Abstract
Purpose
Determine the roles of the phosphatidylinositol 3-kinase (PI3K) isoforms p110α and p110β in PTEN-deficient, estrogen receptor α (ER)-positive breast cancer, and the therapeutic potential of isoform-selective inhibitors.
Experimental Design
Anti-estrogen-sensitive and -resistant PTEN-deficient, ER+ human breast cancer cell lines, and mice bearing anti-estrogen-resistant xenografts were treated with the anti-estrogen fulvestrant, the p110α inhibitor BYL719, the p110β inhibitor GSK2636771, or combinations. Temporal response to growth factor receptor-initiated signaling, growth, apoptosis, predictive biomarkers, and tumor volumes were measured.
Results
p110β primed cells for response to growth factor stimulation. While p110β inhibition suppressed cell and tumor growth, dual targeting of p110α/β enhanced apoptosis and provided sustained tumor response. The growth of anti-estrogen-sensitive cells was inhibited by fulvestrant, but fulvestrant inconsistently provided additional therapeutic effects beyond PI3K inhibition alone. Treatment-induced decreases in phosphorylation of AKT and Rb were predictive of therapeutic response. Short-term drug treatment induced tumor cell apoptosis and proliferative arrest to induce tumor regression, while long-term treatment only suppressed proliferation to provide durable regression.
Conclusions
p110β is the dominant PI3K isoform in PTEN-deficient, ER+ breast cancer cells. Upon p110β inhibition, p110α did not induce significant reactivation of AKT, but combined targeting of p110α/β most effectively induced apoptosis in vitro and in vivo and provided durable tumor regression. Since apoptosis and tumor regression occurred early but not late in the treatment course, and proliferative arrest was maintained throughout treatment, p110α/β inhibitors may be considered short-term cytotoxic agents and long-term cytostatic agents.
Keywords: PI3K, anti-estrogen, PTEN, breast cancer, p110-beta
Introduction
Two-thirds of breast cancers express nuclear hormone receptors for estrogen (ER) and/or progesterone (PR). Patients with hormone receptor-positive breast cancer are treated with anti-estrogen therapies [e.g., tamoxifen, fulvestrant (fulv), and aromatase inhibitors (AIs)] that inhibit ER. While adjuvant anti-estrogen therapies have changed the natural history of hormone-dependent breast cancer, approximately one-third of patients develop metastatic disease that becomes resistant to all available therapies (1).
Activation of the phosphatidylinositol 3-kinase (PI3K) pathway has been implicated in anti-estrogen resistance (2-4). The PI3K product phosphatidylinositol 3,4,5-trisphosphate (PIP3) promotes the recruitment of pleckstrin homology (PH) domain-containing proteins to the plasma membrane, triggering signaling cascades including PDK1/AKT/mechanistic target of rapamycin (mTOR) that drive cell growth, proliferation, survival, and migration. The tumor suppressor phosphatase and tensin homolog (PTEN) dephosphorylates PIP3, antagonizing PI3K. The PI3K pathway is genetically altered in >70% of ER+ breast cancers, most frequently by gain-of-function mutations in PIK3CA (encodes the PI3K subunit p110α; occur in 28-47% of cases), and/or decreased expression or loss-of-function mutations in PTEN (occur in 29-44% of cases) (5-9). Small molecule-mediated inhibition of PI3K, AKT, and/or mTOR suppresses anti-estrogen-resistant growth of ER+ breast cancer cells and xenografts. While mTOR complex 1 (mTORC1) inhibition with everolimus is being used to treat patients with advanced ER+ breast cancer, there is concern that mTORC1 inhibition alleviates feedback inhibition on activators of PI3K, promoting PI3K activation and attenuating therapeutic efficacy (10, 11). Thus, direct inhibitors of PI3K may be more effective.
PI3K inhibitors are being developed for the treatment of breast and other cancers. Unfortunately, pan-PI3K inhibitors that target the p110α, p110β, and p110δ Class IA isoforms of PI3K induce considerable dose-limiting toxicity (12-14). Expression of p110δ is largely restricted to immune and hematopoietic cells, while p110α and p110β are ubiquitously expressed. Isoform-selective PI3K inhibitors are showing improved safety profiles, but the subpopulations of patients with solid tumors most likely to benefit from these agents are only partially defined. p110α is essential for PI3K/AKT signaling and growth of tumors driven by PIK3CA mutations, growth factor receptor tyrosine kinases (RTKs), and/or mutant Ras. In contrast, p110β can be activated by G protein-coupled receptors (GPCRs), RTKs, and Rac1/Cdc42, exists in complex with PTEN, and has been shown to mediate tumorigenesis in some but not all PTEN-deficient cancer models (15-20). PIK3CA mutations predict sensitivity to p110α inhibition in preclinical models (21), and early clinical data from patients with advanced ER+ breast cancer treated with the p110α-selective inhibitor BYL719 show increased benefit when PIK3CA is mutated (22). Since PTEN-deficient cancer cells may rely on p110β to drive PIP3/AKT signaling (23-25), early clinical testing of p110β-selective inhibitors has been focused on patients with cancer types that frequently harbor PTEN alterations (i.e., prostate cancer, squamous cell lung carcinoma, and triple-negative breast cancer). However, it is unclear whether p110β inhibitors will be effective against PTEN-deficient, ER+ breast cancer. p110α is required for vascular endothelial cell migration and angiogenesis (26). There is extensive crosstalk between the ER and PI3K pathways: PI3K inhibition induces ER transcriptional activity; anti-estrogens induce PI3K activation; ER drives transcription of genes encoding RTKs, adaptors, and ligands known to activate p110α (2, 3, 27). Antagonism between the ER and PI3K pathways provides rationale for the ongoing clinical testing of PI3K inhibitors in combination with anti-estrogens. We thus tested the effects of p110α and p110β inhibitors, with and without anti-estrogens in models of PTEN-deficient, ER+ breast cancer.
Materials and Methods
Cell culture
Parental cell lines (ATCC) were cultured in DMEM/10% FBS (Hyclone). Cells were treated with GSK2636771 (gift from GlaxoSmithKline), BYL719 (Chemietek), BKM120, OSI-906 (SelleckChem), and/or fulvestrant (Tocris). Fulv-resistant (FR) ZR75-1 (ZR75-1/FR) and MDA-MB-415 (MDA-MB-415/FR) cells were generated through culture with 1 μM fulv for 4 months.
Xenograft studies
Animal studies were approved by the Dartmouth College IACUC. Female athymic nude (J:Nu) mice (4-5 wk old; obtained from Jackson Laboratory) were injected s.c. with 5-10×106 ZR75-1/FR cells resuspended in matrigel (BD Biosciences). On the same date, mice were implanted s.c. with a 17β-estradiol pellet (0.72 mg, 60-day-release; Innovative Research of America), and fulv treatment was initiated (5 mg/wk s.c.; clinical formulation; gift from Astrazeneca). Tumor volumes were measured twice weekly using calipers (volume=length2xwidth/2). Four weeks after implantation, tumor-bearing mice were randomized to treatment with vehicle, GSK2636771 (30 mg/kg/d, p.o.), BYL719 (25 mg/kg/d, p.o.), or the combination, all with a fulv treatment backbone. Tumors were harvested and cut in pieces for snap-freezing, or formalin fixation and paraffin embedding (FFPE).
Statistics
In vitro cell growth, and tumor IHC and TUNEL data were analyzed by ANOVA with Bonferroni multiple comparison-adjusted post-hoc testing between groups. To estimate treatment-induced tumor growth delay (TGD), the LINEXP non-linear mixed model of tumor regrowth was employed (28), which accounts for inter-tumor heterogeneity in treatment response. The R function ‘nlme’ was used to estimate parameters of non-linear regrowth and compute TGD in each treatment group. p≤0.05 was considered statistically significant.
Additional methods are provided in Supplementary Information.
Results
p110β primes PTEN-deficient, ER+ breast cancer cells for response to growth factor stimulation
We first interrogated the Genomics of Drug Sensitivity in Cancer database (29), in which 672 cancer cell lines were screened for sensitivity to 138 anti-cancer drugs including the p110β-selective inhibitors TGX221 and AZD6482. Among 70 cancer-related genes analyzed, alterations in PTEN significantly predicted sensitization to TGX221 and AZD6482 (Fig. S1), supporting the concept that p110β is critical for growth in PTEN-deficient cancer cells.
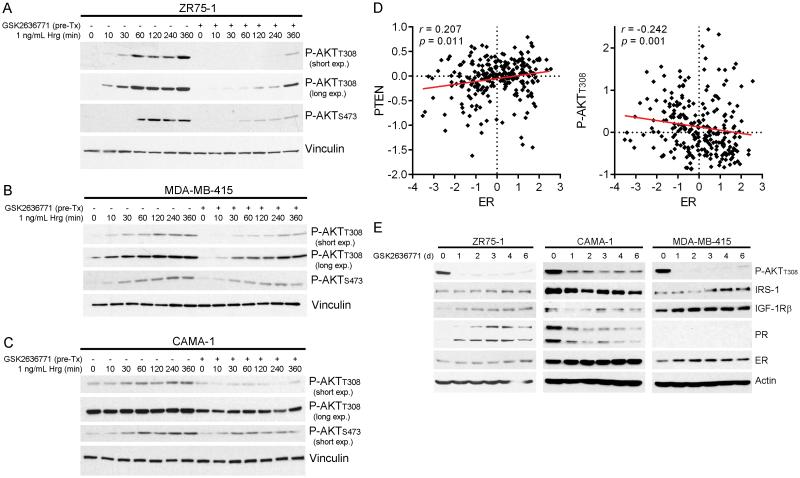
p110β has been found in complex with PTEN in MCF-7 breast and other cancer cells, and p110β produces a basal level of PIP3 that is curbed by PTEN, offering an explanation of how PTEN loss increases levels of PIP3 and AKT activation [Fig. S2 and refs. (15, 16, 30, 31)]. We confirmed the isoform selectivity of the p110β-selective inhibitor GSK2636771 and the p110α-selective inhibitor BYL719 in p110β-driven, PTEN-mutant MDA-MB-415 cells and p110α-driven, PIK3CA-mutant T47D cells, respectively (Fig. S3). While stimulation of 3 PTEN-deficient ER+ breast cancer cell lines with the growth factor heregulin increased phospho-AKT over time, treatment with the p110β-selective inhibitor GSK2636771 delayed AKT phosphorylation (Fig. 1A-C) despite increased phosphorylation/activation of the heregulin receptor HER3 (Fig. S4). These findings support the model proposed by Knight et al. in which p110β is constitutively active and p110α is required for AKT phosphorylation in response to RTK activation, suggesting that p110α and p110β may generate different pools of PIP3 (15, 30, 32).
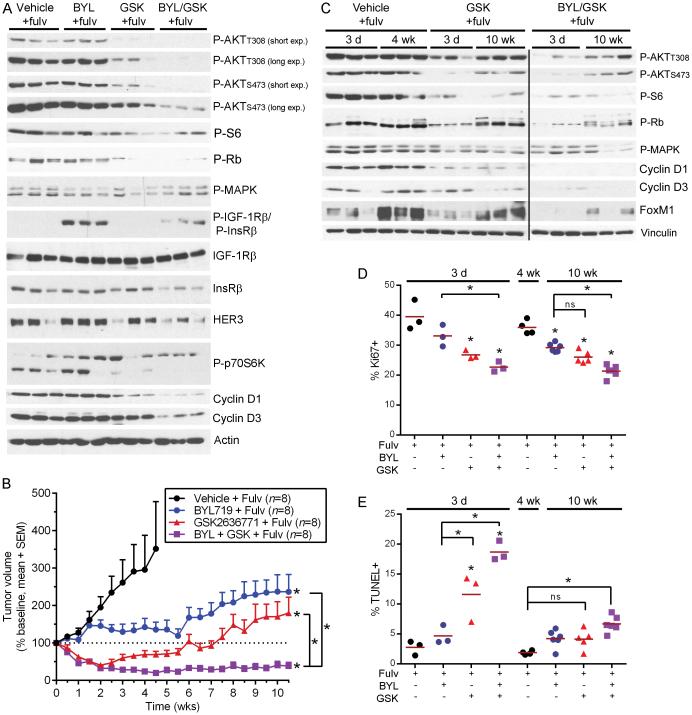
Fig. 1.
p110β inhibition suppresses response to growth factor stimulation. A-C) Cells were serum-starved for 16-24 h, then pretreated with 1 μM GSK2636771 for 1 h followed by stimulation with heregulin (Hrg) +/− GSK2636771. Protein lysates were analyzed by immunoblot. D) RPPA measurements of ER, PTEN, and P-AKTT308 levels in 248 luminal breast tumors were extracted from refs. (5, 36) and compared by Spearman correlation. E) Cells were treated as indicated. Medium and drug were refreshed every 2-3 d, and protein lysates were analyzed as above.
Since the above-described findings do not distinguish between basal and growth factor-induced p110β activation, and both p110α and p110β can be activated by RTKs (20), we tested the effects of isoform-selective inhibitors on signaling response to RTK activation. Cells pretreated with BYL719 and/or GSK2636771 for 1 h were then stimulated with heregulin. Inhibition of either PI3K isoform alone partially decreased growth factor-induced AKT activation in PTEN-deficient cells, while combined p110α/β inhibition was most effective (Fig. S5). Thus, both p110α and p110β are likely activated by RTK signaling in PTEN-deficient, ER+ breast cancer cells. However, a role for p110β in growth factor-induced AKT activation was less evident in PTEN-wild-type, PIK3CA-mutant, ER+ breast cancer cells: p110α inhibition blocked the majority of AKT phosphorylation, while combined p110α/β inhibition elicited modestly greater effects (Fig. S5). In contrast, p110β was reported to have no effect on P-AKT levels in growth conditions in PIK3CA-mutant ER+ breast cancer cells (33); these disparate findings may be due to the use of growth factor stimulation in serum-free conditions in our studies. In PIK3CA-mutant ER+ breast cancer cells, PTEN knockdown by siRNA increased steady-state P-AKT levels driven almost exclusively by p110α (Fig. S6C), suggesting that the p110β predominance observed in intrinsically PTEN-deficient cancer cells may not be inducible by PTEN depletion in PTEN-proficient models.
p110β drives AKT activation in PTEN-deficient, ER+ breast cancer cells.
There is extensive crosstalk and antagonism between the ER and PI3K pathways in ER+ breast cancer (2, 3, 34). Since PTEN levels are inversely correlated with P-AKT in human breast tumors (35), we interrogated a reverse phase protein array database documenting relative levels of ~200 (phospho)proteins in 248 luminal breast tumors (36) to determine whether PTEN-deficient and AKT-activated tumors exhibit ER loss. Indeed, levels of ER and PTEN were positively correlated, and levels of ER and P-AKTT308 were negatively correlated in human luminal breast tumors (Fig. 1D).
We then evaluated the temporal effects of p110β inhibition on markers of ER activation. p110β inhibition with GSK2636771 durably suppressed P-AKT levels in ZR75-1 and MDA-MB-415 cells, and provided partial PI3K inhibition in CAMA-1 cells (Fig. 1E). p110β inhibition increased ER levels and markers of ER activity (IRS-1, IGF-1Rβ, and/or PR) in ZR75-1 and MDA-MB-415 cells, but decreased these markers in CAMA-1 cells, suggesting that ER-PI3K pathway interactions vary between cell lines. Although dual PI3K/mTOR inhibition has been shown to upregulate ER transcriptional activity in ZR75-1 and CAMA-1 cells (34), we cannot exclude the possibility that IRS-1 and IGF-1Rβ, which lie upstream of PI3K, were also modulated in response to a GSK2636771-induced decrease PI3K activity by ER-independent means [e.g., via activation of FoxO transcription factors (37)].
Combined inhibition of p110β and p110α decreases growth of PTEN-deficient, ER+ breast cancer cells.
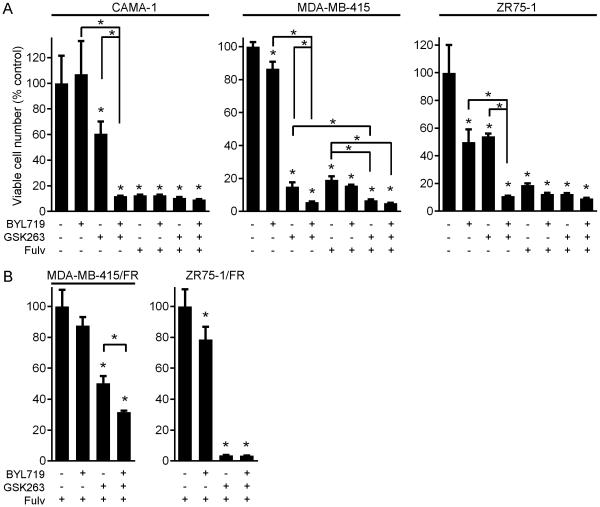
Prior preclinical studies have shown that combined targeting of p110β and p110α sometimes improves anti-cancer effects compared to single-isoform inhibition (23, 38), and that p110α inhibition slows the growth of some PTEN-deficient, PIK3CA-wild-type cancer xenografts (21). We therefore tested the effects of GSK2636771, BYL719, the anti-estrogen fulvestrant (fulv), and combinations on growth of PTEN-deficient, ER+ breast cancer cells. p110β inhibition significantly but incompletely suppressed cell outgrowth in all three cell lines, while single-agent p110α inhibition was only effective against ZR75-1 cells (Figs. 2A, S7A). In all PTEN-deficient ER+ cell lines, combined targeting of p110α and p110β was significantly more effective than either agent alone.
Fig. 2.
Combined targeting of p110α/β abrogates growth in anti-estrogen-sensitive and -resistant, PTEN-deficient, ER+ breast cancer cells. A) Parental cells were treated with 1 μM fulv, 1 μM BYL719, 1 μM GSK2636771, or combinations. Medium and drugs were replenished every 3 days. When control wells reached 50-70% confluence (after 20-40 d), cells were stained with crystal violet. Quantification is shown as mean of triplicates + SD (% control). B) FR cells were analyzed as in (A). *p<0.05 by Bonferroni post-hoc test compared to control, unless otherwise indicated with brackets.
In MDA-MB-415 cells, treatment with the combination of fulv and GSK2636771 was significantly more effective than single agents (Figs. 2A, S7A). However, inhibition of ER with fulv alone dramatically suppressed growth in parental cell lines (Figs. 2A, S7A/C), confounding evaluation of the effects of combined PI3K/ER inhibition. Thus, we developed fulv-resistant (FR) derivatives of ZR75-1 and MDA-MB-415 cells through culture in the presence of 1 μM fulv for 4 months. While ER/p110β inhibition completely blocked outgrowth of ZR75-1/FR cells, combined targeting of ER/p110α/β was more effective than single-isoform targeting in MDA-MB-415/FR cells (Figs. 2B, S7B).
Co-targeting p110α/β maximally suppresses PI3K signaling and induces apoptosis in PTEN-deficient, ER+ breast cancer cells.
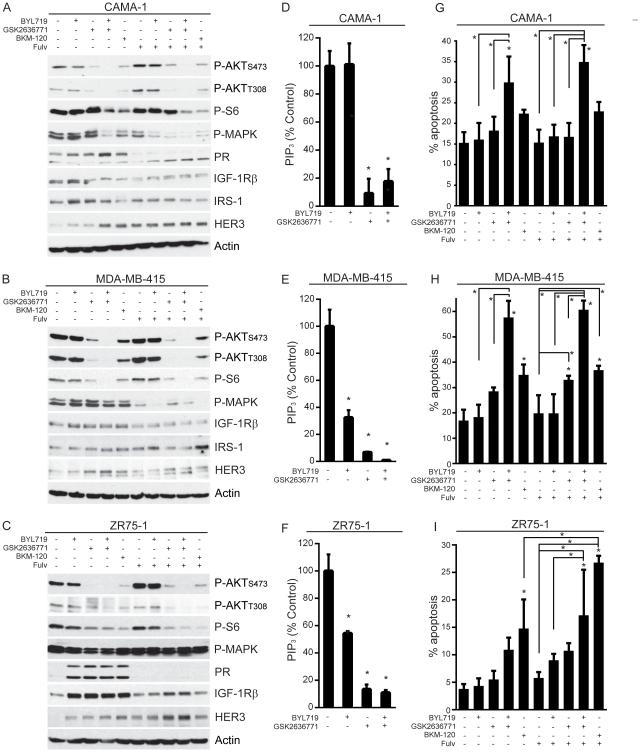
Since dual p110α/β inhibition often more effectively suppressed growth than single-isoform inhibition (Fig. 2), we explored the effects of single- and dual-isoform inhibition on steady-state PI3K/AKT signaling. GSK2636771 drastically decreased P-AKT and PIP3 levels in PTEN-deficient cell lines, while BYL719 less effectively decreased PIP3 levels in MDA-MB-415 and ZR75-1 cells without altering P-AKT (Fig. 3A-F). Combined inhibition of p110α/β further decreased levels of P-AKT and P-S6 (downstream marker of mTORC1 activity), suggesting that p110β inhibition alone incompletely blocks AKT/mTORC1 signaling. Treatment with the pan-PI3K inhibitor BKM120 (1 μM) elicited effects similar to BYL/GSK (1 μM each), but BKM120 was slightly less effective at decreasing P-AKT (Fig. 3A-C), possibly due to the presence of less drug.
Fig. 3.
Combined inhibition of p110α/β suppresses mTORC1 signaling and induces apoptosis in PTEN-deficient, ER+ breast cancer cells. A-C) Cells were pretreated +/− 1 μM fulv for 24 h, then treated +/− fulv and 1 μM PI3K inhibitors for 24 h before lysis. Protein lysates were analyzed by immunoblot using the indicated antibodies. D-F) Cells were treated as indicated for 4 h. Phospholipids were isolated from cell lysates, and relative levels of PIP3 were quantified by ELISA. Data are presented as PIP3 normalized to control, mean of triplicates + SD. G-I) Cells were treated as indicated for 3 d, then analyzed for apoptosis. Proportions of apoptotic cells are shown as mean of triplicates + SD. *p<0.05 by Bonferroni post-hoc test compared to control, unless otherwise indicated with brackets.
PI3K inhibition upregulated markers of ER activity (PR, IGF-1Rβ, and/or IRS-1) in ZR75-1 and MDA-MB-415 cells that were downregulated by fulv treatment. In contrast, PI3K inhibition decreased IRS-1 and IGF-1Rβ in CAMA-1 cells (Fig. 3A-C). The PR upregulation induced by BKM120 or BYL/GSK in CAMA-1 cells (Fig. 3A), which contrasts with the GSK2636771-induced downregulation of PR (Fig. 1E), may be due in-part to the decreased MAPK phosphorylation induced upon more complete PI3K inhibition (Fig. 3A); activated MAPK promotes PR degradation (39). In contrast, fulv increased P-AKT levels that were suppressed by PI3K inhibition. FR cells also showed upregulation of P-AKT that remained primarily p110β-dependent (Fig. S8), indicating that AKT activation in PTEN-deficient, ER+ breast cancer cells remains p110β-driven upon acquisition of anti-estrogen resistance.
Preclinical and early clinical data suggest that mTORC1 inhibition (as measured by decreased P-S6 levels) is correlated with tumor response to single-agent p110α inhibition in PIK3CA-mutant breast cancer (40). We observed that combined inhibition of p110α/β most effectively decreased P-S6 levels in PTEN-deficient, ER+ breast cancer cells (Fig. 3A-C). However, mTORC1 hyperactivation induced by siRNA knockdown of TSC2 did not confer appreciable resistance to PI3K inhibition in growth assays (Fig. S9), indicating that the PI3K-driven signaling events essential for growth lie upstream of TSC2 in these model systems.
While therapeutic kinase inhibitors frequently inhibit cancer cell proliferation, apoptosis is required for regression of solid tumors (41). Dual p110α/β inhibition induced more apoptosis than single-isoform inhibition (Fig. 3G-I). Combined inhibition of AKT and MEK signaling has been shown to be required for significant induction of apoptosis in cancer cells (42). While fulv suppressed MEK activation [measured by MAPK (ERK1/2) phosphorylation] in CAMA-1 and MDA-MB-415 cells (Fig. 3A-B), the addition of fulv did not further increase apoptosis beyond that affected by p110α/β inhibition (Fig. 3G-I). In contrast, fulv augmented BKM120-induced apoptosis without altering MEK activation in ZR75-1 cells, suggesting that continuous MEK inhibition is not required for PI3K inhibitor-induced apoptosis. Interestingly, co-treatment with fulv/BKM120 increased apoptosis compared to BKM120 alone in ZR75-1 cells where the addition of fulv further decreased P-S6 levels (Fig. 3C/I). These data suggest that targeting p110α/β elicits robust anti-cancer effects in vitro, but the therapeutic benefit of co-treatment with an anti-estrogen to block (compensatory) ER activation may be modest.
Decreased phosphorylation of both AKT and Rb in response to PI3K inhibition is predictive of apoptosis in PTEN-deficient, ER+ breast cancer cells.
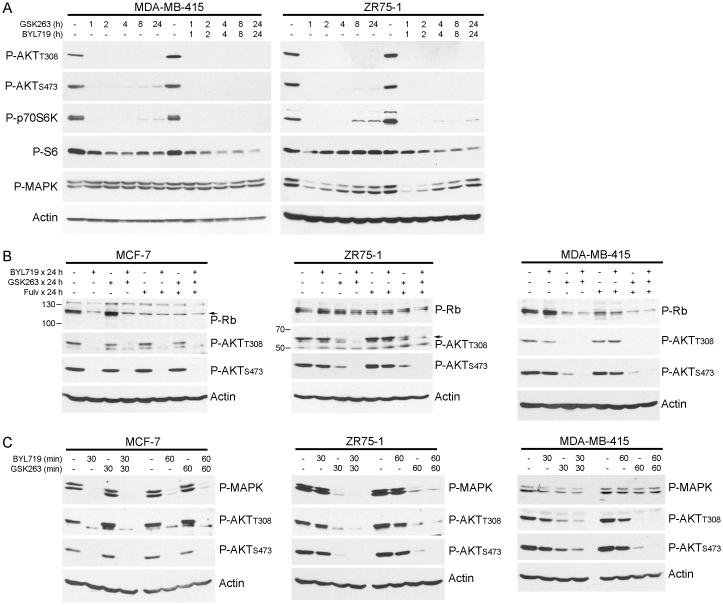
Several signaling alterations induced by PI3K inhibitors have been proposed as predictive biomarkers of therapeutic response/resistance. One mechanism suggests that PTEN-deficient prostate and triple-negative breast cancer cells treated with a p110β inhibitor upregulate IGF-1R-dependent p110α signaling to reactivate PIP3/AKT signaling (38). Similarly, HER2+ breast cancer cells treated with a p110α inhibitor upregulate HER3-driven p110β activation to restore PIP3/AKT signaling (33). While we detected upregulation of IGF-1R and HER3 in response to PI3K inhibition (Fig. 3A-C), we did not observe significant rebound activation of AKT with prolonged p110β inhibition in PTEN-deficient, ER+ breast cancer cells (Figs. 4A, 1E, S10A). Even in cells adapted to growth in the presence of GSK2636771 for 2-4 wk, AKT activation remained primarily p110β-dependent (Fig. S11). However, weak reactivation of p70S6K following ≥8 h of p110β inhibition was correlated with persistent S6 phosphorylation, which was suppressed with the addition of a p110α inhibitor (Figs. 4A, S10A). These data suggest that A) PTEN-deficient, ER+ breast cancer cells do not robustly engage p110α upon p110β inhibition as was observed in other cancer subtypes (38), and B) weak PI3K-driven mTORC1/p70S6K activation is sufficient to maintain steady-state levels of S6 phosphorylation, which is most effectively blocked by combined inhibition of p110α/β. PI3K inhibitor treatments did not alter the protein levels of p110α or p110β (Fig. S10A).
Fig. 4.
PI3K inhibition-induced Rb inactivation predicts subsequent apoptosis in PTEN-deficient, ER+ breast cancer cells. Cells were treated with 1 μM GSK2636771, 1 μM BYL719, or 1 μM fulv as indicated, and protein lysates were analyzed by immunoblot.
A second proposed biomarker links PI3K inhibitor-induced cell cycle arrest with downregulation of cyclin D1 (due to mTORC1 inhibition), in turn decreasing CDK4/CDK6 activities and Rb phosphorylation in PIK3CA-mutant breast cancer cells (43). We confirmed that BYL719-sensitive, PTEN-wild-type, PIK3CA-mutant, ER+ MCF-7 breast cancer cells engage p110α but not p110β to activate AKT, and p110α inhibition decreases P-Rb levels (Fig. 4B). While PI3K inhibition decreased both P-AKT and P-Rb in MDA-MB-415 and CAMA-1 cells, ZR75-1 cells showed persistent Rb phosphorylation despite AKT inhibition (Figs. 4B, S10B). CAMA-1 and MDA-MB-415 cells underwent dramatic apoptosis in response to p110α/β inhibition while effects on ZR75-1 cells were more modest (Fig. 3G-I), indicating that decreased phosphorylation of both AKT and Rb upon PI3K inhibition is a biomarker predictive of apoptotic response in PTEN-deficient, ER+ breast cancer cells.
Single-agent treatment with fulv decreased P-Rb in MDA-MB-415 and CAMA-1 cells (Figs. 4B, S10B), but residual PI3K/AKT signaling likely prevented apoptosis. Both p110α/β inhibition, and combined inhibition of ER and p110α/β, similarly decreased P-AKT and P-Rb levels in MDA-MB-415 and CAMA-1 cells (Figs. 4B, S10B), correlating with similar degrees of apoptosis with or without fulv (Fig. 3G/H). In contrast, ZR75-1 cells showed greater suppression of P-Rb with combined targeting of ER/p110α/β vs. p110α/β (Fig. 4B), in agreement with the requirement for fulv to significantly increase apoptosis in BYL/GSK-treated ZR75-1 cells compared to control, and the increased apoptosis conferred by fulv in BKM-120-treated ZR75-1 cells (Fig. 3I).
A third suggested biomarker predictive of apoptosis in HER2+ breast cancer cells in response to PI3K inhibition is transient inhibition of MAPK (42). We confirmed that short-term (30-60 min) p110α inhibition decreases P-MAPK levels in MCF-7 cells (Fig. 4C). While short-term inhibition of p110β or p110α/β decreased P-AKT and P-MAPK in ZR75-1 cells that did not dramatically apoptose upon p110α/β inhibition (Figs. 4A/C, 3I), p110α/β inhibition did not transiently suppress P-MAPK levels in MDA-MB-415 or CAMA-1 cells that eventually underwent apoptosis (Figs. 4A/C, 3G/H, S10C). Thus, PTEN-deficient, ER+ breast cancer cells do not require transient MAPK inhibition to undergo apoptosis in response to PI3K inhibition.
Combined targeting of p110α/β abrogates anti-estrogen resistance in ER+ breast tumors
Since PI3K inhibitors will most likely be initially clinically implemented for the treatment of ER+ breast cancer in patients with advanced, anti-estrogen-resistant disease in combination with an anti-estrogen, and parental ZR75-1 xenografts are endocrine-sensitive (Fig. S12), we tested the effects of p110α/β inhibition in mice bearing ZR75-1/FR tumors (all mice were treated with fulv since the time of xenografting). Immunoblot analysis of lysates from tumors harvested after 3 d of PI3K inhibitor treatment indicated that inhibition of ER/p110β but not ER/p110α decreased P-AKT and P-S6 levels compared to vehicle/fulv controls, and combined ER/p110α/β inhibition was most effective (Fig. 5A). ER/p110α inhibition induced upregulation of IGF-1Rβ/InsRβ phosphorylation, despite a decrease in total InsRβ and no change in total IGF-1Rβ, suggesting that AKT/mTORC1 signaling is not involved in this mechanism. Growth of cultured ZR75-1/FR cells was unaffected by the IGF-1R/InsR inhibitor OSI-906 +/− BYL719; thus, the functional significance of p110α inhibitor-induced P-IGF-1Rβ/InsRβ remains unclear and may only be apparent in tumors.
Fig. 5.
Dual targeting of p110α/β abrogates anti-estrogen-resistant growth of PTEN-deficient, ER+ breast tumors. A) Lysates of ZR75-1/FR tumors from mice treated with fulv, then harvested after 3 d of treatment with PI3K inhibitors were analyzed by immunoblot. B) Tumor volumes are shown as % baseline (mean + SEM). *p≤0.01 by non-linear mixed modeling compared to “Vehicle/Fulv” group, unless otherwise indicated with brackets. C) Tumors were harvested after 3 d or 10 wk of treatment with fulv/PI3K inhibitors (or 4 wk for Vehicle/Fulv-treated group) and analyzed as in (A). D-E) Tumors were analyzed by (D) IHC for Ki67 or (E) TUNEL. Horizontal red bars indicate mean values. *p≤0.05 by Bonferonni post-hoc test compared to 3-day Vehicle/Fulv controls unless otherwise indicated with brackets.
Inhibition of ER/p110β or ER/p110α/β induced rapid tumor regression, while ER/p110α inhibition had no significant early effect on growth compared to continued ER inhibition alone (Fig. 5B). After ~3 wk of PI3K inhibitor treatment, tumors in fulv/GSK-treated mice resumed growth. In contrast, fulv/BYL/GSK combination treatment prevented tumor regrowth, maintaining tumors at regressed volumes for the duration of the study. Fulv/BYL treatment slowed tumor growth after 2 wk (Fig. 5B); we speculate that p110α inhibition suppressed tumor angiogenesis, as p110α is the critical PI3K isoform in vascular endothelial cells (26). Since tumors in fulv/GSK-treated mice regressed then regrew (Fig. 5B), non-linear mixed modeling was used to predict duration of tumor growth delay (TGD). Fulv/GSK and fulv/BYL treatments provided similar TGD (9 and 8 wk, respectively), while fulv/BYL/GSK increased the projected TGD to 15 wk. All fulv/PI3K inhibitor treatments provided significant TGD compared to fulv/vehicle, and the combination of fulv/BYL/GSK was significantly more effective than fulv/BYL or fulv/GSK (all p≤0.01).
Molecular analysis of tumors from mice treated for 3 d or 10 wk revealed that PI3K inhibitor-induced decreases in P-AKT levels that occurred after short-term treatment were not maintained with long-term treatment (Fig. 5C). mTORC1 drives cap-dependent translation of Cyclin D1, and mTORC1 promotes Cyclin D3 stability (44, 45). Cyclin D1/D3 promote CDK4/CDK6 activities that stabilize the anti-senescence transcription factor FoxM1 (46) and inhibit Rb, causing Rb release from E2F transcription factors that drive cell cycle progression (Fig. 6).The recovered AKT signaling after long-term fulv/GSK and fulv/GSK/BYL treatments correlated with rescued FoxM1 and P-Rb levels but not Cyclin D1/D3 or P-S6, suggesting that CDK4/CDK6 activity was restored independent of mTORC1 signaling (Figs. 5C, 6). TUNEL and Ki67 IHC analyses confirmed that inhibition of ER/p110β increased apoptosis and proliferative arrest, respectively, after 3 d of treatment compared to ER inhibition alone, while ER/p110α/β inhibition was non-significantly more effective (Fig. 5D-E). Following 10 wk of drug treatment, ER/p110α, ER/p110β, and ER/p110α/β inhibition continued to suppress tumor cell proliferation compared to fulv/vehicle control (Figs. 5D, S13), but the proportion of TUNEL-positive cells was significantly increased only by fulv/BYL/GSK treatment compared to fulv/vehicle control (Figs. 5E, S14). Although significantly higher than fulv/vehicle control, apoptosis in the fulv/BYL/GSK treatment group was much less dramatic after 10 wk of treatment compared to 3 d; this significant drop in apoptosis at 10 wk (compared to 3 d) is consistent with partially restored P-AKT and P-Rb (Figs. 5C/E, S14). These data indicate that A) suppression of tumor growth after long-term treatment is mediated primarily by inhibition of proliferation without dramatic apoptosis, B) ER/PI3K inhibitor-induced apoptosis and tumor regression occur early in the treatment course and are correlated with robust decreases in phosphorylation of AKT and Rb, and C) long-term treatment with an anti-estrogen and a p110α/β inhibitor(s) may provide sustained therapeutic response in PTEN-deficient, ER+ breast cancer.
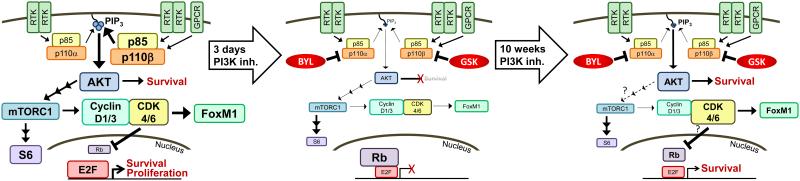
Fig. 6.
Model of response to PI3K inhibition in anti-estrogen-resistant, PTEN-deficient, ER+ breast tumors. Left: PI3K signaling engages AKT, which drives mTORC1-induced translation of mRNAs encoding Cyclin D1/D3. Cyclin D1/D3 bind CDK4/CDK6 to promote Rb phosphorylation, which derepresses E2F transcription factors to drive expression of genes that promote proliferation and suppress apoptosis. CDK6 phosphorylates FoxM1 to promote FoxM1 stability, which suppresses senescence. Middle: Short-term PI3K inhibition with BYL719/GSK2636771 decreases AKT/mTORC1 signaling and Cyclin D1/D3 levels, decreasing CDK4/6 activity, P-Rb, and FoxM1 stability. Unphosphorylated Rb binds E2F proteins to suppress expression of pro-growth and anti-apoptosis genes. Right: After long-term PI3K inhibition, AKT activation is partially restored but mTORC1 remains inhibited, suggesting that AKT signaling becomes disconnected from mTORC1. Despite continued suppression of Cyclin D1/D3, CDK4/6 activity is partially restored as indicated by partial recovery of P-Rb and FoxM1.
Discussion
Herein, we demonstrate that combined inhibition of p110α/β most effectively inhibits AKT/mTORC1 signaling, cell growth and survival, and tumor growth in models of PTEN-deficient, ER+ breast cancer. p110β inhibition suppressed the majority of AKT activation, but suppression of S6 phosphorylation required dual p110α/β inhibition, indicating that PTEN-deficient cancer cells have a large excess of PI3K/AKT signaling and only a small fraction is required to maintain mTORC1 activation. While PI3K inhibition inconsistently increased ER levels and/or markers of ER activity, it is unclear whether anti-estrogen treatment uniformly increases the anti-cancer effects of PI3K inhibition in PTEN-deficient, ER+ breast cancer [as seen in PIK3CA-mutant, ER+ breast cancer (47)].
Among the suggested pharmacodynamic biomarkers predictive of response/resistance to PI3K inhibitors (38, 42, 43), we found that suppression of both P-AKT and P-Rb levels was most predictive of subsequent apoptosis in PTEN-deficient, ER+ breast cancer cells (Figs. 3G-I, 4B, 5, S10B), in agreement with prior observations in PIK3CA-mutant breast cancer models (43). P-AKT and P-Rb may be clinically incorporated as early pharmacodynamic biomarkers to determine whether cancers are likely to respond to PI3K inhibitors, either through IHC comparison of pre- and post-treatment tumor tissues, or through indirect imaging metrics such as [18F]FDG-PET [as a marker of PI3K/AKT activation (12)] and [18F]FLT-PET [as a marker of DNA synthesis]. PI3K inhibition did not transiently interrupt MAPK activation in 2/3 PTEN-deficient, ER+ breast cancer cell lines that subsequently underwent apoptosis (Figs. 3G/H, 4A/C, S10C), conflicting with previous findings from other breast cancer cell lines (42); the transient nature of this signaling interruption also makes it impractical to develop as a clinical diagnostic biomarker. p110β inhibition drastically decreased AKT activation without inducing compensatory activation of p110α in PTEN-deficient, ER+ breast cancer cells (Figs. 1, 4A), contrasting with prior observations in PTEN-deficient prostate and triple-negative breast cancer cells (38). However, residual p110α signaling likely conferred sufficient PI3K/AKT signaling to maintain mTORC1 activation despite p110β inhibition (Figs. 3A-C, 4A, S10A), indicating a need for dual p110α/β inhibition in PTEN-deficient, ER+ breast cancer.
In support of P-AKT and P-Rb as biomarkers predictive of apoptosis in response to PI3K inhibition, ZR75-1/FR tumors in mice treated with fulv/GSK or fulv/BYL/GSK showed apoptosis and decreased P-Rb and P-AKT levels after 3 d but not 10 wk of treatment (Fig. 5C/E). Despite recovering P-AKT levels by 10 wk of treatment, such tumors continued to show decreased P-S6 and Cyclin D1/D3 levels; while this is indicative of low mTORC1 activity and may explain the continued inhibition of tumor cell proliferation, the mechanism underlying the progressive disconnect between AKT and mTORC1 requires further study. Based on these findings, we propose a model (Fig. 6) in which short-term inhibition of ER and PI3K effectively suppresses the PI3K/AKT/mTORC1 axis, resulting in inhibition of CDK4/CDK6 proliferative signaling and induction of tumor cell apoptosis. By 10 wk of treatment, AKT and CDK4/CDK6 activities partially recover, preventing continued apoptosis; however, mTORC1 remains suppressed resulting in continued inhibition of proliferation. Although tumor cell proliferation remained suppressed after 10 wk of treatment with fulv/GSKL and fulv/BYL/GSK, partial recovery of P-Rb and FoxM1 (Fig. 5C) suggest that co-targeting CDK4/CDK6 (to inhibit Rb phosphorylation) may increase therapeutic efficacy. Indeed, combinations of CDK4/CDK6 inhibitors and PI3K inhibitors are currently being evaluated in clinical studies in ER+ breast and other cancers.
Costa et al. recently demonstrated that combined p110α/β inhibition more effectively decreased PIP3 levels and cell viability than single-isoform inhibition in PIK3CA-mutant ER+ breast cancer cells. However, inhibition of p110α maximally suppressed P-AKT levels, suggesting that p110β drives AKT-independent pro-growth signaling in PIK3CA-mutant cells, and that PIP3 may be a more sensitive readout of PI3K activity than P-AKT. In contrast, PIP3 levels correlated with P-AKT levels in HER2+ breast cancer cells (33). In PTEN-deficient ER+ breast cancer cells, we observed that p110α/β inhibition decreased P-AKT, suppressed growth, and induced apoptosis more effectively than single-isoform inhibition, but p110α inhibition did not significantly suppress PIP3 levels beyond the suppression induced by p110β inhibition (Fig. 3A-F). These discrepant results suggest that the most accurate readout of PI3K activity may vary between biological systems, and may not be predictable based on the status of PI3K pathway mutations. Measuring PIP3 levels in cells/tissues is technically challenging, due in part to its labile nature and low levels (48); these issues make routine PIP3 measurement in clinical specimens impractical. P-AKTT308 continues to be the most widely accepted marker of PI3K activity because Thr308 phosphorylation is typically PI3K-dependent, and a more stable, accurate biomarker remains to be developed.
While inhibition of p110α with BYL719 did not alter P-AKT levels in ZR75-1/FR tumors (Fig. 5A), drug treatment slowed tumor growth (Fig. 5B) but only modestly affected cell growth in vitro (Fig. 2B). BYL719 slowed growth of PTEN-deficient PC3 prostate cancer and U87MG glioblastoma xenografts, but P-AKT levels were not appreciably altered in the latter and not tested in the former (21). Thus, p110α inhibition may elicit anti-tumor effects by a non-cancer cell mechanism(s) [e.g., inhibition of angiogenesis (26)]. In addition, other cancer subtypes exhibit patterns of dependence on p110α and/or p110β independent of PI3K pathway mutational status. Weigelt et al. found that PTEN-deficient endometrial cancer cells are not dependent on p110β for growth, survival, or AKT phosphorylation, regardless of the presence or absence of co-existent mutations in PIK3CA or PIK3R1; instead, combined inhibition of p110α/β is required to inhibit growth (49). In non-small cell lung cancer cells, PIK3CA mutations or PTEN deficiency were not associated with sensitivity to inhibitors of p110α or p110β, respectively; instead, combined inhibition of p110α/β was required for significant growth suppression (50). The inconsistency between PTEN deficiency and sensitivity to p110α inhibition supports the clinical exploration of treatment strategies incorporating p110α inhibitors for PTEN-deficient cancers.
We conclude that dual p110α/β inhibition is more effective than single-isoform targeting of PI3K in PTEN-deficient, ER+ breast cancer. While antagonism exists between PI3K and ER activation in this cancer subtype, and anti-estrogen treatment inhibits cell and tumor growth, it is unclear whether addition of an anti-estrogen uniformly increases efficacy beyond that provided by p110α/β inhibition. These results support clinical testing of p110α/β inhibitors, but not p110β inhibitors alone, for the treatment of patients with anti-estrogen-resistant, PTEN-deficient breast cancer.
Supplementary Material
Statement of Translational Relevance.
Phosphatidylinositol 3-kinase (PI3K) is a critical signaling hub that drives cancer cell survival, growth, proliferation, and metastasis. While pan-PI3K inhibitors targeting all Class IA isoforms (p110α, p110β, p110δ) are being tested clinically, toxicity to normal tissues may be reduced with isoform-selective inhibitors. In cancer cells with loss or inactivation of the PI3K antagonist Phosphatase and Tensin Homolog (PTEN), p110β often drives PI3K signaling; thus, p110β-selective inhibitors are being tested clinically for select types of PTEN-deficient cancers. PTEN deficiency occurs in 29-44% of breast cancers expressing estrogen receptor α (ER). Despite crosstalk between the ER and PI3K pathways, and ER-induced expression of genes associated with p110α activation, we found that p110β is the dominant PI3K isoform in PTEN-deficient, ER+ breast cancer cells. However, p110β inhibition only provides temporary protection against growth of anti-estrogen-resistant tumors, while dual targeting of p110α/β induces sustained tumor regression. These data indicate that combined p110α/β inhibition is necessary to achieve a durable response in PTEN-deficient, ER+ breast cancer.
Acknowledgements
We thank Phil DeYoung and Gopi Ganji for reading the manuscript, and Norris Cotton Cancer Center Shared Resources: Transgenic & Genetic Constructs, Pathology Translational Research, and Flow Cytometry.
Financial support
Financial support was provided by the NIH (R00CA142899 to TWM; Dartmouth College Norris Cotton Cancer Center Support Grant P30CA023108) and the American Cancer Society (RSG-13-292-01-TBE to TWM).
Footnotes
Conflicts of interest: none.
References cited
- 1.Early Breast Cancer Trialists' Collaborative G. Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–13. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29:4452–61. doi: 10.1200/JCO.2010.34.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller TW, Perez-Torres M, Narasanna A, Guix M, Stal O, Perez-Tenorio G, et al. Loss of Phosphatase and Tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res. 2009;69:4192–201. doi: 10.1158/0008-5472.CAN-09-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas N Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoman N, Klassen S, McFadden A, Bickis MG, Torlakovic E, Chibbar R. Reduced PTEN expression predicts relapse in patients with breast carcinoma treated by tamoxifen. Mod Pathol. 2005;18:250–9. doi: 10.1038/modpathol.3800296. [DOI] [PubMed] [Google Scholar]
- 7.Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007;104:7564–9. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Tenorio G, Alkhori L, Olsson B, Waltersson MA, Nordenskjold B, Rutqvist LE, et al. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13:3577–84. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale KA, Sahin AA, Liu S, Barrera JA, et al. PI3K Pathway Mutations and PTEN Levels in Primary and Metastatic Breast Cancer. Mol Cancer Ther. 2011 doi: 10.1158/1535-7163.MCT-10-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller TW, Forbes JT, Shah C, Wyatt SK, Manning HC, Olivares MG, et al. Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells. Clin Cancer Res. 2009;15:7266–76. doi: 10.1158/1078-0432.CCR-09-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer IA, Abramson VG, Isakoff SJ, Forero A, Balko JM, Kuba MG, et al. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2014;32:1202–9. doi: 10.1200/JCO.2013.54.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zgheib NB, Xiong Y, Marchion D, Li X, Bicaku E, Stickles X, et al. PLAC1 expression in human cancer cells correlates with carboplatin sensitivity and overall survival from ovarian cancer. Gynecol Oncol. 2012;125:S139–S. [Google Scholar]
- 14.Chon HS, Marchion DC, Xiong Y, Chen N, Bicaku E, Stickles XB, et al. The BCL2 antagonist of cell death pathway influences endometrial cancer cell sensitivity to cisplatin. Gynecol Oncol. 2012;124:119–24. doi: 10.1016/j.ygyno.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–9. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dbouk HA, Pang H, Fiser A, Backer JM. A biochemical mechanism for the oncogenic potential of the p110beta catalytic subunit of phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2010;107:19897–902. doi: 10.1073/pnas.1008739107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmit F, Utermark T, Zhang S, Wang Q, Von T, Roberts TM, et al. PI3K isoform dependence of PTEN-deficient tumors can be altered by the genetic context. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6395–400. doi: 10.1073/pnas.1323004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritsch R, de Krijger I, Fritsch K, George R, Reason B, Kumar MS, et al. RAS and RHO Families of GTPases Directly Regulate Distinct Phosphoinositide 3-Kinase Isoforms. Cell. 2013;153:1050–63. doi: 10.1016/j.cell.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinovsky R, Pochanard P, McNear C, Brachmann SM, Duke-Cohan JS, Garraway LA, et al. p85 Associates with Unphosphorylated PTEN and the PTEN-Associated Complex. Molecular and cellular biology. 2009;29:5377–88. doi: 10.1128/MCB.01649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Utermark T, Rao T, Cheng H, Wang Q, Lee SH, Wang ZC, et al. The p110alpha and p110beta isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes & development. 2012;26:1573–86. doi: 10.1101/gad.191973.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritsch C, Huang A, Chatenay-Rivauday C, Schnell C, Reddy A, Liu M, et al. Characterization of the novel and specific PI3Kalpha inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Molecular cancer therapeutics. 2014;13:1117–29. doi: 10.1158/1535-7163.MCT-13-0865. [DOI] [PubMed] [Google Scholar]
- 22.Mayer IA, Abramson V, Formisano L, Balko JM, Estrada MV, Sanders M, et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kalpha-specific Inhibitor, with Letrozole in ER+/HER2-Negative Metastatic Breast Cancer. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edgar KA, Wallin JJ, Berry M, Lee LB, Prior WW, Sampath D, et al. Isoform-specific phosphoinositide 3-kinase inhibitors exert distinct effects in solid tumors. Cancer Res. 2010;70:1164–72. doi: 10.1158/0008-5472.CAN-09-2525. [DOI] [PubMed] [Google Scholar]
- 24.Ni J, Liu Q, Xie S, Carlson C, Von T, Vogel K, et al. Functional characterization of an isoform-selective inhibitor of PI3K-p110beta as a potential anticancer agent. Cancer Discov. 2012;2:425–33. doi: 10.1158/2159-8290.CD-12-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453:662–6. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 27.Hyder SM, Nawaz Z, Chiappetta C, Stancel GM. Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res. 2000;60:3183–90. [PubMed] [Google Scholar]
- 28.Demidenko E. The Assessment of Tumour Response to Treatment. Journal of the Royal Statistical Society: Series C (Applied Statistics) 2006;55:365–77. [Google Scholar]
- 29.Yang WJ, Soares J, Greninger P, Edelman EJ, Lightfoot H, Forbes S, et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Research. 2013;41:D955–D61. doi: 10.1093/nar/gks1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–47. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabinovsky R, Pochanard P, McNear C, Brachmann SM, Duke-Cohan JS, Garraway LA, et al. p85 associates with unphosphorylated PTEN and the PTEN-associated complex. Mol Cell Biol. 2009 doi: 10.1128/MCB.01649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Vadas O, Perisic O, Anderson KE, Clark J, Hawkins PT, et al. Structure of lipid kinase p110beta/p85beta elucidates an unusual SH2-domain-mediated inhibitory mechanism. Mol Cell. 2011;41:567–78. doi: 10.1016/j.molcel.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa C, Ebi H, Martini M, Beausoleil SA, Faber AC, Jakubik CT, et al. Measurement of PIP3 levels reveals an unexpected role for p110beta in early adaptive responses to p110alpha-specific inhibitors in luminal breast cancer. Cancer cell. 2015;27:97–108. doi: 10.1016/j.ccell.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Creighton CJ, Fu X, Hennessy BT, Casa AJ, Zhang Y, Gonzalez-Angulo AM, et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010;12:R40. doi: 10.1186/bcr2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–91. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Lu Y, Akbani R, Ju Z, Roebuck PL, Liu W, et al. TCPA: a resource for cancer functional proteomics data. Nat Methods. 2013;10:1046–7. doi: 10.1038/nmeth.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz S, Wongvipat J, Trigwell CB, Hancox U, Carver BS, Rodrik-Outmezguine V, et al. Feedback suppression of PI3Kalpha signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kbeta. Cancer Cell. 2015;27:109–22. doi: 10.1016/j.ccell.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1032–7. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elkabets M, Vora S, Juric D, Morse N, Mino-Kenudson M, Muranen T, et al. mTORC1 Inhibition Is Required for Sensitivity to PI3K p110 alpha Inhibitors in PIK3CA-Mutant Breast Cancer. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng LP, Goh PSC. Incidence of discontinuation of angiotensin-converting enzyme inhibitors due to cough, in a primary healthcare centre in Singapore. Singap Med J. 2014;55:146–9. doi: 10.11622/smedj.2014034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Will M, Qin AC, Toy W, Yao Z, Rodrik-Outmezguine V, Schneider C, et al. Rapid induction of apoptosis by PI3K inhibitors is dependent upon their transient inhibition of RAS-ERK signaling. Cancer Discov. 2014;4:334–47. doi: 10.1158/2159-8290.CD-13-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014;26:136–49. doi: 10.1016/j.ccr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Averous J, Fonseca BD, Proud CG. Regulation of cyclin D1 expression by mTORC1 signaling requires eukaryotic initiation factor 4E-binding protein 1. Oncogene. 2008;27:1106–13. doi: 10.1038/sj.onc.1210715. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Morales P, Hernando E, Carrasco-Garcia E, Menendez-Gutierrez MP, Saceda M, Martinez-Lacaci I. Cyclin D3 is down-regulated by rapamycin in HER-2-overexpressing breast cancer cells. Mol Cancer Ther. 2006;5:2172–81. doi: 10.1158/1535-7163.MCT-05-0363. [DOI] [PubMed] [Google Scholar]
- 46.Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20:620–34. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller TW, Balko JM, Fox EM, Ghazoui Z, Dunbier A, Anderson H, et al. ERalpha-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer discovery. 2011;1:338–51. doi: 10.1158/2159-8290.CD-11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salamon RS, Backer JM. Phosphatidylinositol-3,4,5-trisphosphate: tool of choice for class I PI 3-kinases. Bioessays. 2013;35:602–11. doi: 10.1002/bies.201200176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weigelt B, Warne PH, Lambros MB, Reis-Filho JS, Downward J. PI3K pathway dependencies in endometrioid endometrial cancer cell lines. Clin Cancer Res. 2013;19:3533–44. doi: 10.1158/1078-0432.CCR-12-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stamatkin C, Ratermann KL, Overley CW, Black EP. Inhibition of class IA PI3K enzymes in non-small cell lung cancer cells uncovers functional compensation among isoforms. Cancer Biol Ther. 2015;16:1341–52. doi: 10.1080/15384047.2015.1070986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.