.3%. Three of the 6 had improvement to normal hemoglobin levels during that interval.
Four patients experienced periods of grade 1 creatinine elevation (without proteinuria) associated with dehydration or urinary tract obstruction as reported.18 Reducing the diuretic regimen after patient 10 did not increase the serum levels of radioactivity or result in renal toxicity. No late renal, liver, hematologic (excluding anemia as noted above), cardiac, or other toxicity has been observed with monitoring >1 year despite additional therapy after the investigational agent. Although all patients had disease progression in <8 months and proceeded with additional treatment, none refused continued monitoring. There were no clinical signs or symptoms of an immune response, and assays to detect an immune response to 212Pb-TCMC-trastuzumab were negative for all 15 of 15 tested (3 patients had no sample).
Tumor Marker Changes Compared With Clinical Response
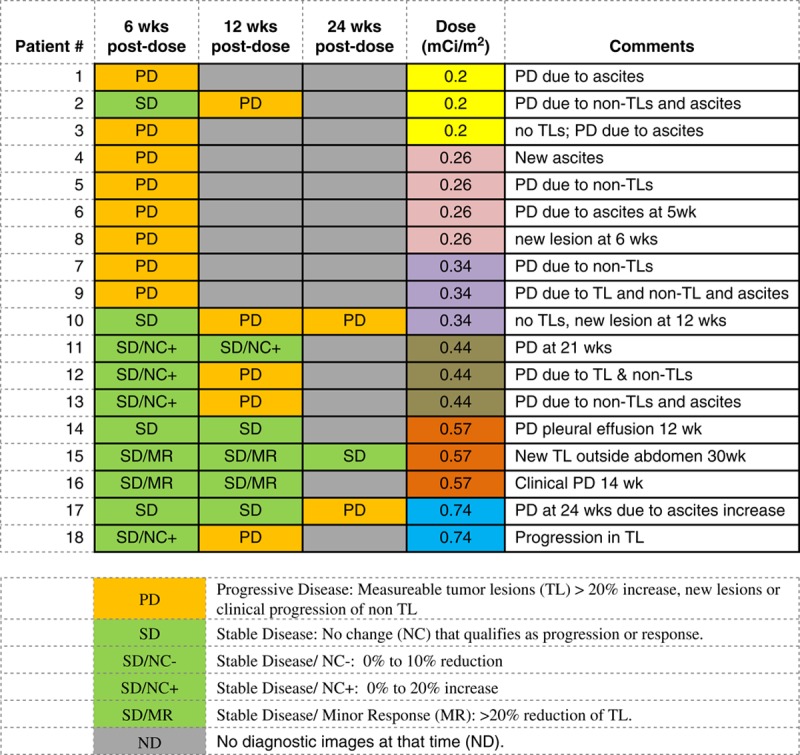
Blood levels of 7 tumor markers were studied and compared with clinical outcome at 6 weeks posttherapy. CEA and CA125 continued to be obtained until progression. CEA increased with disease progression in the 2 colon cancer patients but all the ovarian cancer patients had levels that were within the normal range both pretreatment and posttreatment. Other tumor markers which are often associated with ovarian cancer, including CA125, mesothelin, IL-6, SAA, and HE-4, did not correlate with imaging outcome (Fig. 1). Only a TAG-72 had a pattern similar to that of tumor growth changes. TAG-72 levels covered a wide range from nearly 200% increase to 66% decrease 6 weeks posttherapy. Compared with the imaging changes, the TAG-72 pattern had a steeper slope and higher correlation coefficient with administered level of radioactivity than did tumor growth (R2 for TAG-72 is 0.73 vs. 0.21 for CT) as shown in Figure 2. Five patients did not have measurable tumor lesions (TL) and are not included in Figure 2. Their clinical outcome is included in Table 2, which shows a trend of less tumor growth, including regression, with increasing administered radioactivity. All patients had progression of disease inside and/or outside of the peritoneal cavity before 8 months but the majority lived >1 year, allowing monitoring for late toxicity.
FIGURE 1.
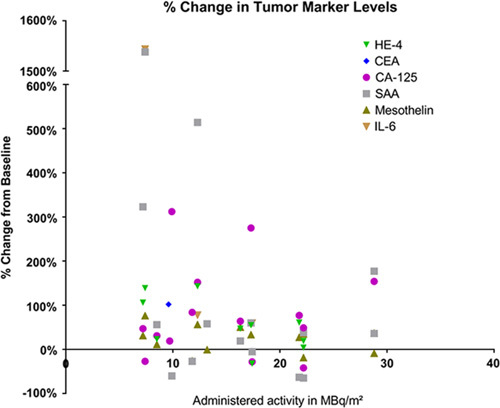
Each data point represents the increase or decrease in serum marker as percent change at 6 weeks compared with baseline for individual patients. The change in markers is compared with administered radioactivity in MBq/m2. Data points are not shown when values were within normal limits.
FIGURE 2.
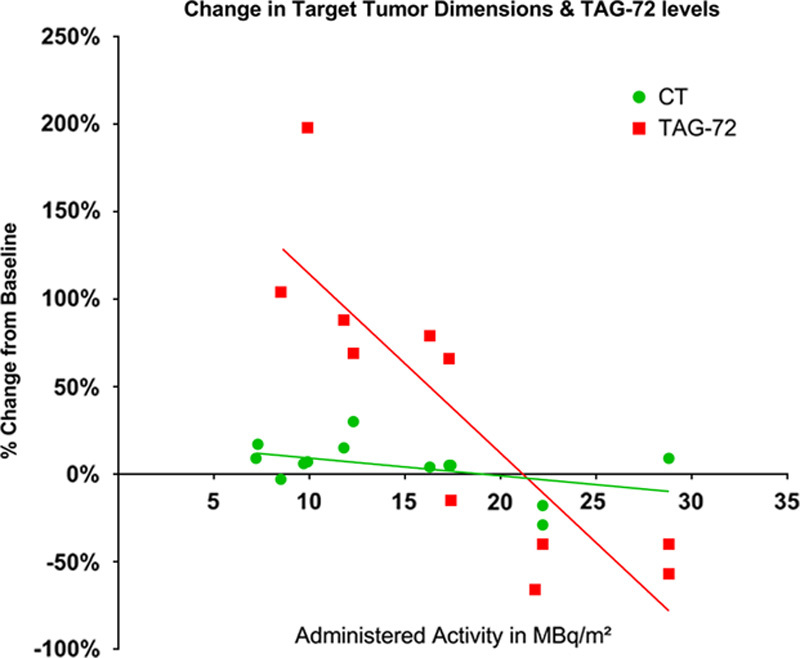
Each data point represents a single patient who had measureable lesions and TAG-72 >6 U/mL at baseline. The data points are expressed as percent change from baseline. The lines represent best fit from regression analysis. TAG-72 indicates tumor-associated glycoprotein 72.
TABLE 2.
Comparison of Posttreatment Disease Status With Administered 212Pb Dose Level
DISCUSSION
The single IP 212Pb-TCMC-trastuzumab infusion was well tolerated with agent-related toxicities limited to grade 1 and the majority of them were asymptomatic, transient laboratory abnormalities. Although there was no visualization of radioactivity outside of the peritoneal cavity, blood collection showed a rate of <1% to 22.9% transit in 24 hours.18 The imaging and blood data allowed dosimetry calculations. These found low radiation exposure to normal organs and a mean tumor milieu to marrow ratio of >1000, as most of the radioactivity decay took place in the peritoneal fluid.17
With >1 year of follow-up in the majority of patients receiving IP 212Pb-TCMC-trastuzumab, no late toxicity has been observed. The paucity of preclinical toxicity data allowed dose escalation for 6 levels, which is the maximum that was planned for this first-in-human study; further increase in dose levels would have soon exceeded preclinical data and required additional nonhuman primate studies.38 On the basis of this initial clinical experience, 212Pb-TCMC-trastuzumab appears safe for further study at the highest dose level tested or even additional dose escalation. As IP α-emitter therapy should be most effective for microscopic disease, the low toxicity should allow it to be further studied in combination with other agents or as an adjuvant after tumor reduction by standard therapies. The transient early toxicities did not appear to be dose related. The grade 1 leukopenia and thrombocytopenia occurred in patients at dose groups 3 and 5. No drops in blood counts to grade 1 were noted in the highest dose group, suggesting factors other than radiation dose alone were associated with the count levels posttherapy. Both of the affected patients had pretreatment levels of only 12% above the lower limits of normal such that a modest drop reached grade 1 level. As the radiation dose to marrow was small at 0.002 to 0.14 cGy/MBq, significant hematologic toxicity would not be expected and was not observed.18 Six of 16 ovarian cancer patients were anemic pretherapy and 3 had improvement to normal levels even during the initial 6 weeks posttherapy. The early posttreatment increase of hemoglobin would not have been expected with higher, marrow-toxic, doses of radiation and suggests incomplete recovery from toxicities of therapy before administration of this investigational agent.
Both methods of index lesion measurement, individual volume versus sum of lesion diameter products, showed a trend of less tumor growth with increasing level of administered radioactivity. The independent review shows more early patients with progression than previously noted when pretreatment and posttreatment comparison used the product of index lesion diameter (Table 2).17,37 However, the later independent review did not necessarily use the same index lesions, which contributed to less than complete concordance of tumor growth results from those previously reported.17 Although the optimal tumor efficacy with α-emitters is proposed to be for microscopic disease, regression was noted among various sized gross lesions.
Many investigators continue to seek serum tumor markers to facilitate diagnosis and monitoring of therapeutic efficacy. Although CA125 has become the standard marker for monitoring antitumor effects in ovarian cancer, checking this alone has not been rigorous enough to become a standard for diagnostic screening.39 Its use in conjunction with other serum markers, plus additional factors, has been more helpful.30,40 Other potential markers under study for ovarian cancer include IL-6, IL-8, kallikrein-10, mesolthelin, HE-4, and p53.41–44 Also, SAA may be useful in serous subtype of ovarian carcinoma.45
In this study, several tumor markers were monitored to determine their potential utility for noninvasive assessment of therapeutic response. Although levels of the standard ovarian cancer tumor marker CA125 did not have a strong relation to outcome, CA125 may have been a better marker had the pretreatment level been obtained closer to the time to treatment. With the measures of this noncancer specific protein usually 2 to 4 weeks before therapy plus intervening manipulation of the peritoneal cavity with catheter insertion and treatment, the posttreatment levels may have been elevated by factors other than tumor burden. One might expect that elevation related to disruption by catheter placement and therapy would have resolved by 6 weeks but further study would be needed to seek relevant information for this determination.
HE-4 has also been studied as a tumor marker of ovarian cancer. It has been more helpful for diagnosis than for monitoring therapeutic response. HE-4 has most frequently been used in conjunction with CA125 and other factors to distinguish ovarian cancer from a benign abdominal process.40,46,47 The mucin-like TAG-72 is generally expressed on adenocarcinomas and less frequently in other types of malignancies.48–50 The only normal tissue with notable TAG-72 expression is secretory endometrium, which was not of concern in this study as none of the patients had an intact uterus.49,50 Tumor shedding of TAG-72 is common and may allow potential noninvasive monitoring of tumor status via blood levels,49 with the assumption that it correlates with tumor burden in an individual patient. TAG-72 had the strongest correlation with CT-monitored tumor changes of the 7 markers reported here. None of the other markers tested showed a good correlation with increasing radioactivity or clinical outcome. Although they have all been associated with ovarian cancer, CA125 is the only marker robust enough to routinely be used and it is not cancer specific. TAG-72 had a relatively robust association with increasing administered radioactivity, having a correlation coefficient of 0.73. On the basis of that, plus its decreasing trend with decreasing tumor growth, a dose/response relation with administered radioactivity is suggested. Additional data are needed to confirm this and to further investigate TAG-72 as a serum marker of response to 212Pb-TCMC-trastuzumab. CEA is a serum marker used as a standard in monitoring response to therapy in colon/rectal cancer. We found it was helpful in the 2 colon cancer patients in this study. CEA may be elevated, and thus is a potential marker for monitoring other gastrointestinal malignancies as well other malignancies of nongastrointestinal origin albeit in a smaller fraction of patients. In this study, all the ovarian cancer patients had normal CEA levels pretreatment and none experienced elevated levels at follow-up, even when disease progression was noted from imaging and other markers (TAG-72).
CONCLUSIONS
IP 212Pb-TCMC-trastuzumab up to 27.4 MBq/m2 appears safe for further study and dose escalation. Serum TAG-72 monitoring is recommended as a potential tumor marker for assessing antitumor effects in patients with ovarian cancer.
ACKNOWLEDGMENTS
The authors thank many for conduct of this study that required an international team: J. Maxwell Austin, Souheil Saddekni, Jacob Estes, Andres Forero, Melissa Baird, Charles Leath, III, Mack Barnes, Desiree Morgan, the late Michael Azure, Jinda Fan, Denise Charlotte Jeffers, Sui Shen, Darrell Fisher, Brenda Sandmaier, Olivier Rixe, Kurt Zinn, Ronda Carlise, Alma Del Grosso, Rebecca Quinn, Robert Oster, Rusty Caranto, Daniel Yoder, Lolinda Brown, Martin Brechbiel, Shakeela Dad, Debbie Soldano, Paul Fanta, Thelma Webb, and Brandy Jonas.
Footnotes
Supported by AREVA Med and NIH CCTS grant 1UL1TR001417.
J.J.T., T.A.R., and E.P.B. are AREVA Med employees. R.F.M. joined the Scientific Advisory Board July 2015. The other authors declare no conflicts of interest.
REFERENCES
- 1.Tewari D, Java JJ, Salani R, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2015;33:1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart JS, Hird V, Sullivan M, et al. Intraperitoneal radioimmunotherapy for ovarian cancer. BJOG. 1989;96:529–536. [DOI] [PubMed] [Google Scholar]
- 3.Meredith R, You Z, Alvarez R, et al. Predictors of long-term outcome from intraperitoneal radioimmunotherapy for ovarian cancer. Cancer Biother Radiopharm. 2012;26:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vergote IB, Vergote-De Vos LN, Abeler VM, et al. Randomized trial comparing cisplatin with radioactive phosphorus or whole-abdomen irradiation as adjuvant treatment of ovarian cancer. Cancer. 1992;69:741–749. [DOI] [PubMed] [Google Scholar]
- 5.Oei AL, Verheijen RH, Seiden MV, et al. Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine HMFG1 without improvement in overall survival. Int J Cancer. 2007;120:2710–2714. [DOI] [PubMed] [Google Scholar]
- 6.Sgouros G, Roeske JC, McDevitt MR, et al. MIRD pamphlet no. 22 (abridged): radiobiology and dosimetry of {alpha}-particle emitters for targeted radionuclide therapy. J Nucl Med. 2010;51:311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elgqvist J, Frost S, Pouget JP, et al. The potential and hurdles of targeted alpha therapy—clinical trials and beyond. Front Oncol. 2014;3:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barendsen GW. Dose-survival curves of human cells in tissue culture irradiated with alpha-, beta-, 20-kV. x- and 200-kV. X-radiation. Nature. 1962;193:1153–1155. [DOI] [PubMed] [Google Scholar]
- 9.Allen BJBT, Brill AB, Fisher DR, et al. MIRD monograph—radiobiology and dosimetry for radiopharmaceutical therapy with alpha-particle emitters. J Nucl Med. 2015;56:1–63.25537988 [Google Scholar]
- 10.Sgouros GB, Shah WA, Watchman A, et al. Relative biological effectiveness for efficacy and toxicity in leukemia patients of the alpha-particle emitter, bismuth-213. Cancer Biother Radiopharm. 2006;21:397. [Google Scholar]
- 11.Jurcic JG, Larson SM, Sgouros G, et al. Targeted alpha particle immunotherapy for myeloid leukemia. Blood. 2002;100:1233–1239. [PubMed] [Google Scholar]
- 12.Allen BJ, Huang CY, Clarke RA. Targeted alpha anticancer therapies: update and future prospects. Biologics. 2014;8:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jurcic JG, Ravandi F, Pagel JM, et al. Phase I trial of targeted alpha-particle immunotherapy with actinium-225-lintuzumab (anti-CD33) and low-dose cytarabine (LDAC) in older patients with untreated acute myeloid leukemia (AML). Blood. 2015;126:3794. [Google Scholar]
- 14.Zalutsky MR, Reardon DA, Akabani G, et al. Clinical experience with alpha-particle emitting 211At: treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med. 2008;49:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen BJ, Raja C, Rizvi S, et al. Intralesional targeted alpha therapy for metastatic melanoma. Cancer Biol Ther. 2005;4:1318–1324. [DOI] [PubMed] [Google Scholar]
- 16.Andersson H, Cederkrantz E, Back T, et al. Intraperitoneal {alpha}-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of 211At-MX35 F(ab’)2—a phase I study. J Nucl Med. 2009;50:1153–1160. [DOI] [PubMed] [Google Scholar]
- 17.Meredith RF, Torgue J, Azure MT, et al. Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother Radiopharm. 2014;29:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meredith RF, Torgue J, Shen S, et al. Dose escalation and dosimetry of first-in-human α radioimmunotherapy with 212 Pb-TCMC-trastuzumab. J Nucl Med. 2014;55:1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cederkrantz E, Andersson H, Bernhardt P, et al. Absorbed doses and risk estimates of (211)At-MX35 F(ab’)2 in intraperitoneal therapy of ovarian cancer patients. Int J Radiat Oncol Biol Phys. 2015;93:569–576. [DOI] [PubMed] [Google Scholar]
- 20.Azure MZK. Intraperitoneal Injection Toxicity Study of 212Pb-TCMC-Trastuzumab in Cynomolgus Monkeys. Birmingham: University of Alabama at Birmingham; 2010. contract No. AREVA01. [Google Scholar]
- 21.Milenic DE, Garmestani K, Brady ED, et al. Alpha-particle radioimmunotherapy of disseminated peritoneal disease using a (212)Pb-labeled radioimmunoconjugate targeting HER2. Cancer Biother Radiopharm. 2005;20:557–568. [DOI] [PubMed] [Google Scholar]
- 22.Milenic DE, Garmestani K, Brady ED, et al. Multimodality therapy: potentiation of high linear energy transfer radiation with paclitaxel for the treatment of disseminated peritoneal disease. Clin Cancer Res. 2008;14:5108–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milenic DE, Wong KJ, Baidoo KE, et al. Targeting HER2: a report on the in vitro and in vivo pre-clinical data supporting trastuzumab as a radioimmunoconjugate for clinical trials. MAbs. 2010;2:5, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milenic DE, Molinolo AA, Solivella MS, et al. Toxicological studies of 212Pb intravenously or intraperitoneally injected into mice for a phase 1 trial. Pharmaceuticals. 2015;8:416–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo Gerfo P, Krupey J, Hansen HJ. Demonstration of an antigen common to several varieties of neoplasia. N Engl J Med. 1971;285:138–141. [DOI] [PubMed] [Google Scholar]
- 26.Soletormos G, Duffy MJ, Othman Abu Hassan S, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bast RC, Jr, Badgwell D, Lu Z, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15(suppl 3):274–281. [DOI] [PubMed] [Google Scholar]
- 28.Moss EL, Hollingworth J, Reynolds TM. The role of CA125 in clinical practice. J Clin Pathol. 2005;58:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yurkovetsky Z, Skates S, Lomakin A, et al. Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol. 2010;28:2159–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotowicz B, Fuksiewicz M, Sobiczewski P, et al. Clinical value of human epididymis protein 4 and the risk of ovarian malignancy algorithm in differentiating borderline pelvic tumors from epithelial ovarian cancer in early stages. Eur J Obstet Gynecol Reprod Biol. 2015;194:141–146. [DOI] [PubMed] [Google Scholar]
- 31.Edgell T, Martin-Roussety G, Barker G, et al. Phase II biomarker trial of a multimarker diagnostic for ovarian cancer. J Cancer Res Clin Oncol. 2010;136:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coticchia CM, Yang J, Moses MA. Ovarian cancer biomarkers: current options and future promise. JNCCN. 2008;6:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiao N, Li H. The value of mesothelin in the diagnosis and follow-up of surgically treated ovarian cancer. Eur J Gynaecol Oncol. 2013;34:163–165. [PubMed] [Google Scholar]
- 34.Anastasi E, Granato T, Falzarano R, et al. The use of HE4, CA125 and CA72-4 biomarkers for differential diagnosis between ovarian endometrioma and epithelial ovarian cancer. J Ovarian Res. 2013;6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Shannessy DJ, Somers EB, Palmer LM, et al. Serum folate receptor alpha, mesothelin and megakaryocyte potentiating factor in ovarian cancer: association to disease stage and grade and comparison to CA125 and HE4. J Ovarian Res. 2013;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen L, Cardenas-Goicoechea SJ, Gordon P, et al. Biomarkers for early detection of ovarian cancer. Womens Health. 2013;9:171–185. [DOI] [PubMed] [Google Scholar]
- 37.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 38.Kasten BB, Azure MT, Schoeb TR, et al. Imaging, biodistribution, and toxicology evaluation of (212)Pb-TCMC-trastuzumab in nonhuman primates. Nucl Med Biol. 2016;43:391–396. [DOI] [PubMed] [Google Scholar]
- 39.Cramer DW, Bast RC, Jr, Berg CD, et al. Ovarian cancer biomarker performance in prostate, lung, colorectal, and ovarian cancer screening trial specimens. Cancer Prev Res. 2011;4:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dikmen ZG, Colak A, Dogan P, et al. Diagnostic performances of CA125, HE4, and ROMA index in ovarian cancer. Eur J Gynaecol Oncol. 2015;36:457–462. [PubMed] [Google Scholar]
- 41.Salmena L, Shaw P, Fans I, et al. Prognostic value of INPP4B protein immunohistochemistry in ovarian cancer. Eur J Gynaecol Oncol. 2015;36:260–267. [PubMed] [Google Scholar]
- 42.Seagle BL, Eng KH, Dandapani M, et al. Survival of patients with structurally-grouped TP53 mutations in ovarian and breast cancers. Oncotarget. 2015;6:18641–18652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamir A, Jag U, Sarojini S, et al. Kallikrein family proteases KLK6 and KLK7 are potential early detection and diagnostic biomarkers for serous and papillary serous ovarian cancer subtypes. J Ovarian Res. 2014;7:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y, Lu M, Zhou Q. Kallikrein expression as a prognostic factor in ovarian cancer: a systematic review and meta-analysis. J BUON. 2015;20:855–861. [PubMed] [Google Scholar]
- 45.Urieli-Shoval S, Finci-Yeheskel Z, Dishon S, et al. Expression of serum amyloid a in human ovarian epithelial tumors: implication for a role in ovarian tumorigenesis. J Histochem Cytochem. 2010;58:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilailak S, Chan KK, Chen CA, et al. Distinguishing benign from malignant pelvic mass utilizing an algorithm with HE4, menopausal status, and ultrasound findings. J Gynecol Oncol. 2015;26:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karlsen MA, Sandhu N, Hogdall C, et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2012;127:379–383. [DOI] [PubMed] [Google Scholar]
- 48.Johnston WW, Szpak CA, Lottich SC, et al. Use of a monoclonal antibody (B72.3) as a novel immunohistochemical adjunct for the diagnosis of carcinomas in fine needle aspiration biopsy specimens. Hum Pathol. 1986;17:501–513. [DOI] [PubMed] [Google Scholar]
- 49.Thor A, Gorstein F, Ohuchi N, et al. Tumor-associated glycoprotein (TAG-72) in ovarian carcinomas defined by monoclonal antibody B72.3. J Natl Cancer Inst. 1986;76:995–1006. [PubMed] [Google Scholar]
- 50.Thor A, Ohuchi N, Szpak CA, et al. Distribution of oncofetal antigen tumor-associated glycoprotein-72 defined by monoclonal antibody B72.3. Cancer Res. 1986;46:3118–3124. [PubMed] [Google Scholar]