Abstract
The use of femtosecond laser surgery improves the precision and reproducibility of corneal incisions and the capsular opening; it also reduces the amount of ultrasound energy required for lens nucleus work-up. The rate of complications reported so far appears to be low. There are a number of contraindications such as a history of cornea and/or glaucoma surgery and certain anatomical features like deep-set eyes, kyphosis, tremor, and obesity. Visual recovery and refractive results of both techniques are excellent. Comparing laser cataract surgery (LCS) with manual cataract surgery (conventional phacoemulsification) based on meta-analysis currently reveals slight differences in refractive and visual outcome. Both methods are extremely successful and safe. LCS is a technique still on the rise, with its full potential not yet tapped.
Keywords: Capsulotomy, Cataract, Capsulorhexis, Effective phaco time, Endothelial cell count, Femtosecond laser, Intraocular lens, Laser cataract surgery, Manual phacoemulsification, Ultrasound, Prostaglandin
Introduction
In most industrialized nations, cataract surgery is by far the most frequently performed surgical intervention, surpassing in numbers other common procedures from all the different surgical subspecialties such as hip or knee replacement, appendectomy, tonsillectomy, and cholecystectomy. The advent of minimal incisions, foldable intraocular lenses (IOL), and the application of ultrasound energy for the fragmentation of the lens, i.e., for phacoemulsification as introduced by Charles Kelman in the 1970s, has made cataract surgery efficient and safe. It can be argued that modern cataract surgery—which is always refractive surgery, striving to provide the patient with an optimal visual acuity without requiring additional correction—is an intervention that in many cases not only restores an organ’s function but also can render it better than it has been for almost a lifetime. This happens, for instance, when a patient myopic since childhood or adolescence becomes emmetropic after implantation of the appropriate IOL.
New methods in medicine often face intense and, arguably, not always fair scrutiny—even the most beneficial inventions like smallpox immunization, anesthesia, and antisepsis had to overcome initial objections. It is even more of a challenge when an innovation has to compete with an established method like phacoemulsification cataract surgery whose efficacy and safety are well established. In cataract and refractive surgery, the expectations are ever increasing with many elderly people leading an active lifestyle and being well informed about the possibilities in modern ophthalmology. Superb postoperative visual function is often expected, sometimes even taken for granted. For many patients, a cataract operation should result in visual acuity of 20/20 without glasses for distance vision. With the advent of multifocal and accommodating IOLs, the highest visual comfort for near, intermediate, and distance vision is advertised. We recommend using exactly that term—laser cataract surgery (LCS)—to describe the new method since the laser is a central element in the procedure and not just an assisting device as intimated in the term femtosecond laser-assisted cataract surgery (FLACS) [1].
Since the introduction of the femtosecond laser into cataract surgery by Zoltan Nagy in 2009 [2], a number of comparisons of the new technique with conventional (manual) phacoemulsification have been published, based on an ever-increasing number of cases. The assessment by the pioneering surgeon Nagy that “femtolaser treatment of the crystalline lens increases safety, efficacy, and predictability of the surgery” [3] has now been tested numerous times. Recently some major meta-analyses have been published by Popovic et al. [4] and by Day et al. [5] that provide valuable overviews—though some questions still remain unanswered and will require further research and clinical experience.
There is probably a differing perception of the merits of each of these two techniques, depending on which one the individual cataract surgeon prefers. It is the aim of this review to provide the reader with samples of the data from the large amount of literature on some crucial aspects of LCS in comparison to conventional phacoemulsification cataract surgery. Some of this information stems from small studies and/or is based on our own experience; other results are taken from larger trials and from meta-analyses.
This review is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by the author.
Efficacy
Capsulotomy
Creating a precise, safe, and reproducible capsulotomy is a prerequisite for success in cataract surgery and for IOL implantation. Compared to manual capsulorhexis [6], the laser has been shown to create a particularly well-shaped and reproducible capsulotomy geometry and circularity [7]. Numerous studies confirmed that these non-invasively created circular capsular openings contribute to an improved IOL–capsule overlap (Fig. 1). Capsulotomies performed by the femtosecond laser reduce the probability of IOL decentration and tilt [8].
Fig. 1.
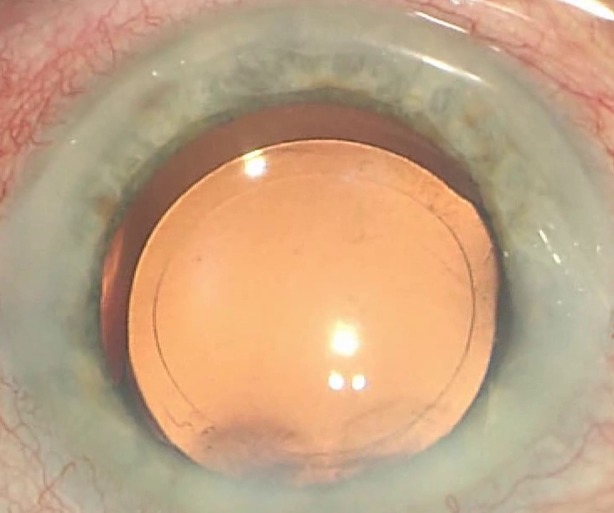
Round, circular, centered capsulotomy in LCS with a 360° overlap on the IOL optic (intraoperative view through the OR microscope)
Regarding the strength of the capsulotomies, Scott et al. reported radial capsular tears in 38 eyes out of a cohort of 8684 cases. That amounts to a rate of 0.43% [9] compared to 2.32% for manual phacoemulsification cataract surgery reported in the literature [10]. Roberts et al. reported an even smaller rate of anterior capsule tears of 0.21% in a cohort of 3355 eyes [11]. In a large study comparing 1852 eyes that underwent LCS and 2228 eyes in the phaco group, Abell et al. found that anterior capsule tears occurred in 1.84% of eyes in the study group and 0.22% of eyes in the control group (P < 0.0001). Anterior capsulotomy tags occurred in 1.62% of study group eyes. The authors claimed that the higher incidence of anterior capsule tears was not related to the learning curve. They concluded that in general, significant intraoperative complications that are likely to affect refractive outcomes and patient satisfaction were low in both groups [12].
There is evidence that the femtosecond laser can successfully be employed when a manual capsulorhexis turns out to be far less than perfect. Manual capsulorhexis, particularly when performed by a surgeon still climbing their learning curve, can be too small which might lead to capsule shrinkage that in turn can cause IOL decentration and decreased vision. In five cases that we have described, it was possible to enlarge a markedly smaller capsulotomy by using the femtosecond laser with a 360° overlap without complications. With the laser platform’s 3-D spectral-domain OCT, it was possible in all cases to identify and target the anterior capsule. This technique has potential to be used routinely in intended as well as unintended cases with a smaller capsule opening [13].
Another rescue mission performed with the femtosecond laser has been described in a number of cases with capsular contraction syndrome—“capsular phimosis”—by Gerten et al. This technique may offer advantages over the existing treatment methods, neodymium:YAG laser capsulotomy and manual extension of the capsulorhexis, though tissue bridges might remain [14].
Refractive Outcome
The earlier the capsular bag diameter stabilizes the better for a more predictable effective lens position, IOL power calculations, and refractive outcomes. Measuring capsular bag shrinkage in 53 eyes that underwent LCS and in 53 fellow eyes that underwent manual phacoemulsification, the LCS group had significantly less capsular bag shrinkage than the standard group at 1, 2, and 3 months, with a mean difference of 0.33 ± 0.25 mm at 3 months [15].
A number of studies have compared the refractive outcome of laser and conventional procedures—in many cases an aspect which is of prime importance for the patient. Roberts et al., for instance, found no significant difference in visual outcomes in a prospective study of 113 LCS procedures versus 105 conventional cases. The absolute mean difference from intended correction was 0.29 ± 0.25 D for the LCS group and 0.31 ± 0.24 D for the standard group (P = 0.5). More than 90% of patients in both groups achieved 20/40 uncorrected distance visual acuity at 3 months [16]. Mihaltz et al. compared the ocular and internal aberrations after femtosecond laser anterior capsulotomy and continuous curvilinear capsulorhexis in cataract surgery. They found no differences between the LCS and manual groups in postoperative sphere (−0.60 ± 1.50 vs −0.50 ± 1.40 D), postoperative cylinder (1.30 ± 1.01 vs 1.10 ± 1.10 D), uncorrected distance visual acuity (0.86 ± 0.15 vs 0.88 ± 0.08), or corrected distance visual acuity (0.97 ± 0.08 vs 0.97 ± 0.06). However, they noted that the laser group had significantly lower values of higher-order aberrations, namely intraocular vertical tilt (vertical deviation in the direction of the beam of light; −0.05 ± 0.36 vs 0.27 ± 0.57), coma (variation in magnification over the entrance pupil; −0.003 ± 0.11 vs. 0.1 ± 0.15), significantly higher Strehl ratios (ratio of peak diffraction intensities of an aberrated versus perfect wavefront; 0.02 ± 0.02 vs. 0.01 ± 0.01), and modulation transfer function values at all measured cycles per degree, compared to the manual capsulorhexis group [17].
In a prospective, randomized cohort study with the aim to analyze postoperative manifest refraction and the deviation from the target refraction, 100 eyes of 100 patients were treated with femtosecond laser cataract surgery; the fellow 100 eyes had conventional phacoemulsification. Six months postoperatively, 196 eyes were included and analyzed. At 6 months, 90 eyes (92%) in the femtosecond laser group and 70 eyes (71%) in the conventional group were within ±0.50 D of the target refractive outcome and 98 eyes (100%) in both groups were within ±1.00 D. Conrad-Hengerer et al. concluded that femtosecond laser cataract surgery is a safe and precise procedure but enhances visual outcomes only minimally. Manually performed cataract removal in standard cases in the hands of an experienced surgeon can obviously provide a similar level of refractive results after 6 months. However, there was an advantage in favor of the laser in the early postoperative visual recovery period (until 1 week) over conventional surgery. Furthermore, the refractive result stabilized earlier in the femtosecond laser-assisted group [18].
Visual Acuity
A number of studies have evaluated best-corrected visual acuity (BCVA) and uncorrected distance visual acuity (UDVA) after both methods. Generally speaking, the differences in this outcome following LCS or manual phacoemulsification were minimal to non-existent. Mastropasqua et al. for instance found a UDVA of 0.35 logMAR in the laser group and of 0.28 logMAR in the standard phacoemulsification group 1 month postoperatively. Six months after surgery, the UDVA was 0.13 and 0.08 logMAR in these two respective groups [19]. Kránitz et al., using Snellen visual acuity, found a UDVA of 0.59 (SD 0.23) and 0.51 (SD 0.29) in the LCS and the standard phacoemulsification cohorts, respectively. At 1 month, UDVA values were 0.69 (SD 0.19) and 0.61 (SD 0.28) in laser-operated eyes and eyes after conventional cataract surgery, respectively; after 1 year, the respective values were 0.63 (SD 0.23) and 0.60 (SD 0.25). They found no statistically significant difference between arms at any time point [20].
Popovic et al. did an extensive literature research that included 14,567 eyes from 15 randomized controlled trials and 22 observational cohort studies. Not entirely unexpected given the high standard of the well-established conventional procedure, no statistically significant difference was detected between LCS and manual phacoemulsification in uncorrected distance visual acuity (weighted mean difference [WMD] −0.02; 95% CI −0.04 to 0.01; P 1/4 0.19), corrected distance visual acuity (WMD −0.01; 95% CI −0.02 to 0.01; P = 0.26), and mean absolute error (WMD −0.02; 95% CI −0.07 to 0.04; P = 0.57). The same applied to total surgery time. Effective phacoemulsification time (EPT) was significantly lower in laser cataract surgery in eight out of eight analyzed studies [4].
Safety
Corneal Incisions
In our report on the histology of corneal femtosecond laser incisions we could demonstrate the extremely precise positioning of intrastromal incisions (Figs. 2 and 3), the minimal tissue bridges left, and the absence of inflammatory cells, pointing to a higher safety standard than possible with a manual incision [21].
Fig. 2.
Preoperative planning of the intrastromal arcuate corneal incision in the femtosecond laser machine
Fig. 3.

Intraoperative position check of the intrastromal incision using 3-D SD LIVE OCT (after docking). A change of length, depth, arc, centration method, and position is still possible
Takács et al. demonstrated in a prospective, randomized study less corneal swelling and endothelial cell damage in patients undergoing LCS compared to a conventional phacoemulsification technique [22]. Alio et al. compared the stability of clear cornea incisions done by the femtosecond laser and found that the actual length, chord length, and surface angle for the primary incision and the length and surface angle for the secondary incisions were stable at 1 day and 1 month following the surgery [23].
Endothelial Cell Count and Ultrasound Exposure
The application of ultrasound energy within the anatomically rather confined structures of the anterior segment is an essential step in phacoemulsification. It is a valuable tool, but not without its risks. High ultrasound power and long ultrasound time (effective phacoemulsification time, EPT) are probably important intraoperative factors leading to endothelial cell loss, sometimes excessive loss, after phacoemulsification in healthy eyes with no previous risk factors, such as a history of intraocular surgery, pseudoexfoliation, or corneal dystrophy [24]. Ultrasound energy has also been implicated in the pathogenesis of cystoid macular edema [25]. With increasing experience with femtosecond laser technology the amount of ultrasound energy usually decreases (Fig. 4).
Fig. 4.
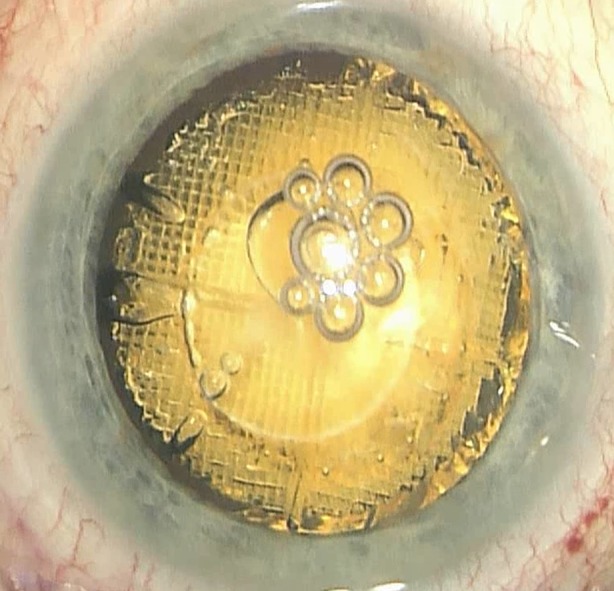
Full fragmentation of the advanced cataractous lens using a two main cut pattern (with multiple repetitions herein) in LCS. Cavitation gas bubbles appear at the end of the lasing
A study from Australia by Abell et al. compared 150 patients undergoing femtosecond laser treatment and 51 patients undergoing conventional phacoemulsification. The study found a mean EPT reduction in the laser group by 83.6% with 30% of patients in this group achieving zero EPT [26]. After femtosecond laser-assisted cataract surgery was introduced in our clinic, the necessity for ultrasound application gradually declined with our growing experience with the femtosecond laser treatment. While phacoemulsification was still needed in 59% of patients (from the 200th to the 400th patient) after the introduction of LCS, in the group comprising the 700th to the 900th patient, only 38% required phacoemulsification for lens fragmentation. After further climbing of the learning curve, phacoemulsification was required in only 9% of patients—number 1200 to number 1400. All 18 eyes that required ultrasound energy had a grade 4 (LOCS III) cataract and the median EPT was 0.4 s. More than 90% of our surgeries are performed with “zero phaco”. In grade 2 cataracts, there is currently no ultrasound application in 100% of cases [27].
A catastrophic cell loss leading to a density of 500 or less cells per mm2 will result in corneal decompensation which in many cases requires a keratoplasty, either penetrating or lamellar. It is well established that ultrasound application during phacoemulsification can lead to endothelial cell damage in cataract surgery due to mechanical trauma from sonic waves and from thermal injury [24]. While improvements of phacoemulsification technology have made the application less perilous for the endothelium, Mencucci et al. nevertheless reported a not insignificant endothelial cell loss between 4% and 25% [28].
There is now sufficient evidence to declare laser cataract surgery the method less traumatic to the corneal endothelium. In a prospective, randomized study of 150 eyes, Conrad-Hengerer et al. reported that the mean endothelial cell loss was 7.9% ± 7.8% 1 week postoperatively and 8.1% ± 8.1% 3 months postoperatively in the LCS group and 12.1% ± 7.3% and 13.7% ± 8.4%, respectively, in the control group (P < 0.001). They found a positive correlation between endothelial cell loss at the 3-month postoperative visit (r = 0.43) and the EPT. In the laser group 64.4% of eyes had zero EPT. The femtosecond laser can be considered particularly beneficial in eyes with low preoperative endothelial cell counts, such as in cases of cornea guttata and Fuchs dystrophy [29]. The differences were less pronounced in a study by Krarup et al. with endothelial cell loss at 3 months postoperatively of 11.4% (after laser treatment) and of 13.9% following conventional phacoemulsification [30]. Schargus et al. demonstrated that femtosecond laser treatment allows the cataract surgeon to perform phacoemulsification and intraocular lens implantation without the use of ophthalmic viscosurgical device (OVD) at no additional risk to the corneal endothelium [31].
In the aforementioned meta-analysis by Popovic et al., the analysis of safety parameters revealed that there were no statistically significant differences in the incidence of overall complications between LCS and manual cataract surgery; however, posterior capsular tears were significantly more common in laser cataract surgery (RR 3.73). The authors add: “There may be certain clinical scenarios, such as cases in which a manual capsulorhexis is harder to perform (e.g., subluxated lens), in which LCS may have specific advantages. Furthermore, there may be applications and modifications of the IOL technology in the future that may favor laser over manual cataract surgery. Because of the continual evolution of the femtosecond laser technology, it is likely that there will be continued head-to-head comparisons between these two techniques” [4].
Prostaglandin Release
Soon after the introduction of the femtosecond laser into cataract surgery, first reports of intraoperative miosis in some patients surfaced [32]. The cause of this problem—and it can be a problem since small pupils can increase the difficulty of the surgery and lead to higher complication rates during lens removal [33]—was soon identified: it is the release of prostaglandins by the laser treatment.
It has been well known for some time that prostaglandins appear in the aqueous humor following different mechanical or thermal stimuli. The principal source for prostaglandins in the eye is the non-pigmented epithelial layer of the ciliary body. So we collected aqueous humor from 113 patients who during cataract surgery either had just undergone femtosecond laser treatment or—in the control group of 107 eyes—before commencing conventional phacoemulsification. A large difference was found between the two groups. In the femtosecond laser group the average level of prostaglandin E2 in one part of the study was 182 pg/ml—more than tenfold the concentration of PGE2 in the control group, which was 17.3 pg/ml [34].
The easiest prophylaxis of an excessive prostaglandin release might be speed. If the patient is swiveled around on his treatment bed from under the laser platform to the adjoining position under the operating microscope immediately, the prostaglandins released by capsulotomy hardly have the time to exert their effect on the muscularis sphincter pupillae. This is one argument—besides, for instance, hygienic considerations—in favor of performing laser treatment and the following steps such as lens removal and IOL in the same operating room instead of doing the former in a separate “laser suite” [35].
There is, however, beyond the speed factor a proven pharmacological prophylaxis: administering non-steroidal anti-inflammatory drugs (NSAID), one eye drop three times on the day of surgery before initiating treatment reliably prevents miosis. NSAID or steroidal pretreatment might be advisable to decrease a possible risk of inflammation and hence intraoperative miosis. It is a highly effective precaution: of the last 500 eyes that received these drugs in our clinic, none became miotic [34].
Inflammation
The prostaglandin release can trigger an alteration of the blood–aqueous barrier and lead to postoperative inflammation that can clinically manifest as a mild iritis and with increased cells and protein in the anterior chamber. The latter effect causes a flare which can be quantified as an indicator of inflammation by laser flare photometry. Abell et al. demonstrated that postoperative aqueous flare was significantly greater in eyes that had undergone manual cataract surgery at 1 day and at 4 weeks postoperatively than in eyes after LCS [36]. Conrad-Hengerer et al. published similar results: when comparing 104 eyes that underwent laser cataract surgery with 104 fellow eyes which had manual phacoemulsification, laser flare photometry showed higher levels in the standard group at the first postoperative visit 2 h after surgery compared with the laser group. In the same study, retinal thickness was measured by spectral-domain optical coherence tomography. No significant differences could be detected, indicating that LCS did not obviously influence the incidence of postoperative macular edema [29]. A different tendency was reported by a group from Australia, though, with seven cases of macular edema out of 833 eyes (0.8%) operated on with the laser vs. one eye in a group of 458 conventionally operated cases (0.1%) [37].
LCS in Challenging Cases
The safety and efficacy of LCS (Fig. 5) has been demonstrated in a number of special cases. The accuracy and reproducibility of the laser capsulotomy are particularly valuable in pediatric patients. A posterior capsulotomy of the right size is crucial up to the age of 6 years to prevent posterior capsule opacification (PCO) and for the implantation of an IOL that is fixated in the capsular bag. Because of the infant lens capsule’s high elasticity, manual anterior and posterior capsulorhexes are challenging to perform and frequently lead to an oversized capsule opening. Capsulotomy performed by the laser has been proven safe and effective, with tissue bridges remaining in 6 eyes out of 22 successful capsulotomies [38]. The age-dependent deviation from the capsulotomy’s target diameter which was observed in the initial operations of children with congenital cataract can be overcome by the Bochum formula. Applying this formula has become crucial in achieving a precalculated diameter and allowing precise adjustment of the posterior capsulotomy to the anterior capsulotomy [39].
Fig. 5.
Screenshot of the planning in LCS (with main incision, two sideports, two arcuate incisions, capsulotomy, and lens fragmentation)
Brunescent cataracts usually require an increased phacoemulsification time and are at higher risk for thermal and mechanical injury to the cornea and corneal edema. In a study on 240 eyes, LCS was more effective than phacoemulsification in fragmenting the advanced cataract in so far as requiring far less EPT. In eyes with LOCS III grade 3 cataracts, EPT ranged from 0.46 to 3.10 s (mean 1.38) in the phaco group while it was zero in the laser group. In eyes with grade 4 brunescent cataracts, EPT was 2.12 to 19.29 s (mean 6.85) in the phaco group and 0 to 6.75 s (mean 1.35) in the laser group [40].
A comparable situation exists with intumescent cataracts which usually pose a challenge to the surgeon since they tend to have increased intralenticular pressure due to liquefaction of the cortex. To release this pressure, a mini-capsulotomy (Fig. 6) technique where a smaller capsulotomy is initially performed to release the intralenticular pressure followed by re-docking on the laser machine and a second larger capsulotomy has been developed [41], which seems to render operating on white cataracts safe—and probably more so than manual capsulorhexis with its potential for complications [42].
Fig. 6.
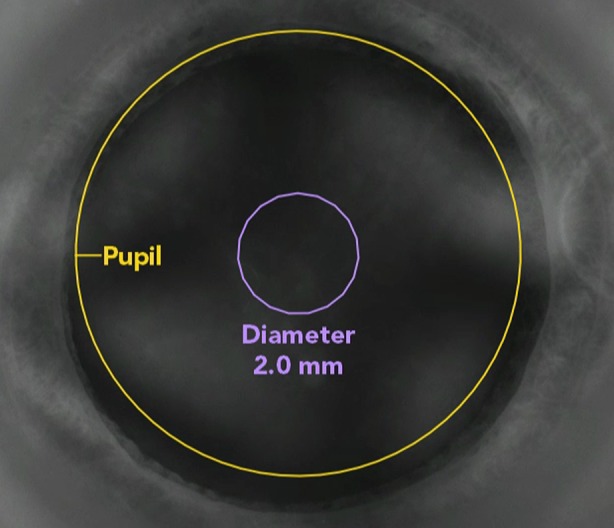
Mini-capsulotomy on intraoperative laser system screen view demonstrating its centration, position, and size
Contraindications to LCS
A number of patients will not be able to undergo laser cataract surgery because of some anatomical features. Deep-set eyes, a prominent nose, and prominent eyebrows may render contact between the globe and the laser’s interface impossible. Heavily overweight patients may not fit onto the treatment bed and lowering the interface will in cases of obesity not result in coupling to the cornea’s surface. Skeletal anomalies like a pronounced kyphosis might prevent patients from lying down properly under the treatment unit. A tremor and restless legs syndrome are also contraindications. LCS should not be performed in eyes with previous glaucoma or cornea surgery. Corneal scars are mentioned as contraindications; depending on their extent, experienced surgeons might consider this an obstacle that can be overcome.
Discussion
Femtosecond laser treatment shows great promise in increasing the accuracy and precision of the cuts compared to the manual procedure. Favorable refractive and functional outcomes and good safety profiles have been reported. The Cochrane analysis comparing LCS with standard phacoemulsification cataract surgery concluded that in the evaluated studies there was a small difference in postoperative refraction prediction error (mean absolute error) in favor of laser-assisted surgery but the confidence intervals for this estimate included a clinically insignificant effect. The general conclusion of the analysis was that evidence from the 16 randomized controlled trials (RCTs) included in the Cochrane review could not determine the equivalence or superiority of laser-assisted cataract surgery compared to standard manual phacoemulsification for the chosen outcomes because of the low to very low certainty of the evidence available from these studies [5].
LCS provides new options in the treatment of advanced pathologies. Furthermore, there are evolving techniques to reduce the likelihood of postoperative capsule opacification as recently described by Gregory Kramer, Liliana Werner, and Nick Mamalis [43] that certainly can be employed in laser-assisted operations as well as in manual cataract surgery. Preventing this most common complication after cataract surgery to a certain degree is probably also possible by employing the laser to perform a primary posterior capsulotomy (Fig. 7) [44].
Fig. 7.
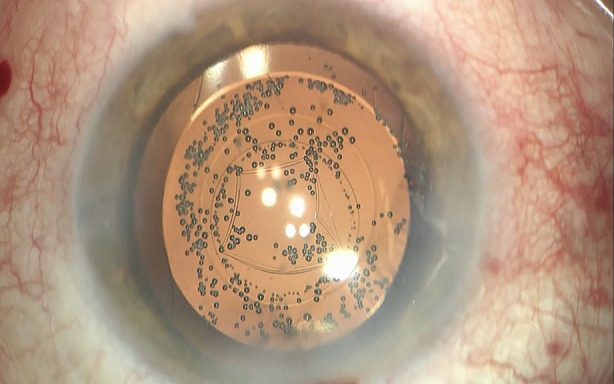
Primary posterior laser-assisted capsulotomy with the IOL in the capsular bag and some cavitation bubbles (intraoperative view through the OR microscope at the end of the surgery)
The two major meta-analyses published very recently give proof that both techniques, LCS and manual phacoemulsification, are highly effective and safe with differences in some parameters minor or non-existent. Besides the publication by Popovic et al. that was mentioned earlier, the Cochrane review by Day et al. found little evidence of any important difference in postoperative visual acuity between laser-assisted and standard phacoemulsification arms. There was a small advantage for laser-assisted cataract surgery at 6 months in corrected distance visual acuity (CDVA). The mean difference (MD) was −0.03 logMAR and was considered clinically insignificant. None of the analyzed trials were powered to investigate for differences in complication rates [5].
With growing experience, LCS has—like manual phacoemulsification—become a procedure that can be used in most of the patients. Patients representing challenging cases like those with Marfan syndrome [45], intumescent cataract as well as pediatric cases do not have to be turned away although these interventions require a high degree of surgical skills.
There are a number of contraindications, however, and some patients are without doubt better served by manual cataract surgery.
A field where LCS lags behind conventional phacoemulsification is the economic side of this frequent intervention. Abell et al., using a computer-based econometric modeling, concluded in 2014 that laser cataract surgery at the time of publication is not cost-effective compared to phacoemulsification [46]. This approach has, however, some weaknesses and does not take into account, for instance, costs that arise from complications like corneal decompensation and the expenses for glasses etc. to correct remaining refractive errors after cataract surgery. Like with any new technology, it is likely that the price of laser cataract surgery will decrease over time.
LCS has a large clinical potential that has not yet been fully tapped with new applications like intraoperative biomorphometry on the horizon.
Conclusion
According to a number of studies femtosecond laser surgery has the potential to improve the precision and reproducibility of corneal incisions and the capsular opening. It has been documented that LCS reduces the amount of ultrasound energy required for lens removal. The reported rate of complications is low and there are limited contraindications. Visual recovery and refractive results are promising. There are, however, a couple of challenges facing wider acceptance of the femtosecond laser in cataract surgery. The economic aspect is of importance. The higher costs are a barrier to wider acceptance by surgeons and clinical centers. However, to increase acceptance the next generation of the lasers systems needs to be smaller, more mobile, and less dependent on a narrowly defined room temperature than the current ones. LCS is still a young technology in progress and surgeons can offer their patients two safe and efficient techniques in operating cataracts: LCS and—not “versus”, not “or”—phacoemulsification.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. This review is based on a presentation given at the ESCRS 2016 winter conference in Copenhagen titled “Laser assisted vs. traditional phaco cataract surgery”.
Disclosures
H. B. Dick and T. Schultz have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/F887F0600575E4B2.
References
- 1.Dick HB. Bladeless custom femtosecond laser-assisted refractive cataract surgery? Let’s give our latest breakthrough surgical technique a name that fits. J Refract Surg. 2015;31:346. doi: 10.3928/1081597X-20150424-04. [DOI] [PubMed] [Google Scholar]
- 2.Nagy ZZ, Takacs A, Filkorn T, Sarayba M. Initial clinical evaluation of intraocular femtosecond laser in cataract surgery. J Refract Surg. 2009;25:1053–1060. doi: 10.3928/1081597X-20091117-04. [DOI] [PubMed] [Google Scholar]
- 3.Nagy ZZ. New technology update: femtosecond laser in cataract surgery. Clin Ophthalmol. 2014;8:1157–1167. doi: 10.2147/OPTH.S36040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popovic M, Campos-Möller X, Schlenker MB, Ahmed IIK. Efficacy and safety of femtosecond laser-assisted cataract surgery compared with manual cataract surgery: a meta-analysis of 14,567 eyes. Ophthalmology. 2016;123:2113–2126. doi: 10.1016/j.ophtha.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Day AC, Gore DM, Bunce C, Evans JR. Laser-assisted cataract surgery versus standard ultrasound phacoemulsification cataract surgery. Cochrane Database Syst Rev 2016; Issue 7. Art. No. CD010735. doi:10.1002/14651858.CD010735.pub2. [DOI] [PMC free article] [PubMed]
- 6.Dick HB, Gerste RD, Schultz T, Waring GO., 3rd Capsulotomy or capsulorhexis in femtosecond laser-assisted cataract surgery? J Cataract Refract Surg. 2013;39:1442. doi: 10.1016/j.jcrs.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Mastropasqua L, Toto L, Calienno R, et al. Scanning electron microscopy evaluation of capsulorhexis in femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39:1581–1586. doi: 10.1016/j.jcrs.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Grewal DS, Schultz T, Basti S, Dick HB. Femtosecond laser-assisted cataract surgery: current status and future directions. Surv Ophthalmol. 2016;61:103–131. doi: 10.1016/j.survophthal.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Scott WJ, Abell R, et al. Re: Abell et al.: Anterior capsulotomy integrity after femtosecond laser-assisted cataract surgery (Ophthalmology 2014;121:17–24) Ophthalmology. 2014;121:e35–e36. doi: 10.1016/j.ophtha.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Abell RG, Davies PE, Phelan D, et al. Anterior capsulotomy integrity after femtosecond laser-assisted cataract surgery. Ophthalmology. 2014;121:17–24. doi: 10.1016/j.ophtha.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Roberts TV, Lawless M, Sutton G, Hodge C. Anterior capsule integrity after femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2015;41:1109–1110. doi: 10.1016/j.jcrs.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 12.Abell RG, Darian-Smith E, Kan JB, et al. Femtosecond laser-assisted cataract surgery versus standard phacoemulsification cataract surgery: outcomes and safety in more than 4000 cases at a single center. J Cataract Refract Surg. 2015;41:47–52. doi: 10.1016/j.jcrs.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Dick HB, Schultz T. Femtosecond laser-assisted capsulotomy rescue for capsulorhexis enlargement. J Cataract Refract Surg. 2014;40:1588–1590. doi: 10.1016/j.jcrs.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Gerten G, Schultz M, Oberheide U. Treating capsule contraction syndrome with a femtosecond laser. J Cataract Refract Surg. 2016;42:1255–1261. doi: 10.1016/j.jcrs.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Dick HB, Conrad-Hengerer I, Schultz T. Intraindividual capsular bag shrinkage comparing standard and laser-assisted cataract surgery. J Refract Surg. 2014;30:228–233. doi: 10.3928/1081597X-20140320-01. [DOI] [PubMed] [Google Scholar]
- 16.Roberts TV, Lawless M, Chan CC, et al. Femtosecond laser cataract surgery: technology and clinical practice. Clin Exp Ophthalmol. 2013;41:180–186. doi: 10.1111/j.1442-9071.2012.02851.x. [DOI] [PubMed] [Google Scholar]
- 17.Mihaltz K, Knorz MC, Alio JL, et al. Internal aberrations and optical quality after femtosecond laser anterior capsulotomy in cataract surgery. J Refract Surg. 2011;27:711–716. doi: 10.3928/1081597X-20110913-01. [DOI] [PubMed] [Google Scholar]
- 18.Conrad-Hengerer I, Al Sheikh M, Hengerer FH, Schultz T, Dick HB. Comparison of visual recovery and refractive stability between femtosecond laser-assisted cataract surgery and standard phacoemulsification: six-month follow-up. J Cataract Refract Surg. 2015;41:1356–1364. doi: 10.1016/j.jcrs.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 19.Mastropasqua L, Toto L, Mastropasqua A, et al. Femtosecond laser versus manual clear corneal incision in cataract surgery. J Refract Surg. 2014;30:27–33. doi: 10.3928/1081597X-20131217-03. [DOI] [PubMed] [Google Scholar]
- 20.Kránitz K, Miháltz K, Sándor GL, Takacs A, Knorz MC, Nagy ZZ. Intraocular lens tilt and decentration measured by Scheimpflug camera following manual or femtosecond laser-created continuous circular capsulotomy. J Refract Surg. 2012;28:259–263. doi: 10.3928/1081597X-20120309-01. [DOI] [PubMed] [Google Scholar]
- 21.Schultz T, Tischoff I, Ezeanosike E, Dick HB. Histological sections of corneal incisions in OCT-guided femtosecond laser cataract surgery. J Refract Surg. 2013;29:863–864. doi: 10.3928/1081597X-20131029-01. [DOI] [PubMed] [Google Scholar]
- 22.Takacs AI, Kovacs I, Mihaltz K, et al. Central corneal volume and endothelial cell count following femtosecond laser-assisted refractive cataract surgery compared to conventional phacoemulsification. J Refract Surg. 2012;28:387–391. doi: 10.3928/1081597X-20120508-02. [DOI] [PubMed] [Google Scholar]
- 23.Alio JL, Abdou AA, Soria F, et al. Femtosecond laser cataract incision morphology and corneal higher-order aberration analysis. J Refract Surg. 2013;29:590–595. doi: 10.3928/1081597X-20130819-01. [DOI] [PubMed] [Google Scholar]
- 24.Walkow T, Anders N, Klebe S. Endothelial cell loss after phacoemulsification: relation to preoperative and intraoperative parameters. J Cataract Refract Surg. 2000;26:727–732. doi: 10.1016/S0886-3350(99)00462-9. [DOI] [PubMed] [Google Scholar]
- 25.Ecsedy M, Mihaltz K, Kovacs I, Takacs A, Filkorn T, Nagy ZZ. Effect of femtosecond laser cataract surgery on the macula. J Refract Surg. 2011;27:717–722. doi: 10.3928/1081597X-20110825-01. [DOI] [PubMed] [Google Scholar]
- 26.Abell RG, Kerr NM, Vote BJ. Toward zero effective phacoemulsification time using femtosecond laser pretreatment. Ophthalmology. 2013;120:942–948. doi: 10.1016/j.ophtha.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 27.Dick HB, Schultz T. On the way to zero phaco. J Cataract Refract Surg. 2013;39:1442–1444. doi: 10.1016/j.jcrs.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Mencucci R, Ponchietti C, Virgil G, Giansanti F, Menchini U. Corneal endothelial damage after cataract surgery: microincision versus standard technique. J Cataract Refract Surg. 2006;32:1351–1354. doi: 10.1016/j.jcrs.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 29.Conrad-Hengerer I, Al Juburi M, Schultz T, et al. Corneal endothelial cell loss and corneal thickness in conventional compared with femtosecond laser-assisted cataract surgery: three-month follow-up. J Cataract Refract Surg. 2013;39:1307–1313. doi: 10.1016/j.jcrs.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 30.Krarup T, Morten Holm L, la Cour M, Kjaerbo H. Endothelial cell loss and refractive predictability in femtosecond laser-assisted cataract surgery compared with conventional cataract surgery. Acta Ophthalmol. 2014;92:617–622. doi: 10.1111/aos.12406. [DOI] [PubMed] [Google Scholar]
- 31.Schargus M, Suckert N, Schultz T, Kakkassery V, Dick HB. Femtosecond laser-assisted cataract surgery without OVD: a prospective intra individual comparison. J Refract Surg. 2015;31:146–152. doi: 10.3928/1081597X-20150220-01. [DOI] [PubMed] [Google Scholar]
- 32.Bali SJ, Hodge C, Lawless M, Roberts TV, Sutton G. Early experience with the femtosecond laser for cataract surgery. Ophthalmology. 2012;119:891–899. doi: 10.1016/j.ophtha.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 33.Artzén D, Lundström M, Behndig A, Stenevi U, Lydahl E, Montan P. Capsule complication during cataract surgery: case–control study of preoperative and intraoperative risk factors: Swedish Capsule Rupture Study Group Report 2. J Cataract Refract Surg. 2009;35:1688–1693. doi: 10.1016/j.jcrs.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Schultz T, Joachim SC, Kuehn M, Dick HB. Changes in prostaglandin levels in patients undergoing femtosecond laser-assisted cataract surgery. J Refract Surg. 2013;29:742–747. doi: 10.3928/1081597X-20131021-03. [DOI] [PubMed] [Google Scholar]
- 35.Dick HB, Gerste RD. Plea for femtosecond laser pre-treatment and cataract surgery in the same room. J Cataract Refract Surg. 2014;40:499–500. doi: 10.1016/j.jcrs.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Abell RG, Allen PL, Vote BJ. Anterior chamber flare after femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39:1321–1326. doi: 10.1016/j.jcrs.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Ewe SY, Oakley CL, Abell RG, Allen PL, Vote BJ. Cystoid macular edema after femtosecond laser-assisted versus phacoemulsification cataract surgery. J Cataract Refract Surg. 2015;41:2373–2378. doi: 10.1016/j.jcrs.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 38.Dick HB, Schultz T. Femtosecond laser–assisted cataract surgery in infants. J Cataract Refract Surg. 2013;39:665–668. doi: 10.1016/j.jcrs.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 39.Dick HB, Schelenz D, Schultz T. Femtosecond laser-assisted pediatric cataract surgery: Bochum formula. J Cataract Refract Surg. 2015;41:821–826. doi: 10.1016/j.jcrs.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 40.Hatch KM, Schultz T, Talamo JH, Dick HB. Femtosecond laser-assisted compared with standard cataract surgery for removal of advanced cataracts. J Cataract Refract Surg. 2015;41:1833–1838. doi: 10.1016/j.jcrs.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 41.Schultz T, Dick HB. Laser-assisted mini-capsulotomy: a new technique for intumescent white cataracts. J Refract Surg. 2014;30:742–745. doi: 10.3928/1081597X-20141021-05. [DOI] [PubMed] [Google Scholar]
- 42.Conrad-Hengerer I, Hengerer FH, Joachim SC, et al. Femtosecond laser-assisted cataract surgery in intumescent white cataracts. J Cataract Refract Surg. 2014;40:44–50. doi: 10.1016/j.jcrs.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 43.Kramer GD, Werner L, Mamalis N. Prevention of postoperative capsular bag opacification using intraocular lenses and endocapsular devices maintaining an open or expanded capsular bag. J Cataract Refract Surg. 2016;42:469–484. doi: 10.1016/j.jcrs.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Dick HB, Schultz T. Primary posterior laser-assisted capsulotomy. J Refract Surg. 2014;30:128–133. doi: 10.3928/1081597X-20140120-09. [DOI] [PubMed] [Google Scholar]
- 45.Schultz T, Ezeanosike E, Dick HB. Femtosecond laser-assisted cataract surgery in pediatric Marfan syndrome. J Refract Surg. 2013;29:650–652. doi: 10.3928/1081597X-20130819-06. [DOI] [PubMed] [Google Scholar]
- 46.Abell RG, Vote BJ. Cost-effectiveness of femtosecond laser-assisted cataract surgery versus phacoemulsification cataract surgery. Ophthalmology. 2014;121:10–16. doi: 10.1016/j.ophtha.2013.07.056. [DOI] [PubMed] [Google Scholar]