Abstract
Background
Compliance with the clinical practice guidelines of sepsis management has been low. The objective of our study was to describe the results of implementing a multifaceted intervention including an electronic alert (e-alert) with a sepsis response team (SRT) on the outcome of patients with sepsis and septic shock presenting to the emergency department.
Methods
This was a pre–post two-phased implementation study that consisted of a pre-intervention phase (January 01, 2011–September 24, 2012), intervention phase I (multifaceted intervention including e-alert, from September 25, 2012–March 03, 2013) and intervention phase II when SRT was added (March 04, 2013–October 30, 2013) in a 900-bed tertiary-care academic hospital. We recorded baseline characteristics and processes of care in adult patients presenting with sepsis or septic shock. The primary outcome measures were hospital mortality. Secondary outcomes were the need for mechanical ventilation and length of stay in the intensive unit and in the hospital.
Results
After implementing the multifaceted intervention including e-alert and SRT, cases were identified with less severe clinical and laboratory abnormalities and the processes of care improved. When adjusted to propensity score, the interventions were associated with reduction in hospital mortality [for intervention phase II compared to pre-intervention: adjusted odds ratio (aOR) 0.71, 95% CI 0.58–0.85, p = 0.003], reduction in the need for mechanical ventilation (aOR 0.45, 95% CI 0.37–0.55, p < 0.0001) and reduction in ICU LOS and hospital LOS for all patients as well as ICU LOS for survivors.
Conclusions
Implementing a multifaceted intervention including sepsis e-alert with SRT was associated with earlier identification of sepsis, increase in compliance with sepsis resuscitation bundle and reduction in the need for mechanical ventilation and reduction in hospital mortality and LOS.
Electronic supplementary material
The online version of this article (doi:10.1186/s13613-017-0280-7) contains supplementary material, which is available to authorized users.
Keywords: Sepsis, Shock, Intensive care unit, Hospital mortality, Quality improvement, Patient safety, Health service administration, Emergency department, Sepsis resuscitation bundle
Background
Despite the major burden of sepsis as a leading cause of human death, studies have repeatedly demonstrated that the compliance with the clinical practice guidelines for sepsis management, grouped in the sepsis resuscitation bundle, is low and that this low compliance is associated with increased mortality [1–9]. As a result, the Surviving Sepsis Campaign (SSC) has recommended performance improvement efforts be undertaken [10, 11] and the National Quality Forum has endorsed the bundle implementation [12]. Thus, several institutional, national and international improvement projects have been launched [13–16].
However, transitioning evidence into sustainable clinical improvement in sepsis management has been a complex task [17–19]. Implementation barriers include delayed identification of septic patients, unawareness of or disagreement with guidelines, guideline complexity, lack of resources [17–19], staff shortage and unavailability of specialized setting required to put therapy into routine practice [9]. In the face of this complexity, different implementation strategies have been utilized, including education, posters, reminders, audit and feedback, paper-based and electronic sepsis screening tools, clinical pathways, rapid response teams and sepsis response teams (SRTs) [20–28]. The differential or the synergistic effect of these strategies on changing sepsis management practice has not been studied. However, it has been shown in other settings that these interventions vary in effectiveness. Two systematic reviews showed that educational activities and audits had low leverage on changing physician practices and reminders had a slightly larger effect [29, 30]. Automation and forcing function have higher leverage [31], and a multifaceted approach may be the most effective [29, 30]. Yet, many sepsis improvement projects invested in potentially low-leverage interventions leading to modest change in sepsis bundle compliance and mortality. The use of automation and forced function in sepsis management may be achieved by combining an electronic sepsis alert (e-alert) system for timely sepsis identification with a sepsis response team for timely management. Although there are a few reports on implementing these two interventions individually [32–34], the inclusion of the two as components of a multifaceted intervention, which may have a synergistic effect [26], has not been studied.
The objective of this study was to examine the impact of an improvement project utilizing a multifaceted intervention that includes an e-alert system and SRT on the compliance with the sepsis resuscitation bundle and outcome of adult patients with sepsis and septic shock presenting to the emergency department (ED).
Methods
Setting
The study was conducted in a 900-bed tertiary-care academic hospital accredited by Joint Commission International. The ED receives >200,000 patients per year is staffed by board-certified emergency medicine physicians and has 15 resuscitation beds for high-acuity patients and 49 beds for moderate-acuity patients. The Intensive Care Department staffs 5 ICUs on a 24-h/7-day in-house basis [35], has a rapid response team [36] and provides coverage for boarding patients in the ED who meet ICU admission criteria.
Study design
This was a pre–post implementation study that consisted of a pre-intervention phase and a 2-step implementation (intervention phases I and II). During the study period, there were no major changes in the hospital admission criteria, nursing or medical care plans, or staffing structure. The study was approved by the Institutional Review Board of Ministry of National Guard-Health affairs, and informed consent requirement was waived.
Pre-intervention phase
In this phase (January 01, 2011–September 24, 2012), patients presenting to the ED were triaged based on illness severity according to the Canadian Triage and Acuity Scale [37], evaluated first by the ED nurses and physicians, and then referred to a primary admitting service. Critically ill septic patients were subsequently referred to the intensive care team. Identifying patient as having sepsis and initial resuscitation were based on clinical assessment by the ED physicians, the primary admitting service, the intensive care team or a combination of these services. During this phase, the sepsis bundle had not been implemented.
Intervention phase I
In this phase (September 25, 2012–March 03, 2013), sepsis e-alert and computerized physician order entry (CPOE) sepsis management order-set (based on the SCC sepsis bundle) were implemented and were accompanied by an educational campaign targeting the ED healthcare providers. A clinical pathway was also generated after multidisciplinary discussions (Additional file 1: Figure S1, Figure S2, Table S1). Additionally, weekly text messages were sent to the phones of the ED and ICU physicians about the bundle compliance rates highlighting the element of the bundle that had the lowest compliance.
This phase required considerable planning, which started in October 2011 as a multidisciplinary quality improvement project aimed at improving sepsis management through the implementation of the SSC bundle. Root-cause analysis was conducted to search for the probable causes of low bundle compliance. In addition, the flow of septic patients from the ED to the ICU was mapped to understand related processes and bottlenecks. After analysis, the low compliance was attributed to multiple factors including delays in sepsis recognition, insufficient awareness of sepsis resuscitation bundle and the complexity of the care processes. For the intervention phase I, the project focused on improving early sepsis recognition through building an electronic sepsis screening tool (e-alert) in the hospital electronic health record (EHR) system (QuadraMed® Computerized Patient Record System, Reston, VA, USA) and on simplifying the care process by building a CPOE order-set that incorporated the required orders in groups on one platform. The e-alert was based on identifying abnormal vital signs and certain laboratory tests, all captured from the EHR. We used in designing our e-alert the formal definition of sepsis (previously called severe sepsis) with includes a combination of the Systemic Inflammatory Response Syndrome (SIRS) criteria and one organ dysfunction, because this quality improvement project was performed the new definition for sepsis (Sepsis-3) became available [38.] When a patient demonstrated any two SIRS criteria (temperature >38 °C OR <36 °C; heart rate >90 beats/min; respiratory rate >20 breaths/min; WBC count >12,000/mm3 OR <4000/mm3) AND at least one of the following organ dysfunctions (systolic blood pressure 86–90 mmHg with intravenous fluids or <86 mmHg regardless of fluids; blood oxygen saturation of 85–90% with supplemental oxygen or <85% without oxygen; lactate >2 mmol/L) OR two of the above organ dysfunctions, an e-alert was sent to the nursing work-list prompting her/him to contact the treating medical team. The treating physician would then evaluate the patient, confirm the presence or absence of these conditions and use the CPOE order-set if indicated. The development and testing of both the e-alert and the CPOE order-set went through multiple PDSA (plan, do, study, act) cycles with small-scale testing, documentation and learning in the EHR system development (in-production) domain until the activation date. During these PDSA cycles, certain triggers were added to the e-alert (systolic blood pressure 86–90 mmHg with intravenous fluids or <86 mmHg regardless of fluids; blood oxygen saturation of 85–90% with supplemental oxygen or <85% without oxygen) to improve specificity. We could not use reduced level of consciousness in building the e-alert, because it was recorded as a text and not as a numeric value in our EHR.
Intervention phase II
On March 04, 2013, and through October 30, 2013, a dedicated 24/7 SRT was launched in addition to the interventions already in place from intervention phase I. The SRT consisted of an intensive care registrar physician and a nurse who were trained on sepsis management using didactic lectures and simulation-based learning. The bedside nurse activated the SRT after being prompted by the e-alert or when sepsis was clinically suspected. The SRT assessed the patient for the presence of sepsis, followed the same clinical pathway used in intervention phase I and provided sepsis management as needed in collaboration with the treating team.
Data collection and outcome measures
Starting October 2011, we collected patient-level data prospectively by a dedicated data collector using the 2008 SSC data collection tool [39]. Additional data were collected retrospectively for the period January 2011–September 2011, to expand the baseline data of pre-intervention phase. Sepsis (originally severe sepsis) was defined as SIRS with acute organ dysfunction secondary to documented or suspected infection. The organ dysfunctions that were used to build the e-alert were hypotension, hypoxia and increased lactate as defined above. Septic shock was defined as sepsis (originally severe sepsis) with persistent hypotension after fluid resuscitation with at least 20 mL/kg of crystalloid (or equivalent). We recorded the compliance with the following individual elements of the 2008 SSC resuscitation bundle and the time to intervention: [1] serum lactate obtained within 6 h of presentation, [2] blood cultures taken before the administration of broad-spectrum antibiotics, [3] broad-spectrum antibiotics administered within 3 h of ED admission, [4] 20 mL/kg of crystalloid (or equivalent) administered in patients who were hypotensive or had lactate >4 mmol/L; and in patients who remained hypotensive or had lactate >4 mmol/L, [6] achievement of central venous pressure (CVP) ≥8 mmHg and central venous oxygen saturation (ScvO2) ≥70% and initiation of vasopressors. Based on recent evidence arising from the recent clinical trials on early goal-directed therapy [40–42], we evaluated bundle compliance with the first 4 elements only and we did not include CVP and ScvO2. The primary outcome measure was hospital mortality. Secondary outcome measures were the need for mechanical ventilation during this episode of sepsis, ICU and hospital length of stay (LOS) for all patients for survivors.
Statistical analysis
All statistical analyses were performed using SAS software (version 9.1; SAS Institute, Cary, NC). Continuous variables were presented as means with standard deviations, and categorical variables as absolute and relative frequencies. Mann–Whitney and Chi-square tests were used to compare differences between the phases as appropriate. To account for the differences in baseline characteristics among the three phases, we generated propensity scores using the following variables that were clinically relevant and statistically associated with the different phases (Table 1): pneumonia, hypothermia, acutely altered mental status, hypoxia, leukopenia, increased creatinine, thrombocytopenia, hyperbilirubinemia, coagulopathy and lactate levels. To check the balancing effect of propensity scores, we used the propensity scores as covariates in an analysis of covariance for continuous baseline variables and in multinomial logistic regression for categorical baseline variables. We used multivariate logistic and linear regression analysis to examine the association of implementation phase with mortality and LOS after adjustment to propensity scores. A p value <0.05 was considered to be statistically significant. We constructed control charts with upper and lower control limits for the 4-element bundle compliance and for hospital mortality using CHARTrunner, version 3.6.88 (PQ Systems, Dayton OH, USA).
Table 1.
Baseline characteristics of patients with sepsis and septic shock on presentation in the three study phases: pre-intervention (A), intervention phase I (B) and intervention phase II (C)
| All patients | Pre-intervention A |
Intervention phase I B |
Intervention phase II C |
Crude p value | p value after propensity score adjustment | ||||
|---|---|---|---|---|---|---|---|---|---|
| N = 436 | N = 195 | N = 699 | B vs. A | C vs. B | C vs. A | B vs. A | C vs. B | C vs. A | |
| Source of sepsis, no. (%) | |||||||||
| Pneumonia | 228 (52.3) | 93 (47.7) | 281 (40.2) | 0.29 | 0.06 | <0.0001 | 0.99 | 0.98 | 0.96 |
| Urinary tract infection | 70 (16.1) | 28 (14.4) | 107 (15.3) | 0.59 | 0.74 | 0.74 | 0.78 | 0.90 | 0.38 |
| Acute abdominal infection | 40 (9.2) | 8 (3.6) | 47 (6.7) | 0.03 | 0.18 | 0.13 | 0.05 | 0.37 | 0.17 |
| Soft tissue infection | 8 (1.8) | 8 (4.1) | 32 (4.6) | 0.10 | 0.78 | 0.01 | 0.42 | 0.84 | 0.56 |
| Other infections | 138 (31.7) | 68 (34.9) | 254 (36.3) | 0.43 | 0.71 | 0.11 | 0.92 | 0.60 | 0.48 |
| Signs and symptoms, no. (%) | |||||||||
| Hyperthermiaa | 114 (26.2) | 52 (26.7) | 172 (24.6) | 0.89 | 0.56 | 0.56 | 0.81 | 0.15 | 0.47 |
| Hypothermiaa | 34 (7.8) | 6 (3.1) | 7 (1.0) | 0.02 | 0.04 | <0.0001 | 0.99 | 0.86 | 0.81 |
| Acutely altered mental status | 157 (36.0) | 48 (24.6) | 59 (8.4) | 0.005 | <0.0001 | <0.0001 | 0.98 | 0.67 | 0.71 |
| Chills and rigors | 11 (2.5) | 7 (3.6) | 2 (0.3) | 0.46 | 0.001 | 0.0006 | 0.55 | 0.0007 | 0.02 |
| Tachycardiaa | 354 (81.2) | 168 (86.2) | 624 (89.3) | 0.13 | 0.23 | 0.0001 | 0.76 | 0.44 | 0.49 |
| Tachypneaa | 368 (84.4) | 174 (89.2) | 583 (83.4) | 0.11 | 0.05 | 0.66 | 0.44 | 0.34 | 0.92 |
| Hypotensiona | 368 (84.4) | 111 (56.9) | 265 (37.9) | <0.0001 | <0.0001 | <0.0001 | 0.004 | 0.42 | <0.0001 |
| Hypoxiaa | 101 (23.2) | 79 (40.5) | 227 (32.5) | <0.0001 | 0.04 | 0.0008 | 0.94 | 0.96 | 0.99 |
| Laboratory findings, no. (%) | |||||||||
| Leukocytosisa | 237 (54.4) | 85 (43.6) | 229 (32.8) | 0.01 | 0.005 | <0.0001 | 0.14 | 0.003 | <0.0001 |
| Leukopeniaa | 36 (8.3) | 6 (3.1) | 21 (3.0) | 0.02 | 0.96 | <0.0001 | 0.99 | 0.99 | 0.92 |
| Increased creatininea | 107 (24.5) | 4 (2.1) | 1 (0.1) | <0.0001 | 0.009 | <0.0001 | 0.97 | 0.99 | 0.68 |
| Thrombocytopeniaa | 40 (9.2) | 0 (0.0) | 1 (0.1) | <0.0001 | 1.0 | <0.0001 | 0.43 | 0.99 | 0.70 |
| Hyperbilirubinemiaa | 44 (10.1) | 1 (0.5) | 1 (0.1) | <0.0001 | 0.39 | <0.0001 | 0.82 | 0.94 | 0.68 |
| Hyperlactatemiaa | 168 (38.5) | 70 (35.9) | 320 (45.8) | 0.53 | 0.01 | 0.02 | 0.99 | 0.99 | 0.98 |
| Coagulopathya | 67 (15.4) | 2 (1.0) | 1 (0.1) | <0.0001 | 0.12 | <0.0001 | 0.87 | 0.86 | 0.67 |
| Classification, no. (%) | |||||||||
| Sepsis | 108 (24.8) | 99 (50.8) | 584 (83.6) | <0.0001 | <0.0001 | <0.0001 | 0.93 | 0.78 | 0.68 |
| Septic shock | 328 (75.2) | 96 (49.2) | 115 (16.5) | ||||||
aHyperthermia: temperature >38 °C, hypothermia: temperature <36 °C, tachycardia: heart rate >90/min, tachypnea: respiratory rate >20/min, hypotension: systolic blood pressure <90, mean arterial pressure <65 or systolic blood pressure decrease >40 mmHg from baseline, hypoxia: oxygen requirement to maintain oxygen saturation >90%, leukocytosis: WBC count >12 × 109/L, leucopenia: white blood cell count <4 × 109/L, increased creatinine: creatinine increase >176.8 mmol/L, thrombocytopenia: platelet count <100 × 109/L, hyperbilirubinemia: bilirubin >34.2 mmol/L, hyperlactatemia: lactate >2 mmol/L, coagulopathy: international normalized ratio (INR) >1
Results
Patients baseline characteristics
At the time of identification, patients in the intervention phases I and II were less likely to have hypothermia, altered mental status, hypotension, hypoxemia, leukopenia, elevated creatinine, thrombocytopenia, hyperbilirubinemia and coagulopathy compared to patients in the pre-intervention phase (Table 1). These differences in symptoms and signs, and key laboratory findings between the three phases suggest that the implementation of multifaceted interventions including sepsis e-alert and SRT was associated with earlier identification of sepsis.
Propensity scores
The balancing effect of the generated propensity scores is shown in Table 1. When adjusted to propensity scores, all baseline characteristics were balanced.
Interventions during the course of treatment
Intervention phases I and II were associated with improvement in most measured processes of sepsis care. The frequency of measuring lactate (at any time and within 6 h) and the time to obtaining lactate all improved. Similarly the frequency of obtaining blood cultures (at any time and before antibiotics) and the time to blood cultures improved. Antibiotics administration within 3 h and the time to antibiotics followed a similar pattern. In patients with persistent hypotension or lactate >4 mmol/L, CVP ≥8 mmHg and ScvO2 of ≥70% were achieved more often (Table 2). As a result, the overall compliance with 4-element bundle increased from 19.3 to 44.6 to 69.4% (p values <0.0001 after adjustment to propensity scores) (Table 2; Fig. 1).
Table 2.
Sepsis-related interventions during the treatment period for patients with sepsis or septic shock in the three study phases: pre-intervention (A), intervention phase I (B) and intervention phase II (C)
| Variable | Pre-intervention A |
Intervention phase I B |
Intervention phase II C |
p value | p value after propensity scores adjustments | ||||
|---|---|---|---|---|---|---|---|---|---|
| All patients | N = 436 | N = 195 | N = 699 | B vs. A | C vs. B | C vs. A | B vs. A | C vs. B | C vs. A |
| Lactate measured at any time, no. (%) | 406 (93.1) | 191 (97.9) | 682 (97.6) | 0.01 | 1.0 | 0.0003 | 0.02 | 0.72 | 0.009 |
| Time to lactate (h), mean ± SD | 9.3 ± 22.1 | 2.5 ± 5.3 | 1.1 ± 3.7 | <0.0001 | 0.0008 | <0.0001 | 0.002 | 0.0002 | <0.0001 |
| Lactate (mmol/L), mean ± SD | 3.9 ± 3.6 | 3.3 ± 2.3 | 3.4 ± 5.5 | 0.01 | 0.93 | 0.03 | 0.76 | 0.28 | 0.26 |
| Measure lactate within 6 h, no. (%) | 270 (61.9) | 168 (86.2) | 663 (94.9) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Blood culture obtained at any time, no. (%)a | 238 (54.6) | 119/194 (61.3) | 592/679 (87.2) | 0.11 | <0.0001 | <0.0001 | 0.16 | <0.0001 | <0.0001 |
| Time to blood culture (h), mean ± SD | 2.4 ± 6.7 | 0.9 ± 1.4 | 0.9 ± 2.5 | 0.001 | 0.94 | 0.0008 | 0.02 | 0.84 | 0.0001 |
| Blood culture before antibiotics, no. (%) | 219 (50.2) | 116 (59.5) | 565 (80.8) | 0.02 | <0.0001 | <0.0001 | 0.10 | <0.0001 | <0.0001 |
| Antibiotics administered at any time, no. (%)a | 266/269 (98.9) | 136/137 (99.3) | 439/448 (98.0) | 1.0 | 0.47 | 0.55 | 0.97 | 0.45 | 0.87 |
| Time to antibiotic administration (h), mean ± SD | 4.8 ± 6.7 | 3.0 ± 3.6 | 1.9 ± 3.3 | 0.0004 | 0.002 | <0.0001 | 0.002 | 0.002 | 0.01 |
| Antibiotics within 3 h, no. (%) | 295 (67.7) | 149 (76.4) | 625 (89.4) | <0.0001 | <0.0001 | <0.0001 | 0.01 | 0.0001 | <0.0001 |
| Patients with hypotension/lactate >4 mmol/L | N = 406 | N = 149 | N = 361 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fluids delivered at any time, no. (%)a | 399 (98.3) | 148 (99.3) | 344 (95.3) | 0.69 | 0.02 | 0.02 | 0.51 | 0.15 | 0.51 |
| MAP raised and remained >65 mmHg, no. (%) | 88/399 (22.1) | 53/148 (35.8) | 229/344 (67.8) | 0.001 | <0.0001 | <0.0001 | 0.02 | 0.50 | 0.09 |
| Vasopressors administered, no. (%) | 299/311 (96.1) | 91/95 (95.8) | 101/115 (87.8) | 1.0 | 0.04 | 0.002 | 0.72 | 0.07 | 0.05 |
| Fluids (≥20 mL/kg) and vasopressors in the first 6 h, no. (%) | 364/412 (88.4) | 144/153 (94.1) | 324/370 (87.6) | 0.04 | 0.03 | 0.74 | 0.04 | 0.08 | 0.85 |
| Compliance with the 4 elements bundle | 84 (19.3) | 87 (44.6) | 485 (69.4) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Patients with persistent hypotension/lactate >4 mmol/L | N = 333 | N = 115 | N = 209 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CVP ≥ 8 mmHg achieved, no. (%) | |||||||||
| Within 6 h | 71/358 (19.8) | 24/121 (19.8) | 64/245 (26.1) | 0.99 | 0.18 | 0.07 | 0.47 | 0.0002 | <0.0001 |
| Within 24 h | 190 (57.2) | 51 (44.4) | 98 (46.9) | 0.002 | 0.69 | <0.0001 | 0.06 | 0.004 | 0.66 |
| After 24 h | 42 (12.7) | 6 (5.2) | 7 (3.4) | ||||||
| Time to CVP (h), mean ± SD | 16.3 ± 21.8 | 11.3 ± 10.4 | 8.1 ± 9.5 | 0.01 | 0.05 | <0.0001 | 0.25 | 0.02 | 0.005 |
| ScvO2 ≥ 70% achieved, no. (%) | |||||||||
| Within 6 h | 65/358 (18.2) | 19/121 (15.7) | 56/245 (22.9) | 0.53 | 0.11 | 0.16 | 0.69 | <0.0001 | 0.0006 |
| Within 24 h | 106 (31.9) | 26 (22.6) | 78 (37.3) | 0.03 | 0.57 | 0.006 | 0.003 | <0.0001 | 0.31 |
| After 24 h | 37 (11.1) | 5 (4.4) | 7 (3.6) | ||||||
| Time to ScvO2 (h) mean ± SD | 20.8 ± 52.1 | 9.8 ± 13.7 | 8.2 ± 12.7 | <0.0001 | <0.0001 | <0.0001 | 0.41 | 0.40 | 0.09 |
aPatients who had cultures or received antibiotics prior to meeting sepsis and septic shock criteria were not included
a20 mL/kg of crystalloid fluids CVP central venous pressure, MAP mean arterial pressure, ScvO 2 central venous oxygen saturation. Denominators are equal to ‘N’ in all three phases unless otherwise specified
Fig. 1.
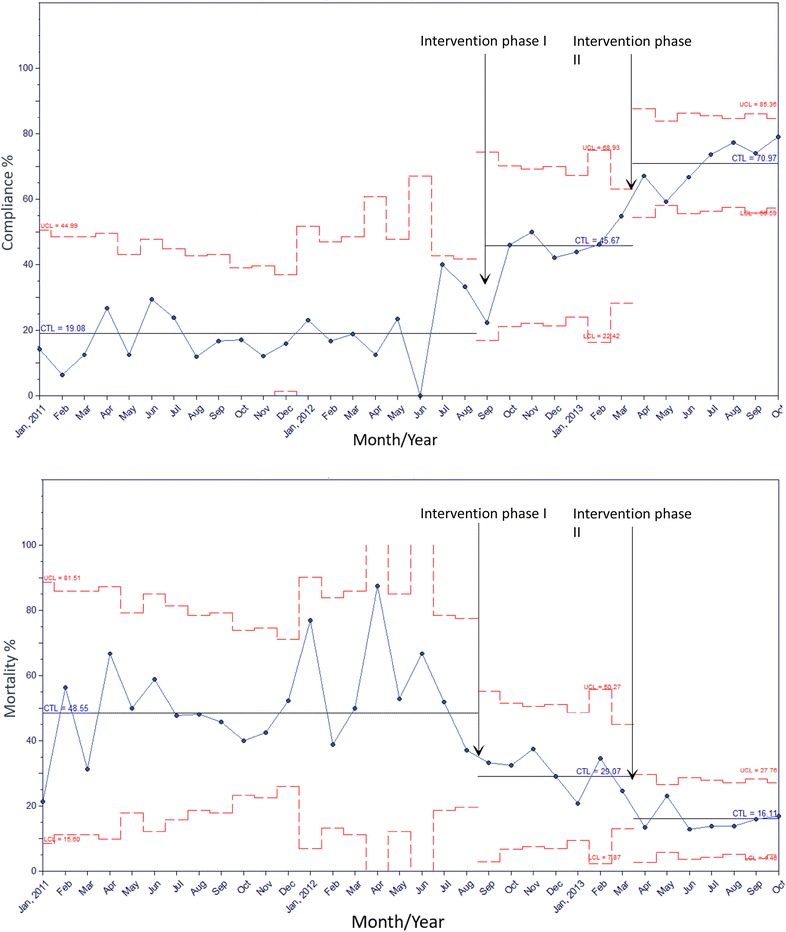
Control chart with upper and lower control limits of the overall compliance with the resuscitation bundle and the mortality in the three phases. The two arrows on the chart show the time of initiation of the intervention phases I and II
Outcomes
Intervention phase II was associated with reduction in crude hospital mortality and ICU LOS (Fig. 1; Table 3). When adjusted to propensity score, intervention phase II was associated with a significant reduction in hospital mortality [adjusted odds ratio (aOR) 0.71, 95% CI 0.58–0.85, p = 0.003] and in the need for mechanical ventilation (aOR 0.45, 95% CI 0.37–0.55, p < 0.0001). In addition, intervention phase II was associated with reduction in ICU LOS and hospital LOS for all patients as well as ICU LOS for survivors (Table 3).
Table 3.
Outcomes among patients with sepsis or septic shock in the three study phases: pre-intervention (A), intervention phase I (B) and intervention phase II (C) with adjustment to propensity scores
| Variable | Pre-intervention A |
Intervention phase I B |
Intervention phase II C |
Propensity scores adjusted OR or correlation coefficient* (95% CI, p value) | ||
|---|---|---|---|---|---|---|
| All patients | N = 436 | N = 195 | N = 699 | B vs. A | C vs. B | C vs. A |
| Hospital mortality, no. (%) | 208 (47.7) | 60 (30.8) | 118 (16.9) | 0.73 (0.48–1.09, 0.13) | 0.78 (0.51–1.17, 0.23) | 0.71 (0.58–0.85, 0.003) |
| ICU LOS (days), mean ± SD | 13.3 ± 17.4 | 8.6 ± 8.3 | 5.1 ± 11.4 | −4.49 (−7.39 to −1.59, 0.002)* | −1.54 (−3.38 to 0.29, 0.09)* | −2.72 (−3.92 to −1.52, <0.0001)* |
| Mechanical ventilation, no. (%) | 265 (60.8) | 54 (27.7) | 78 (11.2) | 0.33 (0.22–0.49, <0.0001) | 0.64 (0.41–0.99, 0.05) | 0.45 (0.37–0.55, <0.0001) |
| ICU LOS among survivors (days), mean ± SD | 15.9 ± 19.5 | 11.9 ± 10.4 | 8.2 ± 13.8 | −5.88 (−11.44 to −0.22, 0.04)* | −1.05 (−5.22 to 3.10, 0.61)* | −3.55 (−6.13 to −0.98, 0.007)* |
| Hospital LOS (days), mean ± SD | 29.3 ± 33.9 | 25.7 ± 40.9 | 15.3 ± 23.5 | −1.37 (−8.28 to 5.53, 0.70)* | −8.42 (−13.29 to −3.54, 0.0007)* | −5.35 (−7.76 to −2.94, <0.0001)* |
| Hospital LOS among survivors (days), mean ± SD | 28.1 ± 30.8 | 24.7 ± 37.7 | 20.6 ± 28.6 | −1.76 (−12.09 to 8.57, 0.73)* | −6.22 (−16.9 to 4.48, 0.25)* | −3.45 (−7.83 to 0.92, 0.12)* |
LOS length of stay, * correlation coefficient (95% CI, p value)
Discussion
Our study demonstrates that the implementation of a multifaceted intervention including a sepsis e-alert with an SRT was associated with improvement in the care process of sepsis management; improvement in the timeliness of lactate measurement, obtaining of blood cultures, antibiotic administration and achievement of CVP and ScvO2 targets and reduction in the need for mechanical ventilation and reduction in hospital mortality and LOS.
Quality improvement projects that implemented sepsis resuscitation bundle [43] have been shown to reduce mortality in several settings [20, 22, 26]. However, the effect varied considerably and was often modest, particularly when instruction-based interventions were used [22]. Our study showed a substantial improvement when a multifaceted approach was used that included an e-alert with SRT. The use of e-alert with SRT exemplifies the use of automation and forcing function in improving adherence to the best practice.
Timely recognition of sepsis is key to improving its management. A sepsis e-alert that is activated as soon as abnormal values are entered in the EHR may serve as a decision support system that bypasses the shortcomings of human processes. In addition, electronic screening for sepsis is more reliable and sustainable than paper-based screening tools as demonstrated in other settings [44]. Our data show that the e-alert identified patients with sepsis cases at earlier stage, which is a major aim of the project. However, early recognition may raise concerns about workload increase and overtreatment. However, in our project, physicians reviewed all cases before initiating treatment; thus reducing the risk of overtreatment. Additionally, it is our belief that the implications of overtreatment, if exists, are small compared to the substantial advantage of timely sepsis management.
Improving compliance with evidence-based time-sensitive therapies may be achieved by using condition-specific teams, as shown with acute myocardial infarction [45], stroke [46] and trauma teams [47]. Such teams are more focused, knowledgeable and experienced thus reducing care variation and increasing reliability. Only few studies have examined SRT implementation. A prospective cohort study examined the impact of ‘team’ vs. ‘non-team’ models on implementing sepsis bundle in multiple Asian countries [21]. In the non-team model, ED physicians completed the bundle in the ED as a standard care [21]. In the team model, the implementation was championed by intensivists with the bundle completed in the ICU and was associated with a greater improvement in the compliance [21]. Another study examined the impact of daily auditing with weekly feedback and SRT activation in patients admitted to the medical ICU with sepsis or septic shock [26]. The compliance rate with the sepsis resuscitation bundle increased from 12.7% at baseline to 37.7 and 53.7% during the weekly feedback and SRT activation periods, respectively (p < 0.001) [26]. The overall hospital mortality rates were 30.3, 28.3 and 22.0% during baseline, weekly feedback and SRT team periods, respectively (p = 0.03) [26]. In this study, the SRT was involved only in managing patients admitted to the medical ICU and required restructuring the existing ICU service, without adding new manpower [26].
There have been a heated debate about using CVP and ScvO2 as treatment goals in patients with sepsis; a debate that manifested intensely among our staff during the course of our project. Three recent trials of early goal-directed therapy demonstrated that routine use of central hemodynamic and oxygen-saturation monitoring compared to their use at-physician discretion did not result in better outcomes [40–42] resulted in removal of these elements from the sepsis resuscitation bundle [48]. Our data show significant improvement in outcomes even with modest increase in the compliance with the CVP and ScvO2 elements, supporting the notion that the key to improving sepsis outcome lies mainly in the early recognition, early fluid administration and early antibiotic therapy [48]. Why bundle compliance fell short of 100% even in the SRT phase?. An important reason is that the 2008 SCC bundle accounts for compliance achievement rather than attempting-to-achieve therapeutic goals within the recommended time [49], which has been revised in the 2012 version. Other reasons may include the complexity and high number of interventions, involvement of multiple players, resistance to change and the long waiting time to access care in our ED.
Our study has demonstrated that early identification and management of sepsis was associated with a reduction the need for mechanical ventilation and vasopressor therapy illustrating the notion that organ dysfunction in sepsis is preventable by early treatment. Some physicians prefer a slower pace of fluid resuscitation than what has been recommended in the SSC guidelines with the perception that a faster approach may precipitate pulmonary edema. While this concern may be valid in selected patients with very poor cardiovascular status, our study shows that early fluid resuscitation is generally associated with reduction in the need for mechanical ventilation.
SRT implementation, which is complex and intensive, should take into account factors that facilitate its sustainability, such as senior and clinical leadership engagement, evidence credibility, staff involvement and training, infrastructure and alignment with the organization strategic aim and culture [50]. SRT implementation, monitoring of compliance and comparing of outcomes require considerable infrastructure, resources and costs, which should be considered before adopting such an intervention. Nevertheless, we believe that the SRT may be a cost-effective intervention given the significant reduction in LOS, although a proper cost-effectiveness analysis is needed.
Our results should be interpreted in light of the study strengths and limitations. Strengths include the prospective design and the use of data to drive improvement. Limitations include being a single-center study and the pre–post nature. The study was limited to the ED intervention and did not include septic patients who were referred from the wards or were already in the ICUs. Because baseline compliance was low, the room for improvement was large. Therefore, it is possible that impact of this intervention could be less in centers with better performance at baseline. In addition, the e-alert system was built internally using the existing EHR and based on the available data and has not been validated externally. However, this will be likely the case for most similar projects, and our data can be used to support the concept of e-alert rather that a particular system. It remains to be studied whether designing an e-alert for sepsis screening using the new Sepsis-3 criteria would be superior to e-alerts based on the SIRS criteria like ours. Due to the nature of the database, we evaluated the timing of antibiotic therapy, but not antibiotic adequacy or adherence to local guidelines. Our data show learning effect, as reflected by improvement in compliance preceding the actual date of each phase on the control chart. Preparation to any intervention, whether the e-alert or the SRT was discussed among the staff who were invited to provide their input into the project; therefore, it is not surprising to see this learning effect. Such phenomenon is not unusual in quality improvement projects. We think that having this learning effect or ‘contamination’ is acceptable as improving the care was the final goal; however, this may have underestimated the difference between the phases. Nevertheless, the intervention phases I and II are distinct phases; with the second phase has manpower implications; therefore, we analyzed them as separate phases. Due to the nature of the SSC database, certain data elements were not available such as age, gender, comorbidities and severity of illness. Although our analysis suggests that the propensity scores were able to balance the baseline differences, the availability of such data elements may have further improved their performance. The possibility of ascertainment bias (detection of milder cases) is inherent in studies that implement screening systems. However, the differences remained significant after adjustment to propensity scores.
In conclusion, the implementation of a multifaceted intervention including a sepsis e-alert system with SRT was associated with improvement in care processes of sepsis and septic shock and reduced need for mechanical ventilation as well as reduced mortality and LOS.
Authors’ contributions
YMA, HA, MS, SS, AM, ST contributed to study conception and design; YMA, HA, SQ, AA, SS, AM, FH, SM, AM, MM, ST helped in acquisition of data; YMA, AA, HA, RH, MS, HT, FH, SM, AM, MM analyzed and interpreted the data; YMA, AA, HA, MS, SA, AM, SM drafted the manuscript; YMA, AA, HA, RH, MS, HT, SQ, AA, SA, AM, FH, SM, AM, MM, ST critically revised the manuscript for important intellectual content; YMA, HMT statistically analyzed the study; YMA, AA, HA, RH, MS, HT, SQ, AA, SA, AM, FH, SM, AM, MM, ST finally approved the version; YMA, HD, RH supervised the study. All authors read and approved the final manuscript.
Acknowledgements
Other Sepsis Improvement Team Members
John Alchin, CCRN5, Syed Kamran Asad, MBBS1, Aslam Patta, MBBS1, Saeed Obbed, MBBS1, Mohammed Almadani, MD1, Tahir Khan, MBBS1, Ijaz Tasawar, MBBS1, Vusala Razayeva, MBBS1, Magdi Ibrahim, MBBS1, Erick Elvena, RN1, Grace Teo, RN1, Komathi Govindan, RN1, Emily Carmona, RN1, Norhaiza Kassim, RN1, Musharaf Sadat, MBBS1, Tarek Dabbagh, MD1,4, Souzan M. Al Owais, RPh, CPHQ2, Basit Baig, MD, MBBS, MSc2, Majed Al Thagafi, MD, FRCPC3,4, Khaled Rajhi, MD3,4, Khalid Al Johani, MD3,4
1Intensive Care Department, King Abdulaziz Medical City
2Department of Quality Management, King Abdulaziz Medical City
3Emergency Medicine Department, King Abdulaziz Medical City
4King Saud bin Abdulaziz University for Health Sciences (KSAU-HS)
5Clinical Nursing, King Abdulaziz Medical City
All from Ministry of National Guard-Health Affairs, Riyadh, Saudi Arabia.
Competing interests
The authors declare that they have no competing interests.
Ethical approval and consent to participate
The study was approved by the Institutional Review Board of Ministry of National Guard-Health affairs and informed consent requirement was waived.
Funding
This study was funded by King Abdullah International Medical Research Center (KAIMRC), Riyadh, Saudi Arabia.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CI
confidence interval
- CPOE
computerized physician order entry
- CVP
central venous pressure
- e-alert
electronic alert
- ED
emergency department
- EHR
electronic health record
- ICU
intensive care department
- LOS
length of stay
- PDSA
plan, do, study and act
- SAS
statistical analysis software
- ScvO2
central venous oxygen saturation
- SRT
sepsis response team
- SSC
surviving sepsis campaign
Additional file
Additional file 1: Figure S1. Sepsis Response Team Clinical Pathway. Figure S2. Sepsis Response Team Checklist. Table S1. Sepsis Response Team Policy and Procedures.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13613-017-0280-7) contains supplementary material, which is available to authorized users.
Contributor Information
Yaseen M. Arabi, Phone: +966-11-8011111, Email: arabi@ngha.med.sa, Email: yaseenarabi@yahoo.com
Hasan M. Al-Dorzi, Email: aldorzih@yahoo.com
Ahmed Alamry, Email: amryah@ngha.med.sa.
Ra’ed Hijazi, Email: hijazir@ngha.med.sa.
Sami Alsolamy, Email: SolamyS@ngha.med.sa.
Majid Al Salamah, Email: SalamahM@ngha.med.sa.
Hani M. Tamim, Email: hani_t@hotmail.com
Saad Al-Qahtani, Email: qahtanis4@ngha.med.sa.
Abdulaziz Al-Dawood, Email: dawooda@ngha.med.sa.
Abdellatif M. Marini, Email: mariniab@ngha.med.sa
Fatimah H. Al Ehnidi, Email: alhunaidifa@ngha.med.sa
Shihab Mundekkadan, Email: mundekkadansh@ngha.med.sa.
Amal Matroud, Email: MatroudA@ngha.med.sa.
Mohamed S. Mohamed, Email: mohamedmo1@ngha.med.sa
Saadi Taher, Email: tahers@ngha.med.sa.
References
- 1.Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12(12):919–924. doi: 10.1016/S1473-3099(12)70239-6. [DOI] [PubMed] [Google Scholar]
- 2.Phua J, Koh Y, Du B, Tang YQ, Divatia JV, Tan CC, et al. Management of severe sepsis in patients admitted to Asian intensive care units: prospective cohort study. BMJ. 2011;342:d3245. doi: 10.1136/bmj.d3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132(2):425–432. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kortgen A, Niederprum P, Bauer M. Implementation of an evidence-based “standard operating procedure” and outcome in septic shock. Crit Care Med. 2006;34(4):943–949. doi: 10.1097/01.CCM.0000206112.32673.D4. [DOI] [PubMed] [Google Scholar]
- 5.Lin SM, Huang CD, Lin HC, Liu CY, Wang CH, Kuo HP. A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock. 2006;26(6):551–557. doi: 10.1097/01.shk.0000232271.09440.8f. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen HB, Corbett SW, Steele R, Banta J, Clark RT, Hayes SR, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007;35(4):1105–1112. doi: 10.1097/01.CCM.0000259463.33848.3D. [DOI] [PubMed] [Google Scholar]
- 7.Micek ST, Roubinian N, Heuring T, Bode M, Williams J, Harrison C, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2006;34(11):2707–2713. doi: 10.1097/01.CCM.0000241151.25426.D7. [DOI] [PubMed] [Google Scholar]
- 8.Sebat F, Musthafa AA, Johnson D, Kramer AA, Shoffner D, Eliason M, et al. Effect of a rapid response system for patients in shock on time to treatment and mortality during 5 years. Crit Care Med. 2007;35(11):2568–2575. doi: 10.1097/01.CCM.0000287593.54658.89. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro NI, Howell MD, Talmor D, Lahey D, Ngo L, Buras J, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med. 2006;34(4):1025–1032. doi: 10.1097/01.CCM.0000206104.18647.A8. [DOI] [PubMed] [Google Scholar]
- 10.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 12.National Quality Forum: severe sepsis and septic shock: management bundle. http://www.qualityforum.org/Home.aspx.
- 13.World Sepsis Day. http://www.world-sepsis-day.org.
- 14.Sepsis UK Core Team. http://sepsistrust.org/meet-the-team/sepsis-uk-core-team/.
- 15.NSW Health System: Clinical Excellence Commission. http://www.cec.health.nsw.gov.au/programs/sepsis.
- 16.College of Emergency Medicine: standards for severe sepsis and septic shock in adults. http://www.collemergencymed.ac.uk/code/document.asp?id=4717.
- 17.Jones AE. Evidence-based therapies for sepsis care in the emergency department: striking a balance between feasibility and necessity. Acad Emerg Med. 2006;13(1):82–83. doi: 10.1111/j.1553-2712.2006.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 18.Carlbom DJ, Rubenfeld GD. Barriers to implementing protocol-based sepsis resuscitation in the emergency department–results of a national survey. Crit Care Med. 2007;35(11):2525–2532. doi: 10.1097/01.ccm.0000298122.49245.d7. [DOI] [PubMed] [Google Scholar]
- 19.Jones AE, Shapiro NI, Roshon M. Implementing early goal-directed therapy in the emergency setting: the challenges and experiences of translating research innovations into clinical reality in academic and community settings. Acad Emerg Med. 2007;14(11):1072–1078. doi: 10.1111/j.1553-2712.2007.tb02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Xiong Y, Schorr C, Dellinger RP. Impact of sepsis bundle strategy on outcomes of patients suffering from severe sepsis and septic shock in china. J Emerg Med. 2013;44(4):735–741. doi: 10.1016/j.jemermed.2012.07.084. [DOI] [PubMed] [Google Scholar]
- 21.Na S, Kuan WS, Mahadevan M, Li CH, Shrikhande P, Ray S, et al. Implementation of early goal-directed therapy and the surviving sepsis campaign resuscitation bundle in Asia. Int J Qual Health Care. 2012;24(5):452–462. doi: 10.1093/intqhc/mzs045. [DOI] [PubMed] [Google Scholar]
- 22.Ferrer R, Artigas A, Levy MM, Blanco J, Gonzalez-Diaz G, Garnacho-Montero J, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299(19):2294–2303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]
- 23.Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, Holanda MS, Ortiz F, Llorca J, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med. 2010;38(4):1036–1043. doi: 10.1097/CCM.0b013e3181d455b6. [DOI] [PubMed] [Google Scholar]
- 24.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36(2):222–231. doi: 10.1007/s00134-009-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giuliano KK, Lecardo M, Staul L. Impact of protocol watch on compliance with the surviving sepsis campaign. Am J Crit Care. 2011;20(4):313–321. doi: 10.4037/ajcc2011421. [DOI] [PubMed] [Google Scholar]
- 26.Schramm GE, Kashyap R, Mullon JJ, Gajic O, Afessa B. Septic shock: a multidisciplinary response team and weekly feedback to clinicians improve the process of care and mortality. Crit Care Med. 2011;39(2):252–258. doi: 10.1097/CCM.0b013e3181ffde08. [DOI] [PubMed] [Google Scholar]
- 27.Laguna-Perez A, Chilet-Rosell E, Delgado Lacosta M, Alvarez-Dardet C, Uris Selles J, Munoz-Mendoza CL. Clinical pathway intervention compliance and effectiveness when used in the treatment of patients with severe sepsis and septic shock at an Intensive Care Unit in Spain. Rev Lat Am Enfermagem. 2012;20(4):635–643. doi: 10.1590/S0104-11692012000400002. [DOI] [PubMed] [Google Scholar]
- 28.Shiramizo SC, Marra AR, Durao MS, Paes AT, Edmond MB, Pavao dos Santos OF. Decreasing mortality in severe sepsis and septic shock patients by implementing a sepsis bundle in a hospital setting. PLoS ONE. 2011;6(11):e26790. doi: 10.1371/journal.pone.0026790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii–iv, 1–72. [DOI] [PubMed]
- 30.Cheung A, Weir M, Mayhew A, Kozloff N, Brown K, Grimshaw J. Overview of systematic reviews of the effectiveness of reminders in improving healthcare professional behavior. Syst Rev. 2012;1:36. doi: 10.1186/2046-4053-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis DA, Taylor-Vaisey A. Translating guidelines into practice. A systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. CMAJ. 1997;157(4):408–416. [PMC free article] [PubMed] [Google Scholar]
- 32.Sawyer AM, Deal EN, Labelle AJ, Witt C, Thiel SW, Heard K, et al. Implementation of a real-time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39(3):469–473. doi: 10.1097/CCM.0b013e318205df85. [DOI] [PubMed] [Google Scholar]
- 33.Meurer WJ, Smith BL, Losman ED, Sherman D, Yaksich JD, Jared JD, et al. Real-time identification of serious infection in geriatric patients using clinical information system surveillance. J Am Geriatr Soc. 2009;57(1):40–45. doi: 10.1111/j.1532-5415.2008.02094.x. [DOI] [PubMed] [Google Scholar]
- 34.Nelson JL, Smith BL, Jared JD, Younger JG. Prospective trial of real-time electronic surveillance to expedite early care of severe sepsis. Ann Emerg Med. 2011;57(5):500–504. doi: 10.1016/j.annemergmed.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Arabi Y, Alshimemeri A, Taher S. Weekend and weeknight admissions have the same outcome of weekday admissions to an intensive care unit with onsite intensivist coverage. Crit Care Med. 2006;34(3):605–611. doi: 10.1097/01.CCM.0000203947.60552.DD. [DOI] [PubMed] [Google Scholar]
- 36.Al-Qahtani S, Al-Dorzi HM, Tamim HM, Hussain S, Fong L, Taher S, et al. Impact of an intensivist-led multidisciplinary extended rapid response team on hospital-wide cardiopulmonary arrests and mortality. Crit Care Med. 2013;41(2):506–517. doi: 10.1097/CCM.0b013e318271440b. [DOI] [PubMed] [Google Scholar]
- 37.Bullard MJ, Unger B, Spence J, Grafstein E. Revisions to the Canadian Emergency Department Triage and Acuity Scale (CTAS) adult guidelines. CJEM. 2008;10(2):136–151. doi: 10.1017/S1481803500009854. [DOI] [PubMed] [Google Scholar]
- 38.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 40.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 41.ARISE Investigators; ANZICS Clinical Trials Group. Peake SL, Delaney A, Bailey M, Bellomo R, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 42.Pro CI, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–873. doi: 10.1097/01.CCM.0000117317.18092.E4. [DOI] [PubMed] [Google Scholar]
- 44.Lecumberri R, Marques M, Diaz-Navarlaz MT, Panizo E, Toledo J, Garcia-Mouriz A, et al. Maintained effectiveness of an electronic alert system to prevent venous thromboembolism among hospitalized patients. Thromb Haemost. 2008;100(4):699–704. doi: 10.1160/th08-05-0337. [DOI] [PubMed] [Google Scholar]
- 45.Bradley EH, Curry LA, Webster TR, Mattera JA, Roumanis SA, Radford MJ, et al. Achieving rapid door-to-balloon times: how top hospitals improve complex clinical systems. Circulation. 2006;113(8):1079–1085. doi: 10.1161/CIRCULATIONAHA.105.590133. [DOI] [PubMed] [Google Scholar]
- 46.Hamidon BB, Dewey HM. Impact of acute stroke team emergency calls on in-hospital delays in acute stroke care. J Clin Neurosci. 2007;14(9):831–834. doi: 10.1016/j.jocn.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 47.Vernon DD, Furnival RA, Hansen KW, Diller EM, Bolte RG, Johnson DG, et al. Effect of a pediatric trauma response team on emergency department treatment time and mortality of pediatric trauma victims. Pediatrics. 1999;103(1):20–24. doi: 10.1542/peds.103.1.20. [DOI] [PubMed] [Google Scholar]
- 48.Lilly CM. The ProCESS trial—a new era of sepsis management. N Engl J Med. 2014;370:1750–1751. doi: 10.1056/NEJMe1402564. [DOI] [PubMed] [Google Scholar]
- 49.Berg GM, Vasquez DG, Hale LS, Nyberg SM, Moran DA. Evaluation of process variations in noncompliance in the implementation of evidence-based sepsis care. J Healthc Qual. 2013;35(1):60–69. doi: 10.1111/j.1945-1474.2011.00168.x. [DOI] [PubMed] [Google Scholar]
- 50.Maher L, Gustafson D, Evans A. NHS sustainability model and guide. NHS Institute for Innovation and Improvement. 2005.


