Abstract
Angiogenesis is naturally balanced by many pro- and anti-angiogenic factors while an imbalance of these factors leads to aberrant angiogenesis, which is closely associated with many diseases. Gene therapy has become a promising strategy for the treatment of such a disordered state through the introduction of exogenous nucleic acids that express or silence the target agents, thereby engineering neovascularization in both directions. Numerous non-viral gene delivery nanoparticles have been investigated towards this goal, but their clinical translation has been hampered by issues associated with safety, delivery efficiency, and therapeutic effect. This review summarizes key factors targeted for therapeutic angiogenesis and anti-angiogenesis gene therapy, non-viral nanoparticle-mediated approaches to gene delivery, and recent gene therapy applications in pre-clinical and clinical trials for ischemia, tissue regeneration, cancer, and wet age-related macular degeneration. Enhanced nanoparticle design strategies are also proposed to further improve the efficacy of gene delivery nanoparticles to modulate angiogenesis.
Keywords: non-viral, gene therapy, polymeric nanoparticles, ischemia, wound healing, tissue engineering, tumor, age-related macular degeneration
Graphical Abstract
1. Introduction
The growth and development of higher order animals requires the establishment of adequate vasculature for nutrient and oxygen transport, waste removal, cell migration, and signal transduction. The formation and maturation of these vessels are mediated by several processes that can be classified broadly into three categories: vasculogenesis, or the de novo synthesis of vessels from angioblasts; angiogenesis, the formation of new blood vessels from pre-existing ones; and arteriogenesis, the maturation of existing collateral vessels to compensate for insufficiencies in primary vessels. Given the greater abundance of literature on the topic, however, most of this review will focus on the process of angiogenesis. Angiogenesis can be sub-divided into sprouting or intussusceptive mechanisms. Most of the available studies of angiogenic factors have focused on sprouting, in which select endothelial cells within a vessel, tips cells, migrate and are followed by adjacent stalk cells to form new branches, or sprouts, that eventually grow and anastomose to mature vessels. In contrast, intussusception occurs when transluminal tissue pillars develop, forming tiny holes that eventually fuse and split the vessel in two. Interestingly, several factors appear to be common to the two processes, suggesting that both should be considered during investigations of angiogenic therapeutics [1, 2].
Regulation in angiogenesis is maintained by a balance of pro- and anti-angiogenic factors. In healthy adults, this balance allows for the maintenance of the vasculature in a quiescent state under normal conditions, but poised for quick dynamic changes in response to perturbations in signaling in the microenvironment caused by situations such as wound healing, growth-related hypoxia, and inflammation. In addition, prolonged or chronic pathological conditions can shift angiogenesis equilibrium as demonstrated by the misappropriation of pro-angiogenic signals in cancer and neovascular age-related macular degeneration. Alternatively, dysfunction of existing vessels, such as atherosclerosis-induced ischemia or thrombotic complications, can lead to a sub-adequate blood supply and a demand for compensatory neovascularization.
The molecular mechanisms of angiogenesis are quite complicated and have been reviewed in detail elsewhere [3, 4] and will be summarized only briefly here followed by more in depth discussions of specific growth factors as they apply to specific gene therapeutics. Under normal circumstances, blood vessels are maintained in a quiescent state characterized by low endothelial cell (EC) proliferation, substantial mural-cell coverage (ie. pericytes and vascular smooth-muscle cells wrap around endothelial cells and stabilize them), and well-developed vascular basement membrane. The direct contact between mural cells and endothelial cells helps to maintain this state through the secretion factors involved in vascular homeostasis, notably angiopoietin-1 (Ang1) and low levels of vascular endothelial growth factor (VEGF). Both of these factors are secreted by mural cells, which maintain vessel stabilization through paracrine and autocrine activation of their corresponding receptors on the surfaces of EC and mural cells respectively.
Following hypoxic insult, inflammatory signaling, wounding, or certain pathological conditions, however, these signals shift from maintenance to the induction of angiogenesis. Pro-angiogenic factors, such as VEGF and fibroblast growth factor (FGF) are released from the surrounding tissues and induce the expression and secretion of matrix metalloproteinases (MMPs) and angiopoietin-2 (Ang2). The MMPs begin to break down the vascular basement membrane surrounding the vessels while Ang2 inhibits the activities of Ang1, leading to a reduction in EC-EC contacts and stimulation of mural cell detachment [5, 6]. The endothelial cells then begin a directional migration to the source of the growth factor gradients. In this process, one cell takes on the lead role as the tip cell in the migration and signals to adjacent ECs via delta-Notch to follow as stalk cells to proliferate and form the body of the growing vessel. Throughout this migration adjacent cells can overtake and replace the tip cell [7]. Upon meeting another vessel or sprout, the vessels are able to merge via anastomosis and form a continuous lumen to establish blood flow. Additional growth factors, such as platelet-derived growth factor (PDGF), are then released from endothelial cells to stimulate the proliferation and recruitment of new mural cells and to re-establish a quiescent state [8].
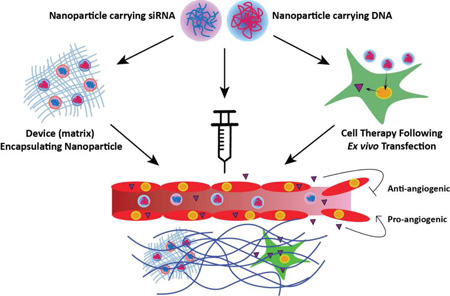
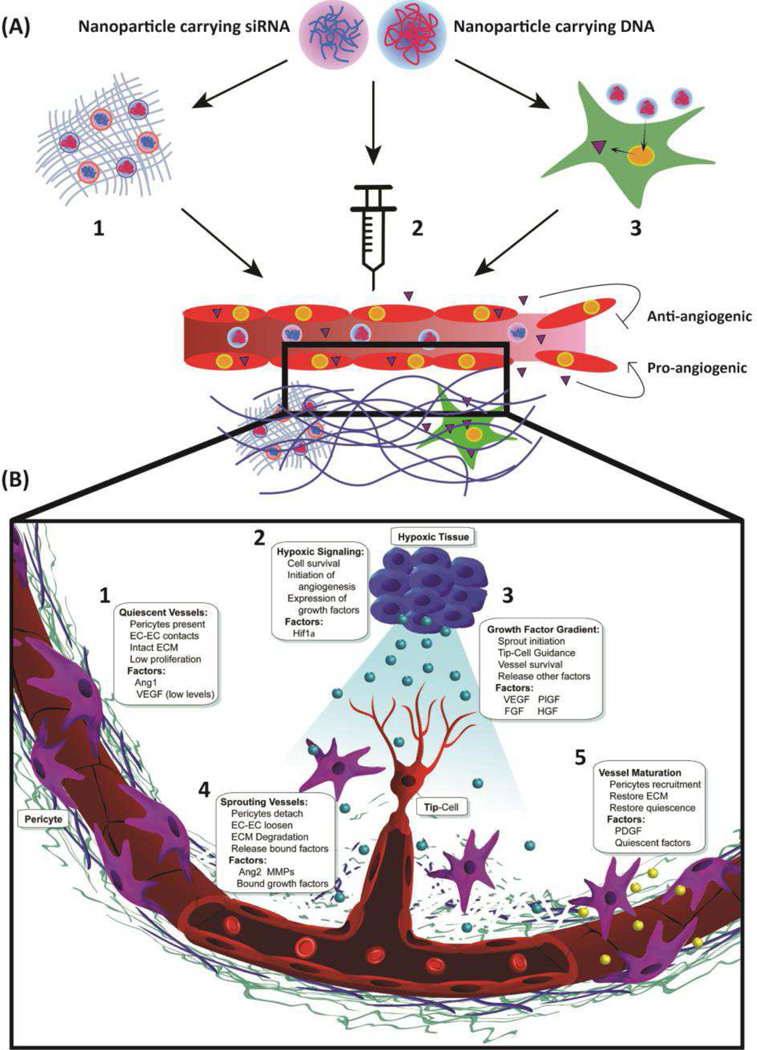
This review focuses on the advancement and applications of non-viral nanoparticles for gene therapy to modulate angiogenesis to treat diseases involving aberrant vasculature (Figure 1, Table 1).
Figure 1.
(A) Schematic showing modulation of angiogenesis by gene therapy using non-viral nanoparticles. Nanoparticles containing nucleic acids related to angiogenic or anti-angiogenic signals are delivered to a target tissue by: 1) Macroscale devices encapsulating nanoparticles that carry nucleic acids (such as a nanoparticle-eluting gel); 2) Systemic administration of nanoparticles carrying nucleic acids; and 3) Cells that are transfected ex vivo with nucleic acid-containing nanoparticles. (B) Schematic detailing the major molecular processes related to angiogenesis using vessel growth in response to tissue hypoxia as an example, including 1) vessel quiescence, 2) hypoxic initiation, 3) growth factor signaling, 4) vessel sprouting, and 5) vessel maturation.
Table 1.
Representative examples of angiogenesis modulation via non-viral nanoparticle-based gene delivery
| Angiogenesis | Disease | Factor | Delivery Vector | Animal Model |
Injection | Result | Reference |
|---|---|---|---|---|---|---|---|
| Pro |
Ischemic Limb |
VEGF165 | Heparin-PEI | Mice | Intramuscular | Over 3-fold ↑ neovasulazation vs. PEI in ischemic limb |
[116] |
| VEGF | PBAE | Mice | exwVoHUVEC transfection |
Limb salvage 50% compared to lipoplex (30%) and PEI (12.5%) |
[140] | ||
| eNOS | Elastin-based polymer | Mice | Intramuscular | ~ 3-fold ↑ perfusion ratio and blood vessel density vs. control |
[120] | ||
|
Myocardial In fact |
Adreno- medulliln |
Dextran | Rats | ex vivo MSC transfection |
2.5-fold ↑ capillary density and 40% ↓ infarct size vs. control |
[131] | |
| VEGF165 | Chitosan-g-PEI | Rats | Intramyocardial | 1.6-fold ↑ejection fraction and vessel density vs. PEI control |
[117] | ||
| Hypoxia- inducible VEGF165 |
Cholesterol chloroformate-bPEI |
Rabbits | Intramyocardial | Myocardial infarct size (36% ↓) vs. vector alone without hypoxia- inducible VEGF165 |
[119] | ||
| VEGF165 | PLGA | Rabbits | Intramyocardial | Capillary density (38% ↑) vs. plasmid only |
[124] | ||
|
Tissue Engineering |
SHh | PBAE | Mice | Transdermal | 15% ↑ wound closure than PBS | [150] | |
| VEGF | PBAE | Mice | ex vivo ASC transfection |
20% ↑ wound closure than non- modified ASC |
[151] | ||
| PDGF-B | PEI | Rats | collagen scaffold with PEI-pPDGF |
40% bone volume and 52-fold ↑ connective density vs. control |
[154] | ||
| Anti | Cancer | sFlt-1 or siVEGFRI |
bPEI-g-PEG-cRGD | Mice | Intravenous | 3-fold / 42% ↓ colon carcinoma umor growth vs. PEI-g-PEG |
[214,216] |
| NF-kB | Tween-SS-bPEI | Mice | Intravenous | Breast tumor volume 6% of saline | [218] | ||
| Endostatin | PVP | Mice | Intramuscular | 60% ↓ metastatic brain tumor growth vs. control by MRI |
[220] | ||
| shVEGF/ DOX | CPP-dendrimer PLL | Mice | Intravenous | VEGF mRNA (65% ↓) and median survival (2-fold ↑) vs. control in orthotopic glioma |
[224] | ||
| siVEGF | guanidine-βCD-PEG- anisamide |
Mice | Intravenous | VEGF mRNA(3.5-fold ↓) / prostate tumor growth(3-fold ↓) vs. control |
[228] | ||
|
Age-related Macular Degenera tion |
Vasostatin | PVP | Rats | Intramuscular | 32.5% / 48% decrease in CNV incidence and area at day 42 |
[247] | |
| shHIF-1α | PLGA | Rats | Intravitreal | Leakage (50%↓) / lesion thickness (40%↓) vs. control |
[248] | ||
| Flt23k | RGD-PLGA | Monkey/ Mice |
Intravenous | CNV area 53% (monkey) and 43% (mice) ↓ vs. sham |
[252] |
2. Nanoparticle technology for gene therapy
Many therapeutics, such as small molecules, proteins, peptides, and nucleic acids, are identified and tested against molecular targets in diseased cells. However, frequently due to their short half-life without modification, they are degraded and/or cleared from the systemic circulation and the body quickly, often before an effective dose can accumulate at the target site. Poor pharmacokinetics requires repeated administration and, sometime due to a narrow therapeutic window, significant detrimental side effects. Some direct modifications to drugs, including binding of albumin and conjugation of poly(ethylene glycol) (PEG) or other macromolecules to the drug molecule, have shown improvement and have been approved by the FDA [9]. The use of particles to carry drugs has also increased bioavailability of the therapeutics by mitigating enzymatic degradation, evading clearance by reticuloendothelial system (RES), and promoting controlled release [10].
Drugs classified as small molecules, antibodies, and peptides can treat diseases by directly interacting with or blocking key molecular signaling molecules and targets to induce therapeutic effect. For example, aflibercept is a recombinant fusion protein that directly traps vascular endothelial growth factor (VEGF) to inhibit angiogenesis [11]. Gene therapy, which introduces nucleic acids as the drug, such as DNA, siRNA or miRNA, has particular advantages compared to other drug therapies. Insertion of a therapeutic gene or deletion of a malfunctioning gene goes beyond tackling the symptoms of a disease and can potentially cure diseases of genetic origin, including cancer [12, 13]. Also, the use of a cell’s own machinery to continuously produce active biomolecules encoded from exogenously delivered DNA can obviate the need for frequent drug dosing and hit targets that are otherwise “undruggable.” However, the challenge with a gene therapy approach is that the nucleic acid cargo has to successfully transduce or transfect the host cells through intracellular barriers in addition to crossing extracellular barriers to reach the target cells, requiring sophisticated engineering of the delivery vehicle.
Gene therapy has seen much growth in two parallel tracks: viral and non-viral. The viral gene delivery method takes advantage of the evolutional ability of viruses to transfer genetic material to the cell. Different types of viruses, such as adeno-associated virus, have been employed and modified to deliver nucleic acids with high and prolonged efficacy [14]. The major hurdles to clinical translation of viral vectors have been limited cargo size, large-scale production, and safety. Because many viruses can integrate exogenous genetic material into a host’s genome, there is a significant concern over insertional mutagenesis and oncogenesis [15]. Similarly, there are safety issues associated with potential immunogenicity and toxicity of certain viruses. In non-viral gene therapy, both synthetic and natural polymers and lipids are utilized to form nanoparticles. While these vectors have advantages of being designed non-toxic, mass producible, and flexible to modification, they generally have poor transfection efficiency. The field of viral gene therapy - including approaches for ex vivo transduction of cells for cellular therapy - has advanced to reduce safety risks and there have been more than 1,500 worldwide viral gene therapy clinical trials [16, 17]. The non-viral community has also advanced gene therapeutics to the clinic, with more than 500 worldwide clinical trials including lipid, polymer, and naked DNA approaches [17]. Despite all of this clinical activity, the FDA has only approved a single therapy thus far as a marketed product for use in the United States, Amgen’s IMLYGIC® (Talimogene Laherparepvec), which uses a modified herpes simplex virus type 1 vector as an oncolytic and to produce granulocyte-macrophage colony-stimulating factor with the goal of producing an anti-tumor immune response [18]. The two non-FDA approved gene therapy products that have been approved by other agencies wordwide are Gendicine, a recombinant adenovirus encoding wildtype-p53 for the treatment of head and neck squamous cell carcinoma and was approved by the China Food and Drug Administration [19], and alipogene tiparvovec (Glybera®), approved by the European Medicines Agency, an adeno-associated virus encoding lipoprotein lipase for treatment of the rare inherited disorder familial lipoprotein lipase deficiency [20]. Thus there is a critical need to develop new vectors or re-engineer existing vectors to have enhanced efficacy and safety and to treat a wide array of human diseases.
Non-viral nanoparticles face several barriers in the process of gene delivery [21]. First, a vector must form a stable complex with the genetic cargo. In the case of polymeric vectors, this is most often achieved through electrostatic interaction between cationic polymer and negatively charged nucleic acids. There are two interchangeable ways to describe this type of nanoparticles in relation to the amount of polymer and genetic material used. N/P is the ratio between the number of protonatable amines in the polymer chain and the number of phosphates in the nucleic acid backbone, while w/w is the mass ratio of the polymer to the genetic cargo. Poly(ethylene imine) (PEI) and poly(L-lysine) (PLL) are two of the most widely investigated polymers that contain primary and secondary amines that impart positive charge at physiological pH to allow DNA binding and condensation of DNA into nanoparticles [22, 23]. Linear cationic polymers, such as PEI, PLL and poly(amidoamine) (PAMAM) are also often modified to a branched dendrimer structure that allows for high density of positive charge or other functionalization [24]. Liposomal formulations and some polymeric nanoparticles, such as poly(lactic-co-glycolic acid) (PLGA) and poly(vinyl pyrrolidone) (PVP) nanoparticles, physically encapsulate nucleic acid cargos. Once the nanoparticles reach the target cell, they must enter the cells through one of several endocytic pathways. The chemical structure of non-viral materials, the presence of targeting ligands, and the physical properties of resulting nanoparticles govern their cellular uptake mechanism, which has been shown to affect the efficiency of successful transfection [25, 26]. Specifically for PEI, the molecular weight as well as the polymer structure, linear versus branched, affect the efficiency of gene delivery overall [27, 28]. The mechanism by which cationic polymeric nanoparticles, such as those formed with PEI, escape the endosomes to enter cytoplasm still remains a hypothesis. The leading hypothesis for PEI has been termed the “proton sponge effect” and is governed by the ability of a vector to buffer endosomal pH, generating high osmotic pressure leading to bursting of the endosomes [29]. Polymers without tertiary amines and less efficiency at endosomal pH buffering, such as PLL, are shown to have limited transfection efficiency without an excipient that can destabilize endosomal membrane [30]. For liposomes, it was proposed that cationic lipids can fuse with endosomal membranes to facilitate endosomal escape [31]. Following endosomal escape, nucleic acids have to be released from the nanoparticles in the cytoplasm in order to be transported into the nucleus (DNA) or to suppress the translation of mRNA (RNAi). The dissociation of nucleic acids depends on unbinding kinetics between the nucleic acid and biomaterial as well as degradation of the polymer, such as through hydrolysis of ester bonds or reduction of disulfide linkages. PEI and PLL, with high charge density but without degradable bonds, are associated with cytotoxicity as well as low transfection. Disulfide linkages are especially important for the rapid release of siRNA and shRNA in the glutathione-rich cytoplasm [32].
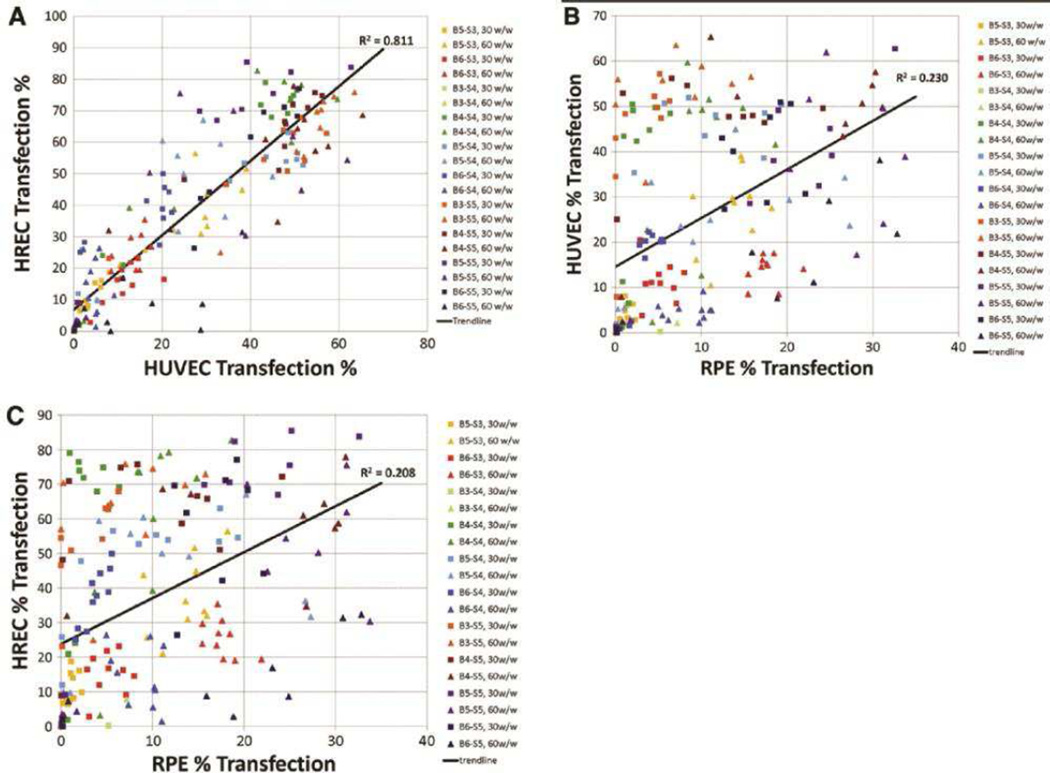
Shortcomings of first generation vectors during in vivo evaluations and clinical trials have led to the development of more versatile delivery systems. Many synthetic approaches have been utilized, including the investigation of polymer libraries to explore structural diversity. For example, the Reineke group has synthesized new gene delivery polymers by polymerizing polycations, (N-[3-(N,N-dimethylamino) propyl] methacrylamide or N-(2-aminoethyl) methacrylamide), with (2-deoxy-2-methacrylamido glucopyranose) [33]. These polymers with varied molecular weights were self-assembled with plasmid DNA to optimize nanoparticles that could have high transfection efficacy, low toxicity, and specificity toward liver transfection. The hydrophilic 2-deoxy-2-methacrylamido glucopyranose unit enhanced particle stability in salt and the N-(2-aminoethyl) methacrylamide) unit, each monomer containing a primary amine, improved gene delivery. Another library approach described in the literature describes the synthesis of a family of 144 polymers that are derivatives of PEI, synthesized from PEIs and acrylates [34]. High-throughput screening of the polymeric nanoparticles formed from these polymers and DNA was utilized to discover optimal formulations that had higher efficacy and lower cytotoxicity than 22 kDa linear PEI. Improved in vivo activity was also observed and the chemical composition of the polymer may have led to potential organ specificity. Other polymer library approaches have also been reported in the literature. For example, 1,536 core-shell nanoparticles were formulated with structurally different, epoxide-containing block copolymers and amine monomers for the intracellular delivery of siRNA [35]. Crosslinkers with potential buffering capacity performed better, presumably to enhance endosomal escape. Poly(β-amino ester)s (PBAEs), tertiary amine-containing polymers capable of binding with nucleic acids and providing pH buffering, can also be synthesized with finely tuned structure to generate a combinatorial library with differential hydrophobicity, buffering capacity, and physicochemical properties that can meet the requirements of successful transfection [36]. Differential structures have been shown to affect DNA binding affinity [37], cellular uptake [26], and pH buffering capacity [38]. Interestingly, screening of a PBAE polymer library also enables discovery of cell type-specific transfection. For example, Shmueli et al. showed that PBAEs with defined structures can selectively transfect endothelial cells from macro- or microvasculature in comparison to epithelial cells (Figure 2) [39]. More recently, a bioreducible variation of PBAEs enabled efficient delivery of siRNA to cancer cells [40].
Figure 2.
Non-viral nanoparticles formulated with a library of PBAE polymers demonstrate high efficacy for transfection of human endothelial cells. Additionally, polymer structure can tune cell-type efficacy and demonstrates (A) a strong correlation in transfection between macrovasculature (HUVEC) and microvasculature (HREC), but (B/C) weaker structural correlation between endothelial and epithelial cells. Adapted from ref [39].
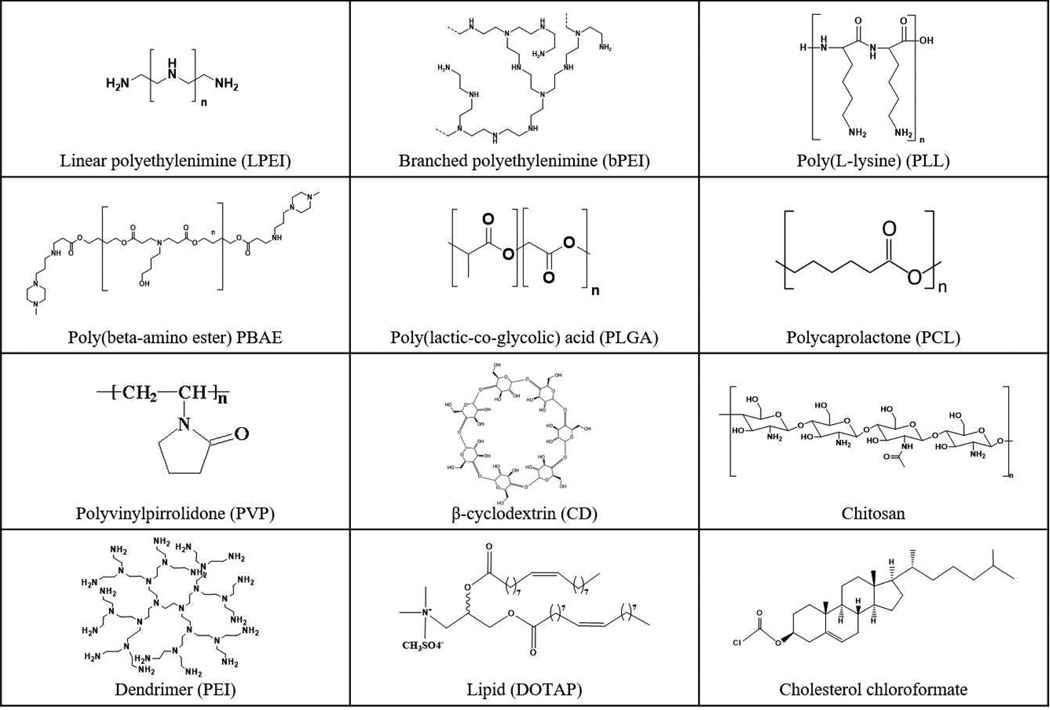
Several important aspects must also be carefully considered at the systemic level when designing next-generation non-viral nanoparticles for gene therapy. First, nanoparticles must be stable in bodily fluids to prevent aggregation and clearance, retaining a small size to facilitate transport and enable cellular uptake. PEGylation is one of the most widely used method to modify the surface of nanoparticles to promote stability [41]. In addition, nanoparticles specifically targeted to the diseased tissue and cells would have an improved therapeutic window with higher dosage at the target site, improving efficacy, and lower dosage elsewhere, reducing side effects. Cellular targeting is most often achieved by exposing diseased tissue-specific ligands on the surface of nanoparticles, but researchers have also shown that nanoparticle biomaterial composition, including the chemical structure of constituent polymers, can direct tissue or cell specificity [42]. Non-canonical peptide motifs with affinity to specific surface receptors, such as apo-transferrin on glioma capillary endothelial cells [43], integrin on endothelial cells [44], and prohibitin on adipocytes [45], have been identified via phage display and computational approaches, the use of which is also of particular interest in the field of nanoparticle targeting. Finally, other features of a gene delivery nanoparticle, such as the promoter on delivered plasmid DNA, can also drive cellular and tissue targeting [46]. Various non-viral gene delivery vectors shown in Figure 3 have been investigated with their standard structures and also in modified forms for enhanced non-viral gene delivery to various targets, including endothelial cells to modulate angiogenesis.
Figure 3.
Chemical structures of various non-viral vectors that can be used to fabricate gene therapy nanoparticles to modulate angiogenesis.
3. Pro-angiogenesis
3.1 Angiogenic Factors
The diffusion of oxygen through tissues is limited to approximately 1 – 2 mm, requiring that most tissues maintain their growth close to established vasculature [47]. However, the disruption of blood flow found in ischemic and infarct regions produces a state of hypoxia in previously vascularized regions. One method by which cells attempt to compensate for this is through hypoxia-inducible factor (HIF) signaling, specifically HIF-1 or 2. Many angiogenic factors are upregulated by HIF, including vascular endothelial growth factor (VEGF), placental growth factor (PlGF), inducible nitric oxide synthase (iNOS), Ang2, and maturation factors like Ang1 and platelet-derived growth factor-BB (PDGF-BB), which are thought to play a critical role in the revascularization process of ischemic tissue. In fact, the overexpression or pharmacological stabilization of HIF-1α in mouse models of myocardial infarction was found to decrease tissue damage and improve infarct revascularization and energy levels of infarct tissue [48–50].
Nitric oxide (NO) is a short lived, pleiotropic signaling molecule involved in a variety of functions including regulation of vascular tone, inflammation, and angiogenesis to name a few. NO generation is catalyzed by homodimeric proteins known as nitric oxide synthases (NOS) [51]. In mammals, there are three isoforms of NOS involved in the formation of NO, neuronal NOS (NOS1 or nNOS), inducible NOS (NOS2 or iNOS) and endothelial NOS (NOS3 or eNOS). NOS1 and NOS3 are named for their constitutive expression in specific tissue, brain neurons for NOS1 and endothelial cells for NOS3, although expression in other tissues has also been observed [52]. In contrast, NOS2 expression is low in most tissues unless induced under specific conditions, including ischemia through HIF-1α. Inhibition to NOS3 or NOS2 was found to inhibit the angiogenesis, collateral formation, and pericyte coverage in rat hindlimb ischemia models [53–55]. NOS is also essential for the activities of several important angiogenic factors, including VEGF, fibroblast growth factor (FGF2), and Ang2, as inhibition of NO was found to ablate their angiogenic responses.
Following the initial functional description of vascular endothelial growth factor A (VEGF-A; originally vascular permeability factor (VPF)) by Senger and Dvorak in 1983, the VEGF family of growth factors quickly become known as key angiogenic factors [56]. Signaling by these growth factors is mediated by a family of receptor tyrosine kinases (RTKs) consisting of VEGFR1 (Flt1), VEGFR2 (KDR or Flk1), and VEGFR3 that show varying specificity to certain members of the VEGF family. VEGF-A is known to possess at least 13 splice isoforms that exhibit different receptor affinities and downstream signaling properties [57, 58]. VEGF165 appears to be the most important as it is expressed to a greater amount than the other splice isoforms and alone is sufficient for the normal development of mice [59]. Given its central role in many vascular processes, it is not surprising that changes in VEGF expression can have profound consequences in ischemic diseases. As noted above, VEGF-A expression in hypoxic tissues is upregulated by the actions of the HIF transcription factor and has also been observed in cells treated with other growth factors, including FGF2, PDGF, and transforming growth factor (TGFβ) [60, 61]. Additionally, VEGF-A mRNA was found to increase within ischemic regions of myocardium from occluded pig hearts [62].
Placental growth factor is a homolog of the VEGF sub-family with increasing implications in pathological angiogenesis [63]. Notably, the loss of PlGF was observed to decrease angiogenesis in wound sites, cancer, and ischemic myocardial, retinal, and peripheral tissues [64]. Conversely, administration of recombinant PlGF in a mouse ischemic hindlimb models was demonstrated to have a greater therapeutic response than VEGF-A by inducing the growth of second- and third-generation collateral vessels [65]. Delivery of a plasmid encoding PlGF in rat models of myocardial infarction was also found to improve revascularization, reduce infarct size, and increase the survival of cardiac myocytes [66]. In part, this effect may be attributable to the stimulation of VEGF, PlGF, Ang1, and Ang2 expression in various regions in or around the infarct zone. Moreover, PlGF-mediated angiogenesis does not produce the excessive permeability and edema associated with VEGF over-expression, allowing for the formation of lasting neovasculature [65].
The fibroblast growth factors (FGFs) consist of a family of 22 factors that share significant sequence similarities. However, FGF1 and FGF2 are well documented in angiogenesis and will remain the focus of this review. Signaling by FGF1 and FGF2 in angiogenesis is primarily mediated through its extracellular interactions with FGF receptors 1 (FGFR1) and 2 (FGFR2) [67–69]. FGFs are thought to be one of the first factors involved in the initiating phases of angiogenesis [70–72] and their activation of FGFRs has also been shown to influence EC survival, proliferation, and migration [73]. Moreover, FGF2 has also been shown to enhance the angiogenic response to VEGF by increasing the expression of VEGFR2 in mouse models and improving VEGF-mediated vessel stability [74]. The activity of FGF has also been demonstrated to increase the expression of HIF-1α, suggesting the presence of a positive feedback loop between FGF2 and HIF-1α expression during hypoxia. In contrast to VEGFR2, however, FGF receptors are expressed in a variety of tissues in addition endothelial and mural cells and, therefore, are less specific to angiogenesis [75]. Moreover, mice lacking either FGF1 or FGF2 remain viable and fertile, suggesting that individual FGFs may possess overlapping or restricted functions [76]. As such, these and other considerations have possibly contributed to the fact that no FGF-specific inhibitors have been approved by the FDA and emphasize potential challenges in targeting FGF-signaling for therapeutic applications.
Hepatocyte growth factor (HGF; or scatter factor (SF)) has been found to influence differentiation, migration, proliferation, scattering, and survival in several cell types, including endothelial cells and pericytes [77, 78]. HGF signal transduction is mediated through the c-MET receptor, the activation of which subsequently leads to the phosphorylation of several downstream effectors common to several receptor tyrosine kinases (RTKs), such as GRB2, SRC, PLCγ, PI3K, and STAT3 (reviewed in [79]). In endothelial cells, HGF signaling was found to stimulate migration, proliferation, tube formation, and rabbit corneal neovascularization, demonstrating the growth factor’s potent angiogenic properties [80]. Based on these and other findings, HGF was subsequently investigated as a potential candidate for therapeutic angiogenesis. Intramuscular injection of naked HGF peptides in rat and rabbit hindlimb ischemia models was found to significantly improve collateral artery growth and blood flow to the limb [81].
Platelet-derived growth factors (PDGFs) improve survival and reduce the permeability of new vessels by recruiting pericytes to the nascent vasculature via a chemotactic gradient [8]. The PDGF family consists of several related members, PDGF-AA, PDGF-BB, PDGF-CC, and PDGF-DD and two receptors, PDGFRα and PDGFRβ. The activities of the PDGF family have been demonstrated to be important in the healing processes of ischemic tissues. Notably, inhibition of PDGFRα and PDGFRβ in models of myocardial infarction was found significantly impart the post-infarct scar formation associated with the tissues repair [82]. Antibody blockade of PDGFRβ in this model was found to inhibit mural cell coverage and prolong the hemorrhaging of vessels within the infarct region. Interestingly, overexposure to PDGF-BB under certain conditions can have detrimental effect on angiogenesis, as the application of exogenous PDGF-BB alone has been shown to destabilize vessels by disrupting existing PDGF-BB gradients and inhibiting mural coverage of neovasculature [83]. However, when coadministered with other angiogenic growth factors, such as VEGF or FGF, PDGF-BB improved wound healing and revascularization [84, 85].
Angiopoietins are a family of secreted glycoproteins that play a variety of roles in angiogenesis. Ang1 and Ang2 are the most studied of these factors and function through their competition for the Tie2 receptor. Ang1 is primarily secreted by perivascular and mural cells and activates EC-bound Tie2 via paracrine signaling. The binding of Ang1 to Tie2 leads to downstream signals responsible for maintaining vessel stability, survival, and quiescence [86–88]. In contrast, Ang2 is a weak Tie2 agonist and is more often thought to compete with Ang1 to deactivate Tie2, which leads to a reduction in EC-EC contacts and stimulates the release of vessel-associated pericytes [5]. In combination with high levels of VEGF, these effects of Ang2 ready the vessel for the formation of VEGF-induced sprouts [89]. Without VEGF, however, the loss of cell-cell contacts and mural cells leads to the destabilization and eventual regression of the vessels. In the treatment of ischemic tissues, intra-muscular injection of plasmids encoding Ang1 in rabbit models of hindlimb ischemia was found to improve revascularization [90]. Also, co-injection of Ang1 and VEGF-A, although still potent in stimulating new vasculature, generate far less permeable vessels [91]. The co-injection of plasmids encoding VEGF-A and Ang1 into the muscles of rabbit hindlimb ischemia models was found to provide a therapeutic benefit in excess compared to either factor delivered alone [92].
The hedgehog (Hh) signaling pathway has been demonstrated to possess pro-angiogenic activity in adult animals by upregulating the expression of NOS2 and netrin-1 [93]. These effects are initiated by the binding of SHh to patched1 (PTCH1), which blocks the inhibition of another membrane protein, smoothened (SMO). Mechanistically, SHh appears to influence angiogenesis indirectly, as SHh protein had no effect on the proliferation and migration of cultured ECs. Alternatively, intramuscular injection or corneal implantation of myristoylated-SHh was found to stimulate neovascularization in mouse hindlimb ischemia and corneal neovascularization models respectively, an affect that the authors attributed to increased expression of VEGF, Ang1, and Ang2 in the mesenchymal tissue [94]. Other in vivo studies, however, suggest a more complicated involvement of SHh signaling in angiogenesis. Specifically, endogenous Hh signaling was found to worsen the recovery of mouse models of myocardial ischemia, as evidenced by the improved recovery observed following treatment with an inhibitor of SMO [95]. In contrast, exogenous SHh treatment in post-ischemia myocardium was found to decrease infarct size, and improve recovery [96].
Adrenomedullin (AM) is a pro-angiogenic, 52 amino acid peptide secreted from endothelial and mural cells in response to hypoxia, hypertension, shear stress, and other inflammatory signals [97–99]. Binding of AM to calcitonin receptor-like receptor (CRLR) leads to the activation of several downstream effectors related to the proliferation (Ras/Raf/Mek/ERK), migration (FAK), survival, (PI3K/AKT), and nitric oxide production in endothelial cells and migration (via PI3K) and vasodilation (by PKG and PKA) in the associated smooth muscle cells (reviewed in [100]). Both CRLR and AM expression is upregulated by HIF-1α and works synergistically with VEGF, demonstrating a clear importance in responding to hypoxia [101, 102]. In addition, adrenomedullin was found to increase endothelial differentiation of bone marrow monocytes and improve the mural cell coverage of new vessels in a rat hindlimb ischemia [103]. Therefore, the ability of adrenomedullin to recruit new ECs and mural cells to the sites of neovasculature implicates it as a strong candidate for angiogenic therapy.
3.2 Applications in Gene Delivery
Although a wide range of biomolecules that promote angiogenesis has been investigated as described in section 3.1, a few potent factors have been most widely used for gene therapy to promote vasculature growth. The two main applications of pro-angiogenic gene therapy utilizing non-viral nanoparticles are for treatment of ischemia and for regenerative medicine / tissue engineering.
3.2.1 Ischemia
According to the World Health Organization, circulatory diseases remain the primary cause death worldwide, accounting for nearly 31% of all fatalities [104]. The main concerns in these conditions are ischemic complications resulting from inadequate blood supply provided to a particular tissue, restricting the available nutrients and oxygen for the tissues metabolic needs. Specific conditions are named after the locations affected: peripheral arterial disease (PAD) for extremities, coronary artery disease in the heart, and cerebral artery diseases in the brain. In severe cases, this lack of blood flow leads to the death of the tissue, known as an infarction, which can be life threatening for particular organs, ie. heart attack (myocardial infarction) or ischemic stroke (cerebral infarction) [105]. Often these diseases begin with the narrowing of arterial vessels, known as stenosis, with little or no clinical manifestations. Although congenital defects can contribute to this vessel narrowing, it most often arises as a result of endothelial dysfunction, such as improper regulation of vascular tone, and eventually leads to the formation of atherosclerotic lesions [106]. These sites are characterized by the accumulation of oxidized low-density lipoprotein plaques and inflammation within the vessel intima. The eventual rupture of these plaques and increased intimal distance within atherosclerotic vessels can lead to occluded vessels or impaired gas exchanged with nearby tissues, further reducing the availability of oxygen and nutrients to affected regions.
The endogenous restoration of blood flow to ischemic tissues is primarily the function of angiogenesis and arteriogenesis. The outgrowth of collateral vessels during arteriogenesis appears to be independent of hypoxia and originates in non-ischemic tissues. Instead, these events are thought to be initiated by the changes in shear stress associated with the perturbed blood flow within atherosclerotic vessels [107]. In contrast, sustained hypoxia instigates the production of pro-angiogenic signals and new capillary networks within the ischemic region. To improve patient outcome, medical intervention, often in the form of angioplasties, bypasses, stents, or anti-coagulant therapy, are usually employed as well [108–110]. However, these approaches are not always sufficient to restore normal tissue function. As a potential alternative, an increasing number of investigations have looked into use of therapeutic angiogenesis in order to establish new vessels within the ischemic regions. Notably, several of these studies have focused the delivery of genes encoding pro-angiogenic factors to achieve sustained expression of these signals within ischemic and infarct sites. Here we review several examples of such treatments using non-viral delivery methods.
Many studies have utilized viruses to deliver genes to promote angiogenesis to ischemic tissue. Adenovirus-mediated delivery of HIF-1α or HGF resulted in improvement of heart function in chronic myocardial ischemia and postinfarct heart failure model in swine and rats [81, 111, 112]. Other variations include a co-delivery of VEGF164 and PDGF-BB in mice ischemic hindlimb [113] and repeated injection of HGF in rat postinfarct heart [114] using adenovirus. Another group studied improved rabbit cardiac function following myocardial infarction upon implantation of a sheet of adipose-derived stem cells overexpressing VEGF by baculovirus transduction [115].
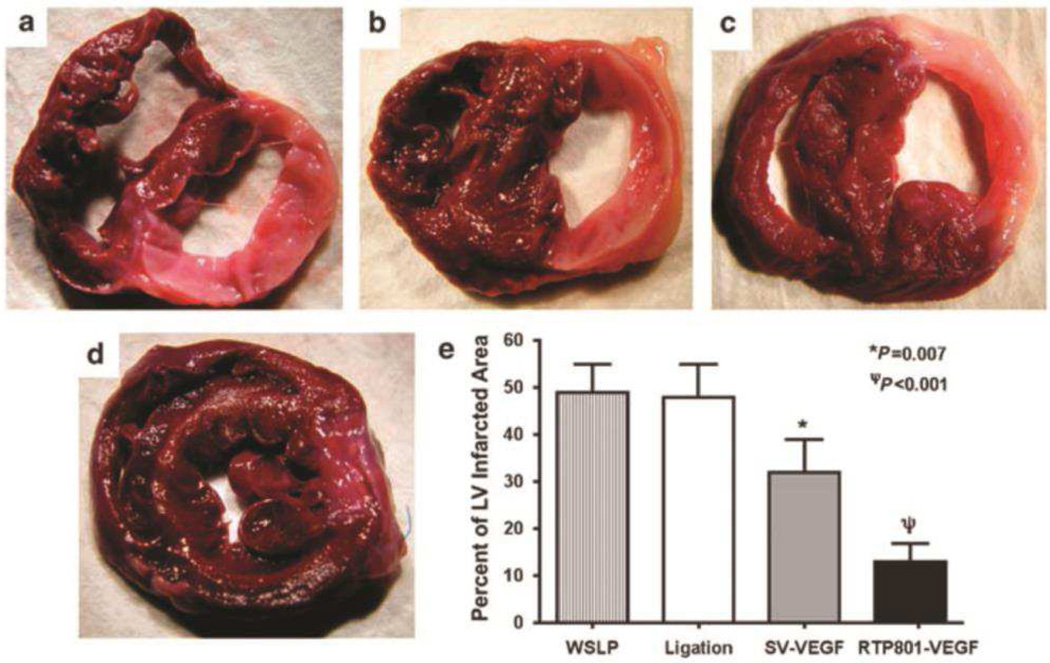
While these therapeutic outcomes using viral vectors are notable, non-viral gene delivery has seen significant success in addressing ischemia. Different combinations of non-viral approaches and nucleic acid cargos have been tested in ischemic animal models. In the majority of studies, nanoparticles formed with polymers or lipids are delivered directly in vivo to transfect target cells. PEI, while widely used in its native form to deliver nucleic acids for various applications, has also been combined with polysaccharides or lipids to create new biomaterials. For example, 250 nm nanoparticles consisting of pVEGF and 1800 Da PEI conjugated with heparin at N/P 10 resulted in over a 3-fold increase in capillary density in mouse ischemic limb model than non-modified PEI [116]. In another study, chitosan-grafted PEI with eprosartan as the targeting moiety to angiotensin II type 1 receptor was used to deliver VEGF165 intramyocardially and showed 7% improvement in ejection fraction and 1.6-fold increase in vessel density than PEI control in rat myocardial ischemia model [117]. Han et al. developed a new system of water soluble lipopolymer (WSLP) by combining branched PEI with hydrophobic lipid anchor cholesterol chloroformate [118]. Using this vector, Yockman et al. successfully delivered hypoxia-inducible VEGF165 plasmid intramyocardially to a rabbit myocardial infarct model in a separate study and reduced the infarct size by 36% compared to ligation only control (Figure 4) [119].
Figure 4.
Size of left ventricular infarct following (a) no injection, or injection of (b) water-soluble lipopolymer (WSLP) alone, (c) WSLP with plasmid encoding constitutively-expressed VEGF, and (d) WSLP with plasmid encoding ischemia-inducible VEGF. Infarcted fibrotic tissue appears whitish-pink. (e) The percentage of the ratio of infarcted to non-infarcted left ventricle. Adapted from ref [119]
Other biomaterials used to create nanoparticles by plasmid DNA condensation include poly-arginine and elastin-like polypeptide [120, 121]. Specifically, Dash et al. developed a hydrogel that contains hollow elastin-based spheres carrying plasmid endothelial nitric oxide synthase (NOS3) and IL-10, which showed approximately 3-fold increase in perfusion ratio of ischemic to non-ischemic limb and in blood vessel surface density in mouse hindlimb ischemia. Meanwhile, encapsulating DNA-condensed nanoparticles in hydrogels, such as agarose, is known to enhance the duration of gene transfection in vivo [122]. The advantage of sustained expression of an angiogenic gene, such as VEGF, is that the gene product can not only initiate vessel formation but also maintain vessels once they are formed [123]. Additional types of nanoparticles that encapsulate plasmid DNA are formulated with synthetic polymers such as PLGA and Pluronic® L64, which has successfully delivered pVEGF165 and pHIF-1α to increase capillary density by approximately 38% in rabbit myocardial and 67% in mouse hindlimb ischemia, respectively [124, 125].
While these non-viral vectors are injected locally at the ischemic site and hence invasive, other studies utilized an ultrasound-targeted microbubble destruction (UTMD) method to deliver therapeutic genes systemically. Without efficient targeting, systemic delivery of VEGF may bring adverse side effects due to undesired angiogenesis in off-target sites [123]. In UTMD-mediated gene therapy, microbubbles can be visualized and destroyed at the target site by ultrasound or contrast echocardiography, releasing the loaded gene at the site of interest to enter local cells with disturbed cell membranes due to cavitation [126]. Lipid microbubbles carrying plasmid DNA encoding VEGF, stem cell factor (SCF), and stromal cell-derived factor-1 (SDF-1), or micro RNA, such as miR-126 that inhibits negative regulators of VEGF signaling, have been investigated in in vivo models of myocardial infarct, ischemic hindlimb, as well as ischemic brain injury after stroke [127–130].
Another popular approach of treating diseases through gene therapy is harnessing cell therapy; cells are transfected ex vivo with non-viral vectors and subsequently the cells are locally injected to serve as either a depot of therapeutic factors or a source of tissue regeneration (Figure 1). For an angiogenesis application, stem cells as well as somatic cells have both been genetically modified. Mesenchymal stem cells (MSCs) transfected with dextran – pAdrenomedullin complex, bile acid-conjugated PEI – hypoxia-inducible pVEGF polyplex, or hollow mesoporous organosilica – pHGF nanoparticles demonstrated increased neovascularization, reduced infarct size, and improved cardiac function in rats following transplantation to myocardial infarct tissue [131–133]. Specifically, dextran-pAdrenomedulin complexed at 2.6 w/w ratio formed nanoparticles with 200 nm size and 12 mV surface charge that not only exhibited 2.5-fold increase in capillary density and 40% reduction in infarct size, but also higher recovery rate of heart function, including left ventricle end-diastolic pressure (EDP) and fraction shortening (FS). Also, PLGA – PEI – pVEGF as well as PBAE – pVEGF nanoparticles were shown to successfully transfect human MSCs for transplantation in mouse ischemic hindlimb [134, 135]. MSCs transfected with PBAE - pVEGFP significantly increased the percentage of limb salvage to 50% while decreasing limb loss to 20%. Endothelial progenitor cells transfected with PEI-coated quantum dot – pVEGF165 nanoparticles were intramuscularly injected in mouse ischemic limbs and resulted in higher blood perfusion level [136]. Somatic cells, such as skeletal myoblasts and endothelial cells, were also utilized as carriers of angiogenic factors. Ye et al. tested myoblasts transfected ex vivo with PEI polyplex as well as liposomes composed of cholesterol and DOTAP to deliver VEGF165 in rat heart suffering acute infarction, and showed increased blood vessel density and ejection fraction [137, 138]. It is important to note that two different types of vessels, arterioles (smooth muscle cells) or capillaries (skeletal muscle cells), can be formed based on differential expression of VEGF from transplanted myoblasts or shear stress in the microenvironment [139]. Cho et al. compared the efficacy of nanoparticles formed with different non-viral vectors to transfect human umbilical vein endothelial cells (HUVECs) with VEGF and transplanted HUVECs to treat mouse limb ischemia. [140]. HUVECs transfected with PBAE-pVEGF showed 50% limb salvage in contrast to 30% with lipo-pVEGF and 12.5% with PEI-pVEGF. As described earlier, the difference in particle size, surface charge, cellular uptake, and endosomal escape efficiency between PBAE, PEI, and lipid nanoparticles could affect the overall transfection efficiency and thus the therapeutic outcome of limb salvage rate. The optimal PBAE nanoparticles used in this study were formulated at 30 w/w ratio of PBAE to pVEGF, which resulted in a 200 nm in size and a −1.26 mV zeta potential, in contrast to PEI nanoparticles that are typically formulated to have a highly positive zeta potential. Meanwhile, two separate studies evaluated PLGA nanoparticles with micro RNA (miR132) using different methods to transfect endothelial cells in order to cell survival and vessel growth. Gomes et al. coated 170-nm PLGA nanoparticles with protamine sulfate that can complex with miR132 and facilitate cellular uptake, and transfected endothelial cells showed 3-fold higher proliferation of cells and blood flow than those transfected with negative control nanoparticles following transplantation in mouse hindlimb ischemia model [141]. In another approach, Devalliere et al. used a double emulsion method to encapsulate spermidine – miR132 into PLGA nanoparticles, which were utilized to transfect HUVECs [142]. When these transfected HUVECs were placed within a subcutaneous collagen gel plug in mice, they mediated 2-fold vessel growth compared to a lipofection control.
3.2.2 Tissue Regeneration
New tissue formation requires sufficient level of oxygen and nutrients. Limitations in diffusional distance of oxygen and nutrients necessitate the matrix to have a highly vascularized network. Hence, tissue growth in a natural matrix, in situ scaffold, or ex vivo artificial organ must be accompanied by angiogenesis. Skin regeneration during the wound healing process and bone regeneration are examples where an increased level of angiogenesis could accelerate and enhance the outcome while preventing necrosis [143].
Viral vector-mediated gene transfer has been shown to be effective in promoting angiogenesis and enhancing wound healing and osteogenesis in numerous studies. For example, adenovirus expressing insulin-like growth factor (IGF-1) and retrovirus expressing cyclooxygenase-2 (Cox-2) induced vessel growth through VEGF- and prostaglandin-dependent pathways in murine wound and rat femoral fracture model, respectively [144, 145]. Adenovirus-mediated VEGF121 expression in rabbit femur re-vascularized the necrotic region and resulted in bone formation [146]. Applications of ex vivo gene transfer combined with cell therapy include adenovirus-mediated angiopoietin-1 gene-modified bone marrow MSCs for use in a rat skin wound model, osteoblasts expressing adenovirus-mediated VEGF and seeded to a chitosan/hydroxyapatite scaffold, and bone marrow cells expressing lentiviral-mediated HIF-1α in a rat bone regeneration model [147–149].
However, lentivirus and retrovirus suffer from potential mutagenesis or uncontrolled angiogenesis from gene insertion into host’s genome while adenovirus does not overcome the limitation of transient expression. Recent efforts are underway to expand the repertoire of non-viral nanoparticles for angiogenic gene therapy in tissue regeneration. Direct intradermal injection of nanoparticles formulated with PBAE and sonic hedgehog (SHh)-encoding plasmid resulted in 100% wound closure area at day 10, significantly greater than 85% for PBS and nonfunctioning plasmid controls [150]. SHh is a morphogen involved in tissue regeneration via activation of angiogenic signaling pathways, as the study shows 2~3-fold increased expression level of VEGF and SDF-1α.
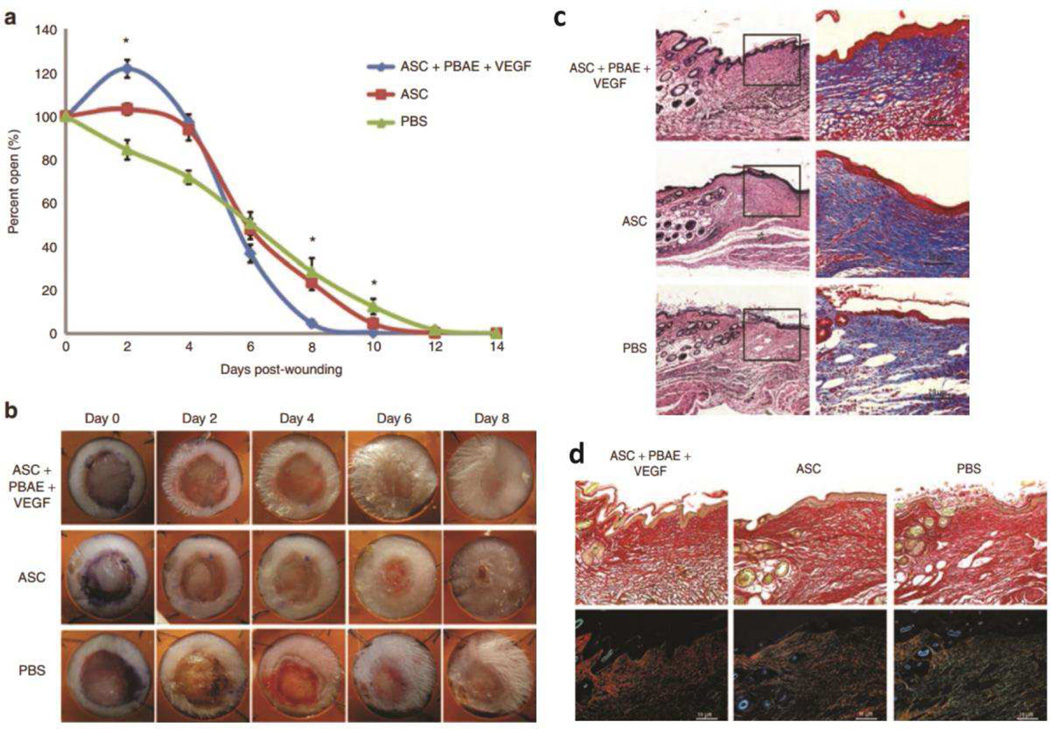
Additionally, ex vivo transfection followed by cell transplantation has also been a popular route of gene therapy for tissue regeneration, similar to applications in ischemic disease models. One of the studies by Dr. Fan Yang’s group utilized adipose-derived stromal cells (ASCs) in a mouse wound-healing model [151]. ASCs transfected with pVEGF using PBAE polymer at 30 w/w ratio of polymer to DNA were injected intradermally at the four quadrants of the wound. The PBAE-pVEGF-transfected ASCs treated wound showed not only accelerated angiogenesis and 20% greater wound closure at day 8 than non-modified ASCs, but also increased cellularity and collagen deposition (Figure 5). Nanoparticles formed at 30 w/w with the specific PBAE structure used were able to transfect less than 10% of ASCs, which was still greater than lipoplexes. Different PBAE structure may transfect ASCs with higher efficiency to enhance therapeutic effect, as shown by transfection screening of PBAE library on ASCs from a separate study [152]. A recent study reported that using PEI-pVEGF nanoparticles at N/P 7 in hyaluronic acid hydrogels with 60-μm pore led to 50% decrease in open diabetic wound area than non-porous hydrogels, possibly due to increased surface area contact for infiltrating host cells with released nanoparticles [153]. In bone tissue engineering, a recent work using a collagen scaffold containing branced PEI (bPEI) complexed with a single gene encoding PDGF-B at N/P 10 ratio showed 40% bone formation in the defect by volume and 50-fold increase in connective density compared to control, leading to bone repair in rats [154]. However, Curtin et al. showed that among collagen scaffolds carrying two genes expressing bone morphogenetic protein 2 (BMP2) and VEGF by bPEI only, nanohydroxyapatite (nHA) only, or mixed vectors, nHA only scaffold resulted in greater than 2-fold increase in bone nucleation area over bPEI (N/P 7) only scaffold condition in vivo [154, 155]. Although branched PEI (bPEI) has greater buffering capacity and charge density than linear PEI, which could lead to higher transfection at the risk of potential cytotoxicity (Figure 3), most studies followed a similar N/P between 7 and 10 to formulate nanoparticles. For many polymeric gene delivery systems, an intermediate N/P ratio is found optimal, where an N/P ratio that is too low (close to neutrality) results in polyplex nanoparticles that are unstable and that aggregate. On the other hand, as the amount of biomaterial is increased, nanoparticles that are more stable and that have improved uptake and transfection form. Yet, at still higher N/P ratios, biomaterial-mediated toxicity can become apparent, especially with highly charged, non-degradable biomaterials such as PEI [156]. While N/P ratio represents a true charge ratio when the polymer amine groups are fully charged, for certain cationic polymers the majority of the amines are tertiary amines rather than primary amines and the polymer is not highly charged at neutral pH. In these cases, the N/P ratio required for charge neutralization of anionic DNA and for the formation of compact polyplex nanoparticle formation can be much higher. These examples demonstrate the potential effectiveness of gene delivery nanoparticles using multiple types of non-viral vectors.
Figure 5.
(a) Percent of open wounds evaluated every 2 days post-wounding and treatment with PBAE/VEGF nanoparticle-transfected ASCs, non-modified ASCs or PBS control. (b) Wounds treated with PBAE-pVEGF nanoparticle transfected ASCs showed accelerated closure with full epithelialization observed by day 8. (c) H&E and Masson’s trichrome staining shows increased cellularity and collagen deposition in the dermis treated with ASC-transplanted groups than PBS. (d) The PBAE-pVEGF-transfected ASC-treated group shows the most abundant mature collagen fibers (red-orange), whereas PBS-treated group showed the highest level of immature collagen fibers (green-yellow). Adapted from ref [151] .
4. Anti-angiogenesis
4.1 Anti-angiogenic Factors
The vascular basement membrane (vBM) is a collection of extracellular macromolecules, including collagen, fibronectin, laminin, and heparan sulfate that stabilizes the structure of vasculature. Under angiogenic conditions, however, the vBM is partially degraded in a tightly regulated fashion to allow for formation of new angiogenic sprouts while still maintaining enough structure to function as a substrate for adhesion and migration of the growing vessel. In addition to its structural role, the proteolytic degradation of the vBM generates various bioactive fragments called matricryptins to regulate endothelial cell proliferation, migration, and survival as well as to help limit excessive angiogenesis during wound healing, inflammation, and disease processes. Several extracellular matrix (ECM) components can contribute to the generation of these fragments, notably, collagens IV (arresten; canstatin; tumstatin) and XVIII (endostatin) and heparan sulfates (endorepellin) [157].
Type IV collagen is a non-fibrillar component that makes-up almost half of all basement membranes and is the source of several anti-angiogenic matricryptins, including arresten (26 kDa), canstatin (24 kDa), and tumstatin (29 kDa) [158]. Treatment of endothelial cells in vitro with any of these three fragments inhibits the migration and proliferation of these cells as well as the ability to form tube-like structures in matrigel substrate. The fragments also demonstrated in vivo efficacy by inhibiting the vascularization of injected matrigel plugs and the growth and metastasis of xenografts in nude mice [159–162]. Endostatin (20 kDa) is another matricryptin derived from collagen, specifically type XVIII. Similar to the collagen type IV matricryptins, endostatin was found to inhibit the migration, but not the proliferation, of endothelial cells in vitro and disrupt tumor vascularization and growth in mice [163].
In addition to the collagens, the breakdown of perlecan, major heparan sulfate proteoglycan, and plasminogen releases the anti-angiogenic fragments endorepellin and angiostatin respectively. Endorepellin is an 80 kDa fragment derived from the C-terminal domain V of perlecan that was found to inhibit EC migration and tube formation [164]. Angiostatin (38 kDa) is a potent anti-angiogenic cleavage fragment derived from plasminogen. Treatment of endothelial cells with angiostatin was found to inhibit migration and induce apoptosis [165, 166].
Mechanistically, all of the above molecules are thought to mediate their effects, at least in part, through interactions with various integrin heterodimers. Arresten binds integrin α1β1 [162], canstatin binds integrins αvβ3 and αvβ5 [167], angiostatin and tumstatin bind αvβ3 [161, 168], endostatin binds αvβ3, αvβ5, and α5β1 [169], and endorepellin binds α2β1 [170]. The disruption of integrin-ECM interactions disrupts cellular adhesion and migration and has been shown to regulate the trafficking and signal duration of growth factor receptors [171]. In addition to the large protein fragments described above, a large number of short anti-angiogenic peptides have been discovered, both endogenous (ie, having natural sequences as in the proteins of origin) and biomimetic. Bioinformatics-based systems biology methodology lead to the discovery of over 100 novel peptides that inhibit proliferation and migration of endothelial cells in vitro [44]. These peptides were derived from different protein domains, such as collagen IV, thrombospondin-1, CXC chemokines, serpin, somatotropin, and tissue inhibitors of matrix metalloproteinases (TIMP). Structure-activity relationship (SAR) investigation yielded biomimetic peptides with optimized anti-angiogenic activity [172]. Selected peptides and their biomimetic derivatives were tested in vivo in cancer xenograft models [173, 174] and in models of ocular neovascularization [175]. Some peptides from different classes exhibit synergy that could be explored therapeutically [176]. Anti-angiogenic peptides for cancer applications have been reviewed in [177]. Importantly, some of the peptides exhibit anti-lymphangiogenic activity and thus could be useful as anti-metastatic agents or immuno-suppressive agents [178, 179]. Each of these peptides and proteins are potential agents that can be used for anti-angiogenesis therapy and that can also be genetically encoded in DNA. Through non-viral nanoparticle-mediated transfection, novel combinations of these therapeutics and others could be expressed at target tissue sites and over time. An advantage of non-viral gene delivery that can facilitate combination therapy with the factors described is that multiple plasmids and large plasmids can be delivered within a single non-viral nanoparticle [180], unlike with viral gene therapy where DNA carrying capacity is more stringently limited.
As mentioned, one of the initial hallmarks of angiogenesis is the breakdown of the vascular basement membrane to allow for the invasion of neovasculature into adjacent tissue. In humans, this activity is catalyzed by a tightly regulated family of 23 zinc-dependent endopeptidases known as matrix metalloproteinases (MMPs) which are secreted in response to growth factor signaling and are essential for initiating the process of angiogenesis (reviewed in [6]). MMP pro-angiogenic functions are controlled by the generation of anti-angiogenic matricryptins (described above) as well as endogenous inhibitors of MMP activity in the form of TIMPs [157, 181]. TIMPs are a family of four proteins (TIMP 1–4; 20–29 kDa) that bind to active sites of MMPs and inhibit their proteolytic activities. As such, TIMPs have been investigated as natural sources of anti-angiogenic therapies and have been shown to inhibit MMP-related vascular effects [182, 183]. TIMPs also possess non-MMP dependent inhibition of angiogenesis through the disruption of growth factor induced signaling [184, 185]. The anti-angiogenic properties of TIMPs have led to investigations of their use in cancer, with several studies demonstrating an inhibition of tumor metastasis [186–188]. However, other reports indicate a poorer prognosis associated with higher levels of TIMPs in some cancers. For instance, higher levels of TIMP1 has been shown to inhibit MMP9-mediated tumor regression by disrupting a pro-inflammatory response, while TIMP4 over-expression in breast cancers was associated with reduced patient survival [189, 190]. TIMPs represent promising novel therapeutic entities that can be genetically encoded for intracellular delivery and expression by gene delivery nanoparticles to treat angiogenesis-dependent diseases.
As described above, the weak kinase activity observed for VEGFR1 has led to the widespread hypothesis that VEGFR1 primarily functions as a sink for VEGFs, thereby attenuating their angiogenic signaling. Interestingly, a soluble form of VEGFR1 (sVEGFR1) secreted by both ECs and monocytes has been observed in human plasma and serum from healthy individuals [191–193]. sVEGFR1, however, still retains its high affinity for VEGF-A and has been shown to inhibit angiogenesis through the regulation of VEGFR2 activation and inhibition of downstream mitogenic activities [194]. Additionally, sVEGFR1 is able to form nonproductive heterodimers with bound VEGFR1 and VEGFR2, further reducing VEGF-A signaling [195]. sVEGFR1 levels were found to decrease in estrogen receptor positive breast cancer through an estrogen-dependent mechanism and this decrease correlated with an increase in angiogenic activity and tumor progression [196]. Furthermore, sVEGFR1 was found to be overexpressed in some patient samples of colorectal cancer and was associated with recurrence-free survival [197].
Pigment epithelial derived factor (PEDF) is a 50 kDa neurotrophic serine protease inhibitor (serpin) originally isolated from retinal pigmented epithelial cells but has since been identified in a variety of tissues [198]. PEDF was also identified as one of the most potent endogenous inhibitors of angiogenesis discovered thus far [199]. PEDF’s inhibition of angiogenesis targets many aspects of endothelial cell biology through interactions with several cellular surface receptors, including a specific transmembrane protein known as PEDFR. Binding of PEDF to PEDFR activates several downstream signaling pathways involved in the stimulation of apoptosis in ECs through NF-κB-, PPARγ-, and p53-mediated processes while inhibiting their proliferation and migration [199–201]. Additionally, PEDF has been found to regulate the signaling of other growth factor receptors by stimulating the cleavage of their receptors or altering factor expression. Consistent with its anti-angiogenic activity, expression of PEDF is low in most tumor tissues. As such, much interest has surrounded its use as a possible cancer therapeutic. In particular, PEDF not only indirectly disrupts tumor growth through its anti-angiogenic properties, it has also been found to directly inhibit cells from a variety of tumors, including prostate, ovarian, and pancreatic carcinomas, melanomas, glioma, and osteosarcomas (reviewed in [202–204]). Specifically, PEDF was found to reduce tumor cells proliferation and invasion through changes in genes expressions (ie Notch and Wnt signaling) and disruption of MMP activity.
4.2 Applications in Gene Delivery
As described in section 4.1, a number of anti-angiogenic factors can be expressed by exogenous DNA to directly inhibit vessel formation. In addition, the process of suppressing angiogenesis can also be achieved by silencing genes that are responsible for vascularization. In such cases, siRNA or shRNA (collectively RNAi) is delivered [205, 206]. Although DNA and RNAi could have the same end goal, they may require different biomaterial design from the delivery perspective. Two significant and widespread diseases where anti-angiogenic therapy would be of great benefit are cancer and neovascular (wet) age-related macular degeneration (AMD). The utility of gene delivery nanoparticles to treat these angiogenesis-dependent diseases is described in the following sections.
4.2.1 Cancer
In order to maintain the necessary nutrient and oxygen concentrations for survival, tissues in excess of a certain volume (typically 1–2 mm3) must establish an adequate blood supply [47]. This a particularly important for tumors, which are often characterized by rapid, misregulated growth and unique metabolic requirements. As such, extensive neovascularization is often a hallmark of many solid tumors and can be present at various stages of the cancer’s development. This shift to a highly angiogenic state, known as the angiogenic switch, occurs when populations of cells within the tumor acquire the ability to bypass normal regulatory mechanisms and shift the tumor microenvironment in favor of excessive angiogenesis [207]. In addition to its contribution to tumor growth, the angiogenic switch is also a critical step in the progression to metastasis. Notably, newly formed vasculature is characterized by low mural coverage, poorly established basement membrane, reduced EC-EC contacts, and hyper-permeability, all of which can facilitate the intravasation and dissemination of tumor cells into circulation [208].
In 1971 Judah Folkman proposed a hypothesis suggesting that inhibition of angiogenesis could maintain tumor dormancy and reduce the occurrence of metastases [209]. Since then, anti-angiogenic therapy has become a significant arm of patient care in the treatment of solid tumors. A major benefit of vascular-based therapies is that EC rarely undergo transformation relative to their malignant counterparts and thus remain a valid target in a variety of cancer types [210]. Additionally, anti-angiogenic therapy has demonstrated promise in the neoadjuvant setting by restricting tumor growth prior to surgical resection [211]. Many of these agents directly target angiogenic growth factors (bevacizumab or aflibercept) or their receptors (tyrosine kinase inhibitors (TKIs); ie. sunitinib and sorafenib). In several vascular tumors, such as colorectal cancer, non-small lung cancer, and renal cell carcinoma, these treatments have been successful in extending disease free progression and overall survival. Moreover, sorafenib remains the only approved therapeutic for the treatment of hepatocellular carcinoma [209]. Even in these cases, however, anti-angiogenic therapy provides only modest benefits with median durations typically on the order of several months and rarely exceeding a year [212, 213].
In order to facilitate the in vivo delivery of anti-angiogenic genetic cargo, several different types of non-viral nanoparticles have been evaluated. PEI, similar to numerous reports of its application in therapeutic angiogenesis, is one of the most widely studied polymers in cancer therapy targeting angiogenesis. Structural modifications and targeting properties are varied to enhance the delivery efficacy of nanoparticles. PEG-grafted PEI with RGD as the targeting ligand (bPEI-g-PEG-cRGD) was developed by Kim et al. to form tumor endothelial-targeted nanoparticles [214]. cRGD motif has been widely used as a targeting ligand that specifically binds to overexpressed integrins on tumor vasculature, which allows higher accumulation of nanoparticles in tumor by systemic delivery [215]. Intravenous injection of DNA encoding sFlt-1 (sVEGFR1) complexed with bPEI-g-PEG-cRGD at N/P 10 in 5% glucose solution showed 7% of the injected dose accumulating in tumor, more than 3-fold inhibition of tumor growth, and increased survival in colon carcinoma model [214]. The same polymer was also used to form 160-nm nanoparticles with siRNA against VEGFR1 at N/P 10 to colon carcinoma in mice, and suppressed tumor growth by 42% at day 11 compared to control plasmid and non-targeted (PEI-g-PEG) nanoparticles [216]. A similar nanoparticle (cRGD-PEG-PEI) synthesized with a different chemistry was also used to complex with pPEDF at N/P 10 in Opti-MEM™ solution for suppressing colorectal cancer growth [217]. These sub-100 nm nanoparticles were able to decrease microvessel density by 2-fold and tumor volume by 67.4%. Another variant of bPEI polymer, Tween®-SS-bPEI, was developed to enhance stability of nanoparticles by amphiphilic property of Tween® and to release shRNA rapidly in the cytoplasm by reduction of disulfide bond. At 14 N/P ratio, the polymer condensed p65 shRNA to block NF-κB pathway, which is also known to facilitate angiogenesis, to 130 nm size, and successfully inhibited the tumor volume of breast cancer xenografts to 6% of saline group at 5 weeks [218]. Based on these studies, both addition of targeting moiety and varying formulation parameters to form smaller nanoparticles, such as N/P ratio or buffer used in nanoparticle formation, improve the efficiency of DNA delivery with PEI, while introducing disulfide bonds to the polymer enhances shRNA delivery to the site of action, the cytoplasm. A dendrimer system has also been investigated as a delivery platform for antiangiogenic plasmid DNA. In a study by Vincent et al., a PAMAM-based dendrimer complexed with pAngiostatin and/or pTIMP-2 between 12 – 36 w/w polymer to nucleic acid mass ratio was intratumorally injected into a subcutaneous human breast carcinoma model in mice and showed 96% inhibition of tumor growth compared to an empty plasmid control [219].
Other non-viral nanoparticles have also shown therapeutic efficacy against primary and metastatic tumors in animals. For example, synthetic polymer PVP can form hydrogen bonds and intercalate with DNA to form positively charged nanoparticles. Intramuscular injection of PVP-pEndostatin nanoparticles showed potent anti-angiogenic activity and retardation of metastatic brain tumor growth by 60% compared to control as measured with MRI [220]. Another example is PEG-conjugated poly(epsilon-caprolactone) tethered to Tat cell-penetrating sequence by disulfide bond to facilitate cytoplasmic plasmid release (mPEG-PCL-SS-Tat) [221]. Tat, having arginine-rich sequence, can be used to calculate N/P ratio for nanoparticle formulation. mPEG-PCL-SS-Tat complexed with siVEGF at N/P 30 to form 40 nm nanoparticles that could take advantage of EPR effect [222] following systemic delivery, and demonstrated 50% decrease in VEGF secretion and approximately 60% suppression of tumor growth in mouse sarcoma.
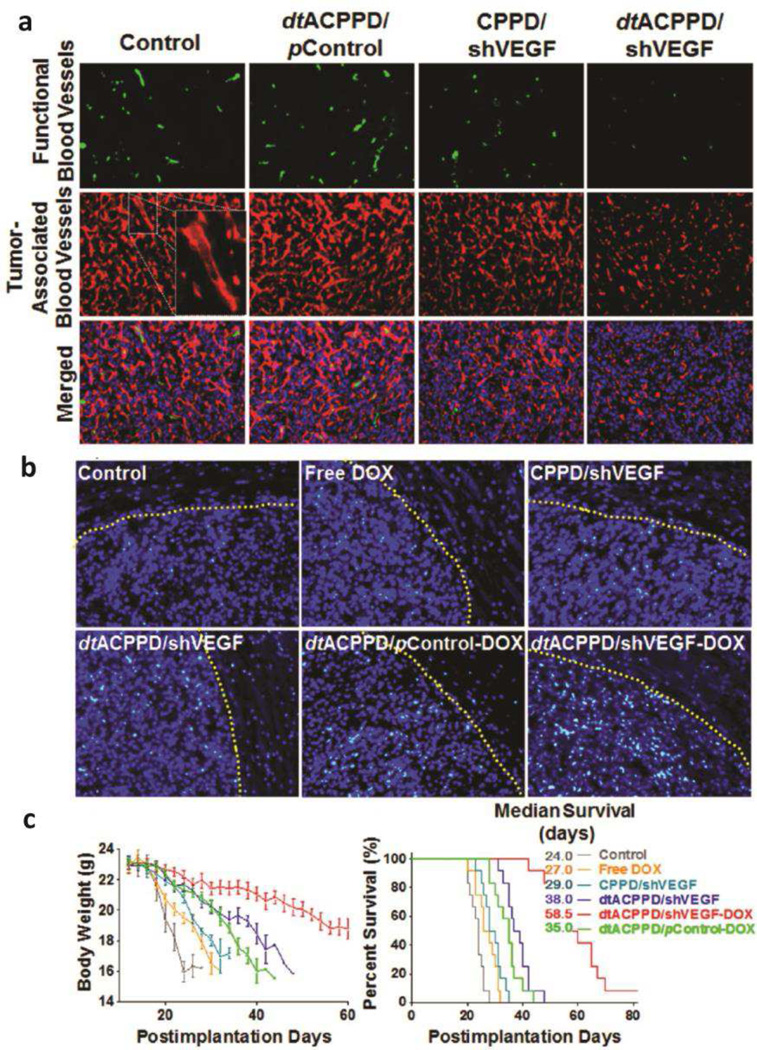
One of the earliest polypeptides used for gene condensation, PLL, was used in a copolymer structure conjugated with PEG to systemically deliver plasmid DNA sFlt-1 to subcutaneous pancreatic tumor [223]. Specifically, thiolated PLL was conjugated to PEG to form disulfide cross-linked micelles that stabilize the structure as well as enable plasmid release in response to reducing environment. Various degree of thiolation was tested against in vivo antitumor activity, and the optimal 11% thiolated micelles resulted in 40% tumor volume suppression compared to control. A related non-viral vector is highly branched, cationic, dendrimer-based PLL (DGL) modified with PEG [224]. As noted above, the disadvantage of PLL is its limitation to escape endosomes once endocytosed, but cell-penetrating peptides (CPPs) facilitate cellular internalization to the cytoplasm. Huang et al. conjugated pH- and MMP-activatable CPP (dtACPP) to DGL-PEG (dtACPPD) that could induce selective internalization of nanoparticles to cells residing in tumor microenvironment [224]. dtACPPD was complexed with doxorubicin (DOX)-intercalated shVEGF at 6 w/w ratio to form near-neutral, 150 nm nanoparticles for drug and gene co-delivery to orthotopic glioma (Figure 6). The nanoparticles successfully inhibited VEGF mRNA expression by 65% in vivo and increased median survival by more than 2-fold compared to untreated, free DOX, and constitutive-CPP bearing DGL-shVEGF.
Figure 6.
pH- and MMP-activatable cell-penetrating peptide conjugated to dendrimer-based PLL-PEG (dtACPPD) successfully delivers shVEGF to enable nanoparticle-mediated anti-angiogenesis gene therapy combined with doxorubicin delivery. (a) Functional blood vessels visualized by lectin (green) and tumor-associated blood vessels by anti-CD34 (red). (b) Histological images of apoptotic cells in glioma tissues using the TUNEL assay (green: apoptotic cells, blue: DAPI). Antitumor efficacy shown by (c) average change in body weight (left) and overall survival (right) of glioma-bearing mice. Original magnification: 200X. Adapted from ref [224]
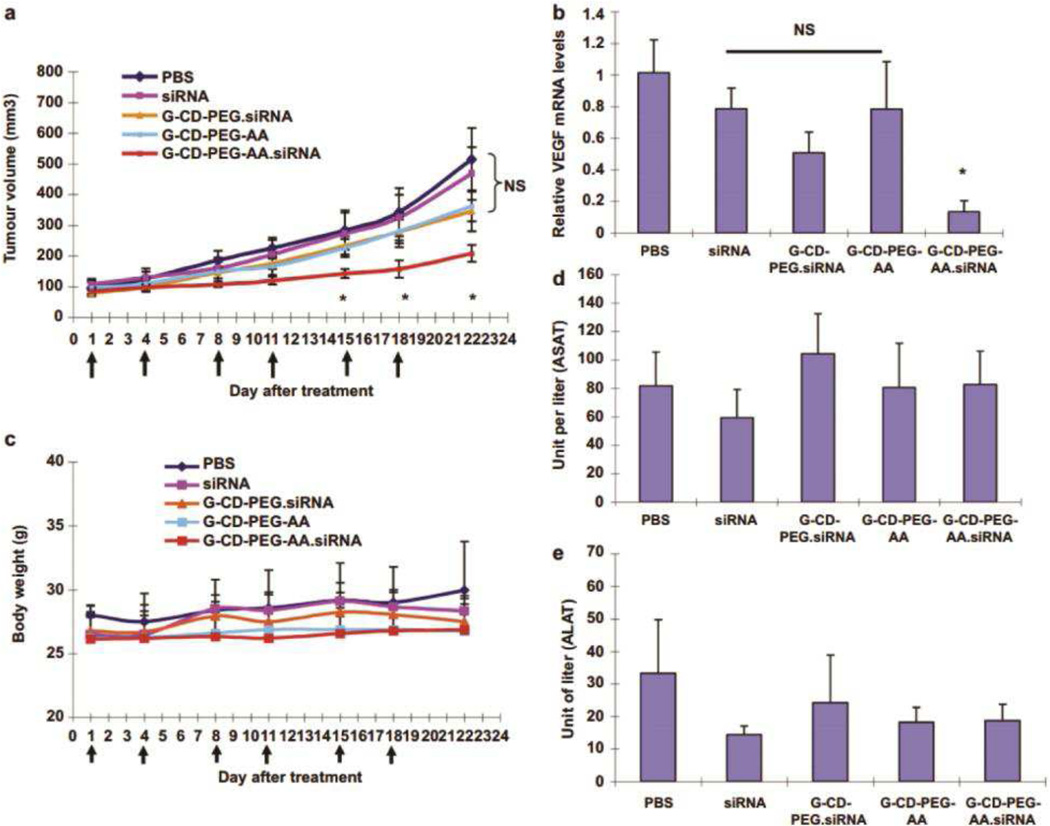
Polysaccharides, such as chitosan and β-cyclodextrin, have also been explored as nanoparticulate gene carriers to tumor vasculature. Low molecular weight chitosan and cyclodextrin provide stability in biological fluids and membrane permeation enhancement capability [225]. Other key features, such as molecular inclusion of cyclodextrin, also simultaneously affect the efficiency of gene delivery by these carbohydrates. Mark Davis’ group developed a targeted cyclodextrin-based nanoparticles for siRNA delivery to tumor (CALAA-01), which was the first of its kind to enter phase 1 clinical trial in 2008 [226]. Low molecular weight (21 kDa) Chitosan-shVEGF complex of 220 nm size formulated at N/P 3 demonstrated effective inhibition of tumor angiogenesis and tumor growth via intratumoral injection or intravenous injection to subcutaneous ectopic and orthotopic hepatocellular carcinoma models, respectively [227]. Guo et al. synthesized PEGylated β-cyclodextrin (βCD) with anisamide (AA) as the targeting moiety against sigma receptor overexpressed in prostate cancer [228]. Specifically, guanidine was first conjugated to βCD to generate a cationic cyclodextrin (G-CD), which was then further modified with PEG and the targeting ligand (G-CD-PEG-AA). Stable 200 nm nanoparticles were formed with G-CD-PEG-AA and siRNA at 75 w/w ratio. A 3-fold suppression of tumor growth was achieved with G-CD-PEG-AA-siVEGF relative to PBS-treated group, as well as greater than 3.5-fold reduction in VEGF mRNA level relative to non-targeted G-CD-PEG-siVEGF (Figure 7).
Figure 7.
Antitumor efficacy following systemic delivery of guanidine-cyclodextrin-PEG-anisamide (G–CD–PEG–AA)-siVEGF complex evaluated by (a) tumor volume, (b) VEGF mRNA knockdown, and (c) body weight concurrent with tumor treatments. Liver enzyme levels, (d) aspartate aminotransferase (ASAT) and (e) alanine aminotransferase (ALAT) (mean ± SD shown; NS = no significance, *p < 0.05). Adapted from ref [228]
Lipid-based nanoparticle formulations have also seen success in animal models to target tumor angiogenesis. A modified lipid termed YSK05, which contains a tertiary amine for pH buffering along with greater ability to fuse with membrane to facilitate endosomal escape, is used to formulate cRGD-coated, PEGylated liposomes [229]. Systemic injection of the targeted liposome inhibited renal carcinoma tumor growth and reduced vessel area by approximately 50% compared to siLuciferase control. Another variation of lipid was developed using disulfide bond and tertiary amine containing lipid as a base. Retinoic acid, which has intrinsic property to transport to peri-nuclear site, maintains nanoparticle structure until releasing at the last step for nuclear transport. Using liposomes formulated with the vector and plasmid DNA sFlt-1, tumor growth was suppressed by 53% and area of endothelium decreased by 50% compared to PBS control in renal cell carcinoma model [230]. In another study, integrin-targeted liposomes containing micro RNA miR132 antagonist were able to decrease the volume of a human breast cancer orthotopic xenograft in mice by approximately 2-fold via restoring p120RasGAP expression in endothelium and decreasing neovascularization [231]. Interestingly and expectedly, water-soluble lipopolymer (WSLP) [119] as well as ultrasound-targeted microbubble destruction (UTMD) of liposomes [126] that were shown to be effective against ischemic diseases were also used in anti-angiogenic gene therapy against prostate [232] and mammary [233] adenocarcinomas, respectively. WSLP-mediated siVEGF delivery resulted in more than 50% reduction in VEGF concentration and tumor growth compared to bPEI-siVEGF nanoparticles, while UTMD-mediated shVEGFR2 delivery also reduced tumor volume by 40% compared to no treatment control.
This highlights an advantage of non-viral gene delivery nanoparticles in that once optimized for delivery to one cell type following a particular route of administration, a plasmid’s sequence, including its encoded gene of interest, can be varied without changing the physicochemical properties of the nanoparticle and its intracellular delivery efficacy to that cell type. Similarly, nanoparticles optimized to deliver siRNA or miRNA to a particular cell type will have similar nanoparticle physicochemical properties and intracellular delivery even if the RNA sequence, and the genetic target for the siRNA or miRNA, was modified. Thus, the same optimized biomaterial nanoparticle formulations for a particular type of nucleic acid cargo, DNA, siRNA, miRNA, or antisense, can be used to deliver a range of different genetic medicines for different physiological effects, without necessarily requiring any re-optimization of the nanoparticle as gene delivery vector. However, when administered to a different microenvironment or following a different route of administration, due to the effect of extracellular delivery barriers, the nanoparticle may need further engineering for enhanced efficacy, such as PEGylation to resist serum proteins in the blood or smaller size to penetrate through certain tissues.
4.2.2 Neovascular Age-related Macular Degeneration (NVAMD)
Age-related macular degeneration is a complex disease that can have two phenotypes leading to vision loss: non-neovascular (or dry) AMD, in which accumulation of extracellular deposits (drusen) results in gradual death of photoreceptors and retinal pigment epithelial (RPE) cells, or neovascular (wet) AMD (NVAMD), in which new blood vessels grow into subretinal space from the choroid. Because vessels in subretinal neovasculature lack tight junctions, fluid can leak out into the retina, resulting in edema, fluid pooling, and vision loss [234]. Anti-angiogenic factors, namely anti-VEGF molecules ranibizumab (Lucentis®) and aflibercept (Eylea®), are currently injected intravitreally in patients with NVAMD to inhibit neovascularization and leakage.
Tissues and RPE cells at the posterior of the eye, which is the target site for NVAMD, can be accessed and targeted by specific routes of administration [235]. Intravitreous injection is a common delivery method, however engineered biomaterial design can enable enhanced nanoparticle diffusion to the posterior and accumulation away from the visual axis to increase therapeutically effective concentration. Subretinal injection can precisely deliver the therapeutics to the RPE at high concentration, but carries a risk of retinal detachment [236]. More recently, injection into suprachoroidal space between choroid and sclera has been shown to be safe, and merits further investigation for therapeutic delivery of nanoparticles, including gene delivery nanoaprticles [237].
Viral vectors have been widely investigated for gene therapy due to their efficiency and potential for long-term expression of transduced genes. Adeno-associated virus (AAV/AAV2) [238, 239], lentivirus [240], and hybrid AAV with serotypes that targets specific cell populations (rAAV) [241] have all shown positive results against CNV in preclinical animal studies. These viruses have transferred genes encoding endogenous angiogenic inhibitors such as soluble VEGF receptor (sFlt-1) [238, 239], pigment epithelium-derived factor (PEDF) [242, 243], endostatin and angiostatin [240], and tissue inhibitor of metalloproteinases-3 (TIMP3) [244]. However, long-term suppression of neovascularization at distant sites due to viruses that made their way into the systemic circulation is a potential safety concern [245].
Studies evaluating in vitro and in vivo transfection of the RPE cell layer with non-viral gene delivery nanoparticles have led to therapeutic efficacy against CNV. The utility of polymeric nanoparticle library approach for ocular gene therapy was demonstrated by Sunshine et al. [246]. 180 nm nanoparticles formed from a library of PBAEs with varying structures were screened and analyzed for transfection against RPE cells in vitro. When the best performing formulation was injected into the subretinal space, high expression was observed 72-h post injection by both RT-PCR and fluorescence microscopy of a genetically-encoded reporter in both retina and RPE/choroid. The specificity and efficiency of PBAE nanoparticles were enhanced through the polymer library screening approach. In a laser-induced CNV model in rats, PVP polymer carrying pVasostatin was able to decrease 32.5% and 48% CNV incidence as well as lesion area for 42 days compared to saline and empty vector controls [247]. In another study using the same model, shHIF-1α was encapsulated in PLGA for intravitreal injection [248]. PLGA allows for controlled release of the nucleic acids for prolonged duration as the material degrades via hydrolysis. At day 14, PLGA - shHIF-1α reduced leakage by 50% and lesion thickness by 40%. Also, PEGylated liposomes modified with hyaluronic acid to reduce immunotoxicity were formulated to deliver siVEGF intravitreally. As a result, no significant retinal toxicity was observed 2 weeks post injection, while CNV area was reduced by more than 50% compared to no treatment control [249]. Other targeted nanoparticles were designed for gene delivery following intravenous injection. RGD-labeled liposomes containing a dominant negative Raf mutant gene successfully accumulated in the CNV lesion via integrin targeting and reduced CNV area by 42% compared with no treatment control [250]. A dendrimer system composed of lipidic α–aminocarboxylic acids and a poly-lysine head was also used to complex with anti-VEGF oligonucleotide at a molar charge ratio of 6 and was administered intravitreally in a laser-induced rat CNV model [251]. The particles penetrated to all retinal cell layers and significantly inhibited CNV by approximately 2-fold at 4 months. RGD/transferrin double-labeled PLGA nanoparticles carrying plasmid for Flt23k intraceptor had a larger size of approximately 400 nm, yet accumulated in the laser-induced CNV eye and not the fellow eye [252]. The accumulation was higher with RGD ligand alone than dual RGD/transferrin ligands. RGD and dual-functionalized nanoparticle treatment exhibited 73% and 56% lower CNV areas compared to non-targeted nanoparticles. More recently, Luo et al. explored the anti-angiogenic functional effect of systemically delivered plasmid Flt23k with PLGA nanoparticles in a different AMD model generated by inducing CNV via knockdown of sFlt-1 [253]. This model is used because laser injury could result in other complications such as laser’s acute injury and retinal burn in addition to pathological state mimicking AMD. As demonstrated by previous studies described above, RGD was an effective targeting ligand against CNV for intravenously injected PLGA nanoparticles of size 400 nm. However, in this study, Luo et al. formed smaller 160 nm RGD-coated PLGA nanoparticles containing plasmid Flt23k using a double emulsion technique that had greater sonication power. These nanoparticles also targeted the retina of the laser-induced CNV eye and regressed 53% CNV area in primates, while regressing 43% in a murine sFlt-1-knockdown model without any significant toxicity (Figure 8). Interestingly, this gene therapy was able to regress CNV lesions more significantly than intravitreal anti-VEGF treatment. The promising results from several polymeric systems in AMD models motivates further research in the field to optimize these non-viral nanoparticles to deliver other anti-angiogenic factors as well.
Figure 8.
(A–D) Significant reduction of CNV lesion volume (A) shown by fluorescein angiograph (FA) (B), immunohistochemistry staining (C) and H&E staining (D) after RGD-PLGA-psFlt23k nanoparticle treatment in a laser-induced CNV non-human primate model. (E–F) Regression of CNV lesion by RGD-PLGA-psFlt23k nanoparticles in a laser-induced CNV mouse model 2 weeks post treatment (E) and in the sFlt-1 knockdown induced CNV mouse model 4 weeks after treatment (F). (G–H) H&E (G) and FA/OCT (H) images of each group in panel F show the sizes of CNV lesions after treatment. (I) Single systemic injection of RGD-PLGA-psFlt23k nanoparticles regressed CNV lesions more than intravitreal anti-VEGF antibody. (J) Partial restoration of visual acuity as determined by optomotor response 4 weeks after RGD-PLGA-psFlt23k nanoparticle treatment. Adapted from ref [253].
5. Clinical trials
Clinical trials of gene therapy targeting angiogenesis have mostly consisted of injection of naked plasmid or using viral vectors, and the number of non-viral approaches is gradually increasing (Table 2). The majority of therapeutic angiogenesis clinical studies focus on the use of plasmid VEGF, which is not surprising based on the large number of preclinical studies that have demonstrated efficacy with this approach. The Regional Angiogenesis With Vascular Endothelial Growth Factor (RAVE) trial was the first large-scale phase 2, placebo-controlled study testing the efficacy of intramuscularly injected adenovirus-mediated VEGF121 gene in patients with peripheral arterial disease (PAD; i.e. ischemic limb) [254]. Another clinical study of 54 patients with critical limb ischemia treated with intramuscular administration of pVEGF165 showed no amputation reduction, but significant improvement in hemodynamics, skin ulcers, and claudication [255]. Liposomal delivery of pVEGF165 and adenovirus-VEGF165 resulted in increased vascularity in a phase 2 clinical trial based in Finland on patients with lower limb ischemia [256], but a 10-year follow-up showed no significant difference in the number of amputations or causes of death [257]. Other clinical trials with VEGF have also demonstrated mixed results. In one phase 2 study of 13 patients, myocardial injection of a VEGF165 plasmid demonstrated improvements with several outcomes over the standard optimal therapy, including benefits to quality of life, improved angina, and decrease in ischemic segments. However, while some of these benefits were maintained over the course of the study, myocardial perfusion progressively returned to pre-treatment conditions over the 6 and 12 month follow-ups [258]. In an additional clinical study of 93 patients with class 3 or 4 conditions (as determined by the Canadian Cardiovascular Society), there were no significant differences between high dose injections of VEGF165 plasmid DNA over placebo [259].
Table 2.
Clinical Trials of Gene Therapy Targeting Angiogenesis
| Angiogenesis | Disease | Factor | Delivery Vector |
Phase | Patient | Country | Result | Reference |
|---|---|---|---|---|---|---|---|---|
| Pro | Ischemia | VEGF121 | Adenovirus | 2 | 105 | USA | Similar end-point (peak walking time, ankle-brachial index, claudication, quality of life (QOL)) between placebo and low /high dose treatment |
[254] |
| VEGF165 | naked | - | 54 | Netherlands | No improvement in pain / amputation rate; significant improvement in hemodynamics and skin ulcers |
[255] | ||
| VEGF165 | Adenovirus / liposome |
2 | 54 | Finland | Catheter-mediated injection; anti-adenovirus increased in 61% patients; increased vascularity distal (both) and ischemic site (adenovirus); long-term (10yr) safety; no diff. in amputations |
[256, 257] | ||
| FGF-1 | naked | 3 | 525 | 30 countries | No significant difference between treated vs. placebo group in amputation / death rates |
[260] | ||
| HGF | naked | - | 106 | - | No significant difference in end-point (amputation, ankle-brachial index, wound healing, pain relief); transcutaneous oxygen tension ↑ in high dose |
[262] | ||
| HGF | naked | - | 40 | Japan | Safe; significant increase in rest pain, ulcer size reduction, and QOL vs. placebo |
[263] | ||
|
Coronary Artery Disease |
VEGF165 | naked | 1/2 | 13 | Brazil | Transitory in myocardial perfusion; 1-yr improvement in treadmill step test, oxygen consumption, and QOL vs. placebo |
[258] | |
| VEGF | naked | - | 93 | Canada | Significant decrease in lesion area; no difference in myocardial perfusion, anginal symptom and treadmill time vs. placebo |
[259] | ||
| FGF-4 | Adenovirus | - | 52 | USA | Significant ↓ in ischemic defect size; significant ↑ in reversible perfusion defect size (w/o 1 outlier). Currently phase 3; status unknown (updated 2013) |
[261] | ||
| HIF-1α | Adenovirus | 1 | 13 | - | Safe; too small sample size to make comparisons | [264] | ||
| Anti | Cancer | Endostatin | Adenovirus | 1 | 15 | China | Safe; anti-adenovirus antibody in serum; ↑ endostatin in serum; ↓ in FGF level vs. placebo |
[265] |
| Endostatin | Adenovirus | 2 | 136 | China | Combination with chemotherapy; benefit for prior chemotherapy-treated patients; longer progression- free survival; ↑ disease control rate in groups treated with endostatin |
[267] | ||
|
Wet AMD |
PEDF | Adenovirus | 1 | 28 | USA | No severe adverse effects at high dose of 10a PU; no mean increase in lesion size at 6 & 12 months |
[243] | |
| sFLT-1 | AAV | 1 | 8 | Australia | Subretinal injection; no adverse effect; lack of fluid in retina; center point thickness ↓; visual acuity ↑ |
[269, 270] | ||
| sFLT-1 | AAV | 1 | Up to 34 | USA | Ongoing | [268] |
Apart from VEGF, other factors have been investigated as potential treatments for ischemia as well. In a phase 3 clinical trial, 525 patients with critical limb ischemia were randomly assigned to receive a non-viral plasmid containing the fibroblast growth factor 1 gene or placebo. No significant differences in amputation or death were observed between the two groups [260]. In contrast, significant improvements over placebo in ischemic defect size were observed in another trial of 52 patients with stable angina and reversible myocardial ischemia following intracoronary injection of adenovirus containing the gene for FGF-4 [261]. Gene therapy using HGF has also been investigated in a few small random, double-blind clinical studies of patients with critical limb ischemia. In one of these trials, 106 patients were separated into four groups, receiving either placebo or low, medium, or high doses of plasmid containing encoding for the HGF gene intramuscularly and monitored for differences in tissue perfusion, as determined by transcutaneous oxygen tension. Six months following treatment, a significant benefit to transcutaneous oxygen tension was observed at all doses relative to the placebo group; however, no differences were observed in secondary endpoint values, such as pain relief, wound healing, or major amputations [262]. In another study, 40 patients with peripheral arterial disease with a Rutherford rating of 4 (non-ulcerous) or (ulcerous) were treated with placebo or intramuscular injection of HGF plasmid. Significant changes in both resting pain (non-ulcerous) and ulcer size (ulcerous) were observed in plasmid-treated patients relative to those treated by placebo [263]. In addition to the standard growth factors, HIF-1α has also been investigated in a small, phase 1 clinical trial of 13 patients with coronary artery disease. Direct myocardial injection of adenovirus containing the HIF-1α gene showed no significant difference in toxicity in comparison to the placebo. However, benefits with this small sample size were minor and non-significant [264].
Whereas gene delivery-based therapies for ischemic complications focus on the administration of pro-angiogenic factors, similar treatments in cancer are primarily concerned with the inhibition and reduction of tumor vessels through the delivery of endogenous, anti-angiogenic peptide sequences. Here we describe two trials investigating the effects of intratumoral injection of adenovirus containing the gene for human endostatin. The first trial was an exploratory investigation consisting of 15 patients with various solid tumors. Noticeable serum levels of endostatin were observed following viral particle injection with tolerable toxicity at all doses. Treatment was also accompanied by a significant decrease in serum FGF2 levels on day 28 [265]. A second trial is a phase 2 study that investigated the efficacy and toxicity of adenovirus containing an endostatin gene in combination with chemotherapy in advanced head and neck cancers. The patients who received prior chemotherapy or 3–4 treatment cycles benefited from endostatin. Longer progression-free survival and increased disease control rate were seen in comparison to the group without endostatin treatment [266, 267].
As described above, wet AMD is characterized by the excessive growth of leaky, abnormal vasculature that can lead to edema and vision loss. The attenuation of this neovasculature through the inhibition of pro-angiogenic factors, such as VEGF, remain the standard of care and emphasizes the potential opportunity for gene delivery of endogenous angiogenic inhibitors. One such inhibitor, PEDF, was first identified in the pigment epithelial cells of the eye and found to possess important anti-angiogenic properties. Expression of this factor is often reduced in AMD, suggesting that the restoration of its expression would provide a therapeutic benefit. In a phase 1 trial, 28 patients with AMD were intravitreally injected with adenoviral particles containing a plasmid encoding for human PEDF. Toxicity included only minor inflammation and treatable increases in ocular pressure, while benefits consisted of inhibition of lesion growth over a period of 12 months, possibly opening opportunities for the future development of long-lasting treatments [243]. In addition, two other phase 1 trials are currently underway investigating the therapeutic benefits of sFlt-1 gene therapy delivered by intravitreal injection of adeno-associated virus particles [268, 269]. One of them had a one year follow-up report showing decreased center point thickness of 352 µm compared to 552 µm in the control group, as well as increased visual acuity [270].
6. Future directions
The short list of clinical trials for non-viral gene delivery vectors to treat angiogenesis-related diseases reveals that 1) this therapeutic approach is still nascent and 2) nanoparticles need further improvement to overcome shortcomings that have hindered their successful clinical translation thus far. Potential future innovations to gene delivery nanoparticle and genetic cargo design are discussed below and could lead to next generation technology able to modulate angiogenesis with enhanced safety and efficacy.
The successful delivery of a gene of interest by a non-viral vector to the target cell is affected by several physicochemical properties of the vector used and the resulting nanoparticle. Although there have been many different approaches to nanoparticle-mediated gene delivery, certain physicochemical property trends have been observed. For example, although nanoparticles over a wide range of sizes, including up to 400 nm as described above, have been used for intracellular non-viral gene delivery, nanoparticles of approximately 100 nm in size or smaller tend to be the most promising due to enhancement in extracellular transport as well as enhanced cellular uptake via clathrin-mediated endocytosis [271] and caveolae-mediated endocytosis [272]. Similarly, neutrally charged particles [41] are found to resist non-specific interactions with off-target biomolecules and cells, best ensuring that the nanoparticles reach their desired target in vivo. When designing future nanoparticle strategies and evaluating them in vitro, an important consideration is that the nanoparticle properties that maximize in vitro transfection may make poor choices for therapeutic delivery. For example, larger particles with high positive surface charge may effectively transfect cells in vitro due to the sedimentation of the large particles and the electrostatic attraction of positively charged particles to the negatively charged cell surface. Yet, these same particles would make poor choices for therapeutic use due to their likelihood for quick aggregation and clearance. An approach that can be useful to mitigate this screening and identification challenge for the design of new nanoparticles is to make the in situ and in vitro analysis conditions as similar to the in vivo conditions as possible, such as by measuring the effect of serum on particle stability and transfection [273] and using novel inverted cell culture conditions to eliminate the role of sedimentation [274].
When considering biomaterial structure to compose next-generation gene delivery nanoparticles, degradable linkages as previously described are key to both alleviate potential cytotoxicity concerns of the biomaterial as well as facilitate nucleic acid release from the nanoparticle carrier. When designing new structures, comprehensive and systematic analysis of structure-activity relationships for the particular biomaterial can be enabling. For example, principal component analysis (PCA) and logical analysis of data (LAD) were performed in two separate studies to correlate specific properties of PBAE nanoparticles to their transfection efficacy [275, 276], demonstrating design principles for this class of materials. Of the many parameters to consider, biomaterial properties such as hydrophobicity and molecular weight have been found to be critical for a range of biomaterials, with a higher degree of hydrophobicity and a higher molecular weight often leading to both improved transfection and increased cytotoxicity, leading to a biphasic response with intermediate hydrophobicity and molecular weight being optimal [39, 275, 277]. Depending on the particular application, optimal design must also consider additional vector parameters relating to the crossing of disease- and tissue-specific barriers, cell-specific targeting and intracellular uptake, and endosomal escape and nucleic acid release rate.
Depending on the disease and treatment method, both local gene therapy and cell therapy following ex vivo gene transfer may require repeated administration to compensate for transient gene expression. While systemic delivery of nanoparticles may facilitate repeated treatments, nanoparticle stability in blood circulation and resistance to clearance can be a challenge. While many groups have studied the effect of PEG molecular weight and its surface density in order to improve nanoparticle stability and half-life [278], there are other strategies to promote resistance to clearance as well. Some groups have focused on investigating biomaterial structural and chemical properties to better condense DNA and improve particle stability [279]. Other innovative approaches to nanoparticle delivery consider aspects such as geometry and anisotropic shapes to extend circulation of the nanoparticle [280, 281]. Additional methods may focus on extending particle circulation by mimicking biology and camouflaging a synthetic particle. For example, the inclusion of “self” biological signals on particles can reduce nonspecific cellular uptake [282].
Biomimetic materials are an exciting area of future growth for enhanced nanoparticles for gene delivery. For example, biological membranes, including the plasma membrane as well as membranes from subcellular compartments such as exosomes, have been evaluated either as the sole vector to form nanovesicles encapsulating nucleic acid or as the surface coating to pre-formulated nanoparticles [283, 284]. Because this material can be salvaged from a host’s cells, the resulting nanoparticles can potentially be able to efficiently evade rapid clearance or immune response from the body [285]. Further, studies have shown that membranes from specific cancer cells contain membrane proteins that can direct specific homing towards target tissues [286]. Research into these materials is still at a nascent stage, but it is already clear that loading of genetic material, such as siRNA by electroporation, can be accomplished, as can membrane coatings of hard nanoparticles. By enhancing circulation time, avoidance of the immune system, and tissue targeting, biomimetic coatings of nanoparticles for gene delivery to treat angiogenesis-related diseases is a promising approach.
From the genetic cargo side, there has been continuous effort in the field to prolong the transient nature of non-viral gene expression from DNA plasmid delivery. One of the most exciting new methods is the use of the CRISPR / Cas-9 system to insert an exogenous gene in the host’s genome site-specifically [287]. This technique not only prolongs the expression of a delivered gene, but minimizes the risk of mutagenesis and tumorigenesis arising from random insertion. By achieving safe and long-term expression of exogenously delivered genes and/or knockdown of endogenous genes, non-viral nucleic acid delivery nanoparticles can be enabled to potentially cure diseases at the genetic level. However, imbalanced growth or reduction of vasculature from uncontrolled long-term gene therapy may lead to other pathological complications. Through pre-clinical and phase I clinical studies, it was shown that high level of exogenous VEGF or its viral expression can cause severe edema, limb loss, and hemangiomas [288]. This may be less of a concern for non-viral systems compared to viral systems because of the nature of transient gene expression, although it is still a concern. There must be a balanced approach in developing biomaterial and biomolecular tools that direct gene expression as the control of gene expression, spatially and over time at the therapeutic site, is paramount. In addition to endothelial cells as a target, mural cells, such as pericytes, are also of critical importance for vessel stabilization. Thus, there is significant potential for future directions that utilize combinatorial treatment strategies that act on both endothelial cells as well as mural cells for both pro-angiogenic and anti-angiogenic therapies [289–293].
Finally, non-canonical biologics also hold promise as potential therapeutics. For example, as mentioned in anti-angiogenesis section, computational biology approaches have enabled identification of cryptic peptide motifs that have strong anti-angiogenic activity as well as biomimetic peptide analogs [177, 294, 295]. Such peptide motifs can be genetically encoded and engineered into a plasmid for delivery singly or in combination with gene delivery nanoparticles.
7. Conclusion
Taken together, the studies described in this review emphasize the potential use of non-viral gene delivery nanoparticles for the modulation of angiogenic processes. Angiogenesis is a critical component in the progression of several disease states and remains a prime candidate for their therapeutic intervention. Although the overall process is markedly complex, growing knowledge in the field has provided the identity of key factors as well as revealed their interplay in a tightly regulated system. Gene therapy allows for the manipulation of these interactions in order to shift the balance of angiogenic signaling, either positively or negatively, away from pathological abnormalities. Currently, the greatest clinical progress has been made with gene-loaded viral particles or injection of free DNA. Nonetheless, the rapid advancements in the field of nanotechnologies have opened opportunities to investigate their use as in vivo gene delivery vectors. Notably, non-viral nanoparticles can provide improved stability, cellular targeting, and internalization over free nucleic acid and are relatively easy to produce, are highly customizable, and can be designed for lower toxicity than viral counterparts. New approaches to biomaterial and nanoparticle construction, including biomaterial libraries as well as rationale design, are promising to further improve the efficacy of gene delivery nanoparticles. Unfortunately, despite much promise in pre-clinical animal models, angiogenesis-based gene therapy has only shown modest benefits thus far in the clinical setting. Challenges in dosing, transfection efficiency, safety, and an incomplete understanding of the biology in certain microenvironments are compromising factors. Therefore, there is a need to further develop our understanding of non-viral gene nanoparticles and the biology of angiogenesis to optimize the selection and delivery of genetic material to target tissues to treat angiogenesis-dependent diseases.
Acknowledgments
This work was supported by the National Institutes of Health [R01EB016721, R01HL101200, R21EY026148 and R01CA138264] and a Samsung Scholarship to JK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds
Poly(ethylene imine); Poly(L-lysine); Poly(beta-amino ester); Poly(lactic-co-glycolic) acid; Poly(vinyl pyrrolidone); -cyclodextrin; chitosan; cholesterol; N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate
References
- 1.Gianni-Barrera R, Bartolomeo M, Vollmar B, Djonov V, Banfi A. Split for the cure: VEGF, PDGF-BB and intussusception in therapeutic angiogenesis. Biochem. Soc. Trans. 2014;42:1637–1642. doi: 10.1042/BST20140234. [DOI] [PubMed] [Google Scholar]
- 2.Logsdon EA, Finley SD, Popel AS, Mac Gabhann F. A systems biology view of blood vessel growth and remodelling. J. Cell. Mol. Med. 2014;18:1491–1508. doi: 10.1111/jcmm.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J. Clin. Invest. 2013;123:3190–3200. doi: 10.1172/JCI70212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ. Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 6.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 2013;3:a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J. Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 10.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 11.Gaya A, Tse V. A preclinical and clinical review of aflibercept for the management of cancer. Cancer Treat. Rev. 2012;38:484–493. doi: 10.1016/j.ctrv.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Merdan T, Kopecek J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv. Drug Deliv. Rev. 2002;54:715–758. doi: 10.1016/s0169-409x(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 13.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 14.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 15.Verma IM. A tumultuous year for gene therapy. Mol. Ther. 2000;2:415–416. doi: 10.1006/mthe.2000.0213. [DOI] [PubMed] [Google Scholar]
- 16.Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 - an update. J. Gene Med. 2013;15:65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 17. [[Accessed September 5, 2016]];Gene therapy clinical trials worldwide, Retrieved from. 2016 Feb; http://www.abedia.com/wiley/index.html.
- 18.Ott PA, Hodi FS. Talimogene Laherparepvec for the Treatment of Advanced Melanoma. Clin. Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2709. [DOI] [PubMed] [Google Scholar]
- 19.Pearson S, Jia H, Kandachi K. China approves first gene therapy. Nat. Biotechnol. 2004;22:3–4. doi: 10.1038/nbt0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller N. Glybera and the future of gene therapy in the European Union. Nat. Rev. Drug Discov. 2012;11:419. doi: 10.1038/nrd3572-c1. [DOI] [PubMed] [Google Scholar]
- 21.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 22.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olins DE, Olins AL, Von Hippel PH. Model nucleoprotein complexes: studies on the interaction of cationic homopolypeptides with DNA. J. Mol. Biol. 1967;24:157–176. doi: 10.1016/0022-2836(67)90324-5. [DOI] [PubMed] [Google Scholar]
- 24.Dufes C, Uchegbu IF, Schatzlein AG. Dendrimers in gene delivery. Adv. Drug Deliv. Rev. 2005;57:2177–2202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Yu B, Zhao X, Lee LJ, Lee RJ. Targeted delivery systems for oligonucleotide therapeutics. AAPS J. 2009;11:195–203. doi: 10.1208/s12248-009-9096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Sunshine JC, Green JJ. Differential polymer structure tunes mechanism of cellular uptake and transfection routes of poly(beta-amino ester) polyplexes in human breast cancer cells. Bioconjug. Chem. 2014;25:43–51. doi: 10.1021/bc4002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godbey WT, Wu KK, Mikos AG. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999;45:268–275. doi: 10.1002/(sici)1097-4636(19990605)45:3<268::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 28.Wightman L, Kircheis R, Rossler V, Carotta S, Ruzicka R, Kursa M, Wagner E. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J. Gene Med. 2001;3:362–372. doi: 10.1002/jgm.187. [DOI] [PubMed] [Google Scholar]
- 29.Lungwitz U, Breunig M, Blunk T, Gopferich A. Polyethylenimine-based non-viral gene delivery systems. Eur. J. Pharm. Biopharm. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem. Rev. 2009;109:259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 31.Wasungu L, Hoekstra D. Cationic lipids, lipoplexes and intracellular delivery of genes. J. Control. Release. 2006;116:255–264. doi: 10.1016/j.jconrel.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Lu Z, Wientjes MG, Au JL. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Wang M, Sprouse D, Smith AE, Reineke TM. Glucose-containing diblock polycations exhibit molecular weight, charge, and cell-type dependence for pDNA delivery. Biomacromolecules. 2014;15:1716–1726. doi: 10.1021/bm5001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas M, Lu JJ, Zhang C, Chen J, Klibanov AM. Identification of novel superior polycationic vectors for gene delivery by high-throughput synthesis and screening of a combinatorial library. Pharm. Res. 2007;24:1564–1571. doi: 10.1007/s11095-007-9279-3. [DOI] [PubMed] [Google Scholar]
- 35.Siegwart DJ, Whitehead KA, Nuhn L, Sahay G, Cheng H, Jiang S, Ma M, Lytton-Jean A, Vegas A, Fenton P, Levins CG, Love KT, Lee H, Cortez C, Collins SP, Li YF, Jang J, Querbes W, Zurenko C, Novobrantseva T, Langer R, Anderson DG. Combinatorial synthesis of chemically diverse core-shell nanoparticles for intracellular delivery. Proc. Natl. Acad. Sci. USA. 2011;108:12996–13001. doi: 10.1073/pnas.1106379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green JJ, Langer R, Anderson DG. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res. 2008;41:749–759. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bishop CJ, Ketola TM, Tzeng SY, Sunshine JC, Urtti A, Lemmetyinen H, Vuorimaa-Laukkanen E, Yliperttula M, Green JJ. The effect and role of carbon atoms in poly(beta-amino ester)s for DNA binding and gene delivery. J. Am. Chem. Soc. 2013;135:6951–6957. doi: 10.1021/ja4002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sunshine JC, Peng DY, Green JJ. Uptake and transfection with polymeric nanoparticles are dependent on polymer end-group structure, but largely independent of nanoparticle physical and chemical properties. Mol. Pharm. 2012;9:3375–3383. doi: 10.1021/mp3004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shmueli RB, Sunshine JC, Xu Z, Duh EJ, Green JJ. Gene delivery nanoparticles specific for human microvasculature and macrovasculature. Nanomedicine. 2012;8:1200–1207. doi: 10.1016/j.nano.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozielski KL, Tzeng SY, Green JJ. A bioreducible linear poly(beta-amino ester) for siRNA delivery. Chem. Commun. (Camb) 2013;49:5319–5321. doi: 10.1039/c3cc40718g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Satterlee A, Huang L. In vivo gene delivery by nonviral vectors: overcoming hurdles? Mol. Ther. 2012;20:1298–1304. doi: 10.1038/mt.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeong GJ, Byun HM, Kim JM, Yoon H, Choi HG, Kim WK, Kim SJ, Oh YK. Biodistribution and tissue expression kinetics of plasmid DNA complexed with polyethylenimines of different molecular weight and structure. J. Control. Release. 2007;118:118–125. doi: 10.1016/j.jconrel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Staquicini FI, Ozawa MG, Moya CA, Driessen WH, Barbu EM, Nishimori H, Soghomonyan S, Flores LG, 2nd, Liang X, Paolillo V, Alauddin MM, Basilion JP, Furnari FB, Bogler O, Lang FF, Aldape KD, Fuller GN, Hook M, Gelovani JG, Sidman RL, Cavenee WK, Pasqualini R, Arap W. Systemic combinatorial peptide selection yields a non-canonical iron-mimicry mechanism for targeting tumors in a mouse model of human glioblastoma. J. Clin. Invest. 2011;121:161–173. doi: 10.1172/JCI44798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karagiannis ED, Popel AS. A systematic methodology for proteome-wide identification of peptides inhibiting the proliferation and migration of endothelial cells. Proc. Natl. Acad. Sci. USA. 2008;105:13775–13780. doi: 10.1073/pnas.0803241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Won YW, Adhikary PP, Lim KS, Kim HJ, Kim JK, Kim YH. Oligopeptide complex for targeted non-viral gene delivery to adipocytes. Nat. Mater. 2014;13:1157–1164. doi: 10.1038/nmat4092. [DOI] [PubMed] [Google Scholar]
- 46.Kim J, Wilson DR, Zamboni CG, Green JJ. Targeted polymeric nanoparticles for cancer gene therapy. J. Drug Target. 2015;23:627–641. doi: 10.3109/1061186X.2015.1048519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 48.Hyvarinen J, Hassinen IE, Sormunen R, Maki JM, Kivirikko KI, Koivunen P, Myllyharju J. Hearts of hypoxia-inducible factor prolyl 4-hydroxylase-2 hypomorphic mice show protection against acute ischemia-reperfusion injury. J. Biol. Chem. 2010;285:13646–13657. doi: 10.1074/jbc.M109.084855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, Thistlethwaite PA. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J. Am. Coll. Cardiol. 2005;46:2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 50.Xi L, Taher M, Yin C, Salloum F, Kukreja RC. Cobalt chloride induces delayed cardiac preconditioning in mice through selective activation of HIF-1alpha and AP-1 and iNOS signaling. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2369–H2375. doi: 10.1152/ajpheart.00422.2004. [DOI] [PubMed] [Google Scholar]
- 51.Cooke JP. NO and angiogenesis, Atheroscler. Suppl. 2003;4:53–60. doi: 10.1016/s1567-5688(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 52.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33:829–837. 837a–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chakrabarti S, Chan CK, Jiang Y, Davidge ST. Neuronal nitric oxide synthase regulates endothelial inflammation. J. Leukoc. Biol. 2012;91:947–956. doi: 10.1189/jlb.1011513. [DOI] [PubMed] [Google Scholar]
- 54.Nematollahi S, Nematbakhsh M, Haghjooyjavanmard S, Khazaei M, Salehi M. Inducible nitric oxide synthase modulates angiogenesis in ischemic hindlimb of rat. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2009;153:125–129. doi: 10.5507/bp.2009.021. [DOI] [PubMed] [Google Scholar]
- 55.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc. Natl. Acad. Sci. USA. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 57.Dehghanian F, Hojati Z, Kay M. New Insights into VEGF-A Alternative Splicing: Key Regulatory Switching in the Pathological Process. Avicenna J. Med. Biotechnol. 2014;6:192–199. [PMC free article] [PubMed] [Google Scholar]
- 58.Mac Gabhann F, Popel AS. Systems biology of vascular endothelial growth factors. Microcirculation. 2008;15:715–738. doi: 10.1080/10739680802095964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, Hicklin D, Anderson DJ, Gardiner T, Hammes HP, Moons L, Dewerchin M, Collen D, Carmeliet P, D’Amore PA. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J. Clin. Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brogi E, Wu T, Namiki A, Isner JM. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation. 1994;90:649–652. doi: 10.1161/01.cir.90.2.649. [DOI] [PubMed] [Google Scholar]
- 61.Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J. Cell Biol. 1998;141:1659–1673. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banai S, Shweiki D, Pinson A, Chandra M, Lazarovici G, Keshet E. Upregulation of vascular endothelial growth factor expression induced by myocardial ischaemia: implications for coronary angiogenesis. Cardiovasc. Res. 1994;28:1176–1179. doi: 10.1093/cvr/28.8.1176. [DOI] [PubMed] [Google Scholar]
- 63.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc. Natl. Acad. Sci. USA. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, VandenDriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 65.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat. Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 66.Iwasaki H, Kawamoto A, Tjwa M, Horii M, Hayashi S, Oyamada A, Matsumoto T, Suehiro S, Carmeliet P, Asahara T. PlGF repairs myocardial ischemia through mechanisms of angiogenesis, cardioprotection and recruitment of myo-angiogenic competent marrow progenitors. PLoS One. 2011;6:e24872. doi: 10.1371/journal.pone.0024872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- 68.Lee SH, Schloss DJ, Swain JL. Maintenance of vascular integrity in the embryo requires signaling through the fibroblast growth factor receptor. J. Biol. Chem. 2000;275:33679–33687. doi: 10.1074/jbc.M004994200. [DOI] [PubMed] [Google Scholar]
- 69.Xu X, Weinstein M, Li C, Naski M, Cohen RI, Ornitz DM, Leder P, Deng C. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development. 1998;125:753–765. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- 70.Luan Q, Sun J, Li C, Zhang G, Lv Y, Wang G, Li C, Ma C, Gao T. Mutual enhancement between heparanase and vascular endothelial growth factor: a novel mechanism for melanoma progression. Cancer Lett. 2011;308:100–111. doi: 10.1016/j.canlet.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 71.Rusnati M, Dell’Era P, Urbinati C, Tanghetti E, Massardi ML, Nagamine Y, Monti E, Presta M. A distinct basic fibroblast growth factor (FGF-2)/FGF receptor interaction distinguishes urokinase-type plasminogen activator induction from mitogenicity in endothelial cells. Mol. Biol. Cell. 1996;7:369–381. doi: 10.1091/mbc.7.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sasaki M, Ito T, Kashima M, Fukui S, Izumiyama N, Watanabe A, Sano M, Fujiwara Y, Miura M. Erythromycin and clarithromycin modulation of growth factor-induced expression of heparanase mRNA on human lung cancer cells in vitro. Mediators Inflamm. 2001;10:259–267. doi: 10.1080/09629350120093731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001;22:201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 74.Murakami M, Nguyen LT, Hatanaka K, Schachterle W, Chen PY, Zhuang ZW, Black BL, Simons M. FGF-dependent regulation of VEGF receptor 2 expression in mice. J. Clin. Invest. 2011;121:2668–2678. doi: 10.1172/JCI44762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J. Histochem. Cytochem. 1997;45:1005–1019. doi: 10.1177/002215549704500710. [DOI] [PubMed] [Google Scholar]
- 76.Miller DL, Ortega S, Bashayan O, Basch R, Basilico C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol. Cell. Biol. 2000;20:2260–2268. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ding S, Merkulova-Rainon T, Han ZC, Tobelem G. HGF receptor up-regulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro. Blood. 2003;101:4816–4822. doi: 10.1182/blood-2002-06-1731. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y, Wilkinson FL, Kirton JP, Jeziorska M, Iizasa H, Sai Y, Nakashima E, Heagerty AM, Canfield AE, Alexander MY. Hepatocyte growth factor and c-Met expression in pericytes: implications for atherosclerotic plaque development. J. Pathol. 2007;212:12–19. doi: 10.1002/path.2155. [DOI] [PubMed] [Google Scholar]
- 79.Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat. Rev. Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 80.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin YN, Inubushi M, Masamoto K, Odaka K, Aoki I, Tsuji AB, Sagara M, Koizumi M, Saga T. Long-term effects of hepatocyte growth factor gene therapy in rat myocardial infarct model. Gene Ther. 2012;19:836–843. doi: 10.1038/gt.2011.128. [DOI] [PubMed] [Google Scholar]
- 82.Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, Frangogiannis NG. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J. Am. Coll. Cardiol. 2006;48:2315–2323. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 83.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 84.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 85.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat. Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 86.Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ. Res. 2000;87:603–607. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 87.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev. Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 88.Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3’-Kinase/Akt signal transduction pathway. Circ. Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 89.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. USA. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shyu KG, Manor O, Magner M, Yancopoulos GD, Isner JM. Direct intramuscular injection of plasmid DNA encoding angiopoietin-1 but not angiopoietin-2 augments revascularization in the rabbit ischemic hindlimb. Circulation. 1998;98:2081–2087. doi: 10.1161/01.cir.98.19.2081. [DOI] [PubMed] [Google Scholar]
- 91.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 92.Shyu KG, Chang H, Isner JM. Synergistic effect of angiopoietin-1 and vascular endothelial growth factor on neoangiogenesis in hypercholesterolemic rabbit model with acute hindlimb ischemia. Life Sci. 2003;73:563–579. doi: 10.1016/s0024-3205(03)00318-7. [DOI] [PubMed] [Google Scholar]
- 93.Ahmed RP, Haider KH, Shujia J, Afzal MR, Ashraf M. Sonic Hedgehog gene delivery to the rodent heart promotes angiogenesis via iNOS/netrin-1/PKC pathway. PLoS One. 2010;5:e8576. doi: 10.1371/journal.pone.0008576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat. Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 95.Bijlsma MF, Leenders PJ, Janssen BJ, Peppelenbosch MP, Ten Cate H, Spek CA. Endogenous hedgehog expression contributes to myocardial ischemia-reperfusion-induced injury. Exp. Biol. Med. (Maywood) 2008;233:989–996. doi: 10.3181/0711-RM-307. [DOI] [PubMed] [Google Scholar]
- 96.Paulis L, Fauconnier J, Cazorla O, Thireau J, Soleti R, Vidal B, Ouille A, Bartholome M, Bideaux P, Roubille F, Le Guennec JY, Andriantsitohaina R, Martinez MC, Lacampagne A. Activation of Sonic hedgehog signaling in ventricular cardiomyocytes exerts cardioprotection against ischemia reperfusion injuries. Sci. Rep. 2015;5:7983. doi: 10.1038/srep07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chun TH, Itoh H, Ogawa Y, Tamura N, Takaya K, Igaki T, Yamashita J, Doi K, Inoue M, Masatsugu K, Korenaga R, Ando J, Nakao K. Shear stress augments expression of C-type natriuretic peptide and adrenomedullin. Hypertension. 1997;29:1296–1302. doi: 10.1161/01.hyp.29.6.1296. [DOI] [PubMed] [Google Scholar]
- 98.Kakishita M, Nishikimi T, Okano Y, Satoh T, Kyotani S, Nagaya N, Fukushima K, Nakanishi N, Takishita S, Miyata A, Kangawa K, Matsuo H, Kunieda T. Increased plasma levels of adrenomedullin in patients with pulmonary hypertension. Clin. Sci. (Lond) 1999;96:33–39. [PubMed] [Google Scholar]
- 99.Nguyen SV, Claycomb WC. Hypoxia regulates the expression of the adrenomedullin and HIF-1 genes in cultured HL-1 cardiomyocytes. Biochem. Biophys. Res. Commun. 1999;265:382–386. doi: 10.1006/bbrc.1999.1674. [DOI] [PubMed] [Google Scholar]
- 100.Nagaya N, Mori H, Murakami S, Kangawa K, Kitamura S. Adrenomedullin: angiogenesis and gene therapy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R1432–R1437. doi: 10.1152/ajpregu.00662.2004. [DOI] [PubMed] [Google Scholar]
- 101.Fernandez-Sauze S, Delfino C, Mabrouk K, Dussert C, Chinot O, Martin PM, Grisoli F, Ouafik L, Boudouresque F. Effects of adrenomedullin on endothelial cells in the multistep process of angiogenesis: involvement of CRLR/RAMP2 and CRLR/RAMP3 receptors. Int. J. Cancer. 2004;108:797–804. doi: 10.1002/ijc.11663. [DOI] [PubMed] [Google Scholar]
- 102.Nikitenko LL, Smith DM, Bicknell R, Rees MC. Transcriptional regulation of the CRLR gene in human microvascular endothelial cells by hypoxia. FASEB J. 2003;17:1499–1501. doi: 10.1096/fj.02-0993fje. [DOI] [PubMed] [Google Scholar]
- 103.Iwase T, Nagaya N, Fujii T, Itoh T, Ishibashi-Ueda H, Yamagishi M, Miyatake K, Matsumoto T, Kitamura S, Kangawa K. Adrenomedullin enhances angiogenic potency of bone marrow transplantation in a rat model of hindlimb ischemia. Circulation. 2005;111:356–362. doi: 10.1161/01.CIR.0000153352.29335.B9. [DOI] [PubMed] [Google Scholar]
- 104. [[Accessed September 4, 2016]];Cardiovascular diseases (CVDs), Retrieved from. 2016 Jun; http://www.who.int/mediacentre/factsheets/fs317/en/
- 105.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 106.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schirmer SH, van Nooijen FC, Piek JJ, van Royen N. Stimulation of collateral artery growth: travelling further down the road to clinical application. Heart. 2009;95:191–197. doi: 10.1136/hrt.2007.136119. [DOI] [PubMed] [Google Scholar]
- 108.King SB, 3rd, Marshall JJ, Tummala PE. Revascularization for coronary artery disease: stents versus bypass surgery. Annu. Rev. Med. 2010;61:199–213. doi: 10.1146/annurev.med.032309.063039. [DOI] [PubMed] [Google Scholar]
- 109.Whayne TF. A review of the role of anticoagulation in the treatment of peripheral arterial disease. Int. J. Angiol. 2012;21:187–194. doi: 10.1055/s-0032-1330232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cassar A, Holmes DR, Jr, Rihal CS, Gersh BJ. Chronic coronary artery disease: diagnosis and management. Mayo Clin. Proc. 2009;84:1130–1146. doi: 10.4065/mcp.2009.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heinl-Green A, Radke PW, Munkonge FM, Frass O, Zhu J, Vincent K, Geddes DM, Alton EW. The efficacy of a ‘master switch gene’ HIF-1alpha in a porcine model of chronic myocardial ischaemia. Eur. Heart J. 2005;26:1327–1332. doi: 10.1093/eurheartj/ehi223. [DOI] [PubMed] [Google Scholar]
- 112.Yang ZJ, Chen B, Sheng Z, Zhang DG, Jia EZ, Wang W, Ma DC, Zhu TB, Wang LS, Li CJ, Wang H, Cao KJ, Ma WZ, Improvement of heart function in postinfarct heart failure swine models after hepatocyte growth factor gene, transfer: comparison of low- medium-and high-dose groups. Mol. Biol. Rep. 2010;37:2075–2081. doi: 10.1007/s11033-009-9665-5. [DOI] [PubMed] [Google Scholar]
- 113.Banfi A, von Degenfeld G, Gianni-Barrera R, Reginato S, Merchant MJ, McDonald DM, Blau HM. Therapeutic angiogenesis due to balanced single-vector delivery of VEGF and PDGF-BB. FASEB J. 2012;26:2486–2497. doi: 10.1096/fj.11-197400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang W, Wang MQ, Wang H, Gao W, Zhang Z, Zhao S, Xu HZ, Chen B, Zhu MX, Wu ZZ, Yang JZ, Wang SL. Effects of Adenovirus-Mediated Hepatocyte Growth Factor Gene Therapy on Postinfarct Heart Function: Comparison of Single and Repeated Injections. Hum. Gene Ther. 2016 doi: 10.1089/hum.2015.119. [DOI] [PubMed] [Google Scholar]
- 115.Yeh TS, Fang YH, Lu CH, Chiu SC, Yeh CL, Yen TC, Parfyonova Y, Hu YC. Baculovirus-transduced, VEGF-expressing adipose-derived stem cell sheet for the treatment of myocardium infarction. Biomaterials. 2014;35:174–184. doi: 10.1016/j.biomaterials.2013.09.080. [DOI] [PubMed] [Google Scholar]
- 116.Jeon O, Yang HS, Lee TJ, Kim BS. Heparin-conjugated polyethylenimine for gene delivery. J. Control. Release. 2008;132:236–242. doi: 10.1016/j.jconrel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 117.Yu K, Wu S, Li H. A chitosan-graft-PEI-eprosartan conjugate for cardiomyocyte-targeted VEGF plasmid delivery in myocardial ischemia gene therapy. J. Exp. Nanosci. 2016;11:81–96. [Google Scholar]
- 118.Han S, Mahato RI, Kim SW. Water-soluble lipopolymer for gene delivery. Bioconjug. Chem. 2001;12:337–345. doi: 10.1021/bc000120w. [DOI] [PubMed] [Google Scholar]
- 119.Yockman JW, Choi D, Whitten MG, Chang CW, Kastenmeier A, Erickson H, Albanil A, Lee M, Kim SW, Bull DA. Polymeric gene delivery of ischemia-inducible VEGF significantly attenuates infarct size and apoptosis following myocardial infarct. Gene Ther. 2009;16:127–135. doi: 10.1038/gt.2008.146. [DOI] [PubMed] [Google Scholar]
- 120.Dash BC, Thomas D, Monaghan M, Carroll O, Chen X, Woodhouse K, O’Brien T, Pandit A. An injectable elastin-based gene delivery platform for dose-dependent modulation of angiogenesis and inflammation for critical limb ischemia. Biomaterials. 2015;65:126–139. doi: 10.1016/j.biomaterials.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 121.Woo J, Bae SH, Kim B, Park JS, Jung S, Lee M, Kim YH, Choi D. Cardiac Usage of Reducible Poly(oligo-D-arginine) As a Gene Carrier for Vascular Endothelial Growth Factor Expression. PLoS One. 2015;10:e0144491. doi: 10.1371/journal.pone.0144491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meilander-Lin NJ, Cheung PJ, Wilson DL, Bellamkonda RV. Sustained in vivo gene delivery from agarose hydrogel prolongs nonviral gene expression in skin. Tissue Eng. 2005;11:546–555. doi: 10.1089/ten.2005.11.546. [DOI] [PubMed] [Google Scholar]
- 123.Storrie H, Mooney DJ. Sustained delivery of plasmid DNA from polymeric scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2006;58:500–514. doi: 10.1016/j.addr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 124.Yi F, Wu H, Jia GL. Formulation and characterization of poly (D,L-lactide-co-glycolide) nanoparticle containing vascular endothelial growth factor for gene delivery. J. Clin. Pharm. Ther. 2006;31:43–48. doi: 10.1111/j.1365-2710.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 125.Song H, Liu S, Li C, Geng Y, Wang G, Gu Z. Pluronic L64-mediated stable HIF-1alpha expression in muscle for therapeutic angiogenesis in mouse hindlimb ischemia. Int. J. Nanomedicine. 2014;9:3439–3452. doi: 10.2147/IJN.S65353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mayer CR, Bekeredjian R. Ultrasonic gene and drug delivery to the cardiovascular system. Adv. Drug Deliv. Rev. 2008;60:1177–1192. doi: 10.1016/j.addr.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 127.Endo-Takahashi Y, Negishi Y, Nakamura A, Ukai S, Ooaku K, Oda Y, Sugimoto K, Moriyasu F, Takagi N, Suzuki R, Maruyama K, Aramaki Y. Systemic delivery of miR-126 by miRNA-loaded Bubble liposomes for the treatment of hindlimb ischemia. Sci. Rep. 2014;4:3883. doi: 10.1038/srep03883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fujii H, Li SH, Wu J, Miyagi Y, Yau TM, Rakowski H, Egashira K, Guo J, Weisel RD, Li RK. Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair. Eur. Heart J. 2011;32:2075–2084. doi: 10.1093/eurheartj/ehq475. [DOI] [PubMed] [Google Scholar]
- 129.Fujii H, Sun Z, Li SH, Wu J, Fazel S, Weisel RD, Rakowski H, Lindner J, Li RK. Ultrasound-targeted gene delivery induces angiogenesis after a myocardial infarction in mice. JACC Cardiovasc. Imaging. 2009;2:869–879. doi: 10.1016/j.jcmg.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 130.Wang HB, Yang L, Wu J, Sun L, Wu J, Tian H, Weisel RD, Li RK. Reduced ischemic injury after stroke in mice by angiogenic gene delivery via ultrasound-targeted microbubble destruction. J. Neuropathol. Exp. Neurol. 2014;73:548–558. doi: 10.1097/NEN.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 131.Jo J, Nagaya N, Miyahara Y, Kataoka M, Harada-Shiba M, Kangawa K, Tabata Y, Transplantation of genetically engineered mesenchymal stem cells improves cardiac function in rats with myocardial, infarction: benefit of a novel nonviral vector. cationized dextran. Tissue Eng. 2007;13:313–322. doi: 10.1089/ten.2006.0133. [DOI] [PubMed] [Google Scholar]
- 132.Moon HH, Joo MK, Mok H, Lee M, Hwang KC, Kim SW, Jeong JH, Choi D, Kim SH. MSC-based VEGF gene therapy in rat myocardial infarction model using facial amphipathic bile acid-conjugated polyethyleneimine. Biomaterials. 2014;35:1744–1754. doi: 10.1016/j.biomaterials.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 133.Zhu K, Wu M, Lai H, Guo C, Li J, Wang Y, Chen Y, Wang C, Shi J. Nanoparticle-enhanced generation of gene-transfected mesenchymal stem cells for in vivo cardiac repair. Biomaterials. 2016;74:188–199. doi: 10.1016/j.biomaterials.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 134.Park JS, Yang HN, Yi SW, Kim JH, Park KH. Neoangiogenesis of human mesenchymal stem cells transfected with peptide-loaded and gene-coated PLGA nanoparticles. Biomaterials. 2016;76:226–237. doi: 10.1016/j.biomaterials.2015.10.062. [DOI] [PubMed] [Google Scholar]
- 135.Yang F, Cho SW, Son SM, Bogatyrev SR, Singh D, Green JJ, Mei Y, Park S, Bhang SH, Kim BS, Langer R, Anderson DG. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc. Natl. Acad. Sci. USA. 2010;107:3317–3322. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang HN, Park JS, Woo DG, Jeon SY, Park KH. Transfection of VEGF(165) genes into endothelial progenitor cells and in vivo imaging using quantum dots in an ischemia hind limb model. Biomaterials. 2012;33:8670–8684. doi: 10.1016/j.biomaterials.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 137.Ye L, Haider H, Tan R, Su L, Law PK, Zhang W, Sim EK. Angiomyogenesis using liposome based vascular endothelial growth factor-165 transfection with skeletal myoblast for cardiac repair. Biomaterials. 2008;29:2125–2137. doi: 10.1016/j.biomaterials.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 138.Ye L, Haider H, Tan R, Toh W, Law PK, Tan W, Su L, Zhang W, Ge R, Zhang Y, Lim Y, Sim EK. Transplantation of nanoparticle transfected skeletal myoblasts overexpressing vascular endothelial growth factor-165 for cardiac repair. Circulation. 2007;116:I113–I120. doi: 10.1161/CIRCULATIONAHA.106.680124. [DOI] [PubMed] [Google Scholar]
- 139.Springer ML, Ozawa CR, Banfi A, Kraft PE, Ip TK, Brazelton TR, Blau HM. Localized arteriole formation directly adjacent to the site of VEGF-induced angiogenesis in muscle. Mol. Ther. 2003;7:441–449. doi: 10.1016/s1525-0016(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 140.Cho SW, Yang F, Son SM, Park HJ, Green JJ, Bogatyrev S, Mei Y, Park S, Langer R, Anderson DG. Therapeutic angiogenesis using genetically engineered human endothelial cells. J. Control. Release. 2012;160:515–524. doi: 10.1016/j.jconrel.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gomes RS, das Neves RP, Cochlin L, Lima A, Carvalho R, Korpisalo P, Dragneva G, Turunen M, Liimatainen T, Clarke K, Yla-Herttuala S, Carr C, Ferreira L. Efficient pro-survival/angiogenic miRNA delivery by an MRI-detectable nanomaterial. ACS Nano. 2013;7:3362–3372. doi: 10.1021/nn400171w. [DOI] [PubMed] [Google Scholar]
- 142.Devalliere J, Chang WG, Andrejecsk JW, Abrahimi P, Cheng CJ, Jane-wit D, Saltzman WM, Pober JS. Sustained delivery of proangiogenic microRNA-132 by nanoparticle transfection improves endothelial cell transplantation. FASEB J. 2014;28:908–922. doi: 10.1096/fj.13-238527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ko HC, Milthorpe BK, McFarland CD. Engineering thick tissues--the vascularisation problem. Eur. Cell. Mater. 2007;14:1–18. doi: 10.22203/ecm.v014a01. discussion 18-19. [DOI] [PubMed] [Google Scholar]
- 144.Balaji S, LeSaint M, Bhattacharya SS, Moles C, Dhamija Y, Kidd M, Le LD, King A, Shaaban A, Crombleholme TM, Bollyky P, Keswani SG. Adenoviral-mediated gene transfer of insulin-like growth factor 1 enhances wound healing and induces angiogenesis. J. Surg. Res. 2014;190:367–377. doi: 10.1016/j.jss.2014.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rundle CH, Strong DD, Chen ST, Linkhart TA, Sheng MH, Wergedal JE, Lau KH, Baylink DJ. Retroviral-based gene therapy with cyclooxygenase-2 promotes the union of bony callus tissues and accelerates fracture healing in the rat. J. Gene Med. 2008;10:229–241. doi: 10.1002/jgm.1148. [DOI] [PubMed] [Google Scholar]
- 146.Liu YG, Zhou Y, Hu X, Fu JJ, Pan Y, Chu TW. Effect of vascular endothelial growth factor 121 adenovirus transduction in rabbit model of femur head necrosis. J. Trauma. 2011;70:1519–1523. doi: 10.1097/TA.0b013e3181f31595. [DOI] [PubMed] [Google Scholar]
- 147.Ding H, Gao YS, Hu C, Wang Y, Wang CG, Yin JM, Sun Y, Zhang CQ. HIF-1alpha transgenic bone marrow cells can promote tissue repair in cases of corticosteroid-induced osteonecrosis of the femoral head in rabbits. PLoS One. 2013;8:e63628. doi: 10.1371/journal.pone.0063628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Koc A, Finkenzeller G, Elcin AE, Stark GB, Elcin YM. Evaluation of adenoviral vascular endothelial growth factor-activated chitosan/hydroxyapatite scaffold for engineering vascularized bone tissue using human osteoblasts: In vitro and in vivo studies. J. Biomater. Appl. 2014;29:748–760. doi: 10.1177/0885328214544769. [DOI] [PubMed] [Google Scholar]
- 149.Li Y, Zheng L, Xu X, Song L, Li Y, Li W, Zhang S, Zhang F, Jin H. Mesenchymal stem cells modified with angiopoietin-1 gene promote wound healing. Stem Cell Res. Ther. 2013;4:113. doi: 10.1186/scrt324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Park HJ, Lee J, Kim MJ, Kang TJ, Jeong Y, Um SH, Cho SW. Sonic hedgehog intradermal gene therapy using a biodegradable poly(beta-amino esters) nanoparticle to enhance wound healing. Biomaterials. 2012;33:9148–9156. doi: 10.1016/j.biomaterials.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 151.Nauta A, Seidel C, Deveza L, Montoro D, Grova M, Ko SH, Hyun J, Gurtner GC, Longaker MT, Yang F. Adipose-derived stromal cells overexpressing vascular endothelial growth factor accelerate mouse excisional wound healing. Mol. Ther. 2013;21:445–455. doi: 10.1038/mt.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mangraviti A, Tzeng SY, Gullotti D, Kozielski KL, Kim JE, Seng M, Abbadi S, Schiapparelli P, Sarabia-Estrada R, Vescovi A, Brem H, Olivi A, Tyler B, Green JJ, Quinones-Hinojosa A. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials. 2016;100:53–66. doi: 10.1016/j.biomaterials.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tokatlian T, Cam C, Segura T. Porous hyaluronic acid hydrogels for localized nonviral DNA delivery in a diabetic wound healing model. Adv. Healthc. Mater. 2015;4:1084–1091. doi: 10.1002/adhm.201400783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Elangovan S, D’Mello SR, Hong L, Ross RD, Allamargot C, Dawson DV, Stanford CM, Johnson GK, Sumner DR, Salem AK. The enhancement of bone regeneration by gene activated matrix encoding for platelet derived growth factor. Biomaterials. 2014;35:737–747. doi: 10.1016/j.biomaterials.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Curtin CM, Tierney EG, McSorley K, Cryan SA, Duffy GP, O’Brien FJ. Combinatorial gene therapy accelerates bone regeneration: non-viral dual delivery of VEGF and BMP2 in a collagen-nanohydroxyapatite scaffold. Adv. Healthc. Mater. 2015;4:223–227. doi: 10.1002/adhm.201400397. [DOI] [PubMed] [Google Scholar]
- 156.Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol. Ther. 2005;11:990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 157.Ricard-Blum S, Vallet SD. Matricryptins Network with Matricellular Receptors at the Surface of Endothelial and Tumor Cells. Front. Pharmacol. 2016;7:11. doi: 10.3389/fphar.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc. Res. Tech. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torre A, Maeshima Y, Mier JW, Sukhatme VP, Kalluri R. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J. Biol. Chem. 2000;275:1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 161.Maeshima Y, Yerramalla UL, Dhanabal M, Holthaus KA, Barbashov S, Kharbanda S, Reimer C, Manfredi M, Dickerson WM, Kalluri R. Extracellular matrix-derived peptide binds to alpha(v)beta(3) integrin and inhibits angiogenesis. J. Biol. Chem. 2001;276:31959–31968. doi: 10.1074/jbc.M103024200. [DOI] [PubMed] [Google Scholar]
- 162.Nyberg P, Xie L, Sugimoto H, Colorado P, Sund M, Holthaus K, Sudhakar A, Salo T, Kalluri R, Characterization of the anti-angiogenic properties of arresten. an alpha1beta1 integrin-dependent collagen-derived tumor suppressor. Exp. Cell Res. 2008;314:3292–3305. doi: 10.1016/j.yexcr.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 164.Mongiat M, Sweeney SM, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J. Biol. Chem. 2003;278:4238–4249. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- 165.Claesson-Welsh L, Welsh M, Ito N, Anand-Apte B, Soker S, Zetter B, O’Reilly M, Folkman J. Angiostatin induces endothelial cell apoptosis and activation of focal adhesion kinase independently of the integrin-binding motif RGD. Proc. Natl. Acad. Sci. USA. 1998;95:5579–5583. doi: 10.1073/pnas.95.10.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Eriksson K, Magnusson P, Dixelius J, Claesson-Welsh L, Cross MJ. Angiostatin and endostatin inhibit endothelial cell migration in response to FGF and VEGF without interfering with specific intracellular signal transduction pathways. FEBS Lett. 2003;536:19–24. doi: 10.1016/s0014-5793(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 167.Magnon C, Galaup A, Mullan B, Rouffiac V, Bouquet C, Bidart JM, Griscelli F, Opolon P, Perricaudet M. Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with alphavbeta3 and alphavbeta5 integrins. Cancer Res. 2005;65:4353–4361. doi: 10.1158/0008-5472.CAN-04-3536. [DOI] [PubMed] [Google Scholar]
- 168.Tarui T, Miles LA, Takada Y. Specific interaction of angiostatin with integrin alpha(v)beta(3) in endothelial cells. J. Biol. Chem. 2001;276:39562–39568. doi: 10.1074/jbc.M101815200. [DOI] [PubMed] [Google Scholar]
- 169.Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K, Vuori K. Interaction of endostatin with integrins implicated in angiogenesis. Proc. Natl. Acad. Sci. USA. 2001;98:1024–1029. doi: 10.1073/pnas.031564998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Woodall BP, Nystrom A, Iozzo RA, Eble JA, Niland S, Krieg T, Eckes B, Pozzi A, Iozzo RV. Integrin alpha2beta1 is the required receptor for endorepellin angiostatic activity. J. Biol. Chem. 2008;283:2335–2343. doi: 10.1074/jbc.M708364200. [DOI] [PubMed] [Google Scholar]
- 171.Bridgewater RE, Norman JC, Caswell PT. Integrin trafficking at a glance. J. Cell Sci. 2012;125:3695–3701. doi: 10.1242/jcs.095810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rosca EV, Koskimaki JE, Pandey NB, Tamiz AP, Popel AS. Structure-activity relationship study of collagen-derived anti-angiogenic biomimetic peptides. Chem. Biol. Drug Des. 2012;80:27–37. doi: 10.1111/j.1747-0285.2012.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee E, Lee SJ, Koskimaki JE, Han Z, Pandey NB, Popel AS. Inhibition of breast cancer growth and metastasis by a biomimetic peptide. Sci. Rep. 2014;4:7139. doi: 10.1038/srep07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rosca EV, Lal B, Koskimaki JE, Popel AS, Laterra J. Collagen IV and CXC chemokine-derived antiangiogenic peptides suppress glioma xenograft growth. Anticancer Drugs. 2012;23:706–712. doi: 10.1097/CAD.0b013e3283531041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cano Mdel V, Karagiannis ED, Soliman M, Bakir B, Zhuang W, Popel AS, Gehlbach PL. A peptide derived from type 1 thrombospondin repeat-containing protein WISP-1 inhibits corneal and choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2009;50:3840–3845. doi: 10.1167/iovs.08-2607. [DOI] [PubMed] [Google Scholar]
- 176.Koskimaki JE, Lee E, Chen W, Rivera CG, Rosca EV, Pandey NB, Popel AS. Synergy between a collagen IV mimetic peptide and a somatotropin-domain derived peptide as angiogenesis and lymphangiogenesis inhibitors. Angiogenesis. 2013;16:159–170. doi: 10.1007/s10456-012-9308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rosca EV, Koskimaki JE, Rivera CG, Pandey NB, Tamiz AP, Popel AS. Anti-angiogenic peptides for cancer therapeutics. Curr. Pharm. Biotechnol. 2011;12:1101–1116. doi: 10.2174/138920111796117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee E, Koskimaki JE, Pandey NB, Popel AS. Inhibition of lymphangiogenesis and angiogenesis in breast tumor xenografts and lymph nodes by a peptide derived from transmembrane protein 45A. Neoplasia. 2013;15:112–124. doi: 10.1593/neo.121638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee E, Rosca EV, Pandey NB, Popel AS. Small peptides derived from somatotropin domain-containing proteins inhibit blood and lymphatic endothelial cell proliferation, migration, adhesion and tube formation. Int. J. Biochem. Cell Biol. 2011;43:1812–1821. doi: 10.1016/j.biocel.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bhise NS, Shmueli RB, Gonzalez J, Green JJ. A novel assay for quantifying the number of plasmids encapsulated by polymer nanoparticles. Small. 2012;8:367–373. doi: 10.1002/smll.201101718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 182.Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ. Res. 1999;85:906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- 183.Ikenaka Y, Yoshiji H, Kuriyama S, Yoshii J, Noguchi R, Tsujinoue H, Yanase K, Namisaki T, Imazu H, Masaki T, Fukui H. Tissue inhibitor of metalloproteinases-1 (TIMP-1) inhibits tumor growth and angiogenesis in the TIMP-1 transgenic mouse model. Int. J. Cancer. 2003;105:340–346. doi: 10.1002/ijc.11094. [DOI] [PubMed] [Google Scholar]
- 184.Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat. Med. 2003;9:407–415. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- 185.Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 186.Shen Q, Lee ES, Pitts RL, Wu MH, Yuan SY. Tissue inhibitor of metalloproteinase-2 regulates matrix metalloproteinase-2-mediated endothelial barrier dysfunction and breast cancer cell transmigration through lung microvascular endothelial cells. Mol. Cancer Res. 2010;8:939–951. doi: 10.1158/1541-7786.MCR-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bloomston M, Shafii A, Zervos EE, Rosemurgy AS. TIMP-1 overexpression in pancreatic cancer attenuates tumor growth, decreases implantation and metastasis, and inhibits angiogenesis. J. Surg. Res. 2002;102:39–44. doi: 10.1006/jsre.2001.6318. [DOI] [PubMed] [Google Scholar]
- 188.Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 2002;21:2245–2252. doi: 10.1038/sj.onc.1205291. [DOI] [PubMed] [Google Scholar]
- 189.Leifler KS, Svensson S, Abrahamsson A, Bendrik C, Robertson J, Gauldie J, Olsson AK, Dabrosin C. Inflammation induced by MMP-9 enhances tumor regression of experimental breast cancer. J. Immunol. 2013;190:4420–4430. doi: 10.4049/jimmunol.1202610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liss M, Sreedhar N, Keshgegian A, Sauter G, Chernick MR, Prendergast GC, Wallon UM. Tissue inhibitor of metalloproteinase-4 is elevated in early-stage breast cancers with accelerated progression and poor clinical course. Am. J. Pathol. 2009;175:940–946. doi: 10.2353/ajpath.2009.081094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Barleon B, Reusch P, Totzke F, Herzog C, Keck C, Martiny-Baron G, Marme D. Soluble VEGFR-1 secreted by endothelial cells and monocytes is present in human serum and plasma from healthy donors. Angiogenesis. 2001;4:143–154. doi: 10.1023/a:1012245307884. [DOI] [PubMed] [Google Scholar]
- 192.Hornig C, Barleon B, Ahmad S, Vuorela P, Ahmed A, Weich HA. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest. 2000;80:443–454. doi: 10.1038/labinvest.3780050. [DOI] [PubMed] [Google Scholar]
- 193.Wu FT, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH, Popel AS. A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J. Cell. Mol. Med. 2010;14:528–552. doi: 10.1111/j.1582-4934.2009.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ahmad S, Hewett PW, Al-Ani B, Sissaoui S, Fujisawa T, Cudmore MJ, Ahmed A. Autocrine activity of soluble Flt-1 controls endothelial cell function and angiogenesis. Vasc. Cell. 2011;3:15. doi: 10.1186/2045-824X-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem. Biophys. Res. Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 196.Elkin M, Orgel A, Kleinman HK. An angiogenic switch in breast cancer involves estrogen and soluble vascular endothelial growth factor receptor 1. J. Natl. Cancer Inst. 2004;96:875–878. doi: 10.1093/jnci/djh140. [DOI] [PubMed] [Google Scholar]
- 197.Yamaguchi T, Bando H, Mori T, Takahashi K, Matsumoto H, Yasutome M, Weich H, Toi M. Overexpression of soluble vascular endothelial growth factor receptor 1 in colorectal cancer: Association with progression and prognosis. Cancer Sci. 2007;98:405–410. doi: 10.1111/j.1349-7006.2007.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.He X, Cheng R, Benyajati S, Ma JX. PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases. Clin. Sci. (Lond) 2015;128:805–823. doi: 10.1042/CS20130463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 200.Aurora AB, Biyashev D, Mirochnik Y, Zaichuk TA, Sanchez-Martinez C, Renault MA, Losordo D, Volpert OV. NF-kappaB balances vascular regression and angiogenesis via chromatin remodeling and NFAT displacement. Blood. 2010;116:475–484. doi: 10.1182/blood-2009-07-232132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ho TC, Chen SL, Yang YC, Liao CL, Cheng HC, Tsao YP. PEDF induces p53-mediated apoptosis through PPAR gamma signaling in human umbilical vein endothelial cells. Cardiovasc. Res. 2007;76:213–223. doi: 10.1016/j.cardiores.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 202.Belkacemi L, Zhang SX. Anti-tumor effects of pigment epithelium-derived factor (PEDF): implication for cancer therapy. A mini-review. J. Exp. Clin. Cancer Res. 2016;35:4. doi: 10.1186/s13046-015-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Becerra SP, Notario V. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nat. Rev. Cancer. 2013;13:258–271. doi: 10.1038/nrc3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Manalo KB, Choong PF, Becerra SP, Dass CR. Pigment epithelium-derived factor as an anticancer drug and new treatment methods following the discovery of its receptors: a patent perspective. Expert Opin. Ther. Pat. 2011;21:121–130. doi: 10.1517/13543776.2011.545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Campochiaro PA. Potential applications for RNAi to probe pathogenesis and develop new treatments for ocular disorders. Gene Ther. 2006;13:559–562. doi: 10.1038/sj.gt.3302653. [DOI] [PubMed] [Google Scholar]
- 206.Devi GR. siRNA-based approaches in cancer therapy. Cancer Gene Ther. 2006;13:819–829. doi: 10.1038/sj.cgt.7700931. [DOI] [PubMed] [Google Scholar]
- 207.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 208.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 209.Furuse J. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Biologics. 2008;2:779–788. doi: 10.2147/btt.s3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Boehm T, Folkman J, Browder T, O’Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 211.Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17:471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, Group SIS. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 213.Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin. Ther. 2006;28:1779–1802. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 214.Kim WJ, Yockman JW, Jeong JH, Christensen LV, Lee M, Kim YH, Kim SW. Anti-angiogenic inhibition of tumor growth by systemic delivery of PEI-g-PEG-RGD/pCMV-sFlt-1 complexes in tumor-bearing mice. J. Control. Release. 2006;114:381–388. doi: 10.1016/j.jconrel.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 215.Marelli UK, Rechenmacher F, Sobahi TR, Mas-Moruno C, Kessler H. Tumor Targeting via Integrin Ligands. Front. Oncol. 2013;3:222. doi: 10.3389/fonc.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kim J, Kim SW, Kim WJ. PEI-g-PEG-RGD/small interference RNA polyplex-mediated silencing of vascular endothelial growth factor receptor and its potential as an anti-angiogenic tumor therapeutic strategy. Oligonucleotides. 2011;21:101–107. doi: 10.1089/oli.2011.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li L, Yang J, Wang WW, Yao YC, Fang SH, Dai ZY, Hong HH, Yang X, Shuai XT, Gao GQ. Pigment epithelium-derived factor gene loaded in cRGD-PEG-PEI suppresses colorectal cancer growth by targeting endothelial cells. Int. J. Pharm. 2012;438:1–10. doi: 10.1016/j.ijpharm.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 218.Xiao J, Duan X, Yin Q, Miao Z, Yu H, Chen C, Zhang Z, Wang J, Li Y. The inhibition of metastasis and growth of breast cancer by blocking the NF-kappaB signaling pathway using bioreducible PEI-based/p65 shRNA complex nanoparticles. Biomaterials. 2013;34:5381–5390. doi: 10.1016/j.biomaterials.2013.03.084. [DOI] [PubMed] [Google Scholar]
- 219.Vincent L, Varet J, Pille JY, Bompais H, Opolon P, Maksimenko A, Malvy C, Mirshahi M, Lu H, Vannier JP, Soria C, Li H. Efficacy of dendrimer-mediated angiostatin and TIMP-2 gene delivery on inhibition of tumor growth and angiogenesis: in vitro and in vivo studies. Int. J. Cancer. 2003;105:419–429. doi: 10.1002/ijc.11105. [DOI] [PubMed] [Google Scholar]
- 220.Oga M, Takenaga K, Sato Y, Nakajima H, Koshikawa N, Osato K, Sakiyama S. Inhibition of metastatic brain tumor growth by intramuscular administration of the endostatin gene. Int. J. Oncol. 2003;23:73–79. [PubMed] [Google Scholar]
- 221.Kanazawa T, Sugawara K, Tanaka K, Horiuchi S, Takashima Y, Okada H. Suppression of tumor growth by systemic delivery of anti-VEGF siRNA with cell-penetrating peptide-modified MPEG-PCL nanomicelles. Eur. J. Pharm. Biopharm. 2012;81:470–477. doi: 10.1016/j.ejpb.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 222.Tanaka K, Kanazawa T, Shibata Y, Suda Y, Fukuda T, Takashima Y, Okada H. Development of cell-penetrating peptide-modified MPEG-PCL diblock copolymeric nanoparticles for systemic gene delivery. Int. J. Pharm. 2010;396:229–238. doi: 10.1016/j.ijpharm.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 223.Oba M, Vachutinsky Y, Miyata K, Kano MR, Ikeda S, Nishiyama N, Itaka K, Miyazono K, Koyama H, Kataoka K. Antiangiogenic gene therapy of solid tumor by systemic injection of polyplex micelles loading plasmid DNA encoding soluble flt-1. Mol. Pharm. 2010;7:501–509. doi: 10.1021/mp9002317. [DOI] [PubMed] [Google Scholar]
- 224.Huang S, Shao K, Liu Y, Kuang Y, Li J, An S, Guo Y, Ma H, Jiang C. Tumor-targeting and microenvironment-responsive smart nanoparticles for combination therapy of antiangiogenesis and apoptosis. ACS Nano. 2013;7:2860–2871. doi: 10.1021/nn400548g. [DOI] [PubMed] [Google Scholar]
- 225.Ortiz Mellet C, Garcia Fernandez JM, Benito JM. Cyclodextrin-based gene delivery systems. Chem. Soc. Rev. 2011;40:1586–1608. doi: 10.1039/c0cs00019a. [DOI] [PubMed] [Google Scholar]
- 226.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 227.Huang Z, Dong L, Chen J, Gao F, Zhang Z, Chen J, Zhang J. Low-molecular weight chitosan/vascular endothelial growth factor short hairpin RNA for the treatment of hepatocellular carcinoma. Life Sci. 2012;91:1207–1215. doi: 10.1016/j.lfs.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 228.Guo J, Ogier JR, Desgranges S, Darcy R, O’Driscoll C. Anisamide-targeted cyclodextrin nanoparticles for siRNA delivery to prostate tumours in mice. Biomaterials. 2012;33:7775–7784. doi: 10.1016/j.biomaterials.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 229.Sakurai Y, Hatakeyama H, Sato Y, Hyodo M, Akita H, Ohga N, Hida K, Harashima H. RNAi-mediated gene knockdown and anti-angiogenic therapy of RCCs using a cyclic RGD-modified liposomal-siRNA system. J. Control. Release. 2014;173:110–118. doi: 10.1016/j.jconrel.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 230.Akita H, Ishiba R, Togashi R, Tange K, Nakai Y, Hatakeyama H, Harashima H. A neutral lipid envelope-type nanoparticle composed of a pH-activated and vitamin E-scaffold lipid-like material as a platform for a gene carrier targeting renal cell carcinoma. J. Control. Release. 2015;200:97–105. doi: 10.1016/j.jconrel.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 231.Anand S, Majeti BK, Acevedo LM, Murphy EA, Mukthavaram R, Scheppke L, Huang M, Shields DJ, Lindquist JN, Lapinski PE, King PD, Weis SM, Cheresh DA. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat. Med. 2010;16:909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kim WJ, Chang CW, Lee M, Kim SW. Efficient siRNA delivery using water soluble lipopolymer for anti-angiogenic gene therapy. J. Control. Release. 2007;118:357–363. doi: 10.1016/j.jconrel.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 233.Fujii H, Matkar P, Liao C, Rudenko D, Lee PJ, Kuliszewski MA, Prud’homme GJ, Leong-Poi H. Optimization of Ultrasound-mediated Anti-angiogenic Cancer Gene Therapy. Mol. Ther. Nucleic Acids. 2013;2:e94. doi: 10.1038/mtna.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ratner M. Next-generation AMD drugs to wed blockbusters. Nat. Biotechnol. 2014;32:701–702. doi: 10.1038/nbt0814-701. [DOI] [PubMed] [Google Scholar]
- 235.Zulliger R, Conley SM, Naash MI. Non-viral therapeutic approaches to ocular diseases: An overview and future directions. J. Control. Release. 2015;219:471–487. doi: 10.1016/j.jconrel.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Qi Y, Dai X, Zhang H, He Y, Zhang Y, Han J, Zhu P, Zhang Y, Zheng Q, Li X, Zhao C, Pang J. Trans-Corneal Subretinal Injection in Mice and Its Effect on the Function and Morphology of the Retina. PLoS One. 2015;10:e0136523. doi: 10.1371/journal.pone.0136523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Einmahl S, Savoldelli M, D’Hermies F, Tabatabay C, Gurny R, Behar-Cohen F. Evaluation of a novel biomaterial in the suprachoroidal space of the rabbit eye. Invest. Ophthalmol. Vis. Sci. 2002;43:1533–1539. [PubMed] [Google Scholar]
- 238.Lukason M, DuFresne E, Rubin H, Pechan P, Li Q, Kim I, Kiss S, Flaxel C, Collins M, Miller J, Hauswirth W, Maclachlan T, Wadsworth S, Scaria A. Inhibition of choroidal neovascularization in a nonhuman primate model by intravitreal administration of an AAV2 vector expressing a novel anti-VEGF molecule. Mol. Ther. 2011;19:260–265. doi: 10.1038/mt.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pechan P, Rubin H, Lukason M, Ardinger J, DuFresne E, Hauswirth WW, Wadsworth SC, Scaria A. Novel anti-VEGF chimeric molecules delivered by AAV vectors for inhibition of retinal neovascularization. Gene Ther. 2009;16:10–16. doi: 10.1038/gt.2008.115. [DOI] [PubMed] [Google Scholar]
- 240.Balaggan KS, Binley K, Esapa M, MacLaren RE, Iqball S, Duran Y, Pearson RA, Kan O, Barker SE, Smith AJ, Bainbridge JW, Naylor S, Ali RR. EIAV vector-mediated delivery of endostatin or angiostatin inhibits angiogenesis and vascular hyperpermeability in experimental CNV. Gene Ther. 2006;13:1153–1165. doi: 10.1038/sj.gt.3302769. [DOI] [PubMed] [Google Scholar]
- 241.Weber M, Rabinowitz J, Provost N, Conrath H, Folliot S, Briot D, Cherel Y, Chenuaud P, Samulski J, Moullier P, Rolling F. Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol. Ther. 2003;7:774–781. doi: 10.1016/s1525-0016(03)00098-4. [DOI] [PubMed] [Google Scholar]
- 242.Raisler BJ, Berns KI, Grant MB, Beliaev D, Hauswirth WW. Adeno-associated virus type-2 expression of pigmented epithelium-derived factor or Kringles 1-3 of angiostatin reduce retinal neovascularization. Proc. Natl. Acad. Sci. USA. 2002;99:8909–8914. doi: 10.1073/pnas.122247299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Campochiaro PA, Nguyen QD, Shah SM, Klein ML, Holz E, Frank RN, Saperstein DA, Gupta A, Stout JT, Macko J, DiBartolomeo R, Wei LL. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum. Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- 244.Takahashi T, Nakamura T, Hayashi A, Kamei M, Nakabayashi M, Okada AA, Tomita N, Kaneda Y, Tano Y. Inhibition of experimental choroidal neovascularization by overexpression of tissue inhibitor of metalloproteinases-3 in retinal pigment epithelium cells. Am. J. Ophthalmol. 2000;130:774–781. doi: 10.1016/s0002-9394(00)00772-8. [DOI] [PubMed] [Google Scholar]
- 245.Prea SM, Chan EC, Dusting GJ, Vingrys AJ, Bui BV, Liu GS. Gene Therapy with Endogenous Inhibitors of Angiogenesis for Neovascular Age-Related Macular Degeneration: Beyond Anti-VEGF Therapy. J. Ophthalmol. 2015;2015:201726. doi: 10.1155/2015/201726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sunshine JC, Sunshine SB, Bhutto I, Handa JT, Green JJ. Poly(beta-amino ester)-nanoparticle mediated transfection of retinal pigment epithelial cells in vitro and in vivo. PLoS one. 2012;7:e37543. doi: 10.1371/journal.pone.0037543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sheu SJ, Chou LC, Bee YS, Chen JF, Lin HC, Lin PR, Lam HC, Tai MH. Suppression of choroidal neovascularization by intramuscular polymer-based gene delivery of vasostatin. Exp. Eye Res. 2005;81:673–679. doi: 10.1016/j.exer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 248.Zhang C, Wang YS, Wu H, Zhang ZX, Cai Y, Hou HY, Zhao W, Yang XM, Ma JX. Inhibitory efficacy of hypoxia-inducible factor 1alpha short hairpin RNA plasmid DNA-loaded poly (D, L-lactide-co-glycolide) nanoparticles on choroidal neovascularization in a laser-induced rat model. Gene Ther. 2010;17:338–351. doi: 10.1038/gt.2009.158. [DOI] [PubMed] [Google Scholar]
- 249.Liu HA, Liu YL, Ma ZZ, Wang JC, Zhang Q. A lipid nanoparticle system improves siRNA efficacy in RPE cells and a laser-induced murine CNV model. Invest. Ophthalmol. Vis. Sci. 2011;52:4789–4794. doi: 10.1167/iovs.10-5891. [DOI] [PubMed] [Google Scholar]
- 250.Salehi-Had H, Roh MI, Giani A, Hisatomi T, Nakao S, Kim IK, Gragoudas ES, Vavvas D, Guccione S, Miller JW. Utilizing targeted gene therapy with nanoparticles binding alpha v beta 3 for imaging and treating choroidal neovascularization. PLoS One. 2011;6:e18864. doi: 10.1371/journal.pone.0018864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Marano RJ, Toth I, Wimmer N, Brankov M, Rakoczy PE. Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: a long-term study into inhibition of laser-induced CNV, distribution, uptake and toxicity. Gene Ther. 2005;12:1544–1550. doi: 10.1038/sj.gt.3302579. [DOI] [PubMed] [Google Scholar]
- 252.Singh SR, Grossniklaus HE, Kang SJ, Edelhauser HF, Ambati BK, Kompella UB. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16:645–659. doi: 10.1038/gt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Luo L, Zhang X, Hirano Y, Tyagi P, Barabas P, Uehara H, Miya TR, Singh N, Archer B, Qazi Y, Jackman K, Das SK, Olsen T, Chennamaneni SR, Stagg BC, Ahmed F, Emerson L, Zygmunt K, Whitaker R, Mamalis C, Huang W, Gao G, Srinivas SP, Krizaj D, Baffi J, Ambati J, Kompella UB, Ambati BK. Targeted intraceptor nanoparticle therapy reduces angiogenesis and fibrosis in primate and murine macular degeneration. ACS Nano. 2013;7:3264–3275. doi: 10.1021/nn305958y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Rajagopalan S, Mohler E, 3rd, Lederman RJ, Saucedo J, Mendelsohn FO, Olin J, Blebea J, Goldman C, Trachtenberg JD, Pressler M, Rasmussen H, Annex BH, Hirsch AT. t. Regional Angiogenesis With Vascular Endothelial Growth Factor, Regional Angiogenesis with Vascular Endothelial Growth Factor (VEGF) in peripheral arterial disease: Design of the RAVE trial. Am. Heart J. 2003;145:1114–1118. doi: 10.1016/S0002-8703(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 255.Kusumanto YH, van Weel V, Mulder NH, Smit AJ, van den Dungen JJ, Hooymans JM, Sluiter WJ, Tio RA, Quax PH, Gans RO, Dullaart RP, Hospers GA. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double-blind randomized trial. Hum. Gene Ther. 2006;17:683–691. doi: 10.1089/hum.2006.17.683. [DOI] [PubMed] [Google Scholar]
- 256.Makinen K, Manninen H, Hedman M, Matsi P, Mussalo H, Alhava E, Yla-Herttuala S. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: a randomized, placebo-controlled, double-blinded phase II study. Mol. Ther. 2002;6:127–133. doi: 10.1006/mthe.2002.0638. [DOI] [PubMed] [Google Scholar]
- 257.Muona K, Makinen K, Hedman M, Manninen H, Yla-Herttuala S. 10-year safety follow-up in patients with local VEGF gene transfer to ischemic lower limb. Gene Ther. 2012;19:392–395. doi: 10.1038/gt.2011.109. [DOI] [PubMed] [Google Scholar]
- 258.Giusti II, Rodrigues CG, Salles FB, Sant’Anna RT, Eibel B, Han SW, Ludwig E, Grossman G, Prates PR, Sant’Anna JR, Filho GF, Markoski MM, Nesralla IA, Nardi NB, Kalil RA. High doses of vascular endothelial growth factor 165 safely, but transiently, improve myocardial perfusion in no-option ischemic disease. Hum. Gene Ther. Methods. 2013;24:298–306. doi: 10.1089/hgtb.2012.221. [DOI] [PubMed] [Google Scholar]
- 259.Stewart DJ, Kutryk MJ, Fitchett D, Freeman M, Camack N, Su Y, Della Siega A, Bilodeau L, Burton JR, Proulx G, Radhakrishnan S, Investigators NT. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol. Ther. 2009;17:1109–1115. doi: 10.1038/mt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, Van Belle E, Committees T. Investigators, Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377:1929–1937. doi: 10.1016/S0140-6736(11)60394-2. [DOI] [PubMed] [Google Scholar]
- 261.Grines CL, Watkins MW, Mahmarian JJ, Iskandrian AE, Rade JJ, Marrott P, Pratt C, Kleiman N, Angiogene GTSG. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J. Am. Coll. Cardiol. 2003;42:1339–1347. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- 262.Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M, Morishita R, Annex BH, Results of a double-blind. placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58–65. doi: 10.1161/CIRCULATIONAHA.107.727347. [DOI] [PubMed] [Google Scholar]
- 263.Shigematsu H, Yasuda K, Iwai T, Sasajima T, Ishimaru S, Ohashi Y, Yamaguchi T, Ogihara T, Morishita R. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17:1152–1161. doi: 10.1038/gt.2010.51. [DOI] [PubMed] [Google Scholar]
- 264.Kilian EG, Sadoni S, Vicol C, Kelly R, van Hulst K, Schwaiger M, Kupatt C, Boekstegers P, Pillai R, Channon K, Hetzer R, Reichart B. Myocardial transfection of hypoxia inducible factor-1alpha via an adenoviral vector during coronary artery bypass grafting. - A multicenter phase I and safety study. Circ. J. 2010;74:916–924. doi: 10.1253/circj.cj-09-0594. [DOI] [PubMed] [Google Scholar]
- 265.Li HL, Li S, Shao JY, Lin XB, Cao Y, Jiang WQ, Liu RY, Zhao P, Zhu XF, Zeng MS, Guan ZZ, Huang W. Pharmacokinetic and pharmacodynamic study of intratumoral injection of an adenovirus encoding endostatin in patients with advanced tumors. Gene Ther. 2008;15:247–256. doi: 10.1038/sj.gt.3303038. [DOI] [PubMed] [Google Scholar]
- 266.Renmiao Z, West China H. L. Chengdu Shi Endor Biological Engineering Technology Co. [[Accessed September 5, 2016]];Recombinant Human Endostatin Adenovirus Combined With Chemotherapy for Advanced Head and Neck Malignant Tumors. 2014 Nov 3; Retrieved from https://clinicaltrials.gov/ct2/show/NCT02283489.
- 267.Ye W, Liu R, Pan C, Jiang W, Zhang L, Guan Z, Wu J, Ying X, Li L, Li S, Tan W, Zeng M, Kang T, Liu Q, Thomas GR, Huang M, Deng W, Huang W. Multicenter randomized phase 2 clinical trial of a recombinant human endostatin adenovirus in patients with advanced head and neck carcinoma. Mol. Ther. 2014;22:1221–1229. doi: 10.1038/mt.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.a.S.C. Genzyme, Sanofi. [[Accessed September 5, 2016]];Safety and Tolerability Study of AAV2-sFLT01 in Patients With Neovascular Age-Related Macular Degeneration (AMD) 2016 Jan 5; Retrieved from https://ClinicalTrials.gov/show/NCT01024998.
- 269.P.W.A. Lions Eye Institute, I. Avalanche Biotechnologies. [[Accessed September 5, 2016]];Safety and Efficacy Study of rAAV.sFlt-1 in Patients With Exudative Age-Related Macular Degeneration. 2014 Apr 9; Retrieved from https://ClinicalTrials.gov/show/NCT01494805.
- 270.Rakoczy EP, Lai M, Magno AL, French M, Chalberg TW, Blumenkranz M, Schwartz SD, Constable IJ. One Year Follow-Up Report on the rAAV.sFlt-1 Phase I Gene Therapy Trial for Exudative Age-Related Macular Degeneration. Investigative Ophthalmology & Visual Science. 2014;55:1309–1309. [Google Scholar]
- 271.Durymanov MO, Beletkaia EA, Ulasov AV, Khramtsov YV, Trusov GA, Rodichenko NS, Slastnikova TA, Vinogradova TV, Uspenskaya NY, Kopantsev EP, Rosenkranz AA, Sverdlov ED, Sobolev AS. Subcellular trafficking and transfection efficacy of polyethylenimine-polyethylene glycol polyplex nanoparticles with a ligand to melanocortin receptor-1. J. Control. Release. 2012;163:211–219. doi: 10.1016/j.jconrel.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–320. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 273.Green JJ, Shi J, Chiu E, Leshchiner ES, Langer R, Anderson DG. Biodegradable polymeric vectors for gene delivery to human endothelial cells. Bioconjug. Chem. 2006;17:1162–1169. doi: 10.1021/bc0600968. [DOI] [PubMed] [Google Scholar]
- 274.Cho EC, Zhang Q, Xia Y. The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nat. Nanotechnol. 2011;6:385–391. doi: 10.1038/nnano.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bishop CJ, Abubaker-Sharif B, Guiriba T, Tzeng SY, Green JJ. Gene delivery polymer structure-function relationships elucidated via principal component analysis. Chem. Commun. (Camb) 2015;51:12134–12137. doi: 10.1039/c5cc04417k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Gubskaya AV, Bonates TO, Kholodovych V, Hammer P, Welsh WJ, Langer R, Kohn J. Logical Analysis of Data in Structure-Activity Investigation of Polymeric Gene Delivery. Macromol. Theory Simul. 2011;20:275–285. doi: 10.1002/mats.201000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Breunig M, Lungwitz U, Liebl R, Fontanari C, Klar J, Kurtz A, Blunk T, Goepferich A. Gene delivery with low molecular weight linear polyethylenimines. J. Gene Med. 2005;7:1287–1298. doi: 10.1002/jgm.775. [DOI] [PubMed] [Google Scholar]
- 278.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Eltoukhy AA, Chen D, Alabi CA, Langer R, Anderson DG. Degradable terpolymers with alkyl side chains demonstrate enhanced gene delivery potency and nanoparticle stability. Adv. Mater. 2013;25:1487–1493. doi: 10.1002/adma.201204346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Meyer RA, Sunshine JC, Perica K, Kosmides AK, Aje K, Schneck JP, Green JJ. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small. 2015;11:1519–1525. doi: 10.1002/smll.201402369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Jiang X, Qu W, Pan D, Ren Y, Williford JM, Cui H, Luijten E, Mao HQ. Plasmid-templated shape control of condensed DNA-block copolymer nanoparticles. Adv. Mater. 2013;25:227–232. doi: 10.1002/adma.201202932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL, Wood MJ. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat. Protoc. 2012;7:2112–2126. doi: 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- 284.Gao W, Zhang L. Coating nanoparticles with cell membranes for targeted drug delivery. J. Drug Target. 2015;23:619–626. doi: 10.3109/1061186X.2015.1052074. [DOI] [PubMed] [Google Scholar]
- 285.Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M. A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta. 2014;1846:75–87. doi: 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 286.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 288.Martino MM, Brkic S, Bovo E, Burger M, Schaefer DJ, Wolff T, Gurke L, Briquez PS, Larsson HM, Gianni-Barrera R, Hubbell JA, Banfi A. Extracellular matrix and growth factor engineering for controlled angiogenesis in regenerative medicine. Front. Bioeng. Biotechnol. 2015;3:45. doi: 10.3389/fbioe.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Chu H, Gao J, Chen CW, Huard J, Wang Y. Injectable fibroblast growth factor-2 coacervate for persistent angiogenesis. Proc. Natl. Acad. Sci. USA. 2011;108:13444–13449. doi: 10.1073/pnas.1110121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Helfrich I, Scheffrahn I, Bartling S, Weis J, von Felbert V, Middleton M, Kato M, Ergun S, Augustin HG, Schadendorf D. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J. Exp. Med. 2010;207:491–503. doi: 10.1084/jem.20091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Jain RK. Molecular regulation of vessel maturation. Nat. Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 292.Raza A, Franklin MJ, Dudek AZ. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am. J. Hematol. 2010;85:593–598. doi: 10.1002/ajh.21745. [DOI] [PubMed] [Google Scholar]
- 293.Samura M, Morikage N, Suehiro K, Tanaka Y, Nakamura T, Nishimoto A, Ueno K, Hosoyama T, Hamano K. Combinatorial Treatment with Apelin-13 Enhances the Therapeutic Efficacy of a Preconditioned Cell-Based Therapy for Peripheral Ischemia. Sci. Rep. 2016;6:19379. doi: 10.1038/srep19379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Rosca EV, Penet MF, Mori N, Koskimaki JE, Lee E, Pandey NB, Bhujwalla ZM, Popel AS. A biomimetic collagen derived peptide exhibits anti-angiogenic activity in triple negative breast cancer. PLoS One. 2014;9:e111901. doi: 10.1371/journal.pone.0111901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Shmueli RB, Ohnaka M, Miki A, Pandey NB, Lima e Silva R, Koskimaki JE, Kim J, Popel AS, Campochiaro PA, Green JJ. Long-term suppression of ocular neovascularization by intraocular injection of biodegradable polymeric particles containing a serpin-derived peptide. Biomaterials. 2013;34:7544–7551. doi: 10.1016/j.biomaterials.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]