Abstract
BACKGROUND/OBJECTIVES
Recent living condition improvements, changes in dietary habits, and reductions in physical activity are contributing to an increase in metabolic syndrome symptoms including diabetes and obesity. Through such societal developments, humankind is continuously exposed to metabolic diseases such as diabetes, and the number of the victims is increasing. This study investigated Cordyceps militaris water extract (CMW)-induced glucose uptake in HepG2 cells and the effect of CMW treatment on glucose metabolism.
MATERIALS/METHODS
Colorimetric assay kits were used to determine the glucokinase (GK) and pyruvate dehydrogenase (PDH) activities, glucose uptake, and glycogen content. Either RT-PCR or western blot analysis was performed for quantitation of glucose transporter 2 (GLUT2), hepatocyte nuclear factor 1 alpha (HNF-1α), phosphatidylinositol 3-kinase (PI3k), protein kinase B (Akt), phosphorylated AMP-activated protein kinase (pAMPK), phosphoenolpyruvate carboxykinase, GK, PDH, and glycogen synthase kinase 3 beta (GSK-3β) expression levels. The α-glucosidase inhibitory activities of acarbose and CMW were evaluated by absorbance measurement.
RESULTS
CMW induced glucose uptake in HepG2 cells by increasing GLUT2 through HNF-1α expression stimulation. Glucose in the cells increased the CMW-induced phosphorylation of AMPK. In turn, glycolysis was stimulated, and glyconeogenesis was inhibited. Furthermore, by studying the mechanism of action of PI3k, Akt, and GSK-3β, and measuring glycogen content, the study confirmed that the glucose was stored in the liver as glycogen. Finally, CMW resulted in a higher level of α-glucosidase inhibitory activity than that from acarbose.
CONCLUSION
CMW induced the uptake of glucose into HepG2 cells, as well, it induced metabolism of the absorbed glucose. It is concluded that CMW is a candidate or potential use in diabetes prevention and treatment.
Keywords: Cordyceps militaris, antidiabetics, glucose transporter, glucokinase, glycogen
INTRODUCTION
Diabetes is a chronic metabolic disease with a high global prevalence. Diabetes-related health management costs continue to increase [1,2], and diabetic subjects fundamentally require pharmacotherapy together with exercise and diet.
Among the pharmacotherapies currently in use for improving diabetic symptoms, sulfonylureas, biguanide class drugs, α-glucosidase inhibitors, and thiazolidinedione class drugs are used as oral hypoglycemic agents. However, most of these drugs carry with them financial burden and side effects such as hypoglycemia, anemia, nephrotoxicity, weight gain, abdominal distension, and diarrhea [3]. For those reasons, researchers are actively investigating the diverse resources of oriental medicine in pursuit of physiologically active substances that can prevent or control diabetes. In addition, various scientific investigations attempting to elucidate the antidiabetic effects of therapies and medicinal plants that have been traditionally used in clinical oriental medicine are in progress [4,5].
Cordyceps militaris has long been known as one of three great herbal medicines (together with ginseng and velvet antler) in China. Approximately 800 kinds of Cordyceps spp. have been discovered domestically and internationally, among which 78 kinds have been collected and classified in Korea [6]. C. militaris is an entomopathogenic fungus that is usually parasitic on larvae or pupae of Lepidoptera and forms orange club-like stromata. Recently, this fungal species has gained wide recognition as a functional health food with physiologically active substances, such as the secondary metabolite cordycepin. Diverse functions of C. militaris have been reported, including a hypoglycemic effect in diabetic mice [7], improvement of liver functions [8,9], and activation of the glycometabolism-related enzyme glucokinase (GK) in liver cells [10,11]. However, among the reported studies of C. militaris, there has been little reported on its antidiabetic effect.
Diabetes occurs as a result of various factors. For the treatment or alleviation of diabetic symptoms, it is important to ensure that a high amount of sugar does not remain in blood. This is achieved by inducing cells of each tissue to quickly absorb and use the glucose molecules that have been absorbed into the system after eating [12]. Glucose gained through food is used mainly in the liver, muscles, and fat tissues. Among these, the liver is involved not only in the use of glucose but also in its storage, functioning as one of the major organs that can provide a fuel source for a starving body that requires energy [13,14]. In order to induce glucose consumption by elevating the rate of utilization of glucose in the liver, it is necessary to increase glucose uptake by enhancing the expression of glucose transporter 2 (GLUT2) [15]. The absorbed glucose is stored or used through the activation of glycolysis-related enzymes such as GK, pyruvate dehydrogenase (PDH), and glycogen synthase (GS) [16,17,18]. The activity of enzymes such as phosphoenolpyruvate carboxykinase (PEPCK), which is related to gluconeogenesis, must be inhibited in order to decrease the glucose level [19]. GLUT2 has a role in transporting glucose into the blood through the small intestine in order to maintain glucose homeostasis and to induce insulin secretion through glucose sensing in the β-cells of the pancreas [20,21]. Accordingly, the regulation of GLUT2 expression is crucial for glucose metabolism in the body. Hepatocyte nuclear factor (HNF)-1α and CCAAT-enhancer-binding proteins beta (C/EBPβ) are transcription factors reported to regulate GLUT2 expression. Various recent studies have been conducted on the human GLUT2 promoter.
AMP-activated protein kinase (AMPK) is reported to use ATP and ADP as energy sources in the metabolic activities of living cells, and to have a key role in maintaining energy homeostasis through AMP production [22,23]. AMPK has been reported to regulate the expression of genes involved in glycolysis and gluconeogenesis to maintain glucose homeostasis in the liver [24,25,26,27]. Phosphatidylinositol 3-kinase (PI3k), a signaling protein capable of acting on various mechanisms in the cell, is reported to be stimulated by G protein-coupled receptors (GPCR) or insulin receptors (IR) [26,28]. In addition, Pl3k is involved in cell growth, cell proliferation, cell differentiation, cell survival, and intracellular trafficking [28]. Protein kinase B (Akt) regulates various functions, such as glycogen synthesis, protein synthesis, and gene expression [28,29,30,31]. Therefore, many recent studies have investigated glucose uptake increases by inducing the expression of GLUT using natural products as well as by inducing the activity and expression of enzymes associated with glycolysis and gluconeogenesis.
In addition, the glucose level can be lowered effectively by inhibiting α-glucosidase in the small intestine thereby controlling the resolution of disaccharide maltose, which as a whole will delay the digestion and absorption of carbohydrates [32].
Therefore, this study investigated the mechanisms involved in both glucose uptake and metabolism of absorbed glucose in HepG2 cells treated with a C. militaris water extract (CMW). In addition, the effect of CMW on the inhibitory activity of α-glucosidase was investigated. The study describes the effect of CMW on the mechanisms involved in blood glucose reduction and suggests the potential applicability of CMW.
MATERIALS AND METHODS
Cordyceps militaris extract
The C. militaris sample used in our study was obtained from Human Herb (Gyeongsan-si, Korea). The sample was dried in a forced convection oven (OF-02G; Jeio Tech, Seoul, Korea), and 30 g of dried sample were completely ground with a blender (FM-681C; Hanil Electric, Seoul, Korea), after which, the powder was resuspended in 10.7 volumes of distilled water. The mixture was extracted at 60℃ in a shaking incubator (KMC 8480SF; Vision Scientific, Daejeon, Korea) for 24 h and then centrifuged. The supernatant obtained was filtered through a 0.45 µm syringe filter and then dried (weight after drying: 10.93 g) in a freeze dryer (BD8512; Ilshin Lab., Gyeonggi-do, Korea). The final solid content (yield: 19.44%) was thawed for the experiment.
Cell culture
HepG2 (hepatocellular carcinoma) cells were obtained from the Korean Cell Line Bank (Seoul, Korea). The cells were incubated in a growth medium comprising 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 25 mM NaHCO3, and 90% minimum essential medium (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Welgene, Daegu, Korea) and 1% penicillin-streptomycin (Welgene) at 37℃ under 5% CO2.
Cytotoxicity assay
The cytotoxicity of CMW was measured by using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) conversion method. In brief, 100 µL aliquots of HepG2 cells (1 × 104 cells/mL) were dispensed in each well of a 96-well plate and incubated for 24 h. The CMW was diluted to different concentrations in growth media to which no FBS or antibiotics had been added. The HepG2 cells were treated with the extracts for 24 h, after which the growth media were removed and MTT reagent (5 mg/mL) was added. The plates were then incubated at 37℃ for 4 h, after which the supernatant was removed. Subsequently, 100 µL of dimethyl sulfoxide was added, and the formazan that formed in each well was dissolved with shaking. After 30 min, the absorbance was measured at 570 nm using a UV/Vis spectrophotometer (Opiwen 2120 UV plus; Mecasys, Daejeon, Korea). The cytotoxicities of the different concentrations were determined through comparison to the absorbance value of the control.
Measure of glucose uptake
The change in glucose uptake by the HepG2 cells following CMW treatment was assessed by using a glucose uptake colorimetric assay kit (BioVision, Milpitas, CA, USA). In brief, the HepG2 cells were added to the wells of a 96-well plate (1 × 105 cells/mL) and incubated for 24 h in growth medium. The growth media were then replaced with medium not containing FBS, and the plates were incubated for another 24 h. The medium was replaced with Krebs-Ringer-phosphate-HEPES buffer containing 2% bovine serum albumin. After 40 min, the cells were treated with 1 µM insulin and 1.0 mg/mL CMW and incubated for 20 min. Then, they were treated with 10 mM 2-deoxyglucose for 20 min. Finally, the cells were treated with 8 µL of assay buffer and 2 µL of enzyme mix A at 37℃ for 1 h. The cell extract was retrieved by using an extraction buffer and then held at 85℃ for 40 min. After cooling, a neutralization buffer was added and the mixture was centrifuged at 500 r/min for 10 s. The supernatant was removed to a microwell plate, and 20 µL of glutathione reductase, 16 µL of 5-5′-dithiobis (2-nitrobenzoic acid), and 2 µL of recycling mix included in the assay kit were added to each of the wells and left for 40 min to react. Absorbance was then measured at 405 nm by using an ELISA microplate reader (EL808; BioTek, Winooski, VT, USA).
Glucokinase activity assay
The GK activity was measured by using a hexokinase colorimetric assay kit (Abcam, Cambridge, MA, USA). In this assay, nicotinamide adenine dinucleotide phosphate (NADP+) is converted into NADPH when the glucose-6-phosphate (G6P) produced by GK activity reacts with G6P dehydrogenase. In order to determine the optimum condition for activity induction, protein was extracted and quantified from HepG2 cells that had been treated with CMW at different concentration levels and times. Each 96 well was filled with 40 µg/50 µL of the extracted protein and then treated with 34 µL of assay buffer, 2 µL of enzyme mix, 2 µL of developer, 2 µL of coenzyme, and 10 µL of hexokinase substrate. The absorbance was measured at 450 nm at 3 min intervals.
Pyruvate dehydrogenase activity assay
The PDH activity was measured by using a commercial assay kit (Novagen; EMD Millipore, Darmstadt, Germany). This assay is based on the conversion of NAD+ into NADH through PDH activity. In order to determine the optimum condition of activity induction, HepG2 cells were treated with CMW at different concentration levels and times. Protein was then extracted from the HepG2 cells and 100 µg/0.2 mL of the extract was added to each well of a 96 well plate. After 3 h of incubation at room temperature, the supernatant was suctioned and rinsed twice using 1× stabilizer. Then, it was treated with 11 µL of 20× reagent mix, 0.2 mL of 1× buffer, 2.25 µL of 100× coupler, and 2.25 µL of 100× reagent dye. The absorbance was measured at 450 nm at 3 min intervals.
Total RNA extraction and cDNA synthesis
HepG2 cells were added to 6-well plates (1 × 106 cells/mL) and incubated for 24 h. The cells were then treated with 0.1 mg/mL CMW in growth medium for 24 h. After the CMW-containing growth medium in each well was removed, 1 mL of QIAzol lysis reagent (Qiagen, Hilden, Germany) was added for lysing the cells. The lysate was then transferred to a tube, and 200 µL of chloroform added and mixed for 15 s. The tube was then centrifuged at 12,000 × g for 15 min. The supernatant was transferred to a tube containing 500 µL of isopropanol, and the mixture was centrifuged at 12,000 × g for 10 min. The supernatant was removed, and 1 mL of a 75% ethanol solution (100% ethanol : 0.1% diethyl pyrocarbonate (DEPC) water, 75:25) was added to each tube. The mixture was centrifuged at 12,000 × g for 5 min, and the supernatant was removed and dried pellet at room temperature. Then, 40 µL of nuclease-free water was added to dissolve the RNA extract. To quantify the total RNA, 995 µL of 0.1% DEPC water was added to 5 µL of the RNA extract and the absorbance was measured at 260 nm.
An amfiRivert Platinum cDNA Synthesis Master Mix (GenDEPOT, Barker, TX, USA) was used to synthesize the first-strand cDNA. The extracted RNA (2 µg) and RNase-free water were combined to a final volume of 9 µL and left at 70℃ for 5 min. In the meantime, 10 µL of 2× cDNA synthesis buffer solution was mixed with 1 µL of cDNA Synthesis Enzyme Mix, and 11 µL of the mixture was added to each PCR tube. The reaction protocol for synthesizing the cDNA was set at 25℃ for 5 min, 42℃ for 60 min, and 70℃ for 15 min.
RT-PCR
In order to measure the mRNA expression of glucose transporter 2 (GLUT2), a PCR was performed with cDNA. The primer sequence applied in the experiment is shown in Table 1. In brief, 0.5 µL each of Go Tag Green Master (Promega, Madison, WI, USA) and 10 µL of forward primer (15 µM) and reverse primer (15 µM) were added to a PCR tube, to which 8 µL of nuclease-free water and 1 µL of synthesized first-stand cDNA were then added and thoroughly mixed. The PCR conditions for each primer are shown in Table 2. The PCR product was loaded onto a 1.2% agarose gel containing 0.002% ethidium bromide for electrophoresis and then verified under UV light. Band density was analyzed and quantified by using the software ImageJ (National Institutes of Health, Bethesda, MD, USA).
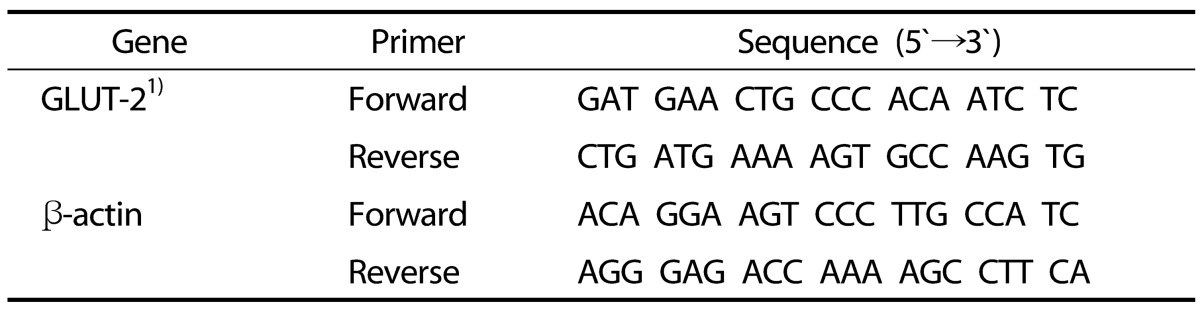
Table 1. PCR primer sequences.
1) GLUT-2, glucose transporter-2.
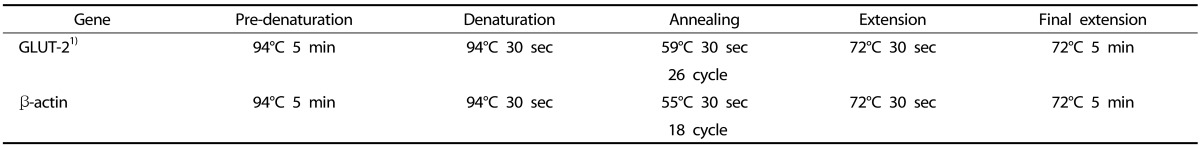
Table 2. PCR condition of each primer.
1) GLUT-2, glucose transporter-2
Western blot analysis
HepG2 cells were added into 6-well plates (1 × 106 cells/mL) and incubated for 24 h. They were then treated with CMW and incubated for another 24 h. Thereafter, the cells were lysed at 4℃ using a lysis buffer (10 mM Tris-HCl, pH 7.4, 0.1 M EDTA, 10 mM NaCl, 0.5% Triton X-100, and protease inhibitor cocktail). The concentration of protein obtained after centrifugation of the cell extract (14,000 r/min, 10 min, 4℃) was measured by using the Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA). The same amount of protein (30 µg) and a sample buffer containing β-mercaptoethanol were mixed at a 1:1 ratio and heated at 100℃ for 3 min. The prepared protein sample was electrophoresed on a sodium dodecyl sulfate-polyacrylamide gel using the Bio-Rad minigel system (Bio-Rad Laboratories), following which the protein was transferred to a polyvinylidene fluoride membrane (0.45 µm; Thermo Scientific, Rockford, IL, USA). The membrane was blocked for 1 h in tris-buffered saline containing 0.1% Tween 20 (TBS-T) and 5% skim milk and then left to react for 1 h in a buffer loaded with hepatocyte nuclear factor-1 alpha (HNF-1α; 5,000:1), glucose transporter 2 (GLUT2; 5,000:1), phosphorylated AMP-activated protein kinase (pAMPK; 1,000:1), phosphoenolpyruvate carboxykinase (PEPCK; 1,000:1), GK (1,000:1), glyceraldehyde-3-phosphate dehydrogenase (5,000:1) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), phosphorylated PDH (pPDH; 10,000:1), PDH (10,000:1) (Abcam, Cambridge, UK), phosphorylated phosphatidylinositol 3-kinase (pPI3K; 1,000:1), PI3K (1,000:1), phosphorylated protein kinase B (pAkt; 1,000:1), Akt (1,000:1), and phosphorylated glycogen synthase kinase-3 beta (pGSK-3β; 1,000:1) (Cell Signaling Technology, Danvers, MA, USA) primary antibodies. The membrane was then rinsed 3 times with the TBS-T buffer, for 5 min each time. This was followed by its immersion in a buffer loaded with horseradish peroxidase-conjugated secondary anti-rabbit IgG antibody (Santa Cruz Biotechnology) for 1 h at room temperature. The membrane was rinsed 3 times with TBS-T (5 min each time) and then photosensitized on an X-ray film using the enhanced chemiluminescence method. The band strength was analyzed and quantified using the software ImageJ.
Measuring glycogen contents
HepG2 cells were cultured with 1 mg/mL of CMW for 24 h. Medium were removed and washed cells with phosphate buffer (PBS). Cell were resuspended in 200 µL of H2O on ice and homogenized by quickly pipetting up and down several times. Cell homogenates were boiled for 10 min to inactivate enzymes and centrifuged for 10 min at 4℃ at 18,000 × g in a cold microcentrifuge to remove insoluble material. A Bradford assay (Bio-Rad Laboratories) was used to quantify the protein concentration. The glycogen content of the processed HepG2 cells was measured and quantified by using a glycogen assay kit (Abcam, Cambridge, UK) following the manufacturer's instructions. Glycerol (50 µL) and the processed samples (50 µL) were added to a 96-well plate, and hydrolysis buffer (50 µL) and hydrolysis enzyme mix (2 µL) were added to all wells as background controls. The plate wells were mixed thoroughly and incubated at room temperature for 30 min followed by the addition of 50 µL of reaction mix (46 µL development buffer, 2 µL development enzyme mix, and 2 µL oxiRed probe) into wells. The plate wells were mixed thoroughly and incubated at room temperature for 30 min and protected from light. Immediately after incubation, a microplate reader (EL808; BioTek, Winooski, VT, USA) set at OD 570 nm was used to measure glycogen content.
α-Glucosidase inhibitory activity
For analysis of the α-glucosidase inhibitory activity of CMW, 200 mg of rat intestinal acetone powder (Sigma, St. Louis, MO, USA) was combined with 4 mL of 50 mM phosphate buffer and sufficiently mixed. The mixture was then ultrasonically ground at 4℃ for 15 min and then centrifuged at 10,000 × g for 30 min at 4℃. The middle layer was used as the α-glucosidase enzyme solution. For the assay, 50 µL of 50 mM phosphate buffer (pH 6.8), 20 µL of 1 U/mL rat α-glucosidase, and 50 µL of CMW were mixed together and left at 37℃ for 5 min. Then, 20 µL of 1 mM p-nitrophenyl-α-D-glucopyranoside were added, and the reaction was carried out at 37℃ for 30 min. The reaction was terminated by the addition of 50 µL of 0.1 M Na2CO3, and the absorbance was measured at 405 nm. The α-glucosidase inhibitor acarbose (Sigma) was used as a control.
Statistical analysis
Data are expressed as mean values ± SD and comparisons were carried out by using Student's unpaired t-test or one-way ANOVA, as appropriate. Mean values were considered significantly different when P < 0.05.
RESULTS
Cytotoxicity measurement
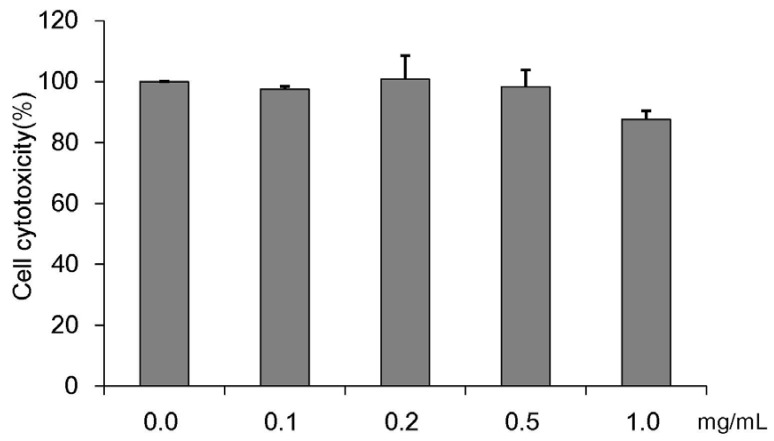
The cell viability measurements determined by using MTT are based on the principle that mitochondria in live cells are able to reduce yellow-colored water-soluble tetrazolium into celadon green-colored non-water-soluble formazan through the dehydrogenase reaction. The cytotoxicity results of CMW tested at concentrations of 0.1, 0.2, 0.5, and 1.0 mg/mL are shown in Fig. 1. No significant apoptosis was observed up to the maximum concentration 1.0 mg/mL. Based on the results, further treatments of HepG2 cells were carried out with 1.0 mg/mL or lower CMW.
Fig. 1. Concentration-dependent effects of Cordyceps Militaris water extract (CMW) on HepG2 cell growth.
Cell viability was analyzed using MTT assay. Data were expressed as mean values ± SD, derived from three independent experiments.
Evaluation of glucokinase activity at various CMW concentrations and treatment times
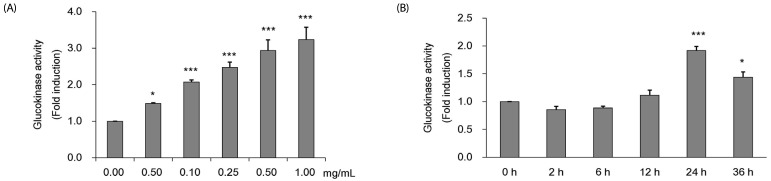
The measured GK activity levels at various CMW concentrations and treatment times are shown in Fig. 2. GK activity increased dose-dependently with CMW concentrations of 0.05, 0.1, 0.25, 0.5, and 1.0 mg/mL (Fig. 2A). Compared with the control (100%), the activity increased by 352% with the 1.0 mg/mL CMW treatment, demonstrating a statistically significant optimum activity increase. The CMW concentration of 1.0 mg/mL was therefore used to treat HepG2 cells for 0, 2, 6, 12, 24, and 36 h. The GK activity from the extracted protein was significantly high at 24 h (192%), and then decreased to 144% at 36 h (Fig. 2B). Based on the test results, it was concluded that the induction condition for optimum GK activity in HepG2 cells was treatment with 1.0 mg/mL CMW for 24 h.
Fig. 2. Measurement of glucokinase (GK) activity of Cordyceps Militaris water extract (CMW) in HepG2 cell.
(A) GK activity of CMW treatment according to concentration dependent manner. (B) GK activity CMW treatment (1.0 mg/mL) on time dependent manner. Data were expressed as mean values ± SD and comparisons of data were carried out using one-way ANOVA, as appropriate. * P < 0.05, *** P < 0.001 compared 0 mg/mL, * P < 0.05, *** P < 0.001 vs. 0h.
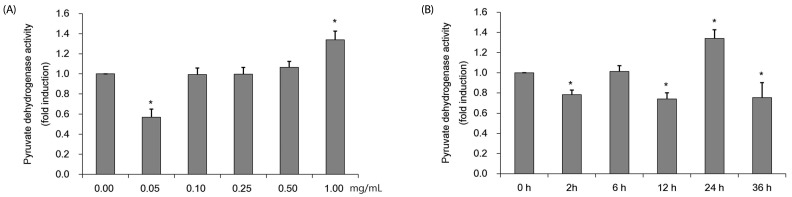
Evaluation of pyruvate dehydrogenase activity at various CMW concentrations and treatment times
The PDH activity was measured by using protein obtained after treating HepG2 cells with various CMW concentrations and durations. Fig. 3A shows the PDH activity in HepG2 cells treated with CMW at concentrations of 0.1, 0.25, 0.5, and 1.0 mg/mL for 24 h. Relative to the control, the PDH activity significantly increased, by approximately 138%, at the maximum CMW concentration of 1.0 mg/mL. The 1.0 mg/mL CMW concentration was therefore used to treat on HepG2 cells for 0, 2, 6, 12, 24, and 36 h, and the protein extracted to determine the PDH activity (Fig. 3B). The optimum PDH activity, a significant increase of approximately 134% over the control level, occurred when treated for 24 h. Based on the test results, the induction condition for optimum PDH activity in HepG2 cells was treatment with 1.0 mg/mL CMW for 24 h.
Fig. 3. Measurement of pyruvate dehydrogenase (PDH) activity of Cordyceps Militaris water extract (CMW) in HepG2 cell.
(A) PDH activity of CMW treatment according to concentration dependent manner. (B) PDH activity CMW treatment (1.0 mg/mL) on time dependent manner. Data were expressed as mean values ± SD and comparisons of data were carried out using one-way ANOVA, as appropriate. * P < 0.05 compared 0 mg/mL, * P < 0.05 vs. 0h.
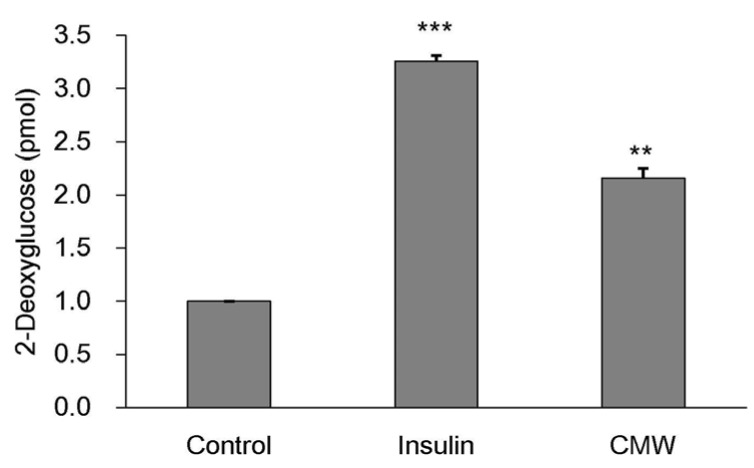
Effect of CMW on glucose uptake by HepG2 cells
To determine the effect of CMW on glucose uptake, we carried out an enzymatic 2DG uptake assay in HepG2 cells. As shown in Fig. 4, glucose uptake in HepG2 cells treated with 1.0 mg/mL CMW for 24 h was significantly increased (168%) over that in the control, but the uptake was lower than that in cells treated with insulin (326%).
Fig. 4. Measurement of glucose uptake and glucose consumption following Cordyceps Militaris water extract (CMW) treatment.
HepG2 cells were incubated for 20 min in a KRPH buffer containing insulin (1uM) and CMW (1 mg/mL). The 2-deoxyglucose (2-DG) assay was performed 20 min later, as detailed in “Methods”. Data were expressed as mean values ± SD and comparisons of data were carried out using one-way ANOVA, as appropriate. ** P < 0.01, *** P < 0.001 vs. control.
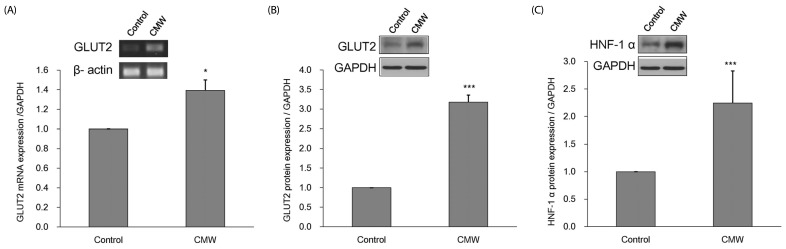
Effect of CMW on GLUT2 expression in HepG2 cells
Changes in GLUT2 expression were analyzed, based on the results showing that CMW treatment induces a glucose influx into HepG2 cells. HepG2 cells were treated with 1.0 mg/mL CMW for 24 h, and the expression levels of GLUT2 mRNA (Fig. 5A) and protein (Fig. 5B) were measured. The GLUT2 mRNA and protein expression levels significantly increased to about 1.39 and 3.18 times, respectively, those of the control. In addition, the expression of HNF-1α protein significantly increased to about 2.24 times that of the control (Fig. 5C).
Fig. 5. Measurement of glucose transporter 2 (GLUT2) mRNA and protein and hepatocyte nuclear factor 1 alpha (HNF-α) protein expression.
(A) Effect of Cordyceps Militaris water extract (CMW) on GLUT2 mRNA expression in HepG2 cells. (B) Effect of CMW treatment on GLUT2 protein expression in HepG2 cells. (C) Effect of CMW treatment on HNF-1α protein expression in HepG2 cells. Data were expressed as mean values ± SD and comparisons of data were carried out using Student's unpaired t-test, as appropriate. * P < 0.05, *** P < 0.001 vs. control.
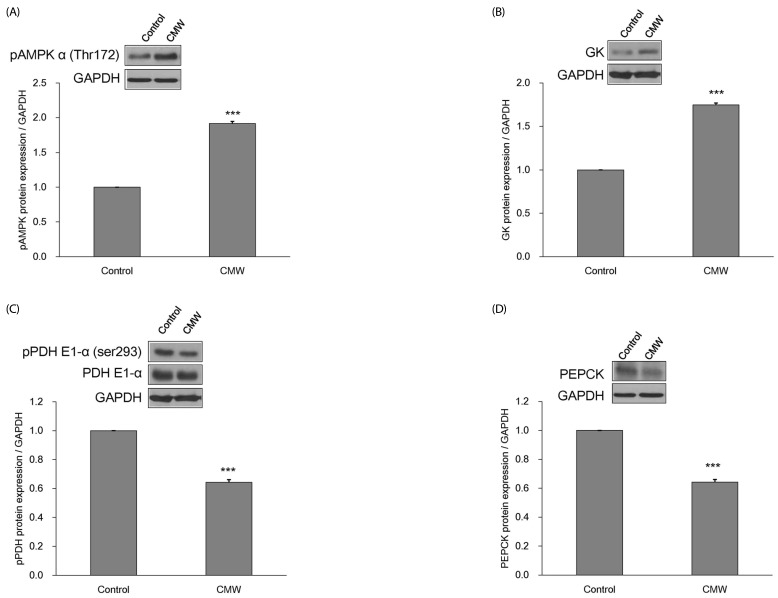
Effect of CMW on glucose metabolism according to AMPK levels in HepG2 cells
The expression levels of pAMPKα (Thr172), GK, pPDH, PDH, and PEPCK proteins were measured in HepG2 cells after treatment with CMW for 24 h (Fig. 6A-6D). Compared with the control, the pAMPKα (Thr172) and GK expression levels significantly increased by about 1.91 and 1.75 times, respectively, whereas the pPDH and PEPCK levels significantly decreased by factors of 0.85 and 0.64, respectively.
Fig. 6. Measurement of glycolysis related enzymes protein expression.
(A) Effect of Cordyceps Militaris water extract (CMW) on phosphorylated AMP-activated protein kinase (pAMPK) protein expression in HepG2 cells. (B) Effect of CMW on glucokinase (GK) protein expression in HepG2 cells. (C) Effect of CMW on phosphorylated pyruvate dehydrogenase (pPDH) protein expression in HepG2 cells. (D) Effect of CMW on phosphoenolpyruvate carboxykinase (PEPCK) protein expression in HepG2 cells. Data were expressed as mean values ± SD and comparisons of data were carried out using Student's unpaired t-test, as appropriate. * P < 0.05, *** P < 0.001 vs. control.
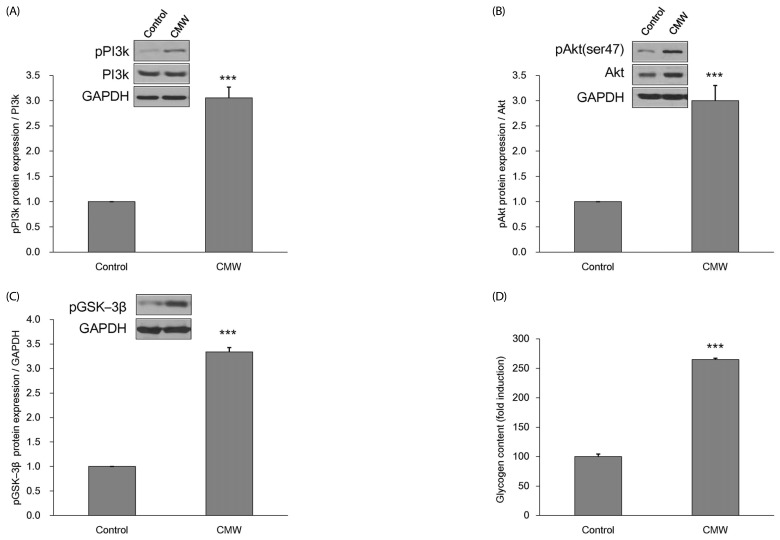
Effect of CMW on glycogen storage in HepG2 cells
The effect of CMW on glycogen conversion within HepG2 cells was examined. The action mechanisms of PI3K, Akt, and GSK-3β were induced by the 24 h CMW treatment (Fig. 7A-C). The expression levels of pPI3k, pAkt, and pGSK-3β proteins were increased by about three times, with statistical significance, compared with the control. Furthermore, the glycogen content within the HepG2 cells had increased by 264.7%, with statistical significance, compared with the control (Fig. 7D).
Fig. 7. Measurement of glucose storage related enzymes protein expression and glycogen contents.
(A) Effect of Cordyceps Militaris water extract (CMW) on phosphorylated phosphatidylinositol 3-kinase (pPI3K) protein expression in HepG2 cells. (B) Effect of CMW on phosphorylated protein kinase B (pAkt) protein expression in HepG2 cells. (C) Effect of CMW on phosphorylated glycogen synthase kinase 3 beta (pGSK-3β) protein expression in HepG2 cells. (D) Effect of CMW treatment on glycogen contents in HepG2 cells. Data were expressed as mean values ± SD and comparisons of data were carried out using Student's unpaired t-test, as appropriate. *** P < 0.001 vs. control.
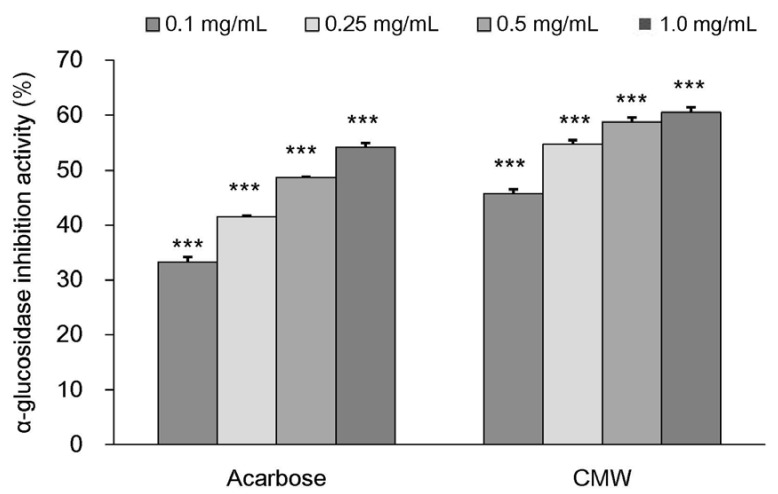
α-Glucosidase inhibitory activity of CMW
The changes in α-glucosidase inhibitory activity of CMW at different concentrations are summarized in Fig. 8. The results showed that, compared to the control the α-glucosidase inhibitory activity of acarbose was 33.27% ± 0.88% with 0.1 mg/mL, 41.5%3 ± 0.21% with 0.25 mg/mL, 48.72% ± 0.060% with 0.5 mg/mL, and 54.21% ± 0.761% with 1.0 mg/mL of acarbose. Compared to the control, the CMW inhibitory activities were 45.77% ± 0.76% with 0.1 mg/mL, 54.76% ± 0.71% with 0.25 mg/mL, 58.74% ± 0.85% with 0.5 mg/mL, and 60.5% ± 0.93% with 1.0 mg/mL of CMW.
Fig. 8. α-Glucosidase inhibitory activity of Cordyceps Militaris water extract (CMW).
Acarbose was used as positive control. Data were expressed as mean values ± SD and comparisons of data were carried out using one-way ANOVA, as appropriate. *** P < 0.001 vs. control.
DISCUSSION
The entomopathogenic fungus C. militaris is reported to have immunosuppressive, anticancer, antioxidative, anti-asthma, and liver function improvement effects, and its application range is expanding [7,8,9,10,11]. This study conducted experiments using CMW in order to investigate the glucose absorption mechanism and glucose metabolism in cells, topics that have not been previously reported. We observed that glucose uptake was induced in CMW-treated HepG2 cells in an insulin-independent manner, and that the expression of several proteins was related to the increase in glucose metabolism. Based on the results, it is expected that CMW will be able to help insulin-independent diabetic patients.
The activities of GK and PDH, the major enzymes for glucose metabolism in HepG2 cells, were measured in order to identify the optimum treatment time and concentration of CMW to use in various glucose metabolism-related experiments. The results showed that 1.0 mg/mL CMW with a 24 h treatment time was the optimum condition for the induction of an optimal glucose metabolic activity level. GK is an important enzyme involved in glucose metabolism, and it is reported to enhance the speed of glucose absorption [28,33]. PDH is an enzyme related to energy generation from glucose and is reported to stimulate the conversion of pyruvate into acetyl-CoA to initiate the TCA cycle [34,35].
Based on the evaluation of CMW-induced glucose uptake, GLUT2 (a major glucose transporter for liver) mRNA and protein expressions were evaluated to determine how glucose absorption into cells takes place. Among the GLUT family genes expressed in human liver tissues, it is reported that GLUT2 expression accounts for the greatest amount (67.4%) of the total expression of all GLUTs [36], indicating that GLUT2 plays a vital role in the maintenance of glucose homeostasis in the liver. In addition, the protein expression of HNF-1α, a transcription factors reported to induce human GLUT2 expression by binding with its promoter [37], was examined. The results showed the expression levels of GLUT2 mRNA and protein, as well as HNF-1α protein, to be increased by CMW, as shown in Fig. 5. This is similar to the results of an experiment reported by Kang et al. [38], in which GLUT2 expression was increased by inducing HNF-1α expression in HepG2 cells using Chinese catbrier leaf extract.
In order to clearly verify the expression control mechanism in liver cells, it is necessary to further investigate the CMW-induced expression of HNF-4α, HNF-3β (FOXA2), sterol-regulatory-element-binding protein 1c, CCAAT element binding protein (C/EBP), and C/EBPβ (all known to be transcription factors acting on GLUT2 promoters) and conduct promoter binding assays. In this study, only the possibility for GLUT2 expression regulation through CMW was investigated, whereas the results of increased GLUT2 expression by HNF-1α were not studied as they are predictable based on the results of various other studies [37,38,39,40,41].
AMPK is a serine/threonine kinase that is reported to be a modulator of lipid and glucose metabolism and to perform crucial regulation activities in diabetes and obesity [24,42,43,44]. AMPK influences various signaling mechanisms in liver cells. In addition, it can detect lowering of the cellular energetic state, thus functioning when energy is required as a result of glucose depletion in cells or when excess energy is supplied externally [45]. Moreover, AMPK regulates the expression of gluconeogenic genes and glucose reproduction, and it increases glucose metabolism in order to maintain glucose homeostasis in the liver [24]. Various reports on AMPK activators for glucose homeostasis are available [24,25,26].
GK, a critical enzyme primarily involved in glucose metabolism, regulates the utilization rate of absorbed glucose. GK has been reported to be associated with AMPK [46]. PDH is mainly regulated by a reversible phosphorylation mechanism, in which the enzyme activity is inhibited upon phosphorylation, thus converting PDH into an inactive form [47]. Conversely, dephosphorylation will convert PDH into an active form [47]. PDH is a critical enzyme that converts pyruvate into acetyl-CoA within mitochondria to generate energy in the TCA cycle [48]. Phosphoenolpyruvate carboxykinase (PEPCK) is a critical enzyme involved in the initiation of glyconeogenesis, in which oxaloacetate is converted into phosphoenolpyruvate (PEP) [49]. Numerous studies have reported that activation of AMPK by natural materials in hepatocytes inhibits PEPCK, thereby inhibiting intracellular glucose production and resulting in antidiabetic effects [19,50,51].
CMW was observed to increase the protein expression of phosphorylated AMPK and GK, control the phosphorylation of PDH, and activate glycolysis. Moreover, it was observed to control glucose reproduction through the decreased protein expression of PEPCK, which is a major factor in gluconeogenesis.
Based on the results showing that glycogen content was induced by CMW treatment, we examined the expression of PI3K, Akt, and pGSK-3β (all proteins related to the mechanism of GS activation) in order to determine how glucose is stored in the form of glycogen. PI3k stimulates Akt, which phosphorylates and activates glycogen synthase kinase 3β (GSK3β), which, in turn, increases GS activity, thereby inducing glycogen storage [31]. GS is reported to be directly affected by GSK-3, AMPK, Akt, and casein kinase 2 (CK2) [32]. Moreover, it is activated by insulin, growth factor, and GPCR. It has been reported that GSK-3β is phosphorylated by activated Akt and, in turn, activates GS [52]. Akt activation activates GK and quickly converts glucose into glucose-6-phosphate. This, in turn, stimulates glycolysis and glycogen storage and has a key role in maintaining glucose homeostasis by controlling glyconeogenesis [53,54]. Furthermore, it has been reported that when glucose is supplied to liver cells, the glucose can be converted into glycogen for storage in an insulin-independent situation [30]. This explains why CMW-stimulated glucose absorption and metabolism occurred in a condition lacking insulin.
The enzyme α-glucosidase is present in the chorion of the mucous membrane of the small intestine. It is essential for the digestion and absorption of carbohydrates, in which polysaccharides are decomposed into monosaccharides. Acarbose, used in this study as a positive control drug, delays the decomposition of disaccharides into monosaccharides in the small intestine [55,56], and is used to improve the symptoms of diabetic patients. When acarbose activity decreases, the decomposition and absorption of polysaccharides are delayed, preventing a marked surge in blood sugar level after a meal [57]. As CMW demonstrates a higher level of α-glucosidase inhibitory activity than acarbose, it represents a functional substance that can be further developed as an acarbose supplement or alternative after additional investigation of its activities and safety [58].
Based on the above results, we conclude that for its induction of glucose uptake in liver cells, CMW stimulates the expression of HNF-1α to activate GLUT2. The glucose supplied into cells is metabolized through glycolysis stimulation, following CMW-induced AMPK phosphorylation and gluconeogenesis inhibition activities. It then produces energy or converted into glycogen for storage. This implies that CMW is a potential candidate material for use in the control of blood sugar levels, as it stimulates the absorption and metabolism of glucose in liver cells. Further experiments into the effects of CMW on the insulin-independent signaling mechanism, along with studies using an experiment model with induced insulin resistance, will determine the value of this fungal extract as a hypoglycemic functional material.
Footnotes
This research was supported by the Basic Science Research Program through the Well-being Bioproducts R&D Regional Innovation Center project (B0009702).
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.Rotshteyn Y, Zito SW. Application of modified in vitro screening procedure for identifying herbals possessing sulfonylurea-like activity. J Ethnopharmacol. 2004;93:337–344. doi: 10.1016/j.jep.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Lee SH, Lee JK, Kim IH. Trends and perspectives in the development of antidiabetic drugs for type 2 diabetes mellitus. Korean J Microbiol Biotechnol. 2012;40:180–185. [Google Scholar]
- 4.Sawada K, Yamashita Y, Zhang T, Nakagawa K, Ashida H. Glabridin induces glucose uptake via the AMP-activated protein kinase pathway in muscle cells. Mol Cell Endocrinol. 2014;393:99–108. doi: 10.1016/j.mce.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Yuan HD, Kim SJ, Quan HY, Huang H, Chung SH. Ginseng leaf extract prevents high fat diet-induced hyperglycemia and hyperlipidemia through AMPK activation. J Ginseng Res. 2010;34:369–375. [Google Scholar]
- 6.Cory JG, Suhadolnik RJ, Resnick B, Rich MA. Incorporation of cordycepin (3′-deoxyadenosine) into ribonucleic acid and deoxyribonucleic acid of human tumor cells. Biochim Biophys Acta. 1965;103:646–653. doi: 10.1016/0005-2787(65)90085-7. [DOI] [PubMed] [Google Scholar]
- 7.Kwon YM, Cho SM, Kim JH, Lee JH, Lee YA, Lee SJ, Lee MW. Hypoglycemic effects of Cordyceps militaris. Korean J Pharmacogn. 2001;32:327–329. [Google Scholar]
- 8.Koh JB, Choi MA. Effect of Cordyceps militaris on lipid metabolism in rats fed cholesterol diet. Korean J Nutr. 2001;34:265–270. [Google Scholar]
- 9.Jo WS, Nam BH, Oh SJ, Choi YJ, Kang EY, Hong SH, Lee SH, Jeong MH. Hepatic protective effect and single-dose toxicity study of water extract of Cordyceps militaris grown upon protaetia dreujtarsis. Korean J Food Sci Technol. 2008;40:106–110. [Google Scholar]
- 10.Kim HS, Roh YJ, Choe M. Cordyceps militaris increases hepatic glucokinase activities. J Korean Soc Food Sci Nutr. 2005;34:158–161. [Google Scholar]
- 11.Kiho T, Hui J, Yamane A, Ukai S. Polysaccharides in fungi. XXXII. Hypoglycemic activity and chemical properties of a polysaccharide from the cultural mycelium of Cordyceps sinensis. Biol Pharm Bull. 1993;16:1291–1293. doi: 10.1248/bpb.16.1291. [DOI] [PubMed] [Google Scholar]
- 12.Choe M, Kim DJ, Lee HJ, You JK, Seo DJ, Lee JH, Chung MJ. A study on the glucose-regulating enzymes and antioxidant activities of water extracts from medicinal herbs. J Korean Soc Food Sci Nutr. 2008;37:542–547. [Google Scholar]
- 13.Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr. 2012;3:286–294. doi: 10.3945/an.112.002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48:1198–1214. doi: 10.2337/diabetes.48.5.1198. [DOI] [PubMed] [Google Scholar]
- 15.Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121–138. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu T, Parker JC, Najafi H, Matschinsky FM. Control of glucose metabolism in pancreatic β-cells by glucokinase, hexokinase and phosphofructokinase; model study with cell lines derived from β-cells. Diabetes. 1988;37:1524–1530. doi: 10.2337/diab.37.11.1524. [DOI] [PubMed] [Google Scholar]
- 17.Matschinsky FM. Glucokinase as glucose sensor and metabolic signal generator in pancreatic β-cells and hepatocytes. Diabetes. 1990;39:647–652. doi: 10.2337/diab.39.6.647. [DOI] [PubMed] [Google Scholar]
- 18.Seoane J, Gómez-Foix AM, O'Doherty RM, Gómez-Ara C, Newgard CB, Guinovart JJ. Glucose 6-phosphate produced by glucokinase, but not hexokinase I, promotes the activation of hepatic glycogen synthase. J Biol Chem. 1996;271:23756–23760. doi: 10.1074/jbc.271.39.23756. [DOI] [PubMed] [Google Scholar]
- 19.Cordero-Herrera I, Martín MA, Bravo L, Goya L, Ramos S. Cocoa flavonoids improve insulin signalling and modulate glucose production via AKT and AMPK in HepG2 cells. Mol Nutr Food Res. 2013;57:974–985. doi: 10.1002/mnfr.201200500. [DOI] [PubMed] [Google Scholar]
- 20.Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annu Rev Nutr. 1999;19:379–406. doi: 10.1146/annurev.nutr.19.1.379. [DOI] [PubMed] [Google Scholar]
- 21.Cerf ME. High fat diet modulation of glucose sensing in the beta-cell. Med Sci Monit. 2007;13:RA12–RA17. [PubMed] [Google Scholar]
- 22.Corton JM, Gillespie JG, Hardie DG. Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol. 1994;4:315–324. doi: 10.1016/s0960-9822(00)00070-1. [DOI] [PubMed] [Google Scholar]
- 23.Winder WW, Thomson DM. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem Biophys. 2007;47:332–347. doi: 10.1007/s12013-007-0008-7. [DOI] [PubMed] [Google Scholar]
- 24.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9:407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Lee ES, Uhm KO, Lee YM, Han M, Lee M, Park JM, Suh PG, Park SH, Kim HS. CAPE (caffeic acid phenethyl ester) stimulates glucose uptake through AMPK (AMP-activated protein kinase) activation in skeletal muscle cells. Biochem Biophys Res Commun. 2007;361:854–858. doi: 10.1016/j.bbrc.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 26.Lin CL, Lin JK. Epigallocatechin gallate (EGCG) attenuates high glucose-induced insulin signaling blockade in human HepG2 hematoma cells. Mol Nutr Food Res. 2008;52:930–939. doi: 10.1002/mnfr.200700437. [DOI] [PubMed] [Google Scholar]
- 27.Ha T, Trung TN, Hien TT, Dao TT, Yim N, Ngoc TM, Oh WK, Bae K. Selected compounds derived from Moutan Cortex stimulated glucose uptake and glycogen synthesis via AMPK activation in human HepG2 cells. J Ethnopharmacol. 2010;131:417–424. doi: 10.1016/j.jep.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 29.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 30.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 31.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Narwal S, Kumar V, Prakash O. α-glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn Rev. 2011;5:19–29. doi: 10.4103/0973-7847.79096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang X, Li J, Xie X, Huang K, Huag H. Effect of TangMaijiaoTai on regulating the glycolipid metabolism and the production of glucokinase and low density lipoprotein receptor protein in diabetic rats with hyperlipidemia. Chin Pharmacol Bull. 2013;29:234–237. [Google Scholar]
- 34.Ferriero R, Brunetti-Pierri N. Phenylbutyrate increases activity of pyruvate dehydrogenase complex. Oncotarget. 2013;4:804–805. doi: 10.18632/oncotarget.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arjunan P, Nemeria N, Brunskill A, Chandrasekhar K, Sax M, Yan Y, Jordan F, Guest JR, Furey W. Structure of the pyruvate dehydrogenase multienzyme complex E1 component from Escherichia coli at 1.85 A resolution. Biochemistry. 2002;41:5213–5221. doi: 10.1021/bi0118557. [DOI] [PubMed] [Google Scholar]
- 36.Johnson JH, Newgard CB, Milburn JL, Lodish HF, Thorens B. The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J Biol Chem. 1990;265:6548–6551. [PubMed] [Google Scholar]
- 37.Cha JY, Kim H, Kim KS, Hur MW, Ahn Y. Identification of transacting factors responsible for the tissue-specific expression of human glucose transporter type 2 isoform gene. Cooperative role of hepatocyte nuclear factors 1alpha and 3beta. J Biol Chem. 2000;275:18358–18365. doi: 10.1074/jbc.M909536199. [DOI] [PubMed] [Google Scholar]
- 38.Kang YH, Lee YS, Kim KK, Kim DJ, Kim TW, Choe M. Study on antioxidative, antidiabetic and antiobesity activity of solvent fractions of Smilax china L. leaf extract. J Nutr Health. 2013;46:401–409. [Google Scholar]
- 39.Narasimhan A, Chinnaiyan M, Karundevi B. Ferulic acid regulates hepatic GLUT2 gene expression in high fat and fructose-induced type-2 diabetic adult male rat. Eur J Pharmacol. 2015;761:391–397. doi: 10.1016/j.ejphar.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 40.Im SS, Kang SY, Kim SY, Kim HI, Kim JW, Kim KS, Ahn YH. Glucose-stimulated upregulation of GLUT2 gene is mediated by sterol response element-binding protein-1c in the hepatocytes. Diabetes. 2005;54:1684–1691. doi: 10.2337/diabetes.54.6.1684. [DOI] [PubMed] [Google Scholar]
- 41.Matsui C, Shoji I, Kaneda S, Sianipar IR, Deng L, Hotta H. Hepatitis C virus infection suppresses GLUT2 gene expression via downregulation of hepatocyte nuclear factor 1α. J Virol. 2012;86:12903–12911. doi: 10.1128/JVI.01418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 43.Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Phielix E, Szendroedi J, Roden M. The role of metformin and thiazolidinediones in the regulation of hepatic glucose metabolism and its clinical impact. Trends Pharmacol Sci. 2011;32:607–616. doi: 10.1016/j.tips.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 47.Berg JM, Tymoczko JL, Stryer L, editors. Biochemistry. 5th ed. New York (NY): W H Freeman; 2002. The glycolytic pathway is tightly controlled; pp. 668–676. [Google Scholar]
- 48.Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans. 2003;31:1143–1151. doi: 10.1042/bst0311143. [DOI] [PubMed] [Google Scholar]
- 49.Cordero-Herrera I, Martín MÁ, Goya L, Ramos S. Cocoa flavonoids attenuate high glucose-induced insulin signalling blockade and modulate glucose uptake and production in human HepG2 cells. Food Chem Toxicol. 2014;64:10–19. doi: 10.1016/j.fct.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem. 2002;277:34933–34940. doi: 10.1074/jbc.M204672200. [DOI] [PubMed] [Google Scholar]
- 51.Kim T, Davis J, Zhang AJ, He X, Mathews ST. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem Biophys Res Commun. 2009;388:377–382. doi: 10.1016/j.bbrc.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 52.Lee J, Kim MS. The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Res Clin Pract. 2007;77(Suppl 1):S49–S57. doi: 10.1016/j.diabres.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 53.Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F, Terracciano L, Heim MH, Rüegg MA, Hall MN. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 2012;15:725–738. doi: 10.1016/j.cmet.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 54.Hao J, Chen C, Huang K, Huang J, Li J, Liu P, Huang H. Polydatin improves glucose and lipid metabolism in experimental diabetes through activating the Akt signaling pathway. Eur J Pharmacol. 2014;745:152–165. doi: 10.1016/j.ejphar.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 55.Mertes G. Efficacy and safety of acarbose in the treatment of type 2 diabetes: data from a 2-year surveillance study. Diabetes Res Clin Pract. 1998;40:63–70. doi: 10.1016/s0168-8227(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 56.Rosak C, Mertes G. Effects of acarbose on proinsulin and insulin secretion and their potential significance for the intermediary metabolism and cardiovascular system. Curr Diabetes Rev. 2009;5:157–164. doi: 10.2174/157339909788920910. [DOI] [PubMed] [Google Scholar]
- 57.Ahn H, Chung L, Choe E. In vitro antioxidant activity and α-glucosidase and pancreatic lipase inhibitory activities of several Korean sanchae. Korean J Food Sci Technol. 2015;47:164–169. [Google Scholar]
- 58.Choi JH, Park YH, Lee SG, Lee SH, Yu MH, Lee MS, Park SH, Lee IS, Kim HJ. Antioxidant activities and α-glucosidase inhibition effects of chicories grown in hydroponics added with Cr3+ or selenium. J Food Hyg Saf. 2014;29:53–59. [Google Scholar]